Abstract
Colorectal cancer begins as a polyp that is a benign growth on the mucosal surface of the colon or rectum. Over a period of 5 to 15 years, polyps can degenerate into a cancer, thus invading the colonic wall. Colorectal screening methods are designed to diagnose and remove polyps before they acquire invasive potential and develop into cancer. Screening for colorectal cancer can prevent and reduce mortality. Given the benefits and effectiveness of screening, guidelines exist from multiple organizations. These guidelines risk-stratify patients to determine the age of screening initiation and the interval for repeat screening. Categories of colorectal cancer risk include average risk, increased risk, and high risk based on individual and family medical history. Screening methods vary widely in the ability to diagnose and treat polyps and in the degree of invasiveness or risk of complication to the patient. Colonoscopy is held as the “gold standard” by which all other methods are compared; however, less-invasive modalities including computed tomographic colonography are increasing in popularity.
Keywords: colorectal polyps, colonoscopy, colorectal screening, CT colonography
Colon and rectal cancer (CRC) is the nation's third leading cause of cancer mortality and one of the most preventable cancers.1 CRC begins as a premalignant polyp that grows on the mucosal surface of the colon or rectum and transforms into a malignancy. For adenomas greater than 1 cm in size, cumulative risk of diagnosis of cancer at the polyp site at 5, 10, and 20 years was 2.5, 8, and 24%, respectively.2 Colorectal (CR) screening for polyp diagnosis and removal can decrease the incidence of, and reduce mortality from, CRC.3 Despite these numbers, screening rates remain low. In a survey by the U.S. Department of Health and Human Services, only 64.8% of adults older than 40 years reported undergoing appropriate screening,4 with disparities noted based on socioeconomic status, ethnicity, age, and geography.
Given the benefits and effectiveness of screening, guidelines exist from multiple organizations. These guidelines risk-stratify patients based on several factors, including age, family history, and other comorbidities and can provide an approach for initiation of screening and continued surveillance. These guidelines also include recommendations for appropriate use of screening methods, including invasive endoscopic techniques and less-invasive radiographic and biochemical techniques.
Initiation and Intervals of Colorectal Screening
CR screening recommendations are based on an individual's risk of developing and accumulating premalignant polyps. Risk stratification depends on the age when CR polyps begin to develop and the interval at which polyps may grow. All national screening guidelines (American Cancer Society [ACS]; United States Multi-Specialty Task Force [MSTF]; American College of Radiology [ACR]; United States Preventive Services Task Force [USPSTF]) stratify patients into three levels of risk: average risk, increased risk, and high risk.
The majority of CRCs are diagnosed in patients with no hereditary component and are considered sporadic cancers.5 Sporadic CRC usually occurs in average-risk individuals. Average-risk individuals are those with no personal history of CRC or premalignant polyps and no history of CRC or premalignant polyps in any first-degree relatives.1 5 The ACS, MSTF, ACR, and the USPSTF currently recommend initial screening for asymptomatic, average-risk patients starting at the age of 50. This recommendation is based on the incidence of CRC being more than 50 times higher in persons aged 60 to 79 years than in those younger than 40 years, and polyp to cancer progression taking between 5 and 10 years.1 6 Symptomatic patients (i.e., melena, change in bowel habits, and weight loss) should undergo CR evaluation at the time symptoms are reported and should not be based on risk stratification. Average-risk individuals have several CR screening options including colonoscopy every 10 years, flexible sigmoidoscopy every 5 years, double-contrast barium enema every 5 years, computed tomographic (CT) colonography every 5 years, or annual fecal occult blood testing (see Fig. 1). The risks and benefits of each modality will be discussed later. Because increasing age confers increasing risk for the development of premalignant polyps in average-risk individuals, repeat CR screening should be performed.7 The interval of repeat screening is dependent on the initial screening method used and any findings at that time but generally should be repeated every 5 to 10 years.5 If during screening examinations an individual is diagnosed with a premalignant polyp, recommendations for interval surveillance will depend on number and size of polyps that were diagnosed.8
Fig. 1.
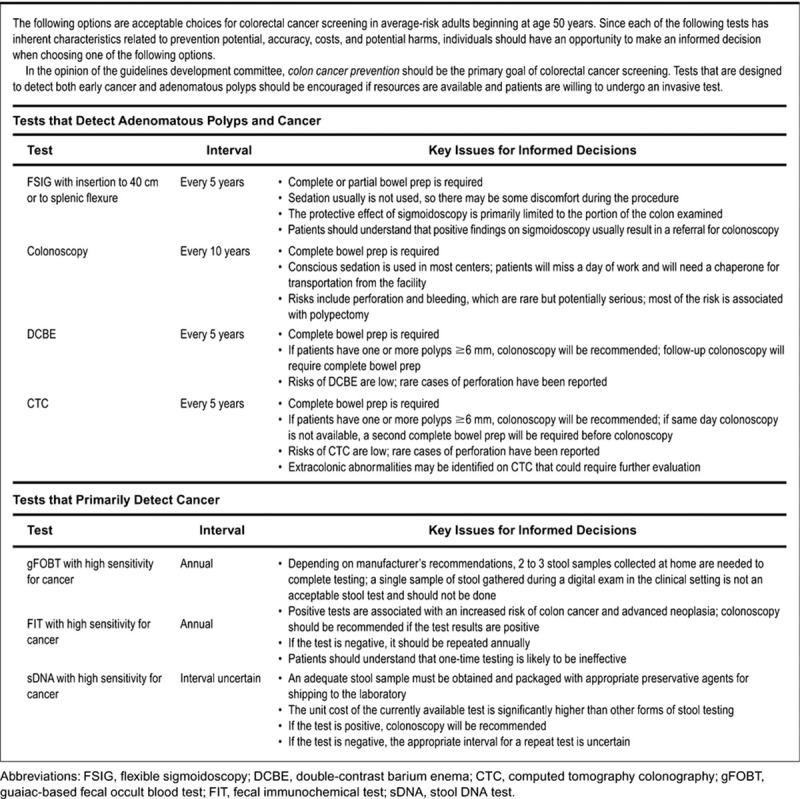
Guidelines for screening for the early detection of colorectal cancer and adenomas for average-risk women and men aged 50 years and older. (Adapted from Levin et al.52)
Individuals with increased risk include patients with a personal history of CRC, those with CRC or adenomatous polyps in a first-degree relative younger than 60 years, or two first-degree relatives of any age.9 10 Individuals with first-degree relatives who have been diagnosed with a CRC or polyps have a twofold increased risk of developing CRC, and three- to fourfold increase if multiple first-degree relatives are diagnosed (thus increasing the concern for a potential hereditary component).11
Patients with increased risk should initiate screening earlier and undergo surveillance at shorter intervals than average-risk individuals (Fig. 2). For patients with personal history, a first-degree relative younger than 60 years, or two or more first-degree relatives of any age with CRC or adenomatous polyps, initial screening should start at the age of 40 years, or 10 years before the youngest case in the immediate family (whichever is earlier), with repeat colonoscopy every 5 years, depending on the findings.12 13 For patients with CRC or adenomatous polyps in a first-degree relative 60 years or older or in two or more second-degree relatives of any age, initial screening should start at the age of 40 years, with repeat screening at intervals recommended for average-risk individuals, depending on the findings.12 13
Fig. 2.
