Abstract
Background & Aims:
Cachexia, which includes muscle wasting, is a frequent complication of pancreatic cancer. There are no therapies that reduce cachexia and increase patient survival, so it is important to learn more about its mechanisms. The zinc transporter ZIP4 promotes growth and metastasis of pancreatic tumors. We investigated its effects on muscle catabolism via extracellular vesicle (EV)-mediated stimulation of mitogen-activated protein kinase 14 (p38MAPK).
Methods:
We studied nude mice with orthotopic tumors grown from human pancreatic cancer cell lines (AsPC-1 and BxPC-3); tumors were removed 8 days after cell injection and analyzed by histology. Mouse survival was analyzed by Kaplan-Meier curves. ZIP4 was knocked down in AsPC-1 and BxPC-3 cells with small hairpin RNAs; cells with empty vectors were used as controls. Muscle tissues were collected from mice and analyzed by histology and immunohistochemistry. Conditioned media from cell lines and 3-dimensional spheroid/organoid cultures of cancer cells was applied to C2C12 myotubes. The myotubes and the media were analyzed by immunoblots, ELISAs, and immunofluorescence microscopy. EVs were isolated from conditioned media and analyzed by immunoblots.
Results:
Mice with orthotopic tumors grown from pancreatic cancer cells with knockdown of ZIP4 survived longer and lost less body weight and muscle mass than mice with control tumors. Conditioned media from cancer cells activated p38MAPK and induced expression of F-box protein 32 and UBR2 in C2C12 myotubes, and also led to loss of myofibrillar protein myosin heavy chain and myotube thinning. Knockdown of ZIP4 in cancer cells reduced these effects. ZIP4 knockdown also reduced pancreatic cancer cell release of HSP70 and HSP90, which are associated with EVs, by decreasing CREB-regulated expression of RAB27B.
Conclusions:
ZIP4 promotes growth of orthotopic pancreatic tumors in mice and loss of muscle mass by activating CREB-regulated expression of RAB27B, required for release of EVs from pancreatic cancer cells. These EVs activate p38MAPK and induce expression of F-box protein 32 and UBR2 in myotubes, leading to loss of myofibrillar myosin heavy chain and myotube thinning. Strategies to disrupt these pathways might be developed to reduce pancreatic cancer progression and accompanying cachexia.
Keywords: muscle loss, pancreas, mouse model, signal transduction
Graphical Abstract
Introduction
Pancreatic cancer has the highest mortality rate of all cancers with the 5-year survival rate only 8% 1 In about 15% of newly diagnosed cases where the tumor is non-metastatic and resectable, most patients will ultimately develop local or distant tumor recurrence within two years of surgery that is usually associated with rapid development of cachexia 2,3. Cachexia is a wasting syndrome characterized by progressive weight loss, muscle loss, fatigue, weakness, and significant loss of appetite 4–6. More than 80% of pancreatic cancer patients suffer from cachexia syndrome, which is not reversible by nutritional support 7,8. In addition, cancer patients with severe cachexia syndrome respond poorly to chemotherapy and radiation therapy, and have a worse outcome with those treatments 9. Overall, the underlying biology of cachexia is poorly understood, previous data suggest that muscle wasting can be caused by tumor derived signaling factors triggering muscle degradation 10–13. However, the detailed molecular mechanism controlling this phenomenon remains elusive.
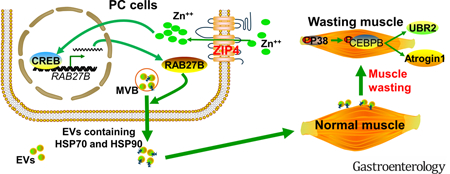
We recently identified a zinc transporter, ZIP4, as a regulator of pancreatic tumor growth and metastasis 14–16. ZIP4 expression also correlates with tumor progression and survival in pancreatic cancer mouse models 14,15, 17–22, suggesting that ZIP4 can regulate tumor function beyond the primary tumor. In the present study, we show that ZIP4 also plays a significant role in pancreatic cancer-associated cachexia by stimulating muscle wasting through promoting the release of HSP70 and HSP90 via extracellular vesicle (EV). EV-associated HSP70 and HSP90, acting as danger-associated molecular patterns (DAMPs) that activate TLR4 23, stimulate p38MAPK-mediated muscle catabolism. By using 2D cell cultures, 3D spheroid/organoid, and a curative mouse pancreatic cancer model we define a previously unidentified role of ZIP4 in promoting pancreatic cancer-associated cachexia. ZIP4 knockdown decreased body weight and muscle mass loss, and increased survival. Moreover, lowering levels of this zinc transporter ameliorated muscle wasting by attenuating tumor release of HSP70 and HSP90 via EVs by suppressing CREB-regulated expression of RAB27B, a GTPase that is required for EV release 24 These data reveal a previously unidentified molecular mechanism of cachexia and muscle wasting in pancreatic cancer and define an uncharacterized role for zinc signaling in this process.
Materials & Methods
3D spheroid/organoid culture
The 3D spheroid culture was based on the slightly modified methylcellulose (MC) hanging drop method by Ware, M. and Godin B25. Briefly, pancreatic cancer cells were resuspended in culture media containing 0.24% methylcellulose (Sigma, M0512) to reach 1×106 cells/ml. Cells were seeded onto the inner lid of 10-cm dishes with 20 μl/drop, the lids were inverted over dishes containing 10 ml phosphate buffer solution. The hanging drops were incubated in 5% CO2, at 37 °C for 5–7 days to form the homogenous 3D cell spheroids. Around 75 spheroids were pooled into one well of a 24-well plate and cultured 24 hrs in complete media for conditioned media collection to treat C2C12 myotubes. The matrigel based 3D organoid were based on the similar method except that the cell suspension was mixed with matrigel (Corning, 354234) to reach final concentration of 5 mg/ml matrigel. The solidified drops were kept on the dish lid for three days. Around 150 matrigel drops were pooled into one well of a 6-well plate with 5 ml complete media. Conditioned media was collected at 48 hrs for treatment with C2C12 myotube.
Orthotopic implantation of pancreatic cancer cells in nude mice
Subconfluent cells (3×106) were inoculated into the tail of the pancreas of 6- to 8-week-old male nude mice (Hsd:Athymic Nude-Foxnlnu, Envigo RMS, Inc). All mice were cared for in accordance with the Office for Protection from Research Risks (OPRR) and Animal Welfare Act Guidelines under an animal protocol approved by the Institutional Animal Welfare Committee.
Surgical removal ofxenograft tumor via Distal Pancreatectomy
On day 8–10 post tumor implantation, orthotopic tumors were removed via distal pancreatectomy. In brief, the tail and the majority of the body of pancreas harboring the pancreatic tumor and the whole spleen were resected as previously described 26. The body weight and overall health status of the mice were monitored daily. At indicated times, tibialis anterior (TA) and extensor digitorum longus (EDL) muscles were collected from the mice immediately after euthanasia and were further analyzed.
