Abstract
Chronic high-fat diet (HFD) consumption causes ovarian dysfunction in rodents. Acute dietary treatment with docosahexaenoic acid (DHA) increases oocyte quality and ovarian reserve at advanced reproductive age. We hypothesized that DHA supplementation after HFD exposure reverses HFD-induced ovarian defects. We conducted a dietary intervention with reversal to chow, DHA-supplemented chow, or DHA-supplemented HFD after HFD consumption. After 10 weeks, HFD-fed mice had impaired estrous cyclicity, decreased primordial follicles, and altered ovarian expression of 24 genes compared to chow controls. Diet reversal to either chow or chow+DHA restored estrous cyclicity, however only chow+DHA appeared to mitigated the impact of HFD on ovarian reserve. All dietary interventions restored HFD-dysregulated gene expression to chow levels. We found no association between follicular fluid DHA levels and ovarian reserve. In conclusion our data suggest some benefit of DHA supplementation after HFD, particularly in regards to ovarian gene expression, however complete restoration of ovarian function was not achieved.
Keywords: High-fat diet, ovary, female fertility, RNA-sequencing, omega-3 fatty acids, obesity
1. Introduction
Obesity is associated with female reproductive dysfunction (1). Assisted reproductive technologies are also less effective in women with obesity (2-4). High-fat diets (HFD) are defined as those that exceed the recommended 35% of calories from fat (5). When HFDs, particularly those rich in saturated fat, are chronically consumed, metabolic and physiological disturbances can develop, including reproductive dysfunction. Indeed, HFD consumption has been shown to cause ovarian dysfunction, diminished ovarian reserve (6,7), altered ovarian gene expression (7), increased ovarian inflammation (6,8,9), and oocyte-specific defects (10,11) in rodents. These oocyte-specific defects, including increased lipid deposition, are not reversed by switching to a standard low-fat diet after HFD consumption, despite the fact that the HFD-induced metabolic disturbances observed in these mice are reversed (11). Although standard low-fat diets have not been able to reverse oocyte-specific defects, it is unknown if other HFD-induced ovarian defects, including premature depletion of the ovarian reserve and aberrant ovarian gene expression, can be reversed with dietary intervention, particularly one including supplementation with omega-3 fatty acids (FA) after HFD feeding.
Omega-3 and omega-6 polyunsaturated fatty acids (PUFA) can regulate reproductive function as they influence both prostaglandin synthesis and steroidogenesis (12). Prostaglandins are synthesized from membrane phospholipids containing omega-6 (1- and 2-series prostaglandins) and omega-3 (3-series prostaglandins) FAs (12), and by altering the omega-6 and omega-3 FAs in the diet, the composition of membrane omega phospholipids is also changed (13). Variations in the composition of omega FAs in membrane phospholipids alter the types of prostaglandins produced, as the different omega-6 and omega-3 PUFAs compete for the same enzymes for prostaglandin synthesis (14,15). Further, the relatively high omega-6 to omega-3 ratio in HFDs (approximately 15:1 (16,17)) has been proposed to play a role in the associated ovarian inflammation (6,8,9). As omega PUFAs are involved in inflammation (omega-6 pro-inflammatory, omega-3 anti-inflammatory) (18), altering their content in the diet after HFD feeding may alleviate the associated ovarian inflammation and improve ovarian function.
A few studies have suggested that higher omega-3 FA levels may improve ovarian function and fertility. For example, a diet supplemented with docosahexaenoic acid (22:6 n3, DHA) at 2% of calories improved the quality of oocytes into advanced reproductive age, prolonging the female mouse reproductive lifespan (19). We have also shown that mice with the constitutively expressed fat-1 transgene (enzymatically converting endogenous omega-6 to omega-3 PUFA (20)), have improved reproductive outcomes including increased primordial follicles at peak reproductive age and decreased ovarian macrophage infiltration with HFD-exposure (21). Others have shown that rats have increased growing follicle numbers when fed a DHA-enriched diet (22) and more ovulated oocytes when they are fed diets enriched with omega-3 FAs (23,24). Increased pregnancy rates in women that consume or have higher omega-3 FA levels have also been observed both with (25-27) and without (28) assisted reproduction technologies. Further, women with high omega-3 FA intakes have embryos with superior morphology, even though they have fewer follicles stimulated during their assisted reproduction cycles (29).
As acute dietary treatment with DHA has been shown to increase egg quality and ovarian reserve at advanced reproductive age (19), we hypothesized that DHA supplementation would similarly restore HFD-induced ovarian defects after HFD feeding. To investigate if DHA supplementation can rescue the negative ovarian phenotype induced by HFD, we performed a dietary intervention with DHA supplemented to both standard chow and HFD after chronic HFD consumption. Further, we examined non-esterified-FA (NEFA) levels in follicular fluid (FF) from women with both normal and diminished ovarian reserve undergoing IVF to evaluate if human NEFA present in FF correlated with human diminished ovarian reserve.
2. Materials and Methods
2.1. Animal Care and Dietary Intervention
The Anschutz Medical Campus Center for Comparative Medicine facilities housed all mice as previously described (30). All procedures were approved by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee (protocol B-109215(7)1D/00126). Mice had free access to water and food, and were monitored daily for wellness, signs of cage aggression, and disease.
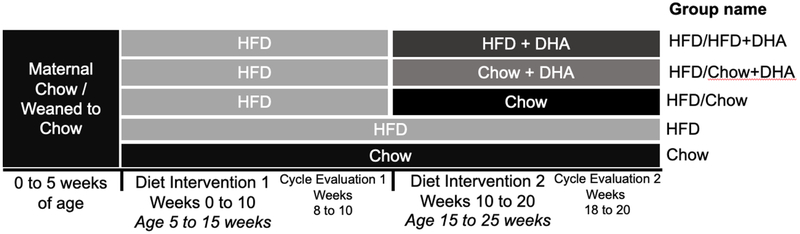
C57BL/6J female mice (4-week-old) were received from Jackson Laboratory (Bar Harbor, ME). After 1-week of acclimation, mice were randomly assigned to receive HFD with 60% fat by calories (HFD, N = 45) (D12492, Research Diets, New Brunswick, NJ) or chow (15.1% fat by calories) (chow, N = 10) (Teklad 2920X, Envigo, Madison, WI) for 10 weeks. After 10 weeks, HFD mice were assigned to continue the 60% HFD (HFD/HFD, N = 15), or were switched to chow (HFD/Chow, N = 10), DHA and arachidonic acid (AA) supplemented chow (HFD/Chow+DHA, N = 10), or DHA and AA supplemented HFD (HFD/HFD+DHA, N = 10) for another 10 weeks. Chow mice remained on chow for the entire study period. DHA (U-84-A, Nu-check Prep, Elysian, MN) and AA (U-72-A, Nu-check Prep) were added to both chow and HFD at a 20:1 ratio by Research Diets. This specific ratio of DHA to AA has been shown to prolong the reproductive lifespan and egg quality at advanced reproductive age in female rodents (19). Weekly body weight and food intake were recorded for all mice. In the HFD/chow+DHA group, one mouse was removed from the study after the diet switch due to extreme weight loss requiring euthanasia, bringing the final sample size of this group to N = 9. Details of the dietary intervention are provided in Figure 1. Detailed information on all diets used in this study are provided in Supplemental Table 1.
Figure 1. Schematic Representation of the Dietary Intervention Study.
Diet 1: Dietary intervention weeks 0 to 10; Diet 2: Dietary intervention weeks 10 to 20.
2.2. Estrous Cycle Evaluation
Mice were subjected to daily estrous cycle stage evaluation during weeks 8 to 10 and 18 to 20 of dietary exposure, as previously described (31). Briefly, vaginal smears were collected daily and evaluated for cycle stage. Cycles were determined to be abnormal if a mouse had irregular progression through the stages, a prolonged cycle (greater than 5 days), or an extended time in a cycle stage (greater than 3 days). All mice were sacrificed in diestrus at 25 weeks of age. One mouse in the HFD/HFD+DHA group was unable to be sacrificed in diestrus due to irregular cyclicity and instead sacrificed in estrus. This mouse was excluded from all analysis where cycle stage is a confounding factor.
2.3. Tissue Collection
Body weight was recorded prior to sacrifice. Blood was collected from the tail vein in mice fasted for at least two hours to assess glucose levels with the ReliOn blood glucose monitoring system (Ultima). Immediately after sacrifice blood was collected by cardiac puncture and serum collected, as previously described (30). Rump to nose length was measured and ovaries were collected, the right ovary used for follicle counts and the left ovary for RNA-sequencing analysis or immunohistochemistry (randomly determined to have at least four ovaries in each group). Abdominal fat and subcutaneous fat were collected from each animal and the wet weight of each depot was recorded. Total fat from each animal was determined as the sum of the two adipose depots. Both fat depots and total fat were normalized to each mouse’s body weight. A body mass index (BMI) value was calculated for each animal using the following equation as previously described (32,33):
2.4. Follicle Counts and Corpus Luteum Analysis
Differential follicle counts on serial sections from the right ovary were determined as previously described (6,34). Additionally, the ratio of primordial (resting) to growing (primary, secondary, and antral follicles) follicles was calculated for each mouse.