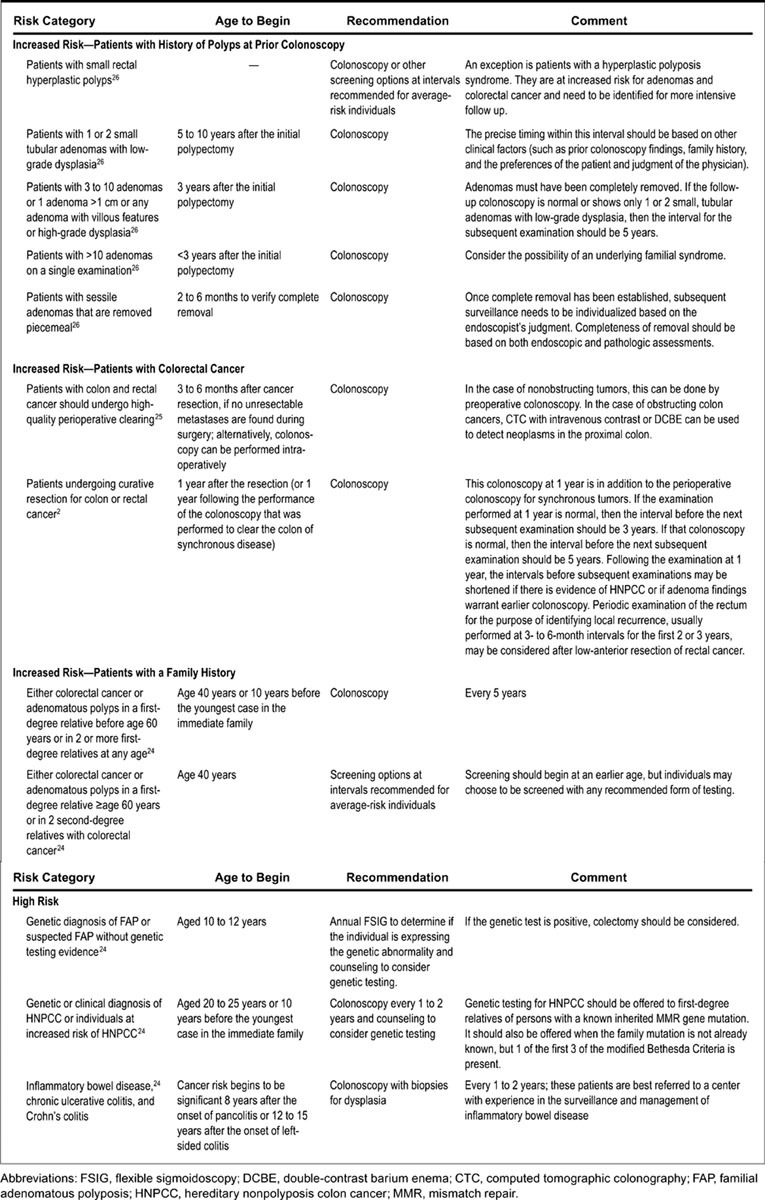
Guidelines for screening and surveillance for the early detection of colorectal adenomas and cancer in individuals at increased risk or at high risk. (Adapted from Levin et al.52)
High-risk individuals include patients with a significant family history of CRC or polyps, those with likely or confirmed hereditary CR cancer syndromes, and those with high-risk medical conditions. Hereditary CR cancer syndromes include familial adenomatous polyposis (FAP), attenuated FAP (aFAP), Lynch syndrome (LS), hereditary nonpolyposis colorectal cancer, and others. Many of these conditions are described in more detail in other sections of this issue. Patients with hereditary syndromes should initiate screening at a younger age and repeat CR screening at shorter intervals. Screening recommendations are based on the suspected or diagnosed hereditary syndrome. For example, FAP is a hereditary polyposis syndrome where germline mutations in the APC gene are inherited in an autosomal-dominant fashion. Patients with FAP will often present in childhood with hundreds to thousands of CR adenomatous polyps. The median age of cancer diagnosis for untreated individuals is 39 years, almost 30 years earlier than the median age for sporadic CR cancer.14 Children of FAP patients should undergo flexible sigmoidoscopy or colonoscopy at 10 to 12 years of age to determine if the child is affected or sooner if symptoms occur. Flexible sigmoidoscopy (FS) should be performed every 1 to 2 years until referral is made for prophylactic surgery in the later teenage years, assuming FAP is diagnosed. If surgery is delayed by more than a year from polyp formation, affected children should undergo colonoscopy for cancer surveillance until surgery is performed.14
aFAP results from a proximal or distal APC mutation or biallelic MUTYH mutations and is characterized by a fewer CR adenomas (10–99) compared with FAP.14 In addition, patients with aFAP may have a higher density of proximal colon polyps and later development of adenomatous polyps and cancers compared with patients with FAP.15 16 Therefore, recommendations are to start CR screening in the late teens to early 20s and should be repeated every 1 to 2 years.14
LS is characterized by a germline mutation in one of four DNA mismatch repair genes14 and is inherited in an autosomal-dominant pattern. The mean age for diagnosis of CRC in patients with LS is 45 years.17 Although LS patients have lifetime polyp burden similar to average-risk patients, the polyp to cancer progression occurs more frequently and at a faster rate.17 18 Therefore, it is recommended that LS patients start CR screening at 20 to 25 years of age, or 10 years before the youngest CRC affected first-degree relative, whichever is earliest. The recommended interval of CR screening is every 1 to 2 years due to the faster progression of polyp to cancer development.5 14 Close surveillance with colonoscopy has been shown to decrease the CRC rate by 62% and decrease mortality by 65% for patients with LS.3 Patients who are unwilling to undergo interval surveillance or whose polyp burden cannot be managed endoscopically should be referred for surgical evaluation.14
Other less common high-risk hereditary syndromes, as discussed later in this issue, exist which mandate earlier initiation and shorter interval CRC screening. Peutz-Jeghers syndrome (PJS) is an autosomal-dominant inherited syndrome characterized by mucocutaneous pigmentation and hamartomatous polyps of the gastrointestinal (GI) tract.14 19 The incidence of this condition is estimated to be between 1:50,000 and 1:200,000 live births.20 Patients with PJS have higher risk of malignancies in both the GI tract, including CRC, and extraintestinal malignancies.21 22 23 The estimated lifetime risk of PJS patients developing CRC is 39%24 25 with a risk of CRC of 3, 5, 15, and 39% at ages 40, 50, 60, and 70 years, respectively.23 Given these high rates of CRC at relatively young ages, it is recommended to start CR screening at age 18 and be repeated in 2- to 5-year intervals.20 26 27 Juvenile polyposis syndrome is an autosomal-dominant inherited condition characterized by the development of juvenile polyps throughout the GI tract, with the vast majority being found in the CR.14 27 28 Juvenile polyps contain areas of adenomatous change and thereby increase patient's risk of CRC. The estimated risk of CRC is 17 to 22% by 35 years of age and approaches 68% by 60 years of age.21 29 Given the increased risk of CRC, the American College of Gastroenterology recommends colonoscopy beginning at the age of 12 years or earlier if symptoms occur, especially rectal bleeding. It should be repeated every 1 to 3 years depending on polyp burden and polyps ≥5 mm should be removed.14 28 Cowden syndrome includes a wide variant of clinical phenotypes, all associated with high prevalence of colonic polyps. Cowden syndrome is caused by mutations in the PTEN gene which increase development of GI hamartomas and gangleoneuromas.14 Individuals are often noted to have numerous hamartomatous polyps throughout the GI tract. Previous evidence has suggested no increased risk of CRC with PTEN mutations.21 However, current data have shown that the lifetime risk of CRC cancer is increased to as high as 9 to 16%.30 31 32 There are no evidence-based screening and surveillance guidelines for patients with PTEN mutations, but expert opinion recommends initiation of CRC screening at the age of 15 years and repeat surveillance every 2 years.14 21 In all, CR screening in high-risk patients depends on the suspected or established diagnosis which confers high lifetime risk for CRC.
