Abstract
Background
Knowledge of the body's response to and recovery from exercise is rapidly increasing. State-of-the-art equipment and facilities allow recreationally active adults to seek innovations to enhance performance and shorten recovery time. Myofascial rolling (MR) is a relatively new practice, providing acute benefits for muscle pain and range of motion (ROM). However, there is no consensus on optimal MR duration.
Purpose
The purpose of this systematic review is to determine the optimal MR duration using a foam roller or a roller massager for muscle pain, ROM, and athletic performance via qualitative review.
Study Design
Systematic Review of the Literature
Methods
A systematic search was conducted using PubMed, EMBASE, EBSCOHost and PEDro (July 2018). Twenty-two studies met the inclusion criteria and were appraised using the PEDro scale. Studies were grouped by outcome measure, with a total number of subjects of n = 328 for pain/soreness, n = 398 for ROM, and n = 241 for performance. Heterogeneity of data prohibited a formal meta-analysis: studies were manually reviewed and classified as providing evidence for benefit of MR (i.e., significant positive effect) or not (i.e., null or negative effect) for each of the studied outcomes.
Results
The most evidence-based benefit of MR is the alleviation of muscle soreness; seven of eight studies assessing pain/soreness resulted in a short-term reduction, and a minimum dose of 90 seconds per muscle appeared beneficial. While ten of 17 studies involving ROM showed acute improvements, the results were inconsistent and highly variable. No significant effects on performance were detected.
Conclusion
Available data indicate that MR for 90 seconds per muscle group may be the minimal duration to achieve a short-term reduction in pain/soreness, with no upper limit found. Results do not support increases in chronic ROM or performance, and data are insufficient to provide a conclusive recommendation for impacting acute ROM. The heterogeneity of the literature highlights the need for additional research to determine optimal dose of MR.
Level of evidence
2a- (Systematic Review with heterogeneity).
Keywords: Athletic performance, dose, movement system, myofascial rolling, pain, range of motion
INTRODUCTION
Performance among high-level athletes is undeniably improving. While there are many factors involved, advancements in technology and understanding of anatomy and physiology are the largest contributors.1-3 These advancements in high-level athletics have downstream effects on the wider population: recreationally active people at all ages and levels seek new information to improve their personal training regimens, whether it is based on clinical recommendation or athlete/celebrity endorsement. When a new training system or recovery tool is introduced and promoted, it is rapidly and widely adopted by people anxious to improve their performance or shorten their subsequent recovery. Importantly, clinicians rely on these therapeutic advancements to expedite their patients’ return to function and improved quality of life.
Myofascial rolling (MR) using a foam roller (FR) or a roller massager (RM) is a relatively new treatment method, accompanied by a recent surge in new literature. MR with a FR involves rolling along the length of the targeted muscle belly on the device, using one's body weight in a laying or seated position to determine the desired treatment pressure. The same concept is applied to MR with a RM, except the individual handles the device and rolls it along the targeted muscle belly, dictating the applied pressure using their upper limbs. A typical FR and a typical RM are shown in Figure 1. Despite the widespread use of MR, there is currently no agreement on the physiological effects of FR or RM-assisted MR, though it has been postulated that applying deep pressure can reduce fascial adhesions,4,5 improve fascial viscosity and mobility,6,7 and alter mechanoreceptor response in the myofascial unit.6,8,9
Figure 1.
Example of a typical foam roller (left) and a roller massager (right).
Research examining the clinical effects of MR has increased considerably over the past decade; however, a true consensus on the benefits and potential risks of MR has not been established. Some available data suggest that MR acutely increases range of motion (ROM) and/or reduces pain or soreness while simultaneously limiting decrements to athletic performance.4,9-11 However, the existing data are highly heterogeneous, making a true quantitative review challenging. Therefore, a qualitative review of quantitative data is needed to determine true effects.
To date, a handful of literature reviews have been conducted on the effectiveness of MR using rollers.4,9-12 While these have indicated that MR is useful in improving pain/soreness and ROM outcomes, there is little information to establish the optimal MR treatment duration. In the clinical setting, practitioners have limited time with their clients: in order to optimize time in training or rehabilitation, clinicians need information on the minimum duration of treatment to confer benefit, and on whether extending the duration of treatment produces more benefit. If a clinician knows that devoting one minute to a treatment versus 10 minutes will yield the same outcome, they can use the extra time for other treatment, increasing rehabilitation efficiency. Consequently, this systematic review aimed to determine the optimal MR duration using a FR or a RM for reducing muscle pain/soreness, increasing ROM, and improving athletic performance.
