Abstract
The incentive salience sensitization (ISS) theory of addiction holds that addictive behavior stems from the ability of drugs to progressively sensitize the brain circuitry that mediates attribution of incentive salience (IS) to reward-predictive cues and its behavioral manifestations. In this article, we establish the plausibility of ISS as an etiological pathway to alcohol use disorder (AUD). We provide a comprehensive and critical review of evidence for: (1) the ability of alcohol to sensitize the brain circuitry of IS attribution and expression; and (2) attribution of IS to alcohol-predictive cues and its sensitization in humans and non-human animals. We point out gaps in the literature and how these might be addressed. We also highlight how individuals with different alcohol subjective response phenotypes may differ in susceptibility to ISS as a pathway to AUD. Finally, we discuss important implications of this neuropsychological mechanism in AUD for psychological and pharmacological interventions attempting to attenuate alcohol craving and cue reactivity.
Keywords: incentive salience, sensitization, alcohol use disorder, cue reactivity, craving, relapse, treatment, etiology, alcohol sensitivity
1. Introduction
Of all substances of abuse, alcohol use is the most common and, arguably, the substance with the greatest combined cost to individuals and society (Nutt et al., 2010, 2007). Although many individuals are able to use alcohol without developing an alcohol use disorder (AUD), results from the 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions III (NESARC-III) suggest that approximately 73% of all non-institutionalized adults in the USA (≥18 years of age) used alcohol at least once in the past year and that 15% those who used alcohol in the past year met diagnostic criteria for AUD (Grant et al., 2017). This means that, in the USA alone, the number of adult alcohol users with active AUD in any given year is larger than the estimated total number of living adults residing in any one state or territory except California (27,432, 000 or 11% of the approximate 254, 000, 000 adults estimated to be living in the USA in 2018; Census Bureau, Population Division). Heavy alcohol use put individuals at greater risk for cancers and cardiovascular disease (Connor, 2017; Wood et al., 2018). The myriad negative medico-legal consequences of excessive alcohol use, such as assault, automobile accidents, gun accidents, rape, suicides, and unwanted pregnancies, create serious costs to society (Bouchery et al., 2011; Hingson et al., 2009; Naimi et al., 2003; Smith et al., 1999; Walsh and Macleod, 1982; Wechsler et al., 2002; Whiteford et al., 2013; Wintemute, 2015). Consequently, there is a great need to understand the biomedical and psychosocial factors that determine excessive alcohol use, including the development of AUD.
An unfortunate reality that hinders this scientific mission is heterogeneity in the clinical presentation and course of AUD (Babor et al., 1992; Cloninger, 1987; Jellinek, 1960). This heterogeneity has been met with numerous attempts to match different “subtypes” of AUD with different treatment approaches, but largely with disappointing results (Allen et al., 1998, 1997). Current approaches to understanding AUD heterogeneity and its consequences for both theory and intervention are informed by broader frameworks for understanding all manner of psychiatric conditions. In particular, the National Institute of Mental Health (NIHM) research domain criteria (RDoC) framework (Insel et al., 2010; Insel and Cuthbert, 2015) has provided an architecture with which multiple causal factors stemming from diverse, biologically grounded systems can be organized for understanding the development and progression of AUD. The RDoC framework emphasizes studying the putative underlying causes (e.g., dysregulated neural circuits) of disorders and aims to generate biologically-meaningful, comprehensive descriptions that might form the basis for new classification schemes, which may be dimensional rather than categorical. The application of the RDoC framework to understanding heterogeneity in AUD has been termed the Alcohol Addiction RDoC (AARDoC) (Litten et al., 2015). The AARDoC organizes research across units of analysis (viz., from genes to brain to behavior, including self-report) relevant to AUD liability and promotive processes, and may lead to useful insights about etiology and treatment of AUD.
One of the research domains proposed for the AARDoC is the extent to which alcohol-associated cues acquire “incentive salience.” 1 Incentive salience is a construct that refers to the motivational significance attributed to exteroceptive and interoceptive stimuli that reliably predict rewards, via Pavlovian conditioning (Bevins and Besheer, 2014; Paulus et al., 2009; Robinson et al., 2014; Robinson and Berridge, 1993; Saunders and Robinson, 2013). Among the exteroceptive stimuli that can come to predict ethanol ingestion and/or intoxication are the sight, smell, and taste of a preferred alcoholic beverage, the implements used to contain, prepare, and/or consume the beverage, and the sounds of accessing and/or transferring a container’s contents. Given that experiencing the diverse interoceptive stimuli involved in or produced by beverage ingestion, including the pharmacological effects of ethanol, is contingent upon the individual’s interaction with or manipulation of the alcoholic beverage, its container, and other implements, these cues are especially likely to acquire incentive salience (Tomie, 1996; Tomie and Sharma, 2013). Nevertheless, context matters—the ability of these alcohol-associated cues to promote alcohol use in daily life is likely greatest when they are encountered at the “right” place and time, around the “right” people, and in the “right” emotional state (Marlatt, 1996; Niaura et al., 1988).
One neurobiological theory of addiction that directly addresses how drug-associated cues come to affect individuals’ behavior is the incentive salience sensitization theory of addiction (ISST) (Berridge et al., 2009; Berridge and Robinson, 2016, 2003; Robinson and Berridge, 2001, 2000, 1993). The ISST holds that different brain circuits are responsible for attributing hedonic versus incentive value to cues and rewards. The ISST posits that addictive behavior stems from the ability of drugs to progressively sensitize the brain circuitry responsible for attributing IS, such that the individual becomes hyper-reactive to the motivational properties of learned drug-predictive stimuli.2 Critically, the drug-induced sensitization of IS attribution to drug-predictive cues is theorized to progress independently of changes in the hedonic value attributed to either the drug or its cues. The dissociation of hedonic and incentive value can help explain why some people may verbalize explicit goals and reasons for abstaining from or moderating alcohol use, and yet: (1) find themselves drawn toward alcoholic beverages or people and places associated with alcohol use (e.g., bars, neighborhood pub, old drinking buddies); or (2) find it difficult to stop drinking after their first drink; or (3) find it nearly impossible to stop thinking about alcohol under certain circumstances (e.g., after stressful events, certain times of day or the week). Through alcohol-induced incentive salience sensitization (ISS), alcohol-associated cues may be increasingly imbued with the power to instigate alcohol seeking and consumption despite a person’s conscious beliefs, goals, and intentions. For this reason, ISS may not only be a mechanism of AUD development but also maintenance and relapse.
In this article, we argue for ISS as a mechanism relevant to AUD in humans. In order to do so, we first provide a summary review of the neural circuitry theorized to mediate attribution of incentive salience (IS) to reward-predictive cues and the behavioral and psychological manifestations of this process. Readers interested in more in-depth coverage of the neurobiology are referred to (Flagel and Robinson, 2017). We then review evidence from work with preclinical non-human animal models for the ability of ethanol, the addictive agent in alcoholic beverages, to induce neuroadaptations that may mediate ISS. Finally, we review evidence for IS attribution to alcohol-associated cues and its sensitization (ISS) in humans and non-human animals.
1.1. Scope and limitations
The current narrative review is not intended as a comprehensive or exhaustive review of all possible scientific evidence that speaks to the potential existence of a unique etiological pathway to AUD captured by the neuropsychological mechanism delineated in the ISST. Rather, we intend to provide an initial and illustrative survey of that body of evidence. The relevance of ISS as a neuropsychological mechanism in AUD could be inferred from its inclusion as an addiction-promotive process in the binge/intoxication and preoccupation stages of the Three Stages of Addiction Cycle model (Koob and Volkow, 2010). However, to our knowledge, there have been no prior reviews of ISS as a mechanism in AUD; although, the idea is not new (Heinz, 2002). All previous reviews and theoretical papers on ISST have focused on food or other drug rewards, or when focused on alcohol, cover only pre-clinical data (Valyear et al., 2017). Many commonalities are observed across appetitive stimuli such as food, drugs of abuse, and sex; nevertheless, there are important differences in how these different classes of appetitive stimuli are processed by the brain (Berridge and Kringelbach, 2015; Sescousse et al., 2013). Furthermore, the unique pharmacology of different drugs of abuse means that they are not interchangeable “addictive” stimuli (Badiani et al., 2011; Ozburn et al., 2015); it is thus important to establish a case for the plausibility of the ISST with respect to the specific drug of abuse.
The evidence we review here spans multiple units of analysis in humans and non-human animal models. The reader should not infer from our omission of any particular, relevant study in this review that said study is inadmissible as evidence supporting or disconfirming predictions derived from ISST. That being said, much of the primary scientific literature reviewed here was discovered using a two-step procedure. In the first step, we entered search terms as Boolean strings in Internet search databases (EBSCOhost Academic Search Complete: MEDLINE, PsycARTICLES, PsycINFO; Google Scholar; PubMed). These Boolean strings were always composed of [(“alcohol” OR “alcoholic” OR “ethanol” OR “ethyl alcohol”)] plus other search terms that varied by the level of analysis and concept (e.g., “adaptation”, “approach”, “attention”, “incentive”, “reactivity”) as well as whether we were interested in discovering studies involving human participants or non-human animal models. In the second step, we mined the bibliographies of any relevant discovered articles for additional hits. Only studies published in peer-reviewed scientific journals by August 2019 were included.
Readers familiar with ISST (Berridge and Robinson, 2016, 2003; Robinson and Berridge, 2001, 2000, 1993) will note the absence of studies on alcohol-related psychomotor sensitization in the present review. This omission was deliberate. An excellent review on this specific body of work recently was conducted by others (Nona et al., 2018).
2. The incentive salience (IS) circuitry
2.1. Attribution of IS
Attribution of IS to reward-predictive cues is mediated by the mesocorticolimbic dopamine system (Saunders et al., 2018), which is comprised of the ventral tegmental area (VTA) complex and its efferent projections. The VTA complex is a midbrain structure that includes neurons in the lateral and posterior aspects of the ventral tegmental area as well as in the medial aspects of the substantia nigra pars compacta (Yetnikoff et al., 2014). Dopamine release from the VTA complex projections into those structures modulates on-going activity as well as short- and long-term plasticity at the synaptic and cellular levels (Calabresi et al., 2007; Greengard et al., 1999; Lisman et al., 2011; Missale et al., 1998; O’Donnell, 2003; Shen et al., 2008; West and Grace, 2002). Conservation of the VTA complex circuitry across vertebrates establishes the relevance of behavioral neuroscience work on IS attribution and ISS in non-human animals to humans (Boyson et al., 1986; Gaspar et al., 1989; Kubikova et al., 2010; Lavoie et al., 1989; Perez-Fernandez et al., 2014; Smeets et al., 1986).
2.2. Manifestation (expression) of IS
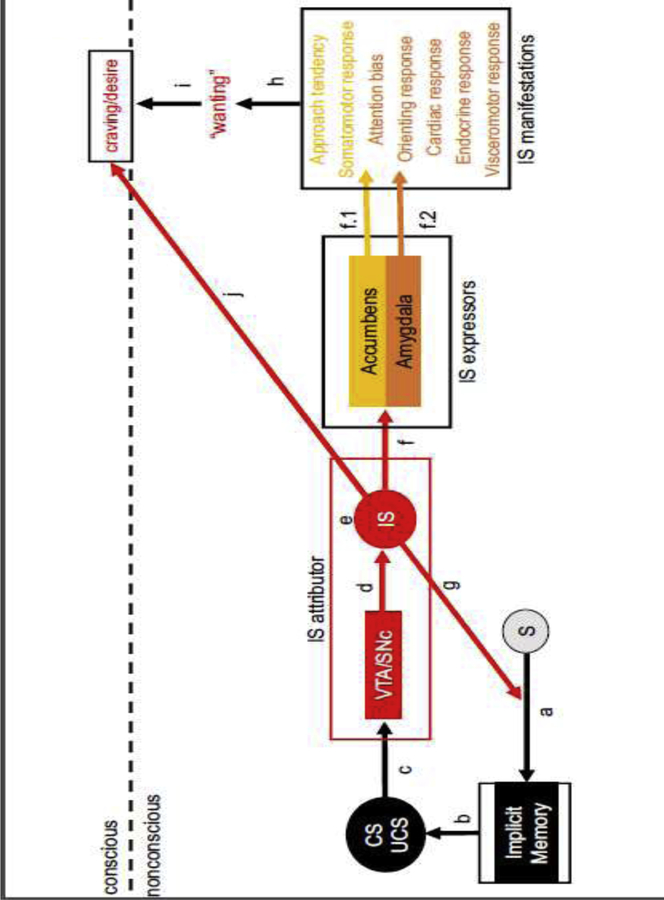
Detection of a reward-predictive cue may automatically activate implicit associations (path a->b in Figure 1) involving attitudes (i.e., evaluations) (Bargh et al., 1992), outcome expectancies, and goals (i.e., motivational tendencies) (Bargh et al., 2001). The attribution of IS to a cue (path c->d->e in Figure 1) may not only reinforce its ability automatically activate implicit associations in long-term memory (path g in Figure 1), but also may amplify the behavioral consequences of implicit associations (path f in Figure 1) including nonconscious attentional biases (Kappenman et al., 2013; Mogg et al., 1997) and nonconscious approach tendencies (Chen and Bargh, 1999). For example, attribution of IS to a cue may amplify its ability to impel approach and action, which may manifest as enhanced evaluative and response planning at the level of neural processing, covertly as a facilitation or priming of response channels leading to pre-potent responses or faster response times, and/or overtly as movement of the individual toward the cue/reward object and/or engagement with it. These skeletomotor manifestations of IS attribution (path f->f.1 in Figure 1) are mediated by an evolutionarily conserved expression circuitry anchored at the nucleus accumbens, a component of the subcortical structure known as the ventral striatum in the rostral forebrain that is situated to integrate diverse functional input from the amygdalar nuclei, cortices, hippocampus, hypothalamus, and thalamus with the IS signal from the VTA complex and to engage the appropriate response systems via the basal ganglia (Cho et al., 2013; Groenewegen et al., 1999; Haber, 2003; Haber et al., 2000; Hasue and Shammah-Lagnado, 2002; Parent and Hazrati, 1995; Reynolds and Zahm, 2005; Zahm et al., 1999).
Figure 1.
The incentive salience (IS) attribution and expression system: a simplified schematic. “S” refers to an exteroceptive or interoceptive stimulus. “CS” and “UCS” refer to the Pavlovian conditional stimulus (cue) and unconditional stimulus (reward), respectively. Inspired by Figure 2 in Robinson & Berridge (1993).
Similarly, attribution of IS to a cue may amplify its ability to capture attention, and this may manifest as enhanced attention at the level of neural processing, an overt orienting response that turns the individual toward the cue/reward object and/or increases visual gaze shifts or fixation on it, and/or a covert orienting response that involves coordinated changes in autonomic physiology. These attentional manifestations of IS attribution (path f->f.2 in Figure 1) are mediated by an evolutionarily conserved expression circuitry anchored at the central nucleus of the amygdala, a subcortical structure in the temporal lobe that is situated to integrate diverse functional input from the cortices, hypothalamus, other amygdalar nuclei, thalamus, and brainstem with the IS signal from the VTA complex, and to engage the appropriate systems in the hypothalamus and brainstem (El-Amamy and Holland, 2007, 2006; Hasue and Shammah-Lagnado, 2002; Lee et al., 2011; Veening et al., 1984; Zahm et al., 1999).
The constellation of endocrine, skeletomotor, and visceromotor IS manifestations may constitute a subconscious biobehavioral appetitive-motivational state of “wanting” (path h in Figure 1). In non-human animals, the cue-triggered “wanting” state is theorized be responsible for the ability of reward-related cues to invigorate actions that are instrumental to seeking or consuming the reward as well as to sustain reward seeking actions in spite of reward omission, punishment, increases in the effort required, or changes to the form the action must take. Given that both IS attribution and expression systems are highly conserved across species, we and others believe that cue-triggered “wanting” is also likely to be conserved across species. Thus, cue-triggered “wanting” may make a person more inclined to act toward the implicitly activated goal, which is to obtain the reward predicted by the cue, and more able to adjust their goal-directed actions according to situational demands.
Some readers may be satisfied to stop here given that much of behavior in humans and non-human animals alike is likely governed by bottom-up, implicit cognitive processes that operate below conscious awareness (Bargh, 2016, 2008; Bargh and Ferguson, 2000; Bargh and Morsella, 2008; Custers and Aarts, 2010; Lewicki et al., 1992). Nevertheless, the subjective experience of desire and craving (at the level of consciousness) is an important phenomenon in humans, and especially relevant to addiction and the ISST. In keeping, human neuroimaging studies of alcohol cue reactivity (reviewed later in Section 4.2.2) tend to find a significant, positive relationship between the level of activation induced by alcohol cues in a person’s IS system and that person’s self-report of the intensity of desire or craving for alcohol induced by those cues ( (Filbey et al., 2008; Fryer et al., 2013; Myrick et al., 2004; Oberlin et al., 2016; Schacht et al., 2013; Tapert et al., 2004; Wiers et al., 2015c); but see: (Ames et al., 2014b; Grüsser et al., 2004; Kim et al., 2014)). Thus, we next propose two ways by which alcohol-associated cue activation of the IS system may drive the emergence of the subjective experience of alcohol-related desire and thought in humans.
According to the Dynamical Model of Desire (Hofmann and Van Dillen, 2018, 2012), which expands on the Elaborated Intrusion Theory of Desire (Kavanagh et al., 2005), subjective experience of a desire for the reward predicted by some cue emerges in a person’s consciousness (enters working memory) when neural representations of the cue or the reward or their association capture (neural) attentional resources in excess of some threshold. Below that threshold, a cue can only affect the person’s behavioral output via bottom-up, implicit mechanisms (e.g., “wanting”). Thus, it is possible that cue-triggered “wanting” becomes conscious craving when the nonconscious, implicitly-activated behavioral tendencies entailed in “wanting” are interrupted (path h->i in Figure 1) because behavioral conflict quickly captures attentional resources (see: Braver, 2012; Saunders et al., 2017; Yeung et al., 2004). In the case of alcohol, this “indirect” pathway to conscious craving may be at work when something impedes successful completion of cue-triggered alcohol seeking (e.g., driving or walking by liquor store, but not going in to make a purchase; reaching for a glass, but finding that it has been taken away or that it is empty). It is in these moments that a person may not only become aware of the nonconscious alcohol seeking behaviors that were interrupted, but also of the altered physiological state in which they find themselves, and conclude that they are (or were) experiencing an urge to drink alcohol (Tiffany and Conklin, 2000).
A “direct” pathway from cue detection and IS attribution to the subjective experience of desire or craving may also exist (path j in Figure 1). Detection of a reward-predictive cue may activate, in parallel to implicit associations, explicit associations in long-term memory, which immediately enter working memory, and thereby bringing into consciousness cue- or reward-related attitudes, expectancies, goals, and other thoughts. Attribution of IS to the representation of the cue in working memory may increase the likelihood that cue-elicited thoughts capture the focus of attention in consciousness. When the reward in question is alcohol, the “direct” pathway may be at work when a person begins to think about alcoholic beverages, alcohol use, or positive alcohol use outcomes after briefly encountering an alcohol-associated exteroceptive cue in the course of daily life.
3. Evidence for ethanol-induced adaptation of the IS circuitry
According to Robinson & Berridge (1993, 2001), the core criteria for establishing that a drug is able to induce ISS are simple: first, the drug must engage the IS system, and second, its repeated administration should induce sensitization, a non-associative learning process, in the IS system (at the neurobiological level) in a gradual or incremental manner. Inquiry into the neurobiological substrates of sensitization in general, and substance-induced sensitization of the IS system in specific, is an active area of research in neuroscience (e.g., (Areal et al., 2019; Hersman et al., 2019; Stevenson et al., 2019; Weber et al., 2019)). However, some details are clear. The adaptations that mediate ISS are persistent changes at the cellular (e.g., changes in dendritic morphology) or inter-cellular level (e.g., changes in pre- and post-synaptic molecular elements such as voltage-gated ion channels, ionotropic neurotransmitter receptor proteins, neurotransmitter synthesis enzymes and transporter proteins, metabotropic neurotransmitter receptor proteins) that necessarily require changes in gene expression.3 Persistent functional cellular adaptations (e.g., increased intrinsic excitability, increased neurotransmitter release) entail persistent circuit-level adaptations (e.g., increased excitatory tone, decreased inhibitory tone), which in turn, entail persistent system-level adaptations.4 Despite all of this, the consequences of a sensitized IS system may only be expressed or manifest in behavior in specific contexts (Leyton, 2007; Vezina and Leyton, 2009). Under certain circumstances, cue- or context-conditioned compensatory (opponent) processes that mediate behavioral tolerance to the effects drugs (e.g., (Weise-Kelly and Siegel, 2001)) may mask the expression of sensitized in cue-conditioned appetitive responses (e.g., (Dalia et al., 1998)).
Consequently, an important issue in establishing ISS as a potential mechanism in AUD is the ability of ethanol exposure to induce persistent adaptations in the IS circuitry, especially adaptations that might underlie non-associative sensitization of the IS system, particularly its functional response to alcohol-associated cues. In this section, we review evidence for adaptations in the IS circuitry of preclinical non-human animal models with ethanol experience that may mediate alcohol cue ISS (Figure 2) in both humans and other animals.
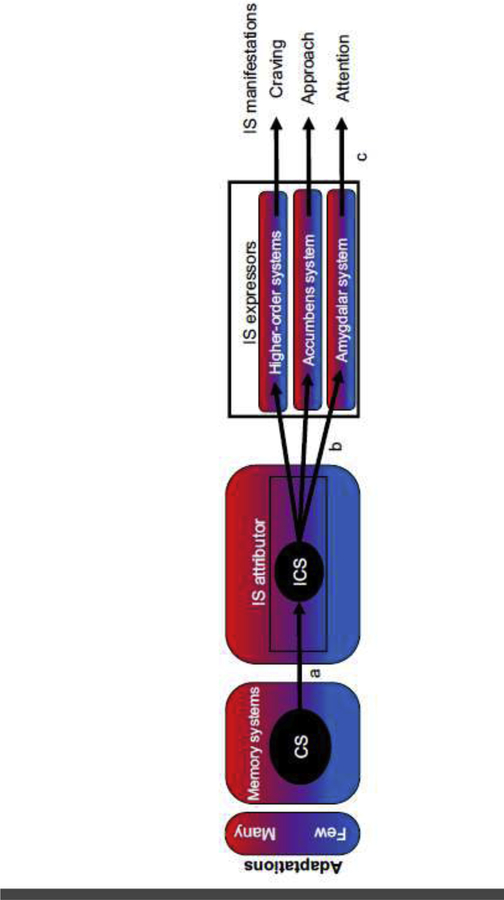
Figure 2.
Paths mediating incentive salience (IS) sensitization (ISS). Components: memory systems, IS attributor, IS expressors, response effector systems. A-type paths: input from memory systems into the IS attributor. B-type paths: IS attributor output to the IS expressors. C-type paths: IS expressors output to response effector systems that are responsible for manifestations of IS in behavioral output across levels of biological organization. The vulnerability of the different paths and IS system components to the adaptations mediating alcohol cue ISS is theorized to increase progressively over the alcohol use history as a function of the frequency, intensity, and pattern of drinking. A 3-color gradient is used to represent an increasing extent or number of adaptations accrued within each component. Adaptations may occur in one component without occurring in another and the accrual rate may vary by component.
In considering this evidence, it is necessary to keep in mind the intensity, frequency, and duration of ethanol exposure. In models involving chronic high-intensity exposure, it is necessary to consider when biological or physiological measurements are made relative to the last exposure, because these exposure paradigms are able to induce dependence, as evidenced by signs of acute withdrawal during the first few days after cessation (e.g., lowered seizure threshold, anxiety-like phenotypes) (Macey et al., 1996; Majchrowicz, 1975). Ideally, measurements would be made at various intervals post-cessation. However, the vast majority of studies make measurements only during acute withdrawal. Although studies describing the brain state in acute withdrawal are important (e.g., this state may be conducive to deeper encoding of cue-alcohol associations via increased homeostatic value of alcohol or negative reinforcement), ISST assumes that the sensitized response to cues is not constrained to instances of acute withdrawal. In fact, there may be an exposure threshold for ethanol-induced adaptation in different brain and body systems found to be altered in people with AUD. Assuming that this threshold is met, adaptations should begin to arise in the IS circuitry early in the exposure history. Later in the exposure history, the adaptations that mediate ISS should persist after instances of acute withdrawal. For this reason, we review preclinical non-human animal model studies making measurements any time after limited (low-intensity) exposure and at two different time-points after extensive (high-intensity) exposure: during acute withdrawal and at least a week after it has resolved. Finally, we comment on the role of intermittency in ethanol exposure.
3.1. Precursor for alcohol cue incentive salience sensitization (ISS)
The earliest ethanol-induced adaptation in the IS circuit is the development of phasic5 dopamine release to ethanol-predictive cues (henceforth: the IS signal), which is necessary for initiation of progressive alcohol cue ISS. We know that outside a self-administration context, i.e., as a purely pharmacological stimulus divorced of its usual motivational significance, ethanol can induce dopamine release in both the prefrontal cortex and nucleus accumbens of people (Boileau et al., 2003; Setiawan et al., 2014; Urban et al., 2010; Yoder et al., 2016, 2007) and rodents alike (Di Chiara and Imperato, 1988; Howard et al., 2008; Imperato and Di Chiara, 1986; Schier et al., 2013; Yim and Gonzales, 2000; Zapata et al., 2006). This occurs putatively as a function of ethanol’s acute pharmacological effects on cells in the VTA complex (Brodie et al., 1999, 1990; Brodie and Appel, 1998; di Volo et al., 2018; Gessa et al., 1985; Mereu et al., 1984; Xiao et al., 2009).
However, over the course of repeated voluntary oral self-administration by ethanol-experienced non-human animals, explicitly or incidentally-conditioned cues, such as a light or a lever or the flavor of alcohol, acquire the ability to trigger dopamine release in the medial prefrontal cortex and the nucleus accumbens before brain ethanol concentrations reach pharmacologically active levels (Bassareo et al., 2017; Carrillo and Gonzales, 2011; Doherty et al., 2016; Doyon et al., 2005, 2003; Fiorenza et al., 2018; Howard et al., 2009; Shnitko and Robinson, 2015). In the same studies, dopamine levels remain either slightly elevated or return to baseline as brain ethanol concentrations continue to rise. This pattern suggests that one of the early adaptations to chronic ethanol experience in its typical motivational context is a decrease in or loss of VTA complex dopamine neuron sensitivity to the pharmacological effects of ethanol (putatively the rewarding stimulus) and acquisition of VTA complex dopamine neuron sensitivity to ethanol-predictive stimuli (the reward-predictive cues). Interestingly, the same pattern of changes in the IS attributor has been documented as arising in 1/3rd of rodents (so-called “sign-trackers”) when they are trained to predict natural reward (food) on the basis of a cue (Flagel et al., 2010). If we accept that the function of the IS attributor is to broadcast whether specific stimuli are “want worthy,” then the development of any CS-elicited activity in the IS attributor (i.e., rapid release of dopamine from axon terminal buttons; with or without loss of the US-elicited activity) represents the first step in the process of alcohol cue ISS (formation of type ‘a’ path in Figure 2).
3.2. ISS-mediating adaptations arising early in the alcohol use history
Limited (low-intensity) exposure to ethanol is also able to induce adaptations in the IS circuitry that have the functional consequence of increasing the neurobiological (and psychological) relevance of the IS signal. A week of voluntary oral self-administration of moderate ethanol dose (ethanol concentration ≈50 mg/dL whole blood 30 min into the drinking episode) appears to be sufficient to decrease basal extracellular dopamine tone in the prefrontal cortex (Doherty et al., 2016) of rats from a genetically heterogeneous population, whereas longer (e.g., 2 months) voluntary oral self-administration histories may be necessary for similar adaptations to arise in the nucleus accumbens (Doyon et al., 2003; Ericson et al., 2019; Howard et al., 2009). These longer histories can also alter the balance of excitatory and inhibitory drive into the dorsal striatum, the nucleus accumbens, and the orbitofrontal cortex depending on the intensity of alcohol consumption (Adermark et al., 2013; Lagström et al., 2019). The functional consequence of lower basal dopamine tone in either the prefrontal cortex or the nucleus accumbens may be an elevated signal-to-noise ratio in cortical and striatal neurons (Kroener et al., 2009; O’Donnell, 2003; West and Grace, 2002) due to lower tonic dopamine auto-receptor activation (Dreyer et al., 2010). An elevated signal-to-noise ratio would have consequences for the neuromodulatory impact of phasic dopamine release on synaptic activity and plasticity as well as excitatory and inhibitory drive onto those synapses in the IS expressors (changes in the type ‘b’ paths or their targets in Figure 2).
A short history of voluntary oral alcohol self-administration in rats from genetically homogenous populations selected for high alcohol preference is also able to increase the number of spontaneously active dopamine neurons in the VTA complex (Morzorati et al., 2010). This suggests that in at least some individuals limited exposure to alcohol can cause a persistent increase in the intrinsic excitability of the IS attributor (which may make it easier for activity in type ‘a’ paths to drive the IS attributor, i.e., to activate type ‘b’ paths in Figure 2). Interestingly, alcohol-naive rats from these more genetically homogeneous populations selected for high alcohol preference and drinking also tend to have lower basal dopamine tone in both the prefrontal cortex and the nucleus accumbens (Engleman et al., 2006; Gongwer et al., 1989; Katner and Weiss, 2006; McBride et al., 1993; Murphy et al., 1982; Quintanilla et al., 2007; Strother et al., 2005), suggesting that selection for high alcohol preference/drinking also selects for neurobiological endophenotypes that may increase vulnerability to alcohol cue ISS.
3.3. ISS-mediating adaptations arising later in the alcohol use history
3.3.1. Adaptations active in acute withdrawal
Depending on alcohol use level and history, abstinence from alcohol can produce an acute withdrawal syndrome, the physical symptoms (e.g., seizures, tremors) of which can begin hours after the last drink yet abate within a few days and the psychological symptoms (e.g., anxiety, depression, trouble sleeping) of which can persist for at least two weeks (Brown et al., 1995; Brown and Schuckit, 1988; Liappas et al., 2002; Schuckit et al., 2015, 1995). During acute withdrawal, cognitive functioning may be especially impaired ((Beatty et al., 2000; Czapla et al., 2015; Loeber et al., 2010; Pitel et al., 2009; Romero-Martínez et al., 2018); for review see: (Bates et al., 2002)) yet alcohol use-directed motivation may be heightened since non-cued craving is at its peak ((Flannery et al., 2003; Martinotti et al., 2008; Schneekloth et al., 2012; Witkiewitz, 2013); but see: (Li et al., 2015)). In non-human animal models, acute withdrawal after extensive (high-intensity) exposure to ethanol is associated with lower basal dopamine tone and greater basal glutamate tone in the nucleus accumbens (Griffin et al., 2015; Hirth et al., 2016; Karkhanis et al., 2015; Uys et al., 2016; Weiss et al., 1996), altered synaptic plasticity in the nucleus accumbens (Jeanes et al., 2011; Renteria et al., 2017b; Spiga et al., 2014), greater excitability in the medial and orbital prefrontal cortices ((Nimitvilai et al., 2016; Pleil et al., 2015), but see: (Renteria et al., 2018) ), and altered excitatory drive and synaptic excitability as well as altered phasic and tonic inhibition in the central and basolateral nuclei of the amygdala (Herman and Roberto, 2016; Läck et al., 2007, 2005; Lindemeyer et al., 2014; Papadeas et al., 2001; Pleil et al., 2015; Roberto et al., 2004a, 2004b; Varodayan et al., 2016). In the amygdala, hippocampus, and nucleus accumbens, the number of brain cells activated during the experience of acute withdrawal grows as a function of the number of previous withdrawal episodes (Borlikova et al., 2006), suggesting that repeated cycles of high intensity exposure and acute withdrawal alter the balance of excitation and inhibition in these structures and induce persistent functional alterations. Finally, although the neurobiology of acute withdrawal from alcohol is primarily informed by post-mortem studies in the mouse and rat brain, a similar neurobiological state, at least in the nucleus accumbens and prefrontal cortices, can be inferred from post-mortem studies of brains in the non-human primate allowed to voluntarily orally self-administer alcohol to intoxication every day for 6 or more months (Acosta et al., 2010; Alexander et al., 2012; Floyd et al., 2004; Hemby et al., 2006; Siciliano et al., 2016a, 2016b, 2015). The functional consequence of these adaptations may be elevated reactivity to alcohol-associated cues in cortical neurons, dysregulated reactivity in amygdalar neurons, and an elevated signal-to-noise ratio in striatal neurons (Kroener et al., 2009; O’Donnell, 2003; West and Grace, 2002) due to lower tonic dopamine auto-receptor activation (Dreyer et al., 2010) that enhances the neuromodulatory impact of event-related dopamine release on synaptic activity and plasticity as well as the thresholds for excitatory and inhibitory drive onto said synapses in the IS expressors (changes in the type ‘b’ paths or their targets in Figure 2). Thus, the brain state in acute withdrawal after chronic high intensity exposure appears to be one that may support sensitized responses to alcohol cues (i.e., amplifies or facilitates the impact of alcohol-associated cues on behavioral output) as well as deeper conditioning of those cues if reinforced with alcohol ingestion and/or intoxication.
