Abstract
The Daily Cognitive Communicative and Sleep Profile (DCCASP) is a seven‐item instrument that captures daily subjective sleep quality, perceived mood, cognitive, and communication functions. The objective of this study was to evaluate the reliability and validity of the DCCASP. The DCCASP was self‐administered daily to a convenience sample of young adults (n = 54) for two two‐week blocks, interspersed with a two‐week rest period. Afterwards, participants completed the Pittsburgh Sleep Quality Index (PSQI). Internal consistency and criterion validity were calculated by Cronbach's α coefficient, Concordance Correlation Coefficient (CCC), and Spearman rank (rs) correlation coefficient, respectively. Results indicated high internal consistency (Cronbach‐s α = 0.864−0.938) among mean ratings of sleep quality on the DCCASP. There were significant correlations between mean ratings of sleep quality and all domains (rs=0.38–0.55, p<0.0001). Criterion validity was established between mean sleep quality ratings on the DCCASP and PSQI (rs=0.40, p<0.001). The DCCASP is a reliable and valid self‐report instrument to monitor daily sleep quality and perceived mood, cognitive, and communication functions over time, amongst a normative sample of young adults. Further studies on its psychometric properties are necessary to clarify its utility in a clinical population. Copyright © 2014 John Wiley & Sons, Ltd.
Keywords: cognitive function, communication, mood, sleep quality, validity
Introduction
Sleep is a complex, vital, and active process that serves a critical function in maintaining health in all humans (Rosenthal, 1998). Sleep plays an essential role in human growth and development, neuroplasticity, immune and endocrine function, and modulation of emotion and mood and aspects of cognition (Meerlo et al., 2006; Touchette et al., 2007; Walker et al., 2005; Wiseman‐Hakes et al., 2009). Sleep is also critical for neurogenesis and recovery from injury (Meerlo et al., 2006, 2009). From birth, humans spend one‐third of their lives asleep (Aldrich, 1999). The importance of sleep is generally not given much thought until it creates a problem for the individual and compromises lifestyle and daily productivity. Sleep problems are present worldwide, with the prevalence of sleep problems reported to be 56% in the United States, 31% in Western Europe, and 23% in Japan (Léger et al., 2008). When sleep is disrupted, it negatively affects physiology, cognition, memory, learning, concentration, behaviour, and overall ability to function (Fulda and Schulz, 2001; Hauri, 1997). Thus, sleep is essential for normal, healthy human functioning across the lifespan.
The International Classification of Sleep Disorders (American Academy of Sleep Medicine (AASM), 2005) lists 84 sleep disorders under eight major categories, including: (1) insomnias; (2) sleep‐related breathing disorders; (3) hypersomnia not due to breathing disorders, (4) circadian rhythm sleep disorders; (5) parasomnias; (6) sleep‐related movement disorders; (7) other sleep disorders; and (8) isolated symptoms, apparently due to adverse effect of drugs, medications and biological substances.
Furthermore, sleep and wake disorders (SWDs) such as insomnia, excessive daytime sleepiness, hypersomnia, and fatigue are common among clinical populations. For example, those with certain neurological and physical impairments, including traumatic brain injury (TBI), Parkinson's disease, fibromyalgia, and rheumatoid arthritis often experience sleep problems throughout various stages of recovery (Green, 2008). Disturbed sleep patterns are also commonly associated with attention deficit hyperactivity disorder and dementia (Stein et al., 2002; Vitiello and Borson, 2001). Cancer patients are also at great risk for developing insomnia and disorders of the sleep–wake cycle (Vena et al., 2004). Disturbed sleep, due to any of the 84 different sleep disorders, can exacerbate other difficulties associated with neurological and physical impairments such as increased pain, reduced participation in therapy, difficulties with new learning, and can negatively affect behavioural, emotional, and social functioning (Beetar et al., 1996; Cantor et al., 2008; Zafonte et al., 1996). Thus, it is clear that SWDs can impede normal physical, cognitive, behavioural and emotional functioning (Cantor et al., 2008).
Cognitive processes, such as sustained attention, concentration, memory, language and information processing are necessary to support successful communication and social interaction (MacDonald and Wiseman‐Hakes, 2010). Sleep–wake disturbances have been shown to negatively impact already‐impaired cognitive and communication abilities post‐TBI, which negatively influence productivity, social integration, emotional functioning, and quality of life (Struchen et al., 2008). Bloomfield et al. (2010) concluded that post‐TBI individuals who reported poor sleep quality demonstrated significantly poorer sustained attention than those who reported good quality of sleep. In addition, disruptions in cognitive processes such as attention, concentration, memory, reasoning, and language processing can affect communication processes, such as the ability to attend to and understand conversation (MacDonald and Wiseman‐Hakes, 2010). Walker (2009) concluded that adequate sleep is critical for new learning or memory encoding, consolidation of declarative memory, and emotional and affective regulation in healthy populations. Together, deficits in cognitive areas due to poor sleep quality and quantity have significant impacts on social interactions, community independence, family interactions, learning and academic performance (Struchen et al., 2008), and vocational re‐entry (MacDonald and Wiseman‐Hakes, 2010). Thus, a brief, reliable, and valid subjective sleep instrument is required to identify SWDs and sleep quality in relation to specific cognitive and communication functions in adults.
