Abstract
Polychlorinated biphenyls (PCBs) have been detected as prevalent environmental contaminants in water, food and biota. Previous studies in vitro have shown that a variety of sorbent materials, including carbon, can sorb PCBs; however, PCB sorbents that can be added to food or drinking water to decrease toxin bioavailability in humans and animals have not been reported. To address this problem, we have developed a broad-acting and highly effective sorbent for PCBs using montmorillonite clays reported to be safe for consumption in animals and humans. In this study, calcium montmorillonite clays were acid processed (APMs) and the interactions of six PCB congeners (PCB 77, 126, 153, 157, 154 and 155) on the surfaces of APMs were characterized. Computational models and isothermal analyses were used to derive surface capacities and affinities, delineate mechanisms and predict the thermodynamics of sorption. To confirm the safety and predict the efficacy of APMs against individual PCBs and common mixtures (Aroclors 1254 and 1260), we have also used a living organism (Hydra vulgaris) that is sensitive to toxins. APMs significantly protected hydra against the toxicity of PCBs and Aroclors. This finding was supported by studies showing tight binding; high capacity, affinity, and enthalpy; and a low therapeutic dose.
Keywords: Polychlorinated biphenyls, Aroclors, Montmorillonite clay, Adsorption, Hydra assay
Capsule
APMs were shown to be broad-acting sorbents that can tightly bind PCBs and mixtures of PCBs, mitigating toxicity in a living organism and reducing potential exposures to humans and animals.
Graphical Abstract
INTRODUCTION
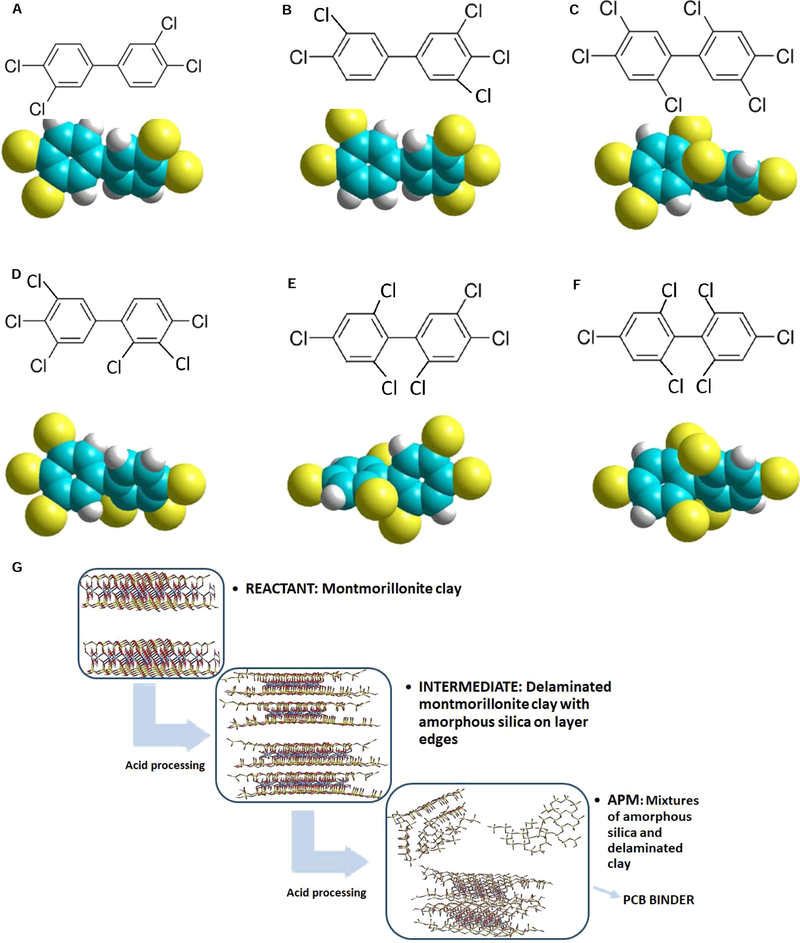
Polychlorinated biphenyls (PCBs) are complex mixtures of isomers and congeners that were marketed as commercial products based on their percentage of chlorine composition. Their physical and chemical properties such as low vapor pressure, low aqueous solubility, and inertness to water, acid, alkali and heat contribute to their extreme persistence in the environment, which results in their bioaccumulation and sustainability in food chains, especially fish and aquatic species (Fadaei et al., 2015). Because of their extensive usage from the 1930s to the 1970s, significant levels of PCBs can be found in all components of the global ecosystem. Their contamination can be enhanced during events such as hurricanes, floods, heavy rain, and storms. These events can result in mobilization and redistribution of PCB contaminated sediments, thus enhancing exposures and adverse health impacts in vulnerable populations at the site of disasters. Coplanar PCB congeners such as PCB 77 (3,3’,4,4’-tetrachlorobiphenyl) and PCB 126 (3,3’,4,4’,5-pentachlorobiphenyl) are non-ortho substituted and dioxin-like PCBs (Fig. 1A and B) (Safe, 1994). Non-coplanar PCB congeners such as PCB 153 (2,2’,4,4’,5,5’-hexachlorobiphenyl, di-ortho-substituted) are ortho substituted that do not exhibit dioxin-like toxicities and these compounds are detected at higher levels than the dioxin-like PCBs (Fig. 1C). PCB 153 is among the most predominant of the PCBs found in human tissue and is sometimes used as an indicator for the total human PCB-burden (Johansson et al., 2006). To investigate the structural mechanism of PCB adsorption, hexachlorobiphenyl PCBs, other than PCB 153, have also been studied, including PCB 157 (2,3,3’,4,4’,5’-hexachlorobiphenyl, mono-ortho-substituted), PCB 154 (2,2’,4,4’,5,6’-hexachlorobiphenyl, tri-ortho-substituted) and PCB 155 (2,2’,4,4’,6,6’-hexachlorobiphenyl, tetra-ortho-substituted) (Fig. 1D–F). These PCB congeners have different toxicological, structural and chemical properties based on variations in the amount and position of chlorine substitutions around the biphenyl rings, and they represent model substances for the different classes of PCBs.
Figure 1.
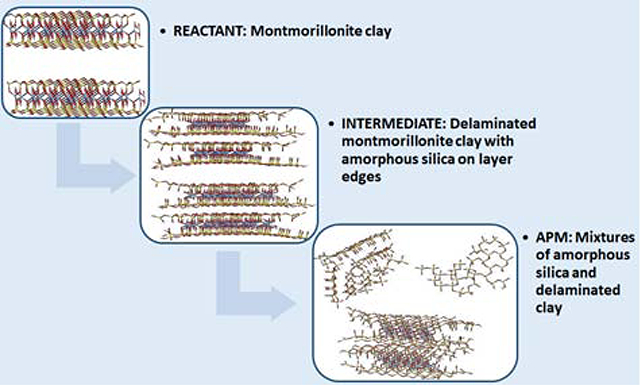
Chemical structures and molecular models of PCB 77 (A), 126 (B), 153 (C), 157 (D), 154 (E), and 155 (F) illustrating the spatial orientation and dihedral angles (carbon = cyan; hydrogen = white; chlorine = yellow). Energy minimized molecular models of parent montmorillonite versus APM products (G) (oxygen = red; silicon = yellow; aluminum = cyan).
