Abstract
Since the emergence of cancer nanomedicine, researchers have had intense interest in developing nanoparticles (NPs) that can specifically target diseased sites while avoiding healthy tissue to mitigate the off-target effects seen with conventional treatments like chemotherapy. Initial endeavors focused on the bioconjugation of targeting agents to NPs, and more recently, researchers have begun to develop biomimetic NP platforms that can avoid immune recognition to maximally accumulate in tumors. In this review, we describe the advantages and limitations of each of these targeting strategies. First, we review developments in bioconjugation strategies, where NPs are coated with biomolecules such as antibodies, aptamers, peptides, and small molecules to enable cell-specific binding. While bioconjugated NPs offer many exciting features and have improved pharmacokinetics and biodistribution relative to unmodified NPs, they are still recognized by the body as “foreign”, resulting in their clearance by the mononuclear phagocytic system (MPS). To overcome this limitation, researchers have recently begun to investigate biomimetic approaches that can hide NPs from immune recognition and reduce clearance by the MPS. These biomimetic NPs fall into two distinct categories: synthetic NPs that present naturally occurring structures, and NPs that are completely disguised by natural structures. Overall, bioconjugated and biomimetic NPs have substantial potential to improve upon conventional treatments by reducing off-target effects through site-specific delivery, and they show great promise for future standards of care. Here, we provide a summary of each strategy, discuss considerations for their design moving forward, and highlight their potential clinical impact on cancer therapy.
Keywords: targeting, nanoparticles, cancer, biomimicry, bioconjugation, delivery
1. Introduction
Conventional treatment strategies for cancer, such as chemotherapy, cause substantial off-target effects that limit their ability to successfully treat patients. To improve upon these traditional approaches, researchers have developed nanoparticle-based medicines that have better pharmacokinetics and biodistribution than free therapeutic agents. However, even “stealth” nanomedicines accumulate substantially at off-target sites; therefore, recent research has been devoted to designing targeted nanoparticles (NPs) that can enable tumor cell-specific binding and uptake, which promotes nanoparticle retention in tumors following initial accumulation mediated by the enhanced permeability and retention effect. These targeted NPs are often designed to specifically bind to receptors that are overexpressed on cancer cells relative to healthy cells. For example, there are several well-known receptors that can be exploited for targeting, like epidermal growth factor receptor (EGFR) [1, 2] and human epidermal growth factor receptor 2 (HER2) [3], but a myriad of novel overexpressed receptors have also been identified that are attractive alternative targets for new nanotherapeutics. Designing NPs to bind to cancer cell-specific receptors may improve upon conventional treatments by enhancing tumor retention to reduce off-target effects, and this strategy shows great promise for future clinical utility. In this review, we discuss the two main categories of targeted NP systems currently under investigation: bio-nanoconjugates and biomimetic NP platforms (Fig. 1). Bio-nanoconjugates are NPs that are coated with biomolecular targeting agents such as antibodies, aptamers, peptides, or small molecules using a chemical linker (Table 1 and Fig 1(a)). Alternatively, biomimetic platforms are NPs that either present naturally occurring cellular structures with inherent targeting capabilities or are completely “disguised” by cellular structures to avoid immune recognition and provide improved delivery (Fig. 1(b)) [4]. Over the past two decades, there has been significant development of both targeting strategies, first in the bioconjugation realm and more recently in biomimicry. This is evidenced by the fact that the number of publications found in PubMed using the search terms “targeted nanoparticle” or “membrane coated nanoparticle” has increased dramatically since the year 2000 (Fig. 2). Our goal in this review is to provide insight into the benefits and limitations of both strategies so that researchers can understand progress in the field and incorporate this knowledge to engineer new and improved NP systems for cancer treatment.
Figure 1.
