Abstract
Background
Achilles tendinopathy is a common overuse injury sustained by athletes (including runners) that often becomes chronic. There is evidence that chronic musculoskeletal pain conditions exhibit signs of nervous system sensitization.
Hypothesis/Purpose
The objective of this study was to compare pain sensitivity (pressure pain threshold [PPT], heat pain threshold [HPT], and heat temporal summation [HTS]) between active healthy adults with and without chronic Achilles tendinopathy in order to determine if signs of peripheral and/or central sensitization exist in chronic Achilles tendinopathy.
Study Design
Cohort study
Methods
Seventeen participants with chronic ( ≥ 3 months) Achilles tendinopathy (39.0 years ± 10.81) and 24 healthy controls (31.83 years ± 8.92) were included. All participants completed the Pain Catastrophizing Scale (PCS). Participants in the Achilles group also completed the Lower Extremity Functional Scale (LEFS) and the Victorian Institute of Sport Assessment-Achilles (VISA-A). Pain processing was quantified using PPT, HPT and HTS tests.
Results
There were no significant differences in PCS scores between groups. In the Achilles tendinopathy group, the mean VISA-A score was 58.5 ± 18.4; the mean LEFS was 63.7 ± 8.0. Primary hyperalgesia (decreased pain threshold at injury site) was detected in the Achilles tendinopathy group, as evidenced by lower PPT (p<0.0001) and lower HPT (p = 0.028). Mechanical secondary hyperalgesia, a sign of central sensitization, was found in the Achilles tendinopathy group at the tibialis anterior (p = 0.042) and non-involved Achilles (p = 0.025), but not at the thenar eminence (p = 0.276). The degree of HTS was not different between groups (p = 0.981).
Conclusion
Active participants with chronic Achilles tendinopathy showed signs of both peripheral and central sensitization; however, widespread hyperalgesia into the upper extremities and elevated temporal summation were not observed. Evidence of differences in pain sensitivity lend support to the theory for a multifactorial model of tendinopathy, which consists of an impaired motor system, local tendon pathology, and changes in the pain/nociceptive system. Physical therapy management of chronic Achilles tendinopathy may need to address potential changes in the nervous system. Interventions used to treat chronic tendinopathies should be investigated for their potential to resolve peripheral and central sensitization.
Level of Evidence
Therapy, level 2b
Keywords: Achilles, movement system, pain, tendinopathy
INTRODUCTION
Achilles tendinopathy (AT) is a common overuse injury sustained by athletes that often becomes chronic.1,2 In recreational runners, the lifetime incidence has been reported to be approximately 52%,3 and intermittent symptoms can reoccur over time.4 Recurrence rates have been reported to be as high as 27% based on a prospective study of elite athletes.5 Insertional AT occurs less frequently, representing approximately 23% of cases as compared to 66% for non-insertional tendinopathy in a retrospective study of athletes with Achilles tendon pain.6 AT is typically characterized by localized pain in the mid-portion (2-6cm proximal to the insertion) or at the insertion of the Achilles tendon at the calcaneus.7 Symptoms are generally provoked with activities that load the Achilles tendon such as running, jumping, uphill walking, or stair climbing.8,9
Symptoms associated with AT are sometimes recalcitrant to conservative management,10 and persistent tendon pain does not always correlate with pathological changes in the tissue via diagnostic imaging.11,12 Sensitization of the nervous system has been proposed as a mechanism to explain continued and persistent tendon pain and can occur in the peripheral and central nervous system.12-15 Signs of peripheral sensitization from quantitative sensory testing (QST) include primary hyperalgesia, defined as the lowering of pain thresholds within the location that is injured/affected by a condition. Signs of central sensitization from QST in humans can be inferred from the presence of secondary hyperalgesia, or the presence of increased temporal summation of pain. Secondary hyperalgesia is defined as the lowering of pain thresholds outside the location that is injured/affected by a condition.15 Whereas, the increased temporal summation of pain is defined as an elevated/exaggerated increase in the pain response over a series of repeated supra-threshold pain stimuli.16,17 There is evidence that people with chronic musculoskeletal pain conditions, such as knee osteoarthritis, low back pain, and subacromial pain syndrome show signs of central sensitization.18-20 Furthermore, deficits in both the sensory and motor systems are observed to occur bilaterally in unilateral tendinopathy suggesting central nervous system involvement.21,22 A recent systematic review found evidence for central sensitization in tendinopathy, but the majority of studies were focused on the upper extremity.15
Recent reports offer conflicting evidence for the presence of changes to the nociceptive system in individuals with chronic Achilles tendon pain.23,24 Tompra and colleagues,23 found that people with chronic AT had primary hyperalgesia and deficient conditioned pain modulation (central/descending inhibition) compared to active controls (runners), whereas Plinsinga and colleagues,24 found no evidence for primary hyperalgesia, secondary hyperalgesia, or mechanical pain (pin prick) temporal summation. Tompra et al,23 reported on pain processing in a population of runners, with a mean weekly running distance of 27.5km, whereas Plinsinga et al,24 reported on minutes of activity per week but did not limit to runners.
The objective of this study was to compare pain sensitivity (pressure pain threshold [PPT], heat pain threshold [HPT], and heat temporal summation [HTS]) between active healthy adults with and without chronic Achilles tendinopathy in order to determine if signs of peripheral and/or central sensitization exist in chronic Achilles tendinopathy. It was hypothesized that signs of both peripheral and central sensitization would be found in participants with chronic AT when compared to active controls.