Inflammatory Bowel Disease
Patients with inflammatory bowel disease (IBD, including ulcerative colitis [UC] or Crohn disease [CD]) have increased risk of CRC compared with the general population.33 A recent meta-analysis of 116 trials of patients with UC estimated the CRC risk to be 2% at 10 years, 8% at 20 years, and 18% at 30 years after disease onset.33 A large population-based study involving 1,655 Swedish patients with CD found the relative risk of development of CRC to be 2.5 times over the general population, which increased to 5.6 times when CD was confined to the colon.34 In a subsequent meta-analysis of 14 studies, Canavan et al found similar results. In any patient with CD, there was an overall relative risk of 2.5 for developing CRC and the risk increased to 4.5 in CD with isolated colonic disease.35 Given the high lifetime risk of CRC in IBD patients, the USPSTF/ACS/ACR screening guidelines recommend high-risk categorization.36 37 In IBD patients, it is recommended for initial colonoscopy starting 8 years after onset of pan-colitis, or 12 to 15 years after onset of left-sided colitis, with ongoing surveillance every 1 to 2 years.1 In a meta-analysis including 11 trials evaluating the effect of CR screening on survival in patients with UC and colonic CD, Collins et al found no clear evidence that surveillance colonoscopy prolongs survival in IBD patients with extensive colitis; however, patients undergoing surveillance tended to have earlier diagnosis of CRC, which corresponded to a better overall prognosis.38 39
Screening/Surveillance/Diagnostic Methods
Screening and surveillance methods for CRC vary widely in effectiveness for polyp and CRC detection, patient compliance, and invasiveness. The two main measures of effectiveness of screening methods are reduction in CRC incidence and mortality.40 The ideal screening technique for CRC should be feasible, accurate, safe, acceptable, and cost-effective.7 The goal of any screening method is to detect those individuals at risk for developing disease or detecting those with disease as early as possible. As most CRC arises from slow growing, premalignant polyps, screening and surveillance methods can also be evaluated on their ability to detect polyps of different sizes. Colon and rectal polyps can be classified into different size categories: ≤5 mm (small), 6 to 9 mm (medium), and ≥10 mm (large). Polyps ≥10 mm in diameter are generally regarded as being clinically significant and those ≤5 mm in diameter as clinically insignificant. Effective CR screening methods are able to detect medium-to-large polyps.7
Endoscopic Screening Methods
Colonoscopy
Colonoscopy is widely considered the gold standard for CR screening, as it allows for both detection and excision of premalignant lesions from the entire colon and rectum. As such, colonoscopy is the method against which all other screening methods are compared.7 Polyps identified at the time of screening/surveillance colonoscopy should be removed for biopsy, which will be described in detail for both colonic and rectal polyps in other articles of this issue. Colonoscopy has been rapidly adopted as a preferred screening tool for CRC, with 20% of Americans aged 50 years and older undergoing colonoscopy in 2000 as compared with 48% in 2008.41 Although only available from observational studies, colonoscopy has been shown to decrease the incidence and mortality from both proximal and distal CRC.42
Colonoscopy sensitivity and specificity for detection of polyps and early CRC is dependent on both patient preparation and endoscopist training. To measure adenoma miss rates, several studies used tandem back-to-back colonoscopies by different endoscopists to assess effectiveness.43 44 The adenoma miss rate varied between 13 and 17%, although the miss rate for advanced adenomas (≥10 mm) was 5.4 to 6.0%. Characteristics associated with an increasing adenoma miss rate included shorter endoscopic withdrawal time, increased number of adenomas found on first colonoscopy, and right-sided adenoma location. With increasing population adherence to screening guidelines and acceptance of colonoscopy, colonoscopy has emerged as an effective method for diagnosis and removal of premalignant CR polyps and diagnosing CRC.