Due to the lipophilic nature of PCBs, they tend to be strongly sorbed by soils and sediments, resulting in widespread PCB contaminated sediments. Studies have reported that activated carbon is the most effective sorbent for PCBs and is used in remediation of PCB contaminated soil, but adsorption isotherms for PCBs by activated carbon in water suggest a weak physisorption activity, although the thermodynamics of this interaction have not been reported (Fairey et al., 2010, McDonough et al., 2008). Additionally, incomplete combustion contributes to the formation of polycyclic aromatic hydrocarbons (PAHs) and other hazardous organic contaminants, which limits the use of carbon as a toxin enterosorbent for human and animal consumption (Mohammad-Khah and Ansari, 2009). Previous studies have shown that soil organic matter (SOM) in clay-based materials is the major sorptive component in soils and sediments for PCBs, however, the primary interaction of PCBs with these clay minerals is thought to be a partitioning mechanism, instead of stable and saturable binding at specific sites (Hiraizumi et al., 1979, Liu et al., 2015). Through physisorption mechanisms, PCBs bound to these types of clays, are attached to the surface of the adsorbent through weak interactions, with an enthalpy (heat of sorption) equal to less than −20 kJ/mol. Olestra™, a nonabsorbable fat substitute for potato chips, has been reported to decrease PCBs in rats and humans (Geusau, et al., 1999, Jandacek et al., 2014). However, it was banned from food markets in the U.K. and Canada due to side effects in the gastrointestinal track and weight gain (Nestle, 1998, Swithers et al., 2011). Therefore, the objective of this study was to develop a toxin binding clay with high capacity and enthalpy for PCBs that can reduce human and animal exposures when included in food or water. PCB binding enterosorbents can be delivered before each meal in the form of powder, pills, capsules, tablets, sachets, vitamins, snacks, or flavored water to decrease the frequent exposure of humans to PCBs through contaminated fish and seafood.
Previously, montmorillonite clays have been shown to be safe for consumption in animals and humans based on multiple animal interventions and six clinical trials in the US and Africa (Phillips et al., 2019). To develop safe and effective sorbents for PCBs, montmorillonite clays were processed with sulfuric acid (APMs), resulting in the enhancement of active surface area and porosity. The treatment of montmorillonite clays with high concentrations of acid facilitates the replacement of interlayer cations with protons, following the gradual dissociation of tetrahedral and octahedral layers in the clay structure. The final APM product is a mixture of delaminated montmorillonite layers along with amorphous silica chains, amorphous silica, and cross-linked silica (Fig. 1G) (Komadel et al., 1990, Madejova et al., 1998, Tyagi et al., 2006). A pH of 3 in the final product is similar to the pH of acidic foods, such as chocolate, cheese, and meat. In fact, acidic food additives can commonly enhance taste and appetite, and increase palatability and consumption (Deshpande et al., 2015). Additionally, sulfuric acid is permitted in food and feed to adjust pH (Government of Canada, 2017). Thus, APMs for short-term treatment at low inclusion levels in the diet of humans and animals should be safe.
In this study, we have characterized the sorption of six different PCBs onto APMs and investigated thermodynamic and structural mechanisms of sorption. Computational models and isothermal analyses were used to delineate mechanisms and to predict the thermodynamics of sorption. Equilibrium isothermal adsorption and dosimetry studies were conducted to determine sorption parameters and to investigate: 1) sorption capacities and affinities, 2) thermodynamics of toxin/surface interactions, 3) estimated dose of sorbent required to maintain toxin threshold limits, and 4) potential structural mechanisms of sorption. We have also used Hydra vulgaris (a living organism) to confirm the safety and predict the efficacy of APMs against individual PCB congeners as well as commercial Aroclors. Aroclors are complex mixtures of PCBs that were extensively used as dielectric fluids in transformers, plasticizers, and heat-exchange fluids. Among these, Aroclors 1254 and 1260 were the most commonly used in the US; they are toxic to laboratory animals (Albro et al., 1981, ATSDR, 2010, EPA, 1989, Mayes et al., 1998), and thus were chosen for studies in the hydra assay.
MATERIALS AND METHODS
Materials and reagents
Parent montmorillonite used in this study is available from BASF in Lampertheim, Germany with a total surface area of approximately 850 m2/g and an external surface area of 70 m2/g (Grant and Phillips, 1998). The generic formula is: (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·nH2O. Samples of the clay contain orthoclase feldspars, calcite, mica, quartz, and sanidine as impurities (Phillips et al., 2008). Coconut shell activated carbon was purchased from General Carbon Corporation (Paterson, NJ). This material was selected because it is widely used for the removal of organic compounds and it is suitable for drinking water and food grade applications. Reagents including high pressure liquid chromatography (HPLC) grade acetonitrile and pH buffers were purchased from VWR (Atlanta, GA). Sulfuric acid (H2SO4, 95–98%) was purchased from Aldrich Chemical Co. (Milwaukee, WI). PCB congeners and Aroclors (with a purity > 99%) were gifts from Dr. Stephen Safe’s laboratory at Texas A&M University (College Station, TX) (Mullin et al., 1981, 1984). Ultrapure deionized water (18.2 MΩ) was generated by an Elga™ automated filtration system in the lab (Woodridge, IL).
Synthesis of APMs was previously described by Wang, et al (2019). Briefly, montmorillonite was treated with sulfuric acid to derive 12 and 18 normality. The clay suspensions were mixed and maintained at 60°C overnight. The slurry was centrifuged at 2000 g for 20 min and rinsed thoroughly with distilled water until constant pH was reached. All products were desiccated at 110°C overnight and sieved through 125 μm to achieve uniform size before use (Neji et al., 2011, Resmi et al., 2012). The acid process results in an increase in surface acidity and an increase in surface area, ranging from 1172 m2/g to 1213 m2/g. It is higher than coconut shell activated carbons (average area of 1100 m2/g) and the parent montmorillonite clay (average area of 850 m2/g). The process also produces permanent mesoporosity in the clay structure and reduces metal ions in the interlayer, which partially delaminates the clay. During the strong acid treatment, a large increase in pore volume and an increase in larger pore size distribution were observed (Amari et al., 2018). FTIR and SEM data for similar products have been reported (Amari et al., 2018; Mekewi et al., 2016; Tyagi et al., 2006).
Adsorption isotherms
The toxin stock solutions were separately prepared by dissolving pure crystals into acetonitrile to yield 15 ppm (μg/mL) PCB solutions. We used acetonitrile for isothermal analysis because: 1) the solubility of PCBs is limited in water, 2) acetonitrile is a “polar aprotic solvent” that does not affect the interlayer structure of montmorillonite clays, and 3) acetonitrile does not interfere with the UV absorbance of PCBs like DMSO. In vitro studies have demonstrated that acetonitrile is an effective solvent for the delivery of lipophilic toxins to clay surfaces, and can be used to determine theoretically “maximum” capacities and affinities of toxin-saturated sites. These studies provide valuable insight and information about potential binding sites and mechanisms of surface interaction for PCBs. Importantly, supplemental (in vivo) studies with PCBs have confirmed the binding efficacy of these toxins that were predicted from isotherm results with acetonitrile.
