Abstract
Faecal microbiota transplant (FMT) has now been established into clinical guidelines for the treatment of recurrent and refractory Clostridioides difficile infection (CDI). Its therapeutic application in inflammatory bowel disease (IBD) is currently at an early stage. To date, there have been four randomized controlled trials for FMT in IBD and a multitude of observational studies. However, significant gaps in our knowledge regarding optimum methods for FMT preparation, technical aspects and logistics of its administration, as well as mechanistic underpinnings, still remain. In this article, we aim to highlight these gaps by reviewing evidence and making key recommendations on the direction of future studies in this field. In addition, we provide an overview of the current evidence of potential mechanisms of FMT in treating IBD.
Keywords: faecal microbiota transplantation, inflammatory bowel disease, microbiota
Introduction
The potential role of faecal microbiota transplant (FMT) has been explored in the treatment of a multitude of diseases, both gastrointestinal (GI) and non-GI, structural and functional in nature. A significant proportion of the initial evidence for its efficacy was derived from observational studies, primarily in recurrent Clostridioides difficile infection (CDI).1–4 Despite the small size and heterogeneity of early studies, the consistently positive findings led to randomized controlled trials (RCTs), which confirmed the therapeutic role of FMT for this indication.5 It is recognized that distinctive alterations of both the composition and function of the gut microbiota characterize other GI diseases as well as other non-GI conditions.6 In particular, some of the initial evidence for its utility in inflammatory bowel disease (IBD) came from subgroup analysis of RCTs of CDI and a handful of observational studies.1–5 Although we may be able to translate our knowledge around principles and methodology from FMT studies in CDI, there are specific uncertainties and challenges for the therapeutic use of FMT for IBD that need to be addressed. In this review, we aim to summarize the current gaps in our knowledge regarding FMT in IBD from a clinical and mechanistic perspective, and to highlight potential areas to target and optimize in future studies.
Current state of evidence for therapeutic use of FMT
The largest evidence base for the therapeutic use of FMT has been built around recurrent and, to a lesser extent, refractory CDI. There have been over 30 observational studies1 and 10 RCTs5 conducted to date concerning the use of FMT in the treatment of CDI. These studies have had variable methodology, reflecting the fact that there previously had been few detailed protocols or guidelines that addressed issues around the technical aspects of processing of stool into FMT, mode of administration into the patient and, perhaps most importantly, the definition of cure and relapse. Although the quality of evidence for refractory CDI is weaker, there is also little consensus on the definition of refractory CDI, with some studies using the terms ‘recurrent’ and ‘refractory’ interchangeably.1–5 Taking these factors into consideration, a meta-analysis of these studies shows that the overall efficacy of FMT for recurrent and refractory CDI is 92%.1 Consequently, FMT has now been established into clinical guidelines for the treatment of recurrent and refractory CDI.2–4
The emergence of next-generation sequencing was one catalyst for further FMT studies. Previously, our understanding of the gut microbiota relied on culturing techniques that were time consuming and required specific conditions to optimize bacterial growth. Such studies were limited by the fact that strictly anaerobic components of the microbiome may be difficult or impossible to culture. Furthermore, culturing techniques are inadequate in aiding our understanding of the other integral elements of the gut microbiota such as the virome, mycobiome and archaea. Next-generation sequencing has allowed us deeper understanding not only of the composition of the gut microbiota, but also regarding functionality. The integration of multiomic platforms may provide us with valuable insights into host–microbiota interactions that are associated with disease and response to therapy.7 As mentioned above, FMT has now been established into clinical guidelines for the treatment or recurrent and refractory CDI; however, gaps still remain. While this review focuses mainly on the gaps in IBD, some areas of interest in recurrent and refractory CDI still include duration of effect, long-term safety, predictive biomarkers of efficacy and sequencing of treatment to first-, second-line or adjunctive therapy.
Summary.
FMT is established into clinical guidelines for the treatment of recurrent and refractory CDI.
Studies exploring FMT in UC
There is a growing evidence base for the use of FMT in the treatment of ulcerative colitis (UC). A systematic review and meta-analysis by Paramsothy and colleagues demonstrated that of the 555 patients (treated to that point), 36% (201/555) of UC patients achieved clinical remission.7 Among the 24 cohort studies (307 patients), the pooled proportion of patients achieving clinical remission was 33% (102/307) [95% confidence interval (CI): 23–43%], with a moderate risk of heterogeneity and no publication bias.7,8 All four RCTs included patients with mild-to-moderate UC. At 8 weeks, 37% of FMT participants achieved remission compared with 18% in the control arm [RR 2.03, 95% CI, 1.07 to 3.86; I2 = 50%].8,9 The pooled rate for achieving the combined clinical and endoscopic remission in these studies was 27.9% for those receiving FMT and 9.5% in the control interventions, with a number needed to treat of 5.10
Summary.
There is emerging evidence that FMT for mild-to-moderately active UC is a safe and efficacious treatment for the induction of remission. Current studies are heterogeneous in study design and have short duration of follow up which makes interpretation of best practice difficult.
Studies exploring FMT in Crohn’s disease
FMT for Crohn’s disease (CD) has been underexplored compared with UC. There are no published RCTs for FMT in CD. A handful of cohort studies contain no more than 30 patients.11 CD is a heterogeneous disease affecting various parts of the GI tract, and CD phenotype may influence the potential efficacy of FMT, especially given the differences in microbiota composition and functionality at different parts of the GI tract. These limited studies using FMT for CD have shown mixed response rates ranging from 0% to 87%.11–14 Further compounding the difficulty in interpreting these studies is the heterogeneity of disease severity. Two studies defined the treatment group as ‘moderate to severe disease’,12,14 one defined the treatment group as ‘mild disease’13 and the other ‘active CD’, without defining severity. There were many other variations between these studies, including the utilization of fresh13,14 or frozen stool, route of FMT delivery, follow-up period and outcome measures. It is therefore difficult to compare studies using FMT for CD due to the heterogeneity in study design, which was highlighted in a meta-analysis.15 Despite the heterogeneity, however, remission rates may be comparable with current therapies. FMT for CD therefore has promise, but to accurately assess the efficacy of FMT in CD, there remains a need for RCTs that carefully define CD phenotype, outcome definitions and follow-up periods. It is feasible that the different distributions of CD may respond differently to FMT, and these need to be studied in isolation.
Summary.
There remains a need for RCTs using FMT for the treatment of Crohn’s disease. A well-defined study cohort is required, taking into account the extent and severity of disease, and perhaps studied in isolation.
Studies exploring FMT in pouchitis
The aetiopathogenesis of pouchitis has been associated with alterations in the pouch microbiota.16 A systematic review has highlighted that medications such as antibiotics achieve remission in chronic pouchitis in about 74% of cases, suggesting that alteration of the bacterial microbiota may play a role in aetiology.17 This observation has led to early attempts at manipulating the gut microbiota to achieve remission through various mechanisms in addition to antibiotics; such techniques have included probiotics, prebiotics and more recently, FMT.18 The first FMT study was undertaken by Landy and colleagues, who delivered nasogastric FMT to eight patients with chronic pouchitis. In this study, none of these patients achieved remission. Despite this failure, there were two patients who regained sensitivity to ciprofloxacin following FMT.18 The second study by Stallmach and colleagues reported outcomes on five patients who received FMT via endoscopic delivery to the jejunum; four of these patients received repeated FMT via enemas. In this cohort, two patients achieved clinical remission within 3 months and two showed clinical improvement.19 A pilot randomized placebo-controlled study looking at the efficacy of oral FMT was halted due to lack of clinical efficacy.20 There therefore remain many unknowns about the utility of FMT in pouchitis. As pouchitis is a relatively rare disease, multicentre trials are required to address utility of FMT. In similarity to other unanswered questions in FMT, key unknowns in treating this cohort include the optimum route of delivery, the ‘dose’ of FMT and the frequency of FMT. As pouchitis is a disease of the remaining distal bowel, it is likely that a topical route may be the optimal route of administration, which may account for the lack of remission rates by the nasogastric route and the capsulized/oral route.20
Summary.
