Abstract
Background
This study aims to evaluate the feasibility and safety of resection of sarcoma liver metastases, and to identify possible prognostic factors for long-term survival.
Methods
All patients who underwent resection of liver metastases of sarcoma in the Netherlands from 1998 to 2014 were included. Study data was retrospectively collected from patient files. Survival rates were calculated using Kaplan-Meier survival analysis.
Results
Some 38 patients treated in 16 hospitals were included (15 male, 23 female). The median age was 57 years (37–80 years). The most common histological subtype was leiomyosarcoma (63%). The predominant site of primary tumour was the abdomen (59%). R0 resection was achieved in 16 patients. Mortality was 3 and 16% of included patients had 1 or more complications. The median follow-up period was 18 months (range 1–161). After liver resection, 1-, 3-, and 5-year survival were 88, 54, and 42% respectively. Median overall survival was 46 months (1–161 months). One- and three-year progression-free survival (PFS) after liver resection were 54 and 19% respectively. Median PFS was 16 months (1–61 months).
Conclusions
Liver surgery for sarcoma metastases is safe and leads to a relatively good survival. The choice for surgical treatment should always be discussed in a multidisciplinary sarcoma and liver team.
Keywords: Liver metastasis, Sarcoma, Liver resection, Hepatic resection
Introduction
Sarcomas are held accountable for less than 1% of all solid malignancies and approximately 80% of all sarcomas originate from soft tissue. Prognosis depends mainly on histological factors and patient characteristics. Of all patients with soft tissue sarcoma (STS), 25–40% will develop distant metastases [1, 2]. Predominant sites of metastases are the lungs and liver. Up to 16% of all patients with retroperitoneal sarcomas and 62% of all patients with visceral sarcomas will develop hepatic metastases [2]. The current standard treatment for patients with metastatic STS (excluding gastrointestinal stromal tumours, Ewing-like sarcomas, and other small blue round cell tumours) is systemic therapy with doxorubicin or ifosfamide, both resulting in poor survival rates [3, 4].
Resection of liver metastases arising from neuroendocrine or colorectal carcinoma in patients with liver-only disease is widely accepted and effective [5, 6, 7]. The role of surgery in the treatment of STS with hepatic metastases remains unclear.
Current literature on this subject consists of small, heterogeneous cohorts, including patients with metastases from gastro intestinal stroma cell tumours (GIST) or other non-colorectal, non-neuroendocrine tumours. These studies demonstrate a possible improved survival after resection of metastases [2, 8, 9, 10, 11, 12]. To date, no data from a population-based national database is reported.
The objective of this study was to evaluate all patients in the Netherlands who underwent liver resection for hepatic metastases of STS since 1998. Primary outcomes were progression-free survival (PFS) and overall survival (OS). Secondary aims were demonstrating the safety of the procedure, and identification of factors that may influence long-term survival.
Materials and Methods
Patients and Data
All patients who underwent a liver resection of metastatic STS between January 1998 and July 2014 in the Netherlands were identified via the Dutch nationwide histology database (PALGA). Since 1991, all reports generated by every pathology department in the Netherlands are collected in this nationwide database [13]. All Ewing-like sarcomas and other small blue round cell tumours were excluded, since these types of sarcomas respond well to chemotherapy. Furthermore, GIST were also excluded. Standard demographic and clinicopathologic data, including histopathological information about the primary tumour and metastases, intraoperative details and use of chemo- and/or radiotherapy, were retrospectively collected from the patient files in 16 different hospitals in the Netherlands. Prior ethical approval was granted for the current study, with the medical Ethic Committee waiving the requirement for informed consent to be obtained for the use of anonymized patient data.
Surgery
Decisions about surgical approaches were tailor-made for every single patient. Hepatic metastases were defined according to Couinaud's liver segments [14]. Radicality was defined according to the Union Internationale Contre le Cancer standards; R0: complete microscopic resection, R1: microscopic residual disease or R2: macroscopic residual hepatic or extrahepatic disease. Liver metastases were considered metachronous when they were diagnosed at least 6 months after diagnosis of the primary tumour.