Currently, there are a number of objective and subjective assessments designed to measure sleep; however, no gold standard for subjective assessments exists. Objective methods such as the polysomnogram, actigraphy, the Multiple Sleep Latency Test, and the Multiple Wake Test have been used to evaluate the architecture, quantity, quality, and timing of sleep and day‐time sleepiness (Kotagal and Goulding, 1996). Since sleep quality varies from one individual to another, subjective reports that track sleep quality and daytime functioning, such as sleep diaries and logs, provide a more accurate measure of individual distress in response to sleep disturbances (Parcell et al., 2006). Currently, there are several subjective sleep questionnaires available to screen for SWDs in the healthy and clinical populations, including the Epworth Sleepiness Scale (ESS; Johns, 1991), the Insomnia Severity Index (ISI; Bastien et al., 2001), and the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1988). All of these measures are widely used in various settings; however, they are general in nature, and none identify a specific relationship between sleep and functional aspects of cognition, communication, or mood.
The PSQI is one measure that has been validated for use with individuals with TBI (Fulda and Schulz, 2001). The PSQI is a self‐administered tool used to measure the construct of “sleep quality” including sleep variables such as sleep efficiency, latency, and duration (Buysse et al., 1988). The PSQI measures subjective sleep quality and disturbances retrospectively over a one‐month interval (Buysse et al., 1988). However, as sleep is a dynamic process, the quality of sleep can change on a daily basis (Totterdell et al., 1994), which would not be captured by the PSQI. The ESS and ISI have similar limitations. In addition, individuals with memory impairments, typical of many neurologcical disorders and impairments such as TBI, may have difficulties recalling accurate and valid information about subjective sleep quality over a long interval. Also, as stated earlier, the PSQI, ESS, and ISI do not address specific mood, cognitive, and communication issues that may be related to altered sleep; however, they do capture general changes in daytime functioning that may accompany and be associated with disrupted sleep. Given these limitations, researchers have begun to develop their own measures to examine sleep and function (e.g. Beetar et al., 1996; Chaput et al., 2009). As such, there is no single sleep instrument devoted to measuring subjective sleep quality in relation to specific daily functions.
The Daily Cognitive–Communication and Sleep Profile (DCCASP; Fung et al., 2011) was developed to address the need for a valid and reliable tool to measure daily fluctuations in subjective sleep quality and cognitive and communication functions, as well as mood, in research and clinical practices. The DCCASP is available both in English and French, and is a brief instrument that is completed by the client, therapist, or significant other who has observed the client function throughout the day. Formal training is not required and a user's manual is available with the measure.
The DCCASP has several advantages over current sleep instruments in research and clinical settings. Firstly, no other sleep measure has been psychometrically validated to monitor both self‐perceived sleep quality and self‐reported daily functions (Carpenter et al., 2007; Pilcher and Ott, 1998). Secondly, studies that have investigated the relationship between sleep quality and mood, fatigue, and concentration often use multiple measures to obtain the required information (e.g. Alapin et al., 2000; Brown et al., 2002). Using research on university students as the sample population, Pilcher and Ott (1998) administered the PSQI and Profile of Mood States (POMS; McNair et al., 1971) separately to determine the correlation between qualities of sleep from the PSQI with depression and fatigue domains from the POMS. This procedure can be tedious and time‐consuming for the individual being evaluated and for researchers and clinicians who administer the assessments. With the introduction of the DCCASP, information regarding the relationship between sleep quality and cognitive and communication functions can be easily tracked and monitored within one measure. The addition of the DCCASP in research will be advantageous, especially with researchers and participants having many priorities and busy daily schedules. For clinicians, one measure that reliably and validly captures relevant information about clients’ self‐reported sleep quality and cognitive communication functions will allow clinicians to be more time‐efficient during heavy caseloads, and minimize the number of assessments that clients need to complete.
Thirdly, the DCCASP can inform clinicians of the need to refer clients for further assessment and/or specialized treatment. The DCCASP can be used as a screening tool by clinicians and family members to identify those with sleeping disturbances and difficulty functioning throughout the day. Once identified, those with sleeping difficulties could be referred to specialists in SWDs. Finally, the DCCASP can be used to examine the longitudinal effects of specific therapeutic interventions for various diagnoses. For instance, the DCCASP was used clinically to longitudinally document functional improvements for an individual with severe TBI throughout difference phases of medication regime (Wiseman‐Hakes et al., 2011).
Utilized as a measure in a pilot study during its early developmental stages, the DCCASP was found to be clinically and statistically sensitive to subtle changes in sleep quality and cognitive and communication function for a young male client with post‐severe TBI (Wiseman‐Hakes et al., 2011). Although the measure was demonstrated to be sensitive to subtle changes in sleep and function, the DCCASP has not been validated for use in any populations. Thus, the aim of this study was to examine the psychometric properties of the DCCASP in a non‐clinical sample of young adults. The purpose of this study was to evaluate the reliability (internal consistency) and validity (criterion validity) of the DCCASP in young adults between the ages of 18 and 30 years.
Methods
Participants
Fifty‐nine university students were recruited for this study using convenience sampling via the Psychology Experiment database and via email to students from Rehabilitation Sciences at the University of Toronto. Participants were included in this study if they met the following criteria: aged between 18 and 30; were fluent in written and spoken English; had no medically diagnosed sleep disorder; had no history or current neurological impairment (e.g. TBI; four participants were included who reported a history of a previous concussion but did not report experiencing any current symptoms); and were not taking psychoaffective, sedative drugs, or other sleeping medications. Once the participants were recruited, written informed consent was obtained from all participants prior to the beginning of the experiment. All participants were provided verbal and written instructions on the study procedure and how to complete the sleep assessments. All participants reported they fully understood the questions presented on the tool. Ethical approval was received by the University of Toronto, Research Ethics Board prior to the onset of the study. All participants were compensated with a gift certificate for a local chain of coffee shop at the end of the study.
