Abstract
Hepatitis B virus (HBV) infection represents a public health threat and a challenge for the medical community. Untimely treatment may lead to liver cirrhosis and even liver cancer. At present, the major treatment for hepatitis B e antigen (HBeAg)-positive chronic hepatitis B patients includes administration of interferon-α (IFN-α), which has anti-viral and immunomodulatory effects. Plasmacytoid dendritic cells (pDCs) and Toll-like receptor-9 (TLR-9) have important roles in anti-viral therapy. However, their predictive value regarding the efficacy of IFN-α treatment of HBeAg-positive chronic hepatitis B (CHB) patients has remained elusive. A total of 178 patients with CHB and HBeAg-positive status, who had not received any previous anti-HBV treatment, were enrolled in the present study. All patients were treated with IFN-α. HBV DNA load, hepatitis B surface antigen and serum alanine aminotransferase were measured prior to and following 48 weeks of treatment. According to HBV levels, the patients were divided into a response group and non-responders group. To determine the amount of pDCs, blood dendritic cell antigen 2 (BDCA-2)- and immunoglobulin-like transcript 7 (ILT7)-expressing cells in liver biopsies were detected using immunohistochemistry. TLR-9 expression in peripheral blood mononuclear cells was determined by reverse transcription-quantitative PCR. There was no significant difference in the proportion of pDCs (BDCA-2; ILT7) and TLR-9 mRNA expression between the response group and the non-responders group prior to IFN-α treatment. After IFN-α treatment, BDCA-2, ILT7 and TLR-9 mRNA expression was obviously increased in the response group compared with that in the non-responders group (P<0.05). Increased expression of BDCA-2, ILT7 and TLR-9 mRNA was negatively correlated with HBV DNA (P<0.05). Increased levels of pDCs and TLR-9 were negatively correlated with HBV DNA, and were thus capable of predicting the IFN-α treatment response in patients with CHB and HBeAg-positive status.
Keywords: plasmacytoid dendritic cells, Toll-like receptor 9, hepatitis B virus, hepatitis B e antigen, efficacy, predict
Introduction
The hepatitis B virus (HBV) infection rate remains high throughout the world. Hundreds of millions of infected individuals have chronic hepatitis B (CHB), which represents a serious threat to public health (1,2). China is a moderately endemic area of HBV infection, the treatment of which is thus one of the serious challenges for China's medical community (3,4). After initial acute infection, certain individuals continue to be infected, the disease gradually turns into CHB. Untimely treatment may lead to cirrhosis and even liver cancer (5). At present, the major therapeutic measures for patients with CHB include nucleotide drugs and IFN-α, the latter of which is more advantageous due to exerting anti-viral and immunomodulatory effects with low drug resistance (6,7). At present, the goal of HBV treatment is to inhibit viral replication for a long time, thereby reducing hepatic inflammatory necrosis, reducing liver tissue fibrosis and improving the prognosis in terms of CHB (8). However, the efficacy of IFN therapy remains limited. The hepatitis B e antigen (HBeAg)-negative rate of patients with HBeAg-positive CHB is low and the treatment cost is high (9,10). Therefore, it is important to effectively evaluate the efficacy of IFN-α in the treatment of patients with HBeAg-positive CHB.
Dendritic cells (DCs) are potent antigen-presenting cells that activate primary T cells and participate in immune response processes, including anti-viral response (11). Plasmacytoid DCs (pDCs), which secrete a large amount of type I IFN, are among the naturally occurring type I IFN cells and therefore have strong anti-viral effects (12,13). Toll-like receptors (TLRs) are pattern recognition receptors that participate in the innate immune response by recognizing pathogen-associated molecular patterns. TLR family members include TLR1-TLR11 (14). TLR9 is a member of the TLR family and has an important role in the anti-viral response. It has been identified that pDCs express TLR9 on their surface, and a TLR9 expression deficit leads to inability of pDCs to secrete type I IFN during viral infection (15,16). However, in the process of IFN-α treatment of HBeAg-positive CHB patients, no previous study has reported on the detection of pDCs and the expression of TLR9, to the best of our knowledge. Therefore, the aim of the present study was to analyze the correlation of pDCs and TLR9 expression with HBV DNA in HBeAg-positive CHB patients receiving IFN therapy in order to evaluate the predictive value regarding treatment response.