To determine the number of corpora lutea (CL) per ovary, every 40th section of a serially sectioned ovary (8 μm sections) was counted as previously described (7). To ensure no CL were counted twice, each CL was followed for its entirety through the ovary and only counted once, even if it appeared in the next section to be counted.
2.5. Serum Assays
Anti-mullerian hormone (AMH) levels were assessed with the rat and mouse ELISA assay (cat # AL-113, Ansh Labs, Webster, TX) and follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, and thyroid stimulating hormone (TSH) levels were assessed with the Milliplex Mouse Pituitary Magnetic Bead Panel (cat # MPTMAG-49K, Millipore Sigma, Burlington, MA) following manufacturer’s protocols. Sensitivity and inter-assay and intra-assay coefficient of variation (CV) for serum assays are provided in Supplemental Table 2. All serum assays were performed on serum obtained from a single blood sample collected at sacrifice.
2.6. Immunohistochemistry
Immunohistochemistry of ovarian CD68 (tissue macrophage marker, Cat # ab125212, Abcam, Cambridge, MA) was performed to assess ovarian inflammation. Ovaries from N = 4-5 mice per group were fixed in 4% paraformaldehyde, embedded in paraffin, and serially sectioned (5-μm sections). Sections were then rehydrated and stained for CD68 as previously described (21). Three ovarian sections for each mouse were stained for CD68 and one negative no primary antibody control was included for each animal to ensure antigen specificity.
The total numbers of macrophages (CD68 positive cells) were counted in three sections taken from the middle of the ovary from each mouse. ImageJ (National Institute of Health, Bethesda, MD) was used to determine the area of each ovarian section counted for macrophages. The number of macrophages for each section was normalized to the area of that ovarian section, and the adjusted counts from the three sections for each animal were averaged to determine the number of macrophages per ovarian mm2 for each experimental animal. 4 to 5 ovaries from each group were analyzed.
2.7. RNA-sequencing
RNA was extracted with the Allprep DNA/RNA/Protein mini kit following manufacture protocols (Cat # 80004, Qiagen, Hilden, Germany) from the left ovary. Four to five animals from each dietary group were randomly selected for RNA-sequencing analysis. Nanodrop 2000 Spectrophotometer (Thermo Scientific, Frederick, MD) was used to assess RNA quality and quantity. RNA samples were submitted to the University of Colorado Denver’s Genomics and Microarray Core (Aurora, CO) where quality was assessed with the Agilent 2100 Bioanalyzer both before and after library preparation with polyA selection using Nugen’s Universal Plus mRNA-seq kit. Sequencing was performed with the NovaSEQ6000 Ilumina sequencing platform as paired end 2 × 150 cycle sequencing at 40 million reads (80 million paired reads) per sample. Samples were run in two batches. Base calling was performed with RTA version 3.3.3. All raw reads from RNA-sequencing had at least 90.1% of bases with a quality score of at least 30 and a mean quality score of at least 35.2. To determine the quality of the raw RNA-sequencing reads, FastQC was used (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). TrimGalore! (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) was used to trim adaptors and to remove poor quality sequences with phred cutoff of 20. FastQC was then used to assess quality of the trimmed reads, and all samples passed quality control. All reads were then aligned to the mouse GRCm38 reference genome with STAR (35) and alignment summary metrics are provided in Supplemental Table 3. FeatureCounts was then used to count the mapped reads (36). Finally, edgeR (37) was used for differential expression (DE) analysis, accounting for the batch each sample was run in and only including genes with at least 5 counts per million. Heat maps were generated with pheatmap in Rstudio using normalized log counts per million values.
2.8. Human Follicular Fluid Fatty Acid Assessment
To begin to correlate our animal data with human data, we analyzed FA levels in leftover FF from patients undergoing IVF at a single academic center (University of Colorado’s Advanced Reproductive Medicine Clinic). All procedures were approved by the Colorado Multiple Institutional Review Board (COMIRB, protocol 16-2496). We recruited patients with diminished ovarian reserve (DOR), and normal ovarian reserve (NOR) controls. DOR was defined as either AMH < 1.0 ng/ml, antral follicle count (AFC) ≤ 10, ≤ 10 oocytes retrieved, or FSH > 10 mIU/ml. NOR controls had either tubal or male factor infertility. Exclusion criteria included polycystic ovary syndrome (PCOS), fish oil supplementation, and age < 21 or > 45 years. Participant characteristics are presented in Supplemental Table 4.
After informed consent was obtained, remaining FF after oocyte retrieval was collected and briefly stored at 4°C until samples were transported to the lab on ice. FF samples were then centrifuged at 500 × g for 5 min at 4°C, and the supernatant was removed and stored at −80°C until FA extraction. Briefly, 250 μL of FF was added to 250 μL of potassium phosphate buffer (pH 7), 10 μL was removed for total protein estimation, and 500 ng of blended stable isotope internal standards (described in (38)) was added to the remaining 490 μL that was acidified with 40 μL of 1M HCl. Total lipids were extracted sequentially with 2 mL of 3:1 (vol/vol) Hexanes:Ethyl Acetate, followed by re-extraction with 2 mL of 100% Hexanes. Both organic extracts were combined and taken to dryness under N2 gas. The NEFA in the total lipid fraction were derivatized and quantified by mass spectrometry as previously described (39), and all NEFA were normalized to total protein levels in the extracted sample (39). To control for confounding fatty acids bound to HSA in the modified human tubal fluid (mHTF) media (40), 250 μL of the unused media was extracted in parallel with FF samples and the FA present in mHTF media were quantitatively subtracted from FA detected in FF samples.
2.9. Statistics
For anthropometrics, blood glucose, dietary intake, serum hormones, follicle counts, CL, and ovarian macrophage infiltration, statistical differences were determined with a one-way ANOVA followed by Tukey’s post-hoc test if the overall ANOVA reached statistical significance. Differences in the number of mice with abnormal estrous cycles between the groups were assessed with a chi-squared test. A p-value of < 0.05 was considered statistically significant. For FF data, student’s two-tailed t test was used to determine differences between the NOR and DOR groups. Correlations of FF FA levels and other parameters were performed with Pearson’s correlations as appropriate. All data are reported as means ± SEM unless otherwise noted. All statistical analysis of anthropometrics, blood glucose, serum hormones, follicle counts, CL, ovarian macrophage infiltration, and FF data were determined with GraphPad Prism7 (GraphPad Software, San Diego, CA). For RNA-sequencing analysis, genes were considered differentially expressed between groups as p < 0.0001, with special consideration given to genes that were also significant after using the false discovery rate to control for multiple comparisons at q < 0.05. Only genes in the GRCm38 mouse reference genome were considered in the analysis and interpretation of DE genes.
3. Results
In order to determine the efficacy of DHA supplementation in improving HFD-induced ovarian dysfunction we performed a study with two periods of dietary intervention (Figure 1). The first dietary intervention consisted of 10 weeks of HFD feeding to induce ovarian dysfunction. The second dietary intervention, or the diet reversal, involved switching mice from HFD to either a standard chow, a chow supplemented with DHA, or a HFD supplemented with DHA for another 10 weeks while a final group remained on HFD as HFD-controls. All mice were compared to mice of the same age fed chow for the entire study period (during both dietary interventions).
3.1. Animal Characteristics and Dietary Intake
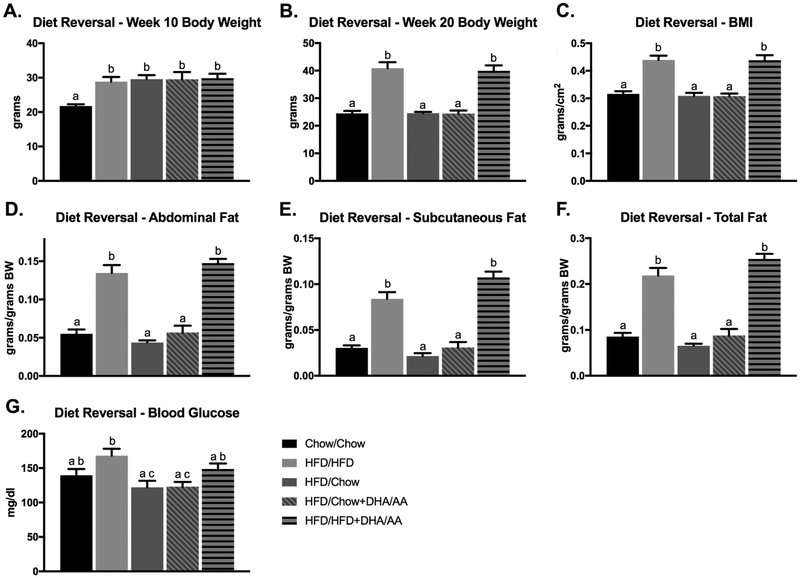
After 10 weeks of HFD exposure, all HFD-fed mice weighed more than chow-fed controls (p < 0.005, Figure 2A). Following 20 weeks of dietary feeding, HFD and HFD/HFD+DHA mice continued to weigh more than chow-fed controls (p < 0.0001), however diet reversal to either chow or chow+DHA after HFD restored body weight to that of chow-fed controls (Figure 2B). Weekly body weights for all mice are presented in Supplemental Figure 1. Consistent with increased body weight, the HFD and HFD/HFD+DHA groups also had elevated BMI, abdominal fat, subcutaneous fat, and total fat compared to chow-fed controls (p < 0.0001), while reversal to chow or chow+DHA restored all these parameters to levels similar to chow-fed controls (Figure 2C-F). Blood glucose levels were only elevated after 20 weeks of dietary intervention in the HFD/HFD mice compared to chow-fed controls (p < 0.05, Figure 2G).
