Abstract
Background
Given that the dysregulation of iron homeostasis leads to genomic instability, iron has been linked to cellular aging. However, epidemiological research on dietary iron intake and cellular aging markers is scarce. The aim of this study was to explore the relationship between dietary iron intake and cellular aging markers and to investigate whether tumor necrosis factor-α (TNFα) mediated this relationship.
Methods
We conducted a cross-sectional analysis with a total of 467 subjects. Detailed dietary data were obtained using 24 h food recalls. Peripheral blood leukocyte telomere length (LTL) and mitochondrial DNA copy number (mtDNAcn) were assessed using real-time PCR assay. The association between dietary iron intake and cellular aging markers and TNFα and superoxide dismutase (SOD) was analyzed by Pearson correlation analysis and regression models adjusted by covariates. Simple mediation models were generated to examine whether TNFα mediated the association between iron intake and cellular aging markers using PROCESS macro Version 3.3.
Results
The study population contained more women than men, but their basic demographic and metabolic characteristics did not differ. After adjusting for age, LTL was the same for men and women, while mtDNAcn was lower in men. Multiple linear regression adjusted for confounding factors found that iron intake was negatively associated with LTL only in women and negatively associated with mtDNAcn only in men. Moreover, iron intake was positively associated with TNFα in both women and men but positively associated with SOD only in men. Path modeling showed that TNFα significantly mediated the indirect detrimental effect of iron intake on LTL only in women; in men, mediation of the indirect effect of iron intake on mtDNAcn by TNFα did not reach significance.
Conclusions
The study found sex-specific negative associations between dietary iron intake and cellular aging markers in that iron intake had deleterious effects on LTL attrition in women and mtDNAcn in men; only the former was partly mediated by TNFα. Consequently, when dietary iron intake and iron supplementation is recommended, the effects of iron imbalance on genomic stability and cellular aging markers must be considered.
1. Background
Telomeres are the cap structures at the ends of chromosomes in eukaryotic cells, rich in the noncoding repeat TTAGGG [1]. Telomeres shorten with each cell division and, to a certain extent, initiate cell senescence and apoptosis. As human cell telomeres shorten with age, peripheral blood leukocyte telomere length (LTL) has come to be considered a reliable marker of cellular aging and age-related diseases [2]. In addition to LTL, peripheral blood mitochondrial DNA copy numbers (mtDNAcn) also decrease with age and are found to be negatively correlated with the health status of elderly people, making mtDNAcn another reliable marker of aging and age-related disease [3].
Along with aging, inflammation and oxidative stress are the most studied factors to accelerate telomere shortening as well as mtDNA mutations and deletions [4, 5]. Furthermore, certain studies have found that diet is also closely related to cellular aging, as LTL has been negatively associated with the proinflammatory capacity of diet (as represented by the Dietary Inflammatory Index™ (DII®)), while positively correlated with the antioxidant capacity of the diet [6–8]. Our previous study also found that various dietary ingredients can have different effects on telomere length [9].
Iron is an essential element supplied mainly by dietary intake. It participates in a variety of physiological processes and must be maintained in a relatively narrow range to sustain metabolic homeostasis and genomic stability [10]. However, iron overload causes iron deposition in tissues and organs that plays a vital role in aging. Iron-fed rats exhibit increased iron levels in tissues and serum, increased serum transferrin saturation, and increased serum levels of TNFα and SOD [11]. The pathophysiological mechanism of the harmful effects of iron overload involves inflammation and oxidative stress caused by labile Fe, resulting in impaired stability and integrity of the genome [10]. The recommended nutrient intake (RNI) for dietary iron in Chinese residents is 9–20 mg/d for adults depending on age and sex. However, the current RNI for iron is mainly prescribed to prevent anemia; it does not take into account the genomic instability involved in the development of cellular aging and age-related diseases.
To date, there are few epidemiological studies on dietary iron intake and cellular aging markers, and the conclusions of those that do exist are inconsistent. An epidemiological survey involving 586 subjects 35–74 years of age found that subjects administered iron supplements had shorter telomeres than those without iron supplementation: 5121 ± 183 bps compared to 5583 ± 87 bps (29.0% difference; p = 0.007) [12]. Another cross-sectional study of 10 568 adults from the United States found that iron intake in the diet was positively correlated with telomere length in the peripheral blood [13]. Moreover, studies on the association between dietary iron and peripheral blood mitochondrial DNA copy number do not exist. Therefore, further population studies on dietary iron intake and genomic stability are necessary to understand the association and determine the most appropriate recommended intake range.