Immunohistochemical staining
Pancreatic tumors were collected and fixed in 4% PFA. The paraffin sections were stained with H&E as previously described 14 Stained slides were assessed using a phase contract microscopy. Cross-sectional area of H&E stained muscle sections were quantified by using the ImageJ software (NIH). Five view-fields with about 150 myofibres per field in each section were measured. Data were expressed as frequency histogram.
Tyrosine release assay
Tyrosine release was measured as previously described previously 27 with the fresh collected extensor digitorum longus (EDL) muscle from the mice.
Myogenic cell culture
Murine C2C12 myoblasts (ATCC) were cultured in growth media at 37°C under 5% CO2. At 85~90% confluence, myoblast differentiation was induced by incubation in differentiation media (DMEM supplemented with 4% horse serum) for 96 hrs to form myotubes. Conditioned media from cultures of pancreatic carcinoma cells or non-tumorigenic mouse fibroblast 10T1/2 that were cultured for 48 hrs were centrifuged to remove floating cells and added to C2C12 cultures (25% final volume in fresh media) when indicated. The conditioned media was replaced every 24 hrs.
Fluorescence microscopy study
C2C12 myotubes were stained with anti-MHC antibody (MF-20, Development Studies Hybridoma Bank at the Univ. of Iowa, Iowa City, IA) and FITC-conjugated secondary antibody, and examined using a Zeiss Axioskop 40 microscope. Myotube diameter was measured in MHC-stained myotubes as previously described 27. Briefly, myotube cultures were photographed under a phase-contrast microscope at 40X. The diameters were measured in a total of 200 myotubes from ≥10 random fields using computerized image analysis (Scion Image, Frederick, MD, USA). Myotubes were measured at 3 points along their length.
Quantification of extracellular HSP70 and HSP90
HSP70 and HSP90α levels in various cell-conditioned media (concentrated 10 folds by centrifugation with 10k filters from Millipore) or mouse sera were analyzed by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instruction (HSP70 EIA, Enzo Life Sciences, PA; HSP90α EIA, Cusabio Biotech, China). HSP70 and HSP90α from fetal bovine serum supplement in the culture media were also measured and subtracted from total HSP70 and HSP90α levels measured in cell conditioned media.
Extracellular Vesicle (EV) isolation and quantitation
EVs in concentrated cell conditioned media (described above) were isolated using the ExoQuickTM kit according to manufacturer’s protocol (System Biosciences, Mountain View, CA). Isolated EVs were quantified by measuring the activity of acetylcholinesterase (AchE) according to a published protocol 28. Briefly, isolated EV preparations (20 μl) were suspended in 100 μl of PBS and incubated with 1.25 mM acetylthiocholine and 0.1 mM 5,5’-dithiobis (2-nitrobenzoic acid) in a final volume of 1 ml for 20 min at room temperature. OD at 412 nm was monitored using the Synergy 2 Multi-Mode Microplate Reader (Biotek Instruments, Winooski, VT). Protein content in the EV preparations was measured by the Lowry assay (Bio-Rad, Hercules, CA). EVs from fetal bovine serum supplement in the culture media were also measured and subtracted from total EV levels measured in cell conditioned media.
Lentiviral expression of RAB27B and transduction of AsPC-1 cells
Human RAB27B (NM_004163) cDNA was inserted into pCDH-CMV-MCS-EF1-Puro vector at XbaI and NotI. Purified transfer plasmid (pCDH-RAB27B, 10 μg) or control vector along with packaging plasmid psPAX2 (10 μg) and envelope plasmid PMD2.G (2.5 μg) were transfected into TLA293T cells in a 10 cm dish using jetPRIME reagent (Polyplus Transfection, Illkirch, France). Viral supernatant fractions were collected at 48 hrs post transfection and filtered through a 0.45 μm filter. The end point dilution method was employed to quantify titers with log dilutions on 100% confluent TLA293T cells. The viral supernatant of selected cells was used to transduce AsPC-1 cells with 8 μg/ml Polybrene for 24 hrs. Transduced AsPc-1 cells were selected by puromycin (1 μg/ml for 5 days in culture media supplemented with 10% FBS). After changing to fresh media, AsPC-1 cells were incubated for 48 hrs. Cells and culture media were analyzed for Rab27 expression and HSP/EV release.
Statistical analysis
Data were analyzed with one-way ANOVA or student t test using the SigmaStat software as indicated. A P value < 0.05 was considered to be statistically significant. Data are presented as the mean ± S.E.
Results
ZIP4 knockdown increases survival after distal pancreatectomy.
To determine the effects of ZIP4 knockdown in combination with tumor resection, we used a recently established mouse model for curative surgical resection of pancreatic cancer using imaging-guided distal pancreatectomy to remove orthotopic pancreatic cancer xenografts 26 Two human pancreatic cancer cell lines AsPC-1 and BxPC-3 were used for xenograft. Control (n=6) and shRNAs ZIP4 (n=7) AsPC-1 cells were implanted orthotopically into nude mice. Resultant tumors were removed on the 8th day post implantation by pancreatectomy/splenectomy. All mice survived surgery and showed no noticeable post-surgical complications. We found that knockdown ZIP4 alone in AsPC-1 cells (AsPC-shZIP4) did not cause significant reduction of primary tumor compared with those from control group (AsPC-shV) at the time of surgery (Fig. 1A), although histological analysis revealed that reduced ZIP4 level leads to decreased pancreatic cancer progression. The majority of the samples from control group were poorly differentiated or sarcomatoid with 60% to 95% in tumor area. Most of tumors (5 of 7) from AsPC-shZIP4 group were moderately or well differentiated and had 10% to 35% smaller tumor area (Supplementary Fig. S1). Using BxPC-3 cells, a less metastatic pancreatic cancer cell line, similar pancreatectomy/splenectomy procedure was performed on the 8th day post tumor implantation. There was no significant difference in primary tumor size and weight between BxPC-shV and BxPC-shZIP4 groups (Fig. 1B), which is consistent with data from AsPC-1 cells.
Figure 1. ZIP4 knockdown in pancreatic cancer cells reduced body weight loss in mice underwent tumor resection.
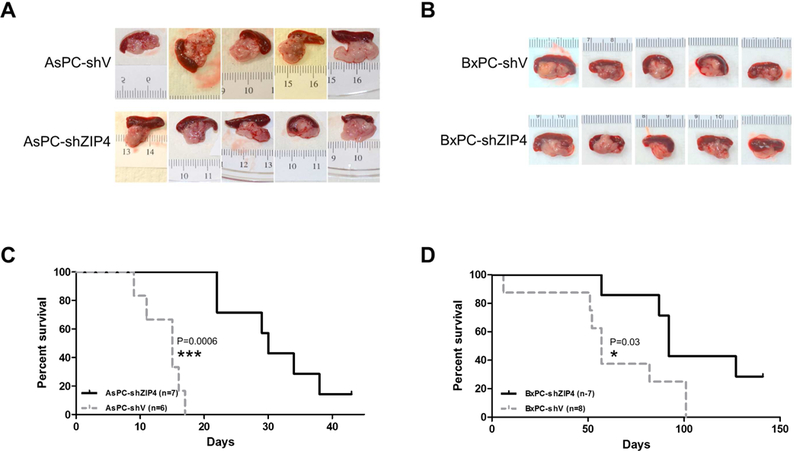
(A) AsPC-shV/shZIP4 and (B) BxPC-shV/shZIP4 cells (3 × 106) were orthotopically inoculated into the nude mice. The primary tumors were removed on day 8 post injection by distal pancreatectomy/splenectomy, and five mice from each group were shown. Survival of the mice was shown in the Kaplan-Meier survival curve for (C) AsPC-shV/shZIP4 groups and (D) BxPC-shV/shZIP4 groups.