Figure 2. Anthropometric and Metabolic Parameters After Dietary Intervention.
Anthropometric (A-F) and metabolic (G) parameters were measured after either 10 or 20 weeks of diet. Data are presented as means ± SEM. Bars with different letters were significantly different (p < 0.05) as determined by a one-way ANOVA followed by Tukey’s post-hoc test. A-B, D-G Chow N=10, HFD N=15, HFD/Chow N=10, HFD/Chow+DHA/AA N=9, HFD/HFD+DHA/AA N=10. C Chow N=10, HFD N=15, HFD/Chow N=10, HFD/Chow+DHA/AA N=8, HFD/Chow+DHA/AA N=10. Body weight (BW).
During the first dietary period, weeks 0 to 10, all mice ingesting HFD consumed more total calories and more calories from total fat than the chow-fed controls (p < 0.05, Table 1). In the first dietary period there was no DHA intake in any of the groups, however AA was consumed by all mice receiving the HFD (Table 1). During the second dietary period, the mice continued to be fed HFD and those switched to HFD+DHA continued to consume more total calories and more calories from total fat than mice in all other groups (p < 0.05, Table 1). During the second dietary period, the HFD/chow+DHA and HFD/HFD+DHA groups consumed on average 6.22 ± 0.55 g/kg BW and 6.55 ± 0.34 g/kg BW DHA per week respectively, while there was no DHA consumption in any of the other groups (Table 1). The chow and HFD/chow groups consumed no AA during the second dietary period, while all other groups had some AA intake (Table 1).
Table 1.
Calories, AA, and DHA Consumed per Mouse per Week.
| Diet 1 | Diet 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Total kcal per week |
Fat kcal per week |
AA per week (g/kg BW) |
DHA per week (g/kg BW) |
Total kcal per week |
Fat kcal per week |
AA per week (g/kg BW) |
DHA per week (g/kg BW) |
|
| Chow | 62.2a ± 2.3 | 9.4a ± 0.4 | 0.0a ± 0.0 | 0.0 ± 0.0 | 59.4a ± 1.0 | 9.0a ± 0.2 | 0.0a ± 0.0 | 0.0a ± 0.0 |
| HFD | 228.1b ± 11.8 | 136.8b ± 7.1 | 1.5b ± 0.1 | 0.0 ± 0.0 | 157.6b ± 8.1 | 94.6b ± 4.9 | 0.79b ± 0.06 | 0.0a ± 0.0 |
| HFD/Chow | 235.0b ± 12.8 | 141.0b ± 7.7 | 1.5b ± 0.1 | 0.0 ± 0.0 | 55.6a ± 7.2 | 8.4a ± 1.1 | 0.0a ± 0.0 | 0.0a ± 0.0 |
| HFD/Chow+DHA | 223.6b ± 9.2 | 134.2b ± 5.5 | 1.5b ± 0.1 | 0.0 ± 0.0 | 59.5a ± 6.3 | 10.1a ± 1.1 | 0.3c ± 0.03 | 6.2b ± 0.6 |
| HFD/HFD+DHA | 197.8b ±12.8 | 118.7b ± 7.7 | 1.3b ± 0.1 | 0.0 ± 0.0 | 153.9b ± 4.7 | 92.4b ± 4.9 | 1.0d ± 0.1 | 6.5b ± 0.3 |
Data reported as mean ± sem. Information for intake during diet 1 contains data from weeks 4 to 10 of dietary feeding and information for intake during diet 2 contains data from weeks 11 to 19 of dietary feeding. In each column, differing letters represent significant differences between the different dietary groups as determined by one-way ANOVA followed by Tukey’s posthoc test when appropriate. Differences were considered significantly different at p < 0.05.
3.2. Estrous Cycle
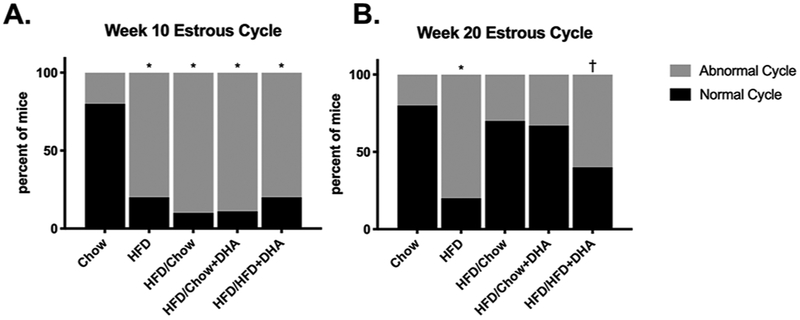
After 10 weeks, all mice fed HFD had a higher prevalence of abnormal estrous cycles than the chow-fed controls (p < 0.05, Figure 3A). After 20 weeks, the HFD mice continued to have a higher prevalence of impaired cyclicity compared to chow-fed controls (p < 0.05, Figure 3B). Undergoing a diet reversal from HFD to either chow or chow+DHA resulted in improvement of the estrous cycle with no difference in the prevalence of mice with abnormal cycles compared to the chow-fed controls (Figure 3B). However, the mice that were switched from the HFD to HFD+DHA did not experience improvement to this extent, still experiencing clinically relevant but not significant increased prevalence of mice with impaired estrous cycles compared to chow-fed controls (p = 0.068, Figure 3B).
Figure 3. Estrous Cyclicity After Diet 1 and 2.
Daily vaginal smears were assessed for the last two weeks of diet 1 (A) and diet 2 (B) to evaluate the estrous cycle. Data are presented as the percent of mice cycling normally or abnormally at each time point. Chi-squared test was used to assess differences between groups. *p < 0.05 †p < 0.07 as compared to chow. A-B Chow N=10, HFD N=15, HFD/Chow N=10, HFD/Chow+DHA/AA N=9, HFD/HFD+DHA/AA N=10.
3.3. Follicle Counts and CL Assessment
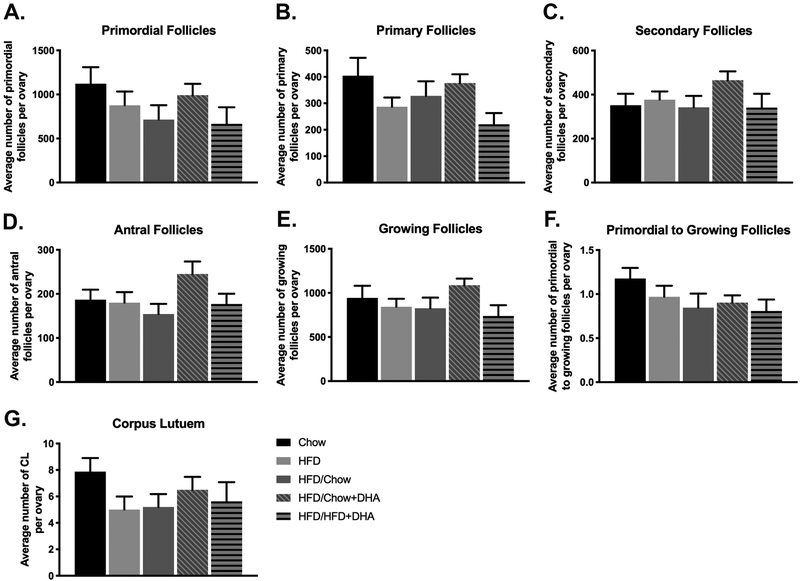
After 20 weeks of the various dietary exposures, there were no statistically significant differences in any of the ovarian follicle populations between the different dietary groups (Figure 4A-F). We observed a non-significant 21.9% decrease in primordial follicles in the mice fed HFD for 20 weeks compared to the chow controls. Mice that were reversed to either chow or HFD+DHA had similar non-significant decreases in primordial follicles with 36.3% and 40.6% fewer primordial follicles respectively relative to chow-fed controls. Mice from the diet reversal to chow+DHA had only 11.6% fewer primordial follicles than the chow-fed controls. Further, reversal to chow+DHA resulted in increased secondary and antral follicles relative to chow controls (32.3% and 31.1% respectively).
Figure 4. Ovarian Follicle and Corpus Luteum Counts.
Serial ovarian sections were used to determine the number of (A) primordial follicles (B) primary follicles (C) secondary follicles (D) antral follicles and (G) CL per ovary after dietary intervention. The number of primary, secondary, and antral follicles for each animal were used to determine the number of growing follicles in each animal (E). The ratio of primordial to growing follicles per ovary is presented in figure 4F. Data are presented as mean ± SEM. A-F Chow N = 8, HFD N = 12, HFD/Chow N = 9, HFD/Chow+DHA N = 9, HFD/HFD+DHA N = 8. G Chow N = 7, HFD N = 12, HFD/Chow N = 10, HFD/Chow+DHA N = 8, HFD/HFD+DHA N = 8.
We also observed no significant differences in the number of CL in the ovaries of our mice (Figure 4G), however, we did observe fewer CL in the HFD-fed mice and in the mice reversed to either chow, chow+DHA, or HFD+DHA compared to chow controls (36.5%, 17.5%, 34.0%, and 28.6% respectively).