Materials and methods
General patient information
A total of 178 patients with HBeAg-positive CHB who were not previously treated for HBV were enrolled at Xiamen Hospital of Traditional Chinese Medicine (Xiamen, China) between January 2014 and December 2015. The patients had a mean age of 34.83±12.3 years (range, 15–62 years). The enrolled patients were treated with recombinant type I IFN-α; a variety of different forms of IFN-α are under development and clinical investigation (17).
The inclusion criteria were as follows: Compliance with diagnostic criteria based on the Guidelines for the Prevention and Treatment of Chronic Hepatitis B published by Chinese Medical Association Liver Diseases Branch and the Chinese Medical Association Infectious Diseases Branch (18); IFN-α treatment (subcutaneous injection at 5MU, 3 times a week) for 48 weeks, and in all of the responder cases, alanine aminotransferase (ALT) levels were normal after 6 months of IFN-α treatment (19). The exclusion criteria were as follows: Combination with other type of hepatitis virus infection or HIV infection; combination with autoimmune diseases, including hyperthyroidism, thyroiditis or systemic lupus erythematosus; combination with severe heart disease, tumor, or dysfunction or failure of a vital organ; recent anti-viral or immunomodulatory therapy; recent liver protection or enzyme-lowering therapy; pregnancy; mental disorders that limit the ability of the patient to cooperate with the research; and poor compliance. The patients were divided into a response group and non-responders group based on treatment outcomes (levels of HBV DNA). A total of 40 healthy individuals with matched age and gender were selected and used as the normal controls. The current study was approved by the Medical Ethics Committee of Xiamen Hospital of Traditional Chinese Medicine (Xiamen, China) and all subjects had provided written informed consent.
Major reagents and instruments
The hepatitis B surface antigen (HBsAg) enhanced chemiluminescence detection kit was purchased from Roche Diagnostics. RNA extraction kit (cat. no. AP-MN-MS-RNA-250) was from Axygen; Corning Inc., and reverse transcription (RT) kit was purchased from Takara Biotechnology Co., Ltd. (cat. no. RR037A). Mouse anti-human BDCA-2 (cat. no. 748001) and ILT7 monoclonal antibodies (cat. no. 562500) were purchased from BD Pharmigen; BD Biosciences. Goat anti-mouse biotin-labeled secondary antibody (cat. no. 31800), diaminobenzidine (DAB) chromogenic reagent and immunohistochemistry (IHC) streptavidin peroxidase conjugated method (SP) kit were purchased from Zymed (Thermo Fisher Scientific, Inc.). The Labsystem Version 1.3.1 microplate reader was purchased from Bio-Rad Laboratories, Inc. The ABI 7700 Fast Quantitative PCR Reactor was from ABI (Thermo Fisher Scientific, Inc.). The Cobas c311 automatic biochemical analyzer was purchased from Roche Diagnostics. The C6015-2 B-ultrasonic instrument was purchased from SonoSite, Inc.
Sample collection
Peripheral blood was collected from patients with CHB prior to and following 48 weeks of treatment. Peripheral blood (8 ml) was drawn into tubes coated with EDTA. Within 30 min, samples were centrifuged at 820 × g for 10 min at room temperature, generating the one-step centrifugation plasma sample (~4 ml). Aliquots of 1 ml of the plasma were transferred to 1.5-ml tubes and centrifuged at 16,000 × g for 10 min at room temperature. Subsequently, white particles (e.g. cellular debris) were removed from the bottom of the tube and the supernatant, the two-step centrifugation plasma sample, was obtained and transferred to a fresh tube (~2 ml). Liver tissue was collected by liver biopsy for IHC staining under the guidance of liver B-ultrasound for localization. A 18G cook biopsy needle was used. The liver tissue specimen was >1.5 cm in length, or with intact hepatic lobules containing >3 portal areas.
Liver routine indicator test
The ALT content in plasma was detected using an automated biochemical analyzer. HBsAg was quantified using chemiluminescence. RT-quantitative (RT-q) PCR was used to detect HBV DNA with a sensitivity of 500 copies/ml.