The aim of this population-based study was to explore the relationship between dietary iron intake and cellular aging markers, as well as to establish mediation models to explore whether TNFα mediated the relationship between them. Moreover, given that men and women differ in physical fitness and dietary habits, the study emphasized the sex differences involved in the association.
2. Methods
2.1. Study Population
The data used for this analysis were derived from a type 2 diabetes program in a Beijing suburb from 2014 to 2015 [9]. All subjects voluntarily submitted written informed consent, including a questionnaire about demographics, lifestyle, and disease and medication history. They also underwent detailed physical exams and a 2-hour oral glucose tolerance test (OGTT) after an overnight fast (>10 h).
The 1999 World Health Organization (WHO) criteria were used to define the glucose tolerance status. Subjects that did not have cellular aging marker, TNFα, or SOD data were not included in the study, leaving a total of 467 individuals that were selected for the experiment (normal glucose tolerance (NGT; n = 187, including 129 women and 58 men), prediabetes (n = 146, including 91 women and 55 men), and diabetes mellitus (DM; n = 134, including 86 women and 48men)) (Figure 1). The study protocol was approved by the Ethics Committee of Peking Union Medical College Hospital.
Figure 1.
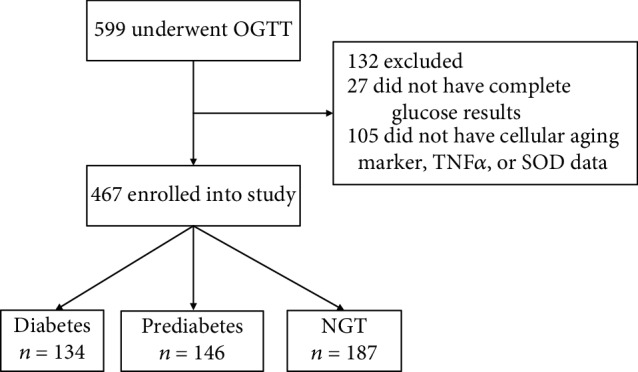
The flow diagram of the population-based study.
2.2. Anthropometric Measurements
While subjects remained in a resting position for 15 minutes, a mercury sphygmomanometer was used to measure upper limb blood pressure; the values obtained from two measurements were averaged. Height and body weight were measured with subjects in light clothes and without shoes, and body mass index (BMI) was derived by dividing the body weight in kilograms by the square of the height in meters.
2.3. Biochemical Measurements
Hemoglobin (HbA1c) analysis was performed by high-performance liquid chromatography (HPLC; intra-assay coefficient of variation (CV) < 3%, interassay CV < 10%). An automated analyzer was used to determine low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and cholesterol (TC) levels. TNFα and SOD were analyzed with kits in compliance with the manufacturer's protocols (Cloud-Clone Corp., Houston, TX, USA).
2.4. Measurement of LTL and mtDNAcn
Previous reports described peripheral blood LTL and mtDNAcn analysis in detail [9, 14]. In short, LTL was determined as the relative ratio of telomere-repeat copy number to single copy number (T/S) using the novel monochrome multiplex quantitative PCR protocol described by Cawthon [15]. The within-plate and between-plate CVs were 18% and 7%, respectively. The relative mtDNAcn was measured by real-time PCR and corrected by the simultaneous measurement of nuclear DNA. The average intra-assay and interassay CVs were 4.2% (range, 1.6%–9.8%) and 4.6% (range, 0.9%–7.8%), respectively.
2.5. Dietary Intake Assessment
Dietary information was collected using 24 h food recalls. The dietary data was reviewed by dietitians and entered into the nutrition calculation software (developed by researchers based on the Microsoft Office Access 2007 database). The food ingredient data was calculated using the China Food Composition Table (2004) database as a guide.
2.6. Statistical Analysis
Continuous data conforming to normal distributions were expressed as mean ± standard deviations (SDs) or standard errors (SEs); data not conforming to normal distributions were transformed. Categorical variables were expressed as percentages or ratios. A general linear model or chi-squared test was used to compare basic characteristics between males and females. Iron intake mg per 1000 kcal energy was also calculated.