There is some potential for a role of FMT in treating pouchitis although the evidence is currently poor. As a rare disease, multicentre studies are likely needed to inform our understanding of the utility in this disease.
Issues in study design: what is needed to help bridge the gaps in FMT IBD studies
Inclusion/exclusion criteria
When considering the RCTs in UC studies, all patients were reported to have mild-to-moderately active UC at baseline; despite this apparent similarity, studies included patients on corticosteroids, with only two mandating a wean of corticosteroids. While all studies allowed patients to be on stable medication in the form of 5-aminosalicylic acid (5-ASA) and immunomodulators, two studies included patients on concurrent biologics. See summary of RCTs in Table 1.
Table 1.
RCTs for UC patients treated with FMT.
| Moayyedi et al.21 | Rossen et al.22 | Paramsothy et al.23 | Costello et al.24 | |
|---|---|---|---|---|
| Patients (n) | 75 | 50 | 81 | 73 |
| Male | 44 | 22 | 47 | 34 |
| Age in years FMT versus placebo | 35.8 versus 42.1 (mean) | 40 versus 41 (median) | 35.6 versus 35.4 (median) | Data not available |
| Patients in the intervention group (n) | 38 | 23 | 40 | 38 |
| Disease severity for inclusion | Mayo score ⩾ 4 | SCCAI ⩾ 4 to ⩽11 | Mayo score 4–10 | Mayo score 3–10 |
| Mayo endoscopic score ⩾ 1 | Mayo endoscopic score ⩾ 1 | Mayo endoscopic score ⩾ 1 | Mayo endoscopic score ⩾ 2 | |
| Physicians global score ⩽ 2 | ||||
| Permitted concomitant therapies | Stable dose: | Stable dose: | Stable dose: | Stable dose: |
| 5-ASAs | Mesalamine | 5-ASAs | 5-ASAs (4 weeks) | |
| Thiopurines | Thiopurines | Immunomodulators including MTX | Immunomodulators including MTX (6 weeks) | |
| Anti-TNFs (12 weeks prior to inclusion) | Biologics 8 weeks | |||
| Glucocorticosteroids (4 weeks prior to inclusion) | Corticosteroids (⩽10 mg/day, 8 weeks prior to inclusion) | Tapering prednisolone (at ⩽20 mg/day) | Tapering prednisolone (at ⩽25 mg/day) | |
| Excluded therapies/other | Probiotics Antibiotics | Anti-TNF or MTX within 8 weeks Cyclosporin within 4 weeks |
Probiotics Antibiotics Anti-TNF Calcineurin inhibitors within 12 weeks Rectal therapies |
Probiotics Antibiotics Anticoagulant therapy |
| Other exclusion criteria | Concomitant infection Pregnancy Severe illness requiring hospitalization |
Severe illness requiring hospitalization | GI infection, proctitis, indeterminant colitis, major comorbidities, major food allergy, IBS, history of bowel cancer and pregnancy | GI infection, previous colonic surgery Pregnancy |
| Definition of remission | Total Mayo < 3 with endoscopic Mayo = 0 | SCCAI ⩽ 2 with ⩾1-point improvement in the endoscopic Mayo score | Total Mayo ⩽ 2 with subscores ⩽ 1 for rectal bleeding, stool frequency, and endoscopic appearance; and a ⩾1-point reduction in endoscopic subscore | Total Mayo ⩽ 2 with endoscopic Mayo ⩽ 1 |
| Definition of clinical remission | Data not available | SCCAI ⩽ 2 | Combined Mayo score ⩽ 1 for both rectal bleeding + stool frequency | SCCAI ⩽ 2 |
| FMT received from/donors | Single donor | Single donor | Blended stool | Blended stool |
| 6 anonymous healthy unrelated adult donors, 1 partner | 13 anonymous healthy unrelated and related adult donors; 1 partner, 1 friend | From 3 to 7 healthy unrelated donors | From healthy unrelated donors | |
| 6 recipients had 2 different donors | Patients received all infusions from the same batch | |||
| Patient preparation | No bowel lavage | Bowel lavage with 2 l macrogol solution, 2 l clear fluids | Bowel lavage, not specified | Bowel lavage with 3 l of polyethylene glycol the evening prior and loperamide 2 mg orally prior to colonoscopy |
| FMT preparation Fresh versus frozen | Administered fresh and frozen | Administered fresh within 6 h of donation | Frozen–thawed FMT | Frozen–thawed FMT |
| Stored at −20°C | Stored at −80°C then home freeze at −20°C | Stored at −80°C | ||
| 21 receiving frozen−thawed stool, 15 received fresh stool; 1 patient was given both fresh and frozen stool on different weeks | ||||
| Placebo preparation | Water | Autologous faecal suspension | Isotonic saline, colourant, odourant | Autologous stool in saline |
| Post-FMT medications | No proton-pump inhibitor, prokinetic or loperamide | No proton-pump inhibitor, prokinetic or loperamide | No proton-pump inhibitor, prokinetic or loperamide | No proton-pump inhibitor or prokinetic Loperamide prior |
| FMT delivery | Retention enema | Nasoduodenal tube | 1 colonoscopy followed by 40 enemas | 1 colonoscopy followed by 2 enemas |
| FMT frequency | Weekly enemas for 7 weeks | 1 at week 0 and then at week 3 | 1 colonoscopy followed by 40 enemas (5 times a week for 8 weeks) | 1 colonoscopy followed by 2 enemas (per week, apart from in week 1) |
| Total number of infusions | 7 | 2 | 41 | 3 |
| Dose | 8.3 g of stool with each enema | 60 g of stool with each treatment | 37.5 g of stool with each treatment | 50 g of stool with colonoscopy; then 2 enemas a week apart with 25 g each |
| Total dose across study period | 58 g | 120 g | 1537 g | 100 g |
| Volume/dose | 50 ml FMT with 8.3 g of stool per enema | 500 ml of FMT with 60 g of stool, divided into in multiple nontransparent syringes | 150 ml isotonic saline with 37.5 g stool was then followed by enemas (volume/dose data not available) | 200 ml of FMT with 50 g stool, with two further 100 ml aliquots of the same faecal suspension (25 g of stool each) |
| Primary endpoint | Remission: total Mayo score < 3, endoscopic Mayo score = 0 | Remission: SCCAI ⩽ 2, endoscopic Mayo score reduction ⩾ 1 | Remission: steroid free: total Mayo ⩽ 2 All Mayo subscores ⩽ 1 plus reduction ⩾ 1 in endoscopic subscore |
Remission: steroid free: total Mayo score ⩽ 2 Endoscopic Mayo score ⩽ 1 |
| Achievement of primary endpoint/clinical remission: FMT versus placebo | 24% (9/38) versus 5% (2/37) | 30.4% (7/23) versus 20% (5/25) | 27% (11/41) versus 8% (3/40) | 32% (12/38) versus 9% (3/35) |
| Significance | p = 0.03 | p = 0.51 | p = 0.021 | p < 0.01 |
| Clinical response FMT versus placebo | 39% (15/38) versus 24% (9/37) | 48% (11/23) versus 52% (13/25) | 54% (22/41) versus 23% (9/40) | 55% (21/38) versus 20% (7/35) |
| Significance | p = 0.16 | p = 0.58 | p < 0.01 | p < 0.01 |
| Follow up | 12 weeks | 12 weeks | 8 weeks | 8 weeks |
| Further reviews | At 12 months | Data not available | Data not available | At 12 months |
| Worsening colitis | 1 placebo (required colectomy) | 3 placebo, 3 FMT | 4 placebo, 2 FMT including 1 colectomy | 2 placebo, 1 FMT |
| Other adverse events and serious adverse events | Colectomy (placebo); CDI (FMT) | Small bowel CD (FMT); CMV infection (placebo) | 6 (2 with FMT, 1 placebo, 3 that progressed to open-label FMT) | CDI requiring colectomy (FMT) |
CD, Crohn’s disease; CDI, Clostridioides difficile infection; CMV, cytomegalovirus; 5-ASA, 5-aminosalicylic acid; FMT, faecal microbiota transplant; GI, gastrointestinal; MTX, methotrexate; RCT, randomized controlled trial; SCCAI, Simple Clinical Colitis Activity Index; TNF, tumour necrosis factor; UC, ulcerative colitis.