RT-qPCR
Total RNA was extracted from peripheral blood mononuclear cells with TRIzol reagent according to the manufacturer's protocol. Next, total RNA was reverse transcribed to complementary DNA using the RT kit. Rox SYBR Master Mix (Eurogentec) was used for qPCR. The primers used were designed using PrimerPremier 6.0 software (Premier Biosoft International). The PCR thermocycling conditions were as follows: 55°C for 1 min, followed by 35 cycles of 92°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec. GAPDH was used as a loading control. The relative expression was calculated using the 2−ΔΔCq method (20). The sequences of the PCR primers (5′-3′) were as follows: GADPH forward, AGTACCAGTCTGTTGCTGG, and reverse, TAATAGACCCGGATGTCTGGT; TLR9 forward, CCAGTCATTCACGGCTCTTGTA and reverse, GCGTCGATGGTTGTGCTAATT.
IHC
The paraffin blocks from liver biopsies were fixed in 10% neutral buffered formalin were processed into 4–5 µm paraffin sections. The pDC cell surface markers BDCA-2 (1:1,000 dilution of an antibody) and ILT7 (1:1,000 dilution of an antibody) were detected by IHC SP method. The tissue slice was dewaxed and incubated in 3% H2O2 (VWR International) for 8 min at room temperature to eliminate endogenous peroxidase activity. After blocking with 5% normal goat serum (Thermo Fisher Scientific, Inc.), the slice was incubated with the corresponding primary antibodies against BDCA-2, ILT7 or TLR-9 at 4°C overnight. After washing with PBS, the slice was incubated in biotin-labeled secondary antibody (1:5,000) at 37°C for 30 min. Next, the slice was incubated in horseradish-labeled streptavidin (0.25 mg/ml) at 37°C for 30 min. The sample was then incubated with DAB Plus (cat. no. K3468; Dako) for 4 min at 37°C, followed by incubation with hematoxylin as the counterstain (Automation Hematoxylin; cat. no. S3301; Dako) at 37°C for 5 min. The slice was then washed with water. Finally, the slice was redyed with hematoxylin for 30 sec at 37°C, sealed and observed under a light microscope (BX63 model; Olympus Corporation).
Statistical analysis
The continuous measurement data conforming to a normal distribution were expressed as the mean ± standard deviation and compared by a student's t-test. All statistical analyses were performed with SPSS 11.5 software (SPSS, Inc.). Enumeration data were compared using the χ2 test. Pearson analysis was adopted for correlation analysis. P<0.05 was considered to indicate statistical significance.
Results
General information
The characteristics of the entire study cohort are provided in Table I. The clinicopathological features of the responders and non-responders are compared in Table II. There was no significant difference in the general clinical data between the two groups, including gender, age and body weight. IFN-α-treated patients had a higher cumulative incidence of HBeAg seroconversion and a lower incidence of cirrhosis and hepatocellular cancer than for untreated controls at the end of follow-up (median, 6.8 years; range, 1.1–16.5 years; Table II).
Table I.
Characteristics of the study cohort (n=178).
| Characteristic | Value |
|---|---|
| Age (years) | 34.83±12.3 |
| Male | 145 (74.0) |
| HBcrAg (log U/ml) | 7.72±1.1 |
| HBV DNA (log IU/ml) | 7.58±1.4 |
| HBsAg (log IU/ml) | 4.02±1.1 |
| HBeAg (log IU/ml) | 2.13±0.99 |
| HBV genotype | |
| A | 42 (21.4) |
| B | 32 (16.3) |
| C | 48 (26.9) |
| D | 49 (27.5) |
| Other/mixed | 6 (3.1) |
| Missing | 1 (0.5) |
Values are expressed as the mean ± standard deviation or n (%). HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBcrAg, hepatitis B core-related antigen.
Table II.
General patient information compared between responders and non-responders.
| Parameter | Non-responders (n=101) | Response (n=77) | P-value |
|---|---|---|---|
| Sex (male/female) | 62/49 | 41/36 | 0.120 |
| Age (years) | 40.7±4.1 | 42.1±5.8 | 0.080 |
| Body weight (kg) | 59.8±6.3 | 60.1±5.3 | 0.230 |
| Cirrhosis | 34 (34%) | 14 (18%) | 0.041 |
| Hepatocellular carcinoma | 13 (13%) | 2 (3%) | 0.011 |
| HBeAg seroconversion | 52 (52%) | 58 (75%) | 0.031 |
| HBsAg clearance | 40 (0.4%) | 2 (3%) | 0.030 |
Mean, 11 years; median, 6.6 years; range, 1.1–16.5 years. Values are expressed as the mean ± standard deviation or n (%). All data were obtained at the end of the follow-up. HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen.