METHODS
Participants with and without chronic AT were recruited through flyers posted in two local running shoe stores, along a popular running trail, and from the local University community. Recruitment of people with AT occurred from September 2012 to October 2013, and controls were recruited from September 2012 to September 2016. The study protocol was approved by the Institutional Review Board of Arcadia University. The protocol was reviewed with each participant and informed consent was obtained prior to data collection. Sample size for this study was based on our access to a sample of convenience and an ability to detect a moderate effect size of 0.7, 1-ß = 0.80, which yielded an estimated sample of 21 participants per group (G*Power 3.31). The effect size was estimated from Achilles pressure pain threshold data on 40 healthy participants and estimating a 3 kg difference in force.
Inclusion criteria for the AT group consisted of active participation in regular physical activity (i.e. running, sports that involved running/jumping) of at least three days per week, self-report of pain at the insertion or mid-portion of the Achilles tendon for at least three months duration, pain with palpation to the involved Achilles tendon and/or its insertion, pain with Achilles tendon loading activities (ie. heel raise or hopping), and Achilles pain with active single-leg heel raises (plantarflexion against bodyweight).25 Participants with unilateral or bilateral symptoms were included. Participants were excluded if they noted a history of a complete or partial tear of the Achilles tendon, a history of surgical intervention to the Achilles tendon, reported the presence of any chronic inflammatory condition, or having a chronic pain condition aside from their Achilles tendon(s). Participants were screened for any issues related to the lumbar spine or lower extremity, including neurodynamic testing, and were excluded if the symptoms in the Achilles region were reproduced with the screening tests.
Inclusion criteria for the healthy control group consisted of no musculoskeletal pain conditions currently or within the past year (based on self-report). Additional inclusion criteria for the control group was self-report of active participation in regular running (at least 5 miles per week), and no pain with a minimum of fifteen single-leg active heel raises (plantarflexion against bodyweight).
Additional exclusion criteria for either group were current use of prescription pain medications, current use of selective-serotonin or norepinephrine reuptake inhibitors, diagnosis of any neurological condition, other orthopaedic injury to the spine or lower extremities within the previous year, loss of sensation to the lower legs, a history of fainting spells, or a loss of protective sensation on the plantar surface of their feet (inability to detect < 10g from a monofilament) associated with or without the presence of diabetes. Participants were asked to refrain from taking over the counter NSAIDS on testing days.
Prior to testing, participants in both groups completed a running history questionnaire and the Pain Catastrophizing Scale (PCS). In addition, participants in the AT group completed the Victorian Institute of Sport Assessment Achilles (VISA-A) and the Lower Extremity Functional Scale (LEFS). The PCS is a 13-item questionnaire, which is a valid and reliable self-report measure designed to quantify an individual's negative behaviors and thoughts in response to actual or potential pain.26-28 It is designed to capture the degree of three pain-related behaviors: rumination, magnification, and helplessness.26 A score of 30 or more on the PCS has been shown to represent a clinically relevant level of catastrophizing.26
The VISA-A is a valid and reliable self-report outcome designed to assess the severity of AT through an eight-item questionnaire, which covers stiffness, pain, and function.29 The final score is expressed as a percentage, with 100% indicating full function. No established cut-off scores for severity of AT on the VISA-A have been reported in the literature. The LEFS is a valid and reliable patient self-report outcome measure for lower extremity conditions which consists of 20 questions, with a maximum score of 80 points.30 These two scales were included to capture the degree of functional deficits in the AT participants from a pathology-specific and region-specific perspective.
Quantitative sensory testing consisted of the following in a standardized order: Pressure Pain Threshold (PPT), Heat Pain Threshold (HPT) and Heat Temporal Summation (HTS). The PPT test sites occurred bilaterally over the Achilles tendon (dermatome S1), tibialis anterior muscle belly (dermatome L4), and over the thenar eminence (dermatome C6). Peripheral sensitization was assessed through the comparison of the PPT of the involved Achilles in the tendinopathy group to the Achilles in the control group, and between the PPT of the involved and non-involved Achilles in the tendinopathy group. We assessed for signs of central sensitization by using comparisons of PPTs at the following sites: 1) non-involved Achilles of the tendinopathy group to Achilles of the control group, 2) the tibialis anterior of the tendinopathy group to tibialis anterior of the control group, and 3) thenar eminence of the tendinopathy group to thenar eminence of the control group. Testing of heat stimuli was performed to look for evidence of peripheral sensitization (e.g. lower HPT over involved Achilles compared to control group) and as a sign of central sensitization (HTS). HTS is a test of central facilitation of pain; and if found to be greater in the AT group, would be an additional sign of central sensitization.16,17