3.4. Hormones Affecting Reproduction
There were no significant differences in the levels of AMH, LH, FSH, prolactin, or TSH between any of the groups (Table 2) after the dietary intervention. When the LH:FSH ratio was examined, we also saw no differences between groups. The LH:FSH ratio was < 1 for all groups exposed to HFD, providing evidence that there was no development of PCOS with HFD feeding (41).
Table 2.
Serum Reproductive Hormone Levels After Dietary Intervention.
| Chow | HFD | HFD/Chow | HFD/Chow+DHA | HFD/HFD+DHA | |
|---|---|---|---|---|---|
|
AMH (ng/ml) |
104.39 ± 9.35 | 74.45 ± 6.99 | 75.76 ± 13.83 | 102.88 ± 5.83 | 87.96 ± 11.47 |
|
LH (ng/ml) |
0.32 ± 0.088 | 0.29 ± 0.056 | 0.20 ± 0.031 | 0.15 ± 0.017 | 0.25 ± 0.056 |
|
FSH (ng/ml) |
2.22 ± 0.38 | 1.66 ± 0.23 | 1.65 ± 0.25 | 1.69 ± 0.17 | 2.10 ± 0.44 |
|
LH:FSH ratio |
0.21 ± 0.092 | 0.18 ± 0.03 | 0.14 ± 0.028 | 0.095 ± 0.011 | 0.24 ± 0.12 |
|
Prolactin (ng/ml) |
73.89 ± 6.72 | 63.07 ± 5.64 | 53.75 ± 5.73 | 54.02 ± 6.63 | 53.62 ± 8.92 |
|
TSH (ng/ul) |
0.96 ± 0.25 | 0.54 ± 0.092 | 0.55 ± 0.18 | 0.40 ± 0.081 | 0.54 ± 0.086 |
Serum was collected after 20 weeks of dietary intervention and assessed for AMH, LH, FSH, prolactin, and TSH levels. Data are presented as mean ± SEM. Differences between groups were determined by a one-way ANOVA, as no significant ANOVA’s were found no post-hoc tests were conducted. AMH: Chow N = 9, HFD N = 10, HFD/Chow N = 9, HFD/Chow+DHA N = 9, HFD/HFD+DHA N = 9. LH, FSH, LH:FSH, prolactin, and TSH: Chow N = 8, HFD N = 7, HFD/Chow N = 7, HFD/Chow+DHA N = 7, and HFD/HFD+DHA N = 8.
3.5. Ovarian Macrophage Infiltration
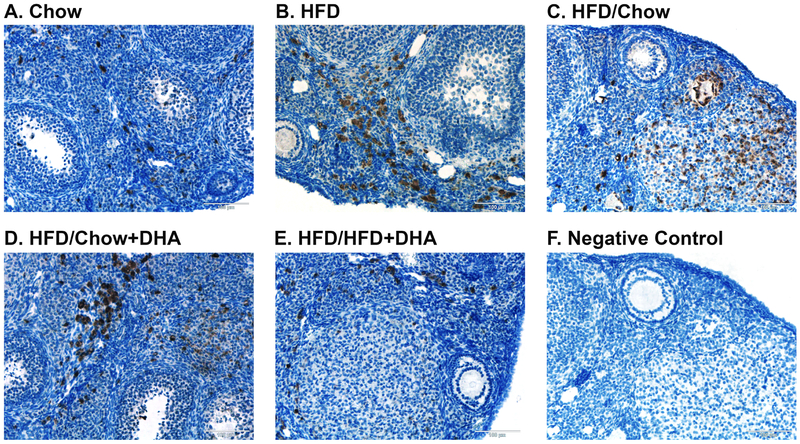
To determine ovarian inflammation we examined infiltrating ovarian macrophages after dietary intervention. Although the HFD-fed mice had 45.6% more macrophages per ovary (143.5 ± 15.7 macrophages/mm2 ovary) than chow-fed controls (98.5 ± 24.7 macrophages/mm2 ovary), this did not reach statistical significance. We also observed non-statistically significant increased infiltration of ovarian macrophages of 56.4% and 41.3% in the mice that were reversed to chow and to chow+DHA after HFD respectively (154.1 ± 13.1 macrophages/mm2 ovary and 139.3 ± 29.8 macrophages/mm2 ovary respectively). Interestingly, the mice reversed to HFD+DHA after HFD feeding had infiltrating macrophages levels similar to the chow-fed controls (110.13 ± 25.2 macrophages/mm2 ovary). Representative images of ovarian macrophage infiltration are presented in Figure 5.
Figure 5. Ovarian Macrophage Infiltration.
Macrophages present in the ovary were identified using the CD68 marker in Chow (N = 5), HFD (N = 5), HFD/Chow (N = 5), HFD/Chow+DHA (N = 4), and HFD/HFD+DHA (N = 5) mice. Representative images are shown for (A) Chow, (B) HFD, (C) HFD/Chow, (D) HFD/Chow+DHA, (E), HFD/HFD+DHA, and (F) negative no-primary antibody control.
3.6. Ovarian Gene Expression
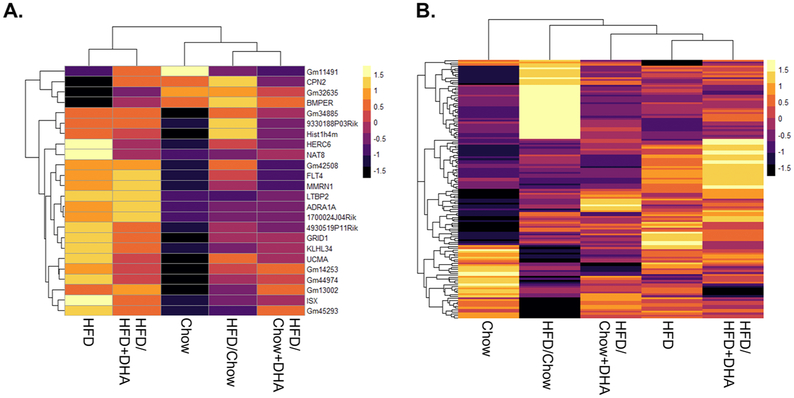
After 20 weeks of HFD, we identified 24 genes with altered ovarian expression compared to the chow-fed controls (p < 0.0001). When we examined the impact of each of our diet reversals on these 24 genes with aberrant expression in the HFD-fed mice, we found that the mice reversed to chow after HFD feeding had 20 of the 24 genes expression levels restored to that of the chow controls, the mice reversed to chow+DHA after HFD had 22 of the 24 genes restored to that of chow controls, and the mice reversed to HFD+DHA had just 16 of the 24 genes expression levels restored to that of chow controls (Figure 6A, Table 3). Interestingly we identified a novel gene, Gm42508, with no known function, that was the only DE gene in all dietary reversal groups compared to chow mice with 6.05, 5.90, 4.42, and 6.25 log2 fold change in the HFD, HFD/Chow, HFD/Chow+DHA, and HFD/HFD+DHA groups respectively. We also found that each of the four dietary interventions induced their own ovarian gene expression profile that was unique from the chow-fed controls (Figure 6B, Supplemental File 1). The mice reversed to chow, to chow+DHA, or to HFD+DHA had 66, 16 and 45 genes with altered expression relative to chow-fed controls respectively (p < 0.0001).
Figure 6. Ovarian Gene Expression Changes Following Dietary Intervention.
RNA-sequencing was conducted on the whole ovary to determine genes with altered expression after dietary intervention. A. Heatmap of the 24 genes (normalized log counts per million) found the be differentially regulated in the HFD mice compared to the chow controls was plotted for each dietary intervention group. B. Heat map of normalized log counts per million of all genes included in RNA-sequencing analysis plotted for each dietary intervention.
Table 3.
