Abstract
Inhibitors of fibroblast growth factor receptor (FGFR) represent an outstanding treatment approach for selected patients with urothelial cancer (UC). These agents are changing the clinical approach to a subgroup of UC, the luminal-papillary subtype, characterized by FGFR mutations, fusions, or amplification. In this review, we provide an overview of the results of recent clinical trials on FGFR tyrosine kinase inhibitors (TKIs) currently in clinical development for the treatment of UC: erdafitinib, rogaratinib, infigratinib, and the monoclonal antibody vofatamab. The Food and Drug Administration recently granted accelerated approval to erdafitinib for patients with advanced UC with alterations of FGFR2 or FGFR3 after progression on platinum-based chemotherapy. We also look at future therapeutic options of combination regimens with immune-checkpoint inhibitors as strategies for improving the antitumor effects of this class of drug, and for preventing or delaying the development of resistance.
Keywords: erdafitinib, FGFR inhibitors, infigratinib, rogaratinib, urothelial cancer, vofatamab
Introduction
Bladder cancer (BC) is a common cancer worldwide whose incidence has increased in recent years. In Europe, the age-standardized incidence rate of BC is 9.0 for men and 2.2 for women.1 BC can be divided into non-muscle-invasive (NMIBC) and muscle-invasive tumors (MIBC). Approximately 90% of all MIBC are urothelial carcinomas (UC). At diagnosis, 75% of UC are NMIBC, while 25% of cases are MIBC or metastatic disease.2 In an estimated 5–8% of cases, UC originates in the renal pelvis or ureter (upper tract urothelial carcinoma, UTUC).3 Patients with advanced UC are not treatable with curative intent. The first-line standard of care is cisplatin-containing chemotherapy such as gemcitabine-cisplatin or M-VAC (methotrexate, vinblastine, doxorubicin, and cisplatin), both of which are characterized by similar efficacy but with a better safety profile for the former.4,5 However, about 30% of patients are not candidates for cisplatin due to renal dysfunction, poor performance status (PS), or other comorbidities.6 Alternative chemotherapeutic regimens, such as carboplatin-based therapies, correlate with inferior outcomes.7,8 Traditionally, the median overall survival (OS) ranged between 14 and 16 months in patients with advanced UC treated with platinum-based regimens, and long-term survival was rare.2,4
After relapse, few options are available for second-line chemotherapy. An important randomized phase III study comparing vinflunine (a third-generation vinca alkaloid) and best supportive care (BSC) with BSC alone in platinum-refractory UC reported an objective response rate (ORR) of 8.6%, a favorable safety profile, and a survival benefit for vinflunine.9 Historically, other potential treatment options include docetaxel or paclitaxel in monotherapy.10,11
Checkpoint blockade immunotherapy recently emerged as a potentially effective treatment for these relapsed patients, with several programmed death 1 (PD-1) and programmed death–ligand 1 (PD-L1) inhibitors rapidly approved by the Food and Drug Administration (FDA), first in a postplatinum chemotherapy setting, and then as front-line treatment for cisplatin-ineligible disease.12–20 As second-line therapy, these drugs have shown an ORR of around 20%,12,13,15–17,19 without the help of predictive biomarkers approved for treating specific subsets of UC patients. Within this context, it is fundamental to find novel therapeutic agents. As part of The Cancer Genome Atlas project, the integrated genomic analysis of 131 MIBC samples revealed a high rate of somatic mutation similar to that of non-small cell lung cancer and melanoma.21,22 Robertson and colleagues proposed a new molecular classification of MIBC based on the integrated analysis of messenger ribonucleic acid (mRNA), long noncoding RNA (lncRNA), and microRNA (miRNA) expression in 412 chemotherapy-naïve samples of high grade MIBC, stratifying MIBC into five distinct subtypes: basal-squamous (35%), luminal-papillary (35%), luminal-infiltrated (19%), luminal (6%), and neuronal (5%).23 The luminal-infiltrated subtype (19%) showed expression of CD274 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA4). Initially, the luminal-infiltrated subtype, corresponding to TGCA subtype II,21 was associated with response to atezolizumab in patients with advanced UC.16 The basal-squamous subtype (35%) is characterized by basal keratin expression, squamous differentiation, high expression of PD-L1 and CTLA4 immune markers, and immune infiltration, making both cisplatin-based and immune checkpoint therapy valid therapeutic approaches.17 The neuronal subtype is characterized by high expression of neuroendocrine/neuronal markers, while the luminal subtype shows high expression of luminal markers. Due to their novelty, optimal therapy for neuronal and luminal subtype has still not been defined. Recently, neuronal and luminal subtypes have been reported to be associated with better survival and response to atezolizumab than the other subtypes.24 In UC, important developments in the molecular characterization of MIBC represent a further step forward in identifying the right treatment for the right patient. The luminal-papillary subtype is characterized by fibroblast growth factor receptor 3 (FGFR3) mutations, fusion with transforming acid coiled-coil containing protein 3 (TACC3), or amplification, suggesting that TKIs of FGFR3 may represent an interesting treatment approach for selected patients.23 In this review, we summarize the landscape of FGFR alterations in patients with UC, the future therapeutic options, and the mechanisms of resistance to FGFR-targeted therapies.
