Abstract
Hepatocellular carcinoma (HCC) has more recently become a leading cause of cancer-associated mortality worldwide. Particularly at an advanced stage, the prognosis is generally poor due to lack of effective treatments. Transarterial chemoembolization (TACE) is now a recognized therapy for advanced HCC, serving to deprive tumors of feeder arteries through induced ischemic necrosis. However, there is also a potential for undesired circulatory toxicity owing to drug reflux from tumor artery to surrounding healthy tissues. Although effective chemotherapeutic drug concentrations are thus lowered, the side effects of systemic chemotherapy are aggravated. The mid-2000 emergence of drug-eluting beads (DEB) loaded with anti-neoplastic drugs has proven particularly advantageous, enabling localized treatment and directed delivery of chemotherapeutics. DEB-TACE (dTACE) augments local infusion of anti-neoplastic agents to prolong agent/tumor contact, expanding upon conventional TACE. At present, data on DEB use in China are limited, particularly in terms of proprietary microspheres (CalliSpheres; Hengrui Medicine Co.). To explore the efficacy and safety of CalliSpheres, A total of 90 patients receiving this means of dTACE for advanced HCC were assessed in the present study. Clinical efficacy was evaluated based on tumor response and overall survival rates using the National Cancer Institute Common Terminology Criteria for Adverse Events to assess tolerability. The satisfactory tumor response and acceptable tolerability demonstrated in the follow-up confirm the promising utility of CalliSpheres in treating patients with advanced HCC.
Keywords: drug-eluting, beads, chemoembolization, advanced hepatocellular carcinoma, CalliSpheres, efficacy, tolerability
Introduction
Hepatocellular carcinoma (HCC) is a common human cancer type and one of the leading causes of cancer-associated mortality worldwide (1,2). The poor prognosis of HCC is largely attributable to a lack of effective therapeutic options (3). Most patients with HCC are diagnosed at advanced stages, precluding effective surgical treatment. As a result, the 5-year survival rate in patients with intra- or extrahepatic metastasis is <5% (4,5).
Transarterial chemoembolization (TACE) is now a recognized therapeutic method for advanced HCC (6), typically involving transarterial chemotherapeutic injection in an ethiodized oil emulsion. This allows for selective, high-concentration delivery of chemotherapeutic drugs to mass lesions while embolizing feeder arteries, combining potent cytotoxic effects with ischemia (7–11). Although such localized hepatic arterial-directed therapy avoids embolic damage to uninvolved perimeters, a potential for agent backflow into the general circulation remains, and the duration of chemotherapeutic action is brief. Ultimately, conventional TACE (cTACE) confers a marginal survival benefit at the cost of systematic toxicity (12).
Having emerged in mid-2000, drug-eluting beads (DEB; bearing anti-neoplastic drugs) for TACE (dTACE) have the capacity for localized and directed chemotherapy (13,14). Compared with cTACE, their delayed post-embolization release yields more sustained and tumor-selective drug delivery, in conjunction with enduring embolization (15). This approach lowers the drug concentration in the bodily circulation, thereby mitigating systemic effects. The resulting outcomes in patients with HCC are encouraging. Prospective phase 2 and 3 trials of DEB loaded with doxorubicin have generated acceptable safety and efficacy profiles with respect to advanced HCC (16,17). In a phase 3 study, dTACE was indicated to enhance the tumor response, improve tolerability and reduce liver toxicity as compared to lipiodol-based cTACE (16,18).
At present, available data on the use of DEB in China are limited, despite the approval of the proprietary microsphere beads used in the present study [CalliSpheres (CB); Hengrui Medicine Co.] in 2016. Although previous toxicology and pharmacokinetic studies have demonstrated the impressive biological safety and durability of CB (18,19), few reports have researched their effects in a clinical setting (20,21). The present analysis was performed to address the efficacy, safety and overall survival (OS) benefits of CB in patients with advanced HCC (stage B or C), as defined by the Barcelona Clinic Liver Cancer (BCLC) criteria (22).