METHODS
Systematic literature review
This review was conducted in accordance with PRISMA guidelines.13 In July 2018, a systematic search of PubMed, EMBASE, and EBSCOHost was conducted, using the following search terms: foam roll; roller massager; time; duration; pressure; pain; myalgia; delayed-onset muscle soreness; range of motion; athletic performance; acute pain; chronic pain; musculoskeletal pain. The terms were then combined with the appropriate Boolean connectors according to a PICO search table: each term under “population”, “intervention”, “comparison”, and “outcome” (PICO) were combined within groups with “OR”, while each term between groups were combined with “AND”. The search strategy did not target a specific population; however, only apparently healthy populations (i.e., no special populations) were included. A manual search of the Physiotherapy Evidence Database (PEDro) was also conducted using the same strategy. No date or language restrictions were implemented when searching the databases.
Exclusion criteria
One reviewer screened titles and abstracts. Studies were excluded if they did not satisfy the following: i) Were published in an English language, peer-reviewed journal; ii) Incorporated FR or RM (or both) as the primary intervention(s); iii) Directly compared the intervention to an independent control group; iv) Considered at least one of the following primary outcome measures: acute pain, chronic pain, muscle soreness, range of motion, athletic/muscular performance; v) Specified the duration of treatment in the Methods; and vi) Studied healthy participants or those with no existing chronic conditions that might influence results of an athletic test (e.g. arthritis or cardiovascular disease). Articles that passed the screening then underwent a full-text review by two reviewers to be further examined for eligibility.
The systematic and manual searches identified 113 articles. From there, 69 studies were removed due to being duplicates (n = 57), reviews (n = 8) or abstracts (n = 4). The remaining 44 studies were considered for full review; after applying the inclusion criteria, 16 studies were removed after abstract review, and six additional studies were removed after full-text review. The flow chart summarizing the systematic review can be found in Figure 2.
Figure 2.
Selection Strategy Flowchart.
Data Quality and Analysis
The quality of included studies was assessed by two independent reviewers according to the Physiotherapy Evidence Database (PEDro) scale, developed to rate the quality of RCTs evaluating physical therapist interventions based on their methodological quality and reliability (Table 1).14-16 Among the available data, the population demographics, intervention type and protocol, outcome measures, and results were extracted for analysis. Studies were grouped by outcome measure for analysis of their interventions’ effect on the study population. Upon grouping, each study's intervention duration and their general study conclusion (positive, negative, or null result) were combined to construct a linear plot illustrating dose-response indications in the literature.
Table 1.
PEDro scores for included studies.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aboodarda et al | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | 9 |
| Bradbury-Squires et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Bushell et al | Y | N | N | Y | Y | N | N | Y | Y | Y | Y | 7 |
| Casanova et al | Y | N | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Cheatham et al | Y | Y | N | Y | Y | N | N | Y | Y | Y | Y | 8 |
| D'Amico & Paolone | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Fleckenstein et al | Y | Y | N | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Jay et al | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Junker & Stoggl | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Kelly & Beardsley | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| MacDonald et al (2014) | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| MacDonald et al (2013) | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Macgregor et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Mohr et al | Y | Y | N | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Monteiro et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Morales-Artacho et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Pearcey et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Phillips et al | Y | Y | N | Y | Y | N | N | Y | Y | Y | Y | 8 |
| Smith et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Sullivan et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Wilke et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
| Young et al | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 |
PEDro scale: 1 = eligibility criteria was specified; 2 = subjects were randomly allocated to groups; 3 = allocation was concealed; 4 = similar groups at baseline; 5 = all subjects were blinded; 6 = all therapists were blinded; 7 = all assessors were blinded; 8 = at least one outcome measure obtained from >85% of allocated subjects; 9 = all subjects received either intervention or control; 10 = results of between-group statistical comparisons reported for at least one outcome; 11 = point measures and measures of variability for at least one outcome were reported
RESULTS
The results of the 22 qualifying studies were grouped according to outcome measure (i.e., ROM, pain/soreness, and performance). Including studies analyzing multiple outcomes, there were eight studies examining pain/soreness, 17 measuring ROM, and 12 that assessed some aspect of athletic performance. In total, 16 studies involved a FR as their main intervention, while the remaining six used a RM. Areas treated by either a FR or a RM and used for subsequent test/retest in the qualifying studies included the gluteals (n = 4), the hip flexors (n = 1), the quadriceps (n = 12), the hamstrings (n = 10), the iliotibial band (n = 4), the adductors (n = 4), and the plantar flexors (n = 8). Similar to previous reviews on this topic, 4, 10, 12 even with separated outcome measures the heterogeneity of studies made data consolidation and meta-analysis invalid.