For the PPT, a handheld digital pressure algometer with a 1cm2 diameter tip (Model FDIX25, Wagner Instruments, Greenwich, CT) was applied with a standardized rate of force application to the Achilles tendons and tibialis anterior muscle at 1 kg/s, and to the thenar eminence at 0.5 kg/s. The tibialis anterior site was determined from a measurement from the midpoint of a measurement from the fibular head to the medial malleolus, and the PPT was tested with the subject in supine. The thenar eminence site was found as the midpoint between the first metacarpal phalangeal joint and the scaphoid tubercle, and the PPT was tested with the subject in supine. Participants were instructed using standardized scripts and asked to identify the first instance of pain (transition from pressure to pain), at which point the algometer was withdrawn and the peak force was recorded. A minimum of a 1-minute rest between trials for PPT testing of the non-Achilles sites was provided to allow for adequate washout, with testing performed in a standardized order of right thenar, left tibialis anterior, left thenar, then right tibialis anterior. Randomizing the order of test sites could have resulted in the same site being tested sequentially, so a standardized order was utilized to allow for sufficient washout time between successive testing to prevent local sensitization from the test stimuli at each specific site. This test was repeated for a total of two trials at each site. Next, the Achilles tendon was tested at the maximum point of tenderness, which was identified via manual palpation. The most tender point in the tendinopathy group ranged from the insertion to 5cm proximal to the insertion point at the calcaneal tubercle (mean 2.17 ± 1.72cm). In the control group, PPT was measured 2cm proximal to the insertion of the Achilles tendon. The participants were positioned in prone with the ankle stabilized in neutral plantarflexion/dorsiflexion with an inelastic strap. Achilles tendon PPT was performed to the involved side (tendinopathy group) or dominant side (control group) followed by the non-involved or non-dominant side, then repeated for a total of two trials on each limb, with a minimum of a one-minute rest time between sites. The mean peak force from the two trials at each site was used for data analysis. In a prior study, test-retest for Achilles tendon PPT was high (ICC = 0.91, SEM = 1.24 kg/cm2).31
For the HPT, a computer-controlled thermode with a 3-cm2 contact area (TSA-II Neurosensory Analyzer, Medoc, Ramat Yishai, Israel) was secured to the to the involved Achilles tendon of the tendinopathy group or dominant side of the control group with the subject in prone and ankle stabilized in neutral dorsiflexion with a mobilization belt secured around a treatment plinth. The dominant leg was determined by asking the participant which leg they would typically use to kick a ball. The temperature of the thermode was set to increase at 0.5 °C/s from a baseline of 35 °C. Participants were provided with a response indicator. The test was stopped when they perceived a change in sensation from warmth to pain, or when the temperature reached a maximum of 51 °C. The HPT was repeated after one minute rest time, and the mean temperature between trials was used for data analysis. A prior study showed moderate test-retest reliability (ICC = 0.78, SEM = 1.05 °C).31
Prior to the formal HTS test, a modified training sequence of this test was performed to the forearm of the subject to familiarize them to the protocol followed by several minutes of rest. For the HTS, the subject remained in the same testing position as the HPT. The neurosensory analyzer and thermode was utilized to apply 10 consecutive heat pulses at a rate of one pulse every three seconds to the Achilles tendon (in the tendinopathy group, at the site of PPT test; in the control group on the dominant leg at the Achilles tendon site used for the PPT test). The thermode temperature oscillated or pulsed between 42 °C and 52 °C at a rate of 10 °C/s. Participants rated their perception of the pain intensity for each heat pulse via a standardized rating visual analog scale (100mm) when verbally prompted by the investigator. The visual scale was provided with written descriptor anchors attached to a 100mm scale (0mm = no pain, 20mm = pain threshold, 100mm = worst pain imaginable), and was adapted from Staud and colleagues.16 For the HTS test, the first rating (measured in mm) was subtracted from the maximum rating of the series to quantify the amount of heat pain summation. Using this methodology for HTS, a prior study obtained a high reliability (ICC = 0.89; SEM = 6.39mm).31
STATISTICAL METHODS
Data were analyzed with SPPS (IBM Corp. Released 2013. IBM SPSS Statistics for Mac, Version 24.0. Armonk, NY: IBM Corp). Normality, homogeneity, and outliers of the data were examined. Descriptive statistics were calculated for VISA-A and LEFS scores in the AT group. Between group means ( ± standard deviations) for age and PCS were assessed with independent t-tests. Because bilateral PPT data were collected (Achilles tendon, tibialis anterior, and thenar eminence), side-to-side comparisons of PPT data for both tendinopathy and control groups were performed with independent t-tests. When no side-to-side differences were found, right and left data were pooled together for subsequent between-group analyses. This occurred for the control group Achilles PPT, the tendinopathy and control group tibialis anterior PPT, and the tendinopathy and control group thenar eminence PPT. Between group (tendinopathy vs. control) comparisons were made using an analysis of covariance (ANCOVA) with sex and age as the covariates for PPT, HPT, and HTS testing. Both age and sex differences have previously been documented for various QST measures, so ANCOVA was used for between group comparisons.32 All bilateral cases were excluded from analyses that used the non-involved Achilles PPT as part of the comparison. Homogeneity was checked and effect sizes were calculated. For all statistical calculations, p values < 0.05 were considered significant. Effect sizes from the ANCOVA's were calculated using the partial eta-squared (η2) method in SPSS; guidelines for interpretation of the magnitude of this effect size calculation are: small ≥ 0.01, medium ≥ 0.05, and large ≥ 0.14.33
RESULTS
Forty-one participants met inclusion criteria and agreed to participate in the study. All of the healthy individuals who were screened met inclusion criteria to participate. One subject was excluded from the AT group, because of a history of chronic lower back issues. There were 17 participants (8 male, 9 female) in the AT group and 24 participants (11 male, 13 female) in the control group. Three people in the tendinopathy group presented with bilateral symptoms. The mean age of the tendinopathy group was different (t(39) = 2.32, p = 0.026) from the mean age of the control group (Table 1); therefore, all between group comparisons included age as a covariate. The duration of symptoms of those in the AT group ranged from three months to eight years, with a mean of 19.76 ( ± 30.28) months. Participants in both groups had reported continuous participation in aerobic lower-extremity loading activities for a minimum of three days per week for at least the past 8 weeks. There was no significant difference in PCS found between groups; both groups had scores indicative of non-clinically relevant levels of catastrophizing (tendinopathy group mean = 10.0 ± 8.94; control group mean = 7.83 ± 4.65; t(39) = 1.02, p = .318). Participants in the tendinopathy group had a mean score of 58.5 ± 18.4 out of 100 on the VISA-A, and a mean score of 63.6 ± 8.0 out of 80 on the LEFS.
Table 1.