Patients and methods
Patients
A total 90 patients with advanced HCC were assessed, each receiving dTACE between August 2016 and December 2017 at the First Affiliated Hospital of Shandong First Medical University (Shandong, China). The clinical characteristics of the cohort are listed in Table I. Follow-up was achieved through outpatient visits or telephone interviews performed until death or closing of the study (March 31, 2019). The median duration of follow-up was 13.20 months (range, 3–24 months). All patients included met the following eligibility criteria: i) Tissue confirmation of diagnosis, with BCLC staging; ii) Eastern Cooperative Oncology Group performance status of 0 or 1 and lesser degrees of hepatic functional impairment (Child-Pugh Class A or B) (23); iii) tumor bulk amenable to modified Response Evaluation Criteria in Solid Tumors (mRECIST) (24); and iv) life expectancy prediction ≥3 months, which was calculated based on dyspnea, anorexia, Karnofsky performance status score, clinical prediction of survival, total WBC and lymphocyte percentage (25). The exclusion criteria were as follows: i) Child-Pugh Class C HCC; ii) contraindications regarding angiographic or visceral catheterization; iii) coagulation disorders; iv) thrombus within main portal vein; v) widespread peripheral metastases; and vi) life expectancy <3 months. The present study adhered to the provisions of the Declaration of Helsinki and was approved by the Institutional Review Board of the First Affiliated Hospital of Shandong First Medical University (Jinan, China). Each subject provided written informed consent.
Table I.
Clinical characteristics of the patients with advanced hepatocellular carcinoma (n=90).
| Characteristics | Value |
|---|---|
| Age (years) | 57.4±11.04 |
| Sex | |
| Male | 69 |
| Female | 21 |
| Etiology | |
| HBV | 82 |
| HCV | 6 |
| Maximal tumor diameter (cm) | |
| ≤5 | 12 |
| >5 | 78 |
| Multiplicity of tumor | |
| Single | 16 |
| Multiple | 74 |
| Child-Pugh class | |
| A | 81 |
| B | 9 |
| Serum AFP (ng/dl) | |
| <200 | 48 |
| ≥200 | 42 |
| BCLC stage | |
| B | 72 |
| C | 18 |
| ECOG | |
| 0 | 84 |
| 1 | 6 |
Values are expressed as the mean ± standard deviation or n. HBV, hepatitis B virus; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group.
Raw material
The CB microspheres (100–300 µm; 4 ml, Hengrui Medicine Co.) were hydrated in 7-ml sterile vials, where 4 ml microspheres were admixed with 40 mg epirubicin (Pfizer) and reconstituted in 2 ml sterile water from lyophilized powder for intravenous injection (concentration of epirubicin loaded into the microspheres, 20 mg/ml). This solution was held in a 20-ml syringe for 30 min with shaking every 5 min. The epirubicin-loaded beads were then added to non-ionic iodinated contrast medium (Hengrui Medicine Co.), reconstituted 1:1 in saline.
dTACE treatment
The dTACE procedure requires transfemoral arterial access and selective catheterization of feeder arteries to tumors via 3-F microcatheters. CB infusion of feeder arteries proceeded slowly (1 ml/min). Care was taken to avoid reflux of material into non-target vessels. The treatment endpoint was stasis of flow in segmental or subsegmental arterial branches (26). At least two chemoembolizations of target lesions were administered and sessions were performed at intervals of 4–6 weeks.
Tumor response
To assess the tumor response at 3 months after dTACE treatment, the mRECIST guidelines were applied to abdominal/pelvic CT or MRI examination results (27). The objective response rate was the sum of complete and partial therapeutic responses and the objective disease control rate was calculated as the sum of complete response (CR), partial response (PR) and stable disease (SD) (28). Pertinent laboratory parameters, namely alpha-fetoprotein (AFP), albumin, bilirubin, aspartate transaminase (AST), alanine aminotransferase (ALT) and prothrombin time (PT), were also assessed bi-monthly to evaluate treatment responses.
Safety
The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE; version 4.0) were used to monitor the tolerability of dTACE (24,29). After DEB treatment, adverse events were recorded for 48 h during hospitalization and then monthly during outpatient visits. The endpoint was liver toxicity, as indicated by various liver function tests (albumin, bilirubin, AST, ALT and PT).