Five participants were omitted from data analyses – three participants withdrew from the study and data was missing from two participants. The remaining sample comprised of 54 participants, which included 45 females and nine males. The mean age of participants was 21.9 ± 3.2 years. Of these participants, 23 students were in the undergraduate level, and 31 students were in the master level. Participants had an average of 6.39 ± 1.27 hours of sleep per night, with hours of sleep ranging from three to nine hours per night (Table 1).
Table 1.
Hours of sleep per night for undergraduate and master male and female university students
| n (54) | Mean | Median | Standard deviation | Range | |
|---|---|---|---|---|---|
| Undergraduate level | 23 | 6.52 | 6.50 | 1.52 | 3–9 |
| Masters level | 31 | 6.30 | 6.50 | 1.05 | 3–8.5 |
| Combined sample | 54 | 6.39 | 6.50 | 1.27 | 3–9 |
| Females | 45 | 6.43 | 6.50 | 1.30 | 3–9 |
| Males | 9 | 6.22 | 6.00 | 1.12 | 4.5–8 |
Measurement
The present study employed two assessment tools: the DCCASP and the PSQI. The DCCASP is graded with a seven‐point Likert scale, with a rating of 1 being the worst function and 7 being the best function, across the following seven domains of sleep, cognitive, communication functioning and mood: (1) sustained attention/vigilance/executive attention to spoken or written communication, (2) verbal memory (retention of spoken or written information), (3) speed of language processing and learning of new verbally mediated information, (4) sleep quality, (5) level of fatigue, (6) daytime sleepiness, and (7) mood (Table 2).
Table 2.
The seven domains and seven items of the Daily Cognitive–Communication and Sleep Profile (DCCASP) (Wiseman‐Hakes et al., 2011)
| Item Rating | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Sleep quality | How was your sleep quality for last night? | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Worst possible sleep | Best possible sleep | ||||||
| Mood | How was your mood for today? | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Overall anxious, irritable, unhappy, restless, and easily annoyed, overwhelmed by my day | Overall calm, reasonably content, able to handle what the day threw at me. | I felt great today | |||||
| Fatigue | How tired did you feel today? | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| I felt really tired, low energy, unable to do my daily tasks | Fair level of energy, able to do daily tasks with minimal difficulty | I had lots of energy today | |||||
| Daytime wakefulness | Did you fall asleep during the day today? | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| I fell asleep even though I didn't want to. I may not have been in my bed, but couldn't keep my eyes open | I felt sleepy and fell asleep even though I didn't do anything too tiring | I felt sleepy and had to lie down and rest during the day after activities | I had a nap today because I could, but could have managed without | I felt sleepy but was able to stay awake | I did not feel sleepy other than usual dips after lunch and late afternoon Was able to stay awake all day | I was completely alert throughout the day | |
| Attention | Rate your attentiveness and concentration for today? | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| I couldn't concentrate today (following a conversation, reading watching television, couldn't block out things that distracted me, (e.g. pain, thoughts, ringing in the ears, background noises, conversations) | Really hard to pay attention. I kept focused on 1 thing briefly but got distracted | Hard to pay attention but I can follow tasks and get back on track if distracted. May need help to get refocused Can only do one thing at a time | Some difficulty paying attention and following task(s). I occasionally have to get back on track if distracted, or do one thing at a time | Fairly easy to block out distractions, I can pay attention for moderate periods of time (e.g. long enough to complete my daily tasks) | Able to pay attention to more than one thing at a time for a period of time, or to focus for a period of time | No difficulties paying attention today even for long periods of time or for multi‐tasking | |
| Memory | Rate your memory abilities for today? | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Can't remember activities, events, or conversations from today | If reminded, can remember the gist of some activities, events, or conversations from today | If reminded, can remember details of activities, events or conversation | With no reminders can remember the gist of some activities, events or conversations from today | Can remember several details of activities, events or conversations from today | Can remember almost all the details of activities events or conversations from today | My memory was just fine today | |
| Language processing | Rate your communication and conversation abilities for today? | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Was unable to carry on a conversation today. My conversation partner must always speak slower and simplify the topic | Can carry on a conversation with one person, but need extra time to think and respond. At times conversation partner must speak slower and simplify the topic | Can carry on a conversation. Helps when conversation partner speaks slower. Can carry on multispeaker conversations briefly if topic stays the same, but I need extra time to think and respond | Can carry on a conversation. Partner doesn't need to speak slower. Some difficulty following and responding to multi‐speaker conversations, but I can do it | Can keep up with and reply to a multi person or single person conversation with minimal difficulty | No difficulties following any conversations, meetings, phone‐calls even with multiple speakers | ||
The PSQI consists of 19 items, providing a global score and seven subscores on sleep quality, sleep onset latency, sleep duration, sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The first four items require participants to input their usual bedtimes, wake times, sleep latency, and sleep duration. The remaining items ask participants whether they experience certain symptoms over the past month and are graded using a four‐point Likert scale.