Population Characteristics
The combined population of participants in the 22 studies was n = 644. One study17 did not disclose the sex of their subjects (n = 40) and was therefore not included in analysis of sexual distribution. Of the remaining 604 subjects, 62.9% were male (n = 380) and 37.1% were female (n = 224). Separated by outcome measure, there were n = 328 subjects (63.4% male, n = 208 and 36.6% female, n = 120) in studies relating to pain/soreness,18–25 n = 398 for ROM (72.3% male, n = 259 and 27.7% female, n = 139),8,19,20,22,23,26-36 and n = 241 (60.2% male, n = 145 and 39.8% female, n = 96) for athletic performance.8,19,21,23,24,26,28-30,32,34,37 The typical study participant across all outcomes was a recreationally active adult (e.g. moderately active two to three times per week), aged between 18 to 47 years (mean + /- standard deviation [SD] = 25.0 + /- 5.54 years). One study18 did not disclose the ages of their participants (n = 150) beyond describing them as university-aged; they were therefore omitted from the age distribution analysis. For pain/soreness, ages ranged from 19 to 47 years (26.0 + /- 5.34 years), with 93% of the participants (n = 306) being at least recreationally active on a regular basis. ROM participant ages ranged from 18 to 47 years (24.9 + /- 5.98 years), the large majority (93.0%, n = 370) of which were also at least recreationally active on a regular basis. Finally, subjects in studies relating to athletic performance had no age range available (24.0 + /- 4.05 years), with 97.5% of the participants (n = 235) being at least recreationally active on a regular basis.
Dose-Response Analysis
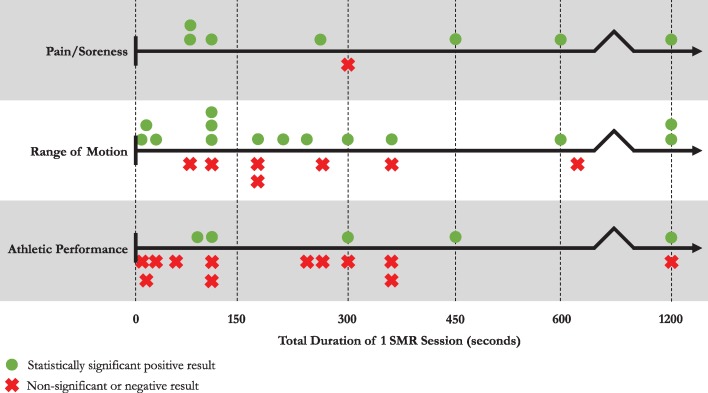
Appendices 1-3 describe the design and results of the 22 included studies relating to (1) pain/soreness, (2) ROM, and (3) athletic performance. The results column includes only the results that are pertinent to the selected outcomes of this review; they are not an exhaustive list of all the outcome measures assessed by the original study authors. Figures 3 and 4 illustrate the linear distribution of studies grouped by outcome based on their MR intervention duration. Green circles denote statistically significant positive results and red X's denote null or statistically negative results. In Figure 3, MR durations in each study have been summed to generate a value equal to the total duration of one MR session across all assessed muscle groups. Conversely, Figure 4 displays the duration of MR per muscle group, as some studies involved the rolling of multiple muscle groups for a set amount of time each.
Figure 3.
Effect of total Self-Myofascial Release (SMR) duration using a foam roller or a roller massager, in seconds, on each of the three indicated outcome measures. Each green circle or red X denotes a unique study result. Some studies appear in multiple outcomes, and some studies have multiple SMR durations per outcome.
Figure 4.
Effect of Self-Myofascial Release (SMR) duration per muscle group using a foam roller or a roller massager in seconds on each of the three indicated outcome measures. Each green circle or red X denotes a unique study result. Some studies appear in multiple outcomes, and some studies have multiple SMR durations per outcome.
Pain/Soreness
Overall, results for the recovery from muscle pain/soreness indicated that the use of MR for any duration would improve a subject's outcome. When analyzed by total time spent treating the subject (Figure 3), no dose-response is present and the single non-significant result21 can be considered an outlier. However, when separated by total time treating a single muscle group (Figure 4), the data points shift slightly and a minimum dose can be observed. Foam rolling a single muscle group for under 45 seconds may indeed be insufficient for adequate recovery from muscle pain or acute/chronic muscle soreness. Further, the positive result seen at 45 seconds per muscle group24 draws conclusions based upon magnitude-based inferences38 (% likelihood) as opposed to including effect sizes (Cohen's d) or tests of significance (p-value) like many of the other experiments. More robust results were seen in studies that intervened for durations between 90 and 600 seconds per muscle group,18-20,22,23,25 suggesting that a minimum dose of 90 seconds is most reliable and is best suited for recovery of muscle pain/soreness.