24 genes DE with HFD and their Restoration to Chow Levels with Diet Reversal
| Gene Symbol | HFD v. Chow |
HFD/Chow v. Chow |
HFD/Chow+DHA v. Chow |
HFD/HFD+DHA v. Chow |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Log2 Fold Change |
p-value | Log2 Fold Change |
p-value | Restored to Chow levels |
Log2 Fold Change |
p-value | Restored to Chow levels |
Log2 Fold Change |
p-value | Restored to Chow levels |
|
| Isx | 1.50 | 5.84E-7* | 0.19 | >0.0001 | Yes | 0.055 | >0.0001 | Yes | 0.93 | >0.0001 | Yes |
| Gm42508 | 6.05 | 2.07E-6* | 5.90 | 4.26E-6* | No | 4.42 | 7.0E-4 | No | 6.25 | 1.37E-6* | No |
| Bmper | −0.68 | 6.36E-6* | 0.26 | >0.0001 | Yes | 0.025 | >0.0001 | Yes | −0.29 | >0.0001 | Yes |
| Grid1 | 0.71 | 5.07E-6* | 0.31 | >0.0001 | Yes | 0.37 | >0.0001 | Yes | 0.56 | 6.8E-4 | No |
| Gm13002 | 2.47 | 1.80E-5 | 1.69 | >0.0001 | Yes | 2.58 | 6.30E-6 | No | 2.59 | 1.11E-5 | No |
| 1700024J04Rik | 2.11 | 4.09E-5 | 1.00 | >0.0001 | Yes | 0.84 | >0.0001 | Yes | 2.19 | 3.26E-5 | No |
| Cpn2 | −5.62 | 9.88E-5 | −0.34 | >0.0001 | Yes | −2.91 | >0.0001 | Yes | −0.75 | >0.0001 | Yes |
| Mmrn1 | 0.69 | 2.08E-4 | 0.24 | >0.0001 | Yes | 0.11 | >0.0001 | Yes | 0.78 | 6.28E-5 | No |
| Gm11491 | −1.73 | 5.06E-4 | −1.56 | >0.0001 | Yes | −1.40 | >0.0001 | Yes | −0.47 | >0.0001 | Yes |
| Herc6 | 0.46 | 4.53E-4 | 0.23 | >0.0001 | Yes | 0.093 | >0.0001 | Yes | 0.16 | >0.0001 | Yes |
| Ltbp2 | 0.68 | 5.05E-4 | 0.044 | >0.0001 | Yes | 0.16 | >0.0001 | Yes | 0.77 | 1.7E-4 | No |
| Gm14253 | 1.18 | 4.35E-4 | 0.96 | >0.0001 | Yes | 0.97 | >0.0001 | Yes | 0.84 | >0.0001 | Yes |
| Adra1a | 1.27 | 4.54E-4 | 0.23 | >0.0001 | Yes | 0.19 | >0.0001 | Yes | 1.47 | 1.0E-4 | No |
| Gm45293 | 1.71 | 3.87E-4 | 0.13 | >0.0001 | Yes | 1.13 | >0.0001 | Yes | 1.27 | >0.0001 | Yes |
| 4930519P11Rik | 1.87 | 2.78E-4 | 1.07 | >0.0001 | Yes | 0.42 | >0.0001 | Yes | 1.39 | >0.0001 | Yes |
| Hist1h4m | 2.23 | 3.48E-4 | 3.08 | 4.34E-7* | No | 1.51 | >0.0001 | Yes | 1.88 | >0.0001 | Yes |
| Nat8 | 2.25 | 3.94E-4 | 0.077 | >0.0001 | Yes | 0.25 | >0.0001 | Yes | 0.33 | >0.0001 | Yes |
| Ucma | 1.89 | 5.99E-4 | 1.17 | >0.0001 | Yes | 0.82 | >0.0001 | Yes | 1.24 | >0.0001 | Yes |
| Gm32635 | −1.32 | 9.77E-4 | −0.11 | >0.0001 | Yes | −0.35 | >0.0001 | Yes | −0.50 | >0.0001 | Yes |
| Flt4 | 0.37 | 9.85E-4 | 0.22 | >0.0001 | Yes | 0.079 | >0.0001 | Yes | 0.45 | 1.9E-4 | No |
| Klhl34 | 1.02 | 9.56E-4 | 0.42 | >0.0001 | Yes | 0.49 | >0.0001 | Yes | 0.70 | >0.0001 | Yes |
| Gm44974 | 1.62 | 8.42E-4 | 0.89 | >0.0001 | Yes | 1.02 | >0.0001 | Yes | 1.15 | >0.0001 | Yes |
| 9330188P03Rik | 1.74 | 9.20E-4 | 2.38 | 4.54E-6* | No | 1.18 | >0.0001 | Yes | 1.58 | >0.0001 | Yes |
| Gm34885 | 1.95 | 7.69E-4 | 1.95 | 8.0E-4 | No | 1.64 | >0.0001 | Yes | 1.78 | >0.0001 | Yes |
Log2 Fold Change values and p-values for the 24 DE genes in HFD mice compared to chow controls are listed. The log2 Fold Change compared to chow controls for each of the three dietary interventions and the corresponding p-value for each of HFD-induced-DE genes are provided to show which were restored to chow levels in each dietary intervention.
Corresponding FDR adjusted p-value <0.05.
To understand how the various dietary exposures influenced ovarian specific function, we identified 15 genes with documented relation to the ovary in the literature (Table 4). Common DE genes included five involved in folliculogenesis (Bmper, Flt4, Bdnf, Crhr1, and Agt) (42-46), three involved in ovulation (Ccr3, Ptx3, and Agt) (46-48), three involved in steroidogenesis (Crhr1. Agt, and Bdnf) (49-51), one involved in luteinization (Agt) (46), three involved in luteolysis (Flt4, Lyve1, and Agt) (52,53) (51), four reproductive hormone responsive genes (Ltbp2, Nr1d1, Sfrp5, and Usp53) (54-57), two involved in cumulus cell expansion (Bdnf and Ptx3) (58,59), three involved in oocyte maturation (Bdnf, Crhr1, and Agt) (60-62), two known to be upregulated in PCOS (Adra1a and Srd5a1) (63,64), and Hp, which is increased in IVF patients with better outcomes (65). Interestingly, two genes involved in circadian rhythms, Nr1d1 and Nr1d2, were upregulated in the DHA-supplemented chow reversal group compared to chow controls. As the ovarian clock and its synchronization with the peripheral clock is proposed to be important to ovarian function (66), the upregulation of these genes in the chow+DHA reversal group may be involved in the improved ovarian function observed in these mice.
Table 4.
Dietary Intervention Induced Differentially Expressed Genes Involved in Ovarian Function
| Gene | Log2 Fold Change | Ovarian role | Ovarian cells expressed in |
|||
|---|---|---|---|---|---|---|
| HFD v.Chow |
HFD/Chow v. Chow |
HFD/ Chow+DHA v. Chow |
HFD/ HFD+DHA v. Chow |
|||
|
Bmper BMP-binding endothelial regulator |
−0.68 | nd | nd | nd | Folliculogenesis | Unknown |
|
Flt4 FMS-like tyrosine kinase 4 |
0.37 | nd | nd | 0.45 | Folliculogenesis Luteolysis |
Oocytes |
|
Bdnf brain derived neurotropic factor |
nd | 1.15 | nd | nd | Folliculogenesis, cumulus cell explansion, steroidogenesis, oocyte maturation | Granulosa, cumulus, oocytes |
|
Ccr3 chemokine motif receptor 3 |
nd | −1.45 | nd | nd | Ovulation | Luteal |
|
Ptx3 pentrazin related gene |
nd | nd | nd | 0.98 | Ovulation, cumulus cell expansion | Granulosa, theca, cumulus |
|
Crhr1 corticotropin releasing hormone receptor 1 |
nd | 2.33 | nd | nd | Steroidogenesis, folliculogenesis, oocyte maturartion | Theca and luteal |
|
Agt angiotensinogen |
nd | −0.76 | nd | nd | Steroidogenesis, folliculogenesis, ovulation, luteinization, luteolysis, oocyte maturation | Granulosa, theca, cumulus, stromal, luteal, and surface epithelial |
|
Lyve1 lymphatic vessel endothelial hyaluronan receptor 1 |
nd | nd | nd | 1.13 | Luteolysis | Theca and luteal |
|
Ltbp2 latent transforming growth factor beta binding protein 2 |
0.68 | nd | nd | 0.77 | Hormone responsive | Theca |
|
Nr1d1 nuclear receptor subfamily 1, group D, member 1 |
nd | nd | 0.70 | 0.70 | Hormone responsive | Granulosa |
|
Sfrp5 secreted frizzled-related sequence protein 5 |
nd | nd | nd | 3.29 | Hormone responsive | Unknown |
|
Usp53 ubiquitin specific peptidase 53 |
nd | 0.76 | nd | Nd | Hormone responsive | Unknown |
|
Adra1a adrenergic receptor, alpha 1a |
1.27 | nd | nd | 1.47 | Upregulated in PCOS | Stromal and luteal |
|
Srd5a1 steroid 5 alpha-reductase 1 |
nd | −0.41 | nd | nd | Upregulated in PCOS | Granulosa and theca |
|
Hp haptoglobulin |
nd | nd | nd | 2.61 | Upregulated in IVF patients with better outcomes | Unknown |
Genes that were differentially expressed in the ovary after dietary intervention that are related to ovarian function are listed along with the log2 fold change difference in expression, and the general ovarian function of the gene. No difference (nd). N = 4-5 for all groups.
Although total ovarian RNA was used for RNA-sequencing, we searched the Ovarian Kaleidoscope Database (67) to determine ovarian cell type specific expression of all DE genes and have provided these data in Table 4. DE gene were found to be expressed in all cell types in the ovary, however the most DE genes were found to be expressed in granulosa, theca, and/or luteal cells.
3.7. Fatty Acid Levels in Human Follicular Fluid
As expected, DOR patients had lower AMH levels, lower AFC, fewer oocytes retrieved, and higher FSH levels compared to NOR controls (Supplemental Table 4). There were no differences in either age or BMI between the DOR and NOR groups (Supplemental Table 4).