Data collection
A demographics survey was administered at the beginning of the study to obtain information about age, sex, and level of current education. The duration of the study for each participant was three two‐week blocks (block 1, rest period, and block 2), for a total of six weeks. Participants completed the DCCASP daily for the first two‐week block (block 1), followed by a two‐week rest period. The DCCASP was then administered for another two weeks (block 2). The PSQI was administered upon the completion of the DCCASP in block 2. An electronic version of both the DCCASP and PSQI was emailed to participants to complete at home. A blank DCCASP form was emailed to participants at the beginning of each week to capture their daily ratings. Participants were instructed to complete the DCCASP each evening before they went to bed to rate their sleep quality the night before and functioning during the current day. Participants were offered to be emailed daily to remind them to complete the tool prior to the start of the study. Some participants did not opt for the daily reminders as they reported they would remember to complete it each day. At the end of each week, participants emailed their completed DCCASP forms back to the researcher. At the end of block 2, the PSQI was emailed to the participants to report their characteristics of sleep over the past month and participants were asked to email the assessment back within one week.
Data analysis
Internal consistency
Internal consistency was evaluated to determine whether daily ratings within each domain of the DCCASP correlated with one another. Internal consistency of each domain was assessed with the Cronbach's α coefficient for both genders combined and for each gender individually.
Criterion validity
To establish criterion validity for the Sleep Quality domain of the DCCASP, mean scores for all participants were calculated from the Sleep Quality domain on the DCCASP over the two periods (four weeks in total). The mean was also calculated for the Subjective Sleep Quality on the PSQI from all participants. The Spearman rank (r s) correlation coefficient was then calculated from the mean sleep quality on the DCCASP and PSQI.
Lag delay
Cross‐correlation was assessed to determine possible lag delay between the Sleep Quality domain and the other domains of the DCCASP. This analysis helped determine whether quality of sleep over night would immediately influence the next day's function or whether the effect on function was carried forward to following days.
Linear regression model
Next, a linear regression model was employed to determine whether ratings from each domain were related to sleep quality, weekday, week one to four, blocks one and two, gender, and/or the days of week. A Generalized Estimation Equation method was employed in this regression model to account for repeated measures (Wiseman‐Hakes et al., 2011).
All statistical analyses were conducted using SAS 9.3 software (SAS Institute, Cary, NC, USA), except for criterion validity, which was conducted using the SPSS version 9.0 (SPSS, Chicago, IL, USA). Statistical significance for each psychometric property was observed and accepted at p < 0.0001, except for criterion validity, which was accepted at p < 0.001.
Results
Reliability
Internal consistency, as estimated by Cronbach's α, was high and ranged from 0.864 to 0.938 across the seven domains of the DCCASP when both genders were combined. The Cronbach's α for all DCCASP domains ranged from 0.848 to 0.940 for females and from 0.815 to 0.933 for males.
Re‐administration of the DCCASP following the rest period was required to assess for test–retest reliability. Test–retest Concordance Correlation Coefficient (CCC) of each domain of the DCCASP was moderate, ranging from 0.548 to 0.742. A summary of the Cronbach's α for each domain can be found in Table 3.
Table 3.
Internal consistency (Cronbach's α) of all domains, and correlation (r s) between sleep quality and all domains of the DCCASP
| Cronbach's α | ||||
|---|---|---|---|---|
| Domain | All | Males | Females | r s * |
| Sleep quality | 0.890 | 0.917 | 0.883 | — |
| Mood | 0.899 | 0.896 | 0.901 | 0.525 |
| Fatigue | 0.893 | 0.897 | 0.893 | 0.546 |
| Daytime wakefulness | 0.864 | 0.929 | 0.847 | 0.469 |
| Attention | 0.870 | 0.815 | 0.878 | 0.479 |
| Memory | 0.930 | 0.933 | 0.931 | 0.380 |
| Language processing | 0.938 | 0.935 | 0.940 | 0.445 |
All significant at p < 0.0001.
Criterion validity
Adequate criterion validity for the Sleep Quality domain of the DCCASP was established by the Sleep Quality domain of the PSQI, with an r s of 0.398 (p < 0.001).
Cross‐correlation
Cross‐correlation analysis showed that there was no lag effect between sleep quality and all of the domains of the DCCASP.
The impact of sleep quality on other domains was visually represented by plotting each domain with sleep quality and fitting a non‐parametric curve (Figure 1). The average of each domain was calculated from all participants and plotted against mean ratings of sleep quality across the 28 days to demonstrate how the fluctuations of sleep quality was related to each domain (Figure 2). Figure 1 displays the non‐parametric curve fitted to the data and shows a positive relationship between sleep quality and all domains of the DCCASP. For the domains Mood (Figure 2a), Fatigue (Figure 2b), Daytime wakefulness (Figure 2c), Attention (Figure 2d), the fluctuations of sleep quality tightly correlated with those of the domains listed earlier. The fluctuations of sleep quality correlated less tightly with the domain of Memory (Figure 2e) and Language processing (Figure 2f); however, this was to be expected with a non‐clinical sample (see Discussion section for details).
Figure 1.
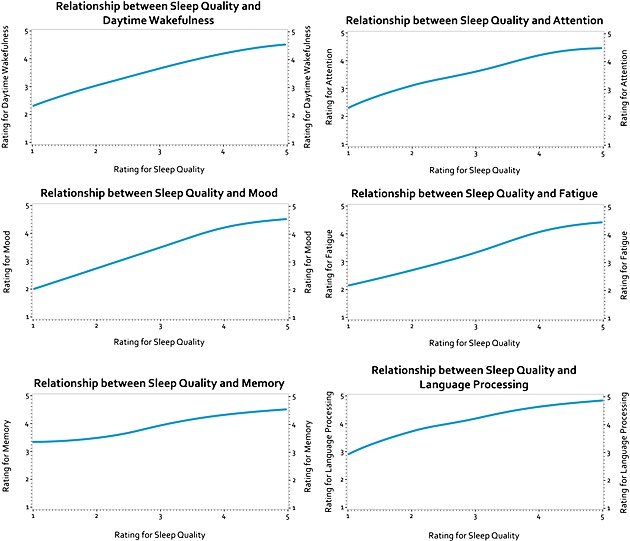
Relationships between sleep quality and the other DCCASP domains. The impact of sleep quality on each domain using a fitted non‐parametric curve.