In terms of a true dose-response trend (i.e. longer MR sessions leading to more lasting effects), the heterogeneity of studies made this difficult to discern. For example, the study with the longest single-muscle MR duration (hamstrings for one set of 600 seconds)22 measured the effects up to 60 minutes post-MR. While they achieved a statistically significant reduction in muscle soreness versus both their control and within-subject control groups, another study intervened with only 120 seconds per muscle (five hip and thigh muscles, bilaterally)23 and observed a decrease in muscle soreness lasting 72 hours post-MR. However, these studies differed in many areas, including the SMR tool they used (FR versus RM), fitness level of their study population, muscle groups that they treated, muscle soreness data collection method (algometer versus rating scale), and statistics that they analyzed (p-value versus Cohen's d). It is worth noting, meanwhile, that when considering the total duration of a single MR session (Figure 3), the study with the longest total MR duration (1200 seconds)23 also resulted in the longest lasting significant effect (72 hours). Otherwise, most studies assessing muscle pain and soreness18,19,24,25 noted the acute and transient nature of their results, suggesting that MR is an effective option only for short-term relief.
Range of Motion
The results for MR's effect on ROM are much less clear. In total, 10 of 17 studies reported statistically significant ROM improvements of some kind,17,20,22,23,26,28-30,32,35 with no ideal MR duration apparent. Similarly, when split by studies that tested ROM by active versus passive means, five of nine studies measuring active ROM showed significant improvements22,28-30,35 while five of eight studies measuring passive ROM studies noted the same.17,20,23,26,32 Among the methods used to assess ROM, the kneeling lunge test (for the hip flexors) was the most common with five studies opting for that technique;8,23,26,28,32 however, the fact that a total of ten different methods were used in 17 different studies to assess ROM about various joints demonstrates that the variation is too large to directly compare results. In terms of MR duration, the results were spread evenly across all time points. There was also a near equal split of positive and negative trials appearing on both sides of the MR duration line when considering both the total MR session duration and the total time spent on one muscle (Figures 3 & 4). From this, it is not possible to provide conclusive support of an optimal MR duration, and further trials are needed to rigorously test and retest (using similar testing protocols) the effect of MR on ROM.
Athletic Performance
Athletic performance was minimally affected by MR. While ROM was difficult to interpret due to its wide array of testing techniques, assessing MR's effect on performance is challenging as there are multiple operational definitions of “athletic performance” and many ways to measure it. Included among the outcome measures are dynamic tests like the vertical jump test or an 800-meter run test. Likewise, tests of maximal voluntary contraction (MVC) and sub-maximal electromyography (EMG) were also used as measures of athletic performance, exacerbating the challenge of combining data. Assessing results as either a significant positive result, a non-significant result, or a significant negative result (as in Figures 3 & 4), demonstrates that MR using either a FR or a RM does not typically provide an individual with a performance increase. Both positive and negative results are spread out along the duration line, so it is not possible to discern a recommended MR duration time for optimal athletic performance. Three of the four studies that reported a performance increase post-MR8,23,24 noted that the effects lasted up to 72 hours post treatment (the fourth did not perform follow-up testing)32. Conversely, the results of another study19 directly contradicted this finding, showing no effect on performance versus control at any time point for 72 hours. One study,37 however, analyzed the effects of two different FR durations of the hamstring (60 or 120 seconds) on the maximum number of subsequent consecutive knee extension repetitions. While both FR durations resulted in a decrease in repetitions versus control, they noted a dose-response: the longer the FR duration, the fewer repetitions their subjects were able to perform.