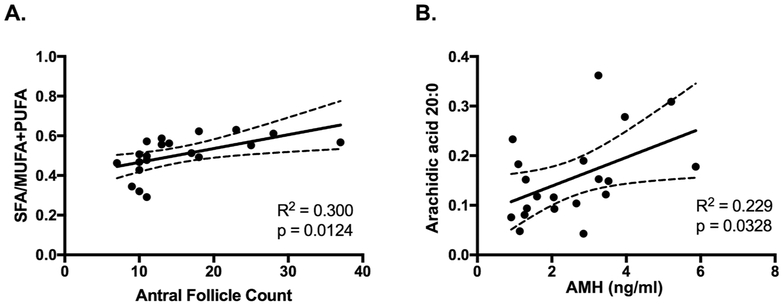
Because our rodent experiments modeled various dietary fat exposures and DHA supplementation, we examined a comprehensive profile of FF FAs by quantitative lipid mass spectrometry. Only the saturated FA arachidic acid (C20:0) present in the NEFA fraction of FF was significantly decreased in the DOR group (p = 0.01, Supplemental Table 5). We observed no difference in the level of any of the bioactive omega-6 or omega-3 FAs between the DOR and NOR groups in the NEFA quantified in FF. Interestingly, we observed a positive correlation between the ratio of saturated FAs to MUFAs plus PUFAs and AFC (r = 0.55, p = 0.012), and a positive correlation between the levels of arachidic acid and AMH (r = 0.48, p = 0.033) (Figure 7).
Figure 7. Correlations Between FF NEFA and Reproductive Measures in IVF Patients.
The ratio of SFA/MUFA+PUFA was positively correlated with the number of antral follicles (A) and the amount of arachidic acid was positively correlated with AMH levels (B) in patients undergoing IVF. N = 20.
4. Discussion
Herein we investigated if dietary intervention, particularly one including DHA supplementation, after chronic HFD exposure could restore HFD-induced ovarian dysfunction. We found that DHA supplementation had no additional benefit to weight loss in improving estrous cyclicity. DHA supplementation to HFD inhibited HFD-induced ovarian macrophage infiltration. Both DHA supplementation and weight loss provided benefit in restoring HFD-induced dysregulation of ovarian gene expression, and when the two were combined they conferred the most benefit to restoration of ovarian gene expression. However, the expression of one gene, Gm42508, was unable to be restored to chow levels by any dietary intervention, suggesting that Gm42508 plays a key role in HFD-induced ovarian dysfunction. Finally, when examining human follicular fluid, we found no association between DHA levels and ovarian reserve.
Interestingly, the only HFD-induced ovarian insult we assessed that showed improvement by all diet reversals was ovarian gene expression. The most benefit was provided in the group reversed to chow+DHA after HFD, suggesting that both weight loss and DHA supplementation have separate roles in restoring ovarian gene expression. Yet, one gene, Gm42508, which was increased with HFD feeding, was not restored to chow levels by any diet reversal, suggesting dietary intervention alone is not sufficient for complete restoration of ovarian gene expression. Gm42508 does not have a known function, but has been shown to be upregulated in female septal astrocytes compared to female lateral astrocytes (68). Future work is needed to investigate Gm42508’s role in the ovary and determine how HFD is impacting its expression and possibly ovarian function.
Although all of our diet reversals were able to at least partially restore HFD-induced gene dysregulation, each of the three dietary interventions also invoked a unique ovarian gene expression profile, highlighting the impact diet has on ovarian gene expression. Others have also shown that changes in diet alter ovarian gene expression including HFDs (69), omega-3 FA supplementation (70), and maternal low protein diets (71). Interestingly, we have previously shown that HFD alters ovarian gene expression regardless of obesity (7). Collectively these data suggest that in regards to ovarian gene expression, diet is an important contributing factor, and perhaps may be a more important than body weight. More work is needed to understand the relationship between diet and ovarian gene expression and how diet induced ovarian gene expression changes impact ovarian function.
We have previously shown that mice with the constitutively expressed fat-1 transgene (20) have increased primordial follicle numbers compared to wild-type mice when fed either standard chow or a 60% HFD (21). However, in the current study we did not see a benefit of DHA supplementation after HFD on the ovarian reserve. Fat-1 mice have elevated omega-3 FA levels starting in the early embryonic period (20,72) and perhaps this lifelong exposure provides protection to ovarian follicles in the face of HFD, while an acute supplementation with DHA is unable to confer the same benefits to the ovarian reserve. It is important to note that our HFD-fed mice did not develop the levels of primordial follicle depletion that we have previously observed (6,7), leaving little room to observe any benefit of DHA.
In contrast to our hypothesis that DHA supplementation would restore HFD-induced ovarian dysfunction by alleviating HFD-induced ovarian inflammation, we only observed improved ovarian inflammation (reduction of infiltrating ovarian macrophages) in the mice receiving HFD supplemented with DHA. However, the mice did not have improved estrous cyclicity or improved ovarian reserve. On the other hand, mice reversed to chow supplemented with DHA did not have decreased ovarian inflammation, but did have improved estrous cyclicity and ovarian reserve similar to controls. It is important to consider that macrophages have important functions in the ovary in addition to accumulating at the site of inflammation and participating in ovarian inflammatory responses (73). Ovarian macrophages play a role in the phagocytosis of regressing CL and atretic follicles as well as ovulation (73,74). Thus, the relationship between ovarian macrophage levels and ovarian function is not as clear as a simple correlation with inflammation. Perhaps the elevated ovarian macrophages in the mice reversed to chow+DHA are there to exert their ovarian function in excess to overcome HFD-induced ovarian dysfunction. On the other hand, the reduced macrophages in the mice reversed to DHA supplemented HFD may be reflecting an improvement in ovarian inflammation, but were now no longer present at high enough levels to overcome HFD-induced ovarian dysfunction. Future work is needed to further understand this relationship.
When considering the results of the DHA supplementation on improving HFD-induced ovarian dysfunction, it is important to consider how this supplementation in mice would translate to human consumption. The DHA supplemented chow diet contains 2.18% of total calories from DHA, while the DHA supplemented HFD provides 1.38% of total calories from DHA. A human consuming a 2000 calorie/day diet would need to consume 4.9 g DHA/day to mimic the DHA supplemented chow or 3 g DHA/day to mimic the DHA supplemented HFD. As these are high levels of DHA, supplementation at the pharmaceutical level would be required to meet these needs. It is interesting to point out that although the DHA supplemented chow and HFD were both supplemented at 8 g DHA/kg diet, the chow diet contains less fat, and thus, the DHA content makes up a larger percentage of both fat calories and total calories of the chow diet than DHA does in the DHA supplemented HFD. This may explain some of the differences observed between the DHA supplemented chow and DHA supplemented HFD ovarian outcomes.
Finally, we did not observe any associations between FF DHA levels and ovarian reserve. However, it is important to note that we did not measure serum DHA levels or DHA intake in our participants, making it impossible to know if there were actual differences in DHA intake between the NOR and DOR groups. As women of child-bearing age in the US consume small amounts of DHA per day (88.1 ± 3.0 mg) (75), it is possible any differences in intake would be too small to resolve with our limited sample. Surprisingly, we found that the saturated FA arachidic acid was increased in the FF of NOR women and was positively associated with AMH levels. This was a surprising finding, as we did not expect higher levels of a saturated FA to be associated with better ovarian reserve. Others have found association with follicular fluid arachidic acid levels and fertility, however these data shown the opposite relationship, with cows from genetic backgrounds indicating low fertility have lower levels of arachidic acid in their follicular fluid (76). We also observed a positive correlation between the ratio of saturated to unsaturated FAs in FF and AFC. These data suggest a benefit of higher FF saturated NEFA levels in regards to ovarian reserve status, which is perplexing to the mouse data presented herein and our prior work showing high saturated fat intake is associated with poor ovarian reserve status (6,7). Future work is needed to investigate the relationship of FF FA, particularly saturated FAs and ovarian reserve.
4.1. Conclusions
In conclusion, our data suggests some benefit of DHA supplementation after HFD, particularly in regards to ovarian gene expression, however complete restoration of ovarian function was not achieved. Interestingly, each diet reversal induced its own gene expression profile, suggesting diet has a strong influence on ovarian gene expression. Although our dietary interventions did provide some improvement of ovarian function after HFD, complete restoration was not achieved. Other dietary and non-dietary interventions need to be considered to improve HFD-induced ovarian dysfunction.
Supplementary Material
Supplemental Figure 1. Weekly Body Weights During Dietary Intervention. Weekly body weights in all mice were recorded. Data is presented as means ± SEM. Chow N = 10, HFD N = 15, HFD/Chow N = 10, HFD/Chow+DHA N = 9, HFD/HFD+DHA N = 10.
Highlights:
Diet reversal with DHA supplementation after HFD restores ovarian gene expression
DHA supplemented to chow after HFD appears to prevent ovarian reserve decline
DHA has no additional benefit to reversal to chow on improving estrous cyclicity
5. Acknowledgments
Conflicts of interest: none. M.E.S-W was supported by ABOG/American Association of Obstetricians and Gynecologists Foundation Research Grant, American Society for Reproductive Medicine Research Grant, and P30DK048520 grant. I.S.L was supported by University of Colorado School of Medicine Resident Research Fund. M.C.R. was supported by grants DK109079 and DK48520. The funding sources had no part aside from funding. We wish to thank Liesl Nel-Themaat, PhD, David Russell, and Melissa Rosario for their help in follicular fluid collection and the research participants for participating in this study.
Abbreviations.