Figure 2.
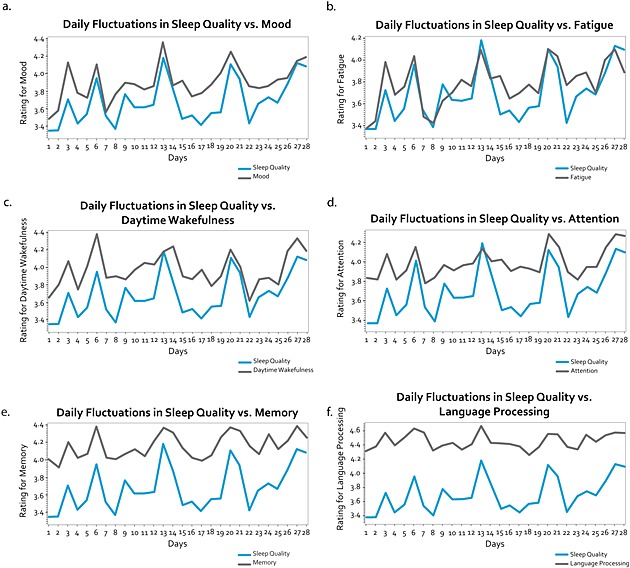
Daily fluctuations sleep quality versus other DCCASP domains. Fluctuations of average sleep quality and mean rating of (a) Mood, (b) Fatigue, (c) Daytime wakefulness, (d) Attention, (e) Memory, and (f) Language processing across the 28 days.
The regression model demonstrated no significance for the relationship between each domain and weekday, week one to four, blocks one and two, gender, and/or the days of week. Sleep Quality was the only factor that had a significant impact on every domain on the DCCASP (p < 0.0001).
Finally, a simple Spearman correlation (r s) between sleep quality and each domain was evaluated. There was a moderate positive correlation between sleep quality and all domains of the DCCASP, with r s ranging from 0.38 to 0.55 (p < 0.0001), as observed in Table 3.
Discussion
Prior to this study, no sleep measures existed that had been validated to monitor daily fluctuations in self‐reported sleep quality in relation to cognitive and communication functions and mood. This paper reports the psychometric properties of a seven‐item scale, the DCCASP. The DCCASP tracks sleep quality and cognitive and communication functions as well as perceived mood, for individuals with significant sleep disturbances over a continuous period of time. The DCCASP is a promising valid tool for measuring self‐reported sleep quality and self‐perceived cognitive and communicative functions in young adults. It can be used to monitor the progression of sleep disturbances and their interaction with daily functioning domain during the course of recovery from various diagnoses, as well as response to treatment.
This study provides preliminary evidence that the DCCASP is able to detect relationships between sleep quality and all domains of the DCCASP in college and university students, which is supported by the literature (Pilcher et al., 1997; Pilcher and Walters, 1997; Trockel et al., 2000). Sleep quality was the only factor to have a statistically significant positive relationship with all domains of the DCCASP. Additionally, sleep quality from the previous night immediately impacted the next day's function, rather than having a delayed or a cumulative effect a few days after. This corroborates current literature, which indicates that sleep quality may directly influence the other domains in clinical populations such as those suffering from TBI (Bloomfield et al., 2010; Lundin et al., 2006; MacDonald and Wiseman‐Hakes, 2010; Mahmood et al., 2004; Struchen et al., 2008). Thus, further research is required to confirm that the DCCASP can detect this trend in a clinical population.
Internal consistency was high for all DCCASP domains, particularly for the Memory and Language processing domains, indicating that participants were consistently rating the same score for these domains on a daily basis. Internal consistency was also determined for each gender to identify differences in normal sleep. Goel et al. (2005) has shown that women have better sleep quality and efficiency over men among young healthy adults. Furthermore, gender differences in sleep may be accounted for by fluctuations in hormone levels such that women experience changes in sleep architecture across their menstrual cycle (Manber and Bootzin, 1997). In our study, preliminary evidence indicated that the Cronbach's α of all domains between the two genders were similar. However, these results may have been limited as the study's sample consisted of more female participants compared to male. Further investigation is warranted to investigate potential gender differences on the self‐ratings of the DCCASP.
The relationship between sleep quality and each of the domains on the DCCASP on a daily basis was demonstrated using a fitted linear regression model. Some domains, such as Attention and Mood, were more tightly correlated with the changes in sleep quality such that fluctuations in these domains followed the fluctuations in sleep quality. Other domains, such as Memory and Language processing, were less sensitive to the fluctuations in sleep quality. This was expected as participants were recruited to represent a non‐clinical/normative population, and processes such as language processing and memory would be more robust and less subjected to changes with sleep patterns. However, in a neurogenic or other clinical population, it is expected that memory and language processing would more closely mirror changes in sleep quality and, that the DCCASP would be sensitive to these changes.