There have been multiple advancements to increase the sensitivity of colonoscopy for detection and removal of premalignant CR polyps. Technological advancements include increased image resolution with high-definition systems and increased tissue magnification from ×50 magnification with a typical colonoscope to ×300 with zoom capabilities.45 In addition, careful review of colonoscopy technique has resulted in higher adenoma detection rates (ADRs). These techniques include fold inspection, washing of residual material, adequate colonic distention, and sufficient withdrawal time.46 Use of chromoendoscopy, or spraying of dye onto the mucosal surface of the colon in a ubiquitous or targeted fashion, has yielded higher ADRs in certain high-risk patients including UC and LS patients.47 48 49 Overall, ongoing improvements in technology and technique will continue to increase CR polyp detection and removal with colonoscopy.
Colonoscopy is an invasive technique associated with clinically important complications. Serious complications include severe abdominal pain, induction of diverticulitis, perforation, hemorrhage, cardiovascular events, sedation complications (used by most for colonoscopy), and even death.36 Complication rates increase when biopsy or polypectomy are performed with colonoscopy.7 50 In a review of 39,286 Medicare patients who underwent colonoscopy, there were 77 perforations (incidence = 1.96/1,000 procedures). The risk of perforation for those who underwent screening colonoscopy (n = 20,163) was 1.3/1,000.7 50 51 Pooled analysis from other studies suggest a risk of serious complication of 2.8/1,000 procedures with 85% of serious complications occurring with associated polypectomy.5 Colonoscopy remains a procedure with rare but serious complications, and therefore informed consent is imperative.
If the initial method for CR screening for an average-risk patient was a colonoscopy, ongoing screening and surveillance depends on findings at initial colonoscopy and any applicable histopathology. If initial screening finds no lesions, repeat colonoscopy can be repeated at 10 years.1 5 8 Patients who have one to two small tubular adenomas detected at initial screening colonoscopy should undergo repeat colonoscopy 5 to 10 years after initial polypectomy, based on physician judgement and other factors.8 Patients with three to ten adenomas, one adenoma larger than 1 cm, or any adenoma with villous features or high-grade dysplasia should undergo repeat colonoscopy 3 years after initial polypectomy.8 If more than 10 adenomas are detected during initial colonoscopy, repeat colonoscopy should be performed in less than 3 years, timing depending on the judgement of the physician. A more in-depth family and personal history should also be obtained in these patients to screen for potential high-risk cancer syndromes. Those individuals with any evidence of sessile adenomas that are removed piecemeal should undergo repeat colonoscopy within 2 to 6 months to evaluate for complete removal.8
Flexible Sigmoidoscopy
FS may be a better tolerated alternative to complete colonoscopy.1 FS examines the distal half of the colon and rectum after a more limited bowel preparation, such as enemas as opposed to full bowel prep as required for colonoscopy. FS can be performed without sedation and can therefore be performed in the office setting. Despite these advantages, there has been an overall decrease in the use of FS in the United States.52
FS has been shown to decrease the incidence of all CRC and to decrease mortality.53 54 Large randomized controlled trials have been performed comparing FS to “usual” care. A meta-analysis of these trials demonstrates that FS is associated with an 18% relative risk reduction in CRC incidence and 28% reduction in mortality for CRC. Importantly, these studies do not compare FS to colonoscopy or stool-based studies.54
FS has been shown to be an accurate and safe method for colon screening.52 In a pooled analysis of simulated FS, this colon screening method has a sensitivity of 72 to 86% for advanced neoplasia (large polyps and CRC). Variance in sensitivity is secondary to the unexamined proximal colon as well as quality in bowel preparation, depth of scope insertion, and endoscopists' skill. The rate of serious complication from FS with polypectomy is 0.34/1,000 procedures. Serious complication includes severe abdominal pain, induction of diverticulitis, perforation, hemorrhage, cardiovascular event, and death.52
Current recommendations for average-risk patients include FS every 5 years with or without fecal occult blood testing annually.36 52 The 5-year interval can be increased to 10 years in programs with proven accuracy and completeness. If patients are found to have an adenoma on FS, recommendations are for adenoma removal followed by subsequent colonoscopic examination of the remainder of the colon or adenoma removal at the time of a subsequent colonoscopic examination.36 In fact, patients with distal colonic adenomas of any size have twice the risk for a proximal advanced adenoma.55 Other risk factors for proximal colonic lesions may include advancing age, female gender, and ethnicity.36 Additional studies are needed before specific CR screening recommendations can be made for these subpopulations. Overall, in comparison to colonoscopy, FS has decreased cost and risk, although FS does not allow examination of the entire colon, so patients with increased risk for more proximal colonic lesions should be referred for colonoscopy.