Analysis of liver function indicators
There was no significant difference in ALT, HBV DNA load and HBsAg between the response group and the non-responders group prior to IFN-α treatment. After treatment, the ALT levels, HBV DNA load and HBsAg expression were obviously different between the two groups (P<0.05; Table III). The amount of pDCs and TLR-9 were higher in normal than in HBeAg-positive CHB patients (Fig. S1)
Table III.
Analysis of liver function indicators.
| Parameter | Non-responders (n=101) | Response (n=77) |
|---|---|---|
| ALT (U/l) | ||
| Prior to treatment | 120±25 | 130±17 |
| After treatment | 79±16a | 32±8a,b |
| HBV DNA (copies/ml) | ||
| Prior to treatment | 0.93±1.92×108 | 0.81±3.21×108 |
| After treatment | 0.31±1.32×108a | 0a,b |
| HBsAg (IU/ml) | ||
| Prior to treatment | 43712.15±5672.23 | 46789.67±6172.42 |
| After treatment | 122564.21±4987.62a | 3982.17±25631.1a,b |
P<0.05 compared with prior to treatment
P<0.05 compared with non-responders group. Values are expressed as the mean ± standard deviation. HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; ALT, alanine amiontransferase.
pDC analysis
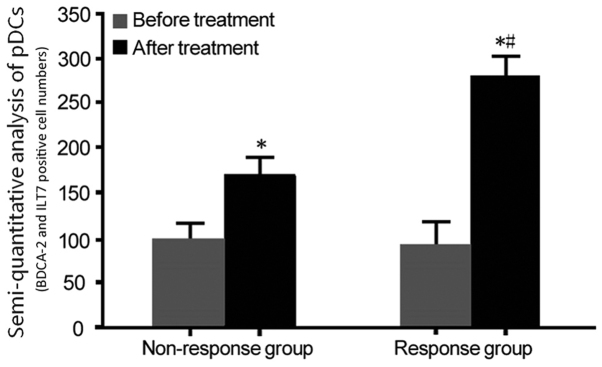
The changes in the levels of pDCs in the response group and non-responders group prior to and after treatment with IFN-α were analyzed. The pDC markers BDCA-2 and ILT7 were detected by IHC analysis, and they were uniformly expressed throughout the cytoplasm of these cells (Fig. 1, arrow). There was no significant difference in amount of pDCs (BDCA-2 and ILT7 expressions) between the response group and the non-responders group prior to IFN-α treatment. After IFN-α treatment, BDCA-2 and ILT7 expression was obviously increased in the response group compared with that in the non-responders group (P<0.05; Fig. 2).
Figure 1.
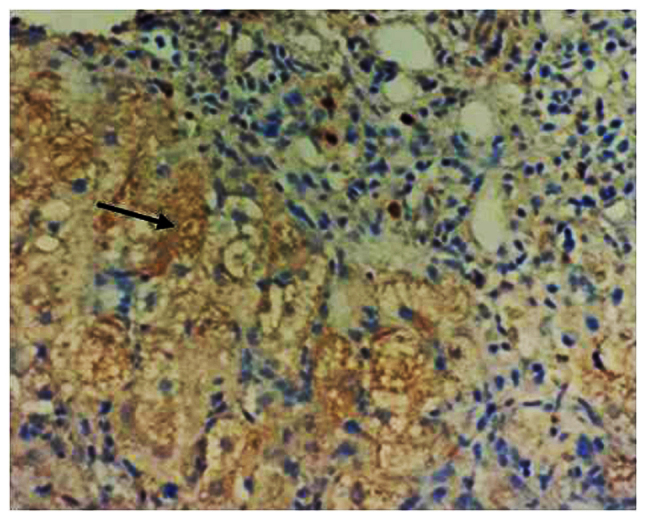
Representative image for the immunohistochemical detection of blood dendritic cell antigen 2- and immunoglobulin-like transcript 7-positive pDC cells. Positive staining was observed in the cytoplasm (black arrow; magnification, ×100).
Figure 2.
Analysis of the amount of pDCs (BDCA-2 and ILT7 positive cell numbers) in the two groups prior to and after treatment. Blood dendritic cell antigen 2 and immunoglobulin-like transcript 7 were detected by immunohistochemistry with the SP method. *P<0.05 compared with before treatment; #P<0.05, compared with non-responders group. pDCs, plasmacytoid dendritic cells; SP, streptavidin peroxidase.