Statistical analysis
All statistical analyses were performed using GraphPad Prism (version 5; GraphPad Software, Inc.) and the level of significance was set at P<0.05. Continuous data were expressed as median values and proportions as percentages. One-way analysis of variance followed by the least significant difference post hoc test was applied to compare differences in laboratory indices. A Kaplan-Meier survival curve was used to examine OS. Spearman's analysis was used assess potential correlations between overall survival time and each clinicopathological parameter.
Results
Clinical characteristics of patients with HCC
A total of 90 patients (69 males and 21 females; mean age, 57.4±11.04 years) who received CB treatments at our hospital were included. The characteristics of this patient population are listed in Table I. Overall, >91.11% of patients exhibited hepatitis B viral positivity and only two tested negative for hepatitis virus. Patients were mostly BCLC stage B (80%), as opposed to 20% in stage C. All were in good physical condition.
Treatment response
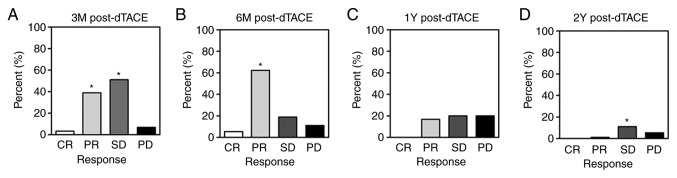
Treatment responses were evaluated at 3 and 6 months, 1 year and 2 years after dTACE. At 3 months, the results were as follows: Complete response, 3 patients (3.33%); partial response, 35 patients (38.89%); stable disease, 46 patients (51.11%); and progressive disease (PD), 6 patients (6.67%) (Fig. 1A). As such, the disease control rate (CR+PR+SD) was 93.33%. At 6 months, 2 of 90 patients (2.22%) had deceased, leaving 88 survivors. Among them, 5 (5.56%) and 56 (62.22%) qualified as having CR and PR, respectively; whereas 17 (18.89%) exhibited SD, and PD was evident in 10 (11.11%). The disease control rate was then 88.89% and the OR rate (CR+PR) was 67.78% (Fig. 1B). In the course of the follow-up, 39 and 75 patients died 1 year and 2 years post-dTACE, corresponding with disease control rates of 36.67 and 12.22%, respectively (Fig. 1C and D). Representative images of dTACE treatment were shown as Fig. 2. A HCC lesion was observed in the right hepatic lobe in artery (Fig. 2A) and venous enhancement MR scanning before dTACE treatment (Fig. 2B). Tumor lesion was clearly visible in the digital subtraction angiography (DSA) image during dTACE treatment (Fig. 2C), which disappeared and 2 years after dTACE (Fig. 2D and E).
Figure 1.
Tumor responses at different follow-up time-points. (A) 3 months post-dTACE (n=90); (B) 6 months post-dTACE (n=88); (C) 1 year post-dTACE (n=51); (D) 2 years after dTACE (n=15). *P<0.05 vs. PD at the same juncture. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; dTACE, drug-eluting bead transarterial chemoembolization; Y, year; M, month.
Figure 2.
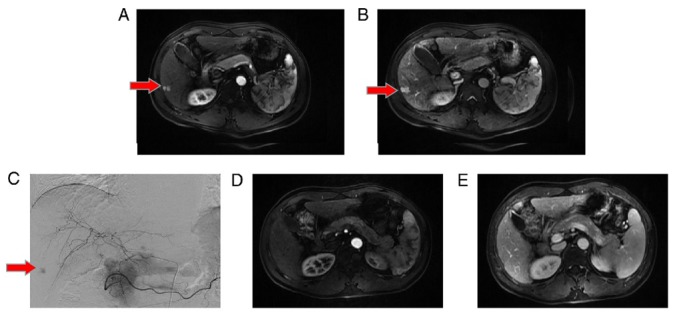
Effects of dTACE on HCC. (A) HCC lesion (indicated by red arrows) located in the right hepatic lobe as observed using artery enhancement MR scanning. (B) HCC lesion (indicated by red arrows) was clearly observed using venous enhancement MR scanning; (C) digital subtraction angiography of HCC lesion (indicated by red arrows) during dTACE; (D) Complete tumor response 2 years later in artery enhancement and (E) venous enhancement MR scanning follow-up. dTACE, drug-eluting bead transarterial chemoembolization; HCC, hepatocellular carcinoma. MR, Magnetic Resonance.