Key genetic alterations
FGFRs (FGFR1, FGFR2, FGFR3, FGFR4) are receptor tyrosine kinases (RTKs) consisting of an extracellular ligand-binding domain [composed of three immunoglobulin (Ig)-like domains], a single-pass transmembrane domain and an intracellular tyrosine-kinase domain, that are not constitutively activated in normal cells. Upon the presence of their natural ligand, FGFRs dimerize and autophosphorylate the tyrosine residue to become activated, and phosphorylate multiple signaling proteins such as phosphatidylinositol 3 kinase (PI3K)-AKT and RAS/mitogen-activated protein kinase (MAPK), stimulating cell growth, differentiation, survival, angiogenesis, and organogenesis, depending on cell type.25–29 The FGFR family is encoded by four different genes, and alternative splicing generates two different isoforms (b/c) of the extracellular domain in each of the FGFR1, FGFR2, and FGFR3 genes, thus making seven distinct FGF receptors.30 As these isoforms are expressed differently in various tissues and cell lines to guarantee differential roles in different tissues and cell lineages, inappropriate expression or mutation of these receptors is involved in the development of malignancies.31–33 FGFR signaling can be constitutively activated in tumor cells through amplification, missense, or fusion mutations in the coding region, and also through dysregulation of the noncoding regions, or through the alterations of epigenetic regulators or upregulation of ligands (Figure 1).34–36 Aberrations in the FGFR signaling pathway, particularly alterations in FGFR1 and FGFR3, have been shown to be involved in UC.37
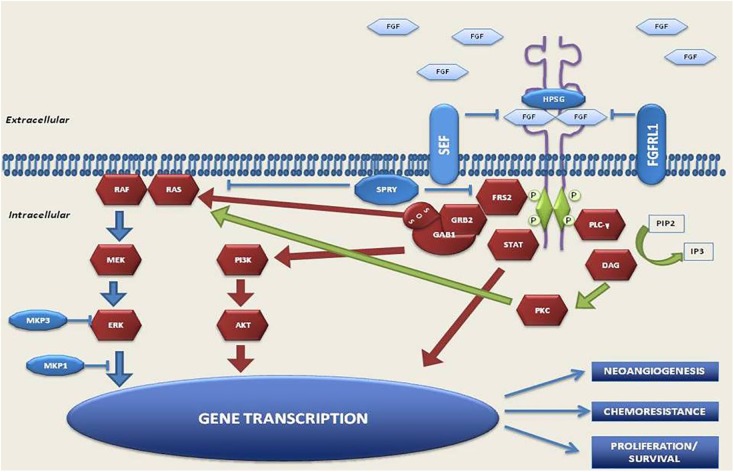
Figure 1.
FGFR pathway.
FGFRs dimerize upon ligand building and trigger a downstream cascade of signaling pathways. Mutation, translocation, gene amplification, and also an increase in circulating FGF ligands, can active FGFR receptors. Downstream signaling can trigger the PI3K/Akt pathway, the MAPK pathway, phosphorylation of the STAT, resulting in DNA transcription.
FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; MAPK, mitogen activated protein kinase; PI3K/Akt, phosphoinositide-3-kinase; STAT, signal transducer and activator of transcription.
FGFR1 amplification is present in around 42% in cancers with an FGFR alteration.34 This gene alteration is reported in 7% of UC patients. The two splicing variants of FGFR1 (FGFR1α and FGFR1β) are expressed equivalently in normal urothelium. However, FGFR1β is the predominant form in UC, and the switch from α to β correlates with increasing tumor stage and grade.38
The luminal-papillary subtype is characterized by FGFR3 mutations,23 and it is interesting to note that urothelial papilloma, a benign tumor, shows frequent (75%) FGFR3 mutations.39 Billerey and colleagues detected FGFR3 alterations in 27 (84%) out of 32 grade (G)1 NMIBCs, 16 (55%) out of 29 G2 tumors, and 5 (7%) of 71 G3 tumors, with a highly significant association (p < 0.0001) between FGFR3 mutations and low-grade disease.40 FGFR3 mutations have also been detected in around 10% of MIBCs.41,42 The most frequent types of FGFR3 aberrations in UCs are activating mutations.21,34 FGFR3S249C, the most common aberration (21%), induces ligand-independent dimerization and activation of receptor, and is more frequently observed in low-grade UCs than in high-grade tumors.43 Another typical alteration in UC is the fusion of FGFR3 to the TACC3 gene, leading to FGFR3 aberrant activation of downstream signaling pathways.44–46 FGFR3 amplifications are less common; however, alternative splicing of FGFR3 is implicated in the proliferation process of UC.47–49 Furthermore, despite a common histologic origin, urothelial bladder carcinoma and UTUC are two different entities with two distinct clinical pathologic profiles. A comparison between high-grade UTUC and UBC revealed that FGFR3 was more commonly altered in UTUC (35.6% versus 21.6%, respectively).50
Molecular targeted agents in clinical trials
To date, several clinical trials are ongoing to evaluate the role of FGFR inhibitors in the treatment of UCs.
Erdafitinib (JNJ-42756493) is an oral FGFR1-4 inhibitor with demonstrated clinical activity, in a phase I trial, in patients with solid tumors, including UC in 12% of patients.51 This study recommended a dose of 10 mg with a 7-day-on/7-day-off schedule. A total of 59 patients were enrolled, including 23 patients with FGFR1-4 alterations, who were predicted to have a constitutively activated FGFR pathway, and 36 patients with unknown FGFR alterations. No responses were recorded in the latter group. Among 23 response-evaluable patients, 4 confirmed responses, and 1 unconfirmed partial response were observed in patients with glioblastoma, UC, and endometrial cancer, while 16 patients had stable disease.51 In the phase II study BLC2001 (ClinicalTrials.gov identifier: NCT02365597), 99 patients were treated with erdafinitib 8 mg/day continuous dosing in 28-day cycles; 12% were chemonaïve, 88% had a history of disease progression or relapse after chemotherapy, 43% had received at least two previous courses of treatment, and 79% had visceral metastases. Confirmed ORR was 40%, and the ORR was 59% among patients treated with prior immune checkpoint inhibitors (ICIs). Response to erdafitinib was seen in patients who had previously not responded to anti PD-L1/PD-1 therapy. The median duration of OS was 13.8 months. Dose adjustments were required in 46% of patients.52 An ongoing phase III study (ClinicalTrials.gov identifier: NCT03390504) is investigating the superiority of single-agent erdafitinib over chemotherapy (vinflunine or docetaxel) and anti-PD1 agent (pembrolizumab) in relapsed/refractory UC with selected FGFR gene alterations. The primary endpoint is overall survival (OS). This open-label trial has an estimated enrollment of 631 patients, and an estimated primary completion date of November 2020.