The isotherm methodology was previously described in our laboratory (Grant and Phillips, 1998). Briefly, 0.001% sorbents were added to an increasing gradient of toxin solutions in disposable glass tubes. The toxin gradient was prepared by mixing a calculated amount of toxin stock solution with a complementary volume of acetonitrile. The 0.001% sorbent was obtained by adding 10 μL of 2.0 mg/mL clay suspension with vigorous stirring. Additionally, we tested 3 control groups including acetonitrile, toxin stock solution and 0.001% sorbent in acetonitrile. In all treatments, tubes were capped and agitated at 1000 rpm on shakers for 2 h at ambient temperature (26 °C) and body temperature (37 °C) for thermodynamic experiments. This was followed by centrifugation at 2000 g for 20 min to separate the clay/toxin complex from solution. The detection and quantitation method using UV-visible scanning spectrophotometry has been well-established for single PCB congeners and Aroclors. This method has been widely used to accurately quantitate PCB 77 at 260.9 nm, PCB 126 at 264.5 nm, PCB 153 and 154 at 207.2 nm, PCB 154 at 280 nm, and PCB 157 at 254.9 nm (Andersson et al., 1997, Grant and Phillips, 1998, Hutzinger et al., 1974, Wang et al., 2017, 2019).
The highest limit of detection (LOD) was 0.5 ppm for six individual PCB congeners, which was below the concentration gradient of PCBs for isothermal analysis (i.e., 0.75 ppm – 15 ppm). Standard toxin solutions were spiked before and after 2 h of agitating and the relative standard deviations (RSD) were < 4%, indicating a high recovery percentage and limited nonspecific binding. The detection methods were validated using standard calibration curves. Standard solutions of PCBs were prepared in acetonitrile at concentration gradients between 0.25 ppm and 20 ppm and these were used to plot standard curves with high linear correlations (r2 > 0.99).
Data calculations and curve fitting
From the equilibrium isotherms, the toxin concentration in solution (x-axis) was determined using a scanning UV-visible spectrophotometer. The amount of adsorbed toxin at each concentration gradient was calculated from the concentration difference between control and test groups. More specifically, the amount bound (mol/kg) was calculated from the difference between free toxin in the test solution and the control groups divided by the mass of the clay added. The Langmuir equation was used to plot equilibrium isotherms for the toxin/clay binding reactions; the heat of toxin sorption to clay surfaces (ΔH) was derived from the Van’t Hoff equation by applying individual Kd values at temperatures of 26 °C and 37 °C as previously described (Wang et al., 2017, 2019).
Dose of sorbents required to maintain threshold limits of PCBs
PCBs (especially those with 4, or fewer, chlorines) can enter drinking water by runoff from landfills or industrial waste discharge or industrial incinerators (ATSDR, 2000, 2011; OEHHA, 2007; USEPA, 2014). The maximum contamination level for PCBs in drinking water has been set as 0.5 ppm by USEPA (comptox.epa.gov/dashboard; EPA, 2012). In dosimetry studies, 1 ppm PCB test samples representing twice the threshold limits, were prepared from stock solutions. Sorbents were added to 2.0 mL of toxin solution resulting in 0.005, 0.02, 0.05, 0.1, 0.5 mg sorbent/mL. Control groups included PCB stock solutions. All groups were agitated at 1000 rpm for 2 h and centrifuged at 2000 g for 20 min. Aliquots of toxin were measured by UV-Visible scanning spectrophotometry and free toxin concentrations were calculated. The toxin sorption percentage was determined by the difference between test and control groups. Algorithms were derived by methods previously described for other toxins by Wang et al. (2019). This procedure was used in this study to predict therapeutic doses of test sorbents for PCBs.
Hydra assay
Earlier work with Hydra vulgaris in culture has shown that this organism can be utilized to screen contaminated water and environmental samples due to its pronounced susceptibility to toxins. In extensive studies in our laboratory, we have used Hydra vulgaris to confirm the toxicity of diverse chemicals (i.e., mycotoxins, pesticides, PAHs, commercial solvents, plasticizers and metals) and to predict the detoxification of contaminated solutions. In preliminary work with hydra, whole-log concentrations of individual chemicals were exposed to determine potency. The minimum effective dose was determined by bracketing the toxic endpoint to the nearest tenth (1/10) of a log (Mayura et al., 1991). Importantly, we have used this organism to select optimal sorbents for food-borne and environmental toxins, prior to safety and efficacy studies in animals and humans. In these studies, the hydra assay has significantly confirmed our in vitro, in vivo, and in silico results.
Hydra vulgaris were obtained from Environment Canada (Montreal) and kept at 18 °C. A morphological method was used to describe adult hydra and classify the adverse effects of toxins (Wilby et al., 1990). In the hydra assay, mature and non-budding hydra of similar size were tested to minimize differences between samples. Toxin treatment groups included 40 ppm PCB 77, 30 ppm PCB 126, 30 ppm PCB 153, 20 ppm Aroclor 1260 and 20 ppm Aroclor 1254 in aqueous hydra media with 1% DMSO (based on predetermined toxicity levels at 92 h). The hydra method was used to confirm the toxicity of PCBs and the ability of test sorbents to protect a living organism from these toxins in aqueous solution. All sorbent/toxin mixtures were shaken at 1000 rpm for 2 h and centrifuged at 2000 g for 20 min, prior to exposure of hydra to toxins in Pyrex dishes. Three adult hydra in each group were added to 4.0 mL of test media and maintained at 18 °C. The monitoring times were at shorter intervals during the first two days and 24 h intervals for the last three days (0, 4, 20, 28, 44, 68, and 92 h). The hydra morphological response was scored from 0–10, where 10 indicated healthy hydra and 0 indicated lethality following treatment. Toxicity was calculated from the average score for morphological changes at a specific time point for each group.
Molecular model protocols
The molecular models for PCBs and APMs were drawn in ISIS Draw 2.0 (MDL Information Systems, Inc., Hayward, California) and then imported into HyperChem 8.0. All chemical structures were energy-minimized using the semiempirical quantum mechanical AM1 method. The unit cell coordinates of muscovite were used to construct the models (Richardson and Richardson, 1982). These coordinates were then converted to orthogonal coordinates and the unit cells defining the clay structure were replicated in three-dimensional space using the symmetry operations for a C2/c space group (Donnay, 1952). The d001 spacing of the clay model was then set to 21 Å (Greenland and Quirk, 1960). The molecular conformations of PCBs were constructed with the fixed dihedral angles between the biphenyl rings, thus allowing strain energies between adjacent atoms to be minimized.
Statistical analysis
Statistical significance was calculated by a two-way t-test. Each experiment was independently triplicated to derive an average and standard deviation. Morphological scores in the hydra assay were included to calculate difference between media control (score 10) and test groups. The t-value was calculated (N = 3) and compared in a p-value table to determine the statistical significance. Results were considered significant at p ≤ 0.05.