TLR-9 expression analysis
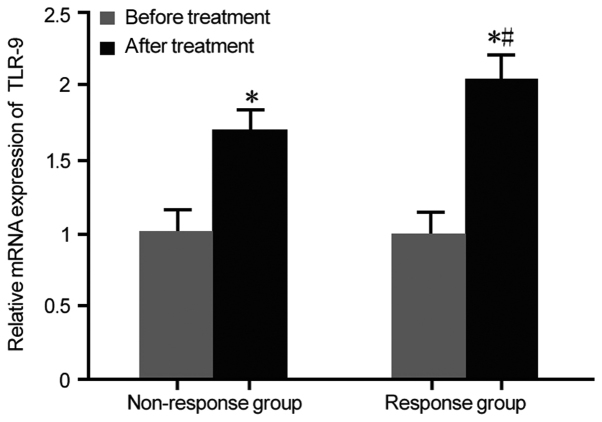
TLR-9 mRNA expression in the response group and non-responders group prior to and after treatment with IFN-α was assessed by RT-qPCR. There was no statistically significant difference in TLR-9 mRNA expression between the response group and the non-responders group prior to IFN-α treatment. After IFN-α treatment, TLR-9 mRNA expression was markedly upregulated in the response group compared with that in the non-responders group (P<0.05; Fig. 3). It was then investigated which type of cell was responsible for the increase in the expression of TLR9 after anti-viral therapy. The expression of TLR9 in the cytoplasm of epithelial cells in the liver biopsies of the response group was higher than that in the non-responders group after anti-viral therapy (Fig. S2)
Figure 3.
TLR-9 expression in the two groups prior to and after treatment. *P<0.05 compared with prior to treatment; #P<0.05 compared with non-responders group. TLR, Toll-like receptor.
Correlation analysis of pDCs or TLR-9 expression with HBV DNA content
The correlation between pDCs or TLR-9 expression and HBV DNA content was then analyzed. It was demonstrated that with the increase of pDCs and TLR-9 expression, the HBV DNA content declined. Therefore, these parameters were negatively correlated with the HBV DNA content (P<0.05), suggesting that pDCs and TLR-9 expressions may predict the IFN-α treatment response in patients with HBeAg-positive CHB (Table IV).
Table IV.
Correlation analysis of pDCs and TLR-9 expression with HBV DNA content.
| Correlation | R-value | P-value |
|---|---|---|
| pDCs vs. HBV DNA | −0.718 | <0.05 |
| TLR-9 vs. HBV DNA | −0.672 | <0.05 |
TLR, Toll-like receptor; HBV, hepatitis B virus; pDCs, plasmacytoid dendritic cells.
Discussion
pDCs are mainly produced by bone marrow hematopoietic stem cells, which are continuously released into peripheral blood as plasma-like cells. They specifically express TLR-7 and TLR-9, and the type I IFN produced may directly exert anti-viral effects. At the same time, the anti-viral ability of natural killer (NK) and B lymphocytes is enhanced, so that pDCs stimulate the innate as well as the acquired immune response, which resembles a bridge between innate immunity and specific immune response (21). It has been observed in vitro that that administration of IFN-α promotes the maturation of pDCs and promotes the production of IL-12, thereby acting on CD40-activated B cells to promote plasma cells and immunoglobulin secretion (22,23). Therefore, pDCs are involved in the activation of T cells, B cells and NK cells. During viral infection, pDCs differentiate into mature DCs and regulate T-cell function. It is of great clinical significance to explore the expression and role of pDCs in infection and treatment of CHB patients. However, there is currently a lack of established specific surface markers for pDCs. Since ILT7 and BDCA-2 are selectively expressed in pDCs, which are human pDC-specific and not expressed in any other mature DCs or peripheral lymphocytes, they were used as specific markers for pDCs in the present study (24,25). The present results confirmed that after IFN-α treatment, the expression of BDCA-2 and ILT7 in the response group was significantly higher than that in the non-responders group, suggesting that the expression of pDCs predicts the IFN-α treatment response in HBeAg-positive CHB patients.