Rogaratinib (BAY 1163877) is a highly selective FGFR1-4 inhibitor with a unique selectivity profile that shows good tolerability and safety. It decreases proliferation in FGFR-addicted cancer cell lines of lung, breast, and colon, as well as UC. The efficacy of rogaratinib is also correlated strongly with FGFR mRNA expression levels.53 A phase I study (ClinicalTrials.gov identifier: NCT01976741) included three dose expansion cohorts, evaluating safety and efficacy in patients with solid tumors overexpressing FGFR1-3, including head and neck squamous-cell carcinoma, non-small cell lung cancer and UC. The ORR was 23% and the disease control rate (DCR) was 60% for UC,54 with a favorable toxicity profile. These encouraging results led to the design of a currently ongoing phase II/III study (ClinicalTrials.gov identifier: NCT03410693) to compare the efficacy and safety of rogaratinib with that of chemotherapy in patients with locally advanced or metastatic high FGFR1- and mRNA-expressing UC previously treated with platinum-based therapy. The primary endpoint is OS, while secondary endpoints are progression-free survival (PFS), ORR, DCR, duration of response (DOR), safety, and tolerability.
Infigratinib (BJG398) is an FGFR1-3 inhibitor that showed activity as a single agent against FGFR3-mutant UC in an early-phase clinical trial.55 In a phase I trial of BGJ398, four out of five patients with advanced UC harboring FGFR3 mutations experienced tumor regression.56 An expansion cohort of 67 patients with FGFR3-altered UC was thus enrolled and treated with single-agent infigratinib administered orally at 125 mg/day in a 3-week-on, 1-week-off schedule. The ORR was 25.4% and DCR was 64.2%.57 Recently, Dizman and colleagues carried out an exploratory analysis in a phase II trial comparing infigratinib in upper tract (UTUC) and lower tract UC (UBC), reporting a substantially different ORR between UTUC and UBC (50% versus 22%, respectively).58
Moss and colleagues demonstrated that UTUC shows distinct mutations and different mutation frequencies compared with UBC, resulting in four different subtypes.59 UTUCs are characterized by a higher mutation frequency of FGFR3 and lower mutation frequency of TP53 than UBCs. Dizman and colleagues found different types of FGFR3 mutations in UTUC and UBC patients. The R248C FGFR3 mutation was present in 50% of patients with UTUC compared with only 22% of UBC patients. S249C was found in 59% of UBC. The R248C mutation is induced by microsatellite instability (MSI) (mutational signature), whereas S249C mutation is induced by APOBEC.60 Thus, the presence of a different type of FGFR mutation might explain the difference in activity.58 However, the trial has a small sample size and results are exploratory, indicating the need for further validation.
Pemigatinib (INCB054828) inhibits FGFR 1, 2, and 3. An ongoing phase II clinical trial (ClinicalTrials.gov identifier: NCT03914794) is investigating the antitumor activity of the drug in NMIBC patients with recurrent tumors treated for 4–6 weeks prior to standard transurethral resection of bladder tumor. The primary endpoint is complete response rate. The study has still not started to recruit.
Debio 1347 is a selective oral inhibitor of FGFR1–3 that has shown elevated antineoplastic activity in tumors harboring FGFR1–3 gene fusions.36,61 A phase II basket trial (ClinicalTrials.gov identifier: NCT03834220) is ongoing to investigate its efficacy in terms of ORR in patients with FGFR1-3 gene fusions treated with at least one prior therapy and no valid alternative treatment option. Secondary endpoints are OS, PFS, DCR, and DOR. Patients receive Debio 1347 orally 80 mg/day continuously. There are three cohorts, with cohort 2 enrolling UC patients. This study estimates a recruitment of 125 patients.62
Dovitinib, another oral TKI, has shown a good safety profile as both a single and combination agent.63 Given the evidence of frequent FGFR3 aberrations in NMIBC, a phase II trial (ClinicalTrials.gov identifier: NCT01732107) was carried out to investigate the clinical and pharmacodynamic activity of dovitinib in patients with BCG-unresponsive NMIBC and with elevated phosphorylated FGFR3 expression. Dovitinib was administered 500 mg orally in a 5-day-on, 2-day-off dosing schedule. However, the study was terminated and the development of dovitinib was stopped because of the absence of clinical activity in this selected subgroup and also because of frequent toxicity.64
Derazantinib (ARQ087) is another inhibitor of FGFR2. A phase Ib/II study (ClinicalTrials.gov identifier: NCT04045613) is now ongoing to evaluate the efficacy of this drug alone or in combination with atezolizumab in patients with advanced UC harboring FGFR gene aberrations who are ineligible for cisplatin or progressed after either first-line treatment or prior treatment with FGFR inhibitors. This study is now recruiting.
Vofatamab (B-701) is a fully human immunoclonal antibody (Ab) that is highly specific for the FGFR3 receptor. The FIERCE-21 study (ClinicalTrials.gov identifier: NCT02401542), a phase Ib/II trial, evaluated vofatamab alone (at 25 mg/kg) or in combination with docetaxel (at 75 mg/m2 q3w) to determine dose and safety. The results of the phase Ib study showed that B-701 combined with standard-dose docetaxel was well-tolerated. Furthermore, FGFR3 mutations/fusions correlated with enhanced activity compared with wild-type tumors.65 The phase II expansion study screened 600 patients and FGFR3 mutations or fusions were seen in 20% of patients assigned to vofatamab alone compared with those receiving vofatamab plus docetaxel. The poster of the study presented at ASCO-GU 2019 meeting revealed that, although vofatamab was well tolerated (both in combination and as single agent), it showed very low single-agent activity (ORR 11%) in heavily pretreated patients.66 Table 1 shows anti-FGFR agents in the advanced phase of development, while Table 2 summarizes ongoing trials.