Group characteristics and self-reported outcome measures of participants included in the study.
| Achilles Tendinopathy Group | Control Group | |
|---|---|---|
| Number of Participants | 17 (8m, 9f) | 24 (11m, 13f) |
| Subject Age | 39.00 ± 10.81 (range 24-55) | 31.83 ± 8.92 (range 23-53) |
| PCS | 10.00 ± 8.94 (range 0-28) | 7.83 ± 4.65 (range 0-16) |
| Numeric Pain Rating With Performing 10 Repetitions of Single Leg Heel Raises | 6.88 ± 2.85 (range 2-10) | N/A |
| VISA-A | 58.47 ± 18.36 (range 19-89) | N/A |
| LEFS | 63.64 ± 8.02 (range 50-78) | N/A |
f = female; LEFS = lower extremity functional scale; m = male; PCS = pain catastrophizing scale; VISA-A = Victorian Institute of Sport Assessment Achilles.
Tables 2 and 3 present the differences between sides for PPT values at the Achilles tendon, tibialis anterior, and thenar eminence for the tendinopathy group and control group, respectively. Table 4 presents the between group differences for PPT, HPT, and HTS values using the combined right and left means from the control group participants values for statistical comparison. After examining the data plots for normality, two outlier cases in the control and one outlier case in the AT group were excluded from analysis for PPT of the Achilles tendon (Table 4).
Table 2.
Comparison of PPT values between sides for the Achilles tendinopathy group (n = 14), excluding bilateral cases (n = 3).
| Achilles Tendinopathy Group INVOLVED Side† | Achilles Tendinopathy Group NON-INVOLVED Side† | Mean Difference Between Sides† | Within group comparison (t-test) | |
|---|---|---|---|---|
| Achilles Mean PPT (kg/cm2) | 6.47 ± 3.06 (4.87, 8.07 ) | 10.45 ± 3.81 (8.45, 12.45) | 3.98 ± 1.31 (1.30, 6.66) | t(26) = -3.040, p = 0.005 |
| Anterior Tibialis Mean PPT (kg/cm2) | 7.93 ± 4.64 (5.50, 10.36) | 7.68 ± 2.79 (6.22, 9.14) | -0.25 ± 1.45 (-3.22, 2.72) | t(26) = .168, p = .868 |
| Thenar Eminence Mean PPT (kg/cm2) | 5.20 ± 2.17 (4.06, 6.34) | 5.67 ± 2.11 (4.56, 6.78) | 0.47 ± 0.81 (-1.19, 2.13) | t(26) = -.590, p = .560 |
CI = confidence interval; kg/cm2 = kilograms per centimeter squared; PPT = pressure pain threshold
Values in parentheses represent the 95% confidence interval
Table 3.
Comparison of PPT values between sides for the Healthy Control group (n = 24).
| Healthy Control Group DOMINANT Side† | Healthy Control Group NONDOMINANT Side† | Mean Difference Between Side† | Within group comparison (t-test) † | |
|---|---|---|---|---|
| Achilles Mean PPT (kg/cm2) | 12.80 ± 5.31 (10.68, 14.92) | 12.60 ± 5.57 (10.37, 14.83) | -0.20 ± 1.57 (-3.36, 2.96) | t(46) = .122, p = .903 |
| Anterior Tibialis Mean PPT (kg/cm2) | 9.92 ± 5.06 (7.90, 11.94) | 9.21 ± 4.81 (7.29, 11.13) | 0.00 ± 1.39 (-2.80, 2.80) | t(46) = .494, p = .624 |
| Thenar Eminence Mean PPT (kg/cm2) | 5.83 ± 2.38 (4.88, 6.78) | 5.45 ± 2.65 (4.39, 6.51) | 0.00 ± 0.77 (-1.54, 1.54) | t(46) = .532, p = .597 |
CI = confidence interval; kg/cm2 = kilograms per centimeters squared; PPT = pressure pain threshold
Values in parentheses represent the 95% confidence interval
Table 4.
Group means (SD) in PPT, HPT, and HTS with sex and age as covariates.
| Achilles Tendinopathy Group Mean† | Healthy Control Group Mean† | Mean Difference Between Groups† | Between Group Comparison (ANCOVA) | |
|---|---|---|---|---|
| PPT Involved Achilles Tendinopathy (n = 17) vs. Control (n = 24) | 6.11 ± 3.08 kg/cm2 (4.65, 7.57) | 12.69 ± 5.39 kg/cm2 (10.53, 14.85) | 6.58 ± 1.45 kg/cm2 (3.64, 9.52) | F(1,37) = 21.83, p<0.0001, Effect size .371‡ |