TLR-9 is only expressed on the surface of human B cells and pDCs. The surface molecules on pDCs include BDCA-2 and ITL7, and they may be upregulated upon activation with TLR-9. The TLR7/9-dependent pathway appears to be a predominant mode of nucleic acid sensing in pDCs, but is essential for TLR9-induced IFN production by pDCs. This indirectly accelerates the maturation, differentiation and proliferation of lymphocytes (26,27). TLR-9 assists pDCs in chemotaxis of lymph nodes and aggregation, which in turn assists in the exertion of the anti-viral effect (28). The direct causal association between pDC-derived IFN and lupus progression/severity is difficult to establish in the human system and is to be elucidated in animal models (29).
In the present study, the expression of TLR-9 was analyzed in the peripheral blood of patients with HBeAg-positive CHB treated with IFN-α, providing a reference for the safe and effective treatment of HBV infection. The present study confirmed that after IFN-α treatment, the mRNA expression of TLR-9 was markedly upregulated in the response group compared with that in the non-responders group, and was negatively correlated with the HBV DNA content, suggesting that the expression of TLR-9 may also predict the treatment effect of IFN-α. In line with this, previous studies suggested that markers of fibrosis were obviously higher in non-responders than in responders (30,31). In the present study, TLR-9 expression was detected in peripheral blood mononuclear cells and not in liver tissues, which is a limitation.
The present study demonstrated changes in the levels of pDCs and the expression of TLR-9 in patients with HBeAg-positive CHB treated with IFN-α, and analyzed their predictive significance regarding treatment response. Further study is required to explore the mechanistic roles of pDCs and TLR-9 in the treatment of CHB patients with IFN-α.
In conclusion, increased levels of pDCs and TLR-9 were negatively correlated with HBV DNA, and may thus predict the IFN-α treatment response in patients with HBeAg-positive CHB. The present study provided a theoretical basis for selecting more effective anti-HBV programs for patients with CHB. However, as the range of follow-up is very wide, further studies with close follow-ups are required to confirm this finding in the future.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- HBV
hepatitis B virus
- HBeAg
hepatitis B e antigen
- IFN-α
interferon-α
- pDCs
plasmacytoid dendritic cells
- TLR-9
Toll-like receptor-9
- ALT
alanine aminotransferase
- BDCA-2
blood dendritic cell antigen 2
- ILT7
immunoglobulin-like transcript 7
Funding
This work was supported by the Xiamen Science and Technology Fund (grant no. 3502Z20144028).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YC, JEY, JMT, QGM and QZZ performed the experiments and analyzed the data. YZ designed the study and wrote the manuscript.
Ethics approval and consent to participate
The current study was approved by the Xiamen Hospital of Traditional Chinese Medicine and consent was obtained for participation.
Patient consent for publication
Informed consent for the publication of data was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tang X, Yan L, Li H, Du L, Shi Y, Huang F, Tang H. Increased expression of phosphoenolpyruvate carboxykinase cytoplasmic isoform by hepatitis B virus X protein affects hepatitis B virus replication. J Med Virol. 2019;91:258–264. doi: 10.1002/jmv.25300. [DOI] [PubMed] [Google Scholar]
- 2.Yambasu EE, Reid A, Owiti P, Manzi M, Murray MJS, Edwin AK. Hidden dangers-prevalence of blood borne pathogens, hepatitis B, C, HIV and syphilis, among blood donors in Sierra Leone in 2016: Opportunities for improvement: A retrospective, cross-sectional study. Pan Afr Med J. 2018;30:44. doi: 10.11604/pamj.2018.30.44.14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrero-Fernández I, Rosado-Sánchez I, Genebat M, Tarancón-Díez L, Rodríguez-Méndez MM, Pozo-Balado MM, Lozano C, Ruiz-Mateos E, Leal M, Pacheco YM. Improved CD4 T cell profile in HIV-infected subjects on maraviroc-containing therapy is associated with better responsiveness to HBV vaccination. J Transl Med. 2018;16:238. doi: 10.1186/s12967-018-1617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M, Du D, Zheng W, Liao M, Zhang L, Liang G, Gong M. Small hepatitis delta antigen selectively binds to target mRNA in hepatic cells: A potential mechanism by which hepatitis D virus down-regulates glutathione S-transferase P1 and induces liver injury and hepatocarcinogenesis. Biochem Cell Biol. 2019;97:130–139. doi: 10.1139/bcb-2017-0321. [DOI] [PubMed] [Google Scholar]