Treatment-related adverse events
Generally, this class of drugs has manageable adverse events (AEs), as shown in Table 1. The most frequent AEs have been observed with FGFR inhibitors (erdafitinib, rogaratinib, and infigratinib), and are based on their mechanism of action. They include hyperphosphatemia, fatigue, diarrhea, and nail toxicity.67,68 The study by Siefker-Radtke and colleagues on erdafitinib did not report any treatment-related deaths.69 In other studies on the same drug, the most common AEs included hyperphosphatemia (65%), asthenia (55%), dry mouth (45%), and nail toxicity (35%).51 Pal and colleagues reported that the most common treatment-toxicities for patients undergoing treatment with infigratinib were hyperphosphatemia (46%), fatigue (37%), decreased appetite (32%), and stomatitis (25%).57 Rogaratinib also has a good safety profile, Joerger and colleagues reporting hyperphosphatemia, diarrhea, and alopecia (grade 1 and 2) as the most common adverse events.54 Retinal disorders have been observed in patients treated with FGFR inhibitors. In a phase 1 trial of rogaratinib, 3/118 patients had G1 retinal pigment epithelial detachment, while one patient experienced a G2 event consistent with serious retinopathy.54 In a phase II trial of erdafitinib, 3% of patients discontinued treatment because of serious retinopathy.69 Thus, an ophthalmological evaluation was implemented at baseline and during treatment in both the phase II/III study (ClinicalTrials.gov identifier: NCT03410693) of rogaratinib and in the ongoing phase III clinical trial of erdafitinib (ClinicalTrials.gov identifier: NCT03390504). All patients treated with dovitinib in the phase II trial (ClinicalTrials.gov identifier: NCT03410693) experienced at least one G4 or G4 toxicity, in particular, fatigue. One patient suffered a subdural intracranial hemorrhage, and another experienced G4 hypertriglyceridemia.64 The development of the drug was subsequently stopped. Vofatamab was well tolerated, with few patients discontinuing treatment for AEs. The majority of AEs were G1 and G2, and included asthenia (19%), diarrhea (9.5%), flushing 14%, chills (9.5%), hypotension (9.5%), decreased appetite (19%), and increased creatinine (9.5%). Unlike FGFR inhibitors, hyperphosphatemia was not reported in patients treated with vofatamab because of the different mechanism of action of the drug.66
Table 1.
Overview of benefits and AEs in clinical trial involving FGFR inhibitors in urothelial carcinoma.
| Drug | Family | Phase | FGFR based eligibility | Dosage | Prior ICIs | Most common AEs | ORR |
|---|---|---|---|---|---|---|---|
| Erdafitinib | FGFR1-4 inhibitor | I | No selection with genomic tests | 10 mg/day 7-days-on/7-days-off | - | Hyperphosphatemia (65%) Asthenia (55%) Dry mouth (45%) Decreased appetite (38%) |
3/8 had PRs (among patients with UC harbouring FGFR2 or FGFR3 fusions) |
| II | Any FGFR alteration | 8 mg daily (up to 9 mg) | 22% | Hyperphosphatemia (69%) Skin and nail AEs (66%) Eye AEs (57%) Stomatitis (47%) |
40% | ||
| Rogaratinib | FGFR1-4 inhibitor | I | FGFR over-expression/ mutation | 800 mg twice daily | 30% | Diarrhea (61%) Nail disorders (52%) Hyperphosphatemia (45%) |
23% |
| Infigratinib | pan-FGFR inhibitor | I | FGFR3 alteration | 125 mg/day 3 weeks on/1 week off | 16% | Diarrhea (61%) Nail disorders (52%) Hyperphosphatemia (45%) |
25.4% |
| Vofatamab | Human anti-FGFR3 monoclonal Ab | Ib/II | FGFR3 alteration | 25 mg/kg q21 | - | Fatigue (12%) Nausea (12%) |
4/10 had SD |
AE, adverse events; FGFR, fibroblast growth factor receptor; ICIs, immune checkpoint inhibitor; ORR, overall response rate; PRs, partial responses, SD, stable disease.
Table 2.
Overview of ongoing trials involving FGFR inhibitors (alone or in combination) in urothelial carcinoma.
| Drug | Therapeutic scheme | FGFR status | Phase | Setting | Estimated Enrollment (n° participants) | Recruitment Status | Primary endpoints |
ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|---|
| Erdafitinib | Erdafitinib + JNJ-63723283 | Selected FGFR gene aberrations | Ib/II | ⩾1 line | 102 | Recruiting | phase Ib: DLT AEs phase II: ORR AEs |
NCT03473743 |
| Erdafitinib versus vinflunine or docetaxel or pembrolizumab | Selected FGFR gene aberrations (based on Fibroblast Growth Factor Receptor inhibitor Clinical Trial Assay) | III | ⩾1 line | 631 | Recruiting | OS | NCT03390504 | |
| Rogaratinib | Rogaratinib + atezolizumab | High FGFR1 or 3 mRNA expression levels | Ib/II | No prior systemic treatment and ineligibility for cisplatin-based | 210 | Recruiting | phase Ib: DLTs, TEAEs TESAEs phase II: PFS |
NCT03473756 |
| Rogaratinib versus chemotherapy | FGFR1 or 3 positive | II/III | ⩾1 line | 175a | Active, not recruiting | OS | NCT03410693 | |
| Vofatamab | Vofatamab + pembrolizumab | - | Ib/II | PD during or following platinum-containing chemotherapy | 74 | Recruiting | DLTs Safety and tolerability of vofatamab + pembrolizumab ORR |
NCT03123055 |
| Vofatamab ± docetaxel, Or versus docetaxe l | FGFR3 mutant/fusion | I/II(b) | ⩾1 line which has not included taxane | 300 | Active, not recruiting | PFS | NCT02401542 |
Actual enrollment.
AEs, adverse events; DLTs, dose-limiting toxicities; OS, overall survival; PD, progression disease; PFS, progression free survival; TEAEs, treatment-emergent adverse events; TESAEs, treatment-emergent serious adverse events.
FGFR inhibitors and tumor microenvironment
The tumor microenvironment (TME) consists not only of cancer cells but also of stromal and immune cells, including fibroblasts, endothelial cells, neutrophils, lymphocytes, monocytes, and macrophages.70,71 It is widely acknowledged that an active interaction between tumor cells and stromal cells is needed for tumor development, and that FGF signaling plays a key role in this network. Consequently, the action of FGFR inhibitors could also rebound on these mechanisms. There is a family of 22 FGF ligands that regulate FGFR tyrosine kinase activity in an autocrine or paracrine tissue-dependent context.72,73 For instance, FGF2 activates human dermal fibroblasts through the downregulation of the TP53 gene. In contrast, BGJ398 induces their senescence through the upregulation of TP53.74 Myeloid-derived suppressor cells (MDSCs) are involved in the process of immune evasion and growth of the tumor through the activation of M2-type tumor-associating macrophages (M2-TAMs) and through the inhibition of CD8+ T cells and natural killer cells (NK).75 FGFR inhibitors promote a reduction of MDSCs in the TME by targeting cytokine-producing cancer-associated fibroblasts (CAFs).36 Migration and proliferation of endothelial cells are promoted directly through the binding of FGF2 and FGFR1, and indirectly through the induction/secretion of vascular endothelial growth factor (VEGF) from endothelial cells.76 Huynh and colleagues recently showed that infigratinib induced vessel normalization by reducing hypoxia-inducible factor 1-alpha (HIF1α) and proangiogenic factors, hypothesizing that this could result in significant antitumor efficacy in hepatocellular carcinoma.77 A better understanding of this autocrine and paracrine action of FGFR could help to identify an effective drug combination.