Correlations between iron intake and cellular aging markers, TNFα, and SOD were explored using Spearman correlation analysis and multiple linear regression. In correlation analysis, iron intake was expressed as mg per 1000 kcal calories. In multiple linear regression, iron intake was also expressed as mg per 1000 kcal calories, and the B coefficients and 95% confidence intervals (CIs) as per 1SD increment of iron intake were calculated. Model 1 was age-adjusted, while model 2 was adjusted by age as well as hypertension status, BMI, and HbA1c, LnTG, HDL-C, and carbohydrate proportions.
To investigate whether TNFα was involved in the relationship between iron intake and cellular aging markers and whether mtDNAcn mediated the relationship between iron intake and SOD, PROCESS macro Version 3.3 [16] was used to generate simple mediation models using ordinary least squares. Mediation hypotheses were tested via a bias-corrected bootstrap method with 5000 samples to calculate confidence intervals (95%). Significance was achieved when an indirect effect was observed and zero was not included in confidence intervals. Iron intake was expressed as mg per 1000 kcal calories, and models were adjusted by age as well as hypertension status, BMI, and HbA1c, LnTG, and HDL-C. SPSS Version 25.0 (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses. All p values are two-tailed, with statistical significance determined when p < 0.05.
3. Results
3.1. Basic Characteristics of the Study Population
Table 1 shows the basic characteristics of the study population. More than half of the study population were women, with no difference in age between the two groups. The serum TNFα level was higher in women (borderline significant) and the serum SOD level was not different between the two groups. The peripheral blood mtDNAcn was significantly lower in men, but the LTL was not different between women and men. Men had greater dietary iron intake than women; conversely, this became slightly lower than women when adjusted for total daily energy intake or when iron intake was expressed as mg per 1000 kilocalories, although there was no statistical difference. There were no significant differences in BMI, blood glucose, blood lipids, or blood pressure between the two groups (Table 1).
Table 1.
Basic characteristics of the study population.
| Parameters | Women | Men | p value |
|---|---|---|---|
| N (%) | 306 (65.5%) | 161 (34.5%) | — |
| Age (years) | 52.2 ± 0.7 | 53.9 ± 0.9 | 0.113 |
| Body mass index (kg/m2) | 26.5 ± 0.3 | 25.9 ± 0.4 | 0.169 |
| LTLa | 28.65 ± 0.05 | 28.74 ± 0.07 | 0.276 |
| Log2 (mtDNAcn)b | 6.65 ± 0.04 | 6.47 ± 0.05 | 0.01 |
| Hypertension (n, %) | 151 (49.7%) | 82 (51.3%) | 0.746 |
| HbA1c (%) | 5.90 ± 0.06 | 5.89 ± 0.09 | 0.942 |
| LnTG (mmol/L) | 0.37 ± 0.04 | 0.45 ± 0.05 | 0.205 |
| HDL-C (mmol/L) | 1.33 ± 0.02 | 1.27 ± 0.03 | 0.101 |
| TNFα (pmol/mL) | 24.46 ± 0.60 | 22.71 ± 0.82 | 0.084 |
| SOD (U/mL) | 2.49 ± 0.07 | 2.47 ± 0.09 | 0.845 |
| Energy intake (kcal/d) | 1414.04 ± 39.99 | 1885.51 ± 54.41 | <0.001 |
| Carbohydrate proportions | 0.66 ± 0.01 | 0.65 ± 0.01 | 0.633 |
| Iron intake (mg/d)c | 14.75 ± 0.83 | 19.76 ± 1.13 | <0.001 |
| Iron intake (mg/d)d | 16.96 ± 0.65 | 15.68 ± 0.90 | 0.258 |
| Iron intake (mg/1000 kcal)e | 10.46 ± 0.31 | 9.97 ± 0.43 | 0.350 |
LTL: leukocyte telomere length; mtDNAcn: mitochondrial DNA copy number; HbA1c: hemoglobin A1c; TG: triglyceride; HDL-C: high-density cholesterol lipoprotein; TNFα: tumor necrosis factor-α; SOD: superoxide dismutase. aLTL was adjusted by age; bmtDNAcn was adjusted by age; ciron intake was not adjusted; diron intake was adjusted by energy intake; eiron intake was calculated as per 1000 kcal energy intake. All variables are presented as means ± SEs.