Noninvasive Screening Methods
Despite the recommendations from multiple national preventive and specialty organizations, low rates of CR screening by means of colonoscopy remain. Colonoscopy and FS may not always be a feasible option for patients, as endoscopy can be time intensive with patient pre-procedure preparation. Common barriers include limited access to providers, cost, lack of appropriate referral, and fear or lack of understanding of the procedure.56 57 58 Given these barriers, more noninvasive tests have been developed as possible screening alternatives for CR polyps and cancer.
Computed Tomographic Colonography
Computed tomographic colonography (CTC, also called “virtual colonoscopy”) is a less-invasive screening modality in which patients, after a complete colonic preparation similar to that required for colonoscopy, undergo transrectal gaseous distension and residual fecal tagging with oral contrast to visualize the colon and rectal wall with thin, 1- to 2-mm multidetector CT for two-dimensional (2D) and 3D images. There have been no studies which demonstrate a decreased incidence of CRC or mortality from CRC as a result of CTC.52 However, the sensitivity of CTC for detecting polyps and CRC is similar to colonoscopy for advanced adenomas and CRC. In the two largest randomized trials, CTC was compared with same-day colonoscopy in average-risk indivudals.59 60 CTC was comparable to colonoscopy for the diagnosis of large adenomas (≥10 mm) and CRC. Sensitivity of CTC for medium polyps (6–9 mm) was variable between the two studies (88.7%, confidence interval [CI]: 82.9–93.1 and 78%, CI: 71–85). Two more recent meta-analyses also found variable sensitivities for CTC detection of medium-sized colonic polyps.61 62 Overall, CTC has a similar advanced ADR compared with colonoscopy.
There are limitations and risks of CTC based on both individual patient characteristics and the technology. Similar to colonoscopy, a high-quality bowel preparation is necessary to visualize the colonic wall, as retained fecal material can mimic or obscure polyps on final imaging. Other patient characteristics which may limit CTC use include obesity as patients weighing more than 450 to 500 pounds may be too large to fit on and move the CT table. The risk of rectal or colonic perforation is very low. The USPSTF reports that in a pooled analysis of six studies, with a total of 30,815 persons, perforations occurred in seven patients and bacteremia in one patient.36 Patients undergoing CTC are exposed to a small amount of radiation not present in most other screening modalities, the long-term effects of which are not known.63 Additionally, patients undergoing CTC were found to have extracolonic findings detected by CT, with as many as 69% of patients having extracolonic findings.64 The significance of these extracolonic findings varied, with 9.3 to 10% of findings categorized as “moderate to high clinical importance” requiring further diagnostic evaluation.66 Compared with colonoscopy, CTC is a safe tool for CR screening for advanced adenomas with the risk of false-positive exams secondary to poor colonic preparation, inexperience with the technology, and distortion from extracolonic pathology (e.g., metallic hip or spine implants).
Current recommendations are to initiate average-risk CR screening with CTC at 50 years of age. Requirements for patient referral to colonoscopy after CTC remain controversial. All experts agree that patients with polyps ≥10 mm should be referred for colonoscopy. However, if a patient is found to have several (1–2) medium-sized CR polyps (i.e., 6–9 mm), consensus opinion is that referral to colonoscopy should be made until more information about the natural history of medium-sized CR polyps is obtained.52 This referral threshold for colonoscopy of polyps ≥6 mm found on CTC will result in a high of one in three or a low of one in eight persons referred.36 Additional information on polyps <6 mm diagnosed on CTC is needed before an official recommendation can be made. Currently, the ACS, MSTF, and ACR recommend screening CTC every 5 years for average-risk individuals,5 while the USPSTF found the current data insufficient to recommend for or against CTC as a screening tool.5 36 Of note, not all insurance companies will pay for CTC as a screening modality, so it is often only covered in the setting of an incomplete colonoscopy.
Double-Contrast Barium Enema
Double-contrast barium enema (DCBE) offers another less-invasive method for radiographic examination of the colon and rectum. It is similar to colonoscopy and CTC in that the patient requires a quality colon preparation to remove all fecal material. In addition, DCBE, like CTC, involves instillation of gas into the colon for distention along with rectal contrast administration to coat the mucosa of the colon and rectum.52 Finally, DCBE, like CTC, can be performed without sedation and has a very low rate of colonic perforation.65 However, use of DCBE for CR screening is declining, as other screening methods become more available. DCBE is time intensive for the radiologist. In addition, DCBE has a lower advanced ADR compared with both CTC and colonoscopy. Unlike other modalities, studies that evaluated DCBE accuracy were not randomized against other well-accepted forms of CR screening, average-risk populations were not assessed, and DCBE has not been shown to directly decrease the incidence or mortality from CRC.52 For these reasons, CTC has been gaining ground as an alternative to DCBE.66 In one of the largest reviews comparing DCBE with CTC for advanced and medium adenoma detection, CTC was found to be more sensitive for the detection of adenomas ≥6 mm as compared with DCBE.66 In a more recent randomized controlled trial67 comparing DCBE with CTC, there were significantly higher detection rates of CRC and advanced adenomas (≥10 mm) with CTC compared with DCBE, with CTC missing 3 of 45 CR cancers and DCBE missing 12 of 85.
DCBE has been a long accepted CR screening method for average-risk patients. Recommendations include initiation of CR screening with DCBE at the age of 50 years in average-risk individuals with repeat exam every 5 years if no abnormalities noted.1 Referral for colonoscopy is made if there are any abnormalities noted on the exam. Overall, DCBE is a safe but labor-intensive form of CR screening.