Mechanisms of resistance
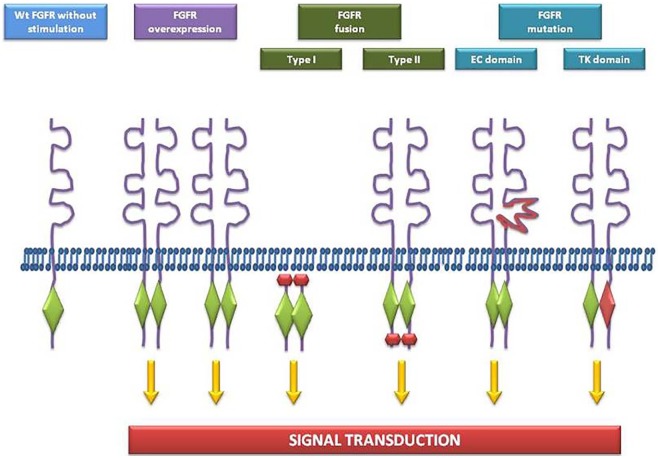
There are currently no clinical reports on mechanisms of resistance against FGFR inhibitors, probably due to the novelty of FGFR as a target for TKIs. However, data on other TKIs suggest that acquired resistance is a central problem associated with FGFR inhibitors. 78–80 Treatment with FGFGR inhibitors leads to the selection of resistant cellular clones that bypass FGFR inhibition, and receive signals for cell survival and replication by activating parallel cellular signaling pathways. A recent review summarized various mechanisms of resistance to FGFR inhibitors (Figure 2), including activation of bypass signaling involving amplification or mutations in proteins appertaining to EGFR, MET, RAS, and PI3K signaling, and gatekeeper mutations conferring resistance by interfering with the binding between the receptor and the targeted agents.81 Another important mechanism is tumor heterogeneity. Inoperable or advanced tumors have undergone clonal evolution, including FGFR-dependent clones and FGFR-independent clones, and such heterogeneity is an important obstacle to the efficacy of FGFR-targeted therapies in patients with liver cancer.82,83 UCs harboring FGFR3S249C alterations are not always sensitive to FGFR inhibitors because of their dependence on EGFR signaling rather than on FGFR3 signaling, which is repressed. Furthermore, UC cells harboring FGFR3-TACC3 fusions acquire resistance to FGFR inhibitors through the upregulation of EGFR/HER3-dependent PI3K-AKT signaling.84,85 Thus, sequencing of cell free circulating tumor (ct) DNA could be used, in addition to biopsy, to monitor the dynamic genomic landscapes of tumors, and detect the evolution of FGFR alterations.86
Figure 2.
Oncogenic FGFR alterations in human cancer.
FGFR, fibroblast growth factor receptor.
Combination therapies
The benefits of FGFR inhibitors in UC have been demonstrated in numerous clinical trials. However, combination strategies involving the concomitant administration of FGFR-targeted therapies with other agents may enhance the antitumor effects of this class of drugs, and also prevent or delay the development of resistance. ICIs are monoclonal antibodies that inhibit the interaction between PD-1 and its ligand PD-L1. In a study by Powles and colleagues, PD-1/PD-L1 inhibition obtained an ORR of 52% in patients with tumor-infiltrating immune cells and high expression of PD-L1 at immunohistochemical analysis.18 Although these results seem encouraging, the majority of patients with UC do not benefit from ICIs. In fact, treatment with ICIs has obtained ORRs ranging from 15% to 21% in platinum-refractory UC patients.12–17 There are still no valid biomarkers to predict tumor response to these treatments, but cytokines and the neutrophil-to-lymphocyte ratio look promising as prognostic and predictive indicators.87,88 However, it has been seen that the presence of an antitumor T-cell response is fundamental for the activity of immunotherapy.89–91 Recently, Sweis and colleagues, leaving from the classification of Robertson and colleagues,23 showed that UC can be divided into T-cell-inflamed and non-T-cell-inflamed subtypes. The latter phenotype correlated with an absence of CD8+ T, and a resistance to ICIs, but was also linked to FGFR3 mutation, providing a rationale for a combination of FGFR inhibitors and anti-PD-1/PD-L1.92 The FIERCE-22 phase II study (ClinicalTrials.gov identifier: NCT03123055) on vofatamab in combination with pembrolizumab was presented at the 2019 Annual American Society of Clinical Oncology (ASCO) meeting. The aim of the study was to prove that targeting FGFR3 (both wild type and mutant FGFR3 patients with UC), makes it possible to turn an immunologically ‘cold’ tumor into a ‘hot’ tumor. In fact, information available on other solid tumors (e.g. melanoma) suggest that the inhibition of an oncogene may induce antigen expression, decrease immunosuppressive cytokine production, and increase T cell clonality and PD-L1 expression. Luminal papillary UCs are enriched in FGFR alterations but lacking in immune infiltration. In the FIERCE-22 study, patients received one cycle of vofatamab (25 mg/kg), followed 2 weeks later by vofatamab (25 mg/kg) and pembrolizumab (200 mg) every 21 days. The ORR was 36% in the overall population, and a response was observed in both wild type (ORR 33%) and mutated (ORR 43%) FGFR3 patients. Furthermore, biopsies performed pre- and posttreatment showed that vofatamab induced immune changes, upregulating genes correlated with an inflammatory response.93 However, data are preliminary, the duration of the response is unknown, and there are still no data on the impact of changes in the immune cell microenvironment and of PD-L1 status modifications on patient outcome. A phase Ib/II study of rogaratinib administered in combination with atezolizumab in UC is currently in progress (ClinicalTrials.gov identifier: NCT03473756). Likewise, a phase Ib-II study (ClinicalTrials.gov identifier: NCT03473743) enrolling advanced UC patients with FGFR gene alterations is ongoing to evaluate the safety and efficacy of erdafitinib plus JNJ-63723283 (an anti-PD-1). A preclinical study by Takamura and colleagues suggested that the combination of BGJ398 and the novel histone deacetylase inhibitor, OBP-801, had a synergic effect on cell growth arrest and apoptosis in high grade UC cells.94
In TME, FGF signaling plays a pivotal role in both the survival and development of treatment resistance in tumor cells.95–97 FGFR inhibitors express their antitumor activities through direct effects on tumor cells harboring FGFR alterations, and through indirect effects on the TME, that is, regulation of angiogenesis, immune evasion, and paracrine tumor proliferation, independently of FGFR alterations.36 Increasing our knowledge about these mechanisms is central to developing new therapeutic strategies.