- 5.Howell J, Pedrana A, Cowie BC, Doyle J, Getahun A, Ward J, Gane E, Cunningham C, Wallace J, Lee A, et al. Aiming for the elimination of viral hepatitis in Australia, New Zealand, the Pacific Islands and Territories: Where are we now and barriers to meeting WHO targets by 2030. J Gastroenterol Hepatol. 2019;34:40–48. doi: 10.1111/jgh.14457. [DOI] [PubMed] [Google Scholar]
- 6.Li MR, Zheng HW, Ma SM, Liu YY, Qie LX, Li JQ, Wang DH, Sun XL, Ren GF, Zheng YH, et al. Correlations between serum hepatitis B surface antigen and hepatitis B core antibody titers and liver fibrosis in treatment-naive CHB patients. J Chin Med Assoc. 2018;81:1052–1059. doi: 10.1016/j.jcma.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Yang G, He H, Ning L, Liu Z, Fu Q, Chen H, Deng H, Wang Z, Luo K. Association of characteristics of HBV quasispecies with hepatitis B surface antigen seroconversion after pegylated interferon-α-2a treatment in child patients. Antivir Ther. 2018;23:567–574. doi: 10.3851/IMP3262. [DOI] [PubMed] [Google Scholar]
- 8.Lutterkort GL, Wranke A, Hengst J, Yurdaydin C, Stift J, Bremer B, Hardtke S, Keskin O, Idilman R, Manns MP, et al. Viral dominance patterns in chronic hepatitis delta determine early response to interferon alpha therapy. J Viral Hepat. 2018;25:1384–1394. doi: 10.1111/jvh.12947. [DOI] [PubMed] [Google Scholar]
- 9.Cao WH, Li MH, Pan CQ, Lu Y, Zhang L, Ran CP, Wu SL, Hua WH, Liu SA, Shen G, et al. Quantitation of plasmacytoid dendritic cells in chronic hepatitis B patients with HBeAg positivity during PEG-IFN and entecavir therapy. J Interferon Cytokine Res. 2018;38:197–205. doi: 10.1089/jir.2018.0014. [DOI] [PubMed] [Google Scholar]
- 10.Karamitros T, Papatheodoridis G, Paraskevis D, Hatzakis A, Mbisa JL, Georgopoulou U, Klenerman P, Magiorkinis G. Impact of interferon-α receptor-1 promoter polymorphisms on the transcriptome of the hepatitis B virus-associated hepatocellular carcinoma. Front Immunol. 2018;9:777. doi: 10.3389/fimmu.2018.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepulveda-Toepfer JA, Pichler J, Fink K, Sevo M, Wildburger S, Mudde-Boer LC, Taus C, Mudde GC. TLR9-mediated activation of dendritic cells by CD32 targeting for the generation of highly immunostimulatory vaccines. Hum Vaccin Immunother. 2019;15:179–188. doi: 10.1080/21645515.2018.1514223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomasello E, Naciri K, Chelbi R, Bessou G, Fries A, Gressier E, Abbas A, Pollet E, Pierre P, Lawrence T, et al. Molecular dissection of plasmacytoid dendritic cell activation in vivo during a viral infection. EMBO J. 2018;37:e98836. doi: 10.15252/embj.201798836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wimmers F, Subedi N, van Buuringen N, Heister D, Vivié J, Beeren-Reinieren I, Woestenenk R, Dolstra H, Piruska A, Jacobs JF, et al. Single-cell analysis reveals that stochasticity and paracrine signaling control interferon-alpha production by plasmacytoid dendritic cells. Nat Commun. 2018;9:3317. doi: 10.1038/s41467-018-05784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank MJ, Reagan PM, Bartlett NL, Gordon LI, Friedberg JW, Czerwinski DK, Long SR, Hoppe RT, Janssen R, Candia AF, et al. In situ vaccination with a TLR 9 agonist and local low dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 2018;8:1258–1269. doi: 10.1158/2159-8290.CD-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atreya R, Reinisch W, Peyrin-Biroulet L, Scaldaferri F, Admyre C, Knittel T, Kowalski J, Neurath MF, Hawkey C. Clinical efficacy of the Toll-like receptor 9 agonist cobitolimod using patient-reported-outcomes defined clinical endpoints in patients with ulcerative colitis. Dig Liver Dis. 2018;50:1019–1029. doi: 10.1016/j.dld.