After tumor removal, mice from both groups (ZIP4 high and low) were observed daily and the overall survival rate was recorded. For the AsPC-shV group (ZIP4 high), all mice died on or before day 17 with the first casualty on day 9 post-surgery. For the AsPC-shZIP4 group (ZIP4 low), the first mouse died on day 22 and two mice were still alive on day 35. Kaplan-Meier survival curves demonstrated that the distal pancreatectomy significantly improved median survival of mice bearing tumors formed by ZIP4-knockdown AsPC-1 cells compared with the control group (P<0.001). Median survival for the AsPC-shZIP4 group was 30 days versus 15 days for the AsPC-shV group (Fig. 1C and Supplementary Table 1). Similar results were found in mice with BxPC-3 xenograft, i.e. surgery plus ZIP4 knockdown significantly prolonged survival compared with the control group (P<0.05). The median survival for BxPC-shZIP4 group was 92 days versus 57 days for BxPC-shV group (Fig. 1D and Supplementary Table 1). These results clearly demonstrate that ZIP4 knockdown combined with surgical resection significantly increases survival of pancreatic cancer in mice, which could not be achieved by surgical resection alone or ZIP4 knockdown alone.
Combination of ZIP4 knockdown and surgical resection ameliorates muscle wasting and cachexia of mice.
In addition to survival, we monitored body weight following surgery, for that in human the tumor recurrence and mortality are always associated with weight loss and cachexia. Body weight of the control AsPC-shV group rapidly decreased where most mice lost more than 10% of their original body weight by day 8 after surgery. These mice usually died when the body mass decreased by 20% and had similar phenotypes as found in the late stage of human pancreatic cancer, such as loss of appetite and mobility. In contrast, most of the mice in the AsPC-shZIP4 group retained at least 90% of their original body weight for three weeks after surgery without obvious alterations in behaviors (Fig. 2A). These results suggest that surgical resection combined with ZIP4 knockdown significantly improves the lifespan of pancreatic cancer through ameliorating cachexia.
Figure 2. Knockdown of ZIP4 reduced muscle wasting in vivo.
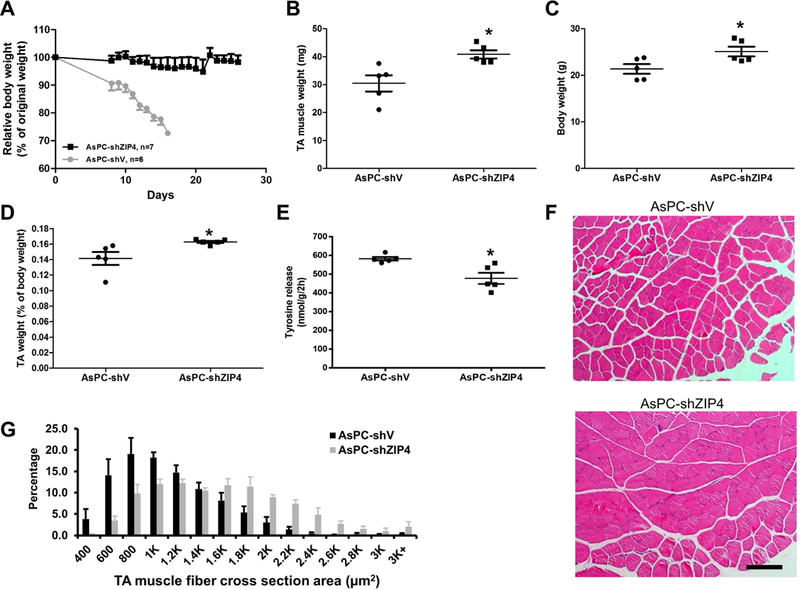
Mice with orthotopic xenograft tumor of AsPC-shV/shZIP4 were subjected to distal pancreatectomy/splenectomy on day 8. On day 18, mice were euthanized and muscle samples were collected for analyses. (A) The body weight data of mice in AsPC-shV/shZIP4 groups. Log-rank (Mantel-Cox) test showed significant loss of body weight in the AsPC-shV group vs. AsPC-shZIP4 group (P<0.05). (B) TA muscle mass. (C) Body weight. (D) TA muscle mass relative to mice body weight. (E) Tyrosine release from EDL. (F) H&E stained TA muscle sections. The scale bar represents 200 μm. (G) Fiber cross-sectional area (CSA). CSA of TA cross-sections was measured as described in materials and methods.
It has been showed that cancer-associated body weight loss is mostly due to muscle wasting 4, 29, we therefore evaluated muscle wasting in mice implanted with AsPC-shV (ZIP4 high) or AsPC-shZIP4 (ZIP4 low) cells. Tibialis anterior (TA) muscle mass was significantly lower in AsPC-shV group compared with that in AsPC-shZIP4 group (Fig. 2B, P<0.05). Since those two groups of mice experienced different levels of body weight loss (Fig. 2C, P<0.05), we normalized muscle mass with the whole mice body weight, and found similar reduction in muscle mass (Fig. 2D, P<0.05). To further confirm muscle wasting, we examined proteolysis by measuring tyrosine release from extensor digitorum longus (EDL), and found that tyrosine release was significantly higher in AsPC-shV group compared with that in AsPC-shZIP4 group, indicating attenuated muscle proteolysis when ZIP4 was lowered in pancreatic cancer (Fig. 2E, P<0.02). Further, histological analysis on TA muscle cross sectional area revealed a significant shrinkage of myofibers in AsPC-shV group. Majority of myofibers in AsPC-shV group had much smaller cross sectional area and larger interstitial space than that in AsPC-shZIP4 group (Fig. 2F and2G). Thus, together these results suggest regulation of muscle proteostasis as the potential cellular mechanism underlying ZIP4 regulation of cachexia.
ZIP4 promotes pancreatic cancer-induced muscle wasting through enhancing p38MAPK-mediated muscle catabolism.