DISCUSSION
This systematic review sought to determine a consensus on MR duration for optimal muscle pain/soreness recovery, ROM, and athletic performance. The results of this review suggest that MR using a FR or a RM for at least 90 seconds per muscle will acutely alleviate muscle soreness; the current literature also suggests that a longer treatment duration extends the duration of analgesic effects, although this effect is non-linear. Nonetheless, more robust data are needed to confirm these findings, as the data studied here are highly heterogeneous. While many studies did report an acute positive effect at varying intervention durations, the long-term effectiveness of MR for ROM remains inconclusive. As the underlying physiological effect of MR is still uncertain, improving subject ROM via MR may vary across individuals, and whether ROM is restricted by pain or by true myofascial stiffness (discussed later in the Nociceptor Involvement section). Finally, the literature indicates that MR has little effect on improving or enhancing athletic performance at any duration, and actually suggests that performance may begin to suffer progressively with longer treatment. Although the effects of MR are inconclusive for acute and chronic ROM, and MR may be detrimental to athletic performance, the authors suggest that MR for approximately 90 seconds per unilateral muscle group may be the most efficient duration to achieve a reduction in muscle pain/soreness.
With the rise in MR popularity over the past decade, research on the topic is recent but remains limited in scope: all 22 studies included in this review were published between 2013 and 2018. Recent reviews examining measurable effects of FR and RM's have come to similar conclusions;4,9,10,12 however, a common theme was the emphasis on determining the optimal duration of MR. Therefore, this review analyzed the topic by separating the literature into three main outcome measures: muscle pain/soreness recovery, ROM, and athletic performance.
The current consensus in the literature regarding MR is that it reduces soreness and improves ROM with limited decrements in performance.4,9-11 The available data indicate that MR is a recovery tool rather than a performance enhancer; consequently, negative plot points (those indicating no effect, not those indicating a negative effect) on Figures 3 and 4 can be considered a positive result assuming the main goal is an reduction in soreness or an increase in ROM. Since data from one study indicate a negative MR dose-response for performance37 and the available evidence indicates that muscle pain and soreness can decrease after approximately 90 seconds of MR, the authors believe an MR duration of roughly 90 seconds per muscle is ideal to maximize the positive benefit of pain/soreness recovery while minimizing the decrement to performance.
Nociceptor Involvement
The mechanism(s) underlying the analgesic effects of MR are ill-defined. Recent findings have identified nociceptors within multi-layered fascia in rats, with researchers postulating that these nociceptors may play a role in chronic muscle pain.39 As is well established, pain is often associated with limited ROM, while non-neurological tension signs in addition to pain further limit ROM.40,41 When considering this in the context of recent data on MR, a persistent question emerges: does MR truly mobilize the myofascial unit, or does it simply dampen the nociceptive response, allowing those with limited ROM due to pain to move beyond their baseline measurements?
The study by Young et al25 (included within the pain/soreness section, Appendix 1) examined this phenomenon, assessing whether or not using a RM on the plantar flexors would reduce spinal excitability, thus increasing a subject's pain-pressure threshold (PPT). They determined that spinal excitability decreased post-RM, with a pressure-dependent response observed (i.e., more pressure led to more neurological inhibition). This response suggested a fast-adaptation: spinal excitability quickly recovered from its response-dampened state in under three minutes. This result appeared as a consistent trend in the majority of studies that observed a significant increase in ROM post-MR; although flexibility improves, the results were transient and only remained significant for a short period of time after MR. One possible interpretation of these findings is that restricted ROM derived from pain/soreness is amenable to improvement via MR, while ROM that is truly restricted by myofascial tightness is non-responsive. Clearly, further research is required to test this hypothesis.
Additionally, studies by both Aboodarda et al18 and Cavanaugh et al42 have found evidence of a global pain modulatory system through their observed effects of roller massaging. Both author's results noted that with three, 30-second RM treatments on the same muscle, there was a significantly smaller increase in pain experienced post-RM intervention in both the ipsilateral and contralateral limb, even though the contralateral limb had not been touched by the intervention. As this directly contradicts early ideas of purported MR mechanisms (i.e., reduced fascial adhesions, improved fascial viscosity and mobility, and altered mechanoreceptor response in the myofascial unit), this further supports the notion that the effects of MR are grounded in cross-over effects of neurological basis. If, conversely, the effects of MR were due to alterations in the mechanical properties of the tissue, one would expect to see ipsilateral effects, local only to the tissue treated. However, as evidence has shown transient, non-local effects in the reduction of pain/soreness, it suggests that MR acts on a neurological basis, with recent evidence suggesting the presence of a global pain modulatory system likely within the central nervous system.