Stool-Based Screening Methods
Fecal testing for the presence of blood is a noninvasive screening modality for CRC that can be guaiac based (gFOBT) or immunochemical (iFOBT). Guaiac FOBT causes oxidation of guaiac and detects peroxidase present in human blood. Immunochemical FOBT uses antibodies against human globulin to detect blood in fecal samples.68 Both gFOBT and iFOBT have the potential to detect CRC and advanced adenomas throughout the colon.37 Stool DNA (sDNA) testing has also been gaining popularity and one of these tests was recently approved by the Food and Drug Administration (FDA) for CR screening. These tests will be discussed separately.
gFOBT is recommended for CR screening in average-risk patients starting at the age of 50 years and should be repeated annually.52 Positive tests should be followed with a colonoscopy. Multiple recommendations are in place to ensure the accuracy of gFOBT. First, peroxidase activity in red meat as well as certain fruits and vegetables can cause false-positive results. In addition, high-dose vitamin C may block peroxidase action and cause false-negative results. Analysis recommendations are for patients to avoid red meat, high-dose vitamin C, and anti-inflammatory medications such as aspirin for several days prior to the study.52 However, a meta-analysis of studies with and without dietary restrictions showed no difference in the rate of positive gFOBT.69 Other recommendations for gFOBT include testing from two to three consecutive fecal samples at home, as test sensitivity increases with each additional stool sample.70 Finally, recommendations are to use the gFOBT annually, as this frequency of testing has been shown to decrease the mortality from CRC.71
When the aforementioned recommendations are followed, the accuracy of gFOBT is variable but optimized. In randomized controlled studies which have referred patients for colonoscopy after a positive gFOBT, the sensitivity of gFOBT ranged from 45 to 54%.68 Specificity for gFOBT is also variable and tends to be lower for higher sensitivity gFOBT. Estimates for gFOBT specificity for CRC and advanced adenomas was 86.7 to 98.1%.72 However, compliance with the recommendations to enhance gFOBT accuracy is poor. A survey of primary care physicians with the United States revealed that up to a third of physicians used the gFOBT as an in-office test on a stool sample obtained from digital rectal exam rather than home-based testing from consecutive bowel movements. Further, up to a third of physicians recommended a repeat gFOBT rather than a colonoscopy after a positive test. When additional testing was recommended, FS was ordered just as often as colonoscopy.73 The gFOBT is safe, and greatest risk for complication occurs when the patient undergoes subsequent colonoscopy for a positive test. However, failure to undergo colonoscopy for a positive gFOBT and failure to repeat gFOBT on an annual basis will result in more missed CR polyps and cancers.
iFOBT is recommended for CR screening in average-risk patients starting at the age of 50 years and should be repeated annually.52 Positive tests should be followed with a colonoscopy. No iFOBT has been studied in a randomized controlled fashion to determine the effect on incidence of, or mortality from, CRC.52 Several factors may increase patient compliance with, and thus the accuracy of, iFOBT compared with gFOBT.52 First, testing does not require dietary modification or abstinence from vitamin C intake. Also, iFOBT may be performed on one stool sample, although two samples may improve outcomes.74 Finally, globin is degraded by digestive enzymes in the upper GI tract, so iFOBT is more specific for bleeding from the colon and rectum than gFOBT.68
A meta-analysis of cohort studies using iFOBT for CRC screening has been performed. iFOBT shows 81% sensitivity and 94% specificity for CRC and 28% sensitivity and 91% specificity for advanced adenomas.75 A cohort study comparing the performance of both iFOBT and gFOBT in patients who had follow-up endoscopic colonoscopy found that the sensitivity for CRC and advanced adenomas was higher for iFOBT (69 and 67%, respectively) than for gFOBT (37 and 31%, respectively).68 Overall, iFOBT is a more accurate, more expensive test than gFOBT but may have greater patient and physician compliance with screening recommendations.
Stool testing for known DNA mutations that occur in the polyp to carcinoma sequence for CRC is an emerging screening method. sDNA testing requires submission of an entire stool specimen for collection of stable human DNA that can be separated and isolated from bacterial DNA. sDNA testing currently identifies specific mutations within genes known to mutate within the polyp to carcinoma sequence including APC, KRAS, p53, microsatellite instability markers, and a marker for DNA integrity.52 Based on current evidence, recommendations for sDNA testing are that it is an acceptable noninvasive option for CRC screening in average-risk individuals, but details for screening interval cannot be clarified at this time.36 Limitations of this technique are that sDNA testing is more sensitive and specific for CRC rather than CR polyps. sDNA testing for CRC revealed a sensitivity of 52 to 91% and specificity of 93 to 97%.52 sDNA testing had superior sensitivity for CRC and adenomas with high-grade dysplasia, that is, Hemoccult II, a gFOBT test.76 In addition, positive sDNA testing requires referral for colonoscopy. Yet, sDNA testing may be positive in the setting of a negative colonoscopy secondary to upper GI cancers. More systematic program testing is required for specific sDNA testing recommendations with regard to CR screening.
Conclusion
CR screening programs are an effective means for decreasing the incidence of and mortality from CRC. Screening programs should stratify individuals based on personal and family medical histories. Risk stratification will determine the age of screening initiation and interval of repeat screening or surveillance. The screening test selected should be agreeable to the patient and physician alike with specific understanding about the risks and benefits of the selected screening method. Additional study will allow for inclusion of newer screening techniques that may be more acceptable to the overall population.