3.3.2. Adaptations that persist into protracted abstinence/withdrawal
Although physical symptoms of withdrawal from alcohol may abate within days, the psychological symptoms such as anxiety and depression can persist, and may be present in some people up to a year after the last drink (Martinotti et al., 2008). Although cognitive functioning may recover over the periods of months to years of protracted abstinence from alcohol ((Beatty et al., 2000; Czapla et al., 2015; Loeber et al., 2010; Pitel et al., 2009; Romero-Martínez et al., 2018), for review see: (Bates et al., 2002)), alcohol use-directed motivation may remain aberrantly elevated, as evidenced by incubation of alcohol cue-induced conscious craving (Li et al., 2015), even if it is not actively expressed in behavior. During protracted abstinence/withdrawal, some, but not all, components of the IS circuitry appear to have a lower threshold for activation. Specifically, non-human animals exhibit elevated basal glutamate tone (Griffin et al., 2014), increased intrinsic excitability, increased excitability at glutamatergic synapses, and altered synaptic plasticity (Marty and Spigelman, 2012; Renteria et al., 2017a; Zhou et al., 2007), but see: (Adermark et al., 2013)) as well as altered astrocytic excitability (Bull et al., 2014) in the nucleus accumbens. Basal dopamine tone in the nucleus accumbens is found to be either increased (Hirth et al., 2016) or decreased (Kashem et al., 2012; Rothblat et al., 2001) or unchanged (Diana et al., 1992), potentially as a function of the exposure paradigm and its ability to induce changes in local dopamine receptor gene expression and/or its regulation (Eravci et al., 1997; Jonsson et al., 2014). In the medial and orbital prefrontal cortices, synaptic excitability, synaptic plasticity, and its biochemical mediators are perturbed (Henniger et al., 2003; Kroener et al., 2012; Renteria et al., 2018). In the central nucleus of the amygdala, altered tonic inhibition persists due changes in the local expression or regulation of inhibitory neurotransmitter clearance mechanisms (Augier et al., 2018). Finally, dopamine neurons in the VTA complex do not appear to exhibit increased baseline spontaneous firing rates (Diana et al., 1992), but do bear biochemical and electrophysiological signatures of enhanced responsivity to excitatory neurotransmission at synapses along their dendrites (Ortiz et al., 1995; Stuber et al., 2008). Overall, even in the absence of the lower signal-to-noise ratio in the nucleus accumbens observed after acute withdrawal, other nodes of the IS circuitry appear to be more easily driven by alcohol-associated cues in protracted abstinence (changes in the type ‘c’ paths in Figure 2). Importantly, the available evidence suggests that long-term ethanol exposure induces adaptations that persist after acute withdrawal into protracted abstinence. Moreover, these ethanol-induced adaptations may support the ability of alcohol-associated cues to affect behavioral output despite non-reinforcement of said cues in protracted abstinence.
3.3.3. On the role of intermittency in the effects of chronic ethanol exposure
Many of the neuroadaptations evident in the acute and protracted withdrawal states after extensive (high-intensity) ethanol exposure that were reported above may hinge upon the intermittency built into many ethanol access/exposure paradigms (e.g., multiple cycles of a 4-day 16-hr/day passive ethanol vapor exposure, every other day 24-hr access or daily 2-hr access schedules). In rodents, these chronic intermittent access/exposure paradigms can induce alcohol addiction-like behavioral phenotypes including: increased alcohol seeking (Augier et al., 2018; Ciccocioppo et al., 2003; Gass et al., 2014; Hauser et al., 2019; Meinhardt et al., 2013; Vendruscolo et al., 2012), development of within-episode drinking patterns that rapidly produce high blood alcohol concentrations (Gilpin et al., 2009; Wilcox et al., 2014), and heavier consumption across episodes (Becker and Lopez, 2004; Bell et al., 2004; Loi et al., 2010; Morales et al., 2015; Simms et al., 2008; Sommer et al., 2008; Wilcox et al., 2014; Wise, 1973) as well as relative insensitivity of alcohol seeking and drinking to alcohol devaluation (Augier et al., 2018; Hopf et al., 2010; Loi et al., 2010; Vendruscolo et al., 2012). Furthermore, rodents that undergo chronic intermittent ethanol access/exposure paradigms exhibit short- and long-term deficits in performance on executive functioning tasks (Gass et al., 2014; Kroener et al., 2012) as well as in learning about aversive, but not appetitive, outcomes (Ripley et al., 2004, 2003; Stephens et al., 2005, 2001). Thus, repeated cycles of intoxication and abstinence may be a critical factor in the progressive loss of control over alcohol use.
3.4. Interim summary 1
In this section, we reviewed evidence for the ability of ethanol, the addictive agent in alcoholic beverages, to induce neuroadaptations that may mediate the ISS process. The evidence, which comes from work in non-human animal models, suggests that several different adaptations capable of mediating the ISS process arise as a function of chronic ethanol exposure (see Figure 3) in the IS attributor, VTA complex, and the IS expressor systems, amygdala and nucleus accumbens, as well as in the medial and orbital prefrontal cortices, which are innervated by the IS attributor and inter-connected with the IS expressor systems. Given that these prefrontal cortices are believed to mediate cognitive control processes (Barbas, 2000; Braver, 2012; Inzlicht et al., 2015; Ridderinkhof, 2004), complex higher-level psychological functions in humans such as emotion regulation and self-control (Ochsner et al., 2012; Robinson et al., 2010) may be vulnerable to dysregulation as ISS progresses.
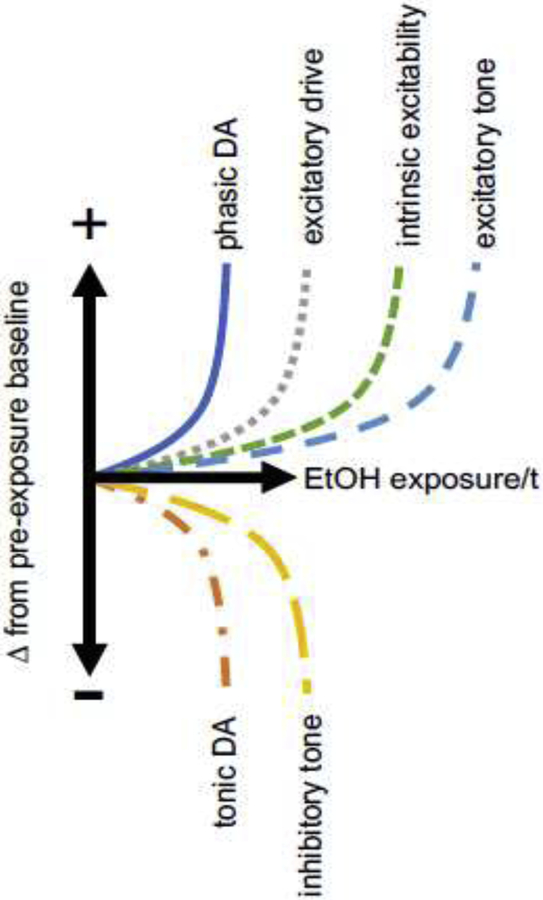
Figure 3.
Example of the kinds of persistent adaptations ethanol (EtOH) exposure might induce over time (t) to mediate alcohol cue ISS and how these might unfold over exposure relative to one another. Conjecture is informed by findings reviewed in the main text. Increased phasic DA release (from synchronized high-frequency action potentials at terminal boutons arising from the IS attributor) must occur at synapses involved in maintaining memory for the alcohol cue or its expression (viz., synapses in memory systems or in the IS expressors). Increased excitatory drive may occur at specific synapses across the IS system components including at the IS attributor. Decreased tonic DA release (from asynchronous low-frequency action potentials at terminal boutons arising from the IS attributor) will naturally affect many more synapses than those immediately involved in alcohol cue memory/expression. Increased excitatory tone, decreased inhibitory tone, and increased intrinsic excitability will naturally affect many cells across the IS system components including at the IS attributor.
4. Evidence for incentive salience (IS) attribution to alcohol cues and its sensitization (ISS)
Based on the behavioral indicators of IS attribution described in (Robinson et al., 2014), if an alcohol-associated cue has been attributed with IS, then: (1) that cue should be able to elicit approach-oriented responses (e.g., attention, approach, conscious craving for alcohol), (2) that cue should be able to serve as a conditional or secondary reinforcer,6 and (3) that cue should be able to induce or invigorate instrumental alcohol seeking actions (e.g., choice, consumption). At the brain-level, the IS-attributed alcohol-associated cue should engage the IS circuitry, i.e., activate the IS attributor and/or expressors (Figure 1).
Applying ISST to AUD, all else being equal, if ISS is a mechanism in AUD, then it holds that an individual’s degree of reactivity to alcohol-associated cues should covary with alcohol use levels. There should be a positive relationship between alcohol consumption or exposure and degrees of reactivity. Consequently, with more exposure: (1) the IS-imbued alcohol-predictive cue should be able to elicit greater levels of alcohol seeking reactions (e.g., attention, approach, conscious craving for alcohol), (2) the cue should have higher conditional rewarding value, and (3) the cue should more easily induce or invigorate instrumental alcohol seeking actions (e.g., choice, consumption). Finally, (4) a similar pattern of sensitization should be evident in brain-level responses to the cue. However, all else may not be equal, and it rarely is. It is important to keep in mind that the reliability of the predicted positive association between alcohol use and cue reactivity level in humans is likely to be low because alcohol use level is subject to the influence of many different trait and state factors at the psychological, social, and cultural levels.
4.1. In non-human animal models
In this section, we review evidence for attribution of IS to alcohol-predictive cues and its sensitization in non-human animal models.
4.1.1. Alcohol cue-related behavioral responses
4.1.1.1. Attentional responses
In discrete alcohol cue conditioning paradigms involving voluntary oral alcohol self-administration by rats, the alcohol-predictive cue can come to elicit an attentional orienting response (Cofresí et al., 2019a, 2019b). However, it remains to be seen whether individual differences in pre-conditioning voluntary alcohol consumption predict conditioned attentional response levels or whether the latter relate to alcohol self-administration in the conditioning task.
4.1.1.2. Approach responses
In the same type of paradigm where alcohol-related attentional responses can be seen in rats, the alcohol-associated discrete cue can also come to elicit an approach response (Cofresí et al., 2019a, 2019b, 2018; Krank, 2003; Krank et al., 2008; Sparks et al., 2014; Srey et al., 2015; Villaruel and Chaudhri, 2016). In keeping with what we might expect from applying ISST to AUD phenotypes, individual differences in pre-conditioning task voluntary alcohol consumption can predict later alcohol cue-conditioned approach levels (Cofresí et al., 2019a). Furthermore, rats with greater magnitude alcohol cue-conditioned approach response also self-administer more alcohol in the cue conditioning paradigm (Cofresí et al., 2019b, 2018). Similarly, alcohol-associated contextual cues, such as distinct places paired with experimenter-administered alcohol, can come to elicit place preference, an approach-like response, in mice (Cunningham et al., 2002a, 2002b; Gremel and Cunningham, 2008) and rats (Bozarth, 1990; Nentwig et al., 2017; Torres et al., 2014). Furthermore, there is a positive association between levels of this approach-like response and levels of voluntary alcohol consumption in rodents (Green and Grahame, 2008).
4.1.1.3. Cue as conditional reinforcer effects
Alcohol-associated cues alone can reinforce learning of new instrumental actions in rats (Milton et al., 2012; Schramm et al., 2016; Srey et al., 2015). Alcohol-associated cues and contexts are also able to cause the return of extinguished instrumental actions that previously produced alcohol and maintain that instrumental responding for some time in the absence of alcohol (Bertholomey et al., 2016; Bienkowski et al., 2004; Burattini et al., 2006; Chaudhri and Sahuque, 2008; Ciccocioppo et al., 2002, 2001; Dayas et al., 2007; Hauser et al., 2019, 2016; Jupp et al., 2011; Katner et al., 1999; Katner and Weiss, 1999; Knight et al., 2016; Martin-Fardon and Weiss, 2017; O’Brien et al., 2011; Radwanska et al., 2008; Randall et al., 2017; Rodd-Henricks et al., 2003, 2002; Zironi et al., 2006).
Evidence for sensitization of conditioned reinforcement comes from studies in rats demonstrating changes in the magnitude of conditioned reinforcement-related effects following termination of access or exposure to alcohol. In the hours to days following termination of access/exposure, alcohol dependent rats will: (1) work harder for alcohol (Gilpin et al., 2009; Gilpin and Koob, 2010; Kissler and Walker, 2015; Roberts et al., 1996; Vendruscolo et al., 2012; Walker and Koob, 2007), (2) self-administer more (Weiss et al., 1996), even if it has been adulterated with bitterant (Vendruscolo et al., 2012), and (3) exhibit larger alcohol-associated cue- and context-induced instrumental response reinstatement effects (Ciccocioppo et al., 2003; Liu and Weiss, 2002a; Weiss et al., 1996). As time post-termination of access/exposure increases from days to weeks or months, alcohol-associated cue- and context-induced instrumental response reinstatement effects can be observed to grow in magnitude ((Bienkowski et al., 2004; Hauser et al., 2019, 2016; Radwanska et al., 2008; Rodd-Henricks et al., 2003, 2002), but see: (Jupp et al., 2011; O’Brien et al., 2011)). This incubation effect is in agreement with known time-dependent increase in the magnitude of spontaneous recovery of extinguished reactivity to alcohol-associated cues (LeCocq et al., 2018; Remedios et al., 2014). From a Pavlovian perspective, these effects demonstrate acute withdrawal and protracted abstinence state-dependent increases in IS attribution to alcohol-associated cues.
4.1.1.4. Pavlovian to instrumental transfer effects
The Pavlovian to instrumental transfer (PIT) construct developed in animal models addresses the ability of incidental exposure to Pavlovian cues to initiate or invigorate an instrumental action. The formal test for PIT involves separately training cue reactivity and instrumental action and then observing behavior during a probe test in which the cue is intermittently presented to the animal while the opportunity for instrumental action is concurrently available. Typically, primary reinforcement is withheld during the test to prevent confounding the effect of cues with the effect of primary reinforcement. In rats, alcohol-associated cues can produce PIT test effects that are specific to alcohol reward as well as PIT test effects that generalize to other rewards ((Alarcón and Delamater, 2018; Corbit et al., 2016; Corbit and Janak, 2016, 2007; Glasner et al., 2005; Krank, 2003; Krank et al., 2008; Lamb et al., 2016); but see: (Lamb et al., 2019, 2016)). Interestingly, alcohol-associated cues do not appear to exert any stronger PIT test effect in rats with a history of physical ethanol dependence than rats without such a history (Glasner et al., 2005). However, in rats without any history of physical dependence, alcohol cues exert a stronger PIT test effect after extensive as opposed to limited voluntary oral self-administration histories ((Corbit and Janak, 2016), but see: (Lamb et al., 2019)).
If we accept that drinking alcohol is itself an action instrumental for the experience of alcohol’s primary reinforcing properties, then we can admit as evidence of PIT in non-human animal models those situations in which conditioned alcohol cue reactivity facilitates alcohol drinking behavior (e.g., initiation of a sip, faster sipping, larger sips). Such PIT-like effects have been observed in rats (Cofresí et al., 2019b, 2018). Here, there is a positive relationship between the degree of Pavlovian alcohol cue reactivity and the speed and intensity of alcohol drinking behavior (Cofresí et al., 2019b, 2018).
4.1.2. Alcohol cue-related brain responses
4.1.2.1. Immediate early gene expression
When neurons and astrocytes are activated, the transcription and translation of immediate early genes (e.g., arc, c-fos, erk) occurs, and this allows researchers to use the relative density of transcripts or translated protein product as an index of regional brain activity (typically, post-mortem) (Herdegen and Leah, 1998; Morgan and Curran, 1989; Sheng and Greenberg, 1990). Using these indices, alcohol cues conditioned by voluntary oral alcohol self-administration have been shown to activate cells in various IS circuit nodes in the rat brain, including the medial and orbital prefrontal cortices, insular cortex, basolateral nucleus of the amygdala, nucleus accumbens, and central nucleus of the amygdala (Barak et al., 2013; Cofresí et al., 2019a; Dayas et al., 2007; Jupp et al., 2011; Radwanska et al., 2008).
These alcohol cues appear gain incentive salience as a function of time since acute withdrawal from alcohol. Specifically, after 6 months since cessation of alcohol access compared to after only 1 month, alcohol cues were able to induce activation of more cells in IS circuit nodes including the medial and orbital prefrontal cortices and central nucleus of the amygdala (Jupp et al., 2011). Other IS circuit nodes (e.g., nucleus accumbens, basolateral nucleus of the amygdala) also exhibited cue-induced activation, but the number of activated cells did not scale with time post-cessation of alcohol access. To our knowledge, no studies have looked at whether alcohol cues activate more cells in these brain regions as a function of alcohol exposure levels per se.
4.1.2.2. Dopamine neurotransmission
Changes in phasic and tonic dopamine release, which differentially affect signaling via different post-synaptic dopamine receptors (Dreyer et al., 2010; Venton et al., 2003), can be measured using fast-scan cyclic voltammetry (Rodeberg et al., 2017) and microdialysis (Zapata et al., 2009), respectively, in awake, freely-behaving non-human animals. Using these two neurochemical monitoring techniques, alcohol-associated cues have been demonstrated to elicit increases in both phasic and tonic dopamine release at IS circuit nodes such as the prefrontal cortices and nucleus accumbens in the rat using (Bassareo et al., 2017; Carrillo and Gonzales, 2011; Doherty et al., 2016; Doyon et al., 2005, 2003; Fiorenza et al., 2018; Gonzales and Weiss, 1998; Howard et al., 2009; Katner and Weiss, 1999; Melendez et al., 2002; Robinson et al., 2009; Shnitko and Robinson, 2015; Weiss et al., 1993).
We were unable to find any published studies that have set out to investigate whether alcohol cues elicit greater dopamine release as a function of alcohol exposure levels. Only one study has looked at whether alcohol-associated cues elicit greater dopamine release as a function of time since acute withdrawal due to termination of alcohol access/exposure. In this single study using microdialysis in rats (Weiss et al., 1996), when alcohol-dependent rats were allowed to orally self-administer alcohol a few hours into acute withdrawal, within 10 minutes, extracellular dopamine levels in the nucleus accumbens reached 200% of the baseline extracellular level observed during acute withdrawal. However, in the same study, the baseline extracellular dopamine level in the nucleus accumbens was found to be lower during those first few hours of acute withdrawal than before the induction of physical dependence. Another study from the same group provides indirect evidence for increased cue-elicited dopamine release in the nucleus accumbens during withdrawal. In this study, the potency and efficacy of systemically-administered dopamine receptor antagonists to inhibit the ability of alcohol-associated cue/contexts to reinstate extinguished instrumental response rates were found to be greater after a history of physical dependence (Liu and Weiss, 2002b). Together, these findings are in keeping with the idea (discussed earlier) that ethanol exposure induces adaptations that enhance the neuromodulatory impact of dopamine in the nucleus accumbens.
4.1.2.3. In vivo neuronal firing
Transient changes in the firing rate of neurons also can be measured in awake, freely-behaving animals by implanting electrodes into the brain regions of interest (Woodward et al., 1999). Alcohol-associated cues have been demonstrated to elicit changes in the firing rate of neurons in the nucleus accumbens of rats from genetically heterogeneous populations (Janak et al., 1999; Robinson and Carelli, 2008; Woodward et al., 1998). In rats from a genetically homogenous population selected for high alcohol preference, alcohol-associated cues may also elicit changes in the firing rate of neurons in the prefrontal cortex (Linsenbardt and Lapish, 2015). To our knowledge, no studies have looked at whether alcohol access-related cues induce greater changes in neuronal firing as a function of alcohol use or exposure levels or as a function of time since acute withdrawal due to termination of alcohol access or exposure.
4.2. In humans
In this section, we review evidence for attribution of IS to alcohol-predictive cues and its sensitization in people.
4.2.1. Alcohol cue-related behavioral responses
4.2.1.1. Attentional responses
In this section, we review the evidence for attentional capture by alcohol-predictive cues, an index of IS attribution. Readers interested in the topic of the attentional capture by cues associated with drugs of abuse (including alcohol) in general, including alternative theoretical and methodological explanations for any measured bias in visual attention, its relationship to drug craving, and its clinical relevance are referred to comprehensive review articles conducted by others (Christiansen et al., 2015b; Field et al., 2016, 2014; Field and Cox, 2008).
The best evidence for or against the ability of alcohol cues to elicit oculomotor behavior (viz., capture visual attention) in humans comes from studies measuring eye movement initiation, latency, and duration. In one such study (Monem and Fillmore, 2016), the duration of gazes toward non-alcoholic beverages decreased across two sessions in a simulated in vivo “recreational” setting whereas the duration of gazes toward alcoholic beverages in the same setting did not such that in session 2, an attentional bias toward the alcoholic beverages was visible at the sample-level. Monem and Fillmore (2016) also found that within-person differences in gaze duration between alcoholic and non-alcoholic beverages in both sessions were positively related to typical alcohol use levels. Similarly, inside complex visual scenes presented on a computer screen, alcoholic beverages can elicit a greater number of cue-directed eye movements and the extent to which they do appears to be relate to alcohol use levels (Roy-Charland et al., 2017). More eye movements tend to be directed toward alcohol use scenes when they are presented alongside other scenes and gaze duration tends to be longer (Vincke and Vyncke, 2017). Similarly, the duration of gazes toward alcoholic beverage pictures and words tend to be longer than the duration of gazes toward concurrently presented non-alcohol pictures and words and this bias tends to be positively related to typical alcohol use ((Ceballos et al., 2015; Christiansen et al., 2015a; Fernie et al., 2012; Field et al., 2011b; Friese et al., 2010; Laude and Fillmore, 2015; Lee et al., 2014; Melaugh McAteer et al., 2015; Miller and Fillmore, 2011, 2010; Rose et al., 2013; Weafer and Fillmore, 2013, 2012), but see: (Schoenmakers et al., 2008)).
Additional evidence for or against the ability of alcohol cues to elicit oculomotor behavior (viz., capture visual attention) in humans comes from tasks where visual attentional capture by alcohol cues can be inferred based on changes in the accuracy and/or latency of other (non-ocular) responses to visual stimuli. For example, the ability and latency to detect alcoholic beverage-related changes within complex visual scenes as well as simple multi-item display grids in the flicker-induced visual change blindness paradigm in the laboratory (Hobson et al., 2013; Jones et al., 2002, 2006, 2003; Schoenmakers et al., 2007) and in the natural environment (Schoenmakers and Wiers, 2010). The ability and latency to detect alcohol-related changes in the flicker-induced change paradigm tend to be, respectively, positively and negatively related to typical alcohol use levels (Hobson et al., 2013; Jones et al., 2002, 2006, 2003; Schoenmakers and Wiers, 2010). Similarly, in the attentional blink paradigm, which measures the efficiency of early visual attention as a decrease in an experimentally-induced stimulus mis-identification rate, greater early visual attention efficiency has been found for both alcoholic beverage pictures and alcohol-related words relative to non-alcohol pictures and words as a positive function of typical alcohol use levels (DePalma et al., 2017; Tibboel et al., 2010). In the modified visual dot probe detection task, an alcoholic beverage picture and non-alcohol beverage picture are presented simultaneously on the left or right-side of a computer screen and a response-target probe is presented shortly after picture offset in the same location as one of the two pictures on the screen. In this task, the latency to respond to the probe tends to be shorter (i.e., people are faster to detect it) when the probe is presented in the same location as the alcoholic beverage picture and this tends to be positively related to typical alcohol use levels ((Christiansen et al., 2015a; Clerkin et al., 2016; Duka and Townshend, 2004; Field et al., 2013, 2007, 2004; Field and Eastwood, 2005; Field and Quigley, 2009; Garland et al., 2012a, 2012c; Manchery et al., 2017; Miller and Fillmore, 2010; Ramirez et al., 2015b, 2015a; Roberts and Fillmore, 2015; Schoenmakers et al., 2007; Shin et al., 2010; Vollstädt-Klein et al., 2012), but see: (Fernie et al., 2012; Field et al., 2005; Jones et al., 2018; Miller and Fillmore, 2011; Schoenmakers et al., 2008; Townshend and Duka, 2007; Wiers et al., 2017)). Additionally, in AUD patients, this measure of attentional capture by alcohol cues appears to “incubate” (i.e., detection of targets in alcohol cued locations becomes increasingly faster) across abstinence (Rinck et al., 2018; Schoenmakers et al., 2010). In the modified color-naming Stroop interference task with words (Stroop, 1935), participants tend to be less accurate and/or slower to identify the color when alcohol-related words are used and this tends to be positively related to typical alcohol use ((Bauer and Cox, 1998; Cox et al., 2003, 2000, 1999; Duka et al., 2002; Fadardi and Cox, 2009, 2006; Field et al., 2013; Grant et al., 2007; Johnsen et al., 1994; Lusher et al., 2004; Modi et al., 2019; Murphy and Garavan, 2011; Ryan, 2002; Sharma et al., 2001; Snelleman et al., 2015; Stautz et al., 2017; Stetter et al., 1995, 1994; Stormark et al., 2000, 1997), but see: (Albery et al., 2015; Christiansen and Bloor, 2014; Duka and Townshend, 2004; Fridrici et al., 2013; Spanakis et al., 2019)) and may “incubate” with repeated cycles of withdrawal (Duka et al., 2002).
Visual attention capture by alcohol cues, across direct and indirect measures, tends to be more consistently related to differences in typical alcohol use than it is related to differences in AUD status, duration, and severity. However, direct and indirect measures of attentional capture by alcohol cues may be confounded by neurocognitive impairments among individuals with AUD (Beatty et al., 2000; Czapla et al., 2015; Loeber et al., 2010; Pitel et al., 2009; Romero-Martínez et al., 2018); for review see: (Bates et al., 2002)). Accounting for these neurocognitive impairments may be necessary in measuring attentional bias among individuals with AUD and when evaluating relationships between attentional bias scores and differences in AUD status, duration, and/or severity (Fadardi and Cox, 2006; Loeber et al., 2009).
4.2.1.2. Approach responses
The best evidence for or against the ability of alcohol cues to elicit skeletomotor behavioral manifestations of IS attribution (viz., approach responses) in humans might be acquired by measuring beverage-directed approach movement initiation and its latency, time spent within proximity, postural changes, or skeletal muscle ‘priming’ following presentation of alcoholic beverages at a distance. Currently, the best available evidence comes from two computer-based tasks using visual proxies for alcoholic beverages and approach v. avoidance responses. The first task is a modified version of the Simon task (De Houwer et al., 2001) in which people use arrow keys to move a manikin toward or away from alcohol-related and control pictures presented on the computer screen (Field et al., 2005). On this task, people tend to be faster to respond when instructed to move the manikin toward rather than away from alcohol pictures ((Barkby et al., 2012; Christiansen et al., 2012; Field et al., 2011a, 2008, 2007, 2005; Pieters et al., 2012; Schoenmakers et al., 2008; van Hemel-Ruiter et al., 2011), but see: (Snelleman et al., 2015; Spruyt et al., 2013)), an alcohol approach bias appears that appears to be positively related to alcohol use level ((Barkby et al., 2012; Christiansen et al., 2012; Field et al., 2011a, 2008, 2005; Pieters et al., 2012), but see: (van Hemel-Ruiter et al., 2011)), and not AUD status (Barkby et al., 2012). The second task is a modified version of the Approach-Avoidance task (Chen and Bargh, 1999) in which participants use a joystick to pull (approach) or push (avoid) pictures of alcoholic and non-alcoholic beverages among other objects presented on the computer screen (Wiers et al., 2009). On this task, people tend to be faster to pull alcoholic beverage pictures toward themselves than they are to push them away (Eberl et al., 2013; Ernst et al., 2013; Fleming and Bartholow, 2014; Leeman et al., 2018; Loijen et al., 2018; Peeters et al., 2013, 2012; Sharbanee et al., 2014; Wiers et al., 2011, 2010, 2009, 2015b, 2014), an alcohol approach bias appears that appears to be positively related to alcohol use level and/or AUD status (Fleming and Bartholow, 2014; Peeters et al., 2013, 2012; Sharbanee et al., 2014; Wiers et al., 2017, 2014).
Arguably, there is a third measure of the approach response in humans. Some researchers have used the Implicit Association Task (IAT) (Greenwald et al., 1998) to assess how strongly the concept of “alcohol” (primed by words or pictures of different brands or types of alcoholic beverages) is linked to other concepts such as “active” (primed by words like ‘energetic’, ‘lively’, ‘cheerful’) or “positive” (primed by words like ‘good’, ‘pleasant’, ‘nice’) (Wiers et al., 2002) as well as “approach” (activated by words like ‘approach’, ‘advance’, ‘closer’) (Palfai and Ostafin, 2003). In many cases, IAT scores were found to be positively related to alcohol use levels ((Houben and Wiers, 2009; Jajodia and Earleywine, 2003; Ostafin and Marlatt, 2008; Ostafin and Palfai, 2006; Palfai and Ostafin, 2003; Wiers et al., 2017, 2002), but see: (Tibboel et al., 2015)). It is an open question, however, whether the IAT truly measures the same construct as the modified Simon Task and modified Approach-Avoidance Task described above (cf. (Wiers et al., 2017). Conceptually, it seems that the IAT might be more likely to reveal the structure of the implicit alcohol-associative memory network whereas the modified Simon Task and modified Approach-Avoidance Task might be more likely to detect expression (manifestation) of IS in human skeletomotor behavior.
4.2.1.3. Autonomic responses
Autonomic responses can be registered in many different physiological units including the activity of the cardiovascular system, hormone-secreting glands (e.g., the adrenal glands, the pancreas), smooth muscle (e.g., lining blood vessels or the gut), and the neurons that innervate them. For simplicity, we chose to review evidence from only a single physiological unit of analysis: heart rate. Heart rate is useful summary index of autonomic response because heart rate is sensitive to the interactive effects of many physiological processes including circulating hormones, nervous system activity, respiratory rate, and smooth muscle activity. Presentation of alcoholic beverages is able to elicit an increase in heart rate (measured as more beats per minute) that is sustained as the person performs the act of ingesting the presented beverage, but dissipates shortly thereafter (Kaplan et al., 1985; Newlin, 1986, 1985; Pomerleau et al., 1983; Staiger and White, 1991; Turkkan et al., 1988). The smell, the taste, and the sight of alcoholic beverages can also increase heart rate when presented in isolation from each other (e.g., smell only, sight only) (McCaul et al., 1989; Payne et al., 1992; Stormark et al., 1995; Turkkan et al., 1989; Witteman et al., 2015). Increases in heart rate have also been observed following personalized alcohol use-related mental imagery (Seo et al., 2013; Sinha et al., 2009). The latter has also been demonstrated to produce greater increases in heart rate among people with AUD (Seo et al., 2013; Sinha et al., 2009) whereas in vivo exposure to alcoholic beverages can do so sometimes (Kaplan et al., 1985), but not others (Thomas et al., 2005). Presentation of alcoholic beverage sights or smells or tastes in isolation can raise heart rate to a greater extent in some people with AUD (Ingjaldsson et al., 2003; Stormark et al., 1995), but not all (McCaul et al., 1989; Turkkan et al., 1989). Presentation of alcohol-related words is able to increase heart rate to the same extent in people with and without AUD (Stormark et al., 2000).
4.2.1.4. Conscious craving/subjective experience of desire for alcohol
The construct of craving has a long history in the study of alcoholism (Drummond, 2001; Edwards and Gross, 1976; Jellinek, 1960; Tiffany and Conklin, 2000). The ability of IS-attributed cues to provoke an explicit desire for alcohol is important not only because “strong urges, cravings, or desires to use alcohol” are now one of the diagnostic criteria for AUD (Diagnostic and statistical manual of mental disorders (DSM-5{®}), 2013), but also because the greater a person’s retrospective subjective experience of alcohol craving in daily life, the greater the number of alcohol-related problems and symptoms of AUD they are likely to be experiencing (Chakravorty et al., 2010; Murphy et al., 2014; Ray et al., 2017; Rohn et al., 2017; Yoon et al., 2006). Consequently, the development of cue-provoked craving for alcohol could be considered one of the earliest signs of alcohol cue ISS. In keeping, presentation of alcoholic beverages is able to provoke self-reported explicit desire for alcohol (viz., conscious craving) measured using single-item visual analog scale or multi-item questionnaires (Amlung and MacKillop, 2014; Blaine et al., 2018; Cooney et al., 1984; Curtin et al., 2005; Field et al., 2005, 2004; Hollett et al., 2017; Kambouropoulos and Staiger, 2004; Kaplan et al., 1985; Kareken et al., 2010a; Kiefer et al., 2015; Kreusch et al., 2017; MacKillop, 2006; MacKillop et al., 2015; MacKillop and Lisman, 2008, 2005; Monti et al., 1987; Ostafin et al., 2008; Pomerleau et al., 1983; Ramirez et al., 2015a, 2015b; Rohsenow et al., 1994; Staiger and White, 1991; Willner et al., 1998). Isolated presentation of alcohol-related smells, tastes, and pictures or videos can also provoke craving ((Bragulat et al., 2008; Christiansen et al., 2017; Courtney et al., 2015; Fey et al., 2017; Field et al., 2007; Field and Eastwood, 2005; Filbey et al., 2008; Lovett et al., 2015; Lukas et al., 2013; Manchery et al., 2017; Mccusker and Brown, 1990; Oberlin et al., 2016, 2013; Ostafin et al., 2008; Payne et al., 1992; Pronk et al., 2015; Schneider et al., 2001; Stauffer et al., 2017; Stormark et al., 1995; Veilleux et al., 2018; Vollstädt-Klein et al., 2012; Witteman et al., 2015; Yoder et al., 2009), but see: (Mucha et al., 2000)) as can personalized alcohol use-related mental imagery (Blaine et al., 2018; Fox et al., 2007; Seo et al., 2013; Sinha et al., 2009).