OS rates
The OS rate during the follow-up was evaluated. The median follow-up duration was 13.20 months (range, 3–24 months). A total of 41 patients (45.56%) were still alive and 49 (54.44%) had deceased at 2 years after dTACE. Causes of death included progressive liver disease (61.90%), myocardial infarction (9.52%), infection (9.52%) and esophageal varices (19.06%). None of the mortalities were treatment-associated. The Kaplan-Meier curve for OS of dTACE recipients is provided in Fig. 3. The median OS time was 13.05 months. (95% confidence interval: 7.41–24.00 months).
Figure 3.
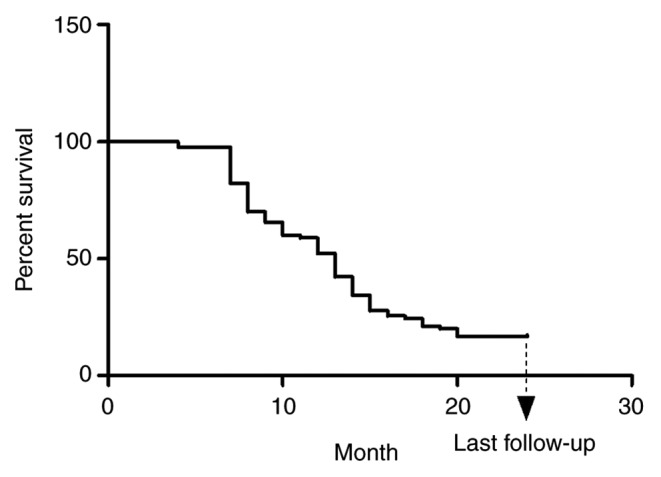
Kaplan-Meier curve of the overall survival (%) after drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma.
Potential associations between OS and clinical parameters were also assessed. In the correlation analysis, a significant association between the BCLC stage and survival was determined (P=0.04; Fig. 4A). Furthermore, this association was significantly higher in female patients (P=0.03; Fig. 4C), but no correlation with male patients has been found (P=0.06; Fig. 4B).
Figure 4.
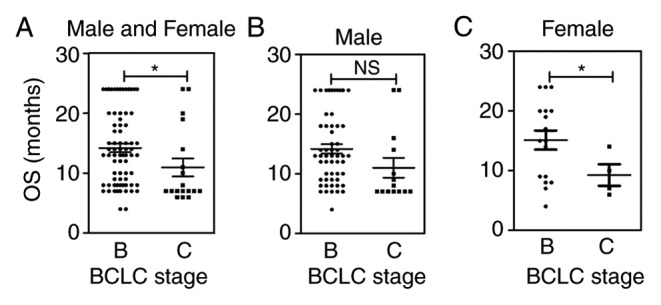
Univariate analysis of BCLC stage relative to survival in (A) all patients and (B) males or (C) females separately. *P<0.05 compared with other stages. NS, no significant difference; BCLC, Barcelona Clinic Liver Cancer; OS, overall survival.
Safety
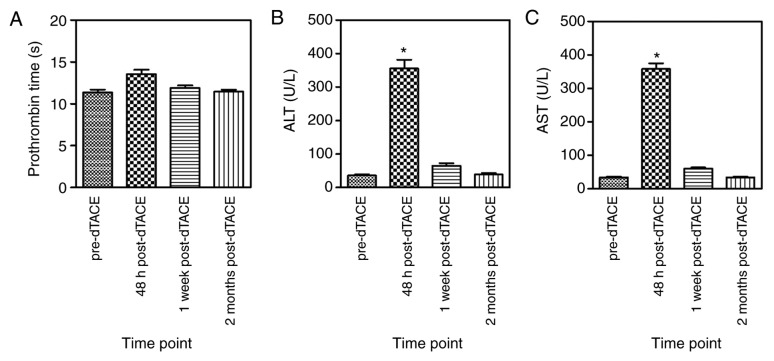
Within 1 month of dTACE administration, no major complications were encountered. Adverse events were primarily graded 2–3. Most adverse events, particularly abdominal pain (38.89%), fever (72.22%) and nausea (35.55%), were characteristic of post-embolization syndrome. AST or ALT elevations within 48 h were significantly more frequent after dTACE compared with pre-TACE, returning to normal within 2 months (Fig. 5). No grade 4 adverse events were observed.