3.2. Correlations between Iron Intake with Cellular Aging Markers, TNFα, and SOD
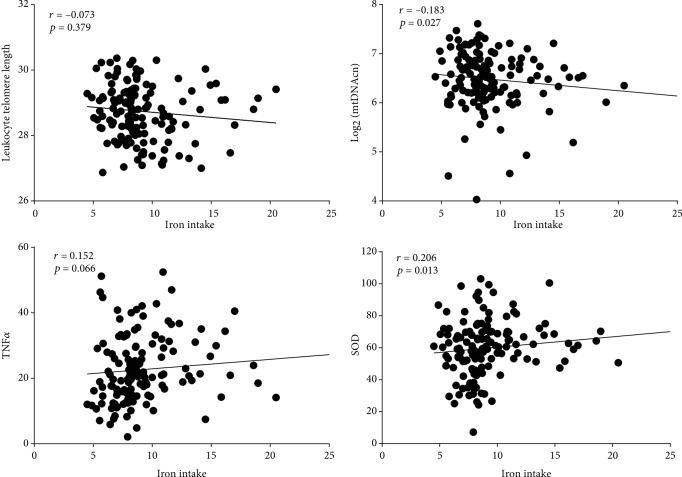
As is shown in Table 2, iron intake was negatively correlated with LTL (r = −0.25, p < 0.001), positively correlated with the serum TNFα level (r = 0.176, p = 0.004), but not correlated with mtDNAcn and serum SOD levels in women (Table 2, Figure 2). However, in men, iron intake was significantly negatively correlated with mtDNA rather than LTL and positively correlated with serum TNFα and SOD levels, with only the latter achieving statistical differences (Table 2, Figure 3).
Table 2.
Correlation analysis between iron intake and cellular aging markers, TNFα, and SOD.
| Parameters | Women | Men | ||
|---|---|---|---|---|
| r | p value | r | p value | |
| LTL | −0.25 | <0.001 | −0.073 | 0.379 |
| Log2 (mtDNAcn) | 0.046 | 0.45 | −0.183 | 0.027 |
| TNFα | 0.176 | 0.004 | 0.152 | 0.066 |
| SOD | 0.023 | 0.700 | 0.206 | 0.013 |
Figure 2.
Correlation dot plots of iron intake and cellular aging markers, TNFα, and SOD in women. The abscissas are iron intake and the ordinates are LTL, mtDNAcn, TNFα, or SOD.
Figure 3.
Correlation dot plots of iron intake and cellular aging markers, TNFα, and SOD in men. The abscissas are iron intake and the ordinates are LTL, mtDNAcn, TNFα, or SOD.
3.3. Regression Analysis of Iron Intake and Cellular Aging Markers, TNFα, and SOD
Table 3 shows the coefficients of multiple linear regression analysis of iron intake and cellular aging markers, TNFα, and SOD. After adjusting for age, hypertension status, BMI, and HbA1c, LnTG, HDL-C, and carbohydrate proportion, iron intake was negatively associated with LTL only in women (B = −0.196, 95% CI: −0.296 to −0.096), while negatively associated with mtDNAcn only in men (B = −0.114, 95% CI: −0.223 to −0.005).
Table 3.
B coefficients and 95% CIs of multiple linear regression analysis of iron intake and cellular aging markers, TNFα, and SOD.
| Parameters | Women | Men | ||||
|---|---|---|---|---|---|---|
| B | 95% CI | p | B | 95% CI | p | |
| LTL | ||||||
| Model 1 | −0.213 | (−0.308, −0.117) | <0.001 | −0.068 | (−0.213, 0.077) | 0.357 |
| Model 2 | −0.196 | (−0.296, -0.096) | <0.001 | −0.102 | (−0.251, 0.047) | 0.177 |
| Log2 (mtDNAcn) | ||||||
| Model 1 | 0.024 | (−0.060, 0.109) | 0.571 | −0.123 | (-0.231, −0.015) | 0.026 |
| Model 2 | 0.054 | (−0.032, 1.141) | 0.217 | −0.114 | (−0.223, −0.005) | 0.04 |
| TNFα | ||||||
| Model 1 | 1.944 | (0.694, 3.193) | 0.002 | 1.856 | (0.137, 3.575) | 0.035 |
| Model 2 | 1.535 | (0.264, 2.807) | 0.018 | 1.947 | (0.151, 3.742) | 0.034 |
| SOD | ||||||
| Model 1 | 0.542 | (−1.991, 3.067) | 0.674 | 4.108 | (1.266, 6.950) | 0.005 |
| Model 2 | 0.995 | (−1.469, 3.459) | 0.427 | 4.238 | (1.033, 7.444) | 0.01 |
Model 1 only adjusted for age; model 2 adjusted for age, hypertension status, BMI, and HbA1c, LnTG, HDL-C, and carbohydrate proportion.