- HFD
High-fat diet
- FA
fatty acid
- PUFA
polyunsaturated fatty acid
- DHA
docosahexaenoic acid
- AA
arachidonic acid
- BMI
body mass index
- AMH
anti-mullerian hormone
- DE
differentially expressed
- IVF
In-vitro fertilization
- FF
follicular fluid
- NEFA
Non-esterified fatty acid
- CL
corpora luteum
- FSH
follicle stimulating hormone
- LH
luteinizing hormone
- TSH
thyroid stimulating hormone
- CV
coefficient of variation
- DOR
diminished ovarian reserve
- NOR
normal ovarian reserve
- MUFA
monounsaturated fatty acid
- AFC
antral follicle count
- PCOS
polycystic ovarian syndrome
- SEM
standard error of the mean
- Bmper
BMP-binding endothelial regulator
- Flt4
FMS-like tyrosine kinase 4
- Bdnf
brain derived neurotropic factor
- Ccr3
chemokine motif receptor 3
- Prx3
pentrazin related gene
- Crhr1
corticotropin releasing hormone receptor 1
- Agt
angiotensinogen
- Lyve1
lymphatic vessel endothelial hyaluronan receptor 1
- Ltbp2
latent transforming growth factor beta binding protein 1
- Nrd1d1
nuclear receptor subfamily 1, group D, member 1
- Sfrp5
secreted frizzled-related sequence protein 5
- Usp53
ubiquitin specific peptidase 53
- Adra1a
adrenergic receptor alpha 1a
- Srd5a1
steroid 5 alpha-reductase 1
- Hp
haptoglobulin
References
- 1.Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol 2015; 29:498–506 [DOI] [PubMed] [Google Scholar]
- 2.Bellver J, Ayllon Y, Ferrando M, Melo M, Goyri E, Pellicer A, Remohi J, Meseguer M. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertility and Sterility 2010; 93:447–454 [DOI] [PubMed] [Google Scholar]
- 3.Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online 2011; 23:421–439 [DOI] [PubMed] [Google Scholar]
- 4.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology--a systematic review. Hum Reprod Update 2007; 13:433–444 [DOI] [PubMed] [Google Scholar]
- 5.Vannice G, Rasmussen H. Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet 2014; 114:136–153 [DOI] [PubMed] [Google Scholar]
- 6.Skaznik-Wikiel ME, Swindle DC, Allshouse AA, Polotsky AJ, McManaman JL. High-Fat Diet Causes Subfertility and Compromised Ovarian Function Independent of Obesity in Mice. Biol Reprod 2016; 94:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohos NM, Cho KJ, Swindle DC, Skaznik-Wikiel ME. High-fat diet exposure, regardless of induction of obesity, is associated with altered expression of genes critical to normal ovulatory function. Mol Cell Endocrinol 2018; 470:199–207 [DOI] [PubMed] [Google Scholar]
- 8.Nteeba J, Ortinau LC, Perfield JW 2nd, Keating AF. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol Reprod Dev 2013; 80:948–958 [DOI] [PubMed] [Google Scholar]
- 9.Akamine EH, Marcal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, Carvalho CR. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol 2010; 206:65–74 [DOI] [PubMed] [Google Scholar]
- 10.Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 2010; 151:5438–5445 [DOI] [PubMed] [Google Scholar]
- 11.Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev 2015; 27:716–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 2007; 77:190–201 [DOI] [PubMed] [Google Scholar]
- 13.Abayasekara DR, Wathes DC. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukot Essent Fatty Acids 1999; 61:275–287 [DOI] [PubMed] [Google Scholar]
- 14.Marshall LA, Szczesniewski A, Johnston PV. Dietary alpha-linolenic acid and prostaglandin synthesis: a time course study. Am J Clin Nutr 1983; 38:895–900 [DOI] [PubMed] [Google Scholar]
- 15.Culp BR, Titus BG, Lands WE. Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandins Med 1979; 3:269–278 [DOI] [PubMed] [Google Scholar]
- 16.da Silva-Santi LG, Antunes MM, Caparroz-Assef SM, Carbonera F, Masi LN, Curi R, Visentainer JV, Bazotte RB. Liver Fatty Acid Composition and Inflammation in Mice Fed with High-Carbohydrate Diet or High-Fat Diet. Nutrients 2016; 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue-Yamauchi A, Itagaki H, Oda H. Eicosapentaenoic acid attenuates obesity-related hepatocellular carcinogenesis. Carcinogenesis 2018; 39:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients 2010; 2:355–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, Pan AH, Guo L, Rodig SJ, Tilly JL, Rueda BR, Puder M. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012; 11:1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004; 427:504. [DOI] [PubMed] [Google Scholar]
- 21.Hohos NM, Cho KJ, Swindle DC, Allshouse AA, Rudolph MC, Skaznik-Wikiel ME. Fat-1 Transgene Is Associated With Improved Reproductive Outcomes. Endocrinology 2018; 159:3981–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mostafa AF, Samir SM, Nagib RM. Omega-3 polyunsaturated fatty acid, docosahexaenoic acid, and its role in exhaustive exercise induced changes in female rat ovulatory cycle. Can J Physiol Pharmacol 2017; [DOI] [PubMed] [Google Scholar]
- 23.Broughton KS, Bayes J, Culver B. High alpha-linolenic acid and fish oil ingestion promotes ovulation to the same extent in rats. Nutr Res 2010; 30:731–738 [DOI] [PubMed] [Google Scholar]
- 24.Trujillo EP, Broughton KS. Ingestion of n-3 polyunsaturated fatty acids and ovulation in rats. J Reprod Fertil 1995; 105:197–203 [DOI] [PubMed] [Google Scholar]
- 25.Chiu YH, Karmon AE, Gaskins AJ, Arvizu M, Williams PL, Souter I, Rueda BR, Hauser R, Chavarro JE, Team ES. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod 2018; 33:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirabi P, Chaichi MJ, Esmaeilzadeh S, Ali Jorsaraei SG, Bijani A, Ehsani M, Hashemi Karooee SF. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis 2017; 16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaaker M, Rahimipour A, Nouri M, Khanaki K, Darabi M, Farzadi L, Shahnazi V, Mehdizadeh A. Fatty acid composition of human follicular fluid phospholipids and fertilization rate in assisted reproductive techniques. Iran Biomed J 2012; 16:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise LA, Wesselink AK, Tucker KL, Saklani S, Mikkelsen EM, Cueto H, Riis AH, Trolle E, McKinnon CJ, Hahn KA, Rothman KJ, Sorensen HT, Hatch EE. Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies. Am J Epidemiol 2018; 187:60–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammiche F, Vujkovic M, Wijburg W, de Vries JH, Macklon NS, Laven JS, Steegers-Theunissen RP. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil Steril 2011; 95:1820–1823 [DOI] [PubMed] [Google Scholar]
- 30.Hohos NM, Cho KJ, Swindle DC, Skaznik-Wikiel ME. High-fat diet exposure, regardless of induction of obesity, is associated with altered expression of genes critical to normal ovulatory function. Mol Cell Endocrinol 2017; [DOI] [PubMed] [Google Scholar]
- 31.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci 2009; Appendix 4:Appendix 4I [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-Restricted C57BL/6J Mice Are Resistant to Diet-Induced Obesity and Insulin Resistance but Have Low Bone Density. Plos One 2012; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjogren K, Hellberg N, Bohlooly-Y M, Savendahl L, Johansson MS, Berglindh T, Bosaeus I, Ohlsson C. Body fat content can be predicted in vivo in mice using a modified dual-energy X-ray absorptiometry technique. Journal of Nutrition 2001; 131:2963–2966 [DOI] [PubMed] [Google Scholar]
- 34.Tilly JL. Ovarian follicle counts--not as simple as 1, 2, 3. Reprod Biol Endocrinol 2003; 1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobin A, Gingeras TR. Mapping RNA-seq Reads with STAR. Curr Protoc Bioinformatics 2015; 51:11 14 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014; 30:923–930 [DOI] [PubMed] [Google Scholar]
- 37.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010; 26:139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudolph MC, Young BE, Jackson KH, Krebs NF, Harris WS, MacLean PS. Human Milk Fatty Acid Composition: Comparison of Novel Dried Milk Spot Versus Standard Liquid Extraction Methods. J Mammary Gland Biol Neoplasia 2016; 21:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudolph MC, Jackman MR, Presby DM, Houck JA, Webb PG, Johnson GC, Soderborg TK, de la Houssaye BA, Yang IV, Friedman JE, MacLean PS. Low Neonatal Plasma N-6/N-3 Pufa Ratios Regulate Offspring Adipogenic Potential and Condition Adult Obesity Resistance. Diabetes 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fredrickson J, Krisher R, Morbeck DE. The impact of the protein stabilizer octanoic acid on embryonic development and fetal growth in a murine model. J Assist Reprod Genet 2015; 32:1517–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banaszewska B, Spaczynski RZ, Pelesz M, Pawelczyk L. Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo- and hyperinsulinemia. Rocz Akad Med Bialymst 2003; 48:131–134 [PubMed] [Google Scholar]