Outcome Variables
Primary outcomes in this study were PFS and OS. PFS was defined as the time between resection of liver metastases and the first diagnostic proven recurrence or progression of disease in liver or any other tissue. OS was defined as the time from first liver resection till the date of death, regardless of the cause of death. Incidentally, the date of death could not be traced; in that case, the last date of follow-up was used. Secondary outcomes included the safety of metastasectomy and the prognostic impact of gender, age, the type of resection (minor; ≤2 segments or major; > 2 segments), radicality (R0, R1 or R2), the extent of PFS, the number of metastases, the time of diagnosis of liver metastases (synchronous or metachronous) on PFS and OS.
Statistical Considerations
PFS and OS were estimated by Kaplan-Meier survival analysis. According to Cox proportional hazards regression methodology, prognostic factors for long-term survival were identified by univariable survival analysis. A p value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences version 23.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Demographics and Histological Characteristics
Thirty-eight patients underwent hepatic resection of metastatic STS between January 1998 and July 2014. Patient characteristics are described in Table 1. Liver resection took place in 16 different hospitals. Twenty-three female and 15 male patients were included in this study. The median age at liver resection was 57 years (range 37–80 years). In total, 5 histological types of sarcoma were described, 7 cases were described as “not otherwise specified”. All diagnoses were confirmed histologically. The median follow-up was 18 months (range 1–161 months) after liver resection.
Table 1.
Patient and tumour characteristics
| Variable | (n = 38) |
|---|---|
| Gender | |
| Male | 15 (40) |
| Female | 23 (60) |
| Age | 57 (37–8 0) |
| Sarcoma subtype | |
| Leiomyosarcoma | 24 (63) |
| Liposarcoma | 3 (8) |
| Hemangiopericytoma | 2 (5) |
| Angiosarcoma | 1 (3) |
| PEComa* | 1 (3) |
| Not otherwise specified | 7 (19) |
| Primary tumour site | |
| Abdomen | 22 (59) |
| Organ (bowel, stomach) | 9 (23) |
| Retroperitoneum | 7 (18) |
| Gynaecologic | 6 (15) |
| Pelvis | 1 (3) |
| Extremity | 5 (13) |
| Head | 2 (5) |
| Other | 9 (24) |
| Interval primary tumour – hepatic metastases | |
| Synchronous | 11 (28) |
| Metachronous | 23 (59) |
| Missing | 4 (10) |
| Number of hepatic metastases | |
| Solitary | 20 (51) |
| Multiple | 14 (36) |
| Missing | 4 (10) |
| Distribution metastases | |
| Unilobar | 28 (74) |
| Bilobar | 8 (21) |
| Missing | 2 (5) |
| Extrahepatic metastases prior to liver resection | |
| Absent | 25 (67) |
| Present | 12 (30) |
| Missing | 1 (3) |
| Type of resection | |
| Minor | 24 (63) |
| Major | 13 (34) |
| Missing | 1 (3) |
For continuous variables data shown represent median (range), all other data is presented as numbers (%).
PEComa, perivascular epithelioid cell tumour.
Preoperative Evaluation of Liver Metastases
All liver metastases were preoperatively diagnosed by ultrasound, CT-, MRI-, PET-scanning or a combination of these means. In 14 patients, multiple liver metastases were found; 20 patients had a solitary metastasis. In 8 patients, metastases were spread bilobar and in 28 patients, unilobar. Liver metastases were synchronous in 11 patients and metachronous in 23. In 4 patients data on the number and location of lesions were missing.
Surgical Procedure
In 3 cases, intraoperative radio frequent ablation (RFA) was combined with liver resection. A minor resection (≤2 segments) was performed in 24 patients and 13 patients underwent a major (> 2 segments) resection. There was no intra-operative mortality; nevertheless, 3 patients had intra-operative complications, including a perforation of the small bowel, tumour rupture and bleeding. In 16 patients, an R0-resection was achieved, 5 patients underwent an R1-resection and in 15 patients, surgery resulted in an R2-resection. In 7 patients, R2-resection was achieved due to extra hepatic disease. In 2 patients, information about radicality was not available.
Postoperative Complications
Six patients encountered postoperative complications, for which 2 patients underwent secondary surgery due to intra-abdominal sepsis. One of these patients died due to sepsis after a gastrointestinal perforation. Median hospital stay was 9 days (range 2–20 days).