As for the inflammation marker, serum TNFα concentration was significantly positively associated with iron intake in both women and men (B = 1.535, 95% CI: 0.264 to 2.807, and B = 1.947, 95% CI: 0.151 to 3.742). In regard to the oxidative stress marker, there was a significantly positive association between iron intake and SOD only in men (B = 4.238, 95% CI: 1.033 to 7.444).
3.4. Mediation Models to Determine the Associations between Iron Intake and Cellular Aging Markers, TNFα, and SOD
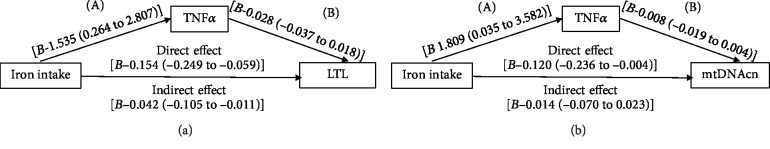
In women, iron intake had direct negative effects on LTL (B −0.154, 95% CI −0.249 to −0.059); TNFα significantly mediated the indirect effect of iron intake on LTL (B −0.042, 95% CI −0.105 to −0.011) (Figure 4(a)). In men, iron intake also had direct negative effects on mtDNAcn (B −0.120, 95% CI −0.236 to −0.004), but the mediation of these indirect effects by TNFα did not reach statistical significance (Figure 4(b)).
Figure 4.
Path models of associations between iron intake, TNFα, and cellular aging markers. (a) The p value for regression (A) was 0.0182, for regression (B) was <0.001, and for direct effect was 0.0016. (b) The p value for regression (A) was 0.0457, for regression (B) was 0.1848, and for direct effect was 0.0429. As for the indirect effect, significance was achieved when zero was not included in confidence intervals and did not have a p value. Models were adjusted for age, hypertension status, BMI, and HbA1c, LnTG, HDL-C, and carbohydrate proportion.
Given that mitochondrial dysfunction may lead to an imbalance in oxidative stress, we further explored whether a decrease in mtDNAcn was associated with an increase in serum SOD concentration. As Figure 5 depicts, we did not find significant indirect effects of iron intake on SOD mediated by a decrease in mtDNAcn.
Figure 5.

Path model of association between iron intake, mtDNAcn, and SOD. In Figure 4, the p value for regression (A) was 0.0153, for regression (B) was 0.4453, and for direct effect was 0.0157. As for the indirect effect, significance was achieved when zero was not included in confidence intervals and did not have a p value. Models was adjusted for age, hypertension status, BMI, and HbA1c, LnTG, HDL-C, and carbohydrate proportion.
4. Discussion
The main findings of the study were that dietary iron intake was negatively associated with peripheral blood cellular aging markers, with sex differences. In women, iron intake was only found to be significantly negatively associated with LTL, whereas in men, iron intake was only found to be negatively associated with mtDNAcn. Furthermore, TNFα partially mediates the detrimental effect of iron intake on telomere shortening only in women.
Although evidence regarding the association between dietary iron and LTL is rare, our results are consistent with a previous study by Xu and colleagues showing that women administered iron supplements had shorter LTL than women without iron supplementation [12]. Moreover, both experiments and epidemiological studies have determined that iron overload is associated with telomere homeostasis [17–20]. A cross-sectional study of 1174 subjects 50–79 years of age found that serum transferrin saturation was inversely correlated with telomere length in the peripheral blood; furthermore, not only subjects with iron overload but also those with normal-high serum transferrin saturation values had shorter peripheral telomeres than subjects with normal-low serum values [18]. The study also found sex differences involved in these relationships in that normally high values of transferrin saturation are associated with shorter telomeres only in women, at least with any statistical significance [18]. Another cross-sectional study of 7336 adults over the age of 20 in the United States found that high ferritin levels were negatively correlated with telomere length only in women with statistical significance (p = 0.003), although there was no statistical difference in sex interaction (p for interaction = 0.12) [17].