- 42.Fenwick MA, Mansour YT, Franks S, Hardy K. Identification and regulation of bone morphogenetic protein antagonists associated with preantral follicle development in the ovary. Endocrinology 2011; 152:3515–3526 [DOI] [PubMed] [Google Scholar]
- 43.Ortega HH, Veiga-Lopez A, Sreedharan S, Velazquez MMD, Salvetti NR, Padmanabhan V. Developmental Programming: Does Prenatal Steroid Excess Disrupt the Ovarian VEGF System in Sheep? Biol Reprod 2015; 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dominguez MA, Cho N, Zhang B, Neal MS, Foster WG. Brain-derived neurotrophic factor expression in granulosa lutein cells. Reprod Biomed Online 2011; 22:17–24 [DOI] [PubMed] [Google Scholar]
- 45.Xu M, Sun J, Wang Q, Zhang Q, Wei C, Lai D. Chronic restraint stress induces excessive activation of primordial follicles in mice ovaries. PLoS One 2018; 13:e0194894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goncalves PB, Ferreira R, Gasperin B, Oliveira JF. Role of angiotensin in ovarian follicular development and ovulation in mammals: a review of recent advances. Reproduction 2012; 143:11–20 [DOI] [PubMed] [Google Scholar]
- 47.Kuwabara Y, Katayama A, Igarashi T, Tomiyama R, Piao H, Kaneko R, Abe T, Mine K, Akira S, Orimo H, Takeshita T. Rapid and transient upregulation of CCL11 (eotaxin-1) in mouse ovary during terminal stages of follicular development. Am J Reprod Immunol 2012; 67:358–368 [DOI] [PubMed] [Google Scholar]
- 48.Yao GD, Liang M, Liang N, Yin MM, Lu MR, Lian J, Wang Y, Sun F. MicroRNA-224 is involved in the regulation of mouse cumulus expansion by targeting Ptx3. Molecular and Cellular Endocrinology 2014; 382:244–253 [DOI] [PubMed] [Google Scholar]
- 49.Calogero AE, Burrello N, Negri-Cesi P, Papale L, Palumbo MA, Cianci A, Sanfilippo S, D'Agata R. Effects of corticotropin-releasing hormone on ovarian estrogen production in vitro. Endocrinology 1996; 137:4161–4166 [DOI] [PubMed] [Google Scholar]
- 50.Siqueira LC, dos Santos JT, Ferreira R, dos Santos RS, dos Reis AM, Oliveira JF, Fortune JE, Goncalves PB. Preovulatory changes in the angiotensin II system in bovine follicles. Reprod Fert Develop 2013; 25:539–546 [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Wang F, Liu Z, Zhao Y, Jiang Y, Chen L, Li C, Zhou X. Brain-derived neurotrophic factor promotes proliferation and progesterone synthesis in bovine granulosa cells. J Cell Physiol 2019; 234:8776–8787 [DOI] [PubMed] [Google Scholar]
- 52.Berisha B, Schilffarth S, Kenngott R, Sinowatz F, Meyer HHD, Schams D. Expression of Lymphangiogenic Vascular Endothelial Growth Factor Family Members in Bovine Corpus Luteum. Anat Histol Embryol 2013; 42:292–303 [DOI] [PubMed] [Google Scholar]
- 53.Nitta A, Shirasuna K, Nibuno S, Bollwein H, Shimizu T, Miyamoto A. Downregulation of lymphatic vessel formation factors in PGF2alpha-induced luteolysis in the cow. J Reprod Dev 2013; 59:296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andersson ML, Eggen RI. Transcription of the fish Latent TGFbeta-binding protein gene is controlled by estrogen receptor alpha. Toxicol In Vitro 2006; 20:417–425 [DOI] [PubMed] [Google Scholar]
- 55.Chen H, Zhao L, Chu G, Kito G, Yamauchi N, Shigeyoshi Y, Hashimoto S, Hattori MA. FSH induces the development of circadian clockwork in rat granulosa cells via a gap junction protein Cx43-dependent pathway. Am J Physiol Endocrinol Metab 2013; 304:E566–575 [DOI] [PubMed] [Google Scholar]
- 56.Maman E, Yung Y, Cohen B, Konopnicki S, Dal Canto M, Fadini R, Kanety H, Kedem A, Dor J, Hourvitz A. Expression and regulation of sFRP family members in human granulosa cells. Molecular Human Reproduction 2011; 17:399–404 [DOI] [PubMed] [Google Scholar]
- 57.Lussier JG, Diouf MN, Levesque V, Sirois J, Ndiaye K. Gene expression profiling of upregulated mRNAs in granulosa cells of bovine ovulatory follicles following stimulation with hCG. Reprod Biol Endocrinol 2017; 15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao X, Du FJ, Liu XL, Ruan QY, Wu ZL, Lei C, Deng YF, Luo C, Jiang JR, Shi DS, Lu FH. Brain-derived neurotrophic factor (BDNF) is expressed in buffalo (Bubalus bubalis) ovarian follicles and promotes oocyte maturation and early embryonic development. Theriogenology 2019; 130:79–88 [DOI] [PubMed] [Google Scholar]
- 59.Scarchilli L, Camaioni A, Bottazzi B, Negri V, Doni A, Deban L, Bastone A, Salvatori G, Mantovani A, Siracusa G, Salustri A. PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem 2007; 282:30161–30170 [DOI] [PubMed] [Google Scholar]
- 60.Kawamura K, Kawamura N, Mulders SM, Gelpke MDS, Hsueh AJW. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. P Natl Acad Sci USA 2005; 102:9206–9211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang B, Wei DL, Cheng YN, Yuan HJ, Lin J, Cui XZ, Luo MJ, Tan JH. Restraint stress impairs oocyte developmental potential in mice: role of CRH-induced apoptosis of ovarian cells. Biol Reprod 2013; 89:64. [DOI] [PubMed] [Google Scholar]
- 62.Barreta MH, Oliveira JF, Ferreira R, Antoniazzi AQ, Gasperin BG, Sandri LR, Goncalves PB. Evidence that the effect of angiotensin II on bovine oocyte nuclear maturation is mediated by prostaglandins E2 and F2alpha. Reproduction 2008; 136:733–740 [DOI] [PubMed] [Google Scholar]
- 63.Manni L, Holmang A, Lundeberg T, Aloe L, Stener-Victorin E. Ovarian expression of alpha (1)- and beta (2)-adrenoceptors and p75 neurotrophin receptors in rats with steroid-induced polycystic ovaries. Auton Neurosci 2005; 118:79–87 [DOI] [PubMed] [Google Scholar]
- 64.Marti N, Galvan JA, Pandey AV, Trippel M, Tapia C, Muller M, Perren A, Fluck CE. Genes and proteins of the alternative steroid backdoor pathway for dihydrotestosterone synthesis are expressed in the human ovary and seem enhanced in the polycystic ovary syndrome. Molecular and Cellular Endocrinology 2017; 441:116–123 [DOI] [PubMed] [Google Scholar]
- 65.Estes SJ, Ye B, Qiu W, Cramer D, Hornstein mD, Missmer SA. A proteomic analysis of IVF follicular fluid in women <or=32 years old. Fertil Steril 2009; 92:1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sellix MT, Menaker M. Circadian clocks in the ovary. Trends Endocrinol Metab 2010; 21:628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leo CP, Vitt UA, Hsueh AJ. The Ovarian Kaleidoscope database: an online resource for the ovarian research community. Endocrinology 2000; 141:3052–3054 [DOI] [PubMed] [Google Scholar]
- 68.Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, London M, Goshen I. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018; 174:59–71 e14 [DOI] [PubMed] [Google Scholar]
- 69.Xie F, Anderson CL, Timme KR, Kurz SG, Fernando SC, Wood JR. Obesity-Dependent Increases in Oocyte mRNAs Are Associated With Increases in Proinflammatory Signaling and Gut Microbial Abundance of Lachnospiraceae in Female Mice. Endocrinology 2016; 157:1630–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otte MV, Moreira F, Bianchi I, Oliveira J Jr., Mendes RE, Haas CS, Anciuti AN, Rovani MT, Gasperin BG, Lucia T Jr. Effects of supplying omega-3 polyunsaturated fatty acids to gilts after weaning on metabolism and ovarian gene expression. J Anim Sci 2019; 97:374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sui SY, Jia YM, He B, Li RS, Li X, Cai DM, Song HG, Zhang RK, Zhao RQ. Maternal Low-protein Diet Alters Ovarian Expression of Folliculogenic and Steroidogenic Genes and Their Regulatory MicroRNAs in Neonatal Piglets. Asian Austral J Anim 2014; 27:1695–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang JX. Fat-1 transgenic mice: a new model for omega-3 research. Prostaglandins Leukot Essent Fatty Acids 2007; 77:263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang ZY, Kong BH, Mosser DM, Zhang X. TLRs, macrophages, and NK cells: Our understandings of their functions in uterus and ovary. Int Immunopharmacol 2011; 11:1442–1450 [DOI] [PubMed] [Google Scholar]
- 74.Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update 2004; 10:119–133 [DOI] [PubMed] [Google Scholar]
- 75.Zhang ZY, Fulgoni VL, Kris-Etherton PM, Mitmesser SH. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001-2014. Nutrients 2018; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore SG, O'Gorman A, Brennan L, Fair T, Butler ST. Follicular fluid and serum metabolites in Holstein cows are predictive of genetic merit for fertility. Reprod Fertil Dev 2017; 29:658–669 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Weekly Body Weights During Dietary Intervention. Weekly body weights in all mice were recorded. Data is presented as means ± SEM. Chow N = 10, HFD N = 15, HFD/Chow N = 10, HFD/Chow+DHA N = 9, HFD/HFD+DHA N = 10.