| PPT Non-Involved Achilles Tendinopathy (n = 13) vs. Control (n = 46) | 9.84 ± 3.19 kg/cm2 (8.11, 11.57) | 11.99 ± 4.24 kg/cm2 (10.76, 13.21) | 2.15 ± 1.27 kg/cm2 (-0.39, 4.69) | F(1,55) = 5.34, p = 0.025 Effect size .089‡ |
| PPT Tibialis Anterior Tendinopathy (n = 34) vs. Control (n = 48) | 7.55 ± 3.72 kg/cm2 (6.30, 8.80) | 9.57 ± 4.90 kg/cm2 (8.18, 10.96) | 2.02 ± 1.00 kg/cm2 (0.03, 4.01) | F(1,78) = 4.78, p = 0.042 Effect size .052‡ |
| PPT Thenar Eminence Tendinopathy (n = 34) vs. Control (n = 48) | 5.15 ± 2.02 kg/cm2 (4.47, 5.83) | 5.64 ± 2.50 kg/cm2 (4.93, 6.35) | 0.49 ± 0.52 kg/cm2 (-0.54, 1.52) | F(1,78) = 1.202, p = 0.276 Effect size .015‡ |
| Heat Pain Threshold Achilles Tendinopathy (n = 17) vs. Control (n = 24) | 44.69 ± 1.99 °C (43.74, 45.64) | 46.45 ± 2.68 °C (45.38, 47.52) | 1.76 ± 0.77 °C (0.21, 3.31) | F(1,37) = 5.202, p = 0.028 Effect size .123‡ |
| Temporal Summation Achilles Tendinopathy (n = 17) vs. Control (n = 24) | 43.18 ± 18.51 mm (34.38, 51.98) | 41.13 ± 18.83 mm (33.39, 48.66) | -2.05 ± 5.93 mm (-14.04, 9.94) | F(1,37) = .001, p = 0.981 Effect size .000‡ |
°C = Celsius; cm = centimeters; CI = confidence interval; kg/cm2 = kilograms per centimeters squared; PPT = pressure pain threshold, SD = standard deviation
Values in parentheses represent the 95% confidence interval
Partial Eta Squared; guidelines for the interpretation of the magnitude of effect size are: small ≥ 0.01, medium ≥ 0.05, and large ≥ 0.14.(Cohen 1988)
Peripheral Sensitization
There was a difference in PPT values between sides of the Achilles tendons in the tendinopathy group, excluding the three bilateral AT participants (involved 6.47 ± 3.09 kg/cm2; non-involved 10.45 ± 3.81 kg/cm2; t(26) = -3.04, p = 0.005; Table 2). In the between-group analysis, the AT group had a lower PPT at the involved Achilles tendon compared to the control group (F(1,37) = 21.83, p < 0.0001, partial η2 = .371) (Table 4). A lower value on PPT testing indicates an increase in sensitivity of the tested structure.
There was a difference found for HPT between the AT group (44.69 ± 1.99 °C) and control group (46.45 ± 2.68 °C) conditions (F(1,37) = 5.20, p = 0.028, partial η2 = .123) (Table 4). A lower temperature on the HPT is indicative of increased sensitivity to heat pain.
Signs of Central Sensitization
The tendinopathy group demonstrated lower PPT values over the non-involved Achilles tendon when compared to Achilles of control group (F(1,55) = 5.34, p = 0.025, partial η2 = .089); this analysis excluded the three participants with bilateral symptoms. Additionally, lower PPT values were found for the tibialis anterior in the tendinopathy group compared to the control group (F(1,78) = 4.78, p = 0.042, partial η2 = .052). No difference was found between the two groups for thenar eminence PPT (F(1,78) = 1.04, p = 0.276, partial η2 = .013) (Table 4).
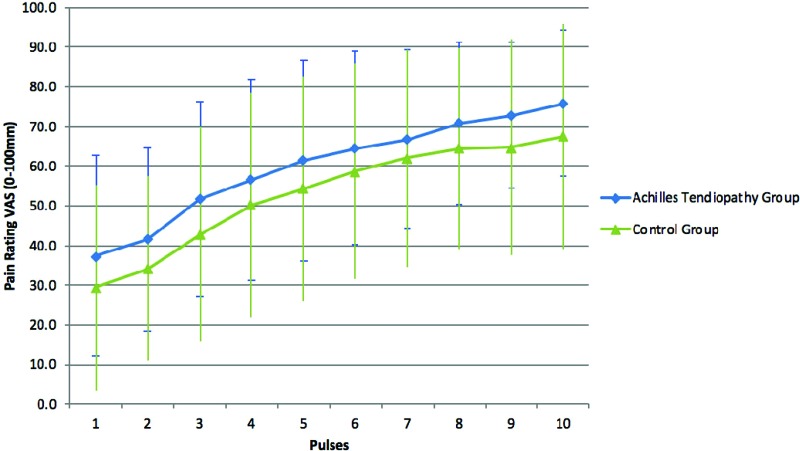
Using the HTS test to look for evidence of enhanced central pain facilitation, no difference was found between groups (AT group (43.18 ± 18.51 mm) and control group (41.13 ± 18.83 mm); F(1,37) = 0.001, p = 0.981, partial η2 = .001) (Table 4). For the HTS, the mean baseline pain rating for the AT group was 37.41 ± 25.36 mm, with a max rating mean of 74.76 ± 17.96 mm. The mean baseline pain rating for the control group was 28.33 ± 25.66 mm, with a max rating mean of 68.71 ± 27.14 mm. Figure 1 depicts the mean (with standard deviation) for both groups for all 10 heat pulses of the temporal summation test.
Figure 1.
Temporal summation data for the Achilles tendinopathy group compared to the control group for each of the 10 heat pulses.