RESULTS AND DISCUSSION
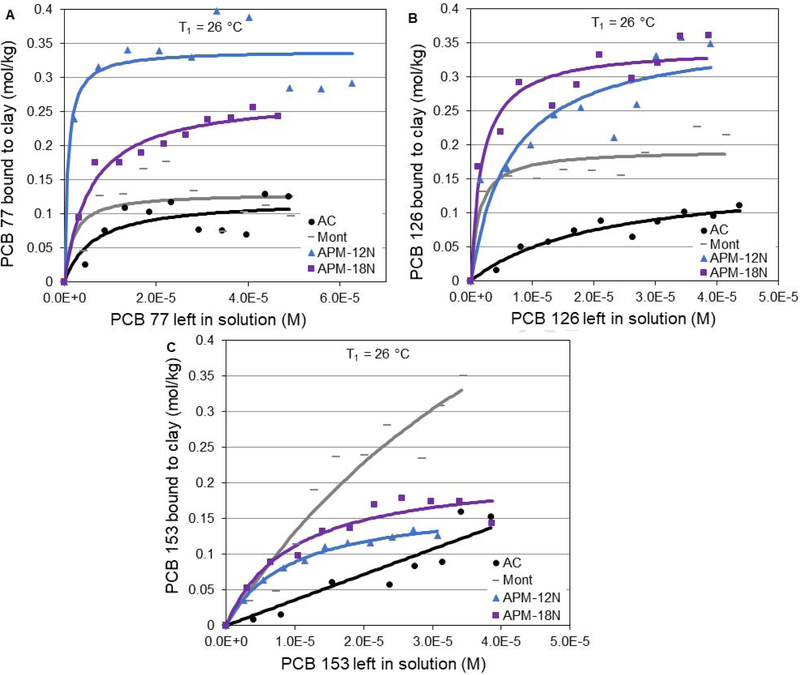
Figure 1 illustrates two common coplanar PCBs, i.e. PCB 77 and 126 (Fig. 1A and B), four hexachlorobiphenyl congeners with variable substitution patterns, i.e. PCB 153, 157, 154, 155 (Fig. 1C–F) and energy minimized molecular models of APMs (Fig. 1G). The isotherms for the sorption of the most dominant PCB congeners found in the environment onto parent montmorillonite, APM clays, and activated carbon at 26 °C are shown in Figure 2. The coplanar PCBs (PCB 77 and 126) exhibited a higher binding capacity (Qmax) and affinity (Kd) than the non-coplanar PCB 153 in the presence of parent montmorillonite clay, or the APMs, or activated carbon. The binding of coplanar PCBs fit the Langmuir model for all three types of sorbents, indicating homogeneous and saturable binding sites for these congeners. Isotherms for the non-coplanar PCBs indicated a partitioning interaction of toxins on the surfaces of montmorillonite clay and activated carbon. Similar PCB sorption trends were previously reported for other organic and inorganic sorbents at lower levels of toxin dissolved in aqueous media, and these studies were consistent with our isotherm results in acetonitrile. The notable difference in sorption effectiveness between the different PCB congeners may be explained by steric effects. The dihedral angles between the biphenyl rings are fixed at the following three values: 44° for PCB congeners without ortho substitution (PCB 77 and 126), 57° for congeners with ortho substitution on one phenyl ring (PCB 157), and 74° for congeners with ortho substitution on both rings (PCB 153, 154 and 155) (Dunnlvant and Elzerman, 1992). This result agrees with previous studies showing that coplanar PCBs showed higher adsorption than non-coplanar PCBs (Liu et al., 2015). Our results (using acetonitrile as the solvent vehicle) showed that the isotherm plots of APMs with PCB 77, 126 and 153 all fit the Langmuir sorption isotherm model (r2 > 0.8), indicating saturable binding sites for PCBs on APM surfaces. The derived Qmax values of APMs increased from the parent montmorillonite and activated carbon for all three PCBs, suggesting improved binding ability of APMs versus parent montmorillonite and carbon; this increase is probably due to 50% higher surface areas (more porosity) and more variations of types of binding sites than that of parent clays and carbon (Wang et al., 2019).
Figure 2.
Langmuir plots of coplanar PCB 77 (A) and PCB 126 (B), and non-coplanar PCB 153 (C) bound to the surfaces of APMs versus parent montmorillonite clays (Mont) and activated carbon (AC) at 26 °C (T1).
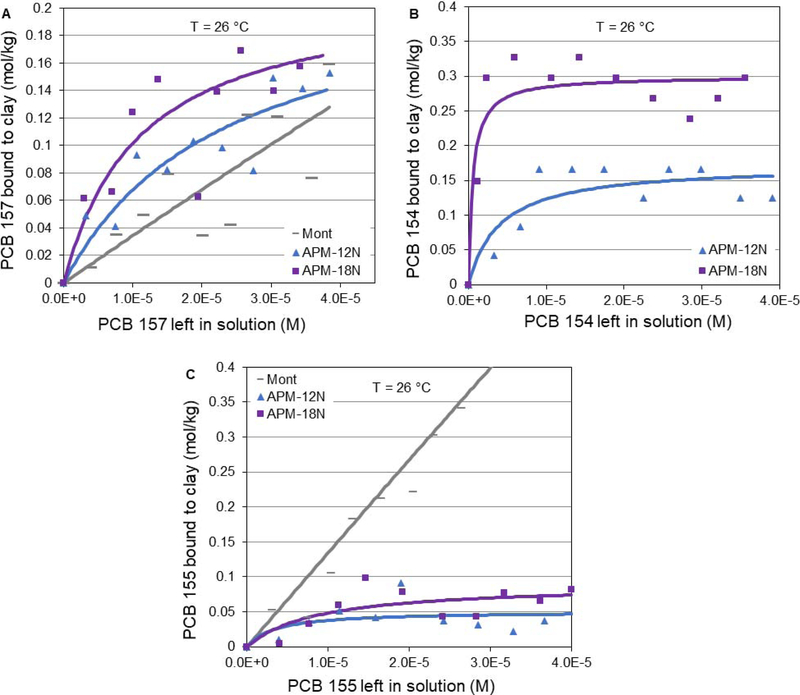
To further investigate the structural mechanism of PCB binding, other hexachlorobiphenyl PCBs were also studied, including PCB 157 (2,3,3’,4,4’,5’-hexachlorobiphenyl, mono-ortho-substituted), PCB 154 (2,2’,4,4’,5,6’-hexachlorobiphenyl, tri-ortho-substituted) and PCB 155 (2,2’,4,4’,6,6’-hexachlorobiphenyl, tetra-ortho-substituted). Although PCB 157 is coplanar, it has mono-ortho-substitution that results in a higher dihedral angle compared to other coplanar PCBs with no ortho substitution (PCB 77 and 126). As illustrated in Figure 3A, isotherms of PCB 157 showed a Freundlich (partitioning) trend onto montmorillonite clay surfaces, indicating less binding sites and tightness compared to the Langmuir trend shown for PCB 77 and 126. APMs increased the binding to a saturable curve that fit the Langmuir model, but its binding capacity was slightly lower than that of PCB 77 and 126. Like PCB 153, PCB 154 also has ortho-substitution on both rings and this results in similar binding isotherm plots with comparable Qmax values (Fig. 3B). PCB 155 has four ortho-chlorine substituents on both rings. Although the dihedral angle is fixed and it is the same as PCB 153 and 154, the isothermal results in Figure 3C showed a significantly decreased Qmax for PCB 155, suggesting that other possible binding mechanisms in addition to the dihedral angle are important. It is possible that the number of ortho chlorine substitution can limit the access to the interlayers or pores of the sorbents due to size of the chlorine atoms. Further studies are warranted to determine the mechanisms of PCB sorption to APMs and parent montmorillonite clays. For all six PCB congeners tested, APM clays showed the highest binding capacities.
Figure 3.
Langmuir plots of hexachlorobiphenyl PCBs, including coplanar PCB 157 (A) and non-coplanar PCB 154 (B) and PCB 155 (C) bound to the surface of APMs versus parent clays (Mont) at 26 °C.