Summary.
It is currently difficult to compare RCTs due to heterogeneity in inclusion/exclusion criteria. Future studies should determine if baseline characteristics, concomitant medications and previous treatments influence the success of FMT.
Primary/secondary endpoints
All studies had clinical response, clinical remission, and endoscopic remission as either a primary or secondary outcome. However, the RCT by Costello and colleagues defined endoscopic and clinical remission with a Mayo endoscopic score ⩽ 1,24 whereas all the other three RCTs had a strict definition of Mayo endoscopic score of 0 at 8 weeks.21–23 It is possible that this choice is a potential confounder and may have contributed to the higher rates of remission and response seen in the RCT by Costello and colleagues. Across the studies, three out of the four showed a positive effect,21,23,24 with Moayyedi and colleagues showing that 24% of patients reached clinical remission at 8 weeks compared with placebo (5%), although none achieved an endoscopic Mayo score of 0.21 On the other hand, Rossen and colleagues demonstrated that 30.4% of patients were in clinical remission after receiving FMT compared with 20% who received placebo at 12 weeks.22 Paramsothy and colleagues’ study demonstrated a positive effect too, with 27% of patients in clinical remission at 8 weeks compared with placebo (8%), and most recently, Costello’s study showed similar effects, with 32% of patients in clinical remission at 8 weeks compared with placebo (9%;23 Table 1 for details).
Summary.
A standardized definition of remission and standardized outcomes are required to compare efficacy of FMT in UC.
Preparation prior to FMT
Of the RCTs in UC, Moayyedi’s study was the only study not to use a bowel lavage pre-FMT, while the other remaining studies, all with variable routes of delivery, used different types of bowel lavage, again, with varying results.21–24
In reality, to perform a successful colonoscopy to the terminal ileum, bowel preparation is almost certainly required. Furthermore, no studies show directly whether bowel lavage has any impact long term. Studies are therefore required to compare treatment effectiveness with or without bowel lavage on a standardized protocol, with long-term follow up. Additionally, the choice of bowel preparation needs clarification and standardization.
Summary.
For colonoscopically delivered FMT, bowel lavage will almost certainly be needed from a technical perspective. Studies are required to identify the bowel lavage of choice. From a mechanistic point of view, gaps remain on the impact bowel lavage has on efficacy regardless of route of delivery. Studies investigating mechanisms and engraftment will help with this understanding.
Pre- and post-FMT medications
Proton-pump inhibitors (PPIs) have been shown to alter the gut microbiota and have been associated with primary and recurrent CDI.25–27 The rationale for the use of PPIs prior to upper GI FMT is to minimize acidity which may impair microbial engraftment.25 None of the four currently published studies of FMT in UC had PPIs as part of their protocol.21–24
The use of prokinetics has been used prior to FMT delivery via the upper GI route, but only in a number of small studies,28 and not in any of the RCTs for UC. However, with the risks of aspiration, the use of prokinetics should be considered where appropriate.2
A single dose/short course of loperamide has been used following FMT (mainly with lower-GI delivery), when treating CDI, with the aim of prolonging exposure of the FMT to the GI tract.29,30 In UC, the only RCT to mention the use of loperamide was Costello’s study,24 administering loperamide 2 mg prior to the index colonoscopy.
The appropriateness of a short course of antibiotics prior to FMT is an area of interest, since it is proposed that this may create an ecological niche that will promote colonization of microbial communities derived from the FMT. None of the 4 RCTs used pre-FMT antibiotics in their protocols,21–24 although meta-analysis of the cohort studies suggests a potential benefit, with 33% of UC patients who received a course of antibiotics before FMT achieving clinical remission compared to 28% who did not (95% CI 17–54% p = 0.026).15
It is again clear that there is limited evidence to support medicating patients pre- and post-FMT with PPI, prokinetics, loperamide and antibiotics. However, the theoretical benefits (at least for a prokinetic in the upper GI route) seems reasonable.
Summary.
The optimum preparation of the recipient is still not yet established. Future studies should attempt to establish the optimum preparation required for the patient to precipitate FMT efficacy with a pragmatic approach likely to involve animal models.
Route, dose/volume and frequency of infusions
All four RCTs published to date used different methods of administering FMT; specifically, using different time lines, intensity, frequency and doses21–24 (See Table 1 for summary of RCT designs).
The studies by Moayyedi and colleagues and Rossen and colleagues delivered FMT via enemas and the nasoduodenal route, respectively, with total doses of FMT at 58 g and 120 g stool reached respectively.21,22 Paramsothy and colleagues’ and Costello and colleagues’ studies, administered FMT via colonoscopy and subsequent enemas, delivering 1537 g and 100 g stool, respectively. 23,24 Both studies differ in intensity and dosing, with Paramsothy and colleagues’ study delivering an overall high dose of FMT over a prolonged period of 8 weeks, compared with Costello and colleagues’ smaller dose given in a more intensive regime in 1 week. Of note, a previous systemic review of a case series using FMT for CDI highlighted an approximate fourfold increase in recurrence rates if less than 50 g of stool was used compared with over 50 g.2 Although each study varies in dose, each RCT in UC delivers a total dose of over 50 g stool.
Each RCT investigating FMT in UC used multiple infusions of FMT; however, their similarities stop there. Each RCT used variable numbers of infusions; specifically, either 2, 3, 7 or 41 infusions. This makes interpreting and comparing efficacy between studies difficult, especially as this is compounded by the differences in dose and FMT delivery as alluded to above.