In theory, as ISS progresses the magnitude of cue-provoked craving for alcohol should increase. In line with this prediction, presentation of alcoholic beverages can provoke greater craving among people with AUD compared to controls ((Pomerleau et al., 1983; Reid et al., 2006; Thomas et al., 2005) but see: (Kaplan et al., 1985; Monti et al., 1987)). People with AUD are also more likely than control participants to report greater increases in craving following presentation of the isolated sight or smell or taste of alcoholic beverages (George et al., 2001; Ingjaldsson et al., 2003; Myrick et al., 2004; Reid et al., 2006; Schneider et al., 2001; Wiers et al., 2015c) and following exposure to personalized alcohol use-related mental imagery (Reid et al., 2006; Seo et al., 2013; Sinha et al., 2009). Among people without AUD, higher levels or more hazardous alcohol use also predict greater craving following presentations of actual beverages or picture of them (Blaine et al., 2018; Curtin et al., 2005; Hollett et al., 2017; Lovett et al., 2015; Pronk et al., 2015; Stauffer et al., 2017; Veilleux et al., 2018). Furthermore, the magnitude of craving produced by alcohol’s interoceptive stimuli is also positively related to both alcohol use and AUD severity (Bujarski et al., 2018, 2017, 2015; Bujarski and Ray, 2014; Duka et al., 1999; King et al., 2016, 2014, 2011, 2002; Morean et al., 2013). Together, these findings suggest sensitization of exteroceptive and interoceptive alcohol cue-elicited craving responses. Finally, it also appears that the level of craving induced by at least exteroceptive alcohol cues can become elevated after at least 2 months of abstinence from alcohol, at least among people with AUD (Li et al., 2015; Monti et al., 1993a), which is in keeping with theorized state-dependent modulation of IS attribution and expression.
4.2.1.5. Cue as conditional reinforcer effects
To our knowledge, currently there are no published reports of direct tests for the conditioned rewarding value of alcohol cues in humans (i.e., alcohol cue-based reinforcement of a new instrumental response, alcohol cue-based reinforcement of a new cue), or even indirect tests such as measuring the extent to which an alcohol-associated cues can reinstate extinguished instrumental actions that previously produced alcohol reward. This precludes any investigation of whether conditional reinforcing value increases as a function of alcohol use level or AUD status, severity, and duration that would be predicted by ISST.
4.2.1.6. Pavlovian to instrumental transfer effects
In people, the alcohol-specific PIT construct developed in non-human animal models of alcohol seeking maps onto situations in which exposure to alcohol-predictive Pavlovian cues increases the likelihood of actions taken to acquire or consume alcoholic beverages. The increases in action likelihood are believed to be at least in part a consequence of cue-triggered Pavlovian alcohol seeking reactions. However, it is important to keep in mind that in non-human animal model paradigms, the subject is typically already in a place associated with alcohol availability, and the instrumental action to obtain or consume alcohol does not require the subject to leave that place. That is, the alcohol-specific PIT construct developed from the non-human animal models may only naturally apply to specific situations in which people may find themselves. Nonetheless, there have been numerous demonstrations of the alcohol-specific PIT construct in the human laboratory. Instrumental responding for alcohol, measured as ingested alcohol volume or ingestion speed in bogus beverage evaluation tasks or number of alcohol beverage-earning responses in computerized tasks, has been shown to increase following isolated presentation of alcoholic beverage cues within specific sensory modalities (Field and Eastwood, 2005; Field and Jones, 2017; Hodgson et al., 1979; Martinovic et al., 2014; Roehrich and Goldman, 1995; Rose et al., 2018; Stein et al., 2000; Van Dyke and Fillmore, 2015), but see: (Carter and Tiffany, 1999; Field et al., 2007, 2005; Jones and Field, 2013; Kersbergen and Field, 2017; Stautz et al., 2017) as well as following presentation of alcoholic beverages and/or the interoceptive stimuli produced by ingestion ((Amlung and MacKillop, 2014; Bigelow et al., 1977; Blaine et al., 2018; Christiansen et al., 2017; Chutuape et al., 1994; Corbin et al., 2008; Farris and Ostafin, 2008; Fernie et al., 2012; Fromme and Dunn, 1992; Hodgson et al., 1979; Holdstock and de Wit, 1998; Johnson and Fromme, 1994; Larsen et al., 2012; Leeman et al., 2009; Ludwig et al., 1978, 1974; MacKillop and Lisman, 2005; Marlatt et al., 1973; Ostafin et al., 2008; Perkins et al., 2003; Rose and Duka, 2006; Stockwell et al., 1982; Wetherill and Fromme, 2009; Williams and Brown, 1985), but see: (Paredes et al., 1973)). In keeping with ISST, these PIT effects have been found to differ in magnitude based on AUD status ((Higgins and Marlatt, 1973; Hodgson et al., 1979; Ludwig et al., 1978; Marlatt et al., 1973; Stockwell et al., 1982), but see: (Bujarski et al., 2018)) as well as individual differences in typical alcohol use levels ((Blaine et al., 2018; Corbin et al., 2008; Leeman et al., 2009; Van Dyke and Fillmore, 2015), but see: (Kersbergen and Field, 2017; Martinovic et al., 2014)).
4.2.2. Alcohol cue-related brain responses
In most cases, functional brain responses to alcohol-related cues in people are measured using simple tasks that involve only repeated presentation of alcohol-related stimuli (e.g., pictures). This allows for unambiguous interpretation of changes in measured brain activity. In part, the simplicity of tasks reflects the technical difficulty of non-invasively measuring activity in the living brain.
4.2.2.1. Brain responses measured using the fMRI-BOLD technique
Behavioral responses to alcohol-associated cues, such as attentional bias and approach tendency, are theorized to be a downstream consequence of cue-induced activation of IS neurocircuitry components. Therefore, it is important to verify the ability of alcohol-associated cues to activate the IS attributor and expressors in the living human brain. Functional magnetic resonance imaging (fMRI) provides a way to visualize cue-induced neuronal activation or de-activation in specific areas of the living human brain using the regional blood oxygen level-dependent (BOLD) contrast imaging technique (Logothetis, 2003; Logothetis and Pfeuffer, 2004). As a previous meta-analysis showed (Schacht et al., 2013), using the fMRI-BOLD technique, alcohol cue-induced activation (i.e., more positive BOLD signals) has been reported in the IS attributor, VTA complex, and the IS expressor systems, amygdala and nucleus accumbens, as well as in the medial and orbital prefrontal cortices, which are innervated by the IS attributor and inter-connected with the IS expressor systems. Specifically, activation has been reported in response to isolated alcohol cues presented in the following sensory modalities: sight (Ames et al., 2014b, 2014a; Braus et al., 2001; Brumback et al., 2015; de Sousa Fernandes Perna et al., 2017; Fryer et al., 2013; Grüsser et al., 2004; Ihssen et al., 2011; Kim et al., 2014; Lee et al., 2013; Lukas et al., 2013; Nikolaou et al., 2013; Schad et al., 2018; Sekutowicz et al., 2019; Tapert et al., 2004; Vollstädt-Klein et al., 2010; Wiers et al., 2015c, 2014; Wrase et al., 2002), smell ((Kareken et al., 2004; Schneider et al., 2001) but see: (Lukas et al., 2013)), and taste (Claus et al., 2011; Courtney et al., 2015; Filbey et al., 2008; Oberlin et al., 2016), as well as their combinations ((George et al., 2001; Myrick et al., 2004), but see: (Bragulat et al., 2008)).
In keeping with an alcohol cue ISS mechanism, typical alcohol use levels and AUD status or severity tend to be positively related to the magnitude of BOLD signals during exposure to isolated alcohol-related sights (Ames et al., 2014a, 2014b; Braus et al., 2001; Brumback et al., 2015; Fryer et al., 2013; Grüsser et al., 2004; Heinz et al., 2004; Ihssen et al., 2011; Kim et al., 2014; Lee et al., 2013; Tapert et al., 2004; Wiers et al., 2015c, 2014); but see: (de Sousa Fernandes Perna et al., 2017; Oberlin et al., 2018; Schad et al., 2018; Vollstädt-Klein et al., 2010; Wiers et al., 2015c)), smells (Kareken et al., 2004; Schneider et al., 2001), and tastes (Claus et al., 2011; Courtney et al., 2015; Filbey et al., 2008) as well as their combinations (George et al., 2001; Myrick et al., 2004). In agreement with a previous meta-analysis (Schacht et al., 2013), we found that across studies, cue-induced BOLD in either the ventral striatum (i.e., nucleus accumbens) or prefrontal cortices (esp. orbital and lateral) were the most frequently associated with clinical or drinking measures.
As mentioned in Section 2.2, many of the studies reviewed here reported a significant positive relationship between the level of activation induced by alcohol cues in a person’s IS system (esp. amygdala, nucleus accumbens, and prefrontal cortical partners) and that person’s self-report of the intensity of desire or craving for alcohol induced by those cues ((Filbey et al., 2008; Fryer et al., 2013; Myrick et al., 2004; Oberlin et al., 2016; Schacht et al., 2013; Tapert et al., 2004; Wiers et al., 2015c); but see: (Ames et al., 2014b; Grüsser et al., 2004; Kim et al., 2014)). Other studies reviewed here did not test the relationship, but implied it in describing their findings (Bragulat et al., 2008; Brumback et al., 2015; George et al., 2001; Heinz et al., 2004; Huang et al., 2018; Kareken et al., 2010b, 2010a, 2004; Lee et al., 2013; Lukas et al., 2013; Schneider et al., 2001; Seo et al., 2013; Vollstädt-Klein et al., 2010). To the extent that it reflects the transformation of “wanting” into a subjective feeling of wanting, then this relationship is important for the ability of ISST to explain changes in the subjective experience of reactivity to alcohol cues in AUD, and warrants further study.
4.2.2.2. Brain responses measured using the PET technique
Alcohol cue-induced activation measured using the fMRI BOLD technique in the brain structures we are referring to as the “IS expressors” could reflect something other than IS signal-related processing; for example, it could reflect processing of a signal from the motivational system involved in avoidance, aversion, and defensive responses. However, since the IS signal is theorized to be encoded by dopamine release from projections that originate in the VTA/SNc complex, one way to ensure that alcohol cue-induced activation of the IS expressors measured with fMRI can reflect IS signal-processing is to measure the IS signal generated upon presentation of said cues. In other words, researchers need to measure cue-induce dopamine release in the IS expressors in the living human brain. Positron emission tomography (PET) can reveal cue-induced neurochemical release in specific areas of the living human brain using radioactive ligands (Phelps and Mazziota, 1985). Using radioactive raclopride, a dopamine receptor antagonist, PET has been used to show cue-induced dopamine release in the ventral striatum as a decrease in raclopride binding potential, which contains the IS expressor system labeled “Accumbens” in Figure 1, in response to the taste of alcohol (Oberlin et al., 2013). However, this does not appear to scale with alcohol use levels or AUD status or severity (Oberlin et al., 2013). It remains to be seen whether this null relationship will replicate in a larger sample or when more intense cues are presented (i.e., combinations of alcohol sight, smell, and taste).
4.2.2.3. Brain responses measured using the EEG-ERP technique
Although both fMRI BOLD and PET inform us about which brain systems (at the circuit and biochemical levels) are engaged by alcohol-associated cues, they do not directly measure the neuronal response to those cues. The fMRI BOLD signal does not reflect neural activation directly but rather reflects changes in a complex hemodynamic response that hinges upon neuro-vascular coupling via perivascular astrocytes (Shetty et al., 2012). The PET signal can reflect neurochemical release, but it is based on changes in binding of the radioactive tracer at both specific and non-specific binding sites, which may be present on both neuronal and non-neuronal cells (Phelps and Mazziota, 1985). Furthermore, both techniques measure biological signal variations that unfold over seconds (by design in fMRI or by signal-to-noise or tracer kinetics-based limitations in PET) rather than on the millisecond timescale of phasic neuronal activity (synaptic transmission) (Zoli et al., 1999). In contrast, the event-related potential (ERP) technique, derived from the scalp-recorded electroencephalogram (EEG), reflects the phasic activity across different neuronal populations following stimulus presentation (Luck and Kappenman, 2017). Studies using the ERP technique assure us that alcohol cue-related activation and dopamine release in nodes of the IS circuit using fMRI-BOLD and PET-raclopride, respectively, reflect alcohol cue-related activation of relevant neuronal populations in a variety of different stimulus presentation paradigms (Bailey and Bartholow, 2016; Bartholow et al., 2018, 2010; Dickter et al., 2014; Fleming and Bartholow, 2014; Herrmann et al., 2001; Kroczek et al., 2018; Martinovic et al., 2014; Martins et al., 2019; Petit et al., 2012; Ryerson et al., 2017; Shin et al., 2010).
In keeping with an alcohol cue ISS mechanism, alcohol use levels are positively related to the magnitude of various components in the ERP waveform (specifically, the P1, P2, N1, N2, P3, LPP, and FSW) elicited by alcohol-related visual stimuli in a variety of stimulus presentation paradigms ((Bailey and Bartholow, 2016; Bartholow et al., 2010, 2003; Fleming and Bartholow, 2014; Herrmann et al., 2001; Kroczek et al., 2018; Petit et al., 2012; Ryerson et al., 2017; Shin et al., 2010); but see: (Martinovic et al., 2014)). Less consistently, AUD status appears to be positively related to the magnitude of some of the ERP waveform components (e.g., P3) elicited by alcohol-related visual stimuli ((Dickter et al., 2014; Matheus-Roth et al., 2016; Namkoong et al., 2004); but see: (Hansenne et al., 2003; Littel et al., 2013; Petit et al., 2015)), in keeping with meta-analytic findings across substance use disorders (Littel et al., 2012). Together, these findings suggest that alcohol use levels and AUD may increase neuronal communication related to attentional (P1, P2, N1) and affective or motivational (P3, LPP) processing of alcohol-related visual stimuli as well as the need to recruit additional cognitive control resources (N2, FSW) in order regulate behavior according to task demands in the presence of task-irrelevant alcohol-related stimuli.
4.2.3. On the origin of alcohol cue-related reactivity
The ISST holds that IS attribution transforms “cold” Pavlovian conditioned reward-predictive stimuli to “hot” Pavlovian conditioned reward-predictive stimuli that act as motivational “magnets” (Berridge et al., 2009; Berridge and Robinson, 2016, 2003; Robinson and Berridge, 2001, 2000, 1993). Thus, a critical feature of the application of ISST to alcohol is that reactivity to alcohol-predictive cues reflects Pavlovian (aka classical) conditioning-like associative learning processes. The vast majority of work in preclinical non-human animal models confirms that alcohol can serve as an unconditional stimulus for appetitive (and aversive) Pavlovian conditioning (e.g., (Cofresí et al., 2019a; Cunningham et al., 2002a, 2002b; Krank, 2003; Krank et al., 2008; Srey et al., 2015)). However, it is important to establish, as best we can, that this is also true for people.
The idea that the ability of the stimulus features of alcoholic beverages to elicit attention and conscious craving for alcohol in people is due to naturally-occurring Pavlovian conditioning processes is supported by at least two controlled conditioning studies. In the first study (Field and Duka, 2002), the sight and smell of a beverage that contained a low dose of ethanol (0.2 g/kg or ≈ 10 mg/dL at 30 min post-ingestion) during training acquired the ability to elicit greater attention (measured as number of stimulus-directed gaze shifts using eye-tracking systems) and self-reported alcohol craving at test relative to the sight and smell of a beverage that did not contain ethanol during training. In the second study (Mayo and de Wit, 2016), pictures of the beverage that contained a moderate dose of ethanol (0.6 g/kg or ≈ 40 mg/dL at 30 min post-ingestion) during training acquired the ability to elicit greater attention (measured as number of stimulus-directed gaze shifts using eye-tracking systems) in a modified visual dot-probe task relative to pictures of the beverage that that did not contain ethanol during training.
The idea that the ability of the stimulus features of alcoholic beverages can come to elicit autonomic state changes in people is due to naturally-occurring Pavlovian conditioning processes is supported by at least one controlled conditioning study. In this study (Staiger and White, 1988), the sight and smell of a beverage that contained a moderate dose of ethanol (0.5 g/kg or ≈ 30 mg/dL at 30 min post-ingestion) during training acquired the ability to elicit anticipatory increases in heart rate (an index of autonomics) at test relative to the sight and smell of a beverage that did not contain ethanol during training.
Some empirical support for the idea that alcohol-related stimuli elicit changes in regional brain activity in people due to naturally-occurring Pavlovian conditioning processes can be derived from a study conducted by David Kareken and colleagues (Oberlin et al., 2018). An arbitrary neutral visual stimulus (a geometric shape) was repeatedly paired with alcohol (18 mg/dL per intravenous infusion) across sessions, and consequently, it acquired the ability to elicit an expectation of subsequent alcohol infusion. This was revealed by the fact that presentation of the newly conditioned stimulus (in the absence of alcohol) produced statistically significant activation (positive fMRI BOLD contrast) in the frontoparietal, orbitofrontal networks, anterior cingulate, and insular cortices as well as sub-threshold activation in the ventral striatum. As discussed by Kareken and colleagues, the study boasts a much larger (n=60) and better characterized sample than previous work (Kareken et al., 2012); thus, the conflicting findings in the latter were likely the result of type 1 error. However, two majors caveats apply to the (Oberlin et al., 2018) study: (1) the pattern of conditioned alcohol cue-elicited regional brain activity may reflect the particular demands of the decoy reaction time task (i.e., goal-directed search for visual stimuli); and (2) the conditioned alcohol cue failed to produce detectable biases in attention as measured by reaction time (although, as the authors argue, their paradigm was designed to measure cue-related brain activity, not cue-related attentional biases). Despite these caveats, the (Oberlin et al., 2018) study provides the strongest direct evidence to date that ethanol can serve as a purely pharmacological unconditional stimulus that supports learning about antecedent conditional stimuli in humans.
4.3. Interim summary 2
In this section, we have reviewed evidence for attribution of IS to learned alcohol-predictive cues. We found that in humans and non-human animal models alike, alcohol-predictive cues are attributed with IS. Specifically, alcohol-predictive cues acquire the ability to: (1) elicit alcohol seeking reactions (e.g., affective state change, attention, approach, and conscious craving for alcohol); (2) serve as conditional or secondary reinforcers (at least in non-human animal models) that can maintain reactivity in the absence of immediate primary reinforcement; (3) induce or invigorate instrumental alcohol seeking actions; and (4) engage the IS circuitry. We have also reviewed the evidence that alcohol cues undergo ISS. We found that in humans and non-human animal models alike, the amount of IS attributed to alcohol-predictive cues appears to increase as a function of alcohol involvement. Specifically, greater alcohol involvement makes alcohol-predictive cues able to: (1) elicit higher levels of alcohol seeking reactions; (2) better serve as conditional reinforcers (at least in non-human animals); (3) more strongly induce or invigorate instrumental alcohol seeking actions (at least in non-human animals); and (4) more strongly activate the IS circuitry (at least in humans). In humans, the in-the-moment intensity of conscious craving for alcohol induced by alcohol cues appears to be positively related to the degree of activation these cues induce in the IS circuitry, especially the amygdala, ventral striatum (nucleus accumbens), and prefrontal cortices, a relationship requiring further study that is important for the ability of ISST to explain changes in the subjective experience of alcohol cue reactivity in AUD. Finally, given that the prefrontal cortices are believed to mediate cognitive control processes (Barbas, 2000; Braver, 2012; Inzlicht et al., 2015; Ridderinkhof, 2004), the present findings also suggest that incidental exposure to alcohol cues may impair on-going alcohol use-unrelated behavior and goal pursuit by drawing away attentional resources available to cognitive control processes or releasing inappropriate behavioral responses that create conflict and require the recruitment of additional cognitive control resources for successful completion of on-going behavior. These kinds of effects from incidental alcohol cues have been reported in human behavioral laboratory tasks (Fryer et al., 2013; Nikolaou et al., 2013; Sommer et al., 2017).
5. Discussion
5.1. General
Nona, Hendershot, and Lê (2018) recently reviewed the evidence for sensitized behavioral, physiological, and/or subjective responses to alcohol intoxication as a mechanism in AUD. In the present review, we set out to examine the evidence for sensitized IS attribution to alcohol-predictive cues (viz., sensitized cue-triggered “wanting”) as a neuropsychological mechanism in AUD. It is important to note that it remains an open question whether the construct of IS attribution developed in non-human animals truly exists in humans, and if so, whether it manifests in the same ways, undergoes sensitization, and affects subjective experience. The answers to these questions have implications for ability of the ISST to explain important behavioral phenomena in alcohol addiction such as the progressive loss of control over use, use despite negative consequences, and the subjective experience of desire and craving including preoccupation with alcohol-related thoughts. It may turn out to be the case that ISST can explain the former, but not the latter. Our review cannot provide definite answers to these questions. Nevertheless, it indicates that across different units of analysis that may reflect manifestations of the IS attribution in humans and non-human animals, the available evidence tends to be consistent with predictions for sensitized alcohol cue-triggered “wanting” (viz., alcohol cue IS sensitization [ISS]) as a mechanism in AUD.
Many predictions for alcohol cue ISS as a mechanism in AUD remain underexamined across levels of biological organization in humans and non-human animals. Some underexamined predictions at the behavioral and neurobiological units of analysis are especially low-hanging fruit worth pointing out to researchers. First, to the extent that the IS attributor can be identified with dopamine cells in the VTA/SNc complex, alcohol-associated cues should be able to activate dopamine cells in the VTA/SNc complex of non-human animals, but we were unable to find direct evidence bearing on this prediction. Although, to the extent that the IS signal can be identified with cue-induced phasic dopamine release from dopamine terminals in the IS expressors, it should be noted that there is a growing body of evidence indicating that the IS signal can occur in the absence of IS attributor activation (Cachope and Cheer, 2014). Second, we do not know whether chronic ethanol exposure is able to shift the balance of excitatory and inhibitory drive or tone on those cells or their intrinsic excitability. Third, a systematic empirical exploration of chronic ethanol exposure profile parameter-dependent functional changes within and between IS system components that could mediate alcohol cue ISS is lacking. Fourth, IS-attributed alcohol cues are predicted to elicit changes in autonomic state that support alcohol seeking reactions and actions at the level of physiology. However, little to no attention has been paid to autonomics when modeling alcohol cue reactivity in non-human animals, and in humans, little attention has been paid to sensitization of cue-induced autonomic changes as a function of alcohol involvement. Fifth, IS-attributed alcohol cues are predicted to serve as secondary or conditional reinforcers that are able to maintain reactivity and behavior in the absence of immediate primary alcohol reinforcement (i.e., post-ingestive psychopharmacology). There is substantial evidence from non-human animal models consistent with this prediction, but no systematic examination of how the level of ethanol exposure determines the degree to which alcohol cues serve as secondary reinforcers for alcohol seeking. This is an important, if underappreciated, behavioral function of IS-attributed cues because it is theorized to mediate the persistence of alcohol cue reactivity. Despite the relevance of this particular property to AUD treatment and relapse, we were unable to find any studies examining the conditioned reinforcing property of alcohol-associated cues in humans. Sixth, given the multitude of different behavioral and brain measures collected as putative indicators of IS attribution and/or advanced as reflecting ISS in humans, factor analytic work is warranted to test which of these measures load onto the same latent construct as well as to determine which measures are the most reliable and/or valid indicators of that construct (Wardle et al., 2018).
It is important to note that gender/sex differences continue to emerge for the acute effects of alcohol and its cues in the human laboratory model literature (Bates et al., 2011; Chaplin et al., 2008; Hartwell and Ray, 2013; Kaplan et al., 1985; Rubonis et al., 1994; Udo et al., 2009). Yet female organisms are often absent in studies from pre-clinical non-human animal model literature. Although this omission is being addressed, much work is ahead for pre-clinical researchers working with non-human animal models. Once the omission has been corrected, a thorough examination of potential gender/sex differences in ISST-derived predictions for this unique etiological pathway to AUD may be conducted in the vein of (Barker and Taylor, 2017).
A major issue worth raising is that the degree to which ‘natural’ alcohol cues (e.g., the sight, smell, and taste of the preferred alcoholic beverage) in humans operate like the conditioned alcohol cues studied in human and non-human animal laboratory models remains to be established. This is an important issue for the applicability and translation of ISST into a unique etiological pathway for AUD (and more broadly, for SUD). ISST addresses a psychological property (IS) that may or may not be attributed to learned conditional predictive stimuli for unconditional rewarding stimuli including drugs of abuse such as alcohol. However, not much attention has been paid to the ability of alcohol to serve as a pharmacological unconditional stimulus (US) for conditional stimulus (CS) or ‘cue’ learning in humans. Our literature review identified only a handful of controlled alcohol cue conditioning studies in humans, each using different administration procedures, different conditioning parameters, and different measures of cue reactivity. Consequently, more controlled conditioning studies are warranted, especially within-subject studies comparing ‘artificial’ alcohol cues conditioned in the human laboratory to ‘natural’ alcohol cues that were putatively conditioned over the person’s alcohol use history.
A related issue is that if ‘natural’ alcohol cues are truly conditioned over a person’s alcohol use history, then these cues are conditioned within specific emotional, physical, social, and temporal contexts. The natural context for alcohol use, and thus, the context in which ‘natural’ cues are conditioned, are difficult to simulate in the human laboratory. Thus, a major caveat applies to human laboratory studies, especially those using functional neuroimaging techniques, which involve the use of equipment—and supine body posture (Harmon-Jones and Peterson, 2009; Price and Harmon-Jones, 2011)–that in many cases opposes any degree of natural physical and motivational context. These studies are typically done in a context that has never been associated with alcohol use, and often explicitly signal the non-availability of alcohol to participants. This experimental setting confounds the interpretation of negative results. In order to study reactivity to ‘natural’ alcohol cues in their ‘natural’ contexts, researchers should consider the combined use of ambulatory assessment and mobile human neuroimaging technology.
A final consideration for evaluating the model-derived predicted relationship between IS attribution and alcohol involvement in humans is that the latter has multiple causes and functions. Additionally, there may be other liability factors that moderate the magnitude of association between and within individuals. Some individuals may be more vulnerable than others to ISS as a pathway to AUD, and samples that contain more of these individuals may show stronger sample-level evidence for alcohol cue ISS.
5.2. Alcohol subjective response (ASR) may moderate AUD risk via alcohol cue ISS pathway
One trait-like liability factor that strongly moderates risk for AUD, alcohol subjective response (ASR) phenotype, may do so by conferring differential vulnerability to ISS as a pathway to AUD. We introduce ASR phenotype and discuss evidence in favor of this idea below.
5.2.1. The link between alcohol subjective response (ASR) phenotype and risks for AUD
The subjective experience of alcohol’s pharmacological effects primarily can be ascribed to two factors. The first, pharmacokinetics, refers to factors that determine the amount of alcohol circulating through the body at any given point in time following ingestion. Variation in pharmacokinetic factors can be due to both state (e.g., amount of food in the stomach, dehydration, other drugs in circulation, acquired metabolic tolerance) and trait factors (e.g., baseline expression level and activity profiles of alcohol-metabolizing enzymes) as well as ingestion route and rate (Cederbaum, 2012). The second, pharmacodynamics, refers to factors that determine the biochemical and physiological effects of alcohol. Variation in pharmacodynamic factors can be due to both state (e.g., current level of certain hormones, other drugs in circulation) and trait factors (e.g., baseline activity level and dynamics in certain brain circuits) (Brown et al., 2007; Haughey et al., 2008; Sharko et al., 2016, 2013; Yoder et al., 2005).
In humans, the subjective experience of alcohol’s pharmacological effects, i.e., the construct of a subjective response to alcohol as a pharmacological stimulus, can be summarized along 3–4 dimensions, some of which appear to develop over the course of a person’s drinking history, viz., as the person gains experience with alcohol as a pharmacological stimulus (Bujarski et al., 2015; Ray et al., 2009). Importantly, even after controlling for inter-individual variation in pharmacokinetics, there is considerable inter-individual variation in self-reported subjective response to the pharmacological effects of alcohol (Bujarski et al., 2015; Gilman et al., 2012; Ray et al., 2007).
There are at least two broadly defined alcohol subjective response (ASR) phenotypes. Specifically, some individuals may have (an initially) low level of subjective response (LLR)—they recollect having required more drinks to feel any effect, dizziness, or stumbling than other individuals in questionnaire-based studies (Schuckit et al., 1997). Similarly, some individuals appear to be less sensitive to alcohol’s sedative-like properties yet more sensitive to its stimulant-like properties (LSedHStim)—they feel less “down” or “sluggish” or “slow thoughts” and more “excited” or “excited” or “up” than other individuals after equivalent alcohol doses in controlled laboratory studies (Davidson et al., 2002; Holdstock and de Wit, 1998; King et al., 2002; Martin et al., 1993; Newlin and Thomson, 1990; Rueger et al., 2009; Rueger and King, 2013) Given differences in how these groups of individuals (LLR versus HLR and LSedHStim versus HSedLStim) were identified, it remains to be seen whether they stem from different or overlapping subpopulations. However, we can tentatively treat LLR and LSedHStim individuals as belonging to one population—the low sensitivity (LS) ASR phenotype population—and HLR and HSedLStim individuals as belonging a different population—the high sensitivity (HS) ASR phenotype population.
The LS ASR phenotypes confer risk for AUD that the HS ASR phenotypes do not (Morean and Corbin, 2010; Quinn and Fromme, 2011). LS individuals present more AUD symptoms than HS individuals (Bartholow et al., 2010; Fleming and Bartholow, 2014; King et al., 2016, 2014). LS individuals tend to drink more frequently, more heavily, and more hazardously than HS individuals (Bartholow et al., 2010; Fleming and Bartholow, 2014; Hinckers et al., 2006; King et al., 2011, 2002; Schuckit et al., 2005; Shin et al., 2010). Compared to HS individuals, LS individuals are also more likely to experience negative legal, social, and occupational consequences of alcohol use (Bartholow et al., 2010; Fleming and Bartholow, 2014; Schuckit et al., 2017, 2005; Schuckit and Smith, 2006). LS individuals are more likely to experience hangovers than HS individuals, but only because LS individuals drink much more—in fact, they are less likely to experience hangover per unit alcohol (Piasecki et al., 2012). Similarly, LS women are more likely to report regretted sexual activity than HS individuals, but only because LS women drink much more—in fact, they are less likely to report regretted sexual activity per unit alcohol (Hone et al., 2017). The unique risk for AUD conferred by LS ASR phenotype may be at least in part explained by ISST/alcohol ISS. The rest of the unique risk conferred by LS ASR phenotype might relate to other psychological mechanisms such as heavy drinking-induced adaptations in the motivations for alcohol use (Cooper et al., 1995) and self-selection into heavy drinking environments and social groups (Schuckit, 1998; Schuckit and Smith, 2000).
To the extent that ASR phenotype is inherited, it may be a cause, rather than consequence, of heavy alcohol use. Twin studies have estimated that its heritability is 0.60 (Heath et al., 1999; Heath and Martin, 1991; Viken et al., 2003). ASR phenotype has also been shown to be relatively stable within individuals (King et al., 2016, 2014). However, there is also evidence for some degree of change in phenotype within individuals (King et al., 2016; Morean and Corbin, 2008), perhaps as a function developmental stage-related changes in alcohol involvement.
5.2.2. The link between ASR phenotype and the alcohol cue ISS pathway to AUD
The biases in attention and approach tendency predicted by ISST applied to alcohol and observed in heavy drinking individuals and individuals with AUD may be a function of ASR phenotype. In keeping with this idea, compared with HS individuals, LS individuals exhibit faster reaction times to alcohol cued locations in the modified dot-probe task controlling for past 30 day alcohol use (Shin et al., 2010). Similarly, LS individuals exhibit greater implicit alcohol approach tendency as measured by the alcohol AAT as well as faster reaction times to alcohol-cued targets in the Cued Go/NoGo Task (CGNT) compared to HS individuals (Fleming and Bartholow, 2014). Unlike HS individuals, LS individuals exhibited lower accuracy on response inhibition (NoGo) probe trials in the CGNT when the cue was an alcoholic beverage image relative to a neutral image, indicating that LS individuals were less able to inhibit pre-potent Go responses in the face of an alcohol cues (Fleming and Bartholow, 2014).
LS individuals also appear to be more susceptible to increases in conscious alcohol craving elicited by real-world drinking-associated contexts (e.g., time of day, weekend, bar/restaurant location, recent tobacco use) than HS individuals (Trela et al., 2018). This may help explain why LS individuals tend to drink more frequently than HS individuals. More hazardous alcohol use patterns among LS individuals may be explained by the fact that these individuals tend to “drink too much, too fast” in their drinking episodes than HS individuals (Trela et al., 2016). It is also likely that “overdrinking,” i.e., drinking more than intended (Bishop and Rodriquez Orjuela, 2018), may be more frequent among LS individuals, although this remains to be determined.