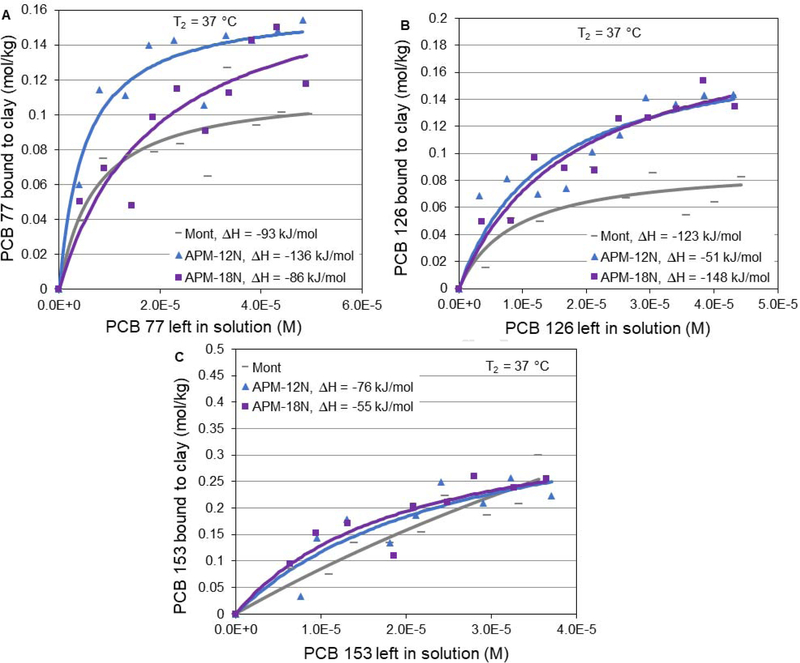
PCBs accumulate in sediments at the bottom of streams, rivers, lakes and coastal areas. These chemicals have also been shown to accumulate in the fatty tissues of fish, shellfish and other animals; in high concentrations, they can pose serious health risks to people who frequently eat contaminated fish and seafood. This problem is magnified during events such as hurricanes, floods, heavy rain, and storms, when PCB contaminated sediments are mobilized and redistributed, thus enhancing exposures and adverse health impacts of these toxin in vulnerable humans and animal populations at the site of disasters. Because fish is an important part of a healthy diet for humans, the ability to limit consumption of PCB-contaminated fish and seafood is highly desirable. Statewide advisories also urge people to limit their consumption of all fish and shellfish from freshwater or coastal areas. For example, Texas Department of State Health Services (DSHS) recently issued a fish possession and consumption ban for various portions of rivers due to high PCB contamination (TCEQ, 2019). Other than seafood, humans are also exposed to PCBs through eating meat and dairy products (Ahmadkhaniha et al., 2017, Chen et al., 2017). PCBs can occur in the fat and viscera of cattle and in milk and eggs. The results of our work suggest that APM could be included in the diet to reduce human and animal exposures to PCBs that are amplified during disasters and flooding. As part of this study, it was important to gain insight into PCB binding mechanisms onto the surfaces of APMs and the thermodynamics of the toxin/surface interactions. To calculate the binding enthalpy of APM clays for PCBs, isotherms were run at 2 different temperatures, i.e., 26 °C (T1) and 37 °C (T2). Calculated enthalpies (ΔH) for APM-12N and APM-18N were equal to −136 kJ/mol and −86 kJ/mol for PCB 77, −51 kJ/mol and −148 kJ/mol for PCB 126, and −76 kJ/mol and −55 kJ/mol for PCB 153, respectively (Fig. 4). These high enthalpy absolute values indicate that the representative coplanar and non-coplanar PCBs are chemisorbed tightly to the APMs, which is consistent with the Langmuir model for the isothermal interaction. A summary of adsorption parameters for six PCB congeners onto surfaces of parent montmorillonite, APMs, and activated carbon is shown in Table 1.
Figure 4.
Langmuir plots of coplanar PCB 77 (A) and PCB 126 (B), and non-coplanar PCB 153 (C) bound to the surfaces of APMs versus parent clays (Mont) at 37 °C (T2).
Table 1.
Summary table of binding parameters for sorbents
| PCB 77 T1 | PCB 77 T2 | PCB 126 T1 | PCB 126 T2 | PCB 153 T1 | PCB 153 T2 | PCB 157 T | PCB 154 T | PCB 155 T | |
|---|---|---|---|---|---|---|---|---|---|
| Mont | Qmax = 0.13 | Qmax = 0.11 | Qmax = 0.19 | Qmax = 0.09 | Kd = 2E5 | Kd = 8E4 | Kd = 1E3 | Qmax = 0 | Kd = 6E2 |
| Kd = 5E5 | Kd = 1E5 | Kd = 8E5 | Kd = 1E5 | Kd = 0 | |||||
| APM-12N | Qmax = 0.34 | Qmax = 0.16 | Qmax = 0.36 | Qmax = 0.18 | Qmax = 0.1 | Qmax = 0.43 | Qmax = 0.23 | Qmax = 0.16 | Qmax = 0.05 |
| Kd = 1E6 | Kd = 2E5 | Kd = 2E5 | Kd = 7E4 | Kd = 1E5 | Kd = 4E4 | Kd = 4E4 | Kd = 3E5 | Kd = 3E5 | |
| APM-18N | Qmax = 0.27 | Qmax = 0.19 | Qmax = 0.34 | Qmax = 0.2 | Qmax = 0.22 | Qmax = 0.39 | Qmax = 0.21 | Qmax = 0.3 | Qmax = 0.09 |
| Kd = 2E5 | Kd = 5E4 | Kd = 5E5 | Kd = 6E4 | Kd = 1E5 | Kd = 5E4 | Kd = 9E4 | Kd = 2E6 | Kd = 1E5 | |
| AC | Qmax = 0.12 | N/A | Qmax = 0.15 | N/A | Kd = 3E2 | N/A | N/A | N/A | N/A |
| Kd = 2E5 | Kd = 5E4 | ||||||||
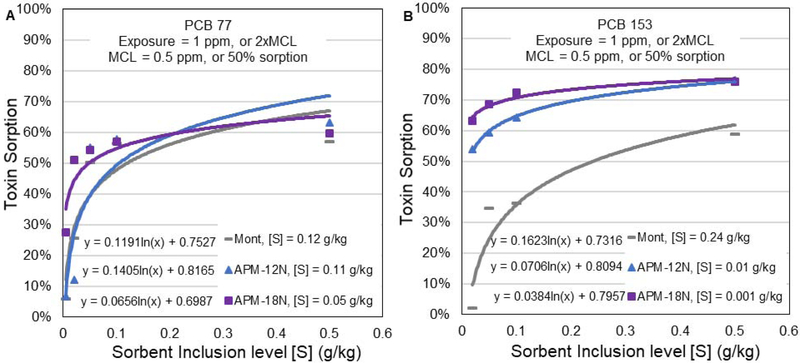
Additionally, we determined a therapeutic dose of APMs required to reduce PCB exposures higher than the action level. The regulatory threshold or maximum contaminant level (MCL) for PCBs in water in the United States is equal to 0.5 ppm. We exposed both coplanar (PCB 77) and non-coplanar (PCB 153) at twice the MCL (1 ppm) to an increasing dose of sorbent treatment from 0.005 g/kg to 0.5 g/kg. An algorithm was derived to calculate the amount of sorbent that would bind 50% of the PCBs, thus maintaining the MCL at 0.5 ppm. The dosimetry results (Fig. 5) showed that the predicted inclusion rates for APM-12N and APM-18N were equal to 0.11 g/kg and 0.05 g/kg (w/w) for PCB 77, and 0.01 g/kg and 0.001 g/kg for PCB 153, respectively. Assuming the average adult has a food intake of 3 kg/day and 3 meals/day, then the appropriate dose of APMs could be delivered in a variety of forms, including powder, snacks, capsules, tablets, vitamin supplements, food, and flavored water. Importantly, APMs can be used to protect people and animals consuming PCB contaminated seafood, meat, dairy products and water at the site of disasters and floods. The low inclusion rates of APMs were consistent with our isothermal results suggesting high capacity binding of PCBs to APM surfaces and confirming that APMs increased binding effectiveness for PCBs more than the parent montmorillonite clay. This data could be helpful to determine dosage requirements for enterosorbent therapy in animals and humans, and other potential applications with APMs during disasters.