Limitations
Several factors limit the conclusiveness of these findings. As demonstrated above, the heterogeneity of MR research is the overarching issue in the relevant literature. When searching for a consensus on the optimal MR duration, no significant number of studies were similar enough for direct comparison; as testing protocols contained too much variation. Studies often analyzed different muscle groups and utilized different muscle soreness/ROM/performance measurements (e.g., testing active versus passive ROM, or measuring pain on a subjective rating scale versus using an algometer). Similarly, there was minimal statistical consistency: not all studies included the same statistics to permit data pooling. This did not allow for true quantitative data analysis, which restricted this review to a qualitative investigation. Another uncertainty among MR research is in regard to the potential lasting effects. Unfortunately, most studies did not assess their subjects with long-term follow-ups to determine if the effects were retained, but rather conducted a same-day test/retest protocol and collected data solely in the acute setting.
CONCLUSION
Available data indicate that MR for 90 seconds per unilateral muscle group may be the minimal duration to achieve a reduction in pain/soreness, with no upper limit found. While the analgesic effects were transient in nature, the longer time spent on MR, the longer the effects seemed to last; however, these results are non-linear and require further investigation to arrive at a consensus. Results do not support increases in chronic ROM or performance, and data are insufficient to provide a conclusive recommendation regarding acute ROM. The heterogeneity of the literature highlights the need for additional research to determine optimal dose of MR.
Appendix 1.Description of Included Studies: Outcomes Relating to Pain/Soreness.
| Author | Tool | Study Population | Muscle(s) Treated | Duration of FR/RM# | Results |
|---|---|---|---|---|---|
| *Aboodarda et al15 | RM |
|
|
|
|
| *Casanova et al16 | RM |
|
|
|
|
| *Cheatham et al17 | FR |
|
|
|
|
| Fleckenstein et al18 | FR |
|
|
|
|
| *Jay et al19 | RM |
|
|
|
|
| *MacDonald et al (2014)20 | FR |
|
|
|
|
| *Pearcey et al21 | FR |
|
|
|
|
| *Young et al22 | RM |
|
|
|
|
- Statistically significant positive conclusion
FR - Foam Roller
RM - Roller Massager
PPT - Pain-Pressure Threshold
reported as s/m/h - Denotes the indicated number of seconds (s), minutes (m), or hours (h)
Appendix 2.
Description of Included Studies: Outcomes Relating to Range of Motion (ROM).
| Author | Tool | Study Population | Muscle(s) Treated | Duration of FR/RM# | Results |
|---|---|---|---|---|---|
| *Bradbury-Squires et al29 | RM |
|
|
|
|
| Bushell et al30 | FR |
|
|
|
|
| Casanova et al16 | RM |
|
|
|
|
| *Cheatham et al17 | FR |
|
|
|
|
| D'Amico & Paolone31 | FR |
|
|
|
|
| *Jay et al19 | RM |
|
|
|
|
| *Junker & Stoggl32 | FR |
|
|
|
|
| Kelly & Beardsley33 | FR |
|
|
|
|
| *MacDonald et al (2014)20 | FR |
|
|
|
|
| *MacDonald et al (2013)34 | FR |
|
|
|
|
| Macgregor et al23 | FR |
|
|
|
|
| *Mohr et al14 | FR |
|
|
|
|
| Morales-Artacho et al24 | FR |
|
|
|
|
| *Phillips et al25 | FR |
|
|
|
|
| *Smith et al26 | FR |
|
|
|
|
| *Sullivan et al27 | RM |
|
|
|
|
| Wilke et al28 | FR |
|
|
|
|
- Statistically significant positive conclusion
FR - Foam Roller
RM - Roller Massager
ROM - Range of Motion
PNF - Proprioceptive Neuromuscular Facilitation
reported as s/m/h - Denotes the indicated number of seconds (s), minutes (m), or hours (h)
Appendix 3.
Description of Included Studies: Outcomes Relating to Performance.