There is also variability in the total volume of FMT suspension and ‘concentration’ of stool used across the RCTs for UC. Regardless of mode of delivery, volume of FMT preparation and ‘concentration’ of stool is heterogenous (see Table 1 for details). This is also mirrored with studies for FMT use in CDI for both upper- and lower-GI modes of delivery.2
These studies suggest promise for FMT in treating UC, but there is huge disparity comparing routes of FMT delivery, as well as dose of FMT and frequency of administration. Furthermore, as studies for CDI showed no clear difference in efficacy between upper- and lower-GI delivery,3 perhaps more studies are required to power for a middle–higher-dose regime delivered via upper GI versus lower GI.
Summary.
Studies are required to identify the optimal route, dose and frequency of FMT in IBD.
Donors
In IBD, the majority of case studies use FMT from a single donor. The four RCTs differ in this aspect. Moayyedi and colleagues’ and Rossen and colleagues’ studies used stool from a single donor, unrelated and related, respectively21,22 Conversely, Paramsothy and colleagues’ and Costello and colleagues’ studies both used FMT from unrelated multiple donors (3–4 and 3–7, respectively), with multiple infusions of 40 and 3, respectively, with both studies showing a positive result.23,24 In Moayyedi and colleagues’ study, one of the donors received antibiotics and did not donate for 4 months. Rossen and colleagues’ study specified the screening using the Dutch Red Cross Questionnaire, screened bloods and stool for bacterial, parasitic and viral pathogens. Donors were not allowed antibiotics within 8 weeks before screening. Paramsothy and colleagues’ and Costello and colleagues’ studies similarly used a rigorous screening process.
In theory, multidonor FMT has the potential to increase gut microbiota diversity to a greater degree than that from a single donor, but a clear drawback is the difficulty identifying the microbiota characteristics that may explain any benefits seen. Specifically, the utilization of single donors allows close interrogation of the microbial community, making it possible to isolate specific organisms or metabolites traceable back to the source. In addition, while transplant of a single healthy donor’s microbiota would be expected to represent transfer of a stable microbial ecosystem,31 mixing of gut microbiota from different donors may result in competition between different established microbial communities (e.g. for limited nutrient resources); this has uncertain effects upon the final microbiota transferred into recipients and consequent efficacy of the FMT. Studies are clearly required to compare single donor versus multidonor application. Further aspects related to optimal donor selection are discussed in the section below, ‘What’s Missing From These Trials’?.
Summary.
Studies are essential to highlight what makes an optimum donor. There may also be a role for donor optimization through diet, medications and pretreatment. Currently, we do not biologically match donor to recipient (which needs to be explored). To enable this, good quality mechanistic studies are required.
Frozen versus fresh FMT
There is also marked heterogeneity with regards to recipients of fresh or frozen stool. Even within Moayyedi and colleagues’ study, there appears to be variability, with 21 patients receiving frozen–thawed stool, 15 patients received fresh stool and 1 patient was given both fresh and frozen stool on different weeks.21 Rossen and colleagues’ study used fresh stool (60 g of stool in 500 ml preparation) divided into nontransparent syringes to conceal contents and was administered within 6 h after production; the procedure was repeated 3 weeks later.30 Paramsothy and colleagues’ and Costello and colleagues’ studies both used frozen–thawed FMT.23,24 What these studies suggest is that FMT for UC is effective using either fresh or frozen FMT, as is the case in treating CDI.32 However, methodological variability makes it difficult to draw conclusions about the comparability of fresh versus frozen FMT in the treatment of UC.
Comparisons have been directly made in RCTs involving FMT and CDI. Lee and colleagues’ study32 concluded that enema-administered frozen FMT (n = 91) was non-inferior for clinical resolution of diarrhoea to fresh FMT (n = 87). A separate RCT33 showed statistically comparable remission rates for recurrent CDI with fresh or frozen FMT delivered colonoscopically (p = 0.233), using fresh FMT stored at −80°C for up to 6 months. Guidelines for CDI treatment advocate that FMT stored frozen at −80°C should be regarded as having a maximum shelf life of 6 months, and used within 6 h of thawing.2 In the three RCTs that used frozen FMT for UC, it is unclear how long frozen FMT was stored prior to usage, but it has been shown that sample preparation can alter both the microbiome and metabolome.34 There are, again, differences with regards to storage temperature, with Moayyedi and colleagues’ study storing FMT at −20°C, while the Paramsothy and colleagues’ and Costello and colleagues’ studies stored FMT at −80°C.
Summary.
Studies are required to compare fresh versus frozen FMT in UC, with a view to identifying optimum administration, as well as defining how long the product can be kept frozen before usage.
Placebo
There is variation in the placebo used for the control arm in the IBD RCTs: aerobically or anaerobically prepared autologous stool,22,24 water21 and saline with an odourant and colourant23 have all been used. Furthermore, there is disparity in opinion about what the ideal placebo is. Studies in CDI have shown that autologous stool results in a much higher rate of response in comparison with nonstool-based placebo. The ‘inertness’ of a colourant is also questionable. Use of water placebo potentially prevents adequate blinding and can lead to several forms of bias.
Variable follow up and lack of long-term data: how long does the efficacy of FMT last?
Follow up across the RCTs is variable and unclear. In Moayyedi and colleagues’ study, the 37 patients were followed up for 12 months.21 Rossen and colleagues’ study describes follow up to 12 weeks.22 Paramsothy and colleagues followed up patients up to 8 weeks, with no long-term outcomes23 or further treatments mentioned. Costello and colleagues, likewise, followed up patients to 8 weeks. Secondary endpoints also included clinical and endoscopic remission at 12 months.22
Summary.
Future FMT–IBD studies require both short-term and long-term follow-up data, with defined endpoints and standardized criteria for clinical and endoscopic remission. Furthermore, how long should patients be followed up in studies centred around induction of remission? Long–term safety complications also need to be factored.
Other long-term considerations
Beyond efficacy, there remain some unknowns regarding other long-term effects of FMT. Donor screening guidelines suggest an extensive screening programme prior to donation of FMT to reduce the risk of transmitting infections to the recipient.2,35 Despite these criteria, there is no established quantifiable risk of transmission of infections in the literature. Specifically, many IBD patients who may be considered for FMT may be immunocompromised and hence, theoretically, may have a higher risk of contracting a transmissible infection. Despite this fact, emerging evidence suggests that even in immunosuppressed patients, FMT can be used safely and effectively21,22,30,33,36 provided the screening of donors has been thorough. However, the risk of administering FMT to IBD patients who may be immunocompromised is currently unknown.
There remains a significant issue regarding the meaning and significance of ‘engraftment’. A large gap remains about the definition of engraftment of the donor microbiome to the recipient. Furthermore, we currently do not know (due to lack of strain-level data) what the microbiome in the recipient looks like after long term-follow up. Additionally, based on a paper which showed a faecal filtrate was effective in CDI treatment, the question about the mechanistic importance of engraftment remains unknown.37
There also remain unknowns regarding microbiota-mediated conditions that may be associated with FMT. Specifically, it has been suggested that phenotypes from the donor may be transferred to the recipient, although no such changes have been convincingly demonstrated in practice at present. On a more significant scale, there is growing evidence that the microbiota may be associated with many disease pathways.38–40
Summary.
The risk of transmission of specific donor features, including infection and phenotype to a patient with IBD, needs to be further established using long-term follow up. Furthermore, studies on the role of FMT in immunocompromised IBD patients is required.