The exaggerated brain response to alcohol cues predicted by ISST applied to alcohol and observed in heavy drinking individuals and individuals with AUD may be a function of ASR phenotype. In the modified dot-probe task, LS individuals exhibit larger amplitude P1, indicating greater early attentional orienting, and a smaller amplitude IIN (ipsilateral invalid negativity), indicating lower attentional re-orienting (away from alcohol-cued locations), than HS individuals (Shin et al., 2010). Unlike HS individuals, LS individuals also exhibit a larger amplitude P3 to pictures of alcoholic beverages than to pictures of other beverages and/or neutral objects in visual categorization and evaluation tasks (Bartholow et al., 2010; Martins et al., 2019), putatively indicating that, at the level of neural processing, alcohol cues were evaluated as having greater affective/motivational significance. Additionally, in the CGNT, in the few response inhibition probe trials on which LS individuals were able to inhibit pre-potent Go response in the face of an alcohol cue, LS individuals exhibited larger amplitude N2, indicating increased stimulus-related conflict, and larger amplitude P3, indicating that more processing was necessary to successfully inhibit the pre-potent Go response in the face of an alcohol cue, relative to HS individuals (Fleming and Bartholow, 2014).
Work in non-human animals provides additional support for the idea that individual differences in sensitivity to alcohol intoxication are linked to individual differences in sensitivity to the appetitive conditioning effects of alcohol, and thus, increased susceptibility to alcohol cue ISS. Rodents bred for LS-like phenotypes tend to be more sensitive to the appetitive conditioning effects of alcohol (Beckstead and Phillips, 2009; Crabbe et al., 1992; Fish et al., 2012; Risinger et al., 1994; Shen et al., 1995), but see: (Files et al., 1996; Sanchez et al., 1996)). Similarly, rodents bred for greater sensitivity to some of the appetitive conditioning effects of alcohol tend to be more sensitive to its other appetitive conditioning effects ((Ciccocioppo et al., 2001, 1999; Murphy et al., 1989; Oster et al., 2006; Toalston et al., 2008), but see: (Stewart et al., 1996)), and more importantly, tend to exhibit LS-like phenotypes (Agabio et al., 2001; Colombo et al., 1998; Murphy et al., 2002; Päivärinta and Korpi, 1993; Waller et al., 1986). There is also some support for this covariation in rodent populations that were not selected for one trait or the other ((Chappell and Weiner, 2008; Spuhler and Deitrich, 1984), but see: (Gauvin et al., 1993; Khanna et al., 1990)). More extensive testing of this covariation is warranted in non-human animals, especially using paradigms that measure different facets of IS attribution to alcohol cues. Additionally, there is uncertainty about the degree to which LS-like phenotypes in non-human animals can model human LS phenotypes given that the latter are defined in terms of subjective responses to alcohol as opposed to objective responses that can be measured across species (Crabbe et al., 2010). Nevertheless, the available evidence from non-human animals is consistent with a link between ASR phenotype (at least its inherited/genetic component) and susceptibility to alcohol cue ISS.
Together, these pieces of evidence suggest that LS individuals may be more susceptible to alcohol cue ISS than HS individuals. However, more work is warranted to test this hypothesis. There are at least three ways in which LS ASR phenotype, alcohol ISS, and AUD could be interrelated. First, LS ASR phenotype may be the overt manifestation of a neurobiological endophenotype that is a trait marker for vulnerability to ISS as a pathway to AUD (or addictions, in general). Second, LS ASR phenotypes foster heavy alcohol use, and heavy alcohol use can induce alcohol ISS, such that alcohol ISS is a downstream consequence of LS ASR phenotype. Third, a combination of the former two. Prospective studies are needed to tease apart these possibilities.
5.3. Treatment implications of alcohol cue ISS as a pathway to AUD
Alcohol cue ISS has implications for certain approaches to treatment and more generally, for treatment outcomes, especially relapse. These implications underscore the need for further investigation of this unique etiological pathway in both humans and non-human animal models.
5.3.1. Post-treatment susceptibility to alcohol cue-related risk for lapses and relapse to AUD
It is highly unlikely that alcohol cue ISS accounts for all cases of ineffective AUD treatment. However, individuals who undergo alcohol cue ISS may comprise a large proportion of the population of people who relapse more quickly or more frequently after treatment. There are 2 lines of evidence supporting this idea. First, the higher levels of cue reactivity among individuals suffering from AUD (reviewed in the paper). Second, the role of cue reactivity in post-AUD treatment lapse/relapse rates.
Post-treatment recovery/relapse rates are strongly determined by alcohol-related cues in at least two ways (Marlatt, 1996). First, individuals with AUD may have learned to cope with stress related to environmental demands (e.g., accidents, evaluations, financial difficulty, interpersonal conflict, social pressure) by using alcohol. The negative feelings induced by these stressors may become conditioned as an alcohol-predictive cues in some individuals (Hogarth and Hardy, 2018; Litt et al., 1990; Rubonis et al., 1994; Stasiewicz et al., 1997; VanderVeen et al., 2016). Second, individuals with AUD may encounter alcohol-predictive cues in their everyday environments (e.g., hidden bottles, passing by a bar, alcoholic beverage advertising, drinking buddies). Cue-triggered behavioral and physiological reactions (e.g., attentional bias, brain circuit activation, heart rate, salivation) measured at treatment time predict subsequent lapses to drinking and relapse to AUD (Braus et al., 2001; Cox et al., 2002; Garland et al., 2012b; Grüsser et al., 2004; Papachristou et al., 2014; Rohsenow et al., 1994), but see (Snelleman et al., 2015). Given the possibility that alcohol cue ISS entails exaggerated cue-triggered Pavlovian alcohol seeking reactions, individuals that underwent this pathway to AUD may be driving the relationship between alcohol cue reactivity and post-treatment lapse/relapse rate.
One way in which treatment may leave individuals vulnerable to the situational re/lapse-risk that cue reactivity creates is that many psychosocial treatment options do not directly address or attempt to reduce cue reactivity, and those that do have limited efficacy. A second way in which this alcohol cue reactivity-based vulnerability may persist is that existing pharmacological treatment options alone may or may not be able to reverse the neuroadaptations that mediate alcohol ISS.
5.3.2. Response to behavioral treatments for alcohol cue-elicited reactivity
Broadly speaking, there are two behavioral treatments for AUD that aim to reduce reactivity to alcohol cues and thereby reduce relapse rates. The first, older treatment is cue exposure therapy (CET). In CET for AUD, individuals are repeatedly presented with the sights, smells, sounds, and tastes experienced during alcohol use without subsequent ingestion and/or intoxication until these cues cease to elicit behavioral, physiological, and/or subjective (craving) reactions (Monti et al., 1993b; Monti and Rohsenow, 2003; Rankin et al., 1983; Vollstädt-Klein et al., 2011). Importantly, decreased drinking and reduced AUD relapse rates have been reported following use of CET as either an adjunct to standard treatment or as a stand-alone treatment (Drummond and Glautier, 1994; Loeber et al., 2006; Monti et al., 2001, 1993b; Rohsenow et al., 2001). The second, more recently developed treatment is cognitive bias modification (CBM). This family of interventions uses modified versions of behavioral tasks typically used to measure alcohol cue-elicited attentional capture and approach tendency—as well as modified versions of tasks used to measure implicit or explicit attitudes toward alcohol and its cues, viz., “liking” responses—to train the opposite behavioral response to the same cues (Wiers et al., 2013). It works: attentional capture and approach tendency are found to be reduced and/or replaced with attentional disengagement and avoidance tendency, respectively, at least when immediately after CBM (Eberl et al., 2013; Fadardi and Cox, 2009; Field et al., 2007; Field and Eastwood, 2005; Rinck et al., 2018; Schoenmakers et al., 2010, 2007; Wiers et al., 2011, 2010). Importantly, decreased drinking and reduced AUD relapse rates have been reported following use of CBM as an adjunct to standard treatment (Cox et al., 2015; Eberl et al., 2013; Rinck et al., 2018; Schoenmakers et al., 2010; Wiers et al., 2015a, 2011).
It would thus seem that two exceptionally effective treatments are available against alcohol cue reactivity. Unfortunately, this is not the case. The treatments do work, but not as well as clinicians, patients, and researchers might like. Recent meta-analyses across existing randomized clinical trials have indicated that CET (Mellentin et al., 2017) and CBM (Boffo et al., 2019) alike have at best a small effect of unknown reliability on AUD treatment outcomes. One explanation for this state of affairs is that even when CET and CBM are successful in reducing alcohol cue reactivity measured in the treatment setting, people may remain at risk for the return of alcohol cue reactivity in their natural environment due to constraints inherent to the learning and memory process on which they both rely.
Although some researchers might see CET and CBM as strikingly different approaches to reducing conditioned cue reactivity, their limited clinical effects likely stem from a shared reliance on training new (alternative) responses to cues for which associations already exist in long-term memory. CBM effects are believed to reflect, at least in part, the training of a specific alternative response to a previously conditioned cue: a response in the opposite direction than the previously-reinforced response (Wiers et al., 2013). Similarly, despite concerns about procedural differences between CET and the non-human animal learning paradigms that inspired it (Conklin and Tiffany, 2002), CET effects may reflect, at least in part, the training a specific alternative response to a previously conditioned cue: omission of the previously-reinforced response (Colwill, 1991; Rescorla, 1997, 1993). Nevertheless, studies of non-human animals in which a response is first conditioned to a cue, and then “treated” by training the animal to omit or suppress that response have shown unequivocally that the original cue reactivity can and does readily reemerge after seemingly successful “treatment.” This is evidenced by the post-“treatment” return of reactivity phenomena known as spontaneous recovery and cue-induced reinstatement (Bouton and Bolles, 1979a, 1979b; Rescorla and Heth, 1975; Robbins, 1990). With the exception of context (place cue)-induced reinstatement (Pitchers et al., 2017; Saunders et al., 2014), these relapse-like effects are especially pronounced with IS-attributed cues in non-human animals (Saunders et al., 2013; Saunders and Robinson, 2011; Yager et al., 2015; Yager and Robinson, 2015, 2013, 2010). The rate of reduction of alcohol cue reactivity within- and between-“treatment” sessions, and the size of post-“treatment” relapse-like return of reactivity phenomena, are likely to be a function of how much ISS has taken place. In part, this may be because more ISS means that IS-attributed cues provide greater conditional reinforcing value, a property of IS that may make it difficult for some individuals to distinguish reinforcement from non-reinforcement of the original cue associations—something which has been demonstrated in non-human animals (Ahrens et al., 2016)—precluding subsequent reinforcement of alternative responses to the cue. Despite these limitations, it is people who progressed to AUD via alcohol cue ISS who may derive the most clinical benefit, at least in the long run, from CBM and CET since reductions in alcohol cue reactivity may be irrelevant to recovery from AUD in people who progressed to AUD via other etiological pathways.
Finally, it is worth mentioning that overcoming these limitations is the focus of an active area of research. Work in this area suggests that it may be possible to enhance learning within-session therapeutic learning for both CBM and CET using transcranial stimulation of the prefrontal cortices (den Uyl et al., 2018, 2017, 2016; Wietschorke et al., 2016) or acute pharmacological augmentation of memory consolidation (Kiefer et al., 2015; MacKillop et al., 2015). Additionally, it may be possible to harness a period of memory lability induced by memory retrieval—the so-called memory reconsolidation window (Nader and Einarsson, 2010; Nader and Hardt, 2007)—to alter or “erase” alcohol cue reactivity by interfering with or disrupting its neurobiological substrates using pharmacological tools (e.g., in non-human animal models: (Barak et al., 2013; Milton et al., 2012; Schramm et al., 2016; von der Goltz et al., 2009); initial evidence in humans: (Das et al., 2018). It may also be possible to “update” alcohol cues to a lower level of IS simply by deploying therapeutic learning during the memory reconsolidation window (e.g., in non-human animal models: (Cofresí et al., 2017); initial evidence in humans: (Das et al., 2015; Hon et al., 2016)).
5.3.3. Response to pharmacological treatments attempting to dampen alcohol craving
To the extent that the subjective experience of craving for alcohol (viz., a strong explicit desire or urge to drink, perceived difficulty in resisting a drink if it were offered) is a core symptom of AUD and/or an important factor in lapse/relapse to AUD, medications that attempt to dampen the subjective experience of craving for alcohol among individuals with AUD (for review, see: (Haass-Koffler et al., 2014)) can play a critical role in harm reduction approaches to management of AUD among non-treatment seeking individuals as well as supporting behavior change and psychosocial treatment engagement among treatment-seeking individuals. In short, anti-craving medications can improve and/or save lives. Among these medications are several pharmaceuticals already approved for AUD treatment (e.g., acamprosate, naltrexone), some pharmaceuticals that are used off-label for AUD treatment (e.g., fluoxetine, bupropion), and others at earlier stages of the medication development pipeline (e.g., aripiprazole).
Given diverse mechanisms of action, different anti-craving medications may differentially disrupt subjective experiences of craving arising from the two IS system pathways to craving (Figure 1: paths [i] and [j]). Alcohol use-related obsessive or intrusive thoughts or desires may reflect hyperactivity in either the indirect path (i) and/or the direct path to craving (j), and anti-craving medications may decrease the level of activity in these IS system pathways such that craving is not experienced. In this light, it might be tempting to see anti-craving medications as the pharmaceutical equivalent to behavioral treatments like CET and CBM. That is, it may be tempting to conceptualize the anti-craving effects of these pharmaceuticals as “response prevention” or “response modification.” Indeed, acutely or chronically disrupting activity in pathways (i) and (j) may prevent alcohol use that would have otherwise occurred as a response to conscious craving for alcohol. However, implicit alcohol “wanting” (Figure 1: paths [f], [f.1], [f.2], and [h]) may still impel alcohol seeking and drinking behaviors in certain contexts despite the absence of conscious craving for alcohol and/or in spite of conscious intentions to abstain from or moderate alcohol use. Moreover, the subjective experience of craving may most often arise from brain systems other than the IS system, such as those involved in mediating non-automatic appraisal/evaluation processes that operate at the level of consciousness. Craving originating in these systems may be entirely unamenable to acute or chronic disruption of IS system activity. Moreover, to the extent that certain brain systems are in place to gate the direct and indirect IS system pathways to craving, then in some individuals alcohol-related obsessive or intrusive thoughts may arise not from hyperactivity in these pathways, but rather from hypoactivity at the gates. Amplifying activity at the gates may require medications that attempt to boost or restore activity in high-level executive function and response control-related brain systems. Nevertheless, to the extent that currently available and future anti-craving medications facilitate reductions in the intensity and/or frequency of alcohol exposure, they may be able to arrest the progression of alcohol cue ISS and begin to reverse its underlying neuroadaptations. In doing so, they may help treat a potential substrate for pathological craving and preoccupation with alcohol. Thus, the efficacy of anti-craving medications and their ultimate clinical benefit may be greater among individuals who have progressed to AUD via alcohol cue ISS.
5.4. Conclusion
There is sufficient empirical evidence to support the idea that the mechanism originally outlined in the incentive salience sensitization theory of addiction (ISST) (Robinson and Berridge, 1993) may be at the core of alcohol use disorder (AUD) etiology for some individuals. Throughout this review article we have referred to this etiological pathway as “alcohol cue ISS” to emphasize that it is the motivational significance and behavioral impact of alcohol-predictive cues that is predicted to undergo sensitization, not the hedonic response to alcohol ingestion. In other words, the mechanism of ISS involves sensitization of alcohol “wanting,” not “liking.” Susceptibility to this etiological pathway may be moderated by individual differences on traits such as differential sensitivity to alcohol ingestion-induced feelings stimulation v. sedation. Individuals who have undergone this etiological pathway may find themselves especially at risk for cue reactivity-based relapse to AUD after treatment. In general, there is need for continued investigation of ISST-predicted mechanisms, across units of analysis, in the development of disordered alcohol use.
Highlights.
Alcohol cues are attributed with incentive salience in humans and non-human animals
Alcohol can render the incentive salience circuitry sensitized to alcohol cues
Humans and non-human animals can exhibit sensitized reactivity to alcohol cues
Incentive salience sensitization may drive alcohol use disorder (AUD) in some people
More work is needed on the role of this neuropsychological mechanism in alcohol use and AUD
Acknowledgements
This work was supported by NIH NIAAA R01-AA-025451 (BDB, TMP) and NIH NIAAA T32-AA-013526 (RUC) as well as the University of Missouri Department of Psychological Sciences Mission Enhancement Fund (RUC). We thank Dr. Terry Robinson for thoughtful comments and suggestions on earlier versions of this manuscript.
Funding Sources: NIH NIAAA R01-AA-025451 (BDB, TMP), NIH NIAAA T32-AA-013526 (RUC), University of Missouri Department of Psychological Sciences Mission Enhancement Fund (RUC)
Role of Funding Source: The funding sources had zero involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interests: None
The incentive salience of alcohol cues has also been proposed as one of the core domains of neurobiologically-informed clinical assessment for AUD (Kwako et al., 2016).
In terms of the RDoC matrix, the ISST is a framework for understanding how two of the primary constructs, Approach Motivation and Reward Learning, in one research domain, the Positive Valence System, might promote addictive behavior. Of the 39 constructs in the RDoC matrix, 7 were identified as being of utmost importance for understanding addictive behavior by a recent Delphi consensus study (Yücel et al., 2019), and of those 7 constructs, 1 was Reward Learning, and 3 were 3 of the 4 sub-constructs that constitute Approach Motivation.
A comprehensive survey of all the genes and molecules that preclinical research on non-human animal models has demonstrated can change as a function of drug exposure, as a function of associative and non-associative learning, and/or as a function of their interaction, is beyond the scope of this review. Suffice it to say that the list of genes is long and ever-growing.
An appreciation for the coordinated nature of functional adaptation across levels of biological organization (e.g., how changes in different molecules can produce changes synaptic plasticity processes) may be garnered from innovative simulation studies such as (Blackwell et al., 2018).
Phasic refers to event-locked dopamine release on a sub-second timescale due to synchronized bursts of high-frequency action potentials (AP) at axon-terminal boutons, and is contrasted with tonic release due to asynchronous low-frequency AP at the same. Tonic release creates the “basal tone” (steady-state extracellular concentration) that regulates clearance mechanisms (e.g., autoreceptors, transporters, degradative enzymes) in the terminal field. Readers desiring more in-depth coverage of the dopamine neurotransmission system are referred to: (Grace, 2000; Marinelli and McCutcheon, 2014; Rice et al., 2011; Vallone et al., 2000).
Conditional or secondary reinforcers are stimuli that support new learning and/or behavioral performance as a function of their learned, meaningful relationship to a primary reinforcer such as resources necessary for survival. When learned cues acquire conditional reinforcing properties, they can motivate behavior in the absence of primary reinforcement and sometimes even in the face of punishment. The most commonly cited “real world” example of a secondary reinforcer is token money. In some societies, token money is able to motivate some individuals to learn and perform new actions, often vigorously and repeatedly, for long stretches of time. The value of token money to a person in such societies is conditional upon learning about the extent to which and ways in which token money can be traded for desired and/or needed goods and services.
References
- Acosta G, Hasenkamp W, Daunais JB, Friedman DP, Grant KA, Hemby SE, 2010. Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Res 1318, 144–154. doi: 10.1016/j.brainres.2009.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Jonsson S, Söderpalm B, Ericson M, 2013. Region-specific depression of striatal activity in Wistar rat by modest ethanol consumption over a ten-month period. Alcohol 47, 289–98. doi: 10.1016/j.alcohol.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Agabio R, Carai MAM, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G, 2001. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol 23, 123–126. doi: 10.1016/S0741-8329(00)00144-0 [DOI] [PubMed] [Google Scholar]
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE, 2016. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behav. Brain Res 296, 418–430. doi: 10.1016/j.bbr.2015.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón DE, Delamater AR, 2018. Outcome-specific Pavlovian-to-instrumental transfer (PIT) with alcohol cues and its extinction. Alcohol. doi: 10.1016/j.alcohol.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albery IP, Sharma D, Noyce S, Frings D, Moss AC, 2015. Testing a frequency of exposure hypothesis in attentional bias for alcohol-related stimuli amongst social drinkers. Addict. Behav. Reports 1, 68–72. doi: 10.1016/j.abrep.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Graef JD, Hammarback JA, Nordskog BK, Burnett EJ, Daunais JB, Bennett AJ, Friedman DP, Suomi SJ, Godwin DW, 2012. Disruptions in serotonergic regulation of cortical glutamate release in primate insular cortex in response to chronic ethanol and nursery rearing. Neuroscience 207, 167–181. doi: 10.1016/j.neuroscience.2012.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Anton RF, Babor TF, Carbonari J, Carroll KM, Connors GJ, Cooney NL, Del Boca FK, DiClemente CC, Donovan D, Kadden RM, Litt MD, Longabaugh R, Mattson ME, Miller WR, Randall CL, Rounsaville BJ, Rychtarik RG, Stout RL, Tonigan JS, Wirtz PW, Zweben A, 1998. Matching patients with alcohol disorders to treatments: Clinical implications from Project MATCH. J. Ment. Heal 7, 589–602. doi: 10.1080/09638239817743 [DOI] [Google Scholar]
- Allen JP, Mattson ME, Miller WR, Tonigan JS, Connors GJ, Rychtarik RG, Cooney NL, 1997. Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH posttreatment drinking outcomes. J. Stud. Alcohol 58, 7–29. doi: 10.15288/jsa.1997.58.7 [DOI] [PubMed] [Google Scholar]
- Ames SL, Grenard JL, He Q, Stacy AW, Wong SW, Xiao L, Xue G, Bechara A, 2014a. Functional imaging of an alcohol-Implicit Association Test (IAT). Addict. Biol 19, 467–81. doi: 10.1111/adb.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames SL, Wong SW, Bechara A, Cappelli C, Dust M, Grenard JL, Stacy AW, 2014b. Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behav. Brain Res 274, 382–9. doi: 10.1016/j.bbr.2014.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, MacKillop J, 2014. Understanding the Effects of Stress and Alcohol Cues on Motivation for Alcohol via Behavioral Economics. Alcohol. Clin. Exp. Res 38, 1780–1789. doi: 10.1111/acer.12423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areal LB, Hamilton A, Martins-Silva C, Pires RGW, Ferguson SSG, 2019. Neuronal scaffolding protein spinophilin is integral for cocaine-induced behavioral sensitization and ERK1/2 activation 11 Medical and Health Sciences 1109 Neurosciences. Mol. Brain 12, 1–16. doi: 10.1186/s13041-019-0434-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augier E, Dulman RS, Licheri V, Augier G, Domi E, Barchiesi R, Farris SP, Natt D, Mayfield RD, Adermark L, Heilig M, 2018. A molecular mechanism for choosing alcohol over an alternative reward. Science (80-.). 360, 1321–1326. doi: 10.1126/science.aao1157 [DOI] [PubMed] [Google Scholar]
- Babor TF, Dolinsky ZS, Meyer RE, Hesselbrock M, Hofmann M, Tennen H, 1992. Types of alcoholics: concurrent and predictive validity of some common classification schemes. Br. J. Addict 87, 1415–1431. doi: 10.1111/j.1360-0443.1992.tb01921.x [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y, 2011. Opiate versus psychostimulant addiction: The differences do matter. Nat. Rev. Neurosci 12, 685–700. doi: 10.1038/nrn3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K, Bartholow BD, 2016. Alcohol words elicit reactive cognitive control in low-sensitivity drinkers. Psychophysiology 53, 1751–1759. doi: 10.1111/psyp.12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, Janak PH, Ron D, 2013. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat. Neurosci 16, 1111–7. doi: 10.1038/nn.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, 2000. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res. Bull 52, 319–330. doi: 10.1016/S0361-9230(99)00245-2 [DOI] [PubMed] [Google Scholar]
- Bargh JA, 2016. Awareness of the prime versus awareness of its influence: Implications for the real-world scope of unconscious higher mental processes. Curr. Opin. Psychol 12, 49–52. doi: 10.1016/j.copsyc.2016.05.006 [DOI] [Google Scholar]
- Bargh JA, 2008. Losing Consciousness: Automatic Influences on Consumer Judgment, Behavior, and Motivation. J. Consum. Res 29, 280–285. doi: 10.1086/341577 [DOI] [Google Scholar]
- Bargh JA, Chaiken S, Govender R, Pratto F, 1992. The Generality of the Automatic Attitude Activation Effect. J. Pers. Soc. Psychol 62, 893–912. doi: 10.1037/0022-3514.62.6.893 [DOI] [PubMed] [Google Scholar]
- Bargh JA, Ferguson MJ, 2000. Beyond behaviorism: On the automaticity of higher mental processes. Psychol. Bull 126, 925–945. doi: 10.1037/0033-2909.126.6.925 [DOI] [PubMed] [Google Scholar]
- Bargh JA, Lee-Chai A, Barndollar K, Gollwitzer PM, Trötschel R, 2001. The automated will: Nonconscious activation and pursuit of behavioral goals. J. Pers. Soc. Psychol 81, 1014–1027. doi: 10.1037/0022-3514.81.6.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargh JA, Morsella E, 2008. The unconscious mind. Perspect. Psychol. Sci 3, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkby H, Dickson JM, Roper L, Field M, 2012. To Approach or Avoid Alcohol? Automatic and Self-Reported Motivational Tendencies in Alcohol Dependence. Alcohol. Clin. Exp. Res 36, 361–368. doi: 10.1111/j.1530-0277.2011.01620.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, 2017. Sex differences in incentive motivation and the relationship to the development and maintenance of alcohol use disorders. Physiol. Behav 0–1. doi: 10.1016/j.physbeh.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Loersch C, Ito TA, Levsen MP, Volpert-Esmond HI, Fleming KA, Bolls P, Carter BK, 2018. University-Affiliated Alcohol Marketing Enhances the Incentive Salience of Alcohol Cues. Psychol. Sci 29, 83–94. doi: 10.1177/0956797617731367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Lust SA, Tragesser SL, 2010. Specificity of P3 Event-related potential reactivity to alcohol cues in individuals low in alcohol sensitivity. Psychol. Addict. Behav 24, 220–228. doi: 10.1037/a0017705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Sher KJ, Wieman LC, Fabiani M, Gratton G, 2003. Effects of alcohol consumption and alcohol susceptibility on cognition: A psychophysiological examination. Biol. Psychol 64, 167–190. doi: 10.1016/S0301-0511(03)00108-X [DOI] [PubMed] [Google Scholar]
- Bassareo V, Cucca F, Frau R, Di Chiara G, 2017. Changes in Dopamine Transmission in the Nucleus Accumbens Shell and Core during Ethanol and Sucrose Self-Administration. Front. Behav. Neurosci 11, 1–14. doi: 10.3389/fnbeh.2017.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D, 2002. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Exp. Clin. Psychopharmacol 10, 193–212. doi: 10.1037//1064-1297.10.3.193 [DOI] [PubMed] [Google Scholar]
- Bates ME, Buckman JF, Vaschillo EG, Fonoberov VA, Fonoberova M, Vaschillo B, Mun E-Y, Mezi A, Mezi I, 2011. The redistribution of power: Neurocardiac signaling, alcohol and gender. PLoS One 6. doi: 10.1371/journal.pone.0028281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Cox WM, 1998. Alcohol-related words are distracting to both alcohol abusers and non-abusers in the Stroop colour-naming task. Addiction 93, 1539. doi: 10.1046/j.1360-0443.1998.9310153910.x [DOI] [PubMed] [Google Scholar]
- Beatty WW, Tivis R, Stott HD, Nixon SJ, Parsons OA, 2000. Neuropsychological deficits in sober alcoholics: Influences of chronicity and recent alcohol consumption. Alcohol. Clin. Exp. Res 24, 149–154. doi: 10.1097/00000374-200002000-00003 [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, 2004. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol. Clin. Exp. Res 28, 1829–1838. doi: 10.1097/01.ALC.0000149977.95306.3A [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Phillips TJ, 2009. Mice selectively bred for high- or low-alcohol-induced locomotion exhibit differences in dopamine neuron function. J. Pharmacol. Exp. Ther 329, 342–349. doi: 10.1124/jpet.108.146316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, Li TK, McBride WJ, 2004. Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcohol. Clin. Exp. Res 28, 1867–1874. doi: 10.1097/01.ALC.0000148101.20547.0A [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML, 2015. Pleasure Systems in the Brain. Neuron 86, 646–664. doi: 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 2016. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol 71, 670–679. doi: 10.1037/amp0000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 2003. Parsing reward. Trends Neurosci 26, 507–13. doi: 10.1016/S0166-2236(03)00233-9 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW, 2009. Dissecting components of reward: “liking”, “wanting”, and learning. Curr. Opin. Pharmacol 9, 65–73. doi: 10.1016/j.coph.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Nagarajan V, Torregrossa MM, 2016. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl) 233, 2277–2287. doi: 10.1007/s00213-016-4278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, 2014. Interoception and learning: Import to understanding and treating diseases and psychopathologies. ACS Chem. Neurosci 5, 624–631. doi: 10.1021/cn5001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, Bogucka-Bonikowska A, Kostowski W, 2004. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur. Neuropsychopharmacol 14, 355–60. doi: 10.1016/j.euroneuro.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Bigelow GE, Griffiths RR, Liebson IA, 1977. Phamacological Influences Upon Human Ethanol Self-Administration, in: Gross MM (Ed.), Alcohol Intoxication and Withdrawal—IIIb. Advances in Experimental Medicine and Biology, Vol 85B Springer, Boston, MA, pp. 523–538. doi: 10.1007/978-1-4615-9038-5_33 [DOI] [PubMed] [Google Scholar]
- Bishop FM, Rodriquez Orjuela JL, 2018. Toward the prevention of alcohol use disorders: Overdrinking (unintentional binge drinking) in a community sample. Heal. Psychol. Open 5, 205510291879270. doi: 10.1177/2055102918792705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KT, Salinas AG, Tewatia P, English B, Kotaleski JH, Lovinger DM, 2018. Molecular Mechanisms Underlying Striatal Synaptic Plasticity: Relevance to Chronic Alcohol Consumption and Seeking. Eur. J. Neurosci 1–16. doi: 10.1111/ejn.13919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha R, 2018. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict. Biol doi: 10.1111/adb.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffo M, Gronau QF, Marsman M, Wiers RW, Zerhouni O, Van B, Nikolaou K, 2019. Cognitive Bias Modification for Behavior Change in Alcohol and Smoking Addiction: Bayesian Meta-Analysis of Individual Participant Data. Neuropsychol. Rev 52–78. doi: 10.1007/s11065-018-9386-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad J-M, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A, 2003. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49, 226–31. doi: 10.1002/syn.10226 [DOI] [PubMed] [Google Scholar]
- Borlikova GG, Le Merrer J, Stephens DN, 2006. Previous experience of ethanol withdrawal increases withdrawal-induced c-fos expression in limbic areas, but not withdrawal-induced anxiety and prevents withdrawal-induced elevations in plasma corticosterone. Psychopharmacology (Berl) 185, 188–200. doi: 10.1007/s00213-005-0301-3 [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD, 2011. Economic costs of excessive alcohol consumption in the U.S., 2006. Am. J. Prev. Med 41, 516–524. doi: 10.1016/j.amepre.2011.06.045 [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC, 1979a. Contextual control of the extinction of conditioned fear. Learn. Motiv 10, 445–466. doi: 10.1016/0023-9690(79)90057-2 [DOI] [Google Scholar]