Progression-Free Survival
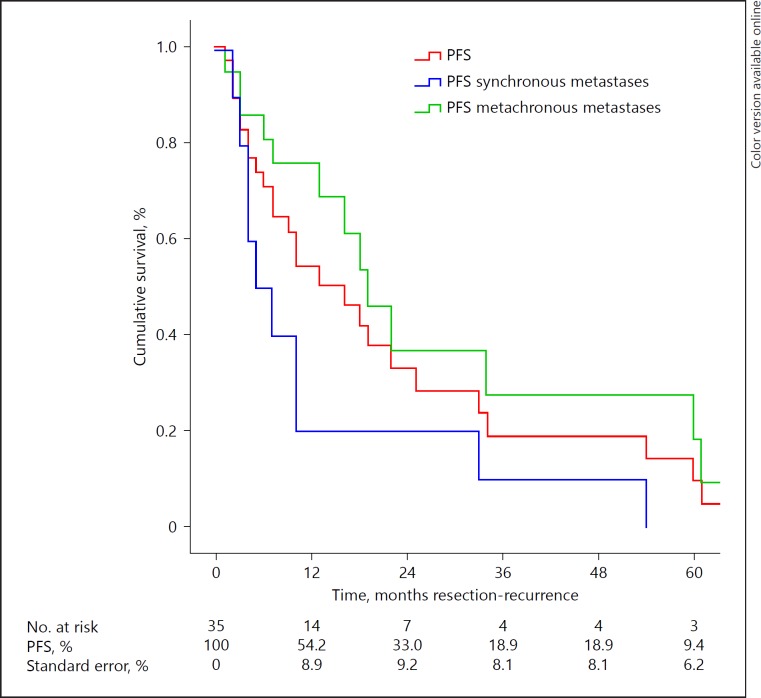
Median PFS was 16 months (range 1–161; Fig. 1). One- and three-year PFS after liver resection were 54.2 and 18.9% respectively. Follow-up was conducted according to the preference of the local centre. One patient did not experience progression of disease after 5 years. Median PFS after resection of metachronous metastases was significantly longer with 19 months (range 1–161 months) versus 5 months (range 2–54 months) for synchronous metastases (p = 0.02). When an R0- or R1-resection could be achieved, median PFS was 16 months (range 2–161 months); for an R2-resection, median PFS was 10 months (range 1–161 months; p = 0.87). Eight patients underwent secondary liver resection. Median PFS was 19 months (range 1–161 months) for patients younger than 60 and 9 months (range 2–34 months) for patients older than 60 (p = 0.06).
Fig. 1.
Progression-free survival (PFS).
PFS was also not significantly different in terms of gender, number of metastases, primary intra- or extra-abdominal tumour, minor/major resection or uni- or bilobar metastases. Factors that may influence PFS are described in Table 2.
Table 2.
Potential prognostic factors for survival
| Variable | (n = 38) | PFS, months | Univariablep value | OS, months | Univariablep value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 15 (40) | 19 | 0.794 | 62 | 0.522 |
| Female | 24 (60) | 10 | 35 | ||
| Age | |||||
| <60 years | 23 (61) | 19 | 0.057 | 62 | 0.196 |
| ≥ 60 years | 15 (29) | 9 | 35 | ||
| Interval primary tumor – hepatic metastases | |||||
| Synchronous | 11 (28) | 5 | 0.021 | 40 | 0.508 |
| Metachronous | 23 (59) | 19 | 20 | ||
| Number of hepatic metastases | |||||
| Solitary | 20 (51) | 10 | 0.367 | 62 | 0.360 |
| Multiple | 14 (36) | 16 | 46 | ||
| Distribution metastases | |||||
| Unilobar | 28 (74) | 13 | 0.684 | 56 | 0.960 |
| Bilobar | 8 (21) | 18 | 20 | ||
| Resection status | |||||
| RO or R1 | 21 (55) | 16 | 0.869 | 77 | 0.192 |
| R2 | 15 (39) | 10 | 20 | ||
| Type of resection | |||||
| Minor | 24 (63) | 10 | 0.854 | 18 | 0.321 |
| Major | 13 (34) | 16 | 56 | ||
| Primary tumour site | |||||
| Intra-abdominal | 22 (58) | 9 | 0.364 | 19 | 0.099 |
| Extra-abdominal | 16 (42) | 17 | Not reached |
PFS, progression-free survival; OS, overall survival.