Conclusion
FGFR alterations are widely present in UC, offering new opportunities for developing targeted and personalized therapies based on FGFR status. Several clinical trials are currently ongoing to demonstrate the clinical efficacy of this class of multi-targeted TKIs, and to assess the potential usefulness of FGFR alterations as a biomarker. On 12 April 2019, the FDA granted accelerated approval to erdafitinib for patients with metastatic BC harboring FGFR2 or FGFR3 alterations in progression after platinum-containing chemotherapy.87 Further research is also warranted to investigate the combination of FGFR pathway inhibitors with other therapies to increase efficacy and overcome resistance mechanisms. Ongoing clinical trials are analyzing the combination of FGFR inhibitors and immune checkpoint inhibitors given the role that the FGF-FGFR pathway plays in TME. We believe that the FGFR pathway could open up new therapeutic avenues in this highly lethal disease, offering hope to a subset of patients by switching from cytotoxic chemotherapy to targeted agents.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Ugo De Giorgi  https://orcid.org/0000-0001-7520-2908
https://orcid.org/0000-0001-7520-2908
Contributor Information
Chiara Casadei, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Nazli Dizman, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Giuseppe Schepisi, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.
Maria Concetta Cursano, Department of Medical Oncology, Campus Bio-Medico University of Rome, Rome, Italy.
Umberto Basso, Medical Oncology Unit, Istituto Oncologico Veneto, IOV-IRCCS, Padova, Italy.
Daniele Santini, Department of Medical Oncology, Campus Bio-Medico University of Rome, Rome, Italy.
Sumanta K. Pal, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
Ugo De Giorgi, Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Via Maroncelli 40, Meldola, 47014, Italy.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018; 103: 356–387. [DOI] [PubMed] [Google Scholar]
- 2. Maffezzini M, Campodonico F. Bladder cancer: ESMO clinical practice guidelines. ESMO Handb Cancer Sr Patient 2010; 2010: 121–127. [Google Scholar]
- 3. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 2000; 164: 1523–1525. [PubMed] [Google Scholar]
- 4. Von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068–3077. [DOI] [PubMed] [Google Scholar]
- 5. Von Der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23: 4602–4608. [DOI] [PubMed] [Google Scholar]
- 6. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012; 30: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol 2012; 23: 406–410. [DOI] [PubMed] [Google Scholar]
- 8. Dreicer R, Manola J, Roth BJ, et al. Phase III trial of methotrexate, vinblastine, doxorubicin, and cisplatin versus carboplatin and paclitaxel in patients with advanced carcinoma of the urothelium: A Trial of the Eastern Cooperative Oncology Group. Cancer 2004; 100: 1639–1645. [DOI] [PubMed] [Google Scholar]
- 9. Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009; 27: 4454–4461. [DOI] [PubMed] [Google Scholar]
- 10. McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 1997; 15: 1853–1857. [DOI] [PubMed] [Google Scholar]
- 11. Vaughn DJ, Broome CM, Hussain M, et al. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 2002; 20: 937–940. [DOI] [PubMed] [Google Scholar]
- 12. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018; 391: 748–757. [DOI] [PubMed] [Google Scholar]
- 13. Powles T, O’Donnell PH, Massard C, et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2017; 35(6 Suppl.): 286–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018; 19: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 2016; 17: 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–562. [DOI] [PubMed] [Google Scholar]
- 19. Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol 2017; 35: 2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017; 18: 1483–1492. [DOI] [PubMed] [Google Scholar]
- 21. Weinstein JN, Akbani R, Broom BM, et al. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014; 507: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013; 499: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017; 171: 540–556.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim J, Kwiatkowski D, McConkey DJ, et al. The cancer genome atlas expression subtypes stratify response to checkpoint inhibition in advanced urothelial cancer and identify a subset of patients with high survival probability. Eur Urol 2019; 75: 961–964. [DOI] [PubMed] [Google Scholar]
- 25. Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev 2014; 34: 280–300. [DOI] [PubMed] [Google Scholar]
- 26. Chen H, Marsiglia WM, Cho MK, et al. Elucidation of a four-site allosteric network in fibroblast growth factor receptor tyrosine kinases. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2010; 141: 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009; 8: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Su YH, Du H, Niu GM, et al. The fibroblast growth factor signaling pathway. Chinese J Tissue Eng Res 2016; 20: 2255–2264. [Google Scholar]
- 30. Porta R, Borea R, Coelho A, et al. FGFR a promising druggable target in cancer: molecular biology and new drugs. Crit Rev Oncol Hematol 2017; 113: 256–267. [DOI] [PubMed] [Google Scholar]
- 31. Naimi B, Latil A, Fournier G, et al. Down-regulation of (IIIb) and (IIIc) isoforms of fibroblast growth factor receptor 2 (FGFR2) is associated with malignant progression in human prostate. Prostate 2002; 52: 245–252. [DOI] [PubMed] [Google Scholar]
- 32. Reis-Filho JS, Simpson PT, Turner NC, et al. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin Cancer Res 2006; 12: 6652–6662. [DOI] [PubMed] [Google Scholar]
- 33. Allerstorfer S, Sonvilla G, Fischer H, et al. FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities. Oncogene 2008; 27: 4180–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Helsten T, Elkin S, Arthur E, et al. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res 2016; 22: 259–267. [DOI] [PubMed] [Google Scholar]
- 35. Tanner Y, Grose RP. Dysregulated FGF signalling in neoplastic disorders. Semin Cell Dev Biol 2016; 53: 126–135. [DOI] [PubMed] [Google Scholar]