- Bouton ME, Bolles RC, 1979b. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J. Exp. Psychol. Anim. Behav. Process 5, 368–378. doi: 10.1037/0097-7403.5.4.368 [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB, 1986. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci 6, 3177–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth M, 1990. Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacol. Biochem. Behav 35, 485–487. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor SJ, Kareken DA, 2008. Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcohol. Clin. Exp. Res 32, 1124–34. doi: 10.1111/j.1530-0277.2008.00693.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grüsser SM, Hermann D, Ruf M, Flor H, Mann KF, Heinz A, 2001. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J. Neural Transm 108, 887–894. doi: 10.1007/s007020170038 [DOI] [PubMed] [Google Scholar]
- Braver TS, 2012. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn. Sci 16, 106–113. doi: 10.1016/j.tics.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Appel SB, 1998. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol. Clin. Exp. Res 22, 236–244. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB, 1999. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol. Clin. Exp. Res 23, 1848–1852. doi: 10.1111/j.1530-0277.1999.tb04082.x [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV, 1990. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508, 65–69. doi: 10.1016/0006-8993(90)91118-Z [DOI] [PubMed] [Google Scholar]
- Brown AK, George DT, Fujita M, Liow J-S, Ichise M, Hibbeln J, Ghose S, Sangare J, Hommer DW, Innis RB, 2007. PET [11C]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol. Clin. Exp. Res 31, 28–32. doi: 10.1111/j.1530-0277.2006.00261.x [DOI] [PubMed] [Google Scholar]
- Brown SA, Inaba RK, Schuckit MA, Stewart A, Christian J, Irwin R, 1995. Alcoholism and Affective Disorder: Clinical Course of Depressive Symptoms. Am J Psychiatry 152, 45–52. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA, 1988. Changes in depression among abstinent alcoholics. J. Stud. Alcohol 49, 412–417. doi: 10.15288/jsa.1988.49.412 [DOI] [PubMed] [Google Scholar]
- Brumback T, Squeglia LM, Jacobus J, Pulido C, Tapert SF, Brown SA, 2015. Adolescent heavy drinkers’ amplified brain responses to alcohol cues decrease over one month of abstinence. Addict. Behav 46, 45–52. doi: 10.1016/j.addbeh.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, David Jentsch J, Roche DJO, Ramchandani VA, Miotto K, Ray LA, 2018. Differences in the subjective and motivational properties of alcohol across alcohol use severity: Application of a novel translational human laboratory paradigm. Neuropsychopharmacology 43, 1891–1899. doi: 10.1038/s41386-018-0086-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Prause N, Ray LA, 2017. Functional significance of subjective response to alcohol across levels of alcohol exposure. Addict. Biol 22, 235–245. doi: 10.1111/adb.12293 [DOI] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Roche DJO, Ray LA, 2015. Factor Structure of Subjective Responses to Alcohol in Light and Heavy Drinkers. Alcohol. Clin. Exp. Res 39, 1193–1202. doi: 10.1111/acer.12737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA, 2014. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: an examination of Koob’s allostatic model in humans. Drug Alcohol Depend 140, 161–7. doi: 10.1016/j.drugalcdep.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Freitas KCC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS, 2014. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology 39, 2835–2845. doi: 10.1038/npp.2014.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burattini C, Gill TM, Aicardi G, Janak PH, 2006. The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience 139, 877–87. doi: 10.1016/j.neuroscience.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Cachope R, Cheer JF, 2014. Local control of striatal dopamine release. Front. Behav. Neurosci 8, 1–7. doi: 10.3389/fnbeh.2014.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M, 2007. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 30, 211–9. doi: 10.1016/j.tins.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Carrillo J, Gonzales RA, 2011. A single exposure to voluntary ethanol self-administration produces adaptations in ethanol consumption and accumbal dopamine signaling. Alcohol 45, 559–66. doi: 10.1016/j.alcohol.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST, 1999. Meta-analysis of cue-reactivity in addiction research. Addiction 94, 327–340. [PubMed] [Google Scholar]
- Ceballos NA, Komogortsev OV, Turner GM, 2015. Ocular Imaging of Attentional Bias Among College Students: Automatic and Controlled Processing of Alcohol-Related Scenes. J. Stud. Alcohol Drugs 70, 652–659. doi: 10.15288/jsad.2009.70.652 [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, 2012. Alcohol Metabolism. Clin. Liver Dis 16, 667–685. doi: 10.1016/j.cld.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S, Kuna ST, Zaharakis N, O’Brien CP, Kampman KM, Oslin D, 2010. Covariates of craving in actively drinking alcoholics. Am. J. Addict 19, 450–457. doi: 10.1111/j.1521-0391.2010.00067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Hong KIA, Bergquist KL, Sinha R, 2008. Gender differences in response to emotional stress: An assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol. Clin. Exp. Res 32, 1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Weiner JL, 2008. Relationship between ethanol’s acute locomotor effects and ethanol self-administration in male Long-Evans rats. Alcohol. Clin. Exp. Res 32, 2088–2099. doi: 10.1111/j.1530-0277.2008.00797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, 2008. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur. J. Neurosci 28, 2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Bargh JA, 1999. Immediate Behavioral Predispositions to Approach or Avoid the Stimulus. Soc. Personal. Soc. Psychol 25, 215–224. [Google Scholar]
- Cho YT, Ernst M, Fudge JL, 2013. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J. Neurosci 33, 14017–30. doi: 10.1523/JNEUROSCI.0170-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen P, Bloor JF, 2014. Individualised but not general alcohol Stroop predicts alcohol use. Drug Alcohol Depend 134, 410–413. doi: 10.1016/j.drugalcdep.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Christiansen P, Cole JC, Goudie AJ, Field M, 2012. Components of behavioural impulsivity and automatic cue approach predict unique variance in hazardous drinking. Psychopharmacology (Berl) 219, 501–510. doi: 10.1007/s00213-011-2396-z [DOI] [PubMed] [Google Scholar]
- Christiansen P, Mansfield R, Duckworth J, Field M, Jones A, 2015a. Internal reliability of the alcohol-related visual probe task is increased by utilising personalised stimuli and eye-tracking. Drug Alcohol Depend 155, 170–174. doi: 10.1016/j.drugalcdep.2015.07.672 [DOI] [PubMed] [Google Scholar]
- Christiansen P, Schoenmakers TM, Field M, 2015b. Less than meets the eye: Reappraising the clinical relevance of attentional bias in addiction. Addict. Behav 44, 43–50. doi: 10.1016/j.addbeh.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Christiansen P, Townsend G, Knibb G, Field M, 2017. Bibi ergo sum: the effects of a placebo and contextual alcohol cues on motivation to drink alcohol. Psychopharmacology (Berl) 234, 827–835. doi: 10.1007/s00213-016-4518-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MAD, Mitchell SH, de Wit H, 1994. Ethanol preloads increase ethanol preference under concurrent random-ratio schedules in social drinkers. Exp. Clin. Psychopharmacol 2, 310–318. doi: 10.1037/1064-1297.2.4.310 [DOI] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F, 2001. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol. Clin. Exp. Res 25, 1414–1419. doi: 10.1111/j.1530-0277.2001.tb02141.x [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F, 2003. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 168, 208–215. doi: 10.1007/s00213-002-1380-z [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, 2002. Effect of selective blockade of μ1 or δ opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27, 391–399. doi: 10.1016/S0893-133X(02)00302-0 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Quitadamo E, Massi M, 1999. Ethanol induces conditioned place preference in genetically selected alcohol-preferring rats. Psychopharmacology (Berl) 141, 235–241. doi: 10.1007/s002130050830 [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, Sabbineni A, Hutchison KE, 2011. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36, 2086–96. doi: 10.1038/npp.2011.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin EM, Magee JC, Wells TT, Beard C, Barnett NP, 2016. Randomized controlled trial of attention bias modification in a racially diverse, socially anxious, alcohol dependent sample. Behav. Res. Ther 87, 58–69. doi: 10.1016/j.brat.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, 1987. Neurogenetic Adaptive Mechanisms in Alcoholism. Science (80-. ) 236, 410–416. [DOI] [PubMed] [Google Scholar]
- Cofresí RU, Grote DJ, Le EVT, Monfils M-H, Chaudhri N, Gonzales RA, Lee HJ, 2019a. Alcohol-associated antecedent stimuli elicit alcohol seeking in non-dependent rats and may activate the insula. Alcohol 76, 91–102. doi: 10.1016/j.alcohol.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofresí RU, Lee HJ, Monfils M-H, Chaudhri N, Gonzales RA, 2018. Characterizing conditioned reactivity to sequential alcohol-predictive cues in well-trained rats. Alcohol 69, 41–49. doi: 10.1016/j.alcohol.2017.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofresí RU, Lewis SM, Chaudhri N, Lee HJ, Monfils M-H, Gonzales RA, 2017. Postretrieval Extinction Attenuates Alcohol Cue Reactivity in Rats. Alcohol. Clin. Exp. Res 41, 608–617. doi: 10.1111/acer.13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofresí RU, Monfils M-H, Chaudhri N, Gonzales RA, Lee HJ, 2019b. Cue-alcohol associative learning in female rats. Alcohol 81, 1–9. doi: 10.1016/j.alcohol.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Vacca G, Gessa GL, 1998. Stimulation of locomotor activity by voluntarily consumed ethanol in Sardinian alcohol-preferring rats. Eur. J. Pharmacol 357, 109–113. doi: 10.1016/S0014-2999(98)00560-3 [DOI] [PubMed] [Google Scholar]
- Colwill RM, 1991. Negative discriminative stimuli provide information about the identity of omitted response-contingent outcomes. Anim. Learn. Behav 19, 326–336. doi: 10.3758/BF03197893 [DOI] [Google Scholar]
- Conklin CA, Tiffany ST, 2002. Applying extinction research and theory to cue-exposure addiction treatments. Addiction 155–167. [DOI] [PubMed] [Google Scholar]
- Connor J, 2017. Alcohol consumption as a cause of cancer. Addiction 112, 222–228. doi: 10.1111/add.13477 [DOI] [PubMed] [Google Scholar]
- Cooney NL, Baker LH, Pomerleau OF, Josephy B, 1984. Salivation to drinking cues in alcohol abusers: Toward the validation of a physiological measure of craving. Addict. Behav 9, 91–94. doi: 10.1016/0306-4603(84)90011-X [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P, 1995. Drinking to Regulate Positive and Negative Emotions: A Motivational Model of Alcohol Use. J. Pers. Soc. Psychol 69, 990–1005. doi: 10.1037/0022-3514.69.5.990 [DOI] [PubMed] [Google Scholar]
- Corbin WR, Gearhardt A, Fromme K, 2008. Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology (Berl). 197, 327–337. doi: 10.1007/s00213-007-1039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Fischbach SC, Janak PH, 2016. Nucleus accumbens core and shell are differentially involved in general and outcome-specific forms of Pavlovian-instrumental transfer with alcohol and sucrose rewards. Eur. J. Neurosci 43, 1229–1236. doi: 10.1111/ejn.13235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH, 2016. Changes in the Influence of Alcohol-Paired Stimuli on Alcohol Seeking across Extended Training. Front. Psychiatry 7, 1–9. doi: 10.3389/fpsyt.2016.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH, 2007. Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcohol. Clin. Exp. Res 31, 766–74. doi: 10.1111/j.1530-0277.2007.00359.x [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA, 2015. The effect of alcohol priming on neural markers of alcohol cue-reactivity. Am. J. Drug Alcohol Abuse 41, 1–9. doi: 10.3109/00952990.2015.1044608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Blount JP, Rozak AM, 2000. Alcohol abusers’ and nonabusers’ distraction by alcohol and concern-related stimuli. Am. J. Drug Alcohol Abuse 26, 489–495. doi: 10.1081/ADA-100100258 [DOI] [PubMed] [Google Scholar]
- Cox WM, Brown MA, Rowlands LJ, 2003. The effects of alcohol cue exposure on non-dependent drinkers’ attentional bias for alcohol-related stimuli. Alcohol Alcohol 38, 45–49. doi: 10.1093/alcalc/agg010 [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Hosier SG, Pothos EM, 2015. Differential effects and temporal course of attentional and motivational training on excessive drinking. Exp. Clin. Psychopharmacol 23, 445–454. doi: 10.1037/pha0000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH, 2002. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend 68, 237–243. doi: 10.1016/S0376-8716(02)00219-3 [DOI] [PubMed] [Google Scholar]
- Cox WM, Yeates GN, Regan CM, 1999. Effects of alcohol cues on cognitive processing in heavy and light drinkers. Drug Alcohol Depend 55, 85–89. doi: 10.1016/S0376-8716(98)00186-0 [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Bell RL, Ehlers CL, 2010. Human and laboratory rodent low response to alcohol: Is better consilience possible? Addict. Biol 15, 125–144. doi: 10.1111/j.1369-1600.2009.00191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Cunningham CL, Belknap JK, 1992. Genetic Determinants of Ethanol Reinforcement. Ann. N. Y. Acad. Sci 654, 302–310. doi: 10.1111/j.1749-6632.1992.tb25976.x [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Clemans JM, Fidler TL, 2002a. Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacol. Biochem. Behav 72, 659–668. doi: 10.1016/S0091-3057(02)00734-7 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ, 2002b. Distal and proximal pre-exposure to ethanol in the place conditioning task: Tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 160, 414–424. doi: 10.1007/s00213-001-0990-1 [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Barnett NP, Colby SM, Rohsenow DJ, Monti PM, 2005. Cue reactivity in adolescents: measurement of separate approach and avoidance reactions. J. Stud. Alcohol 66, 332–343. doi: 10.15288/jsa.2005.66.332 [DOI] [PubMed] [Google Scholar]
- Custers R, Aarts H, 2010. The Unconscious Will : How the Pursuit of Goals Operates Outside of Conscious Awareness. Science (80-. ) 329, 47–51. doi: 10.1126/science.1188595 [DOI] [PubMed] [Google Scholar]
- Czapla M, Simon JJ, Richter B, Kluge M, Friederich H-C, Herpertz S, Mann KF, Herpertz SC, Loeber S, 2015. The impact of cognitive impairment and impulsivity on relapse of alcohol-dependent patients: implications for psychotherapeutic treatment. Addict. Biol doi: 10.1111/adb.12229 [DOI] [PubMed] [Google Scholar]
- Dalia AD, Norman MK, Tabet MR, Schlueter KT, Tsibulsky VL, Norman AB, 1998. Transient amelioration of the sensitization of cocaine-induced behaviors in rats by the induction of tolerance. Brain Res 797, 29–34. doi: 10.1016/S0006-8993(98)00323-0 [DOI] [PubMed] [Google Scholar]
- Das RK, Lawn W, Kamboj SK, 2015. Rewriting the valuation and salience of alcohol-related stimuli via memory reconsolidation. Transl. Psychiatry 5, e645. doi: 10.1038/tp.2015.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Walsh KH, Hannaford J, Lazzarino AI, Kamboj SK, 2018. Nitrous oxide may interfere with the reconsolidation of drinking memories in hazardous drinkers in a prediction-error-dependent manner. Eur. Neuropsychopharmacol 28, 828–840. doi: 10.1016/j.euroneuro.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Davidson D, Hutchison KE, Dagon C, Swift RM, 2002. Assessing the stimulant effects of alcohol in humans. Pharmacol. Biochem. Behav 72, 151–156. doi: 10.1016/S0091-3057(01)00758-4 [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F, 2007. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol. Psychiatry 61, 979–89. doi: 10.1016/j.biopsych.2006.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer J, Crombez G, Baeyens F, Hermans D, 2001. On the generality of the affective Simon effect. Cogn. Emot 15, 189–206. doi: 10.1080/02699930125883 [DOI] [Google Scholar]
- de Sousa Fernandes Perna EB, Theunissen EL, Kuypers KPC, Evers EA, Stiers P, Toennes SW, Witteman J, van Dalen W, Ramaekers JG, 2017. Brain reactivity to alcohol and cannabis marketing during sobriety and intoxication. Addict. Biol 22, 823–832. doi: 10.1111/adb.12351 [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Lindenmeyer J, Wiers RW, 2018. A Clinical Trial with Combined Transcranial Direct Current Stimulation and Attentional Bias Modification in Alcohol-Dependent Patients. Alcohol. Clin. Exp. Res 42, 1961–1969. doi: 10.1111/acer.13841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Rinck M, Lindenmeyer J, Wiers RW, 2017. A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict. Biol 22, 1632–1640. doi: 10.1111/adb.12463 [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Wiers RW, 2016. Electrophysiological and Behavioral Effects of Combined Transcranial Direct Current Stimulation and Alcohol Approach Bias Retraining in Hazardous Drinkers. Alcohol. Clin. Exp. Res 40, 2124–2133. doi: 10.1111/acer.13171 [DOI] [PubMed] [Google Scholar]
- DePalma FM, Ceballos NA, Graham R, 2017. Attentional blink to alcohol cues in binge drinkers versus non-binge drinkers. Addict. Behav 73, 67–73. doi: 10.1016/j.addbeh.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A, 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A 85, 5274–5278. doi: 10.1073/pnas.85.14.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Volo M, Morozova EO, Lapish CC, Kuznetsov A, Gutkin B, 2018. Dynamical Ventral Tegmental Area Circuit Mechanisms of Alcohol-Dependent Dopamine Release. Eur. J. Neuroscia 1–15. doi: 10.1111/ejn.14147 [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders (DSM-5{®}), 2013. American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Diana M, Gessa GL, Rossetti ZL, 1992. Lack of tolerance to ethanol-induced stimulation of mesolimbic dopamine system. Alcohol Alcohol 27, 329–333. [PubMed] [Google Scholar]
- Dickter CL, Forestell C. a, Hammett PJ, Young CM, 2014. Relationship between alcohol dependence, escape drinking, and early neural attention to alcohol-related cues. Psychopharmacology (Berl) 231, 2031–40. doi: 10.1007/s00213-013-3348-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JM, Schier CJ, Vena AA, Dilly GA, Gonzales RA, 2016. Medial Prefrontal Cortical Dopamine Responses During Operant Self-Administration of Sweetened Ethanol. Alcohol. Clin. Exp. Res 40, 1662–1670. doi: 10.1111/acer.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA, 2005. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J. Neurochem 93, 1469–81. doi: 10.1111/j.1471-4159.2005.03137.x [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA, 2003. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol. Clin. Exp. Res 27, 1573–82. doi: 10.1097/01.ALC.0000089959.66222.B8 [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD, 2010. Influence of phasic and tonic dopamine release on receptor activation. J. Neurosci 30, 14273–83. doi: 10.1523/JNEUROSCI.1894-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, 2001. Theories of drug craving, ancient and modern. Addiction 96, 33–46. doi: 10.1080/09652140020016941 [DOI] [PubMed] [Google Scholar]
- Drummond DC, Glautier S, 1994. A controlled trial of cue exposure treatment in alcohol dependence. J. Consult. Clin. Psychol 62, 809–817. [DOI] [PubMed] [Google Scholar]
- Duka T, Jackson A, Smith DC, Stephens DN, 1999. Relationship of components of an alcohol interoceptive stimulus to induction of desire for alcohol in social drinkers. Pharmacol. Biochem. Behav 64, 301–309. doi: 10.1016/S0091-3057(99)00080-5 [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, 2004. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology (Berl) 176, 353–61. doi: 10.1007/s00213-004-1906-7 [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN, 2002. Kindling of withdrawal: a study of craving and anxiety after multiple detoxifications in alcoholic inpatients. Alcohol. Clin. Exp. Res 26, 785–95. [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J, 2013. Approach bias modification in alcohol dependence: Do clinical effects replicate and for whom does it work best? Dev. Cogn. Neurosci 4, 38–51. doi: 10.1016/j.dcn.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Gross MM, 1976. Alcohol dependence: provisional description of a clinical syndrome. Br. Med. J 1, 1058–1061. doi: 10.1136/bmj.1.6017.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC, 2007. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur. J. Neurosci 25, 1557–67. doi: 10.1111/j.1460-9568.2007.05402.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC, 2006. Substantia nigra pars compacta is critical to both the acquisition and expression of learned orienting of rats. Eur. J. Neurosci 24, 270–6. doi: 10.1111/j.1460-9568.2006.04896.x [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM, 2006. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol 38, 5–12. doi: 10.1016/j.alcohol.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Eravci M, Großpietsch T, Pinna G, Schulz O, Kley S, Bachmann M, Wolffgramm J, Götz E, Heyne A, Meinhold H, Baumgartner A, 1997. Dopamine receptor gene expression in an animal model of ‘behavioral dependence’ on ethanol. Mol. Brain Res 50, 221–229. doi: 10.1016/S0169-328X(97)00188-5 [DOI] [PubMed] [Google Scholar]
- Ericson M, Ulenius L, Andrén A, Jonsson S, Adermark L, Söderpalm B, 2019. Different dopamine tone in ethanol high- and low-consuming Wistar rats. Addict. Biol e12761. doi: 10.1111/adb.12761 [DOI] [PubMed] [Google Scholar]
- Ernst LH, Plichta MM, Lutz E, Zesewitz AK, Tupak SV, Dresler T, Ehlis A-C, Fallgatter AJ, 2013. Prefrontal activation patterns of automatic and regulated approach-avoidance reactions - A functional near-infrared spectroscopy (fNIRS) study. Cortex 49, 131–142. doi: 10.1016/j.cortex.2011.09.013 [DOI] [PubMed] [Google Scholar]
- Fadardi JS, Cox WM, 2009. Reversing the sequence: Reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depend 101, 137–145. doi: 10.1016/j.drugalcdep.2008.11.015 [DOI] [PubMed] [Google Scholar]
- Fadardi JS, Cox WM, 2006. Alcohol attentional bias: Drinking salience or cognitive impairment? Psychopharmacology (Berl). 185, 169–178. doi: 10.1007/s00213-005-0268-0 [DOI] [PubMed] [Google Scholar]
- Farris SR, Ostafin BD, 2008. Alcohol consumption primes automatic alcohol-approach associations. Am. J. Drug Alcohol Abuse 34, 703–11. doi: 10.1080/00952990802308247 [DOI] [PubMed] [Google Scholar]
- Fernie G, Christiansen P, Cole JC, Rose AK, Field M, 2012. Effects of 0.4g/kg alcohol on attentional bias and alcohol-seeking behaviour in heavy and moderate social drinkers. J. Psychopharmacol 26, 1017–1025. doi: 10.1177/0269881111434621 [DOI] [PubMed] [Google Scholar]
- Fey W, Moggi F, Rohde KB, Michel C, Seitz A, Stein M, 2017. Development of stimulus material for research in alcohol use disorders. Int. J. Methods Psychiatr. Res 26. doi: 10.1002/mpr.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Caren R, Fernie G, De Houwer J, 2011a. Alcohol Approach Tendencies in Heavy Drinkers: Comparison of Effects in a Relevant Stimulus-Response Compatibility Task and an Approach/Avoidance Simon Task. Psychol. Addict. Behav 25, 697–701. doi: 10.1037/a0023285 [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM, 2008. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol Depend 97, 1–20. doi: 10.1016/j.drugalcdep.2008.03.030 [DOI] [PubMed] [Google Scholar]
- Field M, Duka T, 2002. Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacology (Berl) 159, 325–34. doi: 10.1007/s00213-001-0923-z [DOI] [PubMed] [Google Scholar]
- Field M, Duka T, Eastwood B, Child R, Santarcangelo M, Gayton M, 2007. Experimental manipulation of attentional biases in heavy drinkers: Do the effects generalise? Psychopharmacology (Berl) 192, 593–608. doi: 10.1007/s00213-007-0760-9 [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B, 2005. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology (Berl) 183, 350–357. doi: 10.1007/s00213-005-0202-5 [DOI] [PubMed] [Google Scholar]
- Field M, Hogarth L, Bleasdale D, Wright P, Fernie G, Christiansen P, 2011b. Alcohol expectancy moderates attentional bias for alcohol cues in light drinkers. Addiction 106, 1097–1103. doi: 10.1111/j.1360-0443.2011.03412.x [DOI] [PubMed] [Google Scholar]
- Field M, Jones A, 2017. Elevated alcohol consumption following alcohol cue exposure is partially mediated by reduced inhibitory control and increased craving. Psychopharmacology (Berl) 234, 2979–2988. doi: 10.1007/s00213-017-4694-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Kiernan A, Eastwood B, Child R, 2008. Rapid approach responses to alcohol cues in heavy drinkers. J. Behav. Ther. Exp. Psychiatry 39, 209–218. doi: 10.1016/j.jbtep.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Field M, Marhe R, Franken IHA, 2014. The clinical relevance of attentional bias in substance use disorders. CNS Spectr 19, 225–230. doi: 10.1017/s1092852913000321 [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP, 2005. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol Alcohol. 40, 504–10. doi: 10.1093/alcalc/agh213 [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Mann B, Bennett GA, Bradley BP, 2013. Attentional biases in abstinent alcoholics and their association with craving. Psychol. Addict. Behav 27, 71–80. doi: 10.1037/a0029626 [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP, 2004. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology (Berl) 176, 88–93. doi: 10.1007/s00213-004-1855-1 [DOI] [PubMed] [Google Scholar]
- Field M, Quigley M, 2009. Mild Stress Increases Attentional Bias in Social Drinkers Who Drink to Cope: A Replication and Extension. Exp. Clin. Psychopharmacol 17, 312–319. doi: 10.1037/a0017090 [DOI] [PubMed] [Google Scholar]
- Field M, Werthmann J, Franken IHA, Hofmann W, Hogarth L, Roefs A, 2016. The role of attentional bias in obesity and addiction. Health Psychol 35, 767–780. doi: 10.1037/hea0000405 [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus ED, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE, 2008. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology 33, 1391–401. doi: 10.1038/sj.npp.1301513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Brice GT, Deitrich RA, Draski LJ, 1996. Initiation of Ethanol Self-Administration by the Sucrose-Substitution Method with HAS and LAS Rats. Alcohol. Clin. Exp. Res 20, 677–681. doi: 10.1111/j.1530-0277.1996.tb01671.x [DOI] [PubMed] [Google Scholar]
- Fiorenza AM, Shnitko TA, Sullivan KM, Vemuru SR, Gomez-A A, Esaki JY, Boettiger CA, Da Cunha C, Robinson DL, 2018. Ethanol Exposure History and Alcoholic Reward Differentially Alter Dopamine Release in the Nucleus Accumbens to a Reward-Predictive Cue. Alcohol. Clin. Exp. Res 42, 1051–1061. doi: 10.1111/acer.13636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Robinson JE, Krouse MC, Hodge CW, Reed C, Phillips TJ, Malanga CJ, 2012. Intracranial self-stimulation in FAST and SLOW mice: Effects of alcohol and cocaine. Psychopharmacology (Berl) 220, 719–730. doi: 10.1007/s00213-011-2523-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo LM, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H, 2010. A selective role for dopamine in stimulus–reward learning. Nature 469, 53–57. doi: 10.1038/nature09588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, 2017. Neurobiological basis of individual variation in stimulus-reward learning. Curr. Opin. Behav. Sci 13, 178–185. doi: 10.1016/j.cobeha.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Poole SA, Gallop RJ, Volpicelli JR, 2003. Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. J. Stud. Alcohol 64, 120–6. [DOI] [PubMed] [Google Scholar]
- Fleming KA, Bartholow BD, 2014. Alcohol cues, approach bias, and inhibitory control: Applying a dual process model of addiction to alcohol sensitivity. Psychol. Addict. Behav 28, 85–96. doi: 10.1037/a0031565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, Mccool BA, 2004. Long-Term Ethanol Self-Administration by Cynomolgus Macaques Alters the Pharmacology and Expression of GABAA Receptors in Basolateral Amygdala. Pharmacology 311, 1071–1079. doi: 10.1124/jpet.104.072025.terminalis [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KIA, Sinha R, 2007. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol. Clin. Exp. Res 31, 395–403. doi: 10.1111/j.1530-0277.2006.00320.x [DOI] [PubMed] [Google Scholar]
- Fridrici C, Leichsenring-Driessen C, Driessen M, Wingenfeld K, Kremer G, Beblo T, 2013. The individualized alcohol Stroop task: No attentional bias toward personalized stimuli in alcohol-dependents. Psychol. Addict. Behav doi: 10.1037/a0029139 [DOI] [PubMed] [Google Scholar]
- Friese M, Bargas-Avila J, Hofmann W, Wiers RW, 2010. Here’s Looking at You, Bud: Alcohol-Related Memory Structures Predict Eye Movements for Social Drinkers With Low Executive Control. Soc. Psychol. Personal. Sci 1, 143–151. doi: 10.1177/1948550609359945 [DOI] [Google Scholar]
- Fromme K, Dunn ME, 1992. Alcohol expectancies, social and environmental cues as determinants of drinking and perceived reinforcement. Addict. Behav 17, 167–177. doi: 10.1016/0306-4603(92)90021-M [DOI] [PubMed] [Google Scholar]
- Fryer SL, Jorgensen KW, Yetter EJ, Daurignac EC, Watson TD, Shanbhag H, Krystal JH, Mathalon DH, 2013. Differential brain response to alcohol cue distractors across stages of alcohol dependence. Biol. Psychol 92, 282–291. doi: 10.1016/j.biopsycho.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Boettiger CA, Gaylord S, Chanon VW, Howard MO, 2012a. Mindfulness is inversely associated with alcohol attentional bias among recovering alcohol-dependent adults. Cognit. Ther. Res 36, 441–450. doi: 10.1007/s10608-011-9378-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Franken IHA, Howard MO, 2012b. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology (Berl) 222, 17–26. doi: 10.1007/s00213-011-2618-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Franken IHA, Sheetz JJ, Howard MO, 2012c. Alcohol attentional bias is associated with autonomic indices of stress-primed alcohol cue-reactivity in alcohol-dependent patients. Exp. Clin. Psychopharmacol 20, 225–235. doi: 10.1037/a0027199 [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP, 1989. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J. Comp. Neurol 279, 249–271. doi: 10.1002/cne.902790208 [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WBJ, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ, 2014. Adolescent Alcohol Exposure Reduces Behavioral Flexibility, Promotes Disinhibition, and Increases Resistance to Extinction of Ethanol Self-Administration in Adulthood. Neuropsychopharmacology 39, 2570–2583. doi: 10.1038/npp.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Moore KR, Holloway FA, 1993. Do rat strain differences in ethanol consumption reflect differences in ethanol sensitivity or the preparedness to learn? Alcohol 10, 37–43. doi: 10.1016/0741-8329(93)90051-O [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ, 2001. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch. Gen. Psychiatry 58, 345–352. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G, 1985. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res 348, 201–203. doi: 10.1016/0006-8993(85)90381-6 [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW, 2012. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology 37, 467–77. doi: 10.1038/npp.2011.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF, 2010. Effects of Beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 212, 431–439. doi: 10.1007/s00213-010-1967-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole MD, Weiss F, Koob GF, Richardson HN, 2009. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol. Clin. Exp. Res 33, 2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner S, Overmier J, Balleine BW, 2005. The role of Pavlovian cues in alcohol seeking in dependent and nondependent rats. J. Stud. Alcohol Drugs 66, 53–61. doi: 10.15288/jsa.2005.66.53 [DOI] [PubMed] [Google Scholar]
- Gongwer MA, Murphy JM, McBride WJ, Lumeng L, Li T-K, 1989. Regional brain contents of serotonin, dopamine and their metabolites in the selectively bred high- and low-alcohol drinking lines of rats. Alcohol 6, 317–320. doi: 10.1016/0741-8329(89)90089-X [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F, 1998. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J. Neurosci 18, 10663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, 2000. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 95 Suppl 2, S119–28. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou PS, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS, 2017. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013. JAMA Psychiatry 48, 750–760. doi: 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant VV, Stewart SH, Birch CD, 2007. Impact of positive and anxious mood on implicit alcohol-related cognitions in internally motivated undergraduate drinkers. Addict. Behav 32, 2226–2237. doi: 10.1016/j.addbeh.2007.02.012 [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ, 2008. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol 42, 1–11. doi: 10.1016/j.alcohol.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC, 1999. Beyond the dopamine receptor: The DARPP-32/protein phosphatase-1 cascade. Neuron 23, 435–447. doi: 10.1016/S0896-6273(00)80798-9 [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Mcghee DE, Schwartz JLK, 1998. Measuring Individual Differences in Implicit Cognition: The Implicit Association Test. J. Pers. Soc. Psychol 74, 1464–1480. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL, 2008. Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J. Neurosci 28, 1076–84. doi: 10.1523/JNEUROSCI.4520-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC, 2014. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39, 707–717. doi: 10.1038/npp.2013.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC, 2015. Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front. Pharmacol 6, 1–7. doi: 10.3389/fphar.2015.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P, 1999. Convergence and segregation of ventral striatal inputs and outputs. Ann. N. Y. Acad. Sci 877, 49–63. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann KF, Braus DF, Heinz A, 2004. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 175, 296–302. doi: 10.1007/s00213-004-1828-4 [DOI] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L, Kenna GA, 2014. Pharmacological approaches to reducing craving in patients with alcohol use disorders. CNS Drugs 28, 343–360. doi: 10.1007/s40263-014-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, 2003. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat 26, 317–330. doi: 10.1016/j.jchemneu.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR, 2000. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci 20, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansenne M, Olin C, Pinto E, Pitchot W, Ansseau M, 2003. Event-related potentials to emotional and neutral stimuli in alcoholism. Neuropsychobiology 48, 77–81. doi: 10.1159/000072881 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Peterson CK, 2009. Supine body position reduces neural response to anger evocation: Short report. Psychol. Sci. 20, 1209–1210. doi: 10.1111/j.1467-9280.2009.02416.x [DOI] [PubMed] [Google Scholar]