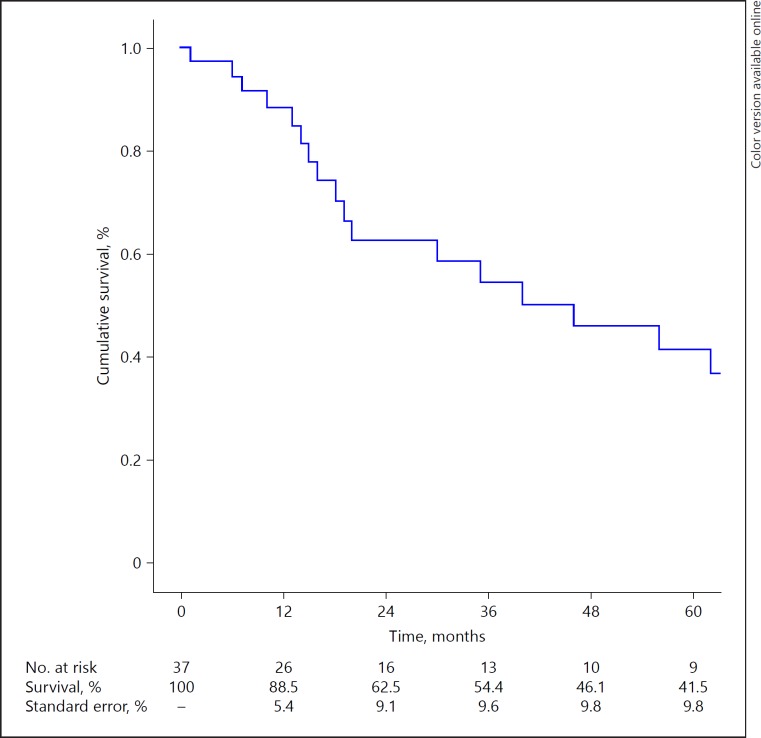
Overall Survival
Median OS was 46 months (1–161 months; Fig. 2). One-year survival, 3-year survival and 5-year survival after liver resection were 88.1, 53.9 and 41.1% respectively. In the R0- or R1-group, 54.6% of the patients were alive after 5 years. Median OS in the R0- or R1-group was 77 months (range 6–142) and median survival in the R2-group was 20 months (range 1–161; p = 0.19). Factors that may influence outcome are described in Table 2. None of the factors mentioned above could be included in multivariable analysis for OS, since all factors had a p value of > 0.05 in univariable analysis.
Fig. 2.
Overall Survival (OS).
Discussion
In the current study, liver resection for metastatic STS resulted in a median OS of 46 months (1–161 months). Median PFS after liver resection was 16 months (1–161 months). Development of metachronous metastases was the only beneficial prognostic factor for PFS. The sample size of the current study clearly illustrates the scarcity of liver resection for hepatic metastases of STS, resulting in a scarcity of studies published on this topic. This study is the first nationwide report of all patients who underwent liver surgery for sarcoma metastases during a period of more than 15 years in the Netherlands.
Current treatment options for patients with STS metastatic to the liver include chemotherapy, trans-arterial chemotherapy embolization, radiofrequency or microwave ablation and hepatectomy. Liver metastasectomy has been previously described to be a curative treatment option in a highly selected group of patients [10, 15, 16, 17, 18].
According to analyses conducted by the European Organization for Research and Treatment of Cancer (EORTC), treatment of patients with hepatic STS metastases with chemotherapy alone, resulted in a median OS of 10 months with a 1-year survival of 42% and a 2-year survival of 13% [15]. Combined chemotherapy (doxorubicin + ifosfamide) has demonstrated not to improve OS [4]. Median OS in our, highly selective, cohort was 46 months with a 1-year survival of 88% and a 2-year survival of 62% respectively. Hepatectomy should therefore be considered a treatment option for a selected group of patients.
During the past decades, liver surgery has become safe, thereby liberalizing its indications [19]. Especially, colorectal liver metastases are frequently operated on in the Netherlands, whereas surgery for non-colorectal metastases is still rare [20]. However, it is pivotal to meticulously select patients who are candidates for potentially curative resection. Discussion in multidisciplinary tumour boards has demonstrated to be essential in selecting patients who might benefit from surgery [21]. The results of the current study are comparable to other cohort studies (Table 3). Only Groeschl et al. [9] and Chua et al. [22] reported a substantially higher OS, with a median OS of 71 and 103 months respectively.
Table 3.