| Author | Tool | Study Population | Muscle(s) Treated | Duration of FR/RM# | Results |
|---|---|---|---|---|---|
| *Bradbury-Squires et al29 | RM |
|
|
|
|
| Casanova et al16 | RM |
|
|
|
|
| D'Amico & Paolone31 | FR |
|
|
|
|
| Fleckenstein et al18 | FR |
|
|
|
|
| *MacDonald et al (2014)20 | FR |
|
|
|
|
| MacDonald et al (2013)34 | FR |
|
|
|
|
| *Macgregor et al23 | FR |
|
|
|
|
| Monteiro et al35 | FR |
|
|
|
|
| *Pearcey et al21 | FR |
|
|
|
|
| Phillips et al25 | FR |
|
|
|
|
| Smith et al26 | FR |
|
|
|
|
| Sullivan et al27 | RM |
|
|
|
|
- Statistically significant positive conclusion
FR - Foam Roller
RM - Roller Massager
RMS - Root Mean Square
EMG - Electromyogram
MVC - Maximal Voluntary Contraction
reported as s/m/h - Denotes the indicated number of seconds (s), minutes (m), or hours (h)
REFERENCES
- 1.Haake SJ. The impact of technology on sporting performance in Olympic sports. J Sports Sci. 2009;27(13):1421-1431. [DOI] [PubMed] [Google Scholar]
- 2.Balmer N Pleasence P Nevill A. Evolution and revolution: gauging the impact of technological and technical innovation on Olympic performance. J Sports Sci. 2012;30(11):1075-1083. [DOI] [PubMed] [Google Scholar]
- 3.Tjønndal A. Sport innovation: developing a typology. Eur J Sport Soc. 2017;14(4):291-310. [Google Scholar]
- 4.Schroeder AN Best TM. Is self myofascial release an effective preexercise and recovery strategyϿ. A literature review. Curr Sports Med Rep. 2015;14(3):200-208. [DOI] [PubMed] [Google Scholar]
- 5.Dommerholt J Finnegan M Hooks T Chou LW. A critical overview of the current myofascial pain literature – October 2017. J Bodyw Mov Ther. 2017;21(4):902-913. [DOI] [PubMed] [Google Scholar]
- 6.Schleip R. Fascial plasticity - a new neurobiological explanation. Part 2. J Bodyw Mov Ther. 2003;7(2):104-116. [Google Scholar]
- 7.Barnes MF. The basic science of myofascial release: morphologic change in connective tissue. J Bodyw Mov Ther. 1997;1(4):231-238. [Google Scholar]
- 8.Macgregor LJ Fairweather MM Bennett RM Hunter AM. The effect of foam rolling for three consecutive days on muscular efficiency and range of motion. Sport Med - Open. 2018;4(26). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beardsley C Škarabot J. Effects of self-myofascial release: a systematic review. J Bodyw Mov Ther. 2015;19(4):747-758. [DOI] [PubMed] [Google Scholar]
- 10.Cheatham SW Kolber MJ Cain M Lee M. The effects of self-myofascial release using a foam roll or roller massager on joint range of motion, muscle recovery, and performance: a systematic review. Int J Sports Phys Ther. 2015;10(6):827-838. [PMC free article] [PubMed] [Google Scholar]
- 11.Kalichman L David C Ben. Effect of self-myofascial release on myofascial pain, muscle flexibility, and strength: A narrative review. J Bodyw Mov Ther. 2017;21(2):446-451. [DOI] [PubMed] [Google Scholar]
- 12.Laimi K Mäkilä A Bärlund E, et al. Effectiveness of myofascial release in treatment of chronic musculoskeletal pain: a systematic review. Clin Rehabil. 2018;32(4):440-450. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A Altman DG Tetzlaff J Mulrow C Gøtzsche PC John PA. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbert R Moseley A Sherrington C. PEDro: a database of randomised controlled trials in physiotherapy. Heal Inf Manag. 1998;28(4):186-188. [DOI] [PubMed] [Google Scholar]
- 15.De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129-133. [DOI] [PubMed] [Google Scholar]
- 16.Macedo L Elkins M Maher C Moseley A Herbert R Sherrington C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. J Clin Epidemiol. 2010;63(8):920-925. [DOI] [PubMed] [Google Scholar]
- 17.Mohr AR Long BC Goad CL. Effect of foam rolling and static stretching on passive hip-flexion range of motion. J Sport Rehabil. 2014;23:296-299. [DOI] [PubMed] [Google Scholar]
- 18.Aboodarda SJ Spence AJ Button DC. Pain pressure threshold of a muscle tender spot increases following local and non-local rolling massage. BMC Musculoskelet Disord. 2015;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casanova N Reis JF Vaz JR, et al. Effects of roller massager on muscle recovery after exercise-induced muscle damage. J Sports Sci. 2018;36(1):56-63. [DOI] [PubMed] [Google Scholar]
- 20.Cheatham SW Stull KR Kolber MJ. Comparison of a vibrating foam roller and a non-vibrating foam roller intervention on knee range of motion and pressure pain threshold: a randomized controlled trial. J Sport Rehabil. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Fleckenstein J Wilke J Vogt L Banzer W. Preventive and regenerative foam rolling are equally effective in reducing fatigue-related impairments of muscle function following exercise. J Sport Sci Med. 2017;16:474-479. [PMC free article] [PubMed] [Google Scholar]