Previous studies identified p38MAPK as a key mediator of lung carcinoma-induced muscle wasting 11. Therefore, we examined whether p38MAPK signaling is involved in ZIP4-induced muscle wasting. C2C12 myotubes were treated with conditioned media derived from two paired pancreatic cancer cells: AsPC-shV/AsPC-shZIP4, and BxPC-shV/BxPC-shZIP4 cells. Conditioned media from AsPC-shV cells strongly induced p38MAPK activation in C2C12 myotubes within 1 hr and upregulated F-box protein 32 (ATROGIN1) within 8 hrs of treatment. These catabolic responses were significantly attenuated when ZIP4 expression was knocked down in AsPC-shZIP4 cells (Fig. 3A and3B). Further incubation of myotubes in the conditioned media for 72 hrs caused a loss of myofibrillar protein myosin heavy chain (MHC) in a ZIP4-dependent manner (Fig. 3C). Consistent with MHC loss, myotubes treated with conditioned media from AsPC-shV became 26% thinner (P<0.05) while the ones treated by AsPC-shZIP4 media were largely unaffected (Fig. 3D). Similar results were found in BxPC-shV/BxPC-shZIP4 cells on p38MAPK and ATROGIN1 (Fig. 3E and3F). In addition, p38MAPK activation and
Figure 3. ZIP4 mediates pancreatic cancer-induced muscle catabolism by activating p38MAPK in vitro.
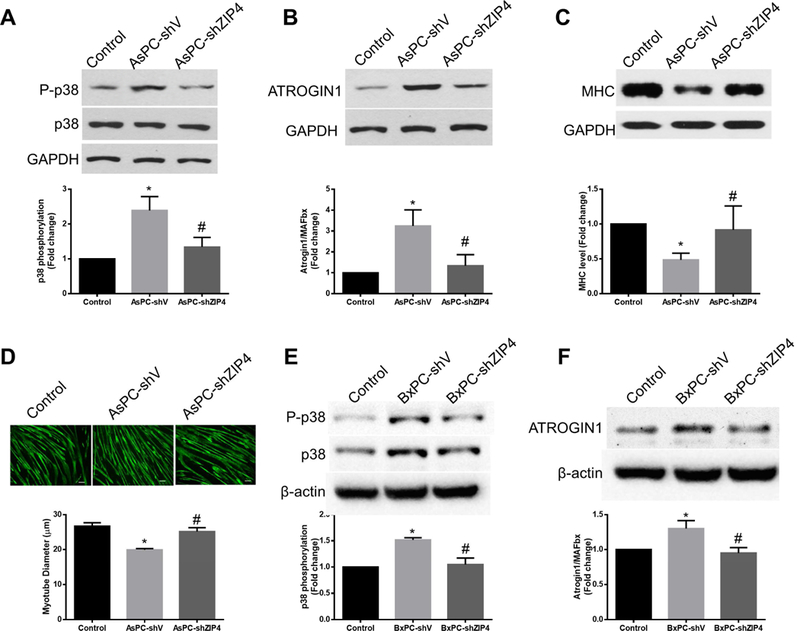
Conditioned media were collected to test their capacity in inducing myotube catabolism. (A) ZIP4 mediates p38MAPK activation induced by conditioned media treatment with C2C12 myotubes for 1 hr. (B) ZIP4 mediates ATROGIN1 upregulation induced by conditioned media treatment with C2C12 myotubes for 8 hrs. (C) ZIP4 mediates myofibrillar protein myosin heavy chain (MHC) loss induced by conditioned media treatment with C2C12 myotubes for 72 hrs. (D) ZIP4 mediates myotube atrophy induced by conditioned media. Immunofluorescence staining of MHC was performed (bar = 100 μm), and diameter of myotubes was determined. (E) ZIP4 mediates p38MAPK activation induced by BxPC-3 cell-conditioned media in C2C12 myotubes.. (F) C2C12 myotubes were treated with BxPC-3 cell-conditioned media for 8 hrs.
ATROGIN1 upregulation were significantly increased in mouse muscle from the ZIP4 high group compared with that from ZIP4 low group, while p38MAPK activation and ATROGIN1 upregulation were much reduced in ZIP4 low group (Fig. 4C). To further confirm the above results from pancreatic cancer cells in normal 2D culture, we examined the muscle cell metabolism using two different 3D spheroid/organoid culture systems: the methylcellulose (MC) based hanging drop 3D cancer spheroids (Supplementary Fig. S2) and the classical matrigel based 3D organoid culture (Supplementary Fig. S3). Conditioned media derived from both 3D systems had the same cachexic effect as observed in 2D cell culture. Conditioned media from ZIP4 high AsPC-1 cell (AsPC-shV) spheroid/organoid culture led to p38MAPK activation and UBR2 upregulation compared with the ZIP4 low cells (AsPC-shZIP4) (Fig. 4A, 4B). We also used the conditioned media from the 2D cell culture and the MC spheroid culture of ZIP4 over-expressing stable cell line (Panc-ZIP4) 30, and observed that increased ZIP4 promoted the activation of p38MAPK pathway in C2C12 myotubes (Supplementary Fig. S4). These results suggest that ZIP4 promotes pancreatic cancer-induced muscle wasting through enhancing p38MAPK-mediated protein degradation.
Figure 4. ZIP4 mediates pancreatic cancer-induced muscle catabolism by activating p38MAPK in vivo.
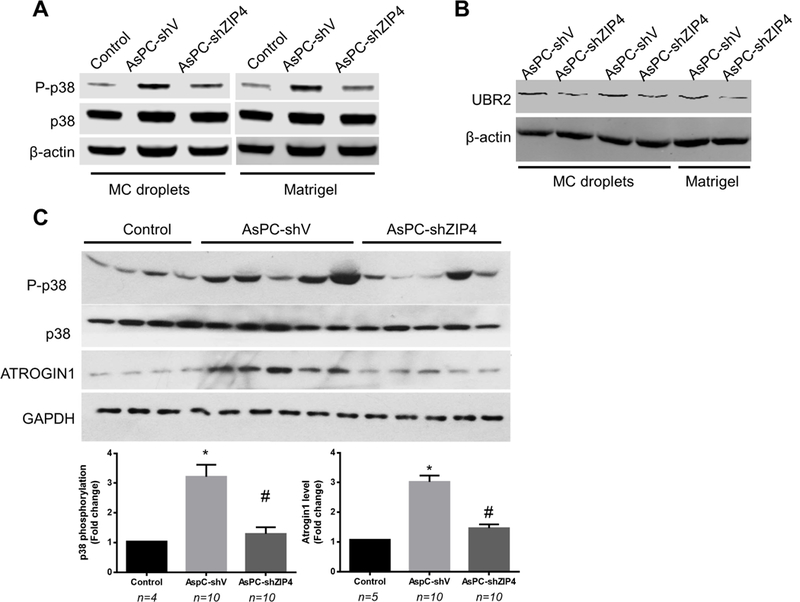
(A) Conditioned media was collected from methylcellulose (MC) based hanging drop 3D cancer spheroids and the classical matrigel based 3D organoid culture from AsPC-shV/shZIP4 cells, then added onto C2C12 myotube cells for 1 hr to check the activation of p38. (B) C2C12 myotubes were treated with the 3D organoid culture media for 8 hrs to check the induction of UBR2. (C) Mice with orthotopic xenograft of AsPC-shV/shZIP4 cells were subjected to distal pancreatectomy/splenectomy on day 8. On day 18, TA lysate was analyzed by western blot analysis for p38MAPKactivity and ATROGIN1 expression. Data are expressed as means ± SE and analyzed by ANOVA. * denotes a difference (P<0.05) from control and # denotes a difference from AsPC-shV (P<0.05)
ZIP4 promotes p38MAPK-mediated muscle catabolism through increasing RAB27B-dependent release of EV-associated HSP70 and HSP90 by pancreatic cancer cells.