Survival after hepatic resection for metastatic sarcoma
| Study | n | Follow-up, median/months | Survival |
|---|---|---|---|
| Jaques et al. [2], 1995 | 14 | 60 | Median OS 30 months, 5-year OS was 0%* |
| Harrison et al. [24], 1997 | 27 | 60 | Median OS 31 months, 5-year OS was 4%* |
| Elias et al. [25], 1998 | 13 | Unknown | 5-Year OS 18%* |
| Chen et al. [26], 1998 | 11 | 53 | Median OS 39 months* |
| Lang et al. [27], 2000 | 26 | Unknown | Median OS after R0 resection: 32 months, 5-year OS 13% |
| DeMatteo et al. [8], 2001 | 22 | 25 | Median OS 30 months |
| Pawlik et al. [16], 2006 | 66 | 35.8 | 1-Year OS 91.2%, 5-year OS 27%** |
| Adam et al. [23], 2006 | 125 | Mean FU: 31 months | Median OS 32 months, 5-year OS 31% |
| Lendoire et al. [28], 2007 | 23 | 28 | 5-Year OS 0% |
| Rehders et al. [29], 2009 | 27 | 84 | Median OS 44 months, 5-year OS 49%^ |
| Chua et al. [22], 2011 | 15 | 122 | Median OS 103 months, 5-year OS: 51%, 10-year OS: 37%^ |
| Marudanayagam et al. [11], 2011 | 36 | 24 | Median OS 24 months, 5-year OS 32%^ |
| Zacherl et al. [30], 2011 | 15 | Unknown | Median OS 34 months, 5-year OS 27%^ |
| Groeschl et al. [9], 2012 | 98 | 32 | Median OS 72 months, 1-year OS: 82%, 5-year OS 32%*** |
| Brudvik et al. [10], 2015 | 50 | 32 | Median OS 45 months, 5-year OS 45%, 10-year OS 23% |
Study population included before 1996.
Study population included 36 Gastrointestinal stromal tumours, 13 patients were treated with RFA only.
Excluded all R1- and R2-resections.
Study population included Sarcomas and Gastrointestinal stromal tumours.
Nevertheless, Chua et al. [22] included only 15 patients with a large OS range (6–200 months) [22] and Groeschl et al. [9] excluded all R1- and R2-resections from their analyses.
Adam et al. [23] identified an age of > 60 years, a PFS of < 12 months, R2-resection and major hepatectomy as factors associated with poor outcome in a large cohort of patients with noncolorectal nonendocrine liver metastases. In accordance with Adam et al. [23], we have shown a trend towards a better PFS and OS after an R0- or R1-resection compared to an R2-resection (Table 2). The statistical insignificance of this difference may be explained by the relatively small sample size. The deteriorated survival rate after an R2-resection is most likely due to surgery with a palliative intent. However, median OS after R2 resection is 20 months (1–161), which is higher than median survival (10 months) described with chemotherapy in EORTC trial [15].
Moreover, patients from our cohort who were treated for synchronous metastases had a significant shorter median PFS than patients treated for metachronous metastases.
Given the low sample size of this study, no firm conclusions can be made in comparison to other historic cohort studies.
Other treatment options for sarcoma metastatic to the liver are RFA, microwave ablation, trans-arterial chemo embolization (TACE), and radioembolization with yttrium 90 microspheres. Pawlik et al. [16] showed that RFA of metastases resulted in higher recurrence rates than resection alone. In our study, only 3 patients received RFA, in combination with surgery for metastases; therefore, comparisons could not be made. The use of TACE for hepatic metastases of STS is reported in a few studies [17, 18]. Median PFS was reported to be 6 months and median OS was 21 months for responders. Therefore, the authors conclude that TACE should be reserved as a salvage option. However, in the study of Maluccio et al. [17] 6 out of 7 leiomyosarcomas treated did not respond to the therapy.
STSs represent a large and heterogenic group of tumours. A drawback of this study is that biological behaviour and response to therapy of the different subtypes could not be determined because of the small number of patients.
As mentioned above and stated in Table 3, most of the previously published studies contain a mixture of information on STS and GIST, or other non-colorectal, non-neuroendocrine tumours [2, 8, 9, 10, 11, 16, 22, 23, 24, 25, 26, 27, 28, 29, 30]. Due to our more homogeneous study group, the outcomes in this study are more specific, making them poorly comparable to the outcomes of older studies (Table 3).