DISCUSSION
Participants with chronic AT showed signs of both peripheral and central sensitization. The presence of secondary hyperalgesia, indicated by reduced PPT at sites not related to the injury in the AT group (the non-involved Achilles and bilaterally at the tibialis anterior), is a sign that central sensitization may have occurred. These signs of central sensitization, however, appear to be modest and not widespread into an upper extremity dermatome. PPTs have been previously studied as a means to detect changes in mechanical sensitivity of a variety of musculoskeletal conditions including tendinopathy.34-39 A systematic review by Plinsinga et al,15 concluded that an association exists between chronic tendon pain and nervous system sensitization; however the majority of the studies were in upper extremity tendinopathies. The findings of the current study also lend support to the theory for a multifactorial model of tendinopathy, which consists of an impaired motor system, local tendon pathology, and changes in the pain system.40
The finding of no difference between AT and controls for PPT of the upper extremity remote site is consistent with prior literature available for AT (thenar eminence,23 and lateral epicondyle24). However, secondary hyperalgesia was observed within the lower extremities (tibialis anterior and non-involved Achilles). The increased sensitivity recorded at the tibialis anterior in this study may be due to the tibialis anterior (dermatome L4) being in closer proximity to the spinal segments where peripheral nociceptive information is entering the dorsal horn and possibly sensitizing local neurons and microglia (Achilles dermatome S1). Evidence from an animal model of overuse indicate that spinal pro-nociceptive agents (substance-P, interleukin-1 beta) are found in elevated levels within the superficial lamina of the spinal cord dorsal horns of the spinal segments related to the limbs performing the repetitive tasks.41-43 The changes in spinal pro-noncioceptive agents have also been related to peripheral musculoskeletal tissue levels of inflammation and behavioral performance.41-43 Elliott and colleagues,43 showed that the significant elevation in spinal cord pronociceptive cytokines also occurred in the dorsal horns associated with the non-reaching forelimb. This “mirror” response also has been described in other animal models of unilateral inflammation and neuropathic pain.44,45 It has been hypothesized that the local release of the pronociceptive cytokines and neuromodulators that occurs within the involved spinal dorsal horns can spread to neighboring spinal segments,43,44 which would support our current findings of increased PPT at the uninvolved Achilles and bilaterally at the tibialis anterior.
The findings of this study are contradictory to those of Plinsinga et al,24 who found no significant group differences in PPT locally (at the involved Achilles) between participants with and without AT. Both the current study and that of Tompra and colleagues,23 found lower involved Achilles PPT compared to a control group. Although Plinsinga and colleagues,24 did not find lower PPT at the involved Achilles tendon, pain with palpation of the involved Achilles tendon (compared to non-involved) is one of the diagnostic hallmarks of AT.25 In the study by Plinsinga et al,24, a standardized location for PPT on the Achilles was utilized, whereas the current study and Tompra et al,23 performed the PPT at the most symptomatic location on the tendon, which was identified during initial screening. In addition, the ankle position was fixed at neutral plantarflexion/dorsiflexion for testing in the current study and in that of Tompra et al,23 but Plinsinga et al,24 did not describe controlling the ankle position during testing. If ankle position was not controlled, this may have affected their ability to detect differences in PPT because the force from the PPT would be partially dissipated and transferred into muscle-tendon unit deformation and talocrural movement.
The current study found an increase in sensitivity to heat (lower HPTs; primary heat hyperalgesia) at the involved Achilles tendon among individuals with chronic AT. Prior reports have found that lower heat pain thresholds occur during inflammatory conditions in experimental human and animals models.46-48 Mechanistically, lowering of HPTs is thought to occur via various tissue inflammatory molecules interacting with the TRPV1 (transient receptor potential-vanilloid 1) receptors found on type II A-delta and C fibers.46 While beyond the scope of this study, the finding of lower HPTs would be consistent with the findings of greater numbers of inflammatory cells and molecules in studies of human AT,49-51 and in animal models of voluntary upper extremity overuse during the early phase (weeks 6 and 12) of increased loading.41-43 The findings of this current study contradict those by Plinsinga et al,24 who did not find a significant difference in HPT at the Achilles tendon between controls and those with AT. Plinsinga and colleagues,24 used a faster rate of rise of temperature of 1 °C/s compared to our 0.5 °C/s protocol. The faster rate of rise of temperature could cause the experimenter to miss smaller differences in HPT. Consistent with this, both tendinopathy and control participants in our study had lower HPTs (44.69 ± 1.99 °C & 46.45 ± 2.68 °C) in comparison to Plinsinga and colleagues,24 (48.51 ± 2.84 °C & 48.19 ± 1.76 °C).
The current study did not find any evidence for enhanced central facilitation of pain as observed through HTS testing.16,17 Elevated temporal summation of pain is considered to be another potential sign of central sensitization.16,17 Tests that employ delivering repetitive stimuli at a rate of at least 0.3 stimuli/sec generate a progressive increase in action potential output from dorsal horn neurons in animal models;52 therefore, this protocol is believed to affect the transmission neurons in the dorsal horn in humans. Temporal summation protocols, however, may also result in enhanced activity in other regions of the of the CNS involved in pain processing.53 Despite the AT group having lower HPTs, the perceptual magnitude of the increase in pain sensation during HTS was not different between groups. Although differences in temporal summation have been previously seen in people with fibromyalgia,16 osteoarthritis,54 temporomandibular disorder,55 chronic post-mastectomy pain,56 and migraine,57 it has rarely been assessed in chronic tendinopathies.
Limitations of this study include a sample of convenience and a relatively small sample size, although it was similar in size to other recent reports.23,24 This study did not assess heat pain threshold outside of the local site of pain or conditioned pain modulation, which would have yielded additional information about changes in pain sensitivity/modulation and allowed additional comparison to other recent studies. Both groups were made up of primarily active individuals, with the tendinopathy group continuing to run through their symptoms; therefore, the results of the study might not apply to a more sedentary population of individuals. Another limitation is the lack of blinding of the assessors, which is a possible risk of bias in this study; however, all tests were performed using standardized instructional sets explaining the test procedures, and rate of application of stimuli were controlled. Lastly, no diagnostic ultrasound or magnetic resonance imaging was available during the time of the study that could have more precisely aided in the diagnosis of AT.
Future research should be directed at describing the changes in quantitative sensory testing using a comprehensive battery of tests with larger sample sizes. Given the prior studies published, careful consideration of the testing methodologies must be given, which may explain some of the discrepancies.23,24 Additional directions of future research should examine the possible role of quantitative sensory testing for the prognosis of outcomes after conservative treatment of AT, and the potential ability of interventions used to treat chronic tendinopathies to resolve peripheral and central sensitization.