To explore the mechanism underlying the detrimental effect of iron intake on telomere attrition, a path model was generated that found that the serum TNFα level partly mediated the association. On the one hand, a cross-sectional study of 1962 elderly people 70-79 years of age found that elevated serum TNFα was positively associated with increased risk of telomere shortening [21] and another cross-sectional study of 840 patients with cardiovascular disease found that polymorphisms in certain TNF loci were associated with elevated hypersensitive C-reactive protein and shortened telomere length in the peripheral blood [22]. On the other hand, basic experiments have determined that high iron ingestion increases the expression of TNFα in rat liver [23], and iron overload induces macrophage polarization imbalance, leading to increased expression of M1 macrophage-associated factors such as TNFα [24]. Therefore, our results are consistent with those reported in the literature and are convincing in terms of the mechanism of action we determined.
In regard to mtDNAcn and iron intake, their reverse association only reached statistical significance in men. Previous studies have also reported the sex difference in that the mitochondrial DNA copy number was found to decrease with age only in men [25]. While no direct evidence regarding the association between iron intake and mtDNAcn exists in the literature, iron has long been closely related to mitochondria [26]. Mitochondria are the centers of iron utilization and accumulation. Both iron deficiency and overload may affect the structure and function of mitochondria. Clinical evidence has manifested that mtDNA copy number is negatively correlated with transferrin in the cerebrospinal fluid of HIV-infected adult patients [27]. As mentioned earlier, increased iron intake was associated with elevated TNFα, which is a proinflammatory pathway leading to cellular inflammation and apoptosis [28] that has been associated with mitochondrial DNA damage. Basic research has established that TNFα acts directly on mitochondria through the TNFα receptor and that TNFα signaling causes PGC1a hypermethylation, resulting in decreased expression of PGC1a, decreased mitochondrial biogenesis, and decreased mitochondrial content [29]. Furthermore, patients with fibromyalgia and severe diabetic retinopathy have reduced mitochondrial DNA and elevated TNFα in their peripheral blood compared to control groups [30, 31]. However, we did not find a significant mediating effect of TNFα on the inverse association between iron intake and mtDNAcn, which is probably attributed to the relatively small sample size and insufficient test performance in this study.
Ultimately, we also found an inverse association between iron intake and SOD in men. Iron overload has been associated with increased oxidative stress [32]. Our results were consistent with experimental research that shows iron-fed rats have increased serum levels of SOD [11]. In order to further explore whether mitochondria participated in the association between iron intake and SOD, the mediation model was generated; it found no significant indirect effect of decreased mtDNAcn. Studies with larger sample sizes as well as mechanistic studies are necessary to further clarify these relationships.
4.1. Strengths and Limitations
This research presented some advantages and disadvantages. The first advantage was that the study consisted of subjects whose glucose metabolism status was available and continuous (from NGT to diabetes), making conclusions representative; the second advantage was that the detailed dietary data made it possible for the study to make up for the gaps in the association between iron intake and cellular aging markers. However, the disadvantage was that the study's sample size was relatively small and that the cross-sectional study did not yield a causal relationship. Moreover, although a single 24 h food recall was not that representative and had some limitations, our dietary composition investigation questionnaires were designed and assessed by clinical dieticians and the investigators were trained, so the validity and reliability of the dietary data can be guaranteed.
5. Conclusions
In conclusion, the study found a sex-specific negative association between dietary iron intake and cellular aging markers in that iron intake had deleterious effects on LTL attrition in women while mtDNAcn decreased in men; furthermore, the former was partly mediated by TNFα. When dietary iron intake and iron supplementation is recommended, the relationship between iron imbalance with genomic stability and cellular aging markers must be fully considered.
Acknowledgments
The authors would like to thank all of the participants in this study. We would like to thank Editage (http://www.editage.cn) for the English language editing. This project was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (CIFMS2016-I2M-4-001).