The strength of our study is the relatively large sample size from a population-based data set. Furthermore, this study provides detailed information on intra- and postoperative complications, which are rare in population-based data sets. However, certain limitations do apply to our current analysis. The current study lacks a proper control group. Also, information on postoperative chemotherapy regimens is lacking. This may explain why synchronous liver metastasis was a prognostic factor for PFS, without influencing OS. Nevertheless, it is the best available evidence since sarcoma, especially with potentially resectable hepatic metastases, is rare and therefore comparative studies and clinical trials are difficult to effectuate. This calls for the need of an international prospective database with results of various treatment modalities for this group of patients to improve the understanding and treatment of the various subtypes of STS.
In conclusion, since current chemotherapy or other treatment options do not lead to cure, resection of sarcoma liver metastases should be considered and discussed in a multidisciplinary sarcoma and liver team for all patients with technically resectable metastases as a potential treatment option. Although this may achieve cure, it remains palliative treatment for the majority of the selected patients.
Collaborators
H.H. Hartgrink (Department of Surgery, Leiden University Medical Centre, Leiden), K.P.J., (Department of Surgery, University Medical Centre Groningen, Groningen), H.J. Heijmans (Department of Surgery, Hospital Group Twente, Hengelo), C. Sietses (department of Surgery, Hospital The Geldersche Vallei, Ede), H. Boutkan (department of surgery, Haga Hospital Leyenburg, The Hague), G. Kazemier (Department of Surgery, VU Medical Centre, Amsterdam), T.M. van Gulik (Department of Surgery, Amsterdam Medical Centre, Amsterdam), M.H.A. Bemelmans (Department of Surgery, Maastricht University Medical Centre, Maastricht), P. van Duijvendijk (Department of Surgery, Gelre Hospitals, Apeldoorn), G.A. Patijn (Department of Surgery, Isala Clinics, Zwolle), K. Bosscha (Department of Surgery, Jeroen Bosch Hospital, Den Bosch), B. van Ramshorst (Department of Surgery, St. Antonius Hospital, Nieuwegein), A.A.M. Huiberts (Department of surgery, Onze Lieve Vrouwen Gasthuis, Amsterdam), J.R.M. van der Sijp (Department of Surgery, Haaglanden Medical Centre, Den Haag), R. Roumen (Department of Surgery, Maxima Medical Centre, Veldhoven), H.J.Y. Rutten (Department of Surgery, Catharina Hospital, Eindhoven) E. van de Harst (Department of Surgery, Maasstad Hospital, Rotterdam).
Disclosure Statement
The authors report no conflicts of interest relevant to this article.
References
- 1.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 2.Jaques D, Coit D, Casper E, Brennan M. Hepatic metastases from soft-tissue sarcoma. Ann Surg. 1995;221:392–397. doi: 10.1097/00000658-199504000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blay JY, van Glabbeke M, Verweij J, Weiss G, Le Cesne AT, Oosterhuis JW, et al. Advanced soft-tissue sarcoma: a disease that is potentially curable for a subset of patients treated with chemotherapy. Cancer. 2003;39:64–69. doi: 10.1016/s0959-8049(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 4.Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain R, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? Coll Surg. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 6.Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776–785. doi: 10.1097/01.sla.0000161981.58631.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SImmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540–547. doi: 10.1097/00000658-200110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groeschl R, Nachmany I, Steel JL, Reddy SK, Glazer ES, de Jong MC, et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer a multi-institutional analysis. J Am Coll Surg. 2012;214:769–777. doi: 10.1016/j.jamcollsurg.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 10.Brudvik KW, Patel SH, Roland CL, Conrad C, Torres KE, Hunt KK, et al. Survival after resection of gastrointestinal stromal tumor and sarcoma liver metastases in 146 patients. J Gastrointest Surg. 2015;19:1476–1483. doi: 10.1007/s11605-015-2845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marudanayagam R, Sandhu B, Perera MT, Bramhall SR, Mayer D, Buckels JA, et al. Liver resection for metastatic soft tissue sarcoma an analysis of prognostic factors. Eur J Surg Oncol. 2011;37:87–92. doi: 10.1016/j.ejso.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald T, Brinkley J, Banks S, Vohra N, Englert ZP, Zervos EE. The benefits of liver resection for non-colorectal, non-neuroendocrine liver metastases: a systematic review. Langenbecks Arch Surg. 2014;399:989–1000. doi: 10.1007/s00423-014-1241-3. [DOI] [PubMed] [Google Scholar]