CONCLUSIONS
Participants with chronic AT showed signs of both peripheral and central sensitization; however, widespread hyperalgesia into the upper extremities and elevated temporal summation was not observed. The evidence from this study and that of Tompra et al,23 support the concept that some changes in pain sensitivity may be present in chronic AT. These findings also lend support to the theory for a multifactorial model of tendinopathy, which consists of an impaired motor system, local tendon pathology, and changes in the pain/nociceptive system.22,40,58
REFERENCES
- 1.Knobloch K Yoon U Vogt PM. Acute and overuse injuries correlated to hours of training in master running athletes. Foot Ankle Int. 2008;29(7):671-676. [DOI] [PubMed] [Google Scholar]
- 2.Kujala UM Sarna S Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135. [DOI] [PubMed] [Google Scholar]
- 3.Scott A Huisman E Khan K. Conservative treatment of chronic Achilles tendinopathy. Can Med Assoc J. 2011;183(10):1159-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silbernagel KG Thomee R Eriksson BI Karlsson J. Full symptomatic recovery does not ensure full recovery of muscle-tendon function in patients with Achilles tendinopathy. Br J Sports Med. 2007;41(4):276-280; discussion 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajhede-Knudsen M Ekstrand J Magnusson H Maffulli N. Recurrence of Achilles tendon injuries in elite male football players is more common after early return to play: an 11-year follow-up of the UEFA Champions League injury study. Br J Sports Med. 2013;47(12):763-768. [DOI] [PubMed] [Google Scholar]
- 6.Kvist M. Achilles tendon injuries in athletes. Ann Chir Gynaecol. 1991;80(2):188-201. [PubMed] [Google Scholar]
- 7.Paavola M Kannus P Jarvinen TA Khan K Jozsa L Jarvinen M. Achilles tendinopathy. J Bone Joint Surg Am. 2002;84-A(11):2062-2076. [DOI] [PubMed] [Google Scholar]
- 8.Abate M Silbernagel KG Siljeholm C, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11(3):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paavola M Kannus P Paakkala T Pasanen M Jarvinen M. Long-term prognosis of patients with achilles tendinopathy. An observational 8-year follow-up study. Am J Sports Med. 2000;28(5):634-642. [DOI] [PubMed] [Google Scholar]
- 10.Cook JL Khan KM Purdam C. Achilles tendinopathy. Man Ther. 2002;7(3):121-130. [DOI] [PubMed] [Google Scholar]
- 11.Malliaras P Cook J. Patellar tendons with normal imaging and pain: change in imaging and pain status over a volleyball season. Clin J Sport Med. 2006;16(5):388-391. [DOI] [PubMed] [Google Scholar]
- 12.Rio E Moseley L Purdam C, et al. The pain of tendinopathy: physiological or pathophysiological? Sports Med. 2014;44(1):9-23. [DOI] [PubMed] [Google Scholar]
- 13.Rees JD Maffulli N Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867. [DOI] [PubMed] [Google Scholar]
- 14.Coronado RA Simon CB Valencia C George SZ. Experimental pain responses support peripheral and central sensitization in patients with unilateral shoulder pain. Clin J Pain. 2014;30(2):143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plinsinga ML Brink MS Vicenzino B van Wilgen CP. Evidence of nervous system sensitization in commonly presenting and persistent painful tendinopathies: A systematic review. J Orthop Sports Phys Ther. 2015;45(11):864-875. [DOI] [PubMed] [Google Scholar]
- 16.Staud R Vierck CJ Cannon RL Mauderli AP Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1-2):165-175. [DOI] [PubMed] [Google Scholar]
- 17.Yarnitsky D Granot M Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155(4):663-665. [DOI] [PubMed] [Google Scholar]
- 18.Gwilym SE Oag HC Tracey I Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br. 2011;93(4):498-502. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj P Bajaj P Graven-Nielsen T Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001;93(2):107-114. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill S Manniche C Graven-Nielsen T Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain. 2007;11(4):415-420. [DOI] [PubMed] [Google Scholar]
- 21.Heales LJ Lim EC Hodges PW Vicenzino B. Sensory and motor deficits exist on the non-injured side of patients with unilateral tendon pain and disability--implications for central nervous system involvement: a systematic review with meta-analysis. Br J Sports Med. 2014;48(19):1400-1406. [DOI] [PubMed] [Google Scholar]
- 22.Chang YJ Kulig K. The neuromechanical adaptations to Achilles tendinosis. J Physiol. 2015;593(15):3373-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tompra N van Dieën JH Coppieters MW. Central pain processing is altered in people with Achilles tendinopathy. Br J Sports Med. 2016;50(16):1004-1007. [DOI] [PubMed] [Google Scholar]
- 24.Plinsinga ML van Wilgen CP Brink MS, et al. Patellar and Achilles tendinopathies are predominantly peripheral pain states: a blinded case control study of somatosensory and psychological profiles. Br J Sports Med. 2018;52(5):284-291. [DOI] [PubMed] [Google Scholar]
- 25.Martin RL Chimenti R Cuddeford T, et al. Achilles Pain, Stiffness, and Muscle Power Deficits: Midportion Achilles Tendinopathy Revision 2018. J Orthop Sports Phys Ther. 2018;48(5):A1-A38. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MGL Bishop S Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995(7):524-532. [Google Scholar]
- 27.Lamé IE Peters ML Kessels AG Van Kleef M Patijn J. Test--retest stability of the Pain Catastrophizing Scale and the Tampa Scale for Kinesiophobia in chronic pain over a longer period of time. J Health Psychol. 2008;13(6):820-826. [DOI] [PubMed] [Google Scholar]
- 28.Sluka KA. Mechanisms and Management of Pain for the Physical Therapist. Second ed. Philadelphia: Wolters Kluwer; 2016. [Google Scholar]