Abbreviations
- LTL:
Leukocyte telomere length
- OGTT:
Oral glucose tolerance test
- PCR:
Polymerase chain reaction
- BMI:
Body mass index
- BP:
Blood pressure
- Wt:
Weight
- Ht:
Height
- HbA1c:
Hemoglobin
- TG:
Triglyceride
- HDL-C:
High-density lipoprotein cholesterol
- SD:
Standard deviation
- TNFα:
Tumor necrosis factor-α
- SOD:
Superoxide dismutase
- NGT:
Normal glucose tolerance
- DM:
Diabetes mellitus
- CV:
Coefficient of variation
- ELISA:
Enzyme linked immunosorbent assay
- CI:
Confidence interval.
Contributor Information
Lingling Xu, Email: llxuwsh@163.com.
Yuxiu Li, Email: liyuxiu@medmail.com.cn.
Data Availability
All the data generated or analyzed during this study are included in the article.
Ethical Approval
This study was approved by the Medical Ethics Committee of PUMCH.
Consent
All patients signed a written informed consent form allowing use of their clinical data and blood samples for research. All patients had signed a written informed consent form to publish their clinical research data. Furthermore, the patients' records and information were anonymized before analysis.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
JY was responsible for data acquisition, analysis and interpretation of the data, and drafting of the manuscript; FP, HBZ, WL, TY, YF, KF, and WBX were responsible for data acquisition and analysis and interpretation of the data. LLX and YXL were responsible for the study concept and design, critical revision of the manuscript for important intellectual content, and study supervision. All authors have read and approved the manuscript for publication.
References
- 1.Riethman H. Human telomere structure and biology. Annual Review of Genomics and Human Genetics. 2008;9(1):1–19. doi: 10.1146/annurev.genom.8.021506.172017. [DOI] [PubMed] [Google Scholar]
- 2.Freitas-Simoes T., Ros E., Sala-Vila A. Telomere length as a biomarker of accelerated aging: is it influenced by dietary intake? Current Opinion in Clinical Nutrition and Metabolic Care. 2018;21(6):430–436. doi: 10.1097/mco.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 3.Mengel-From J., Thinggaard M., Dalgård C., Kyvik K. O., Christensen K., Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Human Genetics. 2014;133(9):1149–1159. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichert S., Stier A. Does oxidative stress shorten telomeres in vivo? A review. Biology Letters. 2017;13(12, article 20170463) doi: 10.1098/rsbl.2017.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong Y., Trabucco S. E., Zhang H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Aging. 2014;39:p. 86. doi: 10.1159/000358901. [DOI] [PubMed] [Google Scholar]
- 6.Shivappa N., Wirth M. D., Hurley T. G., Hébert J. R. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999-2002. Molecular Nutrition & Food Research. 2017;61(4, article 1600630) doi: 10.1002/mnfr.201600630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Calzón S., Zalba G., Ruiz-Canela M., et al. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the PREDIMED-NAVARRA study: cross-sectional and longitudinal analyses over 5 y. The American Journal of Clinical Nutrition. 2015;102(4):897–904. doi: 10.3945/ajcn.115.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Calzón S., Moleres A., Martínez-González M. A., et al. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clinical Nutrition. 2015;34(4):694–699. doi: 10.1016/j.clnu.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Zhou M., Zhu L., Cui X., et al. Influence of diet on leukocyte telomere length, markers of inflammation and oxidative stress in individuals with varied glucose tolerance: a Chinese population study. Nutrition Journal. 2016;15(1):1–10. doi: 10.1186/s12937-016-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prá D., Franke S. I. R., Henriques J. A. P., Fenech M. Iron and genome stability: an update. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2012;733(1-2):92–99. doi: 10.1016/j.mrfmmm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Da Cunha M., Arruda S. Tucum-do-Cerrado (Bactris setosa Mart.) may promote anti-aging effect by upregulating SIRT1-Nrf2 pathway and attenuating oxidative stress and inflammation. Nutrients. 2017;9(11, article 1243) doi: 10.3390/nu9111243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q., Parks C. G., DeRoo L. A., Cawthon R. M., Sandler D. P., Chen H. Multivitamin use and telomere length in women. The American Journal of Clinical Nutrition. 2009;89(6):1857–1863. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazidi M., Kengne A., Banach M. Mineral and vitamin consumption and telomere length among adults in the United States. Polish Archives of Internal Medicine. 2017;127(2):87–90. doi: 10.20452/pamw.3927. [DOI] [PubMed] [Google Scholar]
- 14.Zhou M., Zhu L., Cui X., et al. Reduced peripheral blood mtDNA content is associated with impaired glucose-stimulated islet β cell function in a Chinese population with different degrees of glucose tolerance. Diabetes/Metabolism Research and Reviews. 2016;32(7):768–774. doi: 10.1002/dmrr.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawthon R. M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Research. 2009;37(3, article e21) doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes A. F. PROCESS: a versatile computational tool for observed variable mediation, moderation, and conditional process modeling [white paper] http://www.afhayes.com/public/process2012.pdf.