- 13.Casperie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken. JH Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couinaud C. Intrahepatic distribution of hepatic artery. Acta Anat (Basel) 1954;22:49–81. [PubMed] [Google Scholar]
- 15.van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens – a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 16.Pawlik TM, Vauthey JN, Abdalla EK, Pollock RE, Ellis LM, Curley SA. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg. 2006;141:537–544. doi: 10.1001/archsurg.141.6.537. [DOI] [PubMed] [Google Scholar]
- 17.Maluccio MS, Covey AM, Schubert J, Brody LA, Sofocleous CT, Getrajdman GI, et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer. 2006;107:1617–1623. doi: 10.1002/cncr.22191. [DOI] [PubMed] [Google Scholar]
- 18.Chapiro J, Duran R, Lin M, Mungo B, Schlachter T, Schernthaner R, et al. Transarterial chemoembolization in soft-tissue sarcoma metastases to the liver – the use of imaging biomarkers as predictors of patient survival. Eur J Radiol. 2015;84:424–430. doi: 10.1016/j.ejrad.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Ridder JA, Lemmens VE, Overbeek LI, Nagtegaal ID, de WIlt. JH Liver Resection for metastatic disease; a population-based analysis of trends. Dig Surg. 2016;33:104–113. doi: 10.1159/000441802. [DOI] [PubMed] [Google Scholar]
- 21.de Ridder JA, van der Stok EP, Mekenkamp LJ, Wiering B, Koopman M, Punt CJ, et al. Management of liver metastases in colorectal cancer patients: a retrospective case-control study of systemic therapy versus liver resection. Eur J Cancer. 2016;59:13–21. doi: 10.1016/j.ejca.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Chua T, Chu F, Morris DL. Outcomes of single-centre experience of hepatic resection and cryoablation of sarcoma liver metastases. Am J Clin Oncol. 2011;34:317–320. doi: 10.1097/COC.0b013e3181e1d078. [DOI] [PubMed] [Google Scholar]
- 23.Adam R, Chiiche L, Aloia T, Elias D, Salmon R, Rivoire M, et al. Hepatic resection for noncolorectal nonendocrine liver metastases analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524–535. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison LE, Brennan MF, Newman E, Fortner JG, Picardo A, Blumgart LH, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery. 1997;121:625–632. doi: 10.1016/s0039-6060(97)90050-7. [DOI] [PubMed] [Google Scholar]
- 25.Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, Plaud B, Ducreux M, Spielmann M, et al. Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg. 1998;187:487–493. doi: 10.1016/s1072-7515(98)00225-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Pruitt A, NIcol TL, Gorgulu S, Choti MA. Complete hepatic resection of metastases from leiomyosarcoma prolongs survival. J Gastrointest Surg. 1998;2:151–155. doi: 10.1016/s1091-255x(98)80006-1. [DOI] [PubMed] [Google Scholar]
- 27.Lang H, Nussbaum KT, Kaudel P, Frühauf N, Flemming P, Raab R. Hepatic metastases from leiomyosarcoma: A single-center experience with 34 liver resections during a 15-year period. Ann Surg. 2000;231:500–505. doi: 10.1097/00000658-200004000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lendoire J, Moro M, Andriani O, Grondona J, Gil O, Raffin G, et al. Liver resection for non-colorectal, non-neuroendocrine metastases: analysis of a multicenter study from Argentina. HPB (Oxford) 2007;9:435–439. doi: 10.1080/13651820701769701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehders A, Peiper M, Stoecklein NH, Alexander A, Boelke E, Knoefel WT, et al. Hepatic metastasectomy for soft-tissue sarcomas: is it justified? World J Surg. 2009;33:111–117. doi: 10.1007/s00268-008-9777-4. [DOI] [PubMed] [Google Scholar]
- 30.Zacherl M, Bernhardt GA, Zacherl J, Gruber G, Kornprat P, Bacher H, et al. Surgery for liver metastases originating from sarcoma-case series. Langenbecks Arch Surg. 2011;396:1083–1091. doi: 10.1007/s00423-011-0821-8. [DOI] [PubMed] [Google Scholar]