Pancreatic cancer cells release EVs that carry various signaling molecules to communicate with recipient cells 31. Many of the pancreatic cancer cell-released EVs contain surface heat shock proteins HSP70 and HSP90 32,33, which activate p38MAPK signaling pathway through Toll-like receptor 4 (TLR4) on the recipient cells 23. Thus, we investigated whether ZIP4 promotes muscle wasting by increasing HSP70 and HSP90 release through EVs from AsPC-1 cells. We observed that AsPC-1 cells released markedly higher levels of HSP70 (~22-fold) and HSP90α (~18-fold) into cell culture media as compared to non-tumorigenic human pancreatic duct epithelial cells (HPDE), and ZIP4 knockdown reduced more than half of HSP70 and HSP90 release (Fig. 5A). Analysis of the conditioned media from the spheroid culture of AsPC-1 and Panc-1 stable lines also indicated a strong correlation of HSP90α with ZIP4 level in pancreatic cancer cells (Supplementary Fig. S5A and S5B). To analyze whether AsPC-1 cell-released HSP70 and HSP90 were associated with EVs, we isolated EVs from conditioned media and analyzed the presence of HSP70 and HSP90, as well as other markers of EVs. We detected HSP70 and HSP90 in isolated EVs along with CD9, CD81 and acetylcholinesterase (AchE) (Supplementary Fig. S6), which are proteins often found in EVs 34. Thus, AsPC-1 cells release HSP70 and HSP90-enriched CD9/CD81/AchE-positive EVs. Given that all existing EV isolation methods do not achieve complete purity 34, we quantified isolated EVs by measuring AchE activity 23,35. AsPC-1 cells released markedly higher level of EVs than HPDE cells (~40-fold) and that ZIP4 knockdown reduced EV release by ~55% (Fig. 5B), which was comparable to the reduction in HSP70 and HSP90 release (Fig. 5A). We then treated C2C12 myotubes with EVs isolated from AsPC-1 cells, which induced activation of p38MAPK and upregulation of ubiquitin ligases ATROGIN1 and UBR2, in a ZIP4-dependent manner (Fig. 5C). Similarly, serum HSP90α levels in nude mice xenografted with AsPC-1 cells were reduced by ~56% due to ZIP4 knockdown in the cells (Fig. 5D). These data suggest that ZIP4 promotes AsPC-1 cell-induced muscle wasting by increasing the release of HSP70 and HSP90 via EVs into circulation.
Figure 5. ZIP4 promotes p38MAPK-mediated muscle catabolism through increasing pancreatic cancer cell release of HSP70 and HSP90-positive EVs.
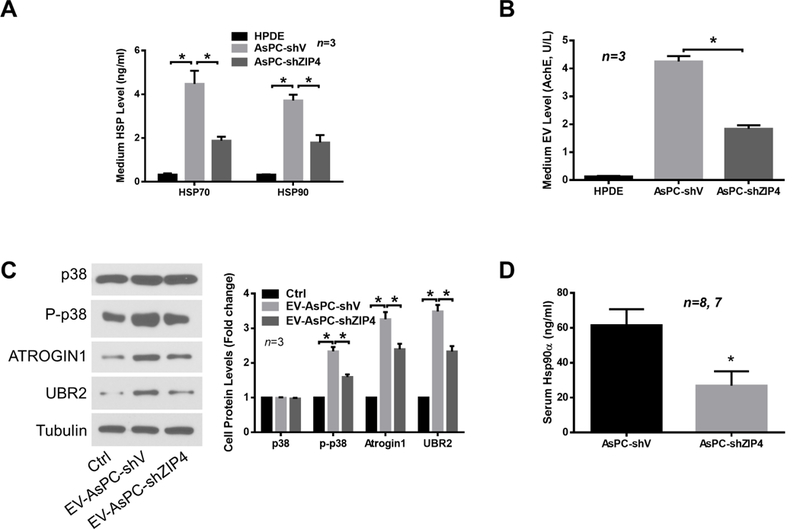
(A) AsPC-1 cells release higher levels of HSP70 and HSP90 in a ZIP4-dependent manner. HSP70 and HSP90 levels in cell conditioned media of indicated cells were measured by ELISA. (B) AsPC-1 cells release higher levels of EVs in a ZIP4-dependent manner as measured by AchE activity. (C) EVs isolated from conditioned media of AsPC-1 cells activates p38MAPK-mediated catabolic response in C2C12 myotubes in a ZIP4-dependent manner. C2C12 myotubes were treated with EVs isolated from AsPC-1 cells and analyzed by western blotting in 1 hr (p38MAPK) or 8 hrs (UBR2 and ATROGIN1). (D) Serum HSP90 levels in nude mice with orthotopic xenograft of AsPC-1 cells are reduced by ZIP4 knockdown. Serum HSP90α was analyzed by ELISA from nude mice grafted with AsPC-shV/shZIP4 cells on day 27 (n = 8 and 7, respectively). * denotes P<0.05.
Finally, we investigated the mechanism through which ZIP4 stimulates EV release by pancreatic cancer cells. Given that RAB27A and RAB27B, which are GTPases present in HSP-positive EVs 36 that are required for and control different steps of EV release 24, we examined the role of these molecules in EV release from AsPC-1 cells. ZIP4 knockdown reduced the expression of RAB27B, but not RAB27A, at both the mRNA and protein levels (Fig. 6A). Suppressed RAB27B expression was also observed in ZIP4-knockdown BxPC-3 cells (Fig. 6B). In addition, mRNA level of RAB27B in the two forms of spheroid culture of AsPC-shV/-shZIP4 cells showed the same pattern as that in 2D culture (Supplementary Fig. S7A, S7B). A similar trend was observed in Panc-V and Panc-ZIP4 MC spheroid culture as well (Supplementary Fig. S7C). These data suggest that ZIP4 specifically upregulates RAB27B, a key regulator of EV release 24,37.Consequently, ZIP4 knockdown reduced AsPC-1 cell release of RAB27B/CD9/CD81/AchE-positive EVs that carry HSP70 and HSP90 (Fig. 6C). To understand how ZIP4 regulates RAB27B expression, we conducted database search for potential transcription factor binding sites in the 5’ promoter of the RAB27B gene. Two potential binding motifs of CREB, a zinc finger transcription factor activated by ZIP4 15, 21, were found. Utilizing the chromatin immunoprecipitation (ChIP) assay, we observed ZIP4-enhanced CREB binding to these two motifs in AsPC-1 cells (Fig. 6D), suggesting that ZIP4 upregulates RAB27B through stimulating CREB-regulated RAB27B transcription. To determine whether RAB27B downregulation is responsible for ZIP4 knockdown-attenuated EV release by AsPC-1 cells, we overexpressed RAB27B in ZIP4-knockdown AsPC-1 cells and observed a restoration of high level release of HSP70 and HSP90 (Fig. 7A). Alteration in cellular RAB27B mRNA levels was verified by qPCR (Supplementary Fig. S8A). Further, overexpression of RAB27B restored high-level release of RAB27B-positive EVs that contains HSP70 and HSP90 by ZIP4-knockdown AsPC-1 cells (Fig. 7B and Supplementary Fig. S8B). Thus, we conclude that ZIP4 promotes release of HSP70 and HSP90-positive EVs by pancreatic cancer cells through upregulating CREB-controlled RAB27B expression.