- Hartwell EE, Ray LA, 2013. Sex moderates stress reactivity in heavy drinkers. Addict. Behav. 38, 2643–2646. doi: 10.1016/j.addbeh.2013.06.016 [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ, 2002. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J. Comp. Neurol. 454, 15–33. doi: 10.1002/cne.10420 [DOI] [PubMed] [Google Scholar]
- Haughey H, Ray LA, Finan P, Villanueva R, Niculescu M, Hutchison KE, 2008. Human γ-aminobutyric acid A receptor α2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes, Brain Behav. 7, 447–454. doi: 10.1111/j.1601-183X.2007.00369.x [DOI] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA, Knight CP, Toalston JE, McBride WJ, Rodd-Henricks ZA, 2016. Parameters of Context-Induced Ethanol (EtOH)-Seeking in Alcohol-Preferring (P) Rats: Temporal Analysis, Effects of Repeated Deprivation, and EtOH Priming Injections. Alcohol. Clin. Exp. Res 40, 2229–2239. doi: 10.1111/acer.13205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA, Knight CP, Waeiss RA, Truitt WA, Johnson PL, Bell RL, McBride WJ, Rodd-Henricks ZA, 2019. Conditioned stimuli affect ethanol-seeking by female alcohol-preferring (P) rats: the role of repeated-deprivations, cue-pretreatment, and cue-temporal intervals. Psychopharmacology (Berl) doi: 10.1007/s00213-019-05264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG, 1999. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol. Med 29, 1069–1081. doi: 10.1017/S0033291799008909 [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, 1991. The inheritance of alcohol sensitivity and of patterns of alcohol use. Alcohol Alcohol. 1, 141–5. [PubMed] [Google Scholar]
- Heinz A, 2002. Dopaminergic dysfunction in alcoholism and schizophrenia - Psychopathological and behavioral correlates. Eur. Psychiatry 17, 9–16. doi: 10.1016/S0924-9338(02)00628-4 [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, 2004. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161, 1783–1789. [DOI] [PubMed] [Google Scholar]
- Hemby SE, O’Connor JA, Acosta G, Floyd DW, Anderson N, McCool BA, Friedman DP, Grant KA, 2006. Ethanol-induced regulation of GABAA subunit mRNAs in prefrontal fields of cynomolgus monkeys. Alcohol. Clin. Exp. Res 30, 1978–1985. doi: 10.1111/j.1530-0277.2006.00254.x [DOI] [PubMed] [Google Scholar]
- Henniger MSH, Wotjak CT, Hölter SM, 2003. Long-term voluntary ethanol drinking increases expression of NMDA receptor 2B subunits in rat frontal cortex. Eur. J. Pharmacol 470, 33–36. doi: 10.1016/S0014-2999(03)01787-4 [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD, 1998. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Brain Res. Rev 28, 370–490. [DOI] [PubMed] [Google Scholar]
- Herman MA, Roberto M, 2016. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict. Biol 21, 72–86. doi: 10.1111/adb.12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Weijers HG, Wiesbeck G. a, Böning J, Fallgatter AJ, 2001. Alcohol cue-reactivity in heavy and light social drinkers as revealed by event-related potentials. Alcohol Alcohol. 36, 588–593. doi: 10.1093/alcalc/36.6.588 [DOI] [PubMed] [Google Scholar]
- Hersman S, Hoffman AN, Hodgins L, Shieh S, Lam J, Parikh A, Fanselow MS, 2019. Cholinergic Signaling Alters Stress-Induced Sensitization of Hippocampal Contextual Learning. Front. Neurosci 13, 1–9. doi: 10.3389/fnins.2019.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins RL, Marlatt GA, 1973. Effects of anxiety arousal on the consumption of alcohol by alcoholics and social drinkers. J. Consult. Clin. Psychol 41, 426–433. doi: 10.1037/h0035366 [DOI] [PubMed] [Google Scholar]
- Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A, 2006. Low Level of Response to Alcohol as Associated with Serotonin Transporter Genotype and High Alcohol Intake in Adolescents. Biol. Psychiatry 60, 282–287. doi: 10.1016/j.biopsych.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W, Weitzman ER, 2009. Magnitude of and Trends in Alcohol-Related Mortality and Morbidity Among U.S. College Students Ages 18–24, 1998–2005. J. Stud. Alcohol Drugs, Suppl. 12–20. doi: 10.15288/jsads.2009.s16.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Broccoli L, Vengeliene V, Roßmanith M, Perreau-Lenz S, Köhr G, Sommer WH, Spanagel R, Hansson AC, 2016. Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc. Natl. Acad. Sci 201506012. doi: 10.1073/pnas.1506012113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson J, Bruce G, Butler SH, 2013. A flicker change blindness task employing eye tracking reveals an association with levels of craving not consumption. J. Psychopharmacol 27, 93–97. doi: 10.1177/0269881112447990 [DOI] [PubMed] [Google Scholar]
- Hodgson RJ, Rankin H, Stockwell T, 1979. Alcohol dependence and the priming effect. Behav Res Ther 17, 379–387. doi:0005-7967(79)90009-3 [pii] [DOI] [PubMed] [Google Scholar]
- Hofmann W, Van Dillen L, 2018. Experiencing and regulating desire, in: Zeigler-Hill V, Shackelford TK (Eds.), The SAGE Handbook of Personality and Individual Diffrences SAGE Publications Ltd, Oakland, pp. 283–299. [Google Scholar]
- Hofmann W, Van Dillen L, 2012. Desire: The New Hot Spot in Self-Control Research. Curr. Dir. Psychol. Sci 21, 317–322. doi: 10.1177/0963721412453587 [DOI] [Google Scholar]
- Hogarth L, Hardy L, 2018. Depressive statements prime goal-directed alcohol-seeking in individuals who report drinking to cope with negative affect. Psychopharmacology (Berl) 235, 269–279. doi: 10.1007/s00213-017-4765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, de Wit H, 1998. Individual differences in the biphasic effects of ethanol. Alcohol. Clin. Exp. Res 22, 1903–11. doi:00000374-199812000-00004 [pii] [PubMed] [Google Scholar]
- Hollett RC, Stritzke WGK, Edgeworth P, Weinborn M, 2017. Changes in the relative balance of approach and avoidance inclinations to use alcohol following cue exposure vary in low and high risk drinkers. Front. Psychol 8, 1–13. doi: 10.3389/fpsyg.2017.00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon T, Das RK, Kamboj SK, 2016. The effects of cognitive reappraisal following retrieval-procedures designed to destabilize alcohol memories in high-risk drinkers. Psychopharmacology (Berl) 233, 851–861. doi: 10.1007/s00213-015-4164-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone LSE, Bartholow BD, Piasecki TM, Sher KJ, 2017. Women’s Alcohol Sensitivity Predicts Alcohol-Related Regretted Sex. Alcohol. Clin. Exp. Res 41, 1630–1636. doi: 10.1111/acer.13447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang S-J, Sparta DR, Bowers MS, Bonci A, 2010. Motivation for Alcohol Becomes Resistant to Quinine Adulteration After 3 to 4 Months of Intermittent Alcohol Self-Administration. Alcohol. Clin. Exp. Res 34, 1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben K, Wiers RW, 2009. Response inhibition moderates the relationship between implicit associations and drinking behavior. Alcohol. Clin. Exp. Res 33, 626–633. doi: 10.1111/j.1530-0277.2008.00877.x [DOI] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA, 2008. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience 154, 1042–53. doi: 10.1016/j.neuroscience.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Gonzales RA, 2009. The Dopamine Response in the Nucleus Accumbens Core – Shell Border Differs From That in the Core and Shell During Operant Ethanol Self-Administration. Alcohol. Clin. Exp. Res 33, 1355–1365. doi: 10.1111/j.1530-0277.2009.00965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mohan A, De Ridder D, Sunaert S, Vanneste S, 2018. The neural correlates of the unified percept of alcohol-related craving: A fMRI and EEG study. Sci. Rep 8, 1–12. doi: 10.1038/s41598-017-18471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DEJ, 2011. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb. Cortex 21, 1408–1415. doi: 10.1093/cercor/bhq220 [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G, 1986. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J. Pharmacol. Exp. Ther 239, 219–228. [PubMed] [Google Scholar]
- Ingjaldsson JT, Thayer JF, Laberg JC, 2003. Craving for alcohol and pre-attentive processing of alcohol stimuli. Int. J. Psychophysiol 49, 29–39. doi: 10.1016/S0167-8760(03)00075-8 [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, 2015. Brain disorders? Precisely. Science (80-. ) 348, 2015–2017. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research Domain Criteria ( RDoC ): Toward a New Classification Framework for Research on Mental Disorders. Am. J. Psychiatry Online 167, 748–751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Bartholow BD, Hirsh JB, 2015. Emotional foundations of cognitive control. Trends Cogn. Sci 19, 126–132. doi: 10.1016/j.tics.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajodia A, Earleywine M, 2003. Measuring alcohol expectancies with the implicit association test. Psychol. Addict. Behav 17, 126–133. doi: 10.1037/0893-164X.17.2.126 [DOI] [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ, 1999. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res 817, 172–184. doi: 10.1016/S0006-8993(98)01245-1 [DOI] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA, 2011. In Vivo Chronic Intermittent Ethanol Exposure Reverses the Polarity of Synaptic Plasticity in the Nucleus Accumbens Shell. J. Pharmacol. Exp. Ther 336, 155–164. doi: 10.1124/jpet.110.171009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinek EM, 1960. Alcoholism: a genus and some of it species. Can. Med. Assoc. J 83, 1341–1345. [PMC free article] [PubMed] [Google Scholar]
- Johnsen BH, Laberg JC, Cox WM, Vaksdal A, Hugdahl K, 1994. Alcoholic Subjects’ Attentional Bias in the Processing of Alcohol-Related Words. Psychol. Addict. Behav 8, 111–115. doi: 10.1037/0893-164X.8.2.111 [DOI] [Google Scholar]
- Johnson CN, Fromme K, 1994. An experimental test of affect, subjective craving, and alcohol outcome expectancies as motivators of young adult drinking. Addict. Behav 19, 631–641. doi: 10.1016/0306-4603(94)90018-3 [DOI] [PubMed] [Google Scholar]
- Jones A, Christiansen P, Field M, 2018. Failed Attempts to Improve the Reliability of the Alcohol Visual Probe Task Following Empirical Recommendations. Psychol. Addict. Behav 32, 922–932. doi: 10.1037/adb0000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Field M, 2013. The effects of cue-specific inhibition training on alcohol consumption in heavy social drinkers. Exp. Clin. Psychopharmacol 21, 8–16. doi: 10.1037/a0030683 [DOI] [PubMed] [Google Scholar]
- Jones BC, Jones BT, Blundell L, Bruce G, 2002. Social users of alcohol and cannabis who detect substance-related changes in a change blindness paradigm report higher levels of use than those detecting substance-neutral changes. Psychopharmacology (Berl) 165, 93–96. doi: 10.1007/s00213-002-1264-2 [DOI] [PubMed] [Google Scholar]
- Jones BT, Bruce G, Livingstone S, Reed E, 2006. Alcohol-related attentional bias in problem drinkers with the flicker change blindness paradigm. Psychol. Addict. Behav 20, 171–177. doi: 10.1037/0893-164X.20.2.171 [DOI] [PubMed] [Google Scholar]
- Jones BT, Jones BC, Smith H, Copley N, 2003. A flicker paradigm for inducing change blindness reveals alcohol and cannabis information processing biases in social users. Addiction 98, 235–244. doi: 10.1046/j.1360-0443.2003.00270.x [DOI] [PubMed] [Google Scholar]
- Jonsson S, Ericson M, Söderpalm B, 2014. Modest long-term ethanol consumption affects expression of neurotransmitter receptor genes in the rat nucleus accumbens. Alcohol. Clin. Exp. Res 38, 722–729. doi: 10.1111/acer.12307 [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence DL, 2011. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin₁ receptors. Br. J. Pharmacol 162, 880–9. doi: 10.1111/j.1476-5381.2010.01088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambouropoulos N, Staiger PK, 2004. Reactivity to alcohol-related cues: Relationship among cue type, motivational processes, and personality. Psychol. Addict. Behav 18, 275–283. doi: 10.1037/0893-164X.18.3.275 [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF, 1985. Reactivity to Alcohol-Related Cues: Physiological and Subjective Responses in Alcoholics and Nonproblem Drinkers. J. Stud. Alcohol 46, 267–272. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, MacNamara A, Proudfit GH, 2013. Electrocortical evidence for rapid allocation of attention to threat in the dot-probe task. Soc. Cogn. Affect. Neurosci 10, 577–583. doi: 10.1093/scan/nsu098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O’Connor SJ, 2010a. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage 50, 267–76. doi: 10.1016/j.neuroimage.2009.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AEK, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li T-K, 2004. Alcohol-Related Olfactory Cues Activate the Nucleus Accumbens and Ventral Tegmental Area in High-Risk Drinkers: Preliminary Findings. Alcohol. Clin. Exp. Res 28, 550–557. doi: 10.1097/01.ALC.0000122764.60626.AF [DOI] [PubMed] [Google Scholar]
- Kareken DA, Grahame NJ, Dzemidzic M, Walker MJ, Lehigh CA, O’Connor SJ, 2012. fMRI of the brain’s response to stimuli experimentally paired with alcohol intoxication. Psychopharmacology (Berl) 220, 787–797. doi: 10.1007/s00213-011-2526-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherill LF, Dzemidzic M, Bragulat V, Cox C, Talavage T, O’Connor SJ, Foroud TM, 2010b. A polymorphism in GABRA2 is associated with the medial frontal response to alcohol cues in an fMRI study. Alcohol. Clin. Exp. Res 34, 2169–2178. doi: 10.1111/j.1530-0277.2010.01293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR, 2015. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend 150, 24–30. doi: 10.1016/j.drugalcdep.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem MA, Ahmed S, Sarker R, Ahmed EU, Hargreaves GA, McGregor IS, 2012. Long-term daily access to alcohol alters dopamine-related synthesis and signaling proteins in the rat striatum. Neurochem. Int 61, 1280–8. doi: 10.1016/j.neuint.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Katner SN, Magalong J, Weiss F, 1999. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology 20, 471–79. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F, 2006. Neurochemical Characteristics Associated With Ethanol Preference in Selected Alcohol-Preferring and -Nonpreferring Rats: A Quantitative Microdialysis Study. Alcohol. Clin. Exp. Res 25, 198–205. doi: 10.1111/j.1530-0277.2001.tb02199.x [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F, 1999. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol. Clin. Exp. Res 23, 1751–1760. doi:00000374-199911000-00007 [pii] [PubMed] [Google Scholar]
- Kavanagh DJ, Andrade J, May J, 2005. Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychol. Rev 112, 446–467. doi: 10.1037/0033-295X.112.2.446 [DOI] [PubMed] [Google Scholar]
- Kersbergen I, Field M, 2017. Alcohol consumers’ attention to warning labels and brand information on alcohol packaging: Findings from cross-sectional and experimental studies. BMC Public Health 17, 1–11. doi: 10.1186/s12889-017-4055-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Shah G, Sharma H, 1990. Comparison of sensitivity and alcohol consumption in four outbred strains of rats. Alcohol 7, 429–434. doi: 10.1016/0741-8329(90)90027-A [DOI] [PubMed] [Google Scholar]
- Kiefer F, Kirsch M, Bach P, Hoffmann S, Reinhard I, Jorde A, von der Goltz C, Spanagel R, Mann KF, Loeber S, Vollstädt-Klein S, 2015. Effects of d-cycloserine on extinction of mesolimbic cue reactivity in alcoholism: a randomized placebo-controlled trial. Psychopharmacology (Berl) 232, 2353–2362. doi: 10.1007/s00213-015-3882-5 [DOI] [PubMed] [Google Scholar]
- Kim SM, Han DH, Min KJ, Kim BN, Cheong JH, 2014. Brain activation in response to craving- and aversion-inducing cues related to alcohol in patients with alcohol dependence. Drug Alcohol Depend 141, 124–131. doi: 10.1016/j.drugalcdep.2014.05.017 [DOI] [PubMed] [Google Scholar]
- King AC, De Wit H, McNamara PJ, Cao D, 2011. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch. Gen. Psychiatry 68, 389–399. doi: 10.1001/archgenpsychiatry.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D, 2016. A Prospective 5-Year Re-examination of Alcohol Response in Heavy Drinkers Progressing in Alcohol Use Disorder. Biol. Psychiatry 79, 489–498. doi: 10.1016/j.biopsych.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, De Wit H, Holdstock L, Schuster A, 2002. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol. Clin. Exp. Res 26, 827–835. doi: 10.1097/00000374-200206000-00012 [DOI] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D, 2014. Alcohol challenge responses predict future alcohol use disorder symptoms: A 6-year prospective study. Biol. Psychiatry 75, 798–806. doi: 10.1016/j.biopsych.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Walker BM, 2015. Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala κ-Opioid Receptors. Neuropsychopharmacology 41, 1–8. doi: 10.1038/npp.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CP, Hauser SR, Deehan GA, Toalston JE, Mcbride WJ, Rodd-Henricks ZA, 2016. Oral Conditioned Cues Can Enhance or Inhibit Ethanol (EtOH)-Seeking and EtOH-Relapse Drinking by Alcohol-Preferring (P) Rats. Alcohol. Clin. Exp. Res 40, 906–915. doi: 10.1111/acer.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–38. doi: 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krank MD, 2003. Pavlovian conditioning with ethanol: sign-tracking (autoshaping), conditioned incentive, and ethanol self-administration. Alcohol. Clin. Exp. Res 27, 1592–8. doi: 10.1097/01.ALC.0000092060.09228.DE [DOI] [PubMed] [Google Scholar]
- Krank MD, O’Neill S, Squarey K, Jacob J, 2008. Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology (Berl) 196, 397–405. doi: 10.1007/s00213-007-0971-0 [DOI] [PubMed] [Google Scholar]
- Kreusch F, Billieux J, Quertemont E, 2017. Alcohol-cue exposure decreases response inhibition towards alcohol-related stimuli in detoxified alcohol-dependent patients. Psychiatry Res 249, 232–239. doi: 10.1016/j.psychres.2017.01.019 [DOI] [PubMed] [Google Scholar]
- Kroczek AM, Haeussinger FB, Hudak J, Vanes LD, Fallgatter AJ, Ehlis A-C, 2018. Cue Reactivity Essentials: Event-Related Potentials During Identification of Visual Alcoholic Stimuli in Social Drinkers. J. Stud. Alcohol Drugs 79, 137–147. doi: 10.15288/jsad.2018.79.137 [DOI] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PEM, Seamans JK, 2009. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One 4. doi: 10.1371/journal.pone.0006507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ, 2012. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One 7, e37541. doi: 10.1371/journal.pone.0037541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Wada K, Jarvis ED, 2010. Dopamine receptors in a songbird brain. J. Comp. Neurol 518, 741–769. doi: 10.1002/cne.22255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D, 2016. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biol. Psychiatry 80, 179–189. doi: 10.1016/j.biopsych.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell AM, DuBois DW, McCool BA, 2007. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol 98, 3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Floyd DW, McCool BA, 2005. Chronic ethanol ingestion modulates proanxiety factors expressed in rat central amygdala. Alcohol 36, 83–90. doi: 10.1016/j.alcohol.2005.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagström O, Danielsson K, Söderpalm B, Ericson M, Adermark L, 2019. Voluntary Ethanol Intake Produces Subregion-Specific Neuroadaptations in Striatal and Cortical Areas of Wistar Rats. Alcohol. Clin. Exp. Res 43, 803–811. doi: 10.1111/acer.14014 [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Ginsburg BC, Greig A, Schindler CW, 2019. Effects of Rat Strain and Method of Inducing Ethanol Drinking on Pavlovian-Instrumental-Transfer with Ethanol-Paired Conditioned Stimuli. Alcohol doi: 10.1016/j.alcohol.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Ginsburg BC, Schindler CW, 2016. Effects of an ethanol-paired CS on responding for ethanol and food: Comparisons with a stimulus in a Truly-Random-Control group and to a food-paired CS on responding for food. Alcohol 57, 15–27. doi: 10.1016/j.alcohol.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H, Engels RCME, Wiers RW, Granic I, Spijkerman R, 2012. Implicit and explicit alcohol cognitions and observed alcohol consumption: Three studies in (semi)naturalistic drinking settings. Addiction 107, 1420–1428. doi: 10.1111/j.1360-0443.2012.03805.x [DOI] [PubMed] [Google Scholar]
- Laude JR, Fillmore MT, 2015. Alcohol cues impair learning inhibitory signals in beer drinkers. Alcohol. Clin. Exp. Res 39, 880–886. doi: 10.1111/acer.12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Smith Y, Parent A, 1989. Dopaminergic innervation of the basal ganglia in the squirrel monkey as revealed by tyrosine hydroxylase immunohistochemistry. J. Comp. Neurol 289, 36–52. doi: 10.1002/cne.902890104 [DOI] [PubMed] [Google Scholar]
- LeCocq MR, Lahlou S, Chahine M, Padillo LN, Chaudhri N, 2018. Modeling Relapse to Pavlovian Alcohol-Seeking in Rats Using Reinstatement and Spontaneous Recovery Paradigms. Alcohol. Clin. Exp. Res 42, 1795–1806. doi: 10.1111/acer.13825 [DOI] [PubMed] [Google Scholar]
- Lee E, Ku J, Jung YC, Lee H, An SK, Kim KR, Yoon KJ, Namkoong K, 2013. Neural evidence for emotional involvement in pathological alcohol craving. Alcohol Alcohol 48, 288–294. doi: 10.1093/alcalc/ags130 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Wheeler DS, Holland PC, 2011. Interactions between amygdala central nucleus and the ventral tegmental area in the acquisition of conditioned cue-directed behavior in rats. Eur. J. Neurosci 33, 1876–84. doi: 10.1111/j.1460-9568.2011.07680.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Cho S, Lee JH, 2014. Approach-avoidance pattern of visual attention in hazardous drinkers with ambivalence. Addict. Behav 39, 669–676. doi: 10.1016/j.addbeh.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Leeman RF, Corbin WR, Fromme K, 2009. Craving predicts within session drinking behavior following placebo. Pers. Individ. Dif 46, 693–698. doi: 10.1016/j.paid.2009.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Nogueira C, Wiers RW, Cousijn J, Serafini K, DeMartini KS, Bargh JA, O’Malley SS, 2018. A Test of Multisession Automatic Action Tendency Retraining to Reduce Alcohol Consumption Among Young Adults in the Context of a Human Laboratory Paradigm. Alcohol. Clin. Exp. Res 42, 803–814. doi: 10.1111/acer.13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki P, Hill T, Czyzewska M, 1992. Nonconscious acquisition of information. Am. Psychol 47, 796–801. [DOI] [PubMed] [Google Scholar]
- Leyton M, 2007. Conditioned and sensitized responses to stimulant drugs in humans. Prog. Neuro-Psychopharmacology Biol. Psychiatry 31, 1601–1613. doi: 10.1016/j.pnpbp.2007.08.027 [DOI] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan Y-L, Wang G-B, Wang F, Ma M-Y, Xue M-M, Luo Y-X, Yang F-D, Bao Y-P, Shi J, Sun H-Q, Lu L, 2015. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict. Biol 20, 513–522. doi: 10.1111/adb.12140 [DOI] [PubMed] [Google Scholar]
- Liappas J, Paparrigopoulos T, Tzavellas E, Christodoulou G, 2002. Impact of alcohol detoxification on anxiety and depressive symptoms. Drug Alcohol Depend 68, 215–220. doi: 10.1016/S0376-8716(02)00195-3 [DOI] [PubMed] [Google Scholar]
- Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, Spigelman I, 2014. Ethanol-Induced Plasticity of GABAA Receptors in the Basolateral Amygdala. Neurochem. Res 39, 1162–1170. doi: 10.1007/s11064-014-1297-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Lapish CC, 2015. Neural Firing in the Prefrontal Cortex During Alcohol Intake in Alcohol-Preferring “P” Versus Wistar Rats. Alcohol. Clin. Exp. Res 39, 1642–1653. doi: 10.1111/acer.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E, 2011. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci 34, 536–47. doi: 10.1016/j.tins.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Kadden RM, Gaupp L, 1990. Reactivity to alcohol cues and induced moods in alcoholics. Addict. Behav 15, 137–146. [DOI] [PubMed] [Google Scholar]
- Littel M, Euser AS, Munafò MR, Franken IHA, 2012. Electrophysiological indices of biased cognitive processing of substance-related cues: A meta-analysis. Neurosci. Biobehav. Rev 36, 1803–1816. doi: 10.1016/j.neubiorev.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Littel M, Field M, Van De Wetering BJM, Franken IHA, 2013. Reduced Cognitive Processing of Alcohol Cues in Alcohol- Dependent Patients Seeking Treatment: An ERP Study. J. Exp. Psychopathol 4, 291–302. doi: 10.5127/jep.027412 [DOI] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF, 2015. Heterogeneity of alcohol use disorder: Understanding mechanisms to advance personalized treatment. Alcohol. Clin. Exp. Res 39, 579–584. doi: 10.1111/acer.12669 [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F, 2002a. Additive Effect of Stress and Drug Cues on Reinstatement of Ethanol Seeking: Exacerbation by History of Dependence and Role of Concurrent Activation of Corticotropin-Releasing Factor and Opioid Mechanisms. J. Neurosci 22, 7856–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F, 2002b. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J. Pharmacol. Exp. Ther 300, 882–889. doi: 10.1124/jpet.300.3.882 [DOI] [PubMed] [Google Scholar]
- Loeber S, Croissant B, Heinz A, Mann KF, Flor H, 2006. Cue exposure in the treatment of alcohol dependence: Effects on drinking outcome, craving and self-efficacy. Br. J. Clin. Psychol 45, 515–529. doi: 10.1348/014466505X82586 [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel Márquez H, Nakovics H, Heinz A, Mann KF, Flor H, 2010. Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol 45, 541–7. doi: 10.1093/alcalc/agq065 [DOI] [PubMed] [Google Scholar]
- Loeber S, Vollstädt-Klein S, Von Der Goltz C, Flor H, Mann KF, Kiefer F, 2009. Attentional bias in alcohol-dependent patients: The role of chronicity and executive functioning. Addict. Biol 14, 194–203. doi: 10.1111/j.1369-1600.2009.00146.x [DOI] [PubMed] [Google Scholar]
- Logothetis NK, 2003. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci 23, 3963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pfeuffer J, 2004. On the nature of the BOLD fMRI contrast mechanism. Magn. Reson. Imaging 22, 1517–1531. doi: 10.1016/j.mri.2004.10.018 [DOI] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MAM, Gessa GL, Colombo G, 2010. Increase in Alcohol Intake, Reduced Flexibility of Alcohol Drinking, and Evidence of Signs of Alcohol Intoxication in Sardinian Alcohol-Preferring Rats Exposed to Intermittent Access to 20% Alcohol. Alcohol. Clin. Exp. Res 34, 2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x [DOI] [PubMed] [Google Scholar]
- Loijen A, Rinck M, Walvoort SJW, Kessels RPC, Becker ES, Egger JIM, 2018. Modification of Automatic Alcohol-Approach Tendencies in Alcohol-Dependent Patients with Mild or Major Neurocognitive Disorder. Alcohol. Clin. Exp. Res 42, 153–161. doi: 10.1111/acer.13529 [DOI] [PubMed] [Google Scholar]
- Lovett DE, Ham LS, Veilleux JC, 2015. Psychometric evaluation of a standardized set of alcohol cue photographs to assess craving. Addict. Behav 48, 58–61. doi: 10.1016/j.addbeh.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES, 2017. Electroencephalography and event-related brain potentials, in: Cacioppo JT, Tassinary LG, Berntson GG (Eds.), Handbook of Psychophysiology Cambridge University Press, New York, NY, US. [Google Scholar]
- Ludwig AM, Bendfeldt F, Wikler A, Cain RB, 1978. ‘Loss of Control’ in Alcoholics. Arch. Gen. Psychiatry 35, 370–373. doi: 10.1001/archpsyc.1978.01770270120012 [DOI] [PubMed] [Google Scholar]
- Ludwig AM, Wikler A, Stark LH, 1974. The first drink. Psychobiological aspects of craving. Arch. Gen. Psychiatry 30, 539–547. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM, 2013. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: A bold FMRI study. Neuroimage 78, 176–185. doi: 10.1016/j.neuroimage.2013.03.055 [DOI] [PubMed] [Google Scholar]
- Lusher J, Chandler C, Ball D, 2004. Alcohol dependence and the alcohol Stroop paradigm: evidence and issues. Drug Alcohol Depend 75, 225–231. doi: 10.1016/j.drugalcdep.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Macey D, Schulteis G, Heinrichs SC, Koob GF, 1996. Time-Dependent Quantifiable Withdrawal From Ethanol in the Rat : Effect of Method of Dependence Induction. Alcohol 13, 163–170. [DOI] [PubMed] [Google Scholar]
- MacKillop J, 2006. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol. Clin. Exp. Res 30, 1315–1321. doi: 10.1111/j.1530-0277.2006.00159.x [DOI] [PubMed] [Google Scholar]
- MacKillop J, Few LR, Stojek MK, Murphy CM, Malutinok SF, Johnson FT, Hofmann SG, McGeary JE, Swift RM, Monti PM, 2015. D-cycloserine to enhance extinction of cue-elicited craving for alcohol: a translational approach. Transl. Psychiatry 5, e544. doi: 10.1038/tp.2015.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Lisman SA, 2008. Effects of a Context Shift and Multiple Context Extinction on Reactivity to Alcohol Cues. Exp. Clin. Psychopharmacol 16, 322–331. doi: 10.1037/a0012686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Lisman SA, 2005. Reactivity to alcohol cues: isolating the role of perceived availability. Exp. Clin. Psychopharmacol 13, 229–237. doi: 10.1037/1064-1297.13.3.229 [DOI] [PubMed] [Google Scholar]
- Majchrowicz E, 1975. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia 43, 245–254. doi: 10.1007/BF00429258 [DOI] [PubMed] [Google Scholar]
- Manchery L, Yarmush DE, Luehring-Jones P, Erblich J, 2017. Attentional bias to alcohol stimuli predicts elevated cue-induced craving in young adult social drinkers. Addict. Behav 70, 14–17. doi: 10.1016/j.addbeh.2017.01.035 [DOI] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE, 2014. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 282, 176–197. doi: 10.1016/j.neuroscience.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, 1996. Taxonomy of high-risk situations for alcohol relapse : evolution and development of a cognitive-behavioral model. Addict. Behav 91. [PubMed] [Google Scholar]
- Marlatt GA, Demming B, Reid JB, 1973. Loss of control drinking in alcoholics: An experimental analogue. J. Abnorm. Psychol 81, 233–241. doi: 10.1037/h0034532 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F, 2017. Perseveration of craving: effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addict. Biol 22, 923–932. doi: 10.1111/adb.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM, 1993. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol. Clin. Exp. Res 17, 140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x [DOI] [PubMed] [Google Scholar]
- Martinotti G, Nicola M. Di, Reina D, Andreoli S, Focà F, Cunniff A, Tonioni F, Bria P, Janiri L, 2008. Alcohol protracted withdrawal syndrome: The role of anhedonia. Subst. Use Misuse 43, 271–284. doi: 10.1080/10826080701202429 [DOI] [PubMed] [Google Scholar]
- Martinovic J, Jones A, Christiansen P, Rose AK, Hogarth L, Field M, 2014. Electrophysiological responses to alcohol cues are not associated with Pavlovian-to-instrumental transfer in social drinkers. PLoS One 9, e94605. doi: 10.1371/journal.pone.0094605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins JS, Bartholow BD, Lynne Cooper M, Irvin KM, Piasecki TM, 2019. Interactive Effects of Naturalistic Drinking Context and Alcohol Sensitivity on Neural Alcohol Cue-Reactivity Responses. Alcohol. Clin. Exp. Res 43, 1777–1789. doi: 10.1111/acer.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty VN, Spigelman I, 2012. Long-Lasting Alterations in Membrane Properties, K+ Currents, and Glutamatergic Synaptic Currents of Nucleus Accumbens Medium Spiny Neurons in a Rat Model of Alcohol Dependence. Front. Neurosci 6, 1–15. doi: 10.3389/fnins.2012.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheus-Roth C, Schenk I, Wiltfang J, Scherbaum N, Müller BW, 2016. Occipital event-related potentials to addiction-related stimuli in detoxified patients with alcohol dependence, and their association with three-month relapse. BMC Psychiatry 16, 1–12. doi: 10.1186/s12888-016-0782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo LM, de Wit H, 2016. Acquisition of Conditioned Responses to a Novel Alcohol-Paired Cue in Social Drinkers. J. Stud. Alcohol Drugs 77, 317–326. doi: 10.15288/jsad.2016.77.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Dyr W, Lumeng L, Li T-K, 1993. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol 10, 387–390. doi: 10.1016/0741-8329(93)90025-J [DOI] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Stitzer ML, 1989. Psychophysiological Effects of Alcohol- related Stimuli: I. The Role of Stimulus Intensity. Alcohol. Clin. Exp. Res 13, 386–391. doi: 10.1111/j.1530-0277.1989.tb00340.x [DOI] [PubMed] [Google Scholar]