What is missing from these trials?
Even in the case of the archetypal success story for FMT of recurrent CDI, there remains a small but appreciable failure rate, with little knowledge on whether the failure of FMT is related to donor or recipient factors, and subsequently, no currently accepted biological means of optimally matching donor and recipient. This issue is even more pronounced in the scenario of FMT for UC, where, in contrast to the scenario of CDI (where almost any donor may be used with high efficacy). There appears to be much more marked variability in success dependent upon the donor used. One often-quoted example of this was the finding from the RCT of Moayyedi and colleagues21 of apparent greater response rates with one donor (‘donor B’) than other donors [i.e. treatment success in 39% (7/18) patients treated with donor B versus 10% (2/20) patients treated with other donors]. In this case, an increased relative abundance of the family Lachnospiraceae and genus Ruminococcus within the stool microbiota of donor B compared with other donors was a hypothesized explanation for donor B’s high efficacy. However, while the concept of a ‘super donor’ remains attractive,41 there remains limited knowledge as to what specific aspects of gut microbiota composition or functionality are most desirable at present.
Summary.
Both the practical drawbacks of FMT and the drive to minimize treatment failure are strong drivers for research to better elucidate mechanistically how and why FMT impacts upon disease activity in UC. Not only might this give further insight into the pathogenesis of IBD, but this may provide knowledge that might be exploitable into the development of targeted ‘microbial therapeutics’ that have the same physiological effects of FMT but avoid some of the difficulties associated with it.
Mechanistic understanding of how FMT works
CDI
A framework of how such research might be performed is provided by such studies undertaken to explore the mechanisms of efficacy of FMT for the treatment of recurrent CDI. One surprising early pilot study within this field used sterile filtered faecal filtrate as an alternative to conventional FMT in five patients with rCDI, and demonstrated comparable treatment efficacy.37 While much prior thinking had focused on the effects of FMT upon microbiota composition, this study reasserted a focus on microbiota functionality, suggesting as it does that FMT’s efficacy in treating rCDI may be explained by restoration of microbial components (including proteins, metabolites, bacteriophages or other soluble mediators), rather than a requirement for viable microorganisms.37
Furthermore, there is also a growing understanding of the role of host–microbiota cometabolites in the efficacy of FMT for rCDI. In a chemostat model of CDI in the distal gut, the five-carbon short-chain fatty acid (SCFA) valerate did not recover after antibiotic cessation alone, but was rapidly restored and maintained after FMT.42 Valerate supplementation was shown to directly impair the vegetative growth of C. difficile (without any adverse effect upon gut commensal bacteria), and to reduce stool C. difficile counts in a mouse model of CDI.42 In addition, CDI has also been shown to be characterized by marked perturbation of host bile-acid metabolism, with enrichment of primary conjugated bile acids and loss of secondary bile acids within the gut,9,43 as well as reduced ileal farnesoid X receptor signalling.44 Such disturbances in bile metabolism are reversed by successful FMT; this is of significance, since primary conjugated bile acids (and particularly, taurocholic acid) are potent triggers for the germination of C. difficile, while secondary bile acids (and particularly, deoxycholic acid) inhibit the vegetative growth of the organism.45 More recently, it has been demonstrated that patients with recurrent CDI have markedly reduced gene load and activity of the microbiota-derived enzyme bile salt hydrolase (BSH), which is responsible for deconjugation of primary bile acids such as taurocholic acid; furthermore, gut BSH functionality is restored through successful FMT.46,47 The demonstration that FMT may enrich the gut microbiota for functionalities related to SCFA production and bile-acid metabolism may be relevant for IBD, given the demonstration of their perturbation in IBD, and that reversal may help aid resolution of colitis via multiple mechanisms.48,49 However, many of these studies have been performed in rodent and in vitro models, and their transferability to human IBD is uncertain.
UC
Regarding mechanistic analysis of human studies of FMT for UC, successful FMT for UC is shown to be associated with gut microbiota enrichment in microbial members known to produce the SCFA butyrate, as well as increased gene copy number of the ButCoA (butyryl coenzyme A transferase) gene.50 However, stool SCFA levels themselves did not change, a finding that was replicated elsewhere.24 Furthermore, a recent analysis of samples taken from the RCT by Paramsothy and colleagues demonstrated that enrichment of the gut microbiota with members of the genus Bacteroides, and species Eubacterium hallii and Roseburia inulinivorans, was associated with remission.23 Importantly, however, it is unclear whether these bacterial taxa were donor derived and engrafted post-FMT, or represent regrowth of pre-existing recipient strains whose growth was suppressed in the intestinal milieu that existed pre-FMT. Remission was also associated with microbiota biosynthetic capacity for SCFA and secondary bile-acid production.51
Overall, our mechanistic understanding of how host–microbiota interactions are influenced by FMT for UC is still in its infancy. Metataxonomic studies demonstrating that enrichment of particular bacterial taxa in the gut microbiota post-FMT is associated with UC remission does not in itself demonstrate donor-to-recipient transmission. For instance, one alternative explanation may be that successful FMT alters an aspect of the gut metabolic or inflammatory milieu that facilitates growth of bacteria already in the recipient microbiota, but whose growth was previously suppressed. Recent developments in standardization of protocols for omic technologies (including cultureomics, metabonomics, metaproteomics and metatranscriptomics), refinements in the databases associated with their use, and the development of bioinformatics expertise that can integrate such complex datasets50–52 mean there is much promise for future studies in this area being able to elucidate with greater clarity the specific effects of FMT upon UC.49
Summary.
Future studies should move away from studies based on amplicon sequencing and towards metagenomics and transcriptomic effects that can provide information about strain engraftment from donors and indicate, rather than impute, functionality.
Understanding of virome and mycobiome
Our understanding of the gut virome and mycobiome in IBD is in its infancy and much less is known about the role of FMT in IBD and its influence on the gut virome and mycobiome.
There is an emerging evidence base for an association between bacteriophages and FMT outcome in recurrent CDI. The gut viromes of recurrent CDI patients receiving FMT rapidly resemble those of the donor, with particular reduction in relative abundance of bacteriophages of the order Caudovirales; this effect is maintained at 12 months post-FMT.52,53 FMT was more likely to be successful if donors had a higher fraction of Caudovirales colonizing their stool virome.53 Furthermore, Broecker and colleagues highlighted that when FMT was used to treat CDI, phages were equally abundant in the treatment-responsive patient and donors,54 while also highlighting that a healthy patient was characterized by a low phage abundance. This observation therefore leads us to conclude that during FMT, a core set of viruses were possibly transferred to the recipient. Furthermore, Draper and colleagues highlighted that in CDI, there is a disturbance in the enteric gut virome (characterized by increased Caudovirales, decreased Microviridae, and increased Anelloviridae abundance when compared with healthy donors) that was restored and persisted with FMT. Despite these findings, they were unable to detect specific viruses responsible for the success of FMT. In a murine experiment, mice were given antibiotics to disturb the gut microbiota, and following this, mice were randomized to receive a faecal virome transplant (FVT) or no FVT. Interestingly, FVT-treated mice were able to restore their bacterial composition to preantibiotic levels, whereas untreated mice were not, suggesting that the virome and bacterial composition of the gut microbiome are intricately linked.55,56
To date, there has been one study that explores the role of the virome in IBD-FMT. In this paediatric UC study, it was demonstrated that a recipient of FMT from an adult donor received a transfer of numerous phages. The authors suggested that the transfer of phages was a characteristic of FMT. They further highlighted that the stability of the phage populations and the long-term clinical significance of phage transfer, however, will require further investigation.57
Less is known about the role of the gut mycobiome in IBD. It has been demonstrated that the fungal microbiota is skewed in IBD, much like the bacterial microbiota, with a specific observed increase in basidiomycota/ascomycota ratio, a decreased proportion of Saccharomyces cerevisiae and an increased proportion of Candida albicans when compared with healthy controls.58 Furthermore, the same authors showed that in patients with IBD, the bacteria–mycobiome interkingdoms are much less balanced than is the case in healthy controls.58 To date, little is known about the role of the mycobiome in FMT-IBD studies and much of our understanding is from the treatment of CDI using FMT. The limited studies have demonstrated that nonresponders to FMT have a dominance of Candida sp. and C. albicans within their stool microbiota, suggesting that C. albicans abundance is associated with unfavourable FMT outcome in humans. This study furthermore showed that there is an enteric mycobiota dysbiosis in CDI subjects, with cure from FMT frequently observed when donor-derived fungal taxa predominate in the recipient mycobiota.59 Interestingly, in a mouse model of CDI, the presence of C. albicans was associated with reduced efficacy of FMT, while use of antifungal therapy helped restore efficacy.59
Summary.