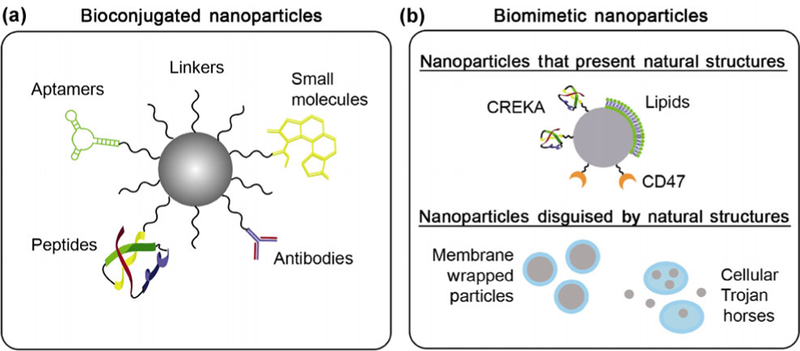
(a) Bio-nanoconjugates consist of nanoparticles physically tethered to targeting agents, which may be antibodies, aptamers, peptides, or small molecules. (b) Biomimetic nanoparticles consist of nanoparticles coated with natural cellular structures to prevent immune recognition, prolong circulation, and enhance tumor delivery. The two types of biomimetic nanoparticles are (i) nanoparticles that present specific cell constituents, and (ii) nanoparticles that are completely disguised by entire cell membranes or loaded within whole cells.
Table 1.
Advantages and limitations of common targeting agents used in nanotherapeutics
| Targeting agent | Antibodies | Aptamers | Peptides | Small molecules |
|---|---|---|---|---|
 |
 |
 |
 |
|
| Advantages | ● Easily conjugated | ● Low cost | ● Systematic development | ● Low cost |
| ● Therapeutic effect | ● Low immunogenicity | ● Small size | ● Easily conjugated | |
| ● High affinity | ● Low cost | ● High stability | ||
| ● Small size | ● Some can mediate endosomal escape | |||
| ● Easily chemically modified | ||||
| Limitations | ● Affinity varies with conjugation approach | ● Rapidly degraded if unmodified | ● Biodistribution varies | ● Non-systematic approaches for development |
| ● High cost | ● Cross-reactivity | ● Toxicology varies | ● Lack high specificity | |
| ● Limited tumor penetration | ● Binding affinity susceptible to environment | |||
| ● High immunogenicity | ||||
| ● Large size | ||||
Figure 2.
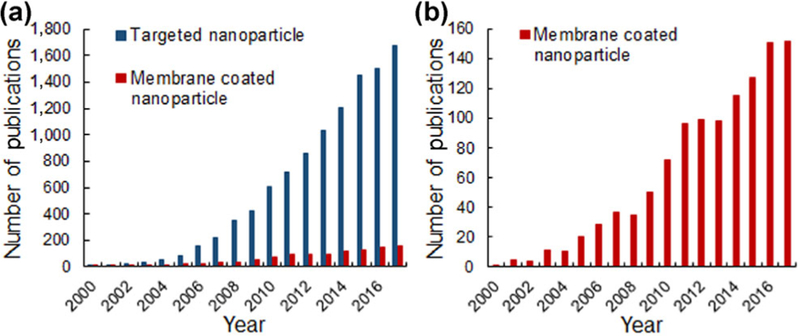
(a) The number of annual publications found in PubMed using the search terms “targeted nanoparticle” and “membrane coated nanoparticle”, since 2000. (b) Magnified scale of the number of publications about “membrane coated nanoparticle” over the last two decades.
2. Bioconjugation strategies
Antibodies, aptamers, peptides, and other small molecules are the most commonly employed targeting agents for NP bioconjugation and site-specific delivery (Table 1) [5, 6]. These targeting moieties are typically attached to the NP surface using a linker that bridges the NP surface with a functional group on the biomolecule (Fig. 3). The most common linker used for bioconjugation is heterobifunctional poly(ethylene) glycol (PEG) because it is easily manipulated to have a variety of end functional groups and lengths that drastically impact targeting success. Many of the biomolecules described in this review can be used not only as targeting agents for NPs, but also as therapeutic payloads [7–9], and this makes bioconjugated NPs very versatile and impactful tools for cancer treatment. Below, we review the progress, benefits, and limitations of bioconjugated NPs made with each of these types of targeting agents. Readers should refer to Table 1 for an overview of the advantages and disadvantages of each targeting agent described in this review.
Figure 3.