- Mccusker CG, Brown K, 1990. Alcohol-predictive cues enhance tolerance to and precipitate “craving” for alcohol in social drinkers. J. Stud. Alcohol 51, 494–499. doi: 10.15288/jsa.1990.51.494 [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH, 2013. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci 33, 2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaugh McAteer A, Curran D, Hanna D, 2015. Alcohol attention bias in adolescent social drinkers: An eye tracking study. Psychopharmacology (Berl) 232, 3183–3191. doi: 10.1007/s00213-015-3969-z [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li T-K, McBride WJ, Murphy JM, 2002. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol. Clin. Exp. Res 26, 318–325. doi: 10.1111/j.1530-0277.2002.tb02540.x [DOI] [PubMed] [Google Scholar]
- Mellentin AI, Skøt L, Nielsen B, Schippers GM, Nielsen AS, Stenager E, Juhl C, 2017. Cue exposure therapy for the treatment of alcohol use disorders: A meta-analytic review. Clin. Psychol. Rev 57, 195–207. doi: 10.1016/j.cpr.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Mereu G, Fadda F, Gessa GL, 1984. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain Res 292, 63–69. doi: 10.1016/0006-8993(84)90890-4 [DOI] [PubMed] [Google Scholar]
- Miller MA, Fillmore MT, 2011. Persistence of attentional bias toward alcohol-related stimuli in intoxicated social drinkers. Drug Alcohol Depend 117, 184–189. doi: 10.1016/j.drugalcdep.2011.01.016 [DOI] [PubMed] [Google Scholar]
- Miller MA, Fillmore MT, 2010. The effect of image complexity on attentional bias towards alcohol-related images in adult drinkers. Addiction 105, 883–890. doi: 10.1111/j.1360-0443.2009.02860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Schramm MJW, Wawrzynski JR, Gore F, Oikonomou-Mpegeti F, Wang NQ, Samuel D, Economidou D, Everitt BJ, 2012. Antagonism at NMDA receptors, but not β-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology (Berl) 219, 751–61. doi: 10.1007/s00213-011-2399-9 [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SRS, Robinson SW, Jaber M, Caron MG, 1998. Dopamine receptors: from structure to function. Physiol. Rev 78, 189–225. doi: 10.1186/1471-2296-12-32 [DOI] [PubMed] [Google Scholar]
- Modi P, Malik L, Punia V, Kumar K, Dogra R, 2019. Attentional biases for alcohol-Stroop test in patients with alcohol dependence. Open J. Psychiatry Allied Sci 10, 155. doi: 10.5958/2394-2061.2019.00034.X [DOI] [Google Scholar]
- Mogg K, Bradley BP, De Bon J, Painter M, 1997. Time course of attentional bias for threat information in non-clinical anxiety. Behav Res Ther 35, 297–303. [DOI] [PubMed] [Google Scholar]
- Monem RG, Fillmore MT, 2016. Measuring Heightened Attention to Alcohol in a Naturalistic Setting: A Validation Study. Exp. Clin. Psychopharmacol 118, 6072–6078. doi: 10.1002/cncr.27633.Percutaneous [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Zwick WR, Abrams DB, Nirenberg MJ, Liepman MR, 1987. Reactivity of alcoholics and nonalcoholics to drinking cues. J. Abnorm. Psychol 96, 122–126. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, 2003. Coping Skills Training and Cue Exposure Treatment, in: Hester R, Miller W (Eds.), Handbook of Alcoholism Treatment Approaches: Effective Alternatives Pearson Education, Inc, pp. 213–236. [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Abrams DB, 1993a. Alcohol cue reactivity: effects of detoxification and extended exposure. J. Stud. Alcohol 54, 235–245. doi: 10.15288/jsa.1993.54.235 [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Abrams DB, 1993b. Cue Exposure With Coping Skills Treatment for Male Alcoholics A Preliminary Investigation. J. Counsulting Clin. Psychol 61, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK, 2001. Naltrexone and Cue Exposure With Coping and Communication Skills Training for Alcoholics: Treatment Process and 1-Year Outcomes. Alcohol. Clin. Exp. Res 25, 1634–1647. doi: 10.1111/j.1530-0277.2001.tb02170.x [DOI] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, Mccool BA, 2015. Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacol. Biochem. Behav 139, 67–76. doi: 10.1016/j.pbb.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, 2010. Subjective response to alcohol: A critical review of the literature. Alcohol. Clin. Exp. Res 34, 385–395. doi: 10.1111/j.1530-0277.2009.01103.x [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, 2008. Subjective alcohol effects and drinking behavior: The relative influence of early response and acquired tolerance. Addict. Behav 33, 1306–1313. doi: 10.1016/j.addbeh.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Morean ME, De Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS, 2013. The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology (Berl) 227, 177–192. doi: 10.1007/s00213-012-2954-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T, 1989. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci 12, 459–62. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Marunde RL, Downey D, 2010. Limited access to ethanol increases the number of spontaneously active dopamine neurons in the posterior ventral tegmental area of nondependent P rats. Alcohol 44, 257–264. doi: 10.1016/j.alcohol.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Stuhlinger M, Mundle G, 2000. Appetitive effects of drug cues modelled by pictures of the intake ritual: generality of cue-modulated startle examined with inpatient alcoholics. Psychopharmacology (Berl) 151, 428–432. doi: 10.1007/s002130000508 [DOI] [PubMed] [Google Scholar]
- Murphy CM, Stojek MK, Few LR, Rothbaum AO, MacKillop J, 2014. Craving as an alcohol use disorder symptom in DSM-5: An empirical examination in a treatment-seeking sample. Exp. Clin. Psychopharmacol 22, 43–49. doi: 10.1037/a0034535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, McBride WJ, Lumeng L, Li T-K, 1989. Operant responding for oral ethanol in the alcohol-preferring P and alcohol-nonpreferring NP lines of rats. Alcohol 6, 127–131. doi: 10.1016/0741-8329(89)90037-2 [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li T-K, 1982. Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol. Biochem. Behav 16, 145–149. doi: 10.1016/0091-3057(82)90026-0 [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK, 2002. Phenotypic and genotypic characterization of the Indiana university rat lines selectively bred for high and low alcohol preference. Behav. Genet doi: 10.1023/A:1020266306135 [DOI] [PubMed] [Google Scholar]
- Murphy P, Garavan H, 2011. Cognitive predictors of problem drinking and AUDIT scores among college students. Drug Alcohol Depend 115, 94–100. doi: 10.1016/j.drugalcdep.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes DJ, Voronin K, George MS, 2004. Differential Brain Activity in Alcoholics and Social Drinkers to Alcohol Cues: Relationship to Craving. Neuropsychopharmacology 29, 393–402. doi: 10.1038/sj.npp.1300295 [DOI] [PubMed] [Google Scholar]
- Nader K, Einarsson EÖ, 2010. Memory reconsolidation: An update. Ann. N. Y. Acad. Sci 1191, 27–41. doi: 10.1111/j.1749-6632.2010.05443.x [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O, 2007. A single standard for memory: the case for reconsolidation. Debates Neurosci 1, 2–16. doi: 10.1007/s11559-007-9005-7 [DOI] [PubMed] [Google Scholar]
- Naimi TS, Lipscomb LE, Brewer RD, Colley Gilbert B, 2003. Pregnancy: Implications for Women and Their Children Binge Drinking in the Preconception Period and the Risk of Unintended Binge Drinking in the Preconception Period and the Risk of Unintended Pregnancy: Implications for Women and Their Children. Pediatr. Blvd 111. [PubMed] [Google Scholar]
- Namkoong K, Lee E, Lee CH, Lee BO, An SK, 2004. Increased P3 amplitudes induced by alcohol-related pictures in patients with alcohol dependence. Alcohol. Clin. Exp. Res 28, 1317–1323. doi: 10.1097/01.ALC.0000139828.78099.69 [DOI] [PubMed] [Google Scholar]
- Nentwig TB, Myers KP, Grisel JE, 2017. Initial subjective reward to alcohol in Sprague-Dawley rats. Alcohol 58, 19–22. doi: 10.1016/j.alcohol.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, 1986. Conditioned compensatory response to alcohol placebo in humans. Psychopharmacology (Berl) 88, 247–251. doi: 10.1016/0741-8329(85)90124-7 [DOI] [PubMed] [Google Scholar]
- Newlin DB, 1985. The Antagonistic Placebo Response to Alcohol Cues. Alcohol. Clin. Exp. Res 9, 411–416. doi: 10.1111/j.1530-0277.1985.tb05573.x [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB, 1990. Alcohol Challenge With Sons of Alcoholics: A Critical Review and Analysis. Psychol. Bull 108, 383–402. doi: 10.1037/0033-2909.108.3.383 [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB, 1988. Relevance of cue reactivity to understanding alcohol and smoking relapse. J. Abnorm. Psychol 97, 133–152. [DOI] [PubMed] [Google Scholar]
- Nikolaou K, Field M, Critchley HD, Duka T, 2013. Acute alcohol effects on attentional bias are mediated by subcortical areas associated with arousal and salience attribution. Neuropsychopharmacology 38, 1365–1373. doi: 10.1038/npp.2013.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ, 2016. Chronic Intermittent Ethanol Exposure Enhances the Excitability and Synaptic Plasticity of Lateral Orbitofrontal Cortex Neurons and Induces a Tolerance to the Acute Inhibitory Actions of Ethanol. Neuropsychopharmacology 41, 1112–1127. doi: 10.1038/npp.2015.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nona CN, Hendershot CS, Lê AD, 2018. Behavioural sensitization to alcohol: Bridging the gap between preclinical research and human models. Pharmacol. Biochem. Behav 173, 15–26. doi: 10.1016/j.pbb.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD, 2010. Drug harms in the UK: A multicriteria decision analysis. Lancet 376, 1558–1565. doi: 10.1016/S0140-6736(10)61462-6 [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Saulsbury W, Blakemore C, 2007. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet 369, 1047–1053. doi: 10.1016/S0140-6736(07)60464-4 [DOI] [PubMed] [Google Scholar]
- O’Brien ES, Legastelois R, Houchi H, Vilpoux C, Alaux-Cantin S, Pierrefiche O, André E, Naassila M, 2011. Fluoxetine, desipramine, and the dual antidepressant milnacipran reduce alcohol self-administration and/or relapse in dependent rats. Neuropsychopharmacology 36, 1518–1530. doi: 10.1038/npp.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, 2003. Dopamine gating of forebrain neural ensembles. Eur. J. Neurosci. 17, 429–435. doi: 10.1046/j.1460-9568.2003.02463.x [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Eiler WJA, Carron CR, Soeurt CM, Plawecki MH, Grahame NJ, O’Connor SJ, Kareken DA, 2018. Pairing neutral cues with alcohol intoxication: new findings in executive and attention networks. Psychopharmacology (Berl) 235, 2725–2737. doi: 10.1007/s00213-018-4968-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Harezlak J, Kudela MA, Tran SM, Soeurt CM, Yoder KK, Kareken DA, 2016. Corticostriatal and Dopaminergic Response to Beer Flavor with Both fMRI and [(11) C]raclopride Positron Emission Tomography. Alcohol. Clin. Exp. Res 1–9. doi: 10.1111/acer.13158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA, 2013. Beer Flavor Provokes Striatal Dopamine Release in Male Drinkers: Mediation by Family History of Alcoholism. Neuropsychopharmacology 38, 1617–1624. doi: 10.1038/npp.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT, 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci 1251, E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ, 1995. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse 21, 289–298. doi: 10.1002/syn.890210403 [DOI] [PubMed] [Google Scholar]
- Ostafin BD, Marlatt GA, 2008. Surfing the Urge: Experiential Acceptance Moderates the Relation Between Automatic Alcohol Motivation and Hazardous Drinking. J. Soc. Clin. Psychol 27, 404–418. doi: 10.1521/jscp.2008.27.4.404 [DOI] [Google Scholar]
- Ostafin BD, Marlatt GA, Greenwald AG, 2008. Drinking without thinking: An implicit measure of alcohol motivation predicts failure to control alcohol use. Behav. Res. Ther. 46, 1210–1219. doi: 10.1016/j.brat.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Ostafin BD, Palfai T, 2006. Compelled to consume: The implicit association test and automatic alcohol motivation. Psychol. Addict. Behav 20, 322–327. doi: 10.1037/0893-164X.20.3.322 [DOI] [PubMed] [Google Scholar]
- Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA, 2006. Effects of multiple alcohol deprivations on operant ethanol self-administration by high-alcohol-drinking replicate rat lines. Alcohol 38, 155–164. doi: 10.1016/j.alcohol.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Janowsky AJ, Crabbe JC, 2015. Commonalities and Distinctions Among Mechanisms of Addiction to Alcohol and Other Drugs. Alcohol. Clin. Exp. Res 39, 1863–1877. doi: 10.1111/acer.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päivärinta P, Korpi ER, 1993. Voluntary ethanol drinking increases locomotor activity in alcohol-preferring AA rats. Pharmacol. Biochem. Behav 44, 127–132. doi: 10.1016/0091-3057(93)90289-6 [DOI] [PubMed] [Google Scholar]
- Palfai T, Ostafin BD, 2003. Alcohol-related motivational tendencies in hazardous drinkers: Assessing implicit response tendencies using the modified-IAT. Behav. Res. Ther 41, 1149–1162. doi: 10.1016/S0005-7967(03)00018-4 [DOI] [PubMed] [Google Scholar]
- Papachristou H, Nederkoorn C, Giesen JCAH, Jansen A, 2014. Cue reactivity during treatment, and not impulsivity, predicts an initial lapse after treatment in alcohol use disorders. Addict. Behav 39, 737–9. doi: 10.1016/j.addbeh.2013.11.027 [DOI] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL, 2001. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor- mediated 36 Cl(−) uptake in mesocorticolimbic regions of rat brain. Alcohol. Clin. Exp. Res 25, 1270–5. [PubMed] [Google Scholar]
- Paredes A, Hood WR, Seymour H, Gollob M, 1973. Loss of control in alcoholism: An investigation of the hypothesis, with experimental findings. Q. J. Stud. Alcohol [PubMed] [Google Scholar]
- Parent A, Hazrati L, 1995. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev 20, 91–127. doi: 10.1016/0165-0173(94)00007-C [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G, 2009. The role of interoception and alliesthesia in addiction. Pharmacol. Biochem. Behav 94, 1–7. doi: 10.1016/j.pbb.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TJ, Rychtarik RG, Rappaport NB, Smith PO, Etscheidt M, Brown TA, Johnson CA, 1992. Reactivity to alcohol-relevant beverage and imaginal cues in alcoholics. Addict. Behav 17, 209–217. [DOI] [PubMed] [Google Scholar]
- Peeters M, Monshouwer K, van de Schoot R, Janssen T, Vollebergh WAM, Wiers RW, 2013. Automatic processes and the drinking behavior in early adolescence: A prospective study. Alcohol. Clin. Exp. Res 37, 1737–1744. doi: 10.1111/acer.12156 [DOI] [PubMed] [Google Scholar]
- Peeters M, Wiers RW, Monshouwer K, van de Schoot R, Janssen T, Vollebergh WAM, 2012. Automatic processes in at-risk adolescents: The role of alcohol-approach tendencies and response inhibition in drinking behavior. Addiction 107, 1939–1946. doi: 10.1111/j.1360-0443.2012.03948.x [DOI] [PubMed] [Google Scholar]
- Perez-Fernandez J, Stephenson-Jones M, Suryanarayana SM, Robertson B, Grillner S, 2014. Evolutionarily conserved organization of the dopaminergic system in lamprey: SNc/VTA afferent and efferent connectivity and D2 receptor expression. J. Comp. Neurol 522, 3775–3794. doi: 10.1002/cne.23639 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Ciccocioppo M, Jacobs L, Doyle T, Caggiula AR, 2003. The subjective and reinforcing effects of visual and olfactory stimuli in alcohol drinking. Exp. Clin. Psychopharmacol 11, 269–75. doi: 10.1037/1064-1297.11.4.269 [DOI] [PubMed] [Google Scholar]
- Petit G, Cimochowska A, Cevallos C, Cheron G, Kornreich C, Hanak C, Schroder E, Verbanck P, Campanella S, 2015. Reduced processing of alcohol cues predicts abstinence in recently detoxified alcoholic patients in a three-month follow up period : An ERP study. Behav. Brain Res 282, 84–94. doi: 10.1016/j.bbr.2014.12.057 [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Maurage P, Noël X, Letesson C, Verbanck P, Campanella S, 2012. Early attentional modulation by alcohol-related cues in young binge drinkers: An event-related potentials study. Clin. Neurophysiol 123, 925–936. doi: 10.1016/j.clinph.2011.10.042 [DOI] [PubMed] [Google Scholar]
- Phelps ME, Mazziota JC, 1985. Positron Emission Tomography : Human Brain Function and Biochemistry. Science (80-. ) 228, 799–809. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Alley KJ, Slutske WS, Wood PK, Sher KJ, Shiffman S, Heath AC, 2012. Low sensitivity to alcohol: Relations with hangover occurrence and susceptibility in an ecological momentary assessment investigation. J. Stud. Alcohol Drugs 73, 925–932. doi: 10.15288/jsad.2012.73.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters S, Burk WJ, Van der Vorst H, Wiers RW, Engels RCME, 2012. The Moderating Role of Working Memory Capacity and Alcohol-Specific Rule-Setting on the Relation Between Approach Tendencies and Alcohol Use in Young Adolescents. Alcohol. Clin. Exp. Res 36, 915–922. doi: 10.1111/j.1530-0277.2011.01688.x [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Phillips KB, Jones JL, Robinson TE, Sarter M, 2017. Diverse Roads to Relapse: A Discriminative Cue Signaling Cocaine Availability Is More Effective in Renewing Cocaine Seeking in Goal Trackers Than Sign Trackers and Depends on Basal Forebrain Cholinergic Activity. J. Neurosci 37, 7198–7208. doi: 10.1523/JNEUROSCI.0990-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Rivier J, Beaunieux H, Vabret F, Desgranges B, Eustache F, 2009. Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcohol. Clin. Exp. Res 33, 490–498. doi: 10.1111/j.1530-0277.2008.00859.x [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL, 2015. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99, 735–749. doi: 10.1016/j.neuropharm.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Fertig JB, Baker LH, Cooney NL, 1983. Reactivity to alcohol cues in alcoholics and non-alcoholics: Implications for a stimulus control analysis of drinking. Addict. Behav 8, 1–10. [DOI] [PubMed] [Google Scholar]
- Price TF, Harmon-Jones E, 2011. Approach motivational body postures lean toward left frontal brain activity. Psychophysiology 48, 718–722. doi: 10.1111/j.1469-8986.2010.01127.x [DOI] [PubMed] [Google Scholar]
- Pronk T, van Deursen DS, Beraha EM, Larsen H, Wiers RW, 2015. Validation of the Amsterdam Beverage Picture Set: A Controlled Picture Set for Cognitive Bias Measurement and Modification Paradigms. Alcohol. Clin. Exp. Res 39, 2047–2055. doi: 10.1111/acer.12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K, 2011. Subjective response to alcohol challenge: A quantitative review. Alcohol. Clin. Exp. Res 35, 1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME, Bustamante D, Tampier L, Israel Y, Herrera-Marschitz M, 2007. Dopamine release in the nucleus accumbens (shell) of two lines of rats selectively bred to prefer or avoid ethanol. Eur. J. Pharmacol 573, 84–92. doi: 10.1016/j.ejphar.2007.06.038 [DOI] [PubMed] [Google Scholar]
- Radwanska K, Wrobel E, Korkosz A, Rogowski A, Kostowski W, Bienkowski P, Kaczmarek L, 2008. Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology 33, 1835–46. doi: 10.1038/sj.npp.1301567 [DOI] [PubMed] [Google Scholar]
- Ramirez JJ, Monti PM, Colwill RM, 2015a. Brief and extended alcohol-cue-exposure effects on craving and attentional bias. Exp. Clin. Psychopharmacol 23, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JJ, Monti PM, Colwill RM, 2015b. Alcohol-cue exposure effects on craving and attentional bias in underage college-student drinkers. Psychol. Addict. Behav 29, 317–322. doi: 10.1037/adb0000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Stewart RT, Besheer J, 2017. Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacol. Biochem. Behav 156, 1–9. doi: 10.1016/j.pbb.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin H, Hodgson RJ, Stockwell T, 1983. Cue exposure and response prevention with alcoholics: a controlled trial. Behav. Res. Ther 21, 435–446. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE, 2017. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am. J. Drug Alcohol Abuse 43, 703–710. doi: 10.1080/00952990.2017.1312423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal AM, Hutchison KE, 2009. Catching the Alcohol Buzz: An Examination of the Latent Factor Structure of Subjective Intoxication. Alcohol. Clin. Exp. Res 33, 2154–2161. doi:doi: 10.1111/j.1530-0277.2009.01053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Meskew-Stacer S, Hutchison KE, 2007. The relationship between prospective self-rating of alcohol sensitivity and craving and experimental results from two alcohol challenge studies. J. Stud. Alcohol Drugs 68, 379–84. doi:http://0-dx.doi.org.lib.exeter.ac.uk/10.15288/jsad.2007.68.379 [DOI] [PubMed] [Google Scholar]
- Reid MS, Flammino F, Starosta A, Palamar J, Franck J, 2006. Physiological and subjective responding to alcohol cue exposure in alcoholics and control subjects: Evidence for appetitive responding. J. Neural Transm 113, 1519–1535. doi: 10.1007/s00702-005-0439-5 [DOI] [PubMed] [Google Scholar]
- Remedios J, Woods C, Tardif C, Janak PH, Chaudhri N, 2014. Pavlovian-conditioned alcohol-seeking behavior in rats is invigorated by the interaction between discrete and contextual alcohol cues: implications for relapse. Brain Behav 4, 278–289. doi: 10.1002/brb3.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, Gremel CM, 2018. Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat. Commun 9, 1–11. doi: 10.1038/s41467-017-02615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Buske TR, Morrisett RA, 2017a. Long-term subregion-specific encoding of enhanced ethanol intake by D1DR medium spiny neurons of the nucleus accumbens. Addict. Biol. 23, 689–698. doi: 10.1111/adb.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Maier EY, Buske TR, Morrisett RA, 2017b. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology 112, 164–171. doi: 10.1016/j.neuropharm.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, 1997. Response-inhibition in Extinction. Q. J. Exp. Psychol. Sect. B Comp. Physiol. Psychol 50, 238–252. doi: 10.1080/713932655 [DOI] [Google Scholar]
- Rescorla RA, 1993. Inhibitory associations between S and R in extinction. Anim. Learn. Behav 21, 327–336. doi: 10.3758/BF03197998 [DOI] [Google Scholar]
- Rescorla RA, Heth CD, 1975. Reinstatement of fear to an extinguished conditioned stimulus. Anim. Behav. Process 104, 88–96. doi: 10.3758/BF03209977 [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS, 2005. Specificity in the Projections of Prefrontal and Insular Cortex to Ventral Striatopallidum and the Extended Amygdala. J. Neurosci 25, 11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Patel JC, Cragg SJ, 2011. Dopamine release in the basal ganglia. Neuroscience 198, 112–37. doi: 10.1016/j.neuroscience.2011.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, 2004. The Role of the Medial Frontal Cortex in Cognitive Control. Science (80-. ) 306, 443–447. doi: 10.1126/science.1100301 [DOI] [PubMed] [Google Scholar]
- Rinck M, Wiers RW, Becker ES, Lindenmeyer J, 2018. Relapse Prevention in Abstinent Alcoholics by Cognitive Bias Modification: Clinical Effects of Combining Approach Bias Modification and Attention Bias Modification. J. Consult. Clin. Psychol 86, 1005–1016. doi: 10.1037/ccp0000321 [DOI] [PubMed] [Google Scholar]
- Ripley TL, Borlikova GG, Lyons S, Stephens DN, 2004. Selective deficits in appetitive conditioning as a consequence of ethanol withdrawal. Eur. J. Neurosci 19, 415–425. doi: 10.1111/j.0953-816X.2003.03114.x [DOI] [PubMed] [Google Scholar]
- Ripley TL, O’Shea M, Stephens DN, 2003. Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear. Eur. J. Neurosci 18, 441–448. doi: 10.1046/j.1460-9568.2003.02759.x [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL, 1994. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 116, 207–216. doi: 10.1007/BF02245064 [DOI] [PubMed] [Google Scholar]
- Robbins SJ, 1990. Mechanisms Underlying Spontaneous Recovery in Autoshaping. J. Exp. Psychol. Behav. Process 16, 235–249. doi: 10.1037/0097-7403.16.3.235 [DOI] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR, 2004a. Increased GABA Release in the Central Amygdala of Ethanol- Dependent Rats. Alcohol. Clin. Exp. Res 24, 10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR, 2004b. Acute and Chronic Ethanol Alter Glutamatergic Transmission in Rat Central Amygdala: an In Vitro and In Vivo Analysis. J. Neurosci 24, 1594–1603. doi: 10.1523/jneurosci.5077-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole MD, Koob GF, 1996. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol. Clin. Exp. Res 20, 1289–1298. [DOI] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, 2015. Attentional bias to alcohol-related stimuli as an indicator of changes in motivation to drink. Psychol. Addict. Behav 29, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Carelli RM, 2008. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur. J. Neurosci 28, 1887–1894. doi: 10.1111/j.1460-9568.2008.06464.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM, 2009. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol. Clin. Exp. Res 33, 1187–96. doi: 10.1111/j.1530-0277.2009.00942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Schmeichel BJ, Inzlicht M, 2010. A Cognitive Control Perspective of Self-Control Strength and Its Depletion. Soc. Personal. Psychol. Compass 4, 189–200. doi: 10.1111/j.1751-9004.2009.00244.x [DOI] [Google Scholar]
- Robinson TE, Berridge KC, 2001. Incentive-sensitization and addiction. Addiction 96, 103–14. doi: 10.1080/09652140020016996 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2000. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95, 91–117. doi: 10.1046/j.1360-0443.95.8s2.19.x [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 1993. The neural basis of drug craving : an incentive-sensitization theory of addiction. Brain Res. Rev 8, 247–291. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT, 2014. On the motivational properties of reward cues: Individual differences. Neuropharmacology 76, 450–459. doi: 10.1016/j.neuropharm.2013.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, 2002. Effects of Ethanol Exposure on Subsequent Acquisition and Extinction of Ethanol Self-Administration and Expression of Alcohol- Seeking Behavior in Adult Alcohol-. Alcohol. Clin. Exp. Res 26, 1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li T-K, McBride WJ, 2003. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology 28, 1614–1621. doi: 10.1038/sj.npp.1300214 [DOI] [PubMed] [Google Scholar]
- Rodeberg NT, Sandberg SG, Johnson JA, Phillips PEM, Wightman RM, 2017. Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in Vivo Fast-Scan Cyclic Voltammetry. ACS Chem. Neurosci 8, 221–234. doi: 10.1021/acschemneuro.6b00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrich L, Goldman MS, 1995. Implicit priming of alcohol expectancy memory processes and subsequent drinking behavior. Exp. Clin. Psychopharmacol 3, 402–410. doi: 10.1037/1064-1297.3.4.402 [DOI] [Google Scholar]
- Rohn MCH, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L, 2017. Differences Between Treatment-Seeking and Nontreatment-Seeking Alcohol-Dependent Research Participants: An Exploratory Analysis. Alcohol. Clin. Exp. Res 41, 414–420. doi: 10.1111/acer.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, Abrams DB, 2001. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6-and 12-month outcomes. Addiction 96, 1161–1174. doi: 10.1080/09652140120060752 [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, Wunschel SM, Abrams DB, 1994. Cue reactivity as a predictor of drinking among male alcoholics. J. Consult. Clin. Psychol 62, 620–626. doi: 10.1037/0022-006X.62.3.620 [DOI] [PubMed] [Google Scholar]
- Romero-Martínez Á, Vitoria-Estruch S, Moya-Albiol L, 2018. Perfil cognitivo de los alcohólicos abstinentes durante un periodo de tiempo prolongado en comparación con un grupo de hombres que no consumen alcohol. Adicciones xx. doi: 10.20882/adicciones.1079 [DOI] [PubMed] [Google Scholar]
- Rose AK, Brown K, Field M, Hogarth L, 2013. The contributions of value-based decision-making and attentional bias to alcohol-seeking following devaluation. Addiction 108, 1241–1249. doi: 10.1111/add.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Brown K, MacKillop J, Field M, Hogarth L, 2018. Alcohol devaluation has dissociable effects on distinct components of alcohol behaviour. Psychopharmacology (Berl) 235, 1233–1244. doi: 10.1007/s00213-018-4839-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Duka T, 2006. Effects of dose and time on the ability of alcohol to prime social drinkers. Behav. Pharmacol 17, 61–70. doi: 10.1097/01.fbp.0000189814.61802.92 [DOI] [PubMed] [Google Scholar]
- Rothblat DS, Rubin E, Schneider J., 2001. Effects of chronic alcohol ingestion on the mesostriatal dopamine system in the rat. Neurosci. Lett 300, 63–66. doi: 10.1016/S0304-3940(01)01548-8 [DOI] [PubMed] [Google Scholar]
- Roy-Charland A, Plamondon A, Homeniuk AS, Flesch CA, Klein RM, Stewart SH, 2017. Attentional bias toward alcohol-related stimuli in heavy drinkers: evidence from dynamic eye movement recording. Am. J. Drug Alcohol Abuse 43, 332–340. doi: 10.1080/00952990.2016.1209511 [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD, 1994. Alcohol cue reactivity and mood induction in male and female alcoholics. J. Stud. Alcohol 55, 487–494. [DOI] [PubMed] [Google Scholar]
- Rueger SY, King AC, 2013. Validation of the Brief Biphasic Alcohol Effects Scale (B-BAES). Alcohol. Clin. Exp. Res 37, 470–476. doi: 10.1111/j.1530-0277.2012.01941.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger SY, McNamara PJ, King AC, 2009. Expanding the utility of the biphasic alcohol effects scale (BAES) and initial psychometric support for the brief-BAES (B-BAES). Alcohol. Clin. Exp. Res 33, 916–924. doi: 10.1111/j.1530-0277.2009.00914.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan F, 2002. Attentional bias and alcohol dependence: A controlled study using the modified Stroop paradigm. Addict. Behav 27, 471–482. doi: 10.1016/S0306-4603(01)00183-6 [DOI] [PubMed] [Google Scholar]
- Ryerson NC, Neal LB, Gable PA, 2017. Attenuating the alcohol allure: attentional broadening reduces rapid motivational response to alcohol pictures. Psychopharmacology (Berl) 234, 1247–1254. doi: 10.1007/s00213-017-4557-1 [DOI] [PubMed] [Google Scholar]
- Sanchez FP, Dickenson L, George FR, 1996. Ethanol self-administration is genetically independent of locomotor stimulation in fast and slow mice. Alcohol 13, 79–84. doi: 10.1016/0741-8329(95)02017-9 [DOI] [PubMed] [Google Scholar]
- Saunders B, Lin H, Milyavskaya M, Inzlicht M, 2017. The emotive nature of conflict monitoring in the medial prefrontal cortex. Int. J. Psychophysiol 119, 31–40. doi: 10.1016/j.ijpsycho.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE, 2014. A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology 39, 2816–2823. doi: 10.1038/npp.2014.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Richard JM, Margolis EB, Janak PH, 2018. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci 21, 1072–1083. doi: 10.1038/s41593-018-0191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE, 2013. Individual variation in resisting temptation: Implications for addiction. Neurosci. Biobehav. Rev 37, 1955–1975. doi: 10.1016/j.neubiorev.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE, 2011. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology 36, 1668–1676. doi: 10.1038/npp.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE, 2013. Cue-Evoked Cocaine “Craving”: Role of Dopamine in the Accumbens Core. J. Neurosci 33, 13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H, 2013. Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addict. Biol 18, 121–133. doi: 10.1111/j.1369-1600.2012.00464.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad DJ, Garbusow M, Friedel E, Sommer C, Sebold M, Hägele C, Bernhardt N, Nebe S, Kuitunen-Paul S, Liu S, Eichmann U, Beck A, Wittchen H-U, Walter H, Sterzer P, Zimmermann US, Smolka MN, Schlagenhauf F, Huys QJM, Heinz A, Rapp MA, 2018. Neural correlates of instrumental responding in the context of alcohol-related cues index disorder severity and relapse risk. Eur. Arch. Psychiatry Clin. Neurosci 1–14. doi: 10.1007/s00406-017-0860-4 [DOI] [PubMed] [Google Scholar]
- Schier CJ, Dilly GA, Gonzales RA, 2013. Intravenous Ethanol Increases Extracellular Dopamine in the Medial Prefrontal Cortex of the Long-Evans Rat. Alcohol. Clin. Exp. Res 37, 740–747. doi: 10.1111/acer.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneekloth TD, Biernacka JM, Hall-Flavin DK, Karpyak VM, Frye MA, Loukianova LL, Stevens SR, Drews MS, Geske JR, Mrazek DA, 2012. Alcohol Craving as a Predictor of Relapse. Am. J. Addict 21, S20–S26. doi: 10.1111/j.1521-0391.2012.00297.x [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Hönig K, Maier W, Gaebel W, Zilles K, 2001. Subcortical correlates of craving in recently abstinent alcoholic patients. Am. J. Psychiatry 1075–1083. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IFM, Goertz AG, Van Kerkhof DHAT, Wiers RW, 2010. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend 109, 30–36. doi: 10.1016/j.drugalcdep.2009.11.022 [DOI] [PubMed] [Google Scholar]
- Schoenmakers TM, Wiers RW, 2010. Craving and attentional bias respond differently to alcohol priming: A field study in the pub. Eur. Addict. Res 16, 9–16. doi: 10.1159/000253859 [DOI] [PubMed] [Google Scholar]
- Schoenmakers TM, Wiers RW, Field M, 2008. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology (Berl) 197, 169–178. doi: 10.1007/s00213-007-1023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers TM, Wiers RW, Jones BT, Bruce G, Jansen A, 2007. Attentional re-training decreases attentional bias in heavy drinkers without generalization. Addiction 102, 399–405. doi: 10.1111/j.1360-0443.2006.01718.x [DOI] [PubMed] [Google Scholar]