- 17.Liu B., Sun Y., Xu G., et al. Association between body iron status and leukocyte telomere length, a biomarker of biological aging, in a nationally representative sample of US adults. Journal of the Academy of Nutrition and Dietetics. 2019;119(4):617–625. doi: 10.1016/j.jand.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin C., Baik I. Transferrin saturation concentrations associated with telomeric ageing: a population-based study. British Journal of Nutrition. 2017;117(12):1693–1701. doi: 10.1017/S0007114517001696. [DOI] [PubMed] [Google Scholar]
- 19.Mainous A. G., 3rd, Wright R. U., Hulihan M. M., et al. Elevated transferrin saturation, health-related quality of life and telomere length. Biometals. 2014;27(1):135–141. doi: 10.1007/s10534-013-9693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M., Zhao P., Yung W., Sheng Y., Ke Y., Qian Z. Tissue iron is negatively correlated with TERC or TERT mRNA expression: a heterochronic parabiosis study in mice. Aging. 2018;10(12):3834–3850. doi: 10.18632/aging.101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Donovan A., Pantell M. S., Puterman E., et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6(5, article e19687) doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangel-Zúñiga O. A., Corina A., Lucena-Porras B., et al. TNFA gene variants related to the inflammatory status and its association with cellular aging: from the CORDIOPREV study. Experimental Gerontology. 2016;83:56–62. doi: 10.1016/j.exger.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Atarashi M., Izawa T., Miyagi R., et al. Dietary iron supplementation alters hepatic inflammation in a rat model of nonalcoholic steatohepatitis. Nutrients. 2018;10(2):p. 175. doi: 10.3390/nu10020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handa P., Thomas S., Morgan-Stevenson V., et al. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. Journal of Leukocyte Biology. 2019;105(5):1015–1026. doi: 10.1002/JLB.3A0318-108R. [DOI] [PubMed] [Google Scholar]
- 25.Reiling E., Ling C., Uitterlinden A. G., et al. The association of mitochondrial content with prevalent and incident type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2010;95(4):1909–1915. doi: 10.1210/jc.2009-1775. [DOI] [PubMed] [Google Scholar]
- 26.Paul B. T., Manz D. H., Torti F. M., Torti S. V. Mitochondria and iron: current questions. Expert Review of Hematology. 2017;10(1):65–79. doi: 10.1080/17474086.2016.1268047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta S. R., Pérez-Santiago J., Hulgan T., et al. Cerebrospinal fluid cell-free mitochondrial DNA is associated with HIV replication, iron transport, and mild HIV-associated neurocognitive impairment. Journal of Neuroinflammation. 2017;14(1):p. 72. doi: 10.1186/s12974-017-0848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vattemi G., Marini M., Ferreri N. R., et al. Overexpression of TNF-α in mitochondrial diseases caused by mutations in mtDNA: evidence for signaling through its receptors on mitochondria. Free Radical Biology & Medicine. 2013;63:108–114. doi: 10.1016/j.freeradbiomed.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Barrès R., Osler M. E., Yan J., et al. Non-CpG methylation of the PGC-1α promoter through DNMT3B controls mitochondrial density. Cell Metabolism. 2009;10(3):189–198. doi: 10.1016/j.cmet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Cordero M. D., Díaz-Parrado E., Carrión A. M., et al. Is inflammation a mitochondrial dysfunction-dependent event in fibromyalgia? Antioxidants & Redox Signaling. 2013;18(7):800–807. doi: 10.1089/ars.2012.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik A. N., Parsade C. K., Ajaz S., et al. Altered circulating mitochondrial DNA and increased inflammation in patients with diabetic retinopathy. Diabetes Research and Clinical Practice. 2015;110(3):257–265. doi: 10.1016/j.diabres.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang T., Han H., Yang Z. Iron, oxidative stress and gestational diabetes. Nutrients. 2014;6(9):3968–3980. doi: 10.3390/nu6093968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in the article.