Further studies are required in order to understand the potential contribution of the gut virome and mycobiome to the efficacy of FMT in IBD.
Immune mechanisms of FMT
It is increasingly recognized that the pathogenesis of IBD is likely the result of a dysregulated immune response to environmental triggers, primarily mediated through the gut microbiota, in a genetically predisposed individual.60 It is intuitive that any effect of FMT in IBD that results in resolution of inflammation is likely to involve mitigation of a pro-inflammatory immunological state. It is therefore surprising that there is a paucity of data exploring immunological changes as a consequence of FMT in clinical studies in IBD. The only RCT for FMT in IBD that performed immunophenotyping failed to show any significant changes in proportions of mucosal T-cell subsets [including regulatory T cells (Tregs) and γδ T cells].24 There was, however, an increase in peripheral gut-homing CD4 T cells (as defined by CD4+ CD45RO+ β7+), but not gut-homing CD4 Tregs, following FMT. A detailed methodology for experimental design and representative plots, however, was not described. None of the case series adopted a focused approach towards immunological analysis; however, some have described specific exploratory changes. An increase in the proportion of colonic mucosal Tregs (CD4+CD25+CD127lo) was described in a pilot with 19 patients with active Crohn’s disease 12 weeks following a single lower-GI infusion of FMT.11 This increase in mucosal Tregs (defined as CD4+FoxP3+) post FMT was also described in a two-patient case series that successfully used FMT for treatment of immune-checkpoint inhibitor-associated colitis.61 A further case series with 19 patients with active UC who underwent a single upper-GI infusion of FMT failed to show any change in a large panel of serum cytokines, including interleukin 10 (IL-10).62 No changes were identified in mucosal dendritic cell and cytokine profiles following a single nasogastric infusion of FMT, in a small cohort of patients with active pouchitis.18 Interestingly, a cohort of 20 patients with UC treated with a single colonic infusion of FMT showed that mucosal CD4+ T-cell production of interferon gamma was negatively associated with remission and correlated with enrichment of the bacteriophage Caudiovirales.63
Immune mechanisms of FMT have been explored in a few nonclinical studies that used animal models of IBD and antibiotic-associated dysbiosis. A seminal study demonstrated that mice with dextran sulphate sodium (DSS)-induced colitis had a significant increase in frequencies of IL-10-producing CD4 T cells and antigen-presenting cells following FMT gavage.64 IL-10 is a key immunoregulatory cytokine produced by T-cell subsets, particularly Tregs. In congruence, there was an increase in colonic IL-10 levels and this change was associated with resolution of inflammation compared with control (non-FMT) DSS-colitis mice. Additionally, there were reduced frequencies of major-histocompatibility-complex-II-expressing antigen-presenting cells, suggestive of a shift towards a tolerogenic state. Similarly, models of antibiotic-associated dysbiosis in mice shown an increase in frequencies of Tregs and IL-10 production following FMT administration.65,66
This limited evidence collectively suggests that FMT potentially mediates its therapeutic effects in IBD by increasing immunoregulatory mechanisms. The causal link, however, is far from being established and certainly it remains to be determined what key players in the constituents of FMT are responsible for this effect. It is possible that specific SCFA-producing Clostridium clusters (that are shown to be associated with clinical response in RCTs) may contribute via various pathways such as via G-protein-coupled receptor-dependent and -independent induction of intestinal Tregs.67,68
Summary.
Studies are required with a focus in understanding immunological changes with FMT.
FMT to maintain clinical remission
All the RCTs to date in UC used FMT as a means of inducing remission, and as already mentioned, in-depth, long-term follow up is lacking. Sood and colleagues have conducted a pilot study assessing the benefits of FMT in maintaining remission up to 48 weeks.69 Although the study does not show a statistical significance (p = 0.111) between those treated with FMT 87.1% (n = 31) versus placebo 66.7% (n = 30), it does show promise, especially as secondary endpoints of endoscopic remission [FMT: 58.1% (18/31) versus placebo: 26.7% (8/30), p = 0.026] and histological remission [FMT: 45.2% (14/31) versus placebo: 16.7% (5/30), p = 0. 033] were achieved in significantly higher number of patients with FMT.69
This study does not, however, specify exactly how patient remission was induced. Patients were treated with an FMT protocol via colonoscopic route at weeks 0, 2, 6, 10, 14, 18 and 22, with no mention of dose and concentration. There is also no mention of whether these patients were treated initially or concomitantly with corticosteroids or biologic therapy.
How patients are selected, induced into remission initially (corticosteroids, biologics, FMT) and long-term follow up with specified/standardized endpoints for clinical and endoscopic remission will need to be defined in studies to come.
Summary.
RCTs to date focus solely on induction of remission. Further studies with standardized inclusion/exclusion criteria and long–term follow up will be needed to validate FMT in maintaining remission.
Diet
The direct role of diet has not yet been established in the development and progression of IBD. There is, however, circumstantial evidence based on global epidemiological increases in IBD associated with shifts in dietary habits and on its effect on modulation of microbiome.70 To date, there has been no published study characterizing or exploring donor or recipient diet in the efficacy of FMT in IBD. An RCT is currently in progress investigating a dietary conditioning method for donor and recipient in the treatment of UC with FMT [ClinicalTrials.gov identifier: NCT02734589].
Others
We have yet to understand mechanisms by which FMT modulates host biology and how this is associated with alleviation of inflammation in IBD. Key gaps in our current knowledge include changes in the host transcriptome and epigenome, and functional consequences of FMT. Through whole transcriptomic sequencing (ribonucleic acid sequencing) or ideally, single-cell sequencing and gene methylation, host biological changes in relation to FMT can help move this field beyond single-association networks.