- Schramm MJW, Everitt BJ, Milton AL, 2016. Bidirectional Modulation of Alcohol-Associated Memory Reconsolidation through Manipulation of Adrenergic Signaling. Neuropsychopharmacology 41, 1103–11. doi: 10.1038/npp.2015.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, 1998. Biological, Psychological and Environmental Predictors of the Alcoholism Risk : A Longitudinal Study *. J. Stud. Alcohol 485–494. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Irwin M, Brown SA, 2015. The history of anxiety symptoms among 171 primary alcoholics. J. Stud. Alcohol 51, 34–41. doi: 10.15288/jsa.1990.51.34 [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, 2006. An evaluation of the level of response to alcohol, externalizing symptoms, and depressive symptoms as predictors of alcoholism. J. Stud. Alcohol 67, 215–227. doi: 10.15288/jsa.2006.67.215 [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, 2000. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J. Stud. Alcohol 61, 827–835. doi: 10.15288/jsa.2000.61.827 [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Anderson KG, Brown SA, Kuperman S, Kramer JR, Hesselbrock VM, Bucholz KK, 2005. Evaluation of a level of response to alcohol-based structural equation model in adolescents. J. Stud. Alcohol 66, 174–184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Anthenelli RM, Schoen L, Kawamura M, Kramer JR, Dick DM, Neale Z, Kuperman S, McCutcheon V, Anokhin AP, Hesselbrock VM, Hesselbrock M, Bucholz KK, 2017. A Prospective Comparison of How the Level of Response to Alcohol and Impulsivity Relate to Future DSM-IV Alcohol Problems in the COGA Youth Panel. Alcohol. Clin. Exp. Res 41, 1329–1339. doi: 10.1111/acer.13407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tipp J, Smith TL, Wiesbeck GA, Kalmijn JA, 1997. The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J. Stud. Alcohol 58, 397–404. doi: 10.15288/jsa.1997.58.397 [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Reich T, Hesselbrock VM, Bucholz KK, 1995. The histories of withdrawal convulsions and delirium tremens in 1648 alcohol dependent subjects. Addiction 90, 1335–1347. doi: 10.1046/j.1360-0443.1995.901013355.x [DOI] [PubMed] [Google Scholar]
- Sekutowicz M, Guggenmos M, Kuitunen-Paul S, Garbusow M, Sebold M, Pelz P, Priller J, Wittchen H-U, Smolka MN, Zimmermann US, Heinz A, Sterzer P, Schmack K, 2019. Neural response patterns during Pavlovian-to-instrumental transfer predict alcohol relapse and young adult drinking. Biol. Psychiatry doi: 10.1016/j.biopsych.2019.06.028 [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KIA, Constable RT, Sinha R, 2013. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry 70, 727–39. doi: 10.1001/jamapsychiatry.2013.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher JC, 2013. Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci. Biobehav. Rev doi: 10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Dagher A, Schlagintweit H, Casey KF, Benkelfat C, Leyton M, 2014. Differential striatal dopamine responses following oral alcohol in individuals at varying risk for dependence. Alcohol. Clin. Exp. Res 38, 126–34. doi: 10.1111/acer.12218 [DOI] [PubMed] [Google Scholar]
- Sharbanee JM, Stritzke WGK, Jamalludin ME, Wiers RW, 2014. Approach-alcohol action tendencies can be inhibited by cognitive load. Psychopharmacology (Berl) 231, 967–975. doi: 10.1007/s00213-013-3318-z [DOI] [PubMed] [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, Wilson MA, 2016. Ethanol-induced anxiolysis and neuronal activation in the amygdala and bed nucleus of the stria terminalis. Alcohol 50, 19–25. doi: 10.1016/j.alcohol.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, Wilson MA, 2013. Individual differences in voluntary ethanol consumption lead to differential activation of the central amygdala in rats: relationship to the anxiolytic and stimulant effects of low dose ethanol. Alcohol. Clin. Exp. Res 37 Suppl 1, E172–80. doi: 10.1111/j.1530-0277.2012.01907.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Albery IP, Cook C, 2001. Selective attentional bias to alcohol related stimuli in problem drinkers and non-problem drinkers. Addiction 96, 285–295. doi: 10.1046/j.1360-0443.2001.96228512.x [DOI] [PubMed] [Google Scholar]
- Shen EH, Harland RD, Crabbe JC, Phillips TJ, 1995. Bidirectional Selective Breeding for Ethanol Effects on Locomotor Activity: Characterization of FAST and SLOW Mice Through Selection Generation 35. Alcohol. Clin. Exp. Res 19, 1234–1245. doi: 10.1111/j.1530-0277.1995.tb01606.x [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ, 2008. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321, 848–51. doi: 10.1126/science.1160575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME, 1990. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4, 477–485. doi: 10.1016/0896-6273(90)90106-P [DOI] [PubMed] [Google Scholar]
- Shetty PK, Galeffi F, Turner DA, 2012. Cellular Links between Neuronal Activity and Energy Homeostasis. Front. Pharmacol 3, 43. doi: 10.3389/fphar.2012.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E, Hopfinger JB, Lust SA, Henry EA, Bartholow BD, 2010. Electrophysiological Evidence of Alcohol-Related Attentional Bias in Social Drinkers Low in Alcohol Sensitivity. Psychol. Addict. Behav 24, 508–515. doi: 10.1037/a0019663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Robinson DL, 2015. Regional Variation in Phasic Dopamine Release during Alcohol and Sucrose Self-Administration in Rats. ACS Chem. Neurosci 6, 147–54. doi: 10.1021/cn500251j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR, 2015. Voluntary Ethanol Intake Predicts κ-Opioid Receptor Supersensitivity and Regionally Distinct Dopaminergic Adaptations in Macaques. J. Neurosci 35, 5959–68. doi: 10.1523/JNEUROSCI.4820-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Lovinger DM, Mateo Y, Jimenez VA, Helms CM, Grant KA, Jones SR, 2016a. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacology (Berl) 233, 1435–1443. doi: 10.1007/s00213-016-4239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Mateo Y, Helms CM, Lovinger DM, Grant KA, Jones SR, 2016b. Chronic ethanol self-administration in macaques shifts dopamine feedback inhibition to predominantly D2 receptors in nucleus accumbens core. Drug Alcohol Depend 158, 159–163. doi: 10.1016/j.drugalcdep.2015.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise RA, Bartlett SE, 2008. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol. Clin. Exp. Res 32, 1816–23. doi: 10.1111/j.1530-0277.2008.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KIA, Bergquist KL, Bhagwagar Z, Siedlarz KM, 2009. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34, 1198–208. doi: 10.1038/npp.2008.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets WJAJ, Hoogland PV, Voorn P, 1986. The distribution of dopamine immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko: An immunohistochemical study with antibodies against dopamine. J. Comp. Neurol 253, 46–60. doi: 10.1002/cne.902530105 [DOI] [PubMed] [Google Scholar]
- Smith GS, Branas CC, Miller TR, 1999. Fatal nontraffic injuries involving alcohol: A metaanalysis. Ann. Emerg. Med 33, 659–668. doi: 10.1016/S0196-0644(99)70195-2 [DOI] [PubMed] [Google Scholar]
- Snelleman M, Schoenmakers TM, van de Mheen D, 2015. Attentional Bias and Approach/Avoidance Tendencies Do Not Predict Relapse or Time to Relapse in Alcohol Dependency. Alcohol. Clin. Exp. Res 39, 1734–1739. doi: 10.1111/acer.12817 [DOI] [PubMed] [Google Scholar]
- Sommer C, Garbusow M, Jünger E, Pooseh S, Bernhardt N, Birkenstock J, Schad DJ, Jabs B, Glöckler T, Huys QJM, Heinz A, Smolka MN, Zimmermann US, 2017. Strong seduction: Impulsivity and the impact of contextual cues on instrumental behavior in alcohol dependence. Transl. Psychiatry 7. doi: 10.1038/tp.2017.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind P. a, Gehlert DR, Barr CS, Heilig M, 2008. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol. Psychiatry 63, 139–45. doi: 10.1016/j.biopsych.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Spanakis P, Jones A, Field M, Christiansen P, 2019. A Stroop in the Hand is Worth Two on the Laptop: Superior Reliability of a Smartphone Based Alcohol Stroop in the Real World. Subst. Use Misuse 54, 692–698. doi: 10.1080/10826084.2018.1536716 [DOI] [PubMed] [Google Scholar]
- Sparks LM, Sciascia JM, Ayorech Z, Chaudhri N, 2014. Vendor differences in alcohol consumption and the contribution of dopamine receptors to Pavlovian-conditioned alcohol-seeking in Long-Evans rats. Psychopharmacology (Berl) 231, 753–64. doi: 10.1007/s00213-013-3292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, Masala N, Cannizzaro C, Biggio G, Sanna E, Diana M, 2014. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc. Natl. Acad. Sci 111, E3745–E3754. doi: 10.1073/pnas.1406768111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruyt A, De Houwer J, Tibboel H, Verschuere B, Crombez G, Verbanck P, Hanak C, Brevers D, Noël X, 2013. On the predictive validity of automatically activated approach/avoidance tendencies in abstaining alcohol-dependent patients. Drug Alcohol Depend 127, 81–86. doi: 10.1016/j.drugalcdep.2012.06.019 [DOI] [PubMed] [Google Scholar]
- Spuhler K, Deitrich RA, 1984. Correlative Analysis of Ethanol-Related Phenotypes in Rat Inbred Strains. Alcohol. Clin. Exp. Res 8, 480–484. doi: 10.1111/j.1530-0277.1984.tb05707.x [DOI] [PubMed] [Google Scholar]
- Srey CS, Maddux J-MN, Chaudhri N, 2015. The attribution of incentive salience to Pavlovian alcohol cues: a shift from goal-tracking to sign-tracking. Front. Behav. Neurosci 9, 54. doi: 10.3389/fnbeh.2015.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger PK, White JM, 1991. Cue reactivity in alcohol abusers: Stimulus specificity and extinction of the responses. Addict. Behav 16, 211–221. doi: 10.1016/0306-4603(91)90014-9 [DOI] [PubMed] [Google Scholar]
- Staiger PK, White JM, 1988. Conditioned alcohol-like and alcohol-opposite responses in humans. Psychopharmacology (Berl) 95, 87–91. doi: 10.1007/BF00212773 [DOI] [PubMed] [Google Scholar]
- Stasiewicz PR, Gulliver SB, Bradizza CM, Rohsenow DJ, Torris R, Monti PM, 1997. Exposure to negative emotional cues and alcohol cue reactivity with alcoholics: a preliminary investigation. Behav. Res. Ther 35, 1143–1149. [PubMed] [Google Scholar]
- Stauffer CS, Dobberteen L, Woolley JD, 2017. American Alcohol Photo Stimuli (AAPS): A standardized set of alcohol and matched non-alcohol images. Am. J. Drug Alcohol Abuse 43, 647–655. doi: 10.1080/00952990.2016.1253093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stautz K, Frings D, Albery IP, Moss AC, Marteau TM, 2017. Impact of alcohol-promoting and alcohol-warning advertisements on alcohol consumption, affect, and implicit cognition in heavy-drinking young adults: A laboratory-based randomized controlled trial. Br. J. Health Psychol 22, 128–150. doi: 10.1111/bjhp.12221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Goldman MS, Del Boca FK, 2000. The influence of alcohol expectancy priming and mood manipulation on subsequent alcohol consumption. J. Abnorm. Psychol 109, 106–115. doi: 10.1037/0021-843X.109.1.106 [DOI] [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, Ripley TL, 2001. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur. J. Neurosci 14, 2023–2031. doi: 10.1046/j.0953-816X.2001.01824.x [DOI] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova GG, Schubert M, Albrecht D, Hogarth L, Duka T, 2005. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol. Psychiatry 58, 392–400. doi: 10.1016/j.biopsych.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Stetter F, Ackermann K, Bizer A, Straube ER, Mann K, 1995. Effects of Disease-Related Cues in Alcoholic Inpatients: Results of a Controlled “Alcohol Stroop” Study. Alcohol. Clin. Exp. Res 19, 593–599. doi: 10.1111/j.1530-0277.1995.tb01553.x [DOI] [PubMed] [Google Scholar]
- Stetter F, Chaluppa C, Ackermann K, Straube ER, Mann K, 1994. Alcoholics’ selective processing of alcohol related words and cognitive performance on a Stroop task, European Psychiatry [Google Scholar]
- Stevenson RA, Hoffman JL, Maldonado-devincci AM, Faccidomo SP, Hodge CW, 2019. MGluR5 activity is required for the induction of ethanol behavioral sensitization and associated changes in ERK MAP kinase phosphorylation in the nucleus accumbens shell and lateral habenula. Behav. Brain Res 367, 19–27. doi: 10.1016/j.bbr.2019.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Murphy JM, McBride WJ, Lumeng L, Li T-K, 1996. Place conditioning with alcohol in alcohol-preferring and -nonpreferring rats. Pharmacol. Biochem. Behav 53, 487–491. doi: 10.1016/0091-3057(95)02102-7 [DOI] [PubMed] [Google Scholar]
- Stockwell TR, Hodgson RJ, Rankin HJ, Taylor C, 1982. Alcohol dependence, beliefs and the priming effect. Behav. Res. Ther 20, 513–522. doi: 10.1016/0005-7967(82)90072-9 [DOI] [PubMed] [Google Scholar]
- Stormark KM, Field NP, Hugdahl K, Horowitz M, 1997. Selective processing of visual alcohol cues in abstinent alcoholics: An approach-avoidance conflict? Addict. Behav 22, 509–519. doi: 10.1016/S0306-4603(96)00051-2 [DOI] [PubMed] [Google Scholar]
- Stormark KM, Laberg JC, Bjerland T, Nordby H, Hugdahl K, 1995. Autonomic cued reactivity in alcoholics: The effect of olfactory stimuli. Addict. Behav 20, 571–584. doi: 10.1016/0306-4603(95)00017-7 [DOI] [PubMed] [Google Scholar]
- Stormark KM, Laberg JC, Nordby H, Hugdahl K, 2000. Selective Attention to Alcohol Stimuli : Automated Processing ?*. J. Stud. Alcohol 18–23. [DOI] [PubMed] [Google Scholar]
- Stroop JR, 1935. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol 18, 643–661. [Google Scholar]
- Strother WN, Lumeng L, Li T-K, McBride WJ, 2005. Dopamine and serotonin content in select brain regions of weanling and adult alcohol drinking rat lines. Pharmacol. Biochem. Behav 80, 229–237. doi: 10.1016/j.pbb.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A, 2008. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol. Clin. Exp. Res 32, 1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA, 2004. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict. Behav 29, 33–50. doi: 10.1016/j.addbeh.2003.07.003 [DOI] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Deas D, 2005. Alcohol cue reactivity in alcohol-dependent adolescents. J. Stud. Alcohol 66, 354–60. doi:http://0-dx.doi.org.lib.exeter.ac.uk/10.15288/jsa.2005.66.354 [DOI] [PubMed] [Google Scholar]
- Tibboel H, De Houwer J, Field M, 2010. Reduced attentional blink for alcohol-related stimuli in heavy social drinkers. J. Psychopharmacol 24, 1349–1356. doi: 10.1177/0269881109106977 [DOI] [PubMed] [Google Scholar]
- Tibboel H, De Houwer J, Spruyt A, Brevers D, Roy E, Noël X, 2015. Heavy social drinkers score higher on implicit wanting and liking for alcohol than alcohol-dependent patients and light social drinkers. J. Behav. Ther. Exp. Psychiatry 48, 185–191. doi: 10.1016/j.jbtep.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA, 2000. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction 95, 145–153. doi: 10.1080/09652140050111717 [DOI] [PubMed] [Google Scholar]
- Toalston JE, Oster SM, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA, 2008. Effects of alcohol and saccharin deprivations on concurrent ethanol and saccharin operant self-administration by alcohol-preferring (P) rats. Alcohol 42, 277–284. doi: 10.1016/j.alcohol.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, 1996. Locating Reward Cue at Response Manipulandum (CAM) Induces Symptoms of Drug Abuse. Neurosci. Biobehav. Rev 20, 505–535. doi: 10.1016/0149-7634(95)00023-2 [DOI] [PubMed] [Google Scholar]
- Tomie A, Sharma N, 2013. Pavlovian Sign-Tracking Model of Alcohol Abuse. Curr. Drug Abuse Rev 6, 1–19. [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’Dell LE, 2014. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol. Clin. Exp. Res 38, 108–15. doi: 10.1111/acer.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend JM, Duka T, 2007. Avoidance of alcohol-related stimuli in alcohol-dependent inpatients. Alcohol. Clin. Exp. Res 31, 1349–1357. doi: 10.1111/j.1530-0277.2007.00429.x [DOI] [PubMed] [Google Scholar]
- Trela CJ, Hayes AW, Bartholow BD, Sher KJ, Heath AC, Piasecki TM, 2018. Moderation of alcohol craving reactivity to drinking-related contexts by individual differences in alcohol sensitivity: An ecological investigation. Exp. Clin. Psychopharmacol 26, 354–365. doi: 10.1037/pha0000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela CJ, Piasecki TM, Bartholow BD, Heath AC, Sher KJ, 2016. The natural expression of individual differences in self-reported level of response to alcohol during ecologically assessed drinking episodes. Psychopharmacology (Berl) 233, 2185–2195. doi: 10.1007/s00213-016-4270-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkkan JS, McCaul ME, Stitzer ML, 1989. Psychophysiological Effects of Alcohol-related Stimuli: II. Enhancement with Alcohol Availability. Alcohol. Clin. Exp. Res 13, 392–398. doi: 10.1111/j.1530-0277.1989.tb00341.x [DOI] [PubMed] [Google Scholar]
- Turkkan JS, Stitzer ML, McCaul ME, 1988. Psychophysiological Effects of Oral Ethanol in Alcoholics and Social Drinkers. Alcohol. Clin. Exp. Res 12, 30–38. doi: 10.1111/j.1530-0277.1988.tb00129.x [DOI] [PubMed] [Google Scholar]
- Udo T, Bates ME, Mun E-Y, Vaschillo EG, Vaschillo B, Lehrer P, Ray S, 2009. Gender differences in acute alcohol effects on self-regulation of arousal in response to emotional and alcohol-related picture cues. Psychol. Addict. Behav 23, 196–204. doi: 10.1037/a0015015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NBL, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O’Malley SS, Krystal JH, Abi-Dargham A, 2010. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [11C]raclopride. Biol. Psychiatry 68, 689–96. doi: 10.1016/j.biopsych.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, McGuier NS, Gass JT, Griffin WC, Ball LE, Mulholland PJ, 2016. Chronic intermittent ethanol exposure and withdrawal leads to adaptations in nucleus accumbens core postsynaptic density proteome and dendritic spines. Addict. Biol 21, 560–574. doi: 10.1111/adb.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E, 2000. Structure and function of dopamine receptors. Neurosci. Biobehav. Rev 24, 125–132. doi: 10.1016/S0149-7634(99)00063-9 [DOI] [PubMed] [Google Scholar]
- Valyear MD, Villaruel FR, Chaudhri N, 2017. Alcohol-seeking and relapse: A focus on incentive salience and contextual conditioning. Behav. Processes 141, 26–32. doi: 10.1016/j.beproc.2017.04.019 [DOI] [PubMed] [Google Scholar]
- Van Dyke N, Fillmore MT, 2015. Operant responding for alcohol following alcohol cue exposure in social drinkers. Addict. Behav 47, 11–6. doi: 10.1016/j.addbeh.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemel-Ruiter ME, de Jong PJ, Wiers RW, 2011. Appetitive and regulatory processes in young adolescent drinkers. Addict. Behav 36, 18–26. doi: 10.1016/j.addbeh.2010.08.002 [DOI] [PubMed] [Google Scholar]
- VanderVeen JD, Plawecki MH, Millward JB, Hays J, Kareken DA, O’Connor SJ, Cyders MA, 2016. Negative urgency, mood induction, and alcohol seeking behaviors. Drug Alcohol Depend 165, 151–158. doi: 10.1016/j.drugalcdep.2016.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Soni N, Bajo M, Luu G, Madamba SG, Schweitzer P, Parsons LH, Roberto M, 2016. Chronic ethanol exposure decreases CB 1 receptor function at GABAergic synapses in the rat central amygdala. Addict. Biol 21, 788–801. doi: 10.1111/adb.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE, 1984. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: A combined retrograde transport-immunohistochemical study. Brain Res 303, 337–357. doi: 10.1016/0006-8993(84)91220-4 [DOI] [PubMed] [Google Scholar]
- Veilleux JC, Lovett DE, Skinner KD, Ham LS, 2018. Non-Alcoholic Beverage Cues as Specific Comparison Images to Alcohol Image Cues. Subst. Use Misuse 53, 773–781. doi: 10.1080/10826084.2017.1365087 [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, 2012. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci 32, 7563–71. doi: 10.1523/JNEUROSCI.0069-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PEM, Sulzer D, Wightman RM, 2003. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J. Neurochem 87, 1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M, 2009. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology 56, 160–168. doi: 10.1016/j.neuropharm.2008.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li T-K, 2003. Subjective intoxication in response to alcohol challenge: Heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol. Clin. Exp. Res 27, 795–803. doi: 10.1097/01.ALC.0000067974.41160.95 [DOI] [PubMed] [Google Scholar]
- Villaruel FR, Chaudhri N, 2016. Individual Differences in the Attribution of Incentive Salience to a Pavlovian Alcohol Cue. Front. Behav. Neurosci 10, 1–13. doi: 10.3389/fnbeh.2016.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincke E, Vyncke P, 2017. Does Alcohol Catch the Eye? Investigating Young Adults’ Attention to Alcohol Consumption. Evol. Psychol 15, 1–13. doi: 10.1177/1474704917730207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Kirsch M, 2011. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol. Psychiatry 69, 1060–1066. doi: 10.1016/j.biopsych.2010.12.016 [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Hermann D, Mann KF, Kiefer F, 2012. Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addict. Biol 17, 807–16. doi: 10.1111/j.1369-1600.2011.00352.x [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann KF, 2010. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105, 1741–9. doi: 10.1111/j.1360-0443.2010.03022.x [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, Spanagel R, 2009. Cue-induced alcohol-seeking behaviour is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology (Berl) 205, 389–397. doi: 10.1007/s00213-009-1544-1 [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF, 2007. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol. Clin. Exp. Res 31, 11–18. doi: 10.1111/j.1530-0277.2006.00259.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li T-K, 1986. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol. Biochem. Behav 24, 617–623. doi: 10.1016/0091-3057(86)90567-8 [DOI] [PubMed] [Google Scholar]
- Walsh E, Macleod DAD, 1982. Breath alcohol analysis in the Accident and Emergency Department. Inj. Br. J. Accid. Surg. 15, 62–66. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Lopez-Gamundi P, Flagel SB, 2018. Measuring appetitive conditioned responses in humans. Physiol. Behav 188, 140–150. doi: 10.1016/j.physbeh.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT, 2013. Acute alcohol effects on attentional bias in heavy and moderate drinkers. Psychol. Addict. Behav 27, 32–41. doi: 10.1037/a0028991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT, 2012. Alcohol-related stimuli reduce inhibitory control of behavior in drinkers. Psychopharmacology (Berl) 222, 489–498. doi: 10.1007/s00213-012-2667-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, McKim DB, Niraula A, Witcher KG, Yin W, Sobol CG, Wang Y, Sawicki CM, Sheridan JF, Godbout JP, 2019. The Influence of Microglial Elimination and Repopulation on Stress Sensitization Induced by Repeated Social Defeat. Biol. Psychiatry 85, 667–678. doi: 10.1016/j.biopsych.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Seibring M, Nelson TF, Lee H, 2002. Trends in college binge drinking during a period of increased prevention efforts: Findings from 4 harvard school of public health college alcohol study surveys: 1993–2001. J. Am. Coll. Health Assoc 50, 203–217. doi: 10.1080/07448480209595713 [DOI] [PubMed] [Google Scholar]
- Weise-Kelly L, Siegel S, 2001. Self-administration cues as signals: Drug self-administration and tolerance. J. Exp. Psychol. Anim. Behav. Process 27, 125–136. doi: 10.1037//0097-7403.27.2.125 [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF, 1993. Oral Alcohol Stimulates Dopamine Release in the Rat Nucleus Accumbens : Genetic and Motivational Determinants. J. Pharmacol. Exp. Ther 267, 250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Koob GF, Schulteis G, Hyytiä P, Lorang MT, Bloom FE, 1996. Ethanol Self-Administration Restores Withdrawal-Associated Deficiencies in Accumbal Dopamine and 5Hydroxytryptamine Release in Dependent Rats. J. Neurosci 16, 3474–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA, 2002. Opposite Influences of Endogenous Dopamine D1 and D2 Receptor Activation on Activity States and Electrophysiological Properties of Striatal Neurons: Studies Combining In Vivo Intracellular Recordings and Reverse Microdialysis. J. Neurosci 22, 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Fromme K, 2009. Subjective Responses to Alcohol Prime Event-Specific Alcohol Consumption and Predict Blackouts and Hangover. J. Stud. Alcohol Drugs 70, 593–600. doi: 10.15288/jsad.2009.70.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJL, Vos T, 2013. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. doi: 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Wiers CE, Gladwin TE, Ludwig VU, Gröpper S, Stuke H, Gawron CK, Wiers RW, Walter H, Bermpohl F, 2017. Comparing three cognitive biases for alcohol cues in alcohol dependence. Alcohol Alcohol 52, 242–248. doi: 10.1093/alcalc/agw063 [DOI] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J, 2011. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol. Sci 22, 490–497. doi: 10.1177/0956797611400615 [DOI] [PubMed] [Google Scholar]
- Wiers RW, Gladwin TE, Hofmann W, Salemink E, Ridderinkhof KR, 2013. Cognitive bias modification and cognitive control training in addiction and related psychopathology: Mechanisms, clinical perspectives, and ways forward. Clin. Psychol. Sci 1, 192–212. doi: 10.1177/2167702612466547 [DOI] [Google Scholar]
- Wiers RW, Houben K, Fadardi JS, van Beek P, Rhemtulla M, Cox WM, 2015a. Alcohol Cognitive Bias Modification training for problem drinkers over the web. Addict. Behav 40, 21–26. doi: 10.1016/j.addbeh.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Wiers RW, Ludwig VU, Gladwin TE, Park SQ, Heinz A, Rinck M, Lindenmeyer J, Walter H, Bermpohl F, 2015b. Effects of cognitive bias modification training on neural signatures of alcohol approach tendencies in male alcohol-dependent patients. Addict. Biol 20, 990–999. doi: 10.1111/adb.12221 [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, van den Wildenberg E, 2009. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes, Brain Behav 8, 101–106. doi: 10.1111/j.1601-183X.2008.00454.x [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F, 2010. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction 105, 279–287. doi: 10.1111/j.1360-0443.2009.02775.x [DOI] [PubMed] [Google Scholar]
- Wiers RW, Shumay E, Volkow ND, Frieling H, Kotsiari a, Lindenmeyer J, Walter H, Bermpohl F, 2015c. Effects of depressive symptoms and peripheral DAT methylation on neural reactivity to alcohol cues in alcoholism. Transl. Psychiatry 5, e648. doi: 10.1038/tp.2015.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Stelzel C, Park SQ, Gawron CK, Ludwig VU, Gutwinski S, Heinz A, Lindenmeyer J, Walter H, Bermpohl F, 2014. Neural Correlates of alcohol-approach bias in alcohol addiction: The spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology 39, 688–697. doi: 10.1038/npp.2013.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Van Woerden N, Smulders FTY, De Jong PJ, 2002. Implicit and explicit alcohol-related cognitions in heavy and light drinkers. J. Abnorm. Psychol 111, 648–658. doi: 10.1037/0021-843X.111.4.648 [DOI] [PubMed] [Google Scholar]
- Wietschorke K, Lippold J, Jacob C, Polak T, Herrmann MJ, 2016. Transcranial direct current stimulation of the prefrontal cortex reduces cue-reactivity in alcohol-dependent patients. J. Neural Transm 123, 1173–1178. doi: 10.1007/s00702-016-1541-6 [DOI] [PubMed] [Google Scholar]
- Wilcox MV, Carlson VCC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA, 2014. Repeated Binge-Like Ethanol Drinking Alters Ethanol Drinking Patterns and Depresses Striatal GABAergic Transmission. Neuropsychopharmacology 39, 579–594. doi: 10.1038/npp.2013.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ, Brown RA, 1985. Measurement of Drinking, Alcoholism and Loss-of-Control Over Alcohol. Aust. Alcohol/Drug Rev 4, 65–79. doi: 10.1080/09595238580000091 [DOI] [Google Scholar]
- Willner P, Field M, Pitts K, Reeve G, 1998. Mood, cue and gender influences on motivation, craving and liking for alcohol in recreational drinkers. Behav. Pharmacol 9, 631–642. doi: 10.1097/00008877-199811000-00018 [DOI] [PubMed] [Google Scholar]
- Wintemute GJ, 2015. Alcohol misuse, firearm violence perpetration, and public policy in the United States. Prev. Med. (Baltim) 79, 15–21. doi: 10.1016/j.ypmed.2015.04.015 [DOI] [PubMed] [Google Scholar]
- Wise RA, 1973. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 210, 203–210. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, 2013. Temptation to Drink as a Predictor of Drinking Outcomes Following Psychosocial Treatment for Alcohol Dependence. Alcohol. Clin. Exp. Res 37, 529–537. doi: 10.1111/j.1530-0277.2012.01950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteman J, Post H, Tarvainen M, de Bruijn A, Perna De Sousa Fernandes E, Ramaekers JG, Wiers RW, 2015. Cue reactivity and its relation to craving and relapse in alcohol dependence: a combined laboratory and field study. Psychopharmacology (Berl) 232, 3685–96. doi: 10.1007/s00213-015-4027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren WMM, Sato S, Njølstad I, Woodward M, Salomaa V, Nordestgaard BG, Yeap BB, Fletcher A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, de la Cámara AG, Völzke H, Dahm CC, Dale CE, Bergmann MM, Crespo CJ, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno-Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Tumino R, Blazer DG, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca-Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundström J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kühn T, Grobbee DE, Barrett-Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Després J-P, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjønneland A, Brenner H, Palmieri L, Dallongeville J, Brunner EJ, Assmann G, Trevisan M, Gillum RF, Ford I, Sattar N, Lazo M, Thompson SG, Ferrari P, Leon DA, Smith GD, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J, Verschuren M, Veikko S, Flecther A, Gómez de la Cámara A, Bergmann M, Rimm EB, Dallongeville J-P, Gillumn RF, Ford IF, Davey Smith G, 2018. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 391, 1513–1523. doi: 10.1016/S0140-6736(18)30134-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DJ, Chang JY, Janak PH, Azarov A, Anstrom K, 1999. Mesolimbic neuronal activity across behavioral states. Ann. N. Y. Acad. Sci 877, 91–112. doi: 10.1111/j.1749-6632.1999.tb09263.x [DOI] [PubMed] [Google Scholar]
- Woodward DJ, Janak PH, Chang JY, 1998. Ethanol action on neural networks studied with multineuron recording in freely moving animals. Alcohol. Clin. Exp. Res 22, 10–22. doi: 10.1111/j.1530-0277.1998.tb03612.x [DOI] [PubMed] [Google Scholar]
- Wrase J, Grüsser SM, Klein S, Diener C, Hermann D, Flor H, Mann KF, Braus DF, Heinz A, 2002. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur. Psychiatry 17, 287–291. doi: 10.1016/S0924-9338(02)00676-4 [DOI] [PubMed] [Google Scholar]
- Xiao C, Shao XM, Olive MF, Griffin WC, Li KY, Krnjević K, Zhou C, Ye JH, 2009. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology 34, 307–318. doi: 10.1038/npp.2008.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE, 2015. Individual Variation in the Motivational and Neurobiological Effects of an Opioid Cue. Neuropsychopharmacology 40, 1269–1277. doi: 10.1038/npp.2014.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE, 2015. Individual variation in the motivational properties of a nicotine cue: sign-trackers vs. goal-trackers. Psychopharmacology (Berl) 232, 3149–3160. doi: 10.1007/s00213-015-3962-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE, 2013. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 217–228. doi: 10.1007/s00213-012-2890-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE, 2010. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav. Brain Res 214, 30–34. doi: 10.1016/j.bbr.2010.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS, 2014. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience 282, 23–48. doi: 10.1016/j.neuroscience.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD, 2004. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol. Rev 111, 931–959. doi: 10.1037/0033-295X.111.4.931 [DOI] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA, 2000. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol 22, 107–15. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, Herring CM, Hile KL, Walters JW, Liang T, Plawecki MH, O’Connor SJ, Kareken DA, 2016. Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend 160, 163–169. doi: 10.1016/j.drugalcdep.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng T-E, O’Connor SJ, Morris ED, 2007. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol. Clin. Exp. Res 31, 965–73. doi: 10.1111/j.1530-0277.2007.00390.x [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O’Connor SJ, Wang C, Zheng Q-H, Mock B, Morris ED, 2005. Dopamine D2 Receptor Availability is Associated with Subjective Responses to Alcohol. Alcohol. Clin. Exp. Res 29, 965–970. doi: 10.1097/01.ALC.0000171041.32716.42 [DOI] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ, Kareken DA, 2009. When what you see isn’t what you get: Alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol. Clin. Exp. Res 33, 139–149. doi: 10.1111/j.1530-0277.2008.00821.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G, Kim SW, Thuras P, Grant JE, Westermeyer J, 2006. Alcohol Craving in Outpatients with Alcohol Dependence: Rate and Clinical Correlates. J. Stud. Alcohol 67, 770–777. doi: 10.15288/jsa.2006.67.770 [DOI] [PubMed] [Google Scholar]
- Yücel M, Oldenhof E, Ahmed SH, Belin D, Billieux J, Bowden-Jones H, Carter A, Chamberlain SR, Clark L, Connor JP, Daglish M, Dom G, Dannon P, Duka T, Fernandez-Serrano MJ, Field M, Franken IHA, Goldstein RZ, Gonzalez R, Goudriaan AE, Grant JE, Gullo MJ, Hester R, Hodgins DC, Le Foll B, Lee RSC, Lingford-Hughes A, Lorenzetti V, Moeller SJ, Munafò MR, Odlaug B, Potenza MN, Segrave R, Sjoerds Z, Solowij N, van den Brink W, van Holst RJ, Voon V, Wiers RW, Fontenelle LF, Verdejo-García A, 2019. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction 114, 1095–1109. doi: 10.1111/add.14424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR, 1999. Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur. J. Neurosci 11, 1119–1126. [DOI] [PubMed] [Google Scholar]
- Zapata A, Chefer VI, Shippenberg TS, 2009. Microdialysis in rodents. Curr. Protoc. Neurosci Chapter 7, Unit7.2. doi: 10.1002/0471142301.ns0702s47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS, 2006. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology 31, 396–405. doi: 10.1038/sj.npp.1300833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P, 2007. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res 1134, 148–161. doi: 10.1016/j.brainres.2006.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH, 2006. Context is a trigger for relapse to alcohol. Behav. Brain Res 167, 150–5. doi: 10.1016/j.bbr.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Syková E, Agnati LF, Fuxe K, 1999. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci 20, 142–150. doi: 10.1016/S0165-6147(99)01343-7 [DOI] [PubMed] [Google Scholar]