References
- 1.Sarfaty M, Wender R. How to increase colorectal cancer screening rates in practice. CA Cancer J Clin. 2007;57(6):354–366. doi: 10.3322/CA.57.6.354. [DOI] [PubMed] [Google Scholar]
- 2.Stryker S J, Wolff B G, Culp C E, Libbe S D, Ilstrup D M, MacCarty R L. Natural history of untreated colonic polyps. Gastroenterology. 1987;93(5):1009–1013. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 3.Winawer S J, Zauber A G, Ho M N. et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Sabatino S A White M C Thompson T D Klabunde C N; Centers for Disease Control and Prevention (CDC). Cancer screening test use - United States, 2013 MMWR Morb Mortal Wkly Rep 20156417464–468. [PMC free article] [PubMed] [Google Scholar]
- 5.Levin B, Lieberman D A, McFarland B. et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Jackson-Thompson J Ahmed F German R R Lai S M Friedman C J. J-T Descriptive epidemiology of colorectal cancer in the United States, 1998-2001 Cancer 2006107(5, Suppl):1103–1111. [DOI] [PubMed] [Google Scholar]
- 7.Health O Assessment T Screening methods for early detection of colorectal cancers and polyps: summary of evidence-based analyses Vol 9. 2009. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3377498&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed]
- 8.Lieberman D A Rex D K Winawer S J Giardiello F M Johnson D A Levin T R; United States Multi-Society Task Force on Colorectal Cancer. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer Gastroenterology 20121433844–857. [DOI] [PubMed] [Google Scholar]
- 9.Lautrup C K, Mikkelsen E M, Lash T L, Katballe N, Sunde L. Familial colorectal cancer risk may be lower than previously thought: a Danish cohort study. Cancer Epidemiol. 2015;39(5):714–719. doi: 10.1016/j.canep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Butterworth A S, Higgins J PT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42(2):216–227. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Johns L E, Houlston R S. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 12.Freedman A N, Slattery M L, Ballard-Barbash R. et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009;27(5):686–693. doi: 10.1200/JCO.2008.17.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park Y, Freedman A N, Gail M H. et al. Validation of a colorectal cancer risk prediction model among white patients age 50 years and older. J Clin Oncol. 2009;27(5):694–698. doi: 10.1200/JCO.2008.17.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syngal S Brand R E Church J M Giardiello F M Hampel H L Burt R W; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes Am J Gastroenterol 20151102223–262., quiz 263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen A L, Bisgaard M L, Bülow S. Attenuated familial adenomatous polyposis (AFAP). A review of the literature. Fam Cancer. 2003;2(1):43–55. doi: 10.1023/a:1023286520725. [DOI] [PubMed] [Google Scholar]
- 16.Burt R W, Leppert M F, Slattery M L. et al. Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology. 2004;127(2):444–451. doi: 10.1053/j.gastro.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Jasperson K W, Tuohy T M, Neklason D W, Burt R W. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jass J R Smyrk T C Stewart S M Lane M R Lanspa S J Lynch H T Pathology of hereditary non-polyposis colorectal cancer Anticancer Res1994–163414(4B)1631–1634. [PubMed] [Google Scholar]
- 19.Beggs A D, Latchford A R, Vasen H F. et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59(7):975. doi: 10.1136/gut.2009.198499. [DOI] [PubMed] [Google Scholar]
- 20.Giardiello F M, Trimbath J D. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol. 2006;4(4):408. doi: 10.1016/j.cgh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Schreibman I R, Baker M, Amos C, McGarrity T J. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005;100(2):476. doi: 10.1111/j.1572-0241.2005.40237.x. [DOI] [PubMed] [Google Scholar]
- 22.Gammon A, Jasperson K, Kohlmann W, Burt R W. Hamartomatous polyposis syndromes. Best Pract Res Clin Gastroenterol. 2009;23(2):219. doi: 10.1016/j.bpg.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hearle N, Schumacher V, Menko F H. et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12(10):3209. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 24.van Lier M GF Wagner A Mathus-Vliegen E M Kuipers E J Steyerberg E W van Leerdam M E High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations Am J Gastroenterol 201010561258–1264., author reply 12655 [DOI] [PubMed] [Google Scholar]
- 25.Giardiello F M, Brensinger J D, Tersmette A C. et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119(6):1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 26.Hemminki A. The molecular basis and clinical aspects of Peutz-Jeghers syndrome. Cell Mol Life Sci. 1999;55(5):735–750. doi: 10.1007/s000180050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlop M Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, juvenile polyposis, and Peutz-Jeghers syndrome Gut 2002;(1). Available at: http://gut.bmj.com/content/51/suppl_5/v21.extract [DOI] [PMC free article] [PubMed]
- 28.Latchford A R, Neale K, Phillips R K, Clark S K. Juvenile polyposis syndrome: a study of genotype, phenotype, and long-term outcome. Dis Colon Rectum. 2012;55(10):1038–1043. doi: 10.1097/DCR.0b013e31826278b3. [DOI] [PubMed] [Google Scholar]
- 29.Brosens L A, van Hattem A, Hylind L M. et al. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56(7):965–967. doi: 10.1136/gut.2006.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanich P P, Owens V L, Sweetser S. et al. Colonic polyposis and neoplasia in Cowden syndrome. Mayo Clin Proc. 2011;86(6):489–492. doi: 10.4065/mcp.2010.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan M-H, Mester J L, Ngeow J, Rybicki L A, Orloff M S, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18(2):400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riegert-Johnson D L, Gleeson F C, Roberts M. et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer Clin Pract. 2010;8(1):6. doi: 10.1186/1897-4287-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eaden J A, Abrams K R, Mayberry J F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekbom A Helmick C Zack M Adami H O Increased risk of large-bowel cancer in Crohn's disease with colonic involvement Lancet (London, England)1990336(8711)357–359.. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1975343 [DOI] [PubMed]
- 35.Canavan C, Abrams K R, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23(8):1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 36.Whitlock E P, Lin J S, Liles E, Beil T L, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 37.Howlader N Noone A Krapcho M et al. SEER Cancer Statistics Review, 1975–2011. Natl Cancer Inst. 2014: based on November 2013 SEER data submission, poste Available at: http://seer.cancer.gov/csr/1975_2011/
- 38.Collins P D. Strategies for detecting colon cancer and dysplasia in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(4):860–863. doi: 10.1097/MIB.0b013e3182802c6a. [DOI] [PubMed] [Google Scholar]