- 29.Robinson JM Cook JL Purdam C, et al. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35(5):335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binkley JM Stratford PW Lott SA Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79(4):371-383. [PubMed] [Google Scholar]
- 31.Stackhouse SK Taylor CM Eckenrode BJ Stuck E Davey H. Effects of noxious electrical stimulation and eccentric exercise on pain sensitivity in asymptomatic individuals. Phys Med Rehabil. 2015;8(5):415-424. [DOI] [PubMed] [Google Scholar]
- 32.Rolke R Baron R Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231-243. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 34.Alburquerque-Sendín F Camargo PR Vieira A Salvini TF. Bilateral myofascial trigger points and pressure pain thresholds in the shoulder muscles in patients with unilateral shoulder impingement syndrome: a blinded, controlled study. Clin J Pain. 2013;29(6):478-486. [DOI] [PubMed] [Google Scholar]
- 35.Coombes BK Bisset L Vicenzino B. Thermal hyperalgesia distinguishes those with severe pain and disability in unilateral lateral epicondylalgia. Clin J Pain. 2012;28(7):595-601. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Carnero J Fernandez-de-Las-Penas C de la Llave-Rincon AI Ge HY Arendt-Nielsen L. Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: a blinded, controlled study. Clin J Pain. 2009;25(7):555-561. [DOI] [PubMed] [Google Scholar]
- 37.Hidalgo-Lozano A Fernández-de-las-Peñas C Alonso-Blanco C Ge HY Arendt-Nielsen L Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Exp Brain Res. 2010;202(4):915-925. [DOI] [PubMed] [Google Scholar]
- 38.Jespersen A Amris K Graven-Nielsen T, et al. Assessment of pressure-pain thresholds and central sensitization of pain in lateral epicondylalgia. Pain Med. 2013;14(2):297-304. [DOI] [PubMed] [Google Scholar]
- 39.van Wilgen CP Konopka KH Keizer D Zwerver J Dekker R. Do patients with chronic patellar tendinopathy have an altered somatosensory profileϿ. A Quantitative Sensory Testing (QST) study. Scand J Med Sci Sports. 2013;23(2):149-155. [DOI] [PubMed] [Google Scholar]
- 40.Coombes BK Bisset L Vicenzino B. A new integrative model of lateral epicondylalgia. Br J Sports Med. 2009;43(4):252-258. [DOI] [PubMed] [Google Scholar]
- 41.Barbe MF Gallagher S Massicotte VS Tytell M Popoff SN Barr-Gillespie AE. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord. 2013;14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott MB Barr AE Clark BD Amin M Amin S Barbe MF. High force reaching task induces widespread inflammation, increased spinal cord neurochemicals and neuropathic pain. Neuroscience. 2009;158(2):922-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott MB Barr AE Clark BD Wade CK Barbe MF. Performance of a repetitive task by aged rats leads to median neuropathy and spinal cord inflammation with associated sensorimotor declines. Neuroscience. 2010;170(3):929-941. [DOI] [PubMed] [Google Scholar]
- 44.Chacur M Milligan ED Gazda LS, et al. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94(3):231-244. [DOI] [PubMed] [Google Scholar]
- 45.Milligan ED Twining C Chacur M, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23(3):1026-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basbaum AI Bautista DM Scherrer G Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janum S Nielsen ST Werner MU Mehlsen J Kehlet H Møller K. Pain perception in healthy volunteers: effect of repeated exposure to experimental systemic inflammation. Innate Immun. 2016;22(7):546-556. [DOI] [PubMed] [Google Scholar]
- 48.Welsh EM Nolan AM. Repeated intradermal injection of low-dose carrageenan induces tachyphylaxis to evoked hyperalgesia. Pain. 1994;59(3):415-421. [DOI] [PubMed] [Google Scholar]
- 49.Legerlotz K Jones ER Screen HR Riley GP. Increased expression of IL-6 family members in tendon pathology. Rheumatology. 2012;51(7):1161-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kragsnaes MS Fredberg U Stribolt K Kjaer SG Bendix K Ellingsen T. Stereological quantification of immune-competent cells in baseline biopsy specimens from achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years. Am J Sports Med. 2014;42(10):2435-2445. [DOI] [PubMed] [Google Scholar]
- 51.Klatte-Schulz F Minkwitz S Schmock A, et al. Different Achilles tendon pathologies show distinct histological and molecular characteristics. Int J Mol Sci. 2018;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price DD Hull CD Buchwald NA. Intracellular responses of dorsal horn cells to cutaneous and sural nerve A and C fiber stimuli. Exp Neurol. 1971;33(2):291-309. [DOI] [PubMed] [Google Scholar]
- 53.Staud R Craggs JG Robinson ME Perlstein WM Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129(1-2):130-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arendt-Nielsen L Nie H Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573-581. [DOI] [PubMed] [Google Scholar]
- 55.Sarlani E Grace EG Reynolds MA Greenspan JD. Evidence for up-regulated central nociceptive processing in patients with masticatory myofascial pain. J Orofac Pain. 2004;18(1):41-55. [PubMed] [Google Scholar]
- 56.Edwards RR Mensing G Cahalan C, et al. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manage. 2013;46(1):30-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weissman-Fogel I Sprecher E Granovsky Y Yarnitsky D. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003;104(3):693-700. [DOI] [PubMed] [Google Scholar]
- 58.Kulig K Chang YJ Winiarski S Bashford GR. Ultrasound-based tendon micromorphology predicts mechanical characteristics of degenerated tendons. Ultrasound Med Biol. 2016;42(3):664-673. [DOI] [PubMed] [Google Scholar]