Criterion validity of the Sleep quality domain of the DCCASP was supported by the Sleep quality domain of the PSQI. The PSQI is currently the most frequently used measure in screening for subjective sleep quality and has been validated among healthy individuals of various ages, adult post‐TBI population, and primary insomnia (Backhaus et al., 2002; Fichtenberg et al., 2001; Grandner et al., 2006). It is important to note that the completion of the DCCASP over the preceding two weeks, prior to the completion of the PSQI, may have affected the information provided on the PSQI. Typically, individuals completing the PSQI are asked to reflect on their sleep patterns over the past month to be able to answer the questions on the PSQI. However, during this study, participants completed the DCCASP on a daily basis, allowing participants to be aware of the impact of their sleep quality on the specific domains of the DCCASP. Therefore, the participants in this study may have become more sensitized to the impact of sleep quality on daily activities, and thus, more accurately rated their responses to the questions on the PSQI following the completion of the DCCASP. Despite this bias, researchers have frequently correlated the PSQI with subjective measurements, such as sleep diaries and logs, and found significant correlations (Backhaus et al., 2002; Grandner et al., 2006). Thus, support of the criterion validity of the DCCASP from the PSQI Sleep quality domain is consistent with the literature, and results from this present study support the use of the DCCASP to monitor daily subjective sleep quality in individuals from a non‐clinical population.
Present results from this study suggest that individuals from a non‐clinical population can give meaningful self‐reports from the DCCASP about sleep quality, general daytime wakefulness, fatigue level, mood, and cognitive and communication functions. Further, since there are only seven questions on the DCCASP, it is quick to administer and participants become more familiar with the questions. As a result, it is likely that participants in this study were able to reflect on the different functions throughout the day and potentially became more aware of how sleep quality may have impacted daytime functioning. This is of benefit to both normative and clinical populations. For example, students are often unaware of the degree to which their sleep and/or sleep deprivation can influence their cognitive functioning such that students who stay up all night for an examination rated their performance as better, yet their actual performance was worse than students who slept eight hours the night before (Pilcher and Walters, 1997). Thus, use of the DCCASP in a clinical population may allow clients to become more reflective and self‐aware of the impact of their sleep quality on daily functions. It also allows clinicians to monitor subjective response to treatments for SWDs. For clients with mild cognitive impairments, the use of the DCCASP as a daily measure also allows them to reflect on functioning of the current day, rather than reflecting on the previous month, as in other measures like the PSQI. The DCCASP also allows the option for others to complete the measure on behalf of the client, particularly for clients who are unable to reflect on their day's functioning due to impaired cognitive functioning. However, this aspect of the DCCASP needs to be further evaluated in a non‐clinical and clinical population.
Although this evaluation indicates that the DCCASP is a promising tool for measuring daily fluctuations in sleep, there are some limitations to this study. The study consisted of a group of university students as the non‐clinical sample. However, it is anticipated that the DCCASP will be useful addition to assessment measures in other clinical populations who present with cognitive–communicative impairments, including patients with TBI, stroke, Parkinson's disease, schizophrenia, and dementia (Bourgeois, 1991; Coelho et al., 1996; Cooper et al., 2007; Mok et al., 2004). Research will be required to evaluate the psychometric properties of the DCCASP in these clinical populations to enhance confidence in its use. Further analyses of the criterion validity of the other domains of the DCCASP are warranted. For example, the Mood domain should be compared with the Beck Depression Inventory (Beck et al., 1961). Analysis of the inter‐rater reliability of the DCCASP would also be beneficial to understand the reliability of using a family member, rehabilitation worker, or therapist to complete the DCCASP for the client. Lastly, since sleep is dynamic and changes from one night to another, test–retest reliability was not a valid construct for the DCCASP, another measure of reliability should be considered in future studies.
In summary, the DCCASP demonstrated sound psychometric properties in monitoring daily fluctuations in self‐reported sleep quality and self‐perceived cognitive and communicative functions in a non‐clinical population. Thus, the DCCASP is a valuable tool to use in evaluating sleep quality and its influence over daily functioning in a normative population.
Declaration of interest statement
The authors do not have any competing conflicts of interests to report.
Acknowledgements
The authors acknowledge and appreciate the financial support from the Toronto Rehabilitation Institute (TRI), the Toronto Rehabilitation Institute Foundation, and a grant to the TRI from the Ontario Ministry of Health and Long‐term Care. Catherine Wiseman‐Hakes was supported by a Canadian Institutes of Health Research (CIHR) Clinical Research Fellowship. Angela Colantonio was supported by a CIHR Research Chair in Gender, Work and Health (#CGW‐126580) and the Saunderson Family Chair in Acquired Brain Injury Research at TRI. The authors also acknowledge Lee Vernich (MSc) for support with data analysis. Dr Catherine Wiseman‐Hakes developed the DCCASP. Finally, the authors thank Sandra Sokoloff for administrative support. This study was conducted in fulfillment of the requirements for the Masters of Science in Occupational Science and Occupational Therapy, Faculty of Medicine, University of Toronto.