- 22.Jay K Sundstrup E Søndergaard SD, et al. Specific and cross over effects of massage for muscle soreness: randomized controlled trial. Int J Sports Phys Ther. 2014;9(1):82-91. [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald GZ Button DC Drinkwater EJ Behm DG. Foam rolling as a recovery tool after an intense bout of physical activity. Med Sci Sports Exerc. 2014;46(1):131-142. [DOI] [PubMed] [Google Scholar]
- 24.Pearcey GEP Bradbury-Squires DJ Kawamoto J-E Drinkwater EJ Behm DG Button DC. Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures. J Athl Train. 2015;50(1):5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young JD Spence A-J Behm DG. Roller massage decreases spinal excitability to the soleus. J Appl Physiol. 2018;124:950-959. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald GZ Penney MDH Mullaley ME, et al. An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J Strength Cond Res. 2013;27(3):812-821. [DOI] [PubMed] [Google Scholar]
- 27.Morales-Artacho AJ Lacourpaille L Guilhem G. Effects of warm-up on hamstring muscles stiffness: cycling vs foam rolling. Scand J Med Sci Sport. 2017;27:1959-1969. [DOI] [PubMed] [Google Scholar]
- 28.Phillips J Diggin D King DL Sforzo GA. Effect of varying self-myofascial release duration on subsequent athletic performance. J Strength Cond Res. 2018;0(0):1-8. [DOI] [PubMed] [Google Scholar]
- 29.Smith JC Pridgeon B Hall MC. Acute effect of foam rolling and dynamic stretching on flexibility and jump height. J Strength Cond Res. 2018;32(8):2209-2215. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan KM Silvey DBJ Button DC Behm DG. Roller-massager application to the hamstrings increases sit-and-reach range of motion within five to ten seconds without performance impairments. Int J Sports Phys Ther. 2013;8(3):228-236. [PMC free article] [PubMed] [Google Scholar]
- 31.Wilke J Niemeyer P Niederer D Schleip R Banzer W. Influence of foam rolling velocity on knee range of motion and tissue stiffness: a randomized, controlled crossover trial. J Sport Rehabil. 2018. [DOI] [PubMed] [Google Scholar]
- 32.Bradbury-Squires DJ Noftall JC Sullivan KM Behm DG Power KE Button DC. Roller-massager application to the quadriceps and knee-joint range of motion and neuromuscular efficiency during a lunge. J Athl Train. 2015;50(2):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bushell JE Dawson SM Webster MM. Clinical relevance of foam rolling on hip extension angle in a functional lunge position. J Strength Cond. 2015;29(9):2397-2403. [DOI] [PubMed] [Google Scholar]
- 34.D’Amico A Paolone V. The effect of foam rolling on recovery between two eight hundred metre runs. J Hum Kinet. 2017;57:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junker DH Stöggl TL. The foam roll as a tool to improve hamstring flexibility. J Strength Cond Res. 2015;29(12):3480-3485. [DOI] [PubMed] [Google Scholar]
- 36.Kelly S Beardsley C. Specific and cross-over effects of foam rolling on ankle dorsiflexion range of motion. Int J Sports Phys Ther. 2016;11(4):544-551. [PMC free article] [PubMed] [Google Scholar]
- 37.Monteiro ER Škarabot J Vigotsky AD Brown AF Gomes TM Novaes J da S. Maximum repetition performance after different antagonist foam rolling volumes in the inter-set rest period. Int J Sports Phys Ther. 2017;12(1):76-84. [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh AH Knight EJ. ‘“Magnitude-based inference”’: a statistical review. Med Sci Sports Exerc. 2015;47(4):874-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mense S Hoheisel U. Evidence for the existence of nociceptors in rat thoracolumbar fascia. J Bodyw Mov Ther. 2016;20(3):623-628. [DOI] [PubMed] [Google Scholar]
- 40.Pearcy M Portek I Shepherd J. The effect of low-back pain on lumbar spinal movements measured by three-dimensional x-ray analysis. Spine (Phila Pa 1976). 1985;10(2):150-153. [DOI] [PubMed] [Google Scholar]
- 41.Holmes B Leggett S Mooney V Nichols J Negri S Hoeyberghs A. Comparison of female geriatric lumbar-extension strength: asymptomatic versus chronic low back pain patients and their response to active rehabilitation. J Spinal Disord. 1996;9(1):17-22. [PubMed] [Google Scholar]
- 42.Cavanaugh MT Döweling A Young JD, et al. An acute session of roller massage prolongs voluntary torque development and diminishes evoked pain. Eur J Appl Physiol. 2017;117(1):109-117. [DOI] [PubMed] [Google Scholar]