Figure 5.
Extrapolation of the sorbent doses required to bind and decrease exposures from 1 ppm PCB 77 (A) and PCB 153 (B).
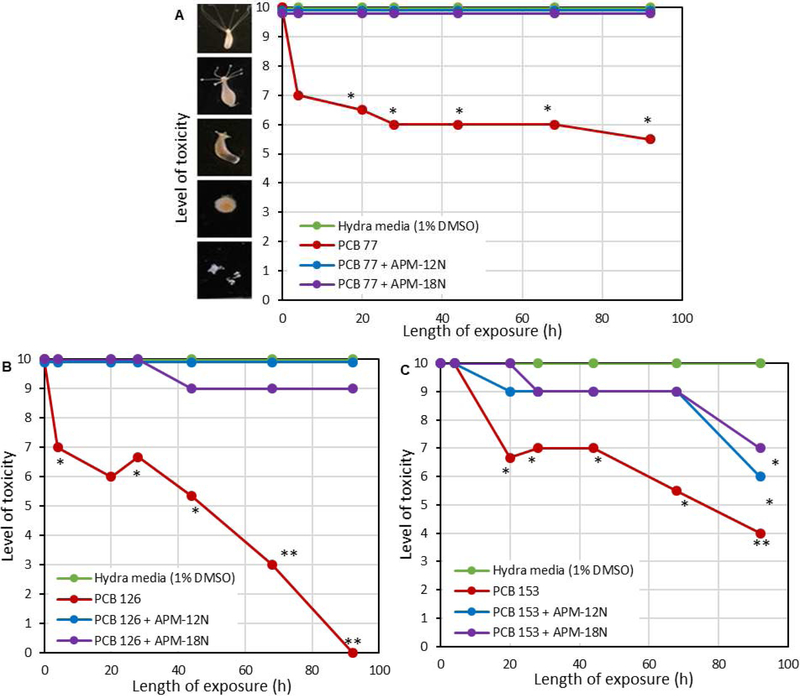
The isothermal results, the safety and the efficacy of the APMs were confirmed in a living organism using the hydra assay in aqueous media (Fig. 6). Individual toxin exposure with 40 ppm PCB 77, 30 ppm PCB 126 and 30 ppm PCB 153 resulted in severe and irreversible toxicity to hydra. Following the inclusion of 0.1% APMs, adult hydra were completely (100%) protected from PCB 77 toxicity. Also, APM sorbents significantly protected the hydra (in vivo) against PCB 126, with protection percentages at the endpoint of the assay equal to 100% and 90% for APM-12N and APM-18N, respectively. At the same inclusion rate, APM sorbents also showed moderate protection against PCB 153 with protection percentages equal to 33% and 50% for APM-12N and APM-18N, respectively. The more significant protection by APMs against PCB 77 and 126 was consistent with the isothermal results indicating that binding was favored for coplanar PCBs more than non-coplanar PCBs. Although there was relatively less protection against PCB 153, the inclusion rate of APM treatment was only 0.1%; the percent protection should be further enhanced with higher inclusion rates of clay based on our previous research.
Figure 6.
Hydra toxicity and protection by montmorillonite clay and APMs at the 0.1% inclusion level against 40 ppm PCB 77(A), 30 ppm PCB 126 (B), and 30 ppm PCB 153 (C). Hydra media and toxin controls are included for comparison. APM sorbents completely protect (100%) against PCB 77. APM sorbents also protect against PCB 126 and 153. The protection percentages for PCB 126 and 153 at the endpoint are as follows: (B) APM-12N: 100%, APM-18N: 90%. (C) APM-12N: 33%, APM-18N: 50%. (* p ≤ 0.05, ** p ≤ 0.01).
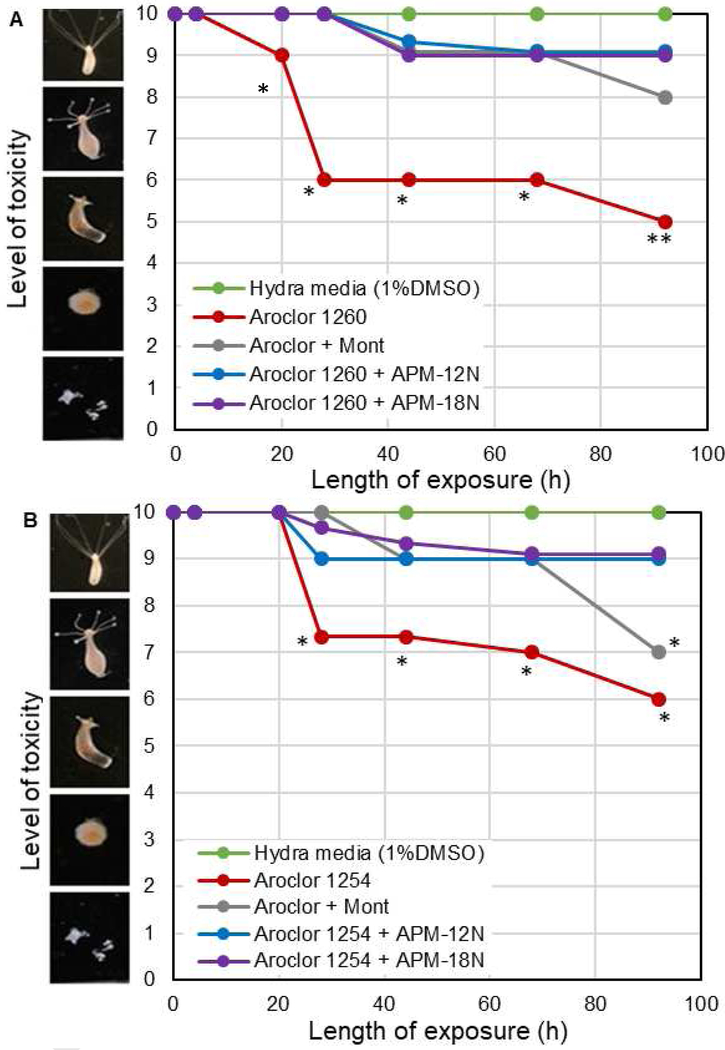
Aroclor 1254 and 1260 were the most widely used industrial PCBs in the United States and both mixtures are toxic based on laboratory animal studies. As shown in the results of the hydra assay in Figure 7, 20 ppm Aroclor 1260 and 1254 were moderately toxic to hydra, and the toxicity was irreversible. APM treatments at 0.2% inclusion rate were able to provide 80% and 75% protection against individual Aroclor 1260 and 1254, respectively, compared to less from parent montmorillonites (60% and 25% protection). Importantly, this result suggests that APMs can effectively sorb Aroclors and possibly other mixtures of PCBs. Dosimetry studies with Aroclor 1254 and 1260 (data not shown) resulted in averages of 60% and 77% binding of 1 ppm Aroclor 1254 and 1260 at inclusion rates equal to 0.005% and 0.1%, respectively. These in vitro results were similar to in vivo findings with hydra (Fig. 7) and demonstrate the ability of APMs to sorb complex PCB mixtures and reduce their toxicity in a living organism. Rodent assays followed by biomonitoring in tissues are needed to further investigate the sorption efficacy of APMs in reducing Aroclor exposures in humans and animals.