References
- Alapin I., Fichten C.S., Libman E., Creti L., Bailes S., Wright J. (2000) How is good and poor sleep in older adults and college students relate to daytime sleepiness, fatigue, and ability to concentrate? Journal of Psychosomatic Research, 49(5), 381–390. [DOI] [PubMed] [Google Scholar]
- Aldrich M.S. (1999) Sleep Medicine. New York, Oxford University Press. [Google Scholar]
- American Academy of Sleep Medicine (AASM) . (2005) International Classification of Sleep Disorders (ICD‐2). Darien, IL, AASM. [Google Scholar]
- Backhaus J., Junghanns K., Broocks A., Riemann D., Hohagen F. (2002) Test–retest and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research, 53, 737–740. [DOI] [PubMed] [Google Scholar]
- Bastien C.H., Vallières A., Morin C.M. (2001) Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2, 297–307. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. (1961) An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Beetar J.T., Guilmette T.J., Sparadeo R. (1996) Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Archives of Physical Medicine and Rehabilitation, 77, 1298–1302. [DOI] [PubMed] [Google Scholar]
- Bloomfield I.L.M., Espie C.A., Evans J.J. (2010) Do sleep difficulties exacerbate deficits in sustained attention following traumatic brain injury? Journal of the International Neuropsychological Society, 16, 17–25. DOI: 10.1017/S1355617709990798 [DOI] [PubMed] [Google Scholar]
- Bourgeois M.S. (1991) Communication treatment for adults with dementia. Journal of Speech, Language, and Hearing Research, 34(4), 831–844. [DOI] [PubMed] [Google Scholar]
- Brown F.C., Buboltz W.C. Jr, Soper B. (2002) Relationship of sleep hygiene awareness, sleep hygiene practices, and sleep quality in university students. Behavioral Medicine, 28(1), 33–38, DOI: 10.1080/08964280209596396 [DOI] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F. III, Monk T.H., Berman S.R., Kupfer D.J. (1988) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cantor J.B., Ashman T., Gordon W., Ginsberg A., Engmann C., Egan M., Spielman L., Dijkers M., Flanagan S. (2008) Fatigue after traumatic brain injury and its impact on participation and quality of life. Journal of Head Trauma Rehabilitation, 23(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Carpenter J.S., Storniolo A.M., Johns S., Monahan P.O., Azzouz F. Elam J.L., … Shelton R.C. (2007) Randomized, double‐blind, placebo‐controlled crossover trials of Venlafaxine for hot flashes after breast cancer. The Oncologist, 12(1), 124–135. DOI: 10.1634/theoncologist.12-1-124 [DOI] [PubMed] [Google Scholar]
- Chaput G., Giguère J.F., Chauny J.M., Denis R., Lavigne G. (2009) Relationship among subjective sleep complaints, headaches, and mood alterations following a mild traumatic brain injury. Sleep Medicine, 10, 713–716. DOI: 10.1016/j.sleep.2008.07.015 [DOI] [PubMed] [Google Scholar]
- Coelho C.A., DeRuyter F., Stein M. (1996) Cognitive–communicative disorders resulting from traumatic brain injury in adults. Journal of Speech, Language, and Hearing Research, 39, s5–s17. [PubMed] [Google Scholar]
- Cooper S.A., Smiley E., Morrison J., Williamson A., Allan L. (2007) Mental ill‐health in adults with intellectual disabilities: Prevalence and associated factors. British Journal of Psychiatry, 190, 27–35. DOI: 10.1192/bjp.bp.106.022483 [DOI] [PubMed] [Google Scholar]
- Fichtenberg N.L., Putnam S.H., Zafonte R.D., Millard A.E. (2001) Insomnia screening in postacute traumatic brain injury: Utility and validity of the Pittsburg Sleep Quality Index. American Journal of Physical Medicine and Rehabilitation, 80, 339–345. [DOI] [PubMed] [Google Scholar]
- Fulda S., Schulz H. (2001) Cognitive dysfunction in sleep disorders. Sleep Medicine Reviews, 5(6), 423–445. DOI: 10.1053/smrv.2001.0157 [DOI] [PubMed] [Google Scholar]
- Fung C., Nguyen M., Wiseman‐Hakes C., Colantonio A. (2011) Preliminary validation of a new instrument for monitoring sleep, wakefulness and daytime function: Daily Cognitive–Communication and Sleep Profile. Sleep Medicine, 12(Suppl 1), S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N., Kim H., Lao R.P. (2005) Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiology International, 22, 905–915. [DOI] [PubMed] [Google Scholar]
- Grandner M.A., Kripke D.F., Yoon I‐Y., Youngstedt S.D. (2006) Criterion validity of the Pittsburg Sleep Quality Index: investigation in a non‐clinical sample. Sleep and Biological Rhythms, 4, 129–136. DOI: 10.1111/j.1479-8425.2006.00207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. (2008) Sleep, occupation and the passage of time. British Journal of Occupational Therapy, 71, 339–347. [Google Scholar]
- Hauri P. (1997) Cognitive deficits in insomnia patients. Acta Neurologica Belgica, 97(2), 113–117. [PubMed] [Google Scholar]
- Johns M.W. (1991) A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep, 14(6), 540–545. [DOI] [PubMed] [Google Scholar]
- Kotagal S., Goulding P.M. (1996) The laboratory assessment of daytime sleepiness in childhood. Journal of Clinical Neurophysiology, 13, 208–218. [DOI] [PubMed] [Google Scholar]
- Léger D., Poursain B., Neubauer D., Uchiyama M. (2008) An international survey of sleeping problems in the general population. Current Medical Research and Opinion, 24(1), 307–317. [DOI] [PubMed] [Google Scholar]
- Lundin A., De Boussard C., Edman G., Borg J. (2006) Symptoms and disability until 3 months after mild TBI. Brain Injury, 20(8), 799–806. DOI: 10.1080/02699050600744327 [DOI] [PubMed] [Google Scholar]