Conclusion
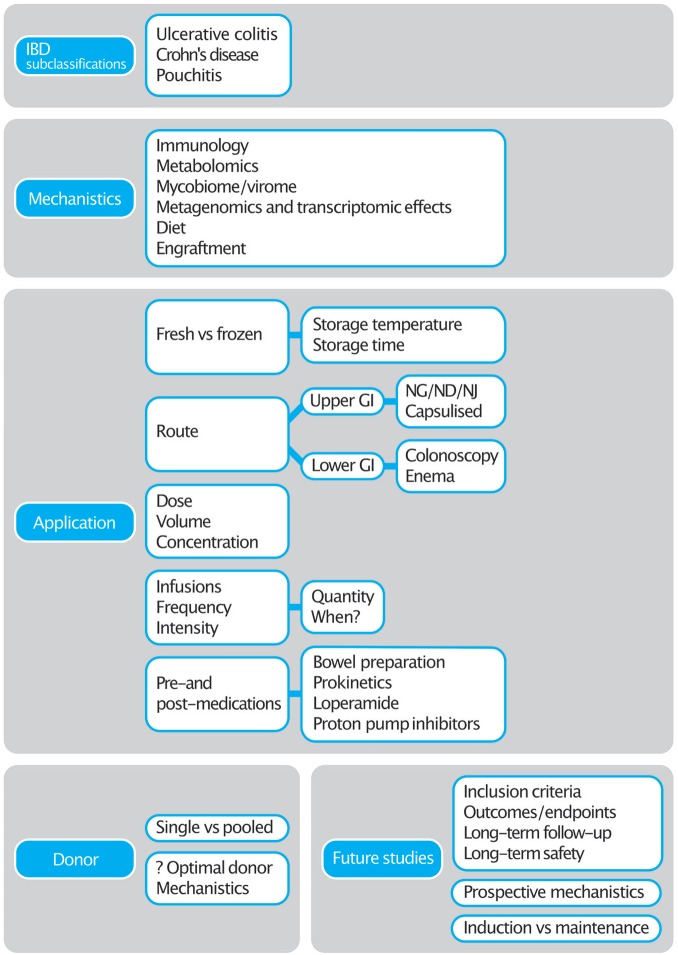
FMT for the treatment of IBD is an area where there are gaps in our knowledge, but where there is promise (see Figure 1). The RCTs that have been conducted to date have helped highlight the numerous challenges and have allowed us to take an informed approach towards designing FMT trials in IBD and beyond. With more focus on mechanistics, further studies can be shaped to understand the complexities behind this exciting and promising technique. There are at least 50 trials registered in IBD (UC and Crohn’s) which will hopefully provide us an answer not just on its efficacy but also, the ideal methodology for FMT preparation and administration.
Figure 1.
Summary of FMT gaps in IBD.
FMT, faecal microbiota transplant; GI, gastrointestinal; IBD, inflammatory bowel disease, ND, nasoduodenal; NG, nasogastric; NJ, nasojejunal.
The fact that FMT has already been established into guidelines for recurrent and refractory CDI, and the positive data already published in observational and RCTs for IBD, give promise that FMT will hopefully soon be able to provide another therapeutic option for patients with a complex and chronic condition.
Acknowledgments
The Department of Metabolism, Digestion and Reproduction at Imperial College receives financial support from the National Institute of Health Research (NIHR) Imperial Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. BHM is the recipient of a Medical Research Council (MRC) Clinical Research Training Fellowship (grant reference: MR/R000875/1) and an NIHR Academic Clinical Lectureship.
MY, JPS, BHM and MNQ were responsible for conception, literature review, writing and revising the manuscript. THI, ALH and JRM gave critical revisions and helped revised the manuscript. All authors agreed to the final version.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Mehmet Yalchin  https://orcid.org/0000-0001-5445-6458
https://orcid.org/0000-0001-5445-6458
Benjamin H Mullish  https://orcid.org/0000-0001-6300-3100
https://orcid.org/0000-0001-6300-3100
Mohammed Nabil Quraishi  https://orcid.org/0000-0003-2338-8397
https://orcid.org/0000-0003-2338-8397
Contributor Information
Mehmet Yalchin, St Mark’s Hospital, Inflammatory Bowel Disease Department, Harrow HA1 UJ, UK.
Jonathan P. Segal, St Mark’s Hospital, Inflammatory Bowel Disease Department, Harrow, UK Department of Metabolism, Digestion and Reproduction, Imperial College London, UK.
Benjamin H. Mullish, Department of Metabolism, Digestion and Reproduction, Imperial College London, UK
Mohammed Nabil Quraishi, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK; Department of Gastroenterology, University Hospitals Birmingham, UK.
Tariq H. Iqbal, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK Department of Gastroenterology, University Hospitals Birmingham, UK.
Julian R. Marchesi, Department of Metabolism, Digestion and Reproduction, Imperial College London, UK School of Biosciences, Cardiff University, UK.
Ailsa L. Hart, St Mark’s Hospital, Inflammatory Bowel Disease Department, Harrow, UK Department of Metabolism, Digestion and Reproduction, Imperial College London, UK.
References
- 1. Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017; 46: 479–493. [DOI] [PubMed] [Google Scholar]
- 2. Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018; 67: 1920–1941. [DOI] [PubMed] [Google Scholar]
- 3. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017; 66: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mullish BH, Quraishi MN, Segal JP, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J Hosp Infect 2018; 100(Suppl. 1): S1–S31. [DOI] [PubMed] [Google Scholar]
- 5. Moayyedi P, Yuan Y, Baharith H, et al. Faecal microbiota transplantation for Clostridium difficile -associated diarrhoea: a systematic review of randomised controlled trials. Med J Aust 2017; 207: 166–172. [DOI] [PubMed] [Google Scholar]
- 6. Sbahi H, Palma JA Di, Jack D, et al. Faecal microbiota transplantation: applications and limitations in treating gastrointestinal disorders. BMJ Open Gastro 2016; 3: e000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Segal JP, Mullish BH, Quraishi MN, et al. The application of omics techniques to understand the role of the gut microbiota in inflammatory bowel disease. Therap Adv Gastroenterol 2019; 12: 175628481882225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imdad A, Nicholson MR, Tanner-Smith EE, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev 2018; 11: CD012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costello SP, Soo W, Bryant RV, et al. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther 2017; 46: 213–224. [DOI] [PubMed] [Google Scholar]
- 10. Narula N, Kassam Z, Yuan Y, et al. Systematic review and meta-analysis. Inflamm Bowel Dis 2017; 23: 1702–1709. [DOI] [PubMed] [Google Scholar]
- 11. Vaughn BP, Vatanen T, Allegretti JR, et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm Bowel Dis 2016; 22: 2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cui B, Feng Q, Wang H, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol 2015; 30: 51–58. [DOI] [PubMed] [Google Scholar]
- 13. Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis 2015; 21: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vermeire S, Joossens M, Verbeke K, et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohn’s Colitis 2016; 10: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohn’s Colitis 2017; 11: 1180–1199. [DOI] [PubMed] [Google Scholar]
- 16. Segal JP, Oke S, Hold GL, et al. Systematic review: ileoanal pouch microbiota in health and disease. Aliment Pharmacol Ther 2018; 47: 466–477. [DOI] [PubMed] [Google Scholar]
- 17. Segal JP, Ding NS, Worley G, et al. Systematic review with meta-analysis: the management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment Pharmacol Ther 2017; 45: 581–592. [DOI] [PubMed] [Google Scholar]
- 18. Landy J, Walker AW, Li JV, et al. Variable alterations of the microbiota, without metabolic or immunological change, following faecal microbiota transplantation in patients with chronic pouchitis. Sci Rep 2015; 5: 12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stallmach A, Lange K, Buening J, et al. Fecal microbiota transfer in patients with chronic antibiotic-refractory pouchitis. Am J Gastroenterol 2016; 111: 441–443. [DOI] [PubMed] [Google Scholar]
- 20. Herfarth H, Barnes EL, Long MD, et al. P591 Low donor microbial engraftment after combined endoscopic and oral faecal microbiota transplant (FMT) in patients with antibiotic dependent pouchitis. J Crohn’s Colitis 2019; 13: S410–S411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015; 149: 102–109.e6. [DOI] [PubMed] [Google Scholar]
- 22. Rossen NG, Fuentes S, Van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015; 149: 110–118.e4. [DOI] [PubMed] [Google Scholar]
- 23. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017; 389: 1218–1228. [DOI] [PubMed] [Google Scholar]
- 24. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis. JAMA 2019; 321: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imhann F, Vich Vila A, Bonder MJ, et al. The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes 2017; 8: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonald EG, Milligan J, Frenette C, et al. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med 2015; 175: 784–791. [DOI] [PubMed] [Google Scholar]