- 36. Katoh M. FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int J Mol Med 2016; 38: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Martino E, Tomlinson DC, Knowles MA. A decade of FGF receptor research in bladder cancer: past, present, and future challenges. Adv Urol 2012; 2012: 429213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomlinson DC, Knowles MA. Altered splicing of FGFR1 is associated with high tumor grade and stage and leads to increased sensitivity to FGF1 in bladder cancer. Am J Pathol 2010; 177: 2379–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Rhijn BWG, Montironi R, Zwarthoff EC, et al. Frequent FGFR3 mutations in urothelial papilloma. J Pathol 2002; 198: 245–251. [DOI] [PubMed] [Google Scholar]
- 40. Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol 2001; 158: 1955–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glaser AP, Fantini D, Shilatifard A, et al. The evolving genomic landscape of urothelial carcinoma. Nat Rev Urol 2017; 14: 215–229. [DOI] [PubMed] [Google Scholar]
- 42. Liu X, Zhang W, Geng D, et al. Clinical significance of fibroblast growth factor receptor-3 mutations in bladder cancer: a systematic review and meta-analysis. Genet Mol Res 2014; 13: 1109–1120. [DOI] [PubMed] [Google Scholar]
- 43. Downes MR, Weening B, van Rhijn BWG, et al. Analysis of papillary urothelial carcinomas of the bladder with grade heterogeneity: supportive evidence for an early role of CDKN2A deletions in the FGFR3 pathway. Histopathology 2017; 70: 281–289. [DOI] [PubMed] [Google Scholar]
- 44. Nelson KN, Meyer AN, Siari A, et al. Oncogenic gene fusion FGFR3-TACC3 is regulated by tyrosine phosphorylation. Mol Cancer Res 2016; 14: 458–469. [DOI] [PubMed] [Google Scholar]
- 45. Williams SV, Hurst CD, Knowles MA. Oncogenic FGFR3 gene fusions in bladder cancer. Hum Mol Genet 2013; 22: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parker BC, Engels M, Annala M, et al. Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J Pathol 2014; 232: 4–15. [DOI] [PubMed] [Google Scholar]
- 47. Di Martino E, Tomlinson DC, Williams SV, et al. A place for precision medicine in bladder cancer: targeting the FGFRs. Future Oncol 2016; 12: 2243–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao Q, Liang WW, Foltz SM, et al. Driver fusions and their implications in the development and treatment of human cancers. Cell Rep 2018; 23: 227–238.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tomlinson DC, L’Hôte CG, Kennedy W, et al. Alternative splicing of fibroblast growth factor receptor 3 produces a secreted isoform that inhibits fibroblast growth factor-induced proliferation and is repressed in urothelial carcinoma cell lines. Cancer Res 2005; 65: 10441–10449. [DOI] [PubMed] [Google Scholar]
- 50. Sfakianos JP, Cha EK, Iyer G, et al. Genomic characterization of upper tract urothelial carcinoma. Eur Urol 2015; 68: 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tabernero J, Bahleda R, Dienstmann R, et al. Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol 2015; 33: 3401–3408. [DOI] [PubMed] [Google Scholar]
- 52. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019; 381: 338–348. [DOI] [PubMed] [Google Scholar]
- 53. Grünewald S, Politz O, Bender S, et al. Rogaratinib: a potent and selective pan-FGFR inhibitor with broad antitumor activity in FGFR-overexpressing preclinical cancer models. Int J Cancer 2019; 145: 1346–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Joerger M, Soo R, Cho BC, et al. Developmental therapeutics Phase I study of the pan-fibroblast growth factor receptor (FGFR) inhibitor BAY 1163877 with expansion cohorts for subjects based on tumor FGFR mRNA expression levels. Ann Oncol 2016; 27(Suppl. 6). [Google Scholar]
- 55. Nogova L, Sequist LV, Garcia JMP, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patientswith advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion stud. J Clin Oncol 2017; 35: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sequist LV, Cassier P, Varga A, et al. Abstract CT326: phase I study of BGJ398, a selective pan-FGFR inhibitor in genetically preselected advanced solid tumors. Cancer Res 2014; CT326–CT326. [Google Scholar]
- 57. Pal SK, Rosenberg JE, Hoffman-Censits JH, et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1–3 inhibitor, in patients with previously treated advanced urothelial carcinoma with FGFR3 alterations. Cancer Discov 2018; 8: 812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dizman N, Rosenberg JE, Hoffman-Censits JH, et al. Infigratinib in upper tract urothelial carcinoma vs urothelial carcinoma of the bladder and association with comprehensive genomic profiling/cell-free DNA results. J Clin Oncol 2019; 37(15 Suppl.): 4510–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moss TJ, Qi Y, Xi L, et al. Comprehensive genomic characterization of upper tract urothelial carcinoma. Eur Urol 2017; 72: 641–649. [DOI] [PubMed] [Google Scholar]
- 60. Shi MJ, Meng XY, Lamy P, et al. APOBEC-mediated mutagenesis as a likely cause of FGFR3 S249C mutation over-representation in bladder cancer. Eur Urol 2019; 76: 9–13. [DOI] [PubMed] [Google Scholar]
- 61. Nakanishi Y, Akiyama N, Tsukaguchi T, et al. The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor. Mol Cancer Ther 2014; 13: 2547–2558. [DOI] [PubMed] [Google Scholar]
- 62. Hyman DM, Goyal L, Grivas P, et al. FUZE clinical trial: a phase 2 study of Debio 1347 in FGFR fusion-positive advanced solid tumors irrespectively of tumor histology. J Clin Oncol 2019; 37(15 Suppl.): TPS3157. [Google Scholar]
- 63. Porta C, Giglione P, Liguigli W, et al. Dovitinib (CHIR258, TKI258): structure, development and preclinical and clinical activity. Futur Oncol 2015; 11: 39–50. [DOI] [PubMed] [Google Scholar]
- 64. Hahn NM, Bivalacqua TJ, Ross AE, et al. A phase II trial of dovitinib in BCG-unresponsive urothelial carcinoma with FGFR3 mutations or overexpression: Hoosier Cancer Research Network trial HCRN 12-157. Clin Cancer Res 2017; 23: 3003–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bellmunt J, Picus J, Kohli M, et al. FIERCE-21: phase Ib/II study of docetaxel + b-701, a selective inhibitor of FGFR3, in relapsed or refractory (R/R) metastatic urothelial carcinoma (mUCC). J Clin Oncol 2018; 36(15 Suppl.): 4534. [Google Scholar]