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Lee JW, Park SK, Lee SB, Yoon YH, Yeon SH, Rha KS, Choi JA, Song CH, Kim YM. Toll-like receptor 9 ligands increase type I interferon induced B-cell activating factor expression in chronic rhinosinusitis with nasal polyposis. Clin Immunol. 2018;197:19–26. doi: 10.1016/j.clim.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Gibbert K, Schlaak JF, Yang D, Dittmer U. IFN-α subtypes: Distinct biological activities in anti-viral therapy. Br J Pharmacol. 2013;168:1048–1058. doi: 10.1111/bph.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou J, Wang G, Wang F, Cheng J, Ren H, Zhuang H, Sun J, Li L, Li J, Meng Q, et al. Guideline of prevention and treatment for chronic hepatitis B (2015 update) J Clin Transl Hepatol. 2017;5:297–318. doi: 10.14218/JCTH.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu S, Zhang H, Dong Y, Wang L, Xu Z, Liu W, Gan Y, Tang H, Chen D, Wang F, Zhao P. Antiviral therapy in hepatitis B virus-infected children with immune-tolerant characteristics: A pilot open-label randomized study. J Hepatol. 2018;68:1123–1128. doi: 10.1016/j.jhep.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Xi Y, Troy NM, Anderson D, Pena OM, Lynch JP, Phipps S, Bosco A, Upham JW. Critical role of plasmacytoid dendritic cells in regulating gene expression and innate immune responses to human rhinovirus-16. Front Immunol. 2017;8:1351. doi: 10.3389/fimmu.2017.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cédile O, Jørgensen LØ, Frank I, Wlodarczyk A, Owens T. The chemokine receptor CCR2 maintains plasmacytoid dendritic cell homeostasis. Immunol Lett. 2017;192:72–78. doi: 10.1016/j.imlet.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Ainola M, Porola P, Takakubo Y, Przybyla B, Kouri VP, Tolvanen TA, Hänninen A, Nordström DC. Activation of plasmacytoid dendritic cells by apoptotic particles-mechanism for the loss of immunological tolerance in Sjögren's syndrome. Clin Exp Immunol. 2018;191:301–310. doi: 10.1111/cei.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruben JM, García-Romo GS, Breman E, van der Kooij S, Redeker A, Arens R, van Kooten C. Human plasmacytoid dendritic cells acquire phagocytic capacity by TLR9 ligation in the presence of soluble factors produced by renal epithelial cells. Kidney Int. 2018;93:355–364. doi: 10.1016/j.kint.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Murayama G, Furusawa N, Chiba A, Yamaji K, Tamura N, Miyake S. Enhanced IFN-α production is associated with increased TLR7 retention in the lysosomes of palasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther. 2017;19:234. doi: 10.1186/s13075-017-1441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torigoe M, Sakata K, Ishii A, Iwata S, Nakayamada S, Tanaka Y. Hydroxychloroquine efficiently suppresses inflammatory responses of human class-switched memory B cells via Toll-like receptor 9 inhibition. Clin Immunol. 2018;195:1–7. doi: 10.1016/j.clim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Han N, Zhang Z, Jv H, Hu J, Ruan M, Zhang C. Culture supernatants of oral cancer cells induce impaired IFN-α production of pDCs partly through the down-regulation of TLR-9 expression. Arch Oral Biol. 2018;93:141–148. doi: 10.1016/j.archoralbio.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 28.White MP, Webster G, Leonard F, La Flamme AC. Innate IFN-γ ameliorates experimental autoimmune encephalomyelitis and promotes myeloid expansion and PDL-1 expression. Sci Rep. 2018;8:259. doi: 10.1038/s41598-017-18543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: Recent progress and open questions. Annu Rev Immunol. 2011;29:163–83. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebensztejn DM, Sobaniec-Lotowska ME, Kaczmarski M, Voelker M, Schuppan D. Matrix-derived serum markers in monitoring liver fibrosis in children with chronic hepatitis B treated with interferon alpha. World J Gastroenterol. 2006;12:3338–3343. doi: 10.3748/wjg.v12.i21.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebensztejn DM, Sobaniec-Łotowska ME, Bauer M, Kaczmarski M, Voelker M, Schuppan D. Serum fibrosis markers as predictors of an antifibrotic effect of interferon alfa in children with chronic hepatitis B. Eur J Gastroenterol Hepatol. 2005;17:843–848. doi: 10.1097/00042737-200508000-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.