- 27. Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012; 107: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 28. Cohen NA, Livovsky DM, Yaakobovitch S, et al. A Retrospective comparison of fecal microbial transplantation methods for recurrent Clostridium difficile infection. Isr Med Assoc J 2016; 18: 594–599. [PubMed] [Google Scholar]
- 29. Hagel S, Stallmach A, Vehreschild M, et al. Fecal microbiota transplant in patients with recurrent Clostridium difficile infection. Dtsch Arztebl Int 2016; 113: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alrabaa S, Jariwala R, Zeitler K, et al. Fecal microbiota transplantation outcomes in immunocompetent and immunocompromised patients: a single-center experience. Transpl Infect Dis 2017; 19: e12726. [DOI] [PubMed] [Google Scholar]
- 31. Jalanka J, Mattila E, Jouhten H, et al. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med 2016; 14: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2016; 315: 142–149. [DOI] [PubMed] [Google Scholar]
- 33. Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection - fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther 2017; 45: 899–908. [DOI] [PubMed] [Google Scholar]
- 34. Papanicolas LE, Choo JM, Wang Y, et al. Bacterial viability in faecal transplants: which bacteria survive? EBioMedicine 2019; 41: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cammarota G, Ianiro G, Kelly CR, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. Epub ahead of print 28 September 2019. DOI: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Bella S, Gouliouris T, Petrosillo N. Fecal microbiota transplantation (FMT) for Clostridium difficile infection: focus on immunocompromised patients. J Infect Chemother 2015; 21: 230–237. [DOI] [PubMed] [Google Scholar]
- 37. Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 2017; 152: 799–811.e7. [DOI] [PubMed] [Google Scholar]
- 38. Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol 2016; 7: 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knauf F, Brewer JR, Flavell RA. Immunity, microbiota and kidney disease. Nat Rev Nephrol 2019; 15: 263–274. [DOI] [PubMed] [Google Scholar]
- 40. Wang B, Yao M, Lv L, et al. The human microbiota in health and disease. Engineering 2017; 3: 71–82. [Google Scholar]
- 41. Wilson BC, Vatanen T, Cutfield WS, et al. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol 2019; 9: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McDonald JAK, Mullish BH, Pechlivanis A, et al. Inhibiting growth of Clostridioides difficile by restoring valerate, produced by the intestinal microbiota. Gastroenterology 2018; 155: 1495–1507.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weingarden AR, Chen C, Bobr A, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Liver Physiol 2014; 306: G310–G319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Monaghan T, Mullish BH, Patterson J, et al. Effective fecal microbiota transplantation for recurrent Clostridioides difficile infection in humans is associated with increased signalling in the bile acid-farnesoid X receptor-fibroblast growth factor pathway. Gut Microbes 2019; 10: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 2008; 190: 2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Allegretti JR, Kearney S, Li N, et al. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther 2016; 43: 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mullish BH, McDonald JAK, Pechlivanis A, et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut. Epub ahead of print 11 February 2019. DOI: 10.1136/gutjnl-2018-317842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gonçalves P, Araújo JR, Di Santo JP. A Cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis 2018; 24: 558–572. [DOI] [PubMed] [Google Scholar]
- 49. Gadaleta RM, Van Erpecum KJ, Oldenburg B, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011; 60: 463–472. [DOI] [PubMed] [Google Scholar]
- 50. Fuentes S, Rossen NG, van der Spek MJ, et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J 2017; 11: 1877–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Paramsothy S, Nielsen S, Kamm MA, et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 2019; 156: 1440–1454.e2. [DOI] [PubMed] [Google Scholar]
- 52. Draper LA, Ryan FJ, Smith MK, et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome 2018; 6: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zuo T, Wong SH, Lam K, et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018; 67: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Broecker F, Klumpp J, Moelling K. Long-term microbiota and virome in a Zürich patient after fecal transplantation against Clostridium difficile infection. Ann N Y Acad Sci 2016; 1372: 29–41. [DOI] [PubMed] [Google Scholar]
- 55. Draper LA, Ryan FJ, Dalmasso M, et al. Autochthonous faecal virome transplantation (FVT) reshapes the murine microbiome after antibiotic perturbation. bioRxiv 2019; 591099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mukhopadhya I, Segal JP, Carding SR, et al. The gut virome: the ‘missing link’ between gut bacteria and host immunity? Therap Adv Gastroenterol 2019; 12: 175628481983662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chehoud C, Dryga A, Hwang Y, et al. Transfer of viral communities between human individuals during fecal microbiota transplantation. MBio 2016; 7: e00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sokol H, Leducq V, Aschard H, et al. Fungal microbiota dysbiosis in IBD. Gut 2017; 66: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zuo T, Wong SH, Cheung CP, et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat Commun 2018; 9: 3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016; 13: 13–27. [DOI] [PubMed] [Google Scholar]
- 61. Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 2018; 24: 1804–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang T, Cui B, Li P, et al. Short-term surveillance of cytokines and C-reactive protein cannot predict efficacy of fecal microbiota transplantation for ulcerative colitis. PLoS One 2016; 11: e0158227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gogokhia L, Buhrke K, Bell R, et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 2019; 25: 285–299.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Burrello C, Garavaglia F, Cribiù FM, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun 2018; 9: 5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ekmekciu I, Von Klitzing E, Fiebiger U, et al. Immune responses to broad-spectrum antibiotic treatment and fecal microbiota transplantation in mice. Front Immunol 2017; 8: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ekmekciu I, Von Klitzing E, Neumann C, et al. Fecal microbiota transplantation, commensal escherichia coli and Lactobacillus johnsonii strains differentially restore intestinal and systemic adaptive immune cell populations following broad-spectrum antibiotic treatment. Front Microbiol 2017; 8: 2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016; 165: 1332–1345. [DOI] [PubMed] [Google Scholar]
- 68. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sood A, Mahajan R, Singh A, et al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohn’s Colitis 2019; 13: 1311–1317. [DOI] [PubMed] [Google Scholar]
- 70. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 2017; 15: 39–49. [DOI] [PubMed] [Google Scholar]