Figure 5.
Pertinent laboratory analytes assessed during the follow-up. (A) PT, (B) ALT and (C) AST levels in the follow-up. *P<0.05 compared with values in the time-point. PT, prothrombin time; ALT, alanine aminotransferase; AST, aspartate transaminase.
Discussion
The results of the present study indicate that the effect of CB in patients with advanced HCC is encouraging. By combining selective infusion of toxic drugs and embolism of tumor-feeding blood vessels, the local concentration and contact time of therapeutic drugs is increased.
TACE has been widely preferred in the treatment of advanced HCC and as a bridge to liver transplantation (30,31). Compared with supportive care, TACE offers therapeutic efficacy and survival benefits by virtue of the potent cytotoxic and ischemic effects achieved through hepatic arterial chemoembolization. However, cTACE involves briefer periods of local drug contact due to washout. To address this problem, DEB have been devised, enabling higher local dosing of chemotherapeutic agents and prolonged contact with tumors. Studies have also demonstrated that compared with cTACE, DEB helps to mitigate concentrations of systemically circulating chemotherapeutics (32,33). More importantly, there are two added benefits of DEB: Coagulative necrosis and inflammatory/fibrotic changes (13). However, necrosis may result from the pharmacologic effects of anti-neoplastic agents released and embolization-induced ischemia and the fibrotic process is mostly incited by the particles themselves (13,34).
The present study provided encouraging outcomes using epirubicin-loaded CB in 90 patients with advanced HCC (CR, n=3; PR, n=35; SD, n=46), conferring a 93.33% disease control rate at 3 months after treatment, and OS at 6 months also improved (CR, n=5; PR, n=56). Only 10 patients progressed and disease control was sustained at a relatively high level (88.89%). The long-term follow-up ranged from 3 to 24 months, wherein the median OS was 13.05 months (95% confidence interval: 7.41–24.00). From the stratification analysis by BCLC stages, OS was significantly associated with the BCLC stage (P=0.04), with higher significance in female patients (P=0.03) but not in males (P=0.06). However, since there were only 21 female patients enrolled into the present study and a total of 15 patients alive in the last follow-up, the limited numbers may not necessarily representative of the entire condition. The treatment response appeared to be more insensitive in male patients compared with females, since the incidence of HCC was higher in males. However, the sample size of patients will need to be increased further to explore this potential correlation in the future. Ultimately, 21 patients died of progressive liver disease, myocardial infarction, infection or esophageal varices. At the last follow-up, 15 patients (16.67%) were still alive and in a stable condition. These cumulative data support the use of CB as a novel treatment, providing improved therapeutic effects in the majority of patients with advanced HCC.
Regarding safety and tolerability, most patients experienced grade 2–3 adverse events as defined by the NCI-CTCAE. The majority of adverse events, however, were in the realm of post-embolization syndrome. Furthermore, elevations of ALT or AST occurred with significant frequency within 48 h after dTACE, albeit with no significant change in liver toxicity compared with pre-dTACE within 2 months (as with cTACE) (35).
The present study had certain limitations that should be acknowledged. The follow-up period was relatively short (3–24 months), with only 15 patients alive and in astable condition at the time of conclusion. Thus, the OS does not directly reflect the specific treatment response. Additional studies are required to assess the efficacy of dTACE in comparison with cTACE, reinforcing the therapeutic potential of CB.
In conclusion, the present results attest to the efficacy and safety of CB in the setting of advanced HCC. dTACE was able to prolong OS and displayed favorable biosafety.
Acknowledgements
The authors would like to thank Mr. Chunbiao Li for his help with the language editing of the manuscript.