Figure 7.
Hydra toxicity and protection by montmorillonite clay and APMs at the 0.1% inclusion level against 20 ppm Aroclor 1260 (A) and 20 ppm Aroclor 1254 (B). Hydra media and toxin controls are included for comparison. APMs showed significant protection against Aroclor toxicity. The protection percentages for Aroclor 1260 and 1254 at the endpoint are as follows: (A) Mont: 60%, APM-12N: 80%, APM-18N: 80%. (B) Mont: 25%, APM-12N: 75%, APM-18N: 75%. (* p ≤ 0.05, ** p ≤ 0.01).
CONCLUSIONS
Based on a combination of in vitro, in vivo and molecular simulation studies, the mechanism of action appears to involve steric hindrance associated with ortho-chloro substituents, which limit or restrict access of the potent compound to the surfaces and pores of montmorillonite clay and activated carbon, reducing their sorption capacities and affinities. This restriction was overcome by APMs with higher surface area, porosity, and chains of amorphous silica at the edges of the clay that provided increased binding sites, and higher binding capacities, affinities and enthalpies for both coplanar and non-coplanar PCBs. Moreover, APMs maintained threshold limits at low dose levels and resulted in effective protection against PCBs and Aroclors.
Besides PCBs, it is possible that APMs, and similar materials, will be broad-acting to reduce exposures to diverse environmental contaminants in food and drinking water. The short-term administration of APMs in the diet of humans and animals can minimize unintended toxin exposures from contaminated food and water supplies.
HIGHLIGHTS.
PCBs and mixtures were chemisorbed onto the surfaces of processed clays (APMs)
APMs showed high capacities, affinities, enthalpies and efficacy for PCBs
APMs (at low doses) protected against PCBs and Aroclors in vivo
Steric effects and ortho chlorine substitution were important for PCB sorption
Low doses of APMs could reduce PCB exposures in humans and animals
Acknowledgments
FUNDING
This work was supported by the Superfund Hazardous Substance Research and Training Program (National Institutes of Health) [P42 ES0277704]; and the United States Department of Agriculture [Hatch 6215].
REFERENCE
- Addendum to the Toxicological Profile for Polychlorinated Biphenyls (PCBs). United State Department of Health & Human Services/Agency for Toxic Substances & Disease Registry: Atlanta, GA, 201; www.atsdr.cdc.gov/toxprofiles/pcbs_addendum.pdf. [PubMed] [Google Scholar]
- Ahmadkhaniha R; Nodehi RN; Rastkari N; Aghamirloo HM Polychlorinated biphenyls (PCBs) residues in commercial pasteurized cows’ milk in Tehran, Iran. J. Environ. Health Sci. Eng 2017, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro PW; Corbett JT; Schroeder JL Quantitative characterization of polychlorinated biphenyl mixtures (aroclors® 1248, 1254 and 1260) by gas chromatograpy using capillary columns. J. Chromatography A, 1981, 205, 103–111. [Google Scholar]
- Amari A; Gannouni H; Khan MI; Almesfer MK; Elkhaleefa AM; Gannouni A Effect of structure and chemical activation on the adsorption properties of green clay minerals for the removal of cationic dye. Appl. Sci, 2018, 8, 2302. [Google Scholar]
- Andersson PL; Haglund P; Tysklind M Ultraviolet absorption spectra of all 209 polychlorinated biphenyls evaluated by principal component analysis. Fresenius J. Anal. Chem, 1997, 357, 1088–1092. [Google Scholar]
- Basic Information about Polychlorinated Biphenyls (PCBs) in Drinking Water. United States Environmental Protection Agency: Washington, DC, 2014; http://water.epa.gov/drink/contaminants/basicinformation/polychlorinated-biphenyls.cfm. [Google Scholar]
- Chen X; Lin Y; Dang K; Puschner B Quantification of polychlorinated biphenyls and polybrominated diphenyl ethers in commercial cows’ milk from california by gas chromatography–triple quadruple mass spectrometry. PloS One, 2017, 12, e0170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SA; Yamada R; Mak CM; Hunter B; Obando AS; Hoxha S; Ja WW Acidic food pH increases palatability and consumption and extends drosophila lifespan. J. Nutr 2015, 145, 2789–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnay JD; In International Tables for X-ray Crystallography; Scheick J Eds.; Kynoch Press: Baltimore: 1952. [Google Scholar]
- Dunnlvant FM; Elzerman AW Quantitative structure-property relationships for aqueous solubilities and henry’s law constants of polychlorinated biphenyls. Environ. Sci. Technol 1992, 26, 1567–1573. [Google Scholar]
- Edition of the Drinking Water Standards and Health Advisories; United State Environmental Protection Agency: Washington, DC, 2012; https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf. [Google Scholar]
- Evaluation of the Toxicology of PCBs; United State Environmental Protection Agency: Washington, DC, 1989; https://semspub.epa.gov/work/01/54773.pdf. [Google Scholar]
- Fadaei H; Watson A; Place A; Connolly J; Ghosh U Effect of PCB bioavailability changes in sediments on bioaccumulation in fish. Environ. Sci. Technol 2015, 49, 12405–12413. [DOI] [PubMed] [Google Scholar]
- Fairey JL; Wahman DG; Lowry GV Effects of natural organic matter on PCB-activated carbon sorption kinetics: implications for sediment capping applications. J. Environ. Qual 2010, 39, 1359–1368. [DOI] [PubMed] [Google Scholar]
- Geusau A; Tschachler E; Meixner M; Sandermann S; Papke O; Wolf C; Valic E; Stingl G; McLachlan M Olestra increases faecal excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Lancet, 1999, 354, 1266–1267. [DOI] [PubMed] [Google Scholar]
- Grant PG; Phillips TD Isothermal adsorption of aflatoxin B1 on HSCAS clay. J. Agric. Food Chem 1998, 46, 599–605. [DOI] [PubMed] [Google Scholar]
- Greenland DJ; Quirk JP Adsorption of 1-n-alkyl pyridinium bromides by montmorillonite. Clays Clay Miner. 1960, 9, 484–499. [Google Scholar]
- Hiraizumi Y; Takahashi M; Nishimura H Adsorption of polychlorinated biphenyl onto sea bed sediment, marine plankton, and other adsorbing agents. Environ. Sci. Technol 1979, 13, 580–584. [Google Scholar]
- Hutzinger O; Safe S; Zitko V In The chemistry of PCB’s; CRC Press: Cleveland: 1974. [Google Scholar]
- Improving water quality in Trinity river: Assessing PCBs in fish tissue; Texas Commission on Environmental Quality: Austin, TX, 2019; https://www.tceq.texas.gov/waterquality/tmdl/77trinity_pcbs.html. [Google Scholar]
- Jandacek RJ; Heubi JE; Buckley DD; Khoury JC; Turner WE; Sjodin A; Olson JR; Shelton C; Helms K; Bailey TD; Carter S; Tso P; Pavuk M Reduction of the body burden of PCBs and DDE by dietary intervention in a randomized trial. J Nutr Biochem, 2014, 25, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C; Tofighi R; Tamm C; Goldni M; Mutti A; Ceccatelli S Cell death mechanisms in AtT20 pituitary cells exposed to polychlorinated biphenyls (PCB 126 and PCB 153) and methylmercury. Toxicol. Lett 2006, 167, 189–190. [DOI] [PubMed] [Google Scholar]
- Komadel P; Schmidt D; Madejova J; Cicel B Alteration of smectites by treatments with hydrochloric acid and sodium carbonate solutions. App. Clay Sci 1990, 5, 113–122. [Google Scholar]
- Liu C; Gu C; Yu K; Li H; Teppen BJ; Johnston CT; Boyd SA; Zhou D Integrating structural and thermodynamic mechanisms for sorption of PCBs by montmorillonite. Environ. Sci. Technol 2015, 49, 2796–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madejova J; Bujdak J; Janek M; Komadel P Comparative FT-IR study of structural modifications during acid treatment of dioctahedral smectites and hectorite. Spectrochim. Acta. Part A: Mol. Biomol. Spectrosc 1998, 54, 1397–1406. [Google Scholar]
- Marketing authorization for food additives that may be used as pH adjusting agents, acid-reacting materials or water correcting agents; https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/lists-permitted.