- 66. Necchi A, Castellano DE, Mellado B, et al. Fierce-21: phase II study of vofatmab (B-701), a selective inhibitor of FGFR3, as salvage therapy in metastatic urothelial carcinoma (mUC). J Clin Oncol 2019; 37(7 Suppl.): 409. [Google Scholar]
- 67. Andre F, Bachelot TD, Campone M, et al. A multicenter, open-label phase II trial of dovitinib, a fibroblast growth factor receptor 1 (FGFR1) inhibitor, in FGFR1- amplified and nonamplified metastatic breast cancer (BC). J Clin Oncol 2017; 29(27 Suppl): 289. [Google Scholar]
- 68. Angevin E, Lopez JA, Pande A, et al. TKI258 (dovitinib lactate) in metastatic renal cell carcinoma (mRCC) patients refractory to approved targeted therapies: a phase I/II dose finding and biomarker study. J Clin Oncol 2009; 27 (15 Suppl.): 3563. [Google Scholar]
- 69. Siefker-Radtke AO, Necchi A, Park SH, et al. First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ-42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt). J Clin Oncol 2018; 36 (15 Suppl.): 4503. [Google Scholar]
- 70. Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 2013; 31: 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19: 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Touat M, Ileana E, Postel-Vinay S, et al. Targeting FGFR signaling in cancer. Clin Cancer Res 2015; 21: 2684–2694. [DOI] [PubMed] [Google Scholar]
- 73. Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn 2008; 237: 18–27. [DOI] [PubMed] [Google Scholar]
- 74. Procopio MG, Laszlo C, Al Labban D, et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol 2015; 17: 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer 2013; 13: 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Choi HJ, Armaiz Pena GN, Pradeep S, et al. Anti-vascular therapies in ovarian cancer: moving beyond anti-VEGF approaches. Cancer Metastasis Rev 2015; 34: 19–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huynh H, Lee LY, Goh KY, et al. Infigratinib mediates vascular normalization, impairs metastasis, and improves chemotherapy in hepatocellular carcinoma. Hepatology 2019; 69: 943–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014; 11: 473–481. [DOI] [PubMed] [Google Scholar]
- 79. Hata AN, Niederst MJ, Archibald HL, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med 2016; 22: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schmitt MW, Loeb LA, Salk JJ. The influence of subclonal resistance mutations on targeted cancer therapy. Vol. 13, Nat Rev Clin Oncol 2016; 13: 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol 2019; 16: 105–122. [DOI] [PubMed] [Google Scholar]
- 82. Shi J-Y, Xing Q, Duan M, et al. Inferring the progression of multifocal liver cancer from spatial and temporal genomic heterogeneity. Oncotarget 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gao Q, Wang ZC, Duan M, et al. Cell culture system for analysis of genetic heterogeneity within hepatocellular carcinomas and response to pharmacologic agents. Gastroenterology 2017; 152: 232–242.e4. [DOI] [PubMed] [Google Scholar]
- 84. Herrera-Abreu MT, Pearson A, Campbell J, et al. Parallel RNA interference screens identify EGFR activation as an escape mechanism in FGFR3-mutant cancer. Cancer Discov 2013; 3: 1058–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang L, Šuštić T, Leite de, Oliveira R, et al. A functional genetic screen identifies the phosphoinositide 3-kinase pathway as a determinant of resistance to fibroblast growth factor receptor inhibitors in FGFR mutant urothelial cell carcinoma [Figure presented]. Eur Urol 2017; 71: 858–862. [DOI] [PubMed] [Google Scholar]
- 86. Barata PC, Koshkin VS, Funchain P, et al. Next-generation sequencing (NGS) of cell-free circulating tumor DNA and tumor tissue in patients with advanced urothelial cancer: a pilot assessment of concordance. Ann Oncol 2017; 28: 2458–2463. [DOI] [PubMed] [Google Scholar]
- 87. Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol 2015; 22: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 88. Schepisi G, Santoni M, Massari F, et al. Urothelial cancer: inflammatory mediators and implications for immunotherapy. BioDrugs 2016; 30: 263–273. [DOI] [PubMed] [Google Scholar]
- 89. Harlin H, Meng Y, Peterson A, et al. Chemokine expression in melanoma metastases associated with. Cancer Res 2009; 69: 3077–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012; 61: 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A 2007; 104: 3967–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sweis RF, Spranger S, Gajewski T. Molecular drivers of the non-T cell-inflamed tumor microenvironment in urothelial bladder cancer. J Clin Oncol 2019; 33(15 Suppl.): 4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Siefker-Radtke AO, Currie G, Abella E, et al. FIERCE-22: clinical activity of vofatamab (V) a FGFR3 selective inhibitor in combination with pembrolizumab (P) in WT metastatic urothelial carcinoma, preliminary analysis. J Clin Oncol 2019; 37(15 Suppl.): 4511. [Google Scholar]
- 94. Takamura T, Horinaka M, Yasuda S, et al. FGFR inhibitor BGJ398 and HDAC inhibitor OBP-801 synergistically inhibit cell growth and induce apoptosis in bladder cancer cells. Oncol Rep 2018; 39: 627–632. [DOI] [PubMed] [Google Scholar]
- 95. Traer E, Martinez J, Javidi-Sharifi N, et al. FGF2 from marrow microenvironment promotes resistance to FLT3 inhibitors in acute myeloid leukemia. Cancer Res 2016; 76: 6471–6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cao Z, Scandura JM, Inghirami GG, et al. Molecular checkpoint decisions made by subverted vascular niche transform indolent tumor cells into chemoresistant cancer stem cells. Cancer Cell 2017; 31: 110–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li Q, Ingram L, Kim S, et al. Paracrine fibroblast growth factor initiates oncogenic synergy with epithelial FGFR/Src transformation in prostate tumor progression. Neoplasia 2018; 20: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]