- MacDonald S., Wiseman‐Hakes C. (2010) Knowledge translation in ABI rehabilitation: a model for consolidating and applying the evidence for cognitive‐communication interventions. Brain Injury, 24(3), 486–508. [DOI] [PubMed] [Google Scholar]
- Mahmood O., Rapport L.J., Hanks R.A., Fichtenberg N.L. (2004) Neuropsychological performance and sleep disturbance following traumatic brain injury. Journal of Head Trauma Rehabilitation, 19(5), 378–390. [DOI] [PubMed] [Google Scholar]
- Manber R., Bootzin R.R. (1997) Sleep and the menstrual cycle. Health Psycholology, 16, 209–214. [DOI] [PubMed] [Google Scholar]
- McNair D.M., Lorr M., Droppelman L.F. (1971) Manual for the Profile of Mood States, San Diego, CA, Educational and Industrial Testing Services. [Google Scholar]
- Meerlo P., Mistlberger R.E., Noiman L., Gould E. (2006) Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proceedings of the National Academy of Sciences USA, 103(50), 19170–19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P., Mistlberger R.E., Jacobs B.L., Heller H.C., McGinty D. (2009) New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Medicine Reviews, 13(3), 187–194. DOI: 10.1016/j.smrv.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok V.C.T., Wong A., Lam W.W.M., Fan Y.H., Tang W.K., Kwok T., … Wong K.S. (2004) Cognitive impairment and functional outcome after stroke associated with small vessel disease. Journal of Neurology, Neurosurgery and Psychiatry, 75, 560–566. DOI: 10.1136/jnnp.2003.015107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcell D.L., Ponsford J.L., Rajaratnam S.M., Redman J.R. (2006) Self‐reported changes to nighttime sleep after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 87, 278–285, DOI: 10.1016/j.apmr.2005.10.024 [DOI] [PubMed] [Google Scholar]
- Pilcher J.J., Ott E.S. (1998) The relationships between sleep and measures of health and well‐being in college students: a repeated measures approach. Behavioral Medicine, 23(4), 170–178. DOI: 10.1080/08964289809596373 [DOI] [PubMed] [Google Scholar]
- Pilcher J.J., Walters A.S. (1997) How sleep deprivation affects psychological variables related to college students’ cognitive performance. Journal of American College Health, 46(3), 121–126. DOI: 10.1080/07448489709595597 [DOI] [PubMed] [Google Scholar]
- Pilcher J.J., Ginter D.R., Sadowsky B. (1997) Sleep quality versus sleep quantity: relationships between sleep and measures of health, well‐being and sleepiness in college students. Journal of Psychosomatic Research, 42(6), 583–596. DOI: 10.1016/S0022-3999(97)00004-4 [DOI] [PubMed] [Google Scholar]
- Rosenthal M. (1998) Physiology and neurochemistry of sleep. American Journal of Pharmaceutical Education, 62, 204–208. [Google Scholar]
- Stein D., Pat‐Horenczyk R., Blank S., Dagan Y., Barak Y., Gumpel T.P. (2002) Sleep disturbances in adolescents with symptoms of attention‐deficit/hyperactivity disorder. Journal of Learning Disabilities, 35, 268–275. [DOI] [PubMed] [Google Scholar]
- Struchen M.A., Clark A.N., Sander A.M., Mills M.R., Evans G., Kurtz D. (2008) Relation of executive functioning and social communication measures to functional outcomes following traumatic brain injury. NeuroRehabilitation, 23(2), 185–198. [PubMed] [Google Scholar]
- Totterdell P., Reynolds S., Parkinson B., Briner R.B. (1994) Associations of sleep with everyday mood, minor symptoms and social interaction experience. Sleep, 17(5), 466–475. [DOI] [PubMed] [Google Scholar]
- Touchette M.P., Petit D., Seguin J.R., Biovin M., Tremblay R.E., Montplaisir J.Y. (2007) Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep, 30(9), 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trockel M.T., Barnes M.D., Egget D.L. (2000) Health‐related variables and academic performance among first‐year college students: implications for sleep and other behaviours. Journal of American College Health, 49(3), 125–131. DOI: 10.1080/07448480009596294 [DOI] [PubMed] [Google Scholar]
- Vena C., Parker K., Cunningham M., Clark J., McMillan S. (2004) Sleep–wake disturbances in people with cancer part I: an overview of sleep, sleep regulation, and effects on disease and treatment. Oncology Nursing Forum, 31, 735–746. [DOI] [PubMed] [Google Scholar]
- Vitiello M.V., Borson S. (2001) Sleep disturbances in patients with Alzheimer's disease: epidemiology, pathophysiology and treatment. CNS Drugs, 15, 777–796. [DOI] [PubMed] [Google Scholar]
- Walker M.P. (2009) The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences, 1156, 168–197. [DOI] [PubMed] [Google Scholar]
- Walker M.P., Stickgold R., Alsop D., Gaab N., Schlaug G. (2005) Sleep‐dependent motor memory plasticity in the human brain. Neuroscience, 133(4), 911–917. [DOI] [PubMed] [Google Scholar]
- Wiseman‐Hakes C., Colantonio A., Gargaro J. (2009) Sleep and wake disorders following traumatic brain injury: a critical review of the literature. Critical Reviews in Physical and Rehabilitation Medicine, 21(3–4), 317–374. DOI: 10.1615/CritRevPhysRehabilMed.v21.i3-4.70 [DOI] [Google Scholar]
- Wiseman‐Hakes C., Victor J.C., Brandys C., Murray B. (2011) Impact of post traumatic hypersomnia on functional recovery of cognition and communication. Brain Injury, 25(12), 1256–1265. DOI: 10.3109/02699052.2011.608215 [DOI] [PubMed] [Google Scholar]
- Zafonte R.D., Mann N.R., Fichtenberg N.L. (1996) Sleep disturbance in traumatic brain injury: pharmacologic options. NeuroRehabilitation, 7, 189–195. [DOI] [PubMed] [Google Scholar]