- Mayes BA; McConnell EE; Neal BH; Brunner MJ; Hamilton SB; Sullivan TM; Peters AC; Ryan MJ; Toft JD; Singer AW; Brown JF; Menton RG; Moore JA Comparative carcinogenicity in sprague-dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicological Science. 1998, 41, 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayura K; Smith EE; Clement BA; Phillips TD Evaluation of the developmental toxicity of chlorinated phenols utilizing hydra atienuata and postimplantation rat embryos in culture. Toxicol. Appl. Pharmacol 1991, 108, 253–266. [DOI] [PubMed] [Google Scholar]
- McDonough KM; Fairey JL; Lowry GV Adsorption of polychlorinated biphenyls to activated carbon: equilibrium isotherms and a preliminary assessment of the effect of dissolved organic matter and biofilm loadings. Water Res. 2008, 42, 575–584. [DOI] [PubMed] [Google Scholar]
- Mekewi MA; Darwish AS; Amin MS; Eshaq Gh.; Bourazan HA Copper nanoparticles supportedonto montmorillonite clays as efficientcatalyst for methylene blue dye degradation. Egypt. J. Petrol 2016, 25. [Google Scholar]
- Mohammad-Khah A; Ansari R Activated charcoal: Preparation, characterization and applications: A review article. Int. J. Chemtech. Res 2009, 1, 859–864. [Google Scholar]
- Mullin M; Sawka G; Safe L; McCrindle S; Safe S Synthesis of the octa- and nonachlorobiphenyl isomers and congeners and their quantitation commercial polychlorinated biphenyls and identification in human breast milk. J. Anal. Toxicol, 1981, 5, 138–142. [DOI] [PubMed] [Google Scholar]
- Mullins MD; Pochini CV; McCrindle S; Romkes M; Safe SH; Safe LM High-resolution PCB analysis: synthesis and chromatographic properties of all 209 PCB congeners. ES&T, 1984, 18, 468–476. [DOI] [PubMed] [Google Scholar]
- Neji SB; Trabelsi M; Frikha MH Esterification of fatty acid over Tunisian acid activated clay: kinetic study. J. Oleo. Sci. 2011, 60, 293–299. [DOI] [PubMed] [Google Scholar]
- Nestle M The selling of olestra. Public Health Rep, 1998, 113, 508–520. [PMC free article] [PubMed] [Google Scholar]
- Phillips TD; Afriyie-Gyawu E; Williams J; Huebner H; Ankrah NA; Ofori-Adjei D; Jolly P; Johnson N; Taylor J; Marroquin-Cardona A; Xu L, Tang L; Wang JS Reducing human exposure to aflatoxin through the use of clay: A review. Food Addit. Contam. Part A 2008, 25, 134–45. [DOI] [PubMed] [Google Scholar]
- Phillips TD; Wang M; Elmore SE; Hearon S; Wang JS NovaSil Clay for the protection of humans and animals from aflatoxins and other contaminants. Clay. Clay Miner, 2019, 67, 99–110. 10.1007/s42860-019-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polychlorinated biphenyls. United States Environmental Protection Agency: Washington, DC, 2018; https://comptox.epa.gov/dashboard/dsstoxdb/results?search=Polychlorinated%20Biphenyls#toxicity-values. [Google Scholar]
- Public Health Goal for Water Soluable Polychlorinated Biphenyls Expected to be Found in Drinking Water. California Office of Environmental Health Hazard Assessment: Sacramento, CA, 2007; www.oehha.ca.gov/water/phg/pdf/PCBphg10052007.pdf. [Google Scholar]
- Resmi G; Thampi SG; Chandrakaran S Removal of lead from wastewater by adsorption using acid-activated clay. Environ. Tech 2012, 33, 291–297. [DOI] [PubMed] [Google Scholar]
- Richardson SM; Richardson JM Crystal structure of a pink muscovite from archer’s post, Kenya: Implications for reverse plechorism in dioctahedral micas. Am. Miner 1982, 67, 69–75. [Google Scholar]
- Safe SH Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol 1994, 24, 87–149. [DOI] [PubMed] [Google Scholar]
- Swithers SE; Ogden SB; Davidson TL Fat substitutes promote weight gain in rats consuming high-fat diets. Behav. Neurosci, 2011, 125, 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toxicological Profile for Polychlorinated biphenyls (PCBs); United State Department of Health & Human Services/Agency for Toxic Substances & Disease Registry: Atlanta, GA, 2010; http://www.atsdr.cdc.gov/toxprofiles/index.asp. [PubMed] [Google Scholar]
- Toxicological Profile for Polychlorinated biphenyls (PCBs); United State Department of Health & Human Services/Agency for Toxic Substances & Disease Registry: Atlanta, GA, 2000; www.atsdr.cdc.gov/toxprofiles/tp17.pdf. [PubMed] [Google Scholar]
- Tyagi B; Chudasama CD; Jasra RV Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim. Acta. Part A: Mol. Biomol. Spectrosc 2006, 64, 273–278. [DOI] [PubMed] [Google Scholar]
- Wang M; Hearon SE; Johnson NM; Phillips TD Development of broad-acting clays for the tight adsorption of benzo[a]pyrene and aldicarb. Appl. Clay Sci 2019, 168, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M; Hearon S; Phillips TD Development of enterosorbents that can be added to food and water to reduce toxin exposures during disasters. J. Environ. Sci. Health. B, 2019, 54, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M; Maki CR; Deng Y; Tian Y; Phillips TD Development of high capacity enterosorbents for aflatoxin B1 and other hazardous chemicals. Chem. Res. Toxicol 2017, 30, 1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilby OK; Tesh JM; Shore PR Application of the hydra regeneration assay: Assessment of the potential teratogenic activity of engine exhaust emissions. Toxicol. In Vitro 1990, 4, 612–613. [DOI] [PubMed] [Google Scholar]