Funding
This study was supported in part by the National Natural Science Foundation of China (grant no. 81803008) and the Natural Science Foundation of Shandong Province (grant no. ZR2019BH041).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GJL and FC conceived and designed the study. ZQZ, WN, HL and SJ performed the dTACE treatment. WZC and PG collated the data and wrote the paper. All authors read and approved the manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Shandong First Medical University (Grant no. S0007; Jinan, China). All subjects provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 3.Ferenci P, Fried M, Labrecque D, Bruix J, Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA, et al. World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): A global perspective. J Gastrointestin Liver Dis. 2010;19:311–317. [PubMed] [Google Scholar]
- 4.Bertino G, Demma S, Ardiri A, Proiti M, Gruttadauria S, Toro A, Malaguarnera G, Bertino N, Malaguarnera M, Malaguarnera M, Di Carlo I. Hepatocellular carcinoma: Novel molecular targets in carcinogenesis for future therapies. Biomed Res Int. 2014;2014:203693. doi: 10.1155/2014/203693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Chen F, Wang H, Zhu J, Zhao R, Xue P, Zhang Q, Bud Nelson M, Qu W, Feng B, Pi J. Camptothecin suppresses NRF2-ARE activity and sensitizes hepatocellular carcinoma cells to anticancer drugs. Br J Cancer. 2017;117:1495–1506. doi: 10.1038/bjc.2017.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 7.Kiefer MV, Albert M, McNally M, Robertson M, Sun W, Fraker D, Olthoff K, Christians K, Pappas S, Rilling W, Soulen MC. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: A 2-center study. Cancer. 2011;117:1498–1505. doi: 10.1002/cncr.25625. [DOI] [PubMed] [Google Scholar]
- 8.Venturini M, Sallemi C, Agostini G, Marra P, Cereda S, Reni M, Aldrighetti L, De Cobelli F, Del Maschio A. Chemoembolization with drug eluting beads preloaded with irinotecan (DEBIRI) vs. doxorubicin (DEBDOX) as a second line treatment for liver metastases from cholangiocarcinoma: A preliminary study. Br J Radiol. 2016;89:20160247. doi: 10.1259/bjr.20160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gusani NJ, Balaa FK, Steel JL, Geller DA, Marsh JW, Zajko AB, Carr BI, Gamblin TC. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): A single-institution experience. J Gastrointest Surg. 2008;12:129–137. doi: 10.1007/s11605-007-0312-y. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, Fischer R. Treatment of unresectable cholangiocarcinoma: Conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24:437–443. doi: 10.1097/MEG.0b013e3283502241. [DOI] [PubMed] [Google Scholar]
- 11.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 12.Rostas JW, Tam AL, Sato T, Scoggins CR, McMasters KM, Martin RCG., II Health-related quality of life during trans-arterial chemoembolization with drug-eluting beads loaded with doxorubicin (DEBDOX) for unresectable hepatic metastases from ocular melanoma. Am J Surg. 2017;214:884–890. doi: 10.1016/j.amjsurg.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Namur J, Citron SJ, Sellers MT, Dupuis MH, Wassef M, Manfait M, Laurent A. Embolization of hepatocellular carcinoma with drug-eluting beads: Doxorubicin tissue concentration and distribution in patient liver explants. J Hepatol. 2011;55:1332–1338. doi: 10.1016/j.jhep.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Delhaye C, Hendlisz A, Vouche M. Update on transarterial approaches to locoregional treatment in hepatocellular carcinoma. Curr Opin Oncol. 2019;31:339–345. doi: 10.1097/CCO.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 15.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, Bruix J. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan YS, He Q, Jin Y, Yao F. Development of CalliSpheres® embolic microshperes. Zhonghua Gan Zang Bing Za Zhi. 2016;24:549–551. doi: 10.3760/cma.j.issn.1007-3418.2016.07.016. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Huang C, Li Z, Yang Y, Bao T, Chen H, Zou Y, Song L. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipodol emulsions and CalliSpheres Beads in rabbit livers. Drug Delivery. 2017;24:1011–1017. doi: 10.1080/10717544.2017.1344336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou GH, Han J, Sun JH, Zhang YL, Zhou TY, Nie CH, Zhu TY, Chen SQ, Wang BQ, Yu ZN, et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres® beads in Chinese hepatocellular carcinoma patients. BMC Cancer. 2018;18:644. doi: 10.1186/s12885-018-4566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Xu J, Zhang W, Chen J, Zhou X, Li Z, Han X. Safety and efficacy of drug-eluting bead transarterial chemoembolization combined with apatinib in patients with advanced hepatocellular carcinoma. Acad Radiol. 2019 Jul 31; doi: 10.1016/j.acra.2019.07.003. doi: 10.1016/j.acra.2019.07.003 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PubMed] [Google Scholar]
- 23.Hinrichs JB, Hasdemir DB, Nordlohne M, Schweitzer N, Wacker F, Vogel A, Kirstein MM, Marquardt S, Rodt T. Health-related quality of life in patients with hepatocellular carcinoma treated with initial transarterial chemoembolization. Cardiovasc Intervent Radiol. 2017;40:1559–1566. doi: 10.1007/s00270-017-1681-6. [DOI] [PubMed] [Google Scholar]
- 24.Aliberti C, Carandina R, Sarti D, Pizzirani E, Ramondo G, Mulazzani L, Mattioli GM, Fiorentini G. Chemoembolization with Drug-eluting microspheres loaded with doxorubicin for the treatment of cholangiocarcinoma. Anticancer Res. 2017;37:1859–1863. doi: 10.21873/anticanres.11522. [DOI] [PubMed] [Google Scholar]
- 25.Song J, Guijie L, Zhuqian Z, Niuwei Interventional chemoembolization with CalliSpheres-loaded microspheres for the treatment of advanced hepatocellular carcinoma. Clin J Inter Rad (Electrionic edition) 2017;5:174–178. (In Chinese) [Google Scholar]
- 26.Kishore S, Friedman T, Madoff DC. Update on embolization therapies for hepatocellular carcinoma. Curr Oncol Rep. 2017;19:40. doi: 10.1007/s11912-017-0597-2. [DOI] [PubMed] [Google Scholar]
- 27.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 28.Purcell Y, Sartoris R, Paradis V, Vilgrain V, Ronot M. Influence of pretreatment tumor growth rate on objective response of hepatocellular carcinoma treated with transarterial chemoembolization. J Gastroenterol Hepatol. 2019 Aug 1; doi: 10.1111/jgh.14816. doi: 10.1111/jgh.14816 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 29.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 30.Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Hepatic resection compared to chemoembolization in intermediate to advanced stage hepatocellular carcinoma: A Meta-analysis of High-quality studies. Hepatology. 2018;68:977–993. doi: 10.1002/hep.29883. [DOI] [PubMed] [Google Scholar]
- 31.Huang YK, Yen CL, Shiu SI, Lee SW, Chang PY, Yeh HZ, Lee TY. Transcatheter arterial chemoembolization after stopping sorafenib therapy for advanced hepatocellular carcinoma. PLoS One. 2017;12:e0188999. doi: 10.1371/journal.pone.0188999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Bai Z, Qin Y, Zhu G, Zhao G, Deng G, Guo J, He S. Efficacy and safety of epirubicin applied in transcatheter arterial chemoembolization for hepatocellular carcinoma: A meta-analysis. J Cancer Res Ther. 2018;14:133–138. doi: 10.4103/jcrt.JCRT_1261_16. [DOI] [PubMed] [Google Scholar]
- 34.Yi PS, Huang M, Zhang M, Xu L, Xu MQ. Comparison of transarterial chemoembolization combined with radiofrequency ablation therapy versus surgical resection for early hepatocellular carcinoma. Am Surg. 2018;84:282–288. [PubMed] [Google Scholar]
- 35.Firouznia K, Ghanaati H, Alavian SM, Azadeh P, Nasiri Toosi M, Haj Mirzaian A, Najafi S, Shakiba M, Jalali AH. Transcatheter arterial chemoembolization therapy for patients with unresectable hepatocellular carcinoma. Hepat Mon. 2014;14:e25792. doi: 10.5812/hepatmon.25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.