- 39.Collins P D, Mpofu C, Watson A J, Rhodes J M. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;(2):CD000279. doi: 10.1002/14651858.CD000279.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Robertson D J, Kaminski M F, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut. 2015;64(6):982–990. doi: 10.1136/gutjnl-2014-308076. [DOI] [PubMed] [Google Scholar]
- 41.Siegel R L, Ward E M, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev. 2012;21(3):411–416. doi: 10.1158/1055-9965.EPI-11-1020. [DOI] [PubMed] [Google Scholar]
- 42.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348(April):g2467. doi: 10.1136/bmj.g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rex D K, Cutler C S, Lemmel G T. et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112(1):24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 44.Ahn S B, Han D S, Bae J H, Byun T J, Kim J P, Eun C S. The miss rate for colorectal adenoma determined by quality-adjusted, back-to-back colonoscopies. Gut Liver. 2012;6(1):64–70. doi: 10.5009/gnl.2012.6.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka S, Kaltenbach T, Chayama K, Soetikno R. High-magnification colonoscopy (with videos) Gastrointest Endosc. 2006;64(4):604–613. doi: 10.1016/j.gie.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Rex D K. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000;51(1):33–36. doi: 10.1016/s0016-5107(00)70383-x. [DOI] [PubMed] [Google Scholar]
- 47.Hüneburg R, Lammert F, Rabe C. et al. Chromocolonoscopy detects more adenomas than white light colonoscopy or narrow band imaging colonoscopy in hereditary nonpolyposis colorectal cancer screening. Endoscopy. 2009;41(4):316–322. doi: 10.1055/s-0028-1119628. [DOI] [PubMed] [Google Scholar]
- 48.Itzkowitz S H Present D H; Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease Inflamm Bowel Dis 2005113314–321. [DOI] [PubMed] [Google Scholar]
- 49.Marion J F, Waye J D, Present D H. et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103(9):2342–2349. doi: 10.1111/j.1572-0241.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 50.Anderson M L, Pasha T M, Leighton J A. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95(12):3418–3422. doi: 10.1111/j.1572-0241.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- 51.Segnan N, Senore C, Andreoni B. et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007;132(7):2304–2312. doi: 10.1053/j.gastro.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 52.Levin B, Lieberman D A, McFarland B. et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 53.Schoen R E, Pinsky P F, Weissfeld J L. et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elmunzer B J, Hayward R A, Schoenfeld P S, Saini S D, Deshpande A, Waljee A K. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9(12):e1001352. doi: 10.1371/journal.pmed.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lilly E According To the Distal Colorectal Findings. Risk Adv Prox Neoplasms Adults Accord To Distal Color Find 2000343(3)169–174.. Available at: http://www.nejm.org/doi/pdf/10.1056/NEJM200007203430302
- 56.Knight J R, Kanotra S, Siameh S, Jones J, Thompson B, Thomas-Cox S. Understanding barriers to colorectal cancer screening in Kentucky. Prev Chronic Dis. 2015;12:E95. doi: 10.5888/pcd12.140586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.May F P Whitman C B Varlyguina K Bromley E G Spiegel B MR Addressing low colorectal cancer screening in African Americans: using focus groups to inform the development of effective interventions J Cancer Educ 2015May: doi: 10.1007/s13187-015-0842-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curbow B A, Dailey A B, King-Marshall E C. et al. Pathways to colonoscopy in the South: seeds of health disparities. Am J Public Health. 2015;105(4):e103–e111. doi: 10.2105/AJPH.2014.302347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson C D, Chen M-H, Toledano A Y. et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pickhardt P J, Choi J R, Hwang I. et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349(23):2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 61.Halligan S, Altman D G, Taylor S A. et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology. 2005;237(3):893–904. doi: 10.1148/radiol.2373050176. [DOI] [PubMed] [Google Scholar]
- 62.Mulhall B P, Veerappan G R, Jackson J L. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005;142(8):635–650. doi: 10.7326/0003-4819-142-8-200504190-00013. [DOI] [PubMed] [Google Scholar]
- 63.Brenner D J, Georgsson M A. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology. 2005;129(1):328–337. doi: 10.1053/j.gastro.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 64.Gluecker T M, Johnson C D, Wilson L A. et al. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology. 2003;124(4):911–916. doi: 10.1053/gast.2003.50158. [DOI] [PubMed] [Google Scholar]
- 65.Blakeborough A, Sheridan M B, Chapman A H. Complications of barium enema examinations: a survey of UK Consultant Radiologists 1992 to 1994. Clin Radiol. 1997;52(2):142–148. doi: 10.1016/s0009-9260(97)80108-0. [DOI] [PubMed] [Google Scholar]
- 66.Sosna J, Sella T, Sy O. et al. Critical analysis of the performance of double-contrast barium enema for detecting colorectal polyps > or = 6 mm in the era of CT colonography. AJR Am J Roentgenol. 2008;190(2):374–385. doi: 10.2214/AJR.07.2099. [DOI] [PubMed] [Google Scholar]
- 67.Halligan S, Wooldrage K, Dadswell E. et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet. 2013;381(9873):1185–1193. doi: 10.1016/S0140-6736(12)62124-2. [DOI] [PubMed] [Google Scholar]
- 68.Analysis A E Fecal Occult Blood Test for Colorectal Cancer Screening: An Evidence-Based Analysis Vol 9. 2009. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3377532&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed]
- 69.Pignone M Campbell M K Carr C Phillips C Meta-analysis of dietary restriction during fecal occult blood testing Eff Clin Pract; 4(4)150–156.. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11525101 [PubMed]
- 70.Lieberman D A Weiss D G; Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon N Engl J Med 20013458555–560. [DOI] [PubMed] [Google Scholar]
- 71.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (Hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 72.Allison J E, Tekawa I S, Ransom L J, Adrain A L. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996;334(3):155–159. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- 73.Nadel M R, Shapiro J A, Klabunde C N. et al. A national survey of primary care physicians' methods for screening for fecal occult blood. Ann Intern Med. 2005;142(2):86–94. doi: 10.7326/0003-4819-142-2-200501180-00007. [DOI] [PubMed] [Google Scholar]
- 74.Nakama H Yamamoto M Kamijo N et al. Colonoscopic evaluation of immunochemical fecal occult blood test for detection of colorectal neoplasia Hepatogastroenterology; 4625228–231.. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10228797 [PubMed]
- 75.Health Quality Ontario . Fecal occult blood test for colorectal cancer screening: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9(10):1–40. [PMC free article] [PubMed] [Google Scholar]
- 76.Imperiale T F Ransohoff D F Itzkowitz S H Turnbull B A Ross M E; Colorectal Cancer Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population N Engl J Med 2004351262704–2714. [DOI] [PubMed] [Google Scholar]