Figure 6. ZIP4 is critical to CREB-regulated RAB27B expression and EV release by pancreatic cancer cells.
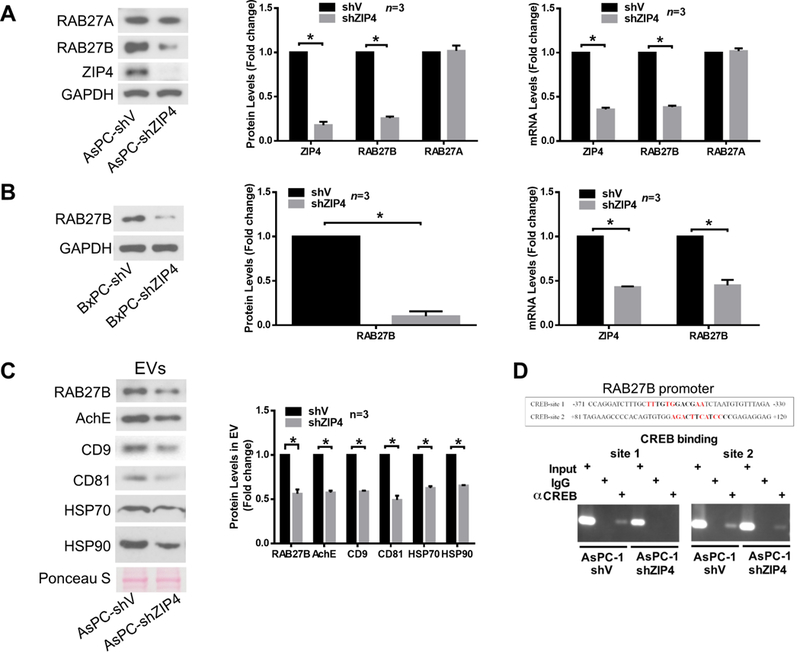
(A) ZIP4 knockdown suppresses RAB27B expression in AsPC-1 cells. RAB27B expression was analyzed by both western blotting and qPCR. (B) ZIP4 knockdown suppresses RAB27B expression in BxPC-3 cells. (C) ZIP4 knockdown decreases HSP70 and HSP90-positive EV release. EVs were isolated from conditioned media of AsPC-shV/shZIP4 cells and analyzed by western blotting for multiple EV markers. Ponseau S staining was used as control for equal loading. (D) ZIP4 knockdown attenuates CREB binding to the RAB27B promoter. ChIP assay was conducted to analyze CREB binding in AsPC-shV/shZIP4 cells to two potential CREB binding motifs (bold letters) identified in the human RAB27B promoter by database search at JASPAR and ConSite. Homologous nucleotides in the binding motifs in mouse RAB27B promoter are shown in red.
Figure 7. RAB27B mediates ZIP4 stimulation of HSP70 and HSP90-positive EV release by pancreatic cancer cells.
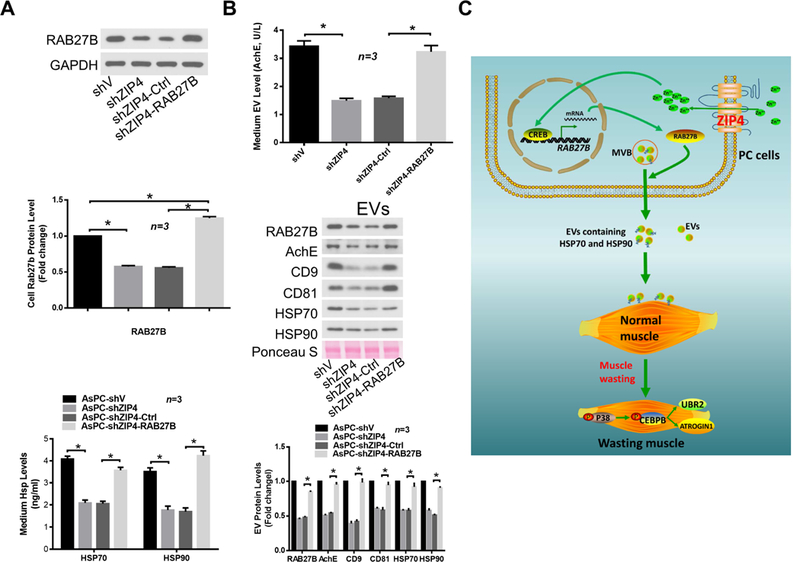
AsPC-shV/shZIP4 cells were transduced with lentivirus encoding RAB27B as described in Materials and Methods. (A) Overexpression of RAB27B restores high-level HSP70 and HSP90 release by ZIP4-knockdown AsPC-shZIP4 cells. RAB27B overexpression was verified by western blotting (left panel). HSP70 and HSP90 release was measured by ELISA (right panel). (B) Overexpression of RAB27B restores high-level EV release by AsPC-shZIP4 cells. EV release levels are shown in left panel. EV markers, HSP70, HSP90 and RAB27B content in isolated EVs were examined by western blotting (middle and right panel). (C) Schematic diagram of the mechanism for ZIP4 promoting pancreatic cancer-associated muscle wasting by stimulating RAB27B-mediated release of HSP70 and HSP90-positive EVs.
Discussion
In this study, we found that ZIP4 knockdown combined with surgical resection significantly increases survival of pancreatic cancer in an orthotopic xenograft mouse model. Cancer cachexia and muscle wasting were also significantly reduced by knocking down ZIP4 in pancreatic cancer cells. Further molecular analysis revealed that ZIP4 promotes cachexia and muscle wasting through stimulating RAB27B-mediated release of HSP70 and HSP90-positive EVs that stimulate p38MAPK-mediated muscle catabolism (Fig. 7C). These results strongly suggest a potential therapeutic strategy using the combination of surgical resection and ZIP4 knockdown to ameliorate pancreatic cancer cachexia and improve patient outcome.
Despite progress on adjuvant therapy in the last decades, surgical resection is still the only curative treatment for human pancreatic cancer; however, a reliable surgical mouse model has only been recently developed to facilitate studies using surgical resection and adjuvant therapies for pancreatic cancer. Specifically, we established an orthotopic xenograft mouse model for imaging-guided curative surgical resection of pancreatic cancer, and found that surgical resection at early stage increases overall survival and quality of life in mice. This model system appears useful for testing and identifying effective adjuvant therapies 26. In the current study, we found that combining surgical resection with knockdown ZIP4, a key master switch gene in pancreatic cancer, significantly prolongs survival in mice. This was done in two different pancreatic cancer cell lines, which have different malignant features. ZIP4 promotes pancreatic cancer cell proliferation, migration, invasion, and tumor growth in vivo 14,21, blocking of ZIP4 expression in pancreatic cancer inhibits tumor growth, and may offer synergistic benefit for other therapies such as surgery and chemotherapy through sensitizing pancreatic cancer cells to other treatments. In this study, we found that ZIP4 knockdown alone did not significantly reduce the primary tumor size and tumor weight at the time of surgery, while reduced ZIP4 combined with surgical resection significantly increased survival of pancreatic cancer-bearing mice, which could not be achieved by surgical resection alone or ZIP4 knockdown alone. The combination therapy also significantly reduced cancer cachexia as evidenced by less body weight loss in the low ZIP4 group, as well as better differentiation and smaller volume of the tumors. These results strongly indicate that ZIP4 plays a critical role in pancreatic cancer progression and prognosis, and may also promote cancer cachexia. ZIP4 knockdown combined with surgical resection effectively controls cancer cachexia and increases survival. Future studies are warranted to include multiple therapies such as chemotherapy in a combinational regimen to treat metastatic pancreatic cancer.
In addition to the routine monolayer pancreatic cancer cell culture, we also used two different approaches of organotypic culture system to make 3D culture of the same pancreatic cancer cells: the methylcellulose (MC) based hanging drop 3D cancer spheroids 25, and the classical matrigel based 3D organoid culture 38, which contains the slow growing cancer cells and mimic the structure of the tumor that could better represent the pancreatic cancer tumor microenvironment than 2D cell culture 39 Results from these spheroid/organoid system correlate with the 2D cell monolayer very well, indicating that there is a direct link between cachexins released by ZIP4 high pancreatic cancer cells and C2C12 myotube catabolism. Cachexia is seen in the majority of human pancreatic cancer patients, and is associated with worse postoperative outcome after pancreatoduodenectomy in human pancreatic cancer.
Cachexia management is a key to the success of pancreatic cancer treatment; however, little is known about the etiology of and there is no established treatment for pancreatic cancer cachexia 40,41 Very few studies have been done to investigate the impact of adjuvant therapy and surgery on cancer cachexia and muscle wasting in pancreatic cancer 42, 43. In this study, we found that tumor removal plus ZIP4 knockdown stabilized body weight. Specifically, knockdown of ZIP4 in pancreatic cancer cells ameliorated muscle wasting in mice as indicated by higher muscle mass, decreased protein degradation, and preserved myofiber cross-sectional area. These results suggest that surgical resection combined with ZIP4 knockdown can effectively reduce cancer cachexia characterized by muscle wasting, which could potentially increase the overall survival and quality of life of pancreatic cancer patients.
There have been few studies on the mechanism of pancreatic cancer-induced muscle wasting, due largely to a lack of appropriate animal models. The orthotopic xenograft model we used here mimics pancreatic cancer in humans more closely without totally destroying pancreas functions, as compared to the original KPC mouse model with knock-in pancreas specific conditional alleles KRASG12D and TP53R172H 44, 45. Utilizing this model, the present study uncovers that pancreatic cancer stimulates p38MAPK-mediated muscle catabolism similar to other cancers shown previously 11, 46 Particularly, in pancreatic cancer high-level expression of ZIP4 promotes the release of HSP70 and HSP90-positive EVs to activate p38MAPK-mediated muscle catabolism. In keeping with this concept, we observed that mice implanted with BxPC-3 cells, which express a lower level of ZIP4 than AsPC-1 cells, survived longer (Fig. 1). Given that not all cancer cells express high levels of ZIP4, the mechanism through which ZIP4 promotes the release of HSP70 and HSP90-positive EVs could be exploited for intervening cachexia associated with specific types of cancer that express high levels of ZIP4 such as pancreatic cancer.
Although intracellular HSP70 and HSP90 are molecular chaperons that protect cells against stressful stimuli, extracellular HSP70 and HSP90 are recognized as danger-associated molecular patterns (DAMPs) that activates Toll-like receptors 47 Activation of TLR4 in skeletal muscle by lung carcinoma 48 or extracellular HSP70 and HSP90 23 leads to activation of p38MAPK-mediated muscle protein degradation pathways. The cancer milieu is highly complex. Many humoral factors had been shown to contribute to cancer cachexia including inflammatory cytokines that promote muscle catabolism 49. We discovered recently that HSP70 and HSP90-mediated TLR4 activation is required for the elevation of circulating inflammatory cytokines in mice bearing lung carcinoma, indicating that cancer-associated increase of circulating inflammatory cytokines is primarily an HSP70 and HSP90-mediated host response to tumor 23.
Importantly, we show here that pancreatic cancer release of EVs is augmented by ZIP4 through CREB-mediated upregulation of RAB27B. The previously uncharacterized ZIP4-CREB-RAB27B signaling pathway identified in pancreatic cancer cells may explain why pancreatic cancer is more potent than many other cancers in its capacity of inducing muscle wasting, i.e., due to high-level expression of ZIP4. Little is known about the regulation of EV release by cancer cells. In this regard, our findings that ZIP4 promotes EV release by upregulating RAB27B expression is highly significant. RAB27B is a GTPase that is required for EV release to the extracellular space through the multivesicular body (MVB) 24 Particularly, RAB27B is expressed in cancer-released EVs and required for HSP90a release 50. However, how RAB27B is regulated remained unknown. The current study uncovers an uncharacterized role of ZIP4 in regulating cancer cell release of EVs by regulating RAB27B expression through zinc-sensitive transcription factor CREB.
Elevated expression of RAB27B has been observed in pancreatic, breast, bladder and liver cancers, and is a prognostic marker associated with aggressive behavior 36, 51–53. Our data indicate that elevation in RAB27B promotes release of HSP70 and HSP90-positive EVs that causes cachexia. Cancer-released EVs serve multiple purposes through various signaling molecules they carry, including promoting metastasis 54, 55 and modulating immune cell activities 28. In addition, pancreatic cancer stimulates lipolysis through releasing adrenomedullin via EVs, which may contribute to the fat tissue loss associated with cachexia 56. Thus, RAB27B may promote the aggressive behaviors of cancer by increasing release of EVs that have multiple functions.
Taken together, our data uncover a role of ZIP4 in promoting pancreatic cancer-associated muscle wasting by stimulating RAB27B-mediated release of HSP70 and HSP90-positive EVs. On the basis of above findings, we propose that a combination of surgical resection and ZIP4 suppression could be a promising therapeutic strategy for metastatic pancreatic cancer.
Supplementary Material
Acknowledgments
Grant support:
This work was supported in part by the National Institutes of Health (NIH) grant R01CA203108 (Li M and Li Y-P), the William and Ella Owens Medical Research Foundation (Li M), and by NIH R01 AR063786 (Li Y-P).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–10. [DOI] [PubMed] [Google Scholar]
- 3.Kiagia M, Syrigos KN, Saif MW. Quality of life in patients with pancreatic cancer. JOP 2014;15:317–8. [DOI] [PubMed] [Google Scholar]
- 4.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95. [DOI] [PubMed] [Google Scholar]
- 5.Martignoni ME, Kunze P, Friess H. Cancer cachexia. Mol Cancer 2003;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin L Diagnostic criteria for cancer cachexia: data versus dogma. Curr Opin Clin Nutr Metab Care 2016;19:188–98. [DOI] [PubMed] [Google Scholar]
- 7.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–66. [DOI] [PubMed] [Google Scholar]
- 8.Ronga I, Gallucci F, Riccardi F, et al. Anorexia-cachexia syndrome in pancreatic cancer: recent advances and new pharmacological approach. Adv Med Sci 2014;59:1–6. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto Y, Hanna DL, Zhang W, et al. Molecular Pathways: Cachexia Signaling-A Targeted Approach to Cancer Treatment. Clin Cancer Res 2016;22:3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YP, Chen Y, John J, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 2005;19:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang G, Jin B, Li YP. C/EBPbeta mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. EMBO J 2011;30:4323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bye A, Wesseltoft-Rao N, Iversen PO, et al. Alterations in inflammatory biomarkers and energy intake in cancer cachexia: a prospective study in patients with inoperable pancreatic cancer. Med Oncol 2016;33:54. [DOI] [PubMed] [Google Scholar]
- 13.Fukawa T, Yan-Jiang BC, Min-Wen JC, et al. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med 2016;22:666–71. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Zhang Y, Liu Z, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A 2007;104:18636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Yang J, Cui X, et al. A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth. EMBO Mol Med 2013;5:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Chen C, Yao Q, et al. ZIP4 upregulates the expression of neuropilin-1, vascular endothelial growth factor, and matrix metalloproteases in pancreatic cancer cell lines and xenografts. Cancer Biol Ther 2010;9:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X, Zhang Y, Yang J, et al. ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer. Cell Cycle 2014;13:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Wallace MB, Yang J, et al. ZIP4 is a novel diagnostic and prognostic marker in human pancreatic cancer: a systemic comparison between EUS-FNA and surgical specimens. Current molecular medicine 2014;14:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Zhang Y, Cui X, et al. Gene profile identifies zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Current molecular medicine 2013;13:401–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Zhang Y, Bharadwaj U, et al. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res 2009;15:5993–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Bharadwaj U, Logsdon CD, et al. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. Clin Cancer Res 2010;16:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Sun X, Yang J, et al. ZIP4 silencing improves bone loss in pancreatic cancer. Oncotarget 2015;6:26041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Liu Z, Ding H, et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun 2017;8:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature cell biology 2010;12:19–30; sup pp 1–13. [DOI] [PubMed] [Google Scholar]
- 25.Ware MJ, Colbert K, Keshishian V, et al. Generation of Homogenous Three-Dimensional Pancreatic Cancer Cell Spheroids Using an Improved Hanging Drop Technique. Tissue Eng Part C Methods 2016;22:312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni X, Yang J, Li M. Imaging-guided curative surgical resection of pancreatic cancer in a xenograft mouse model. Cancer Lett 2012;324:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle A, Zhang G, Abdel Fattah EA, et al. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J 2011;25:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalmin F, Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. The Journal of clinical investigation 2010;120:457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruggeman AR, Kamal AH, LeBlanc TW, et al. Cancer Cachexia: Beyond Weight Loss. J Oncol Pract 2016;12:1163–1171. [DOI] [PubMed] [Google Scholar]
- 30.Liu M, Yang J, Zhang Y, et al. ZIP4 Promotes Pancreatic Cancer Progression by Repressing ZO-1 and Claudin-1 through a ZEB1-Dependent Transcriptional Mechanism. Clin Cancer Res 2018;24:3186–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuzhat Z, Kinhal V, Sharma S, et al. Tumour-derived exosomes as a signature of pancreatic cancer - liquid biopsies as indicators of tumour progression. Oncotarget 2017;8:17279–17291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gastpar R, Gehrmann M, Bausero MA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer research 2005;65:5238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogata M, Naito Z, Tanaka S, et al. Overexpression and localization of heat shock proteins mRNA in pancreatic carcinoma. Journal of Nippon Medical School = Nippon Ika Daigaku zasshi 2000;67:177–85. [DOI] [PubMed] [Google Scholar]
- 34.Lotvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto Y, Kano M, Akutsu Y, et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol Rep 2016;36:2535–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrix A, Maynard D, Pauwels P, et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst 2010;102:866–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostenfeld MS, Jeppesen DK, Laurberg JR, et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res 2014;74:5758–71. [DOI] [PubMed] [Google Scholar]
- 38.Froeling FE, Marshall JF, Kocher HM. Pancreatic cancer organotypic cultures. J Biotechnol 2010;148:16–23. [DOI] [PubMed] [Google Scholar]
- 39.Baker LA, Tiriac H, Clevers H, et al. Modeling pancreatic cancer with organoids. Trends Cancer 2016;2:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller TC, Burmeister MA, Bachmann J, et al. Cachexia and pancreatic cancer: are there treatment options? World J Gastroenterol 2014;20:9361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palesty JA, Dudrick SJ. What we have learned about cachexia in gastrointestinal cancer. Dig Dis 2003;21:198–213. [DOI] [PubMed] [Google Scholar]
- 42.Bachmann J, Heiligensetzer M, Krakowski-Roosen H, et al. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193–201. [DOI] [PubMed] [Google Scholar]
- 43.Elmaci I, Altinoz MA. A Metabolic Inhibitory Cocktail for Grave Cancers: Metformin, Pioglitazone and Lithium Combination in Treatment of Pancreatic Cancer and Glioblastoma Multiforme. Biochem Genet 2016;54:573–618. [DOI] [PubMed] [Google Scholar]
- 44.Habbe N, Shi G, Meguid RA, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A 2008;105:18913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook N, Olive KP, Frese K, et al. K-Ras-driven pancreatic cancer mouse model for anticancer inhibitor analyses. Methods Enzymol 2008;439:73–85. [DOI] [PubMed] [Google Scholar]
- 46.Puppa MJ, Gao S, Narsale AA, et al. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2014;28:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang G, Liu Z, Ding H, et al. Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways. Sci Rep 2017;7:2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKay CJ, Glen P, McMillan DC. Chronic inflammation and pancreatic cancer. Best Pract Res Clin Gastroenterol 2008;22:65–73. [DOI] [PubMed] [Google Scholar]
- 50.Hendrix A, Sormunen R, Westbroek W, et al. Vacuolar H+ ATPase expression and activity is required for Rab27B-dependent invasive growth and metastasis of breast cancer. Int J Cancer 2013;133:843–54. [DOI] [PubMed] [Google Scholar]
- 51.Zhao H, Wang Q, Wang X, et al. Correlation Between RAB27B and p53 Expression and Overall Survival in Pancreatic Cancer. Pancreas 2016;45:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho JR, Chapeaublanc E, Kirkwood L, et al. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS One 2012;7:e39469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong WW, Mou Q, Chen J, et al. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J Gastroenterol 2012;18:1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eustace BK, Sakurai T, Stewart JK, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nature cell biology 2004;6:507–14. [DOI] [PubMed] [Google Scholar]
- 55.McCready J, Sims JD, Chan D, et al. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC cancer 2010;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sagar G, Sah RP, Javeed N, et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 2016;65:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