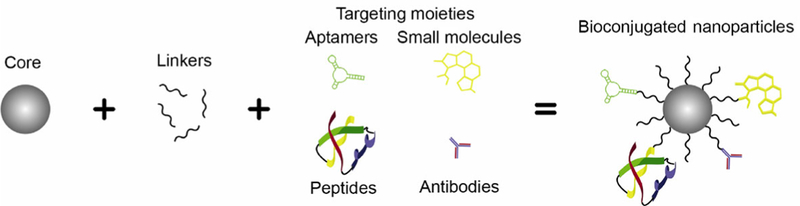
Bioconjugated nanoparticles consist of a nanoparticle core (often composed of gold or polymers), a linker (typically PEG), and a targeting moiety.
2.1. Antibody targeting agents
Antibodies were among the first biomolecules explored as targeting agents for nanotherapies [10, 11]. Antibodies are beneficial targeting moieties because they have high specificity for their target, their production is well-established because some antibodies are already used as standalone therapeutics (as described later in this section), and they can easily be exchanged to target a variety of different cell surface proteins important for disease progression [12]. Additionally, the bioconjugation of antibodies to NPs typically involves simple chemical reactions. For example, for polymer particles, 1-ethyl-3-(3- dimethylaminopropyl)carbodiimide (EDC) chemistry is commonly used to form an amide bond between exposed carboxylic acid functional groups on the surface of the NP and amine functional groups on the conserved fragment (Fc) of the antibody. With other common NP cores, like gold-based NPs, antibodies can be attached via heterobifunctional PEG linkers, where one end of the PEG molecule contains a sulfur group to form a gold–thiol bond with the particle surface, and the other end contains a group that can react with primary amines on the antibody, as described before. Further, for magnetic NPs such as iron oxide (which are particularly useful for contrast enhancement in dual imaging and therapeutic strategies), antibodies can be attached through an amidation reaction that uses EDC chemistry similar to that used for polymer particles [13]. Importantly, directionality of the antibody may play a role in its binding affinity, as the antigen-binding sites need to be accessible for efficient targeting [14, 15]. With the conjugation methods described previously, antibodies are randomly oriented, which reduces selective binding efficiency, may have a low coupling efficiency, and can result in NP aggregation [16]. With directional conjugation methods, antibodies are directly linked to the NP by the Fc region of the antibody, leaving the antigen-binding region unhindered [14–17]. With these strategies, fewer antibodies are needed per NP to achieve the same targeting effect, thus reducing cost and enhancing performance [15, 17].
One benefit of utilizing antibodies as targeting agents is that numerous formulations designed to bind a variety of overexpressed cell surface receptors are readily available. Two common targets for antibody-based NPs are EGFR, found in multiple epithelial cancers, and HER2, found in about 20% of breast cancers [18]. EGFR-targeting antibodies have been incorporated into NPs for the detection or treatment of lung, breast, and bladder cancer, among others [19, 20]. Herceptin, a HER2-targeting breast cancer therapy, is currently used in the clinic as an unconjugated therapeutic. Recently, however, this antibody and other anti-HER2 antibodies have been incorporated into NPs to enable cancer cell-specific binding and uptake for small interfering RNA (siRNA) delivery [21, 22], drug delivery [23], and photothermal therapy [10]. EGFR and HER2 are two examples of targets commonly employed for cancer therapies, but it is important to note that antibodies can be easily interchanged to target any overexpressed biomarker that is pertinent to a specific cancer.
Perhaps one of the most exciting features of antibodies is that, in addition to providing cell-specific binding, they can also confer a therapeutic benefit on the NPs. This is because, upon binding to their targeted receptor, antibodies (and antibody–NP conjugates) can competitively block signaling ligands from binding the receptor, leading to inhibition of the downstream signaling pathway [24, 25]. A major benefit of utilizing antibody–NP conjugates over freely delivered antibodies to elicit such signal cascade interference is that antibody–NP conjugates demonstrate multivalency, in which they have increased binding affinity for their target compared to free antibodies [6, 24, 26]. We and others have shown that this increased valency correlates with enhanced signal cascade interference [24, 27]. Antibody–NP conjugates that continue to explore the benefits of combining multivalent targeting and signal cascade interference with the ability to specifically deliver a payload may be highly suited for the safe and effective treatment of a wide variety of cancers.
Overall, antibodies show substantial promise as targeting agents for NP delivery to tumors, but they are also limited by their large size, high cost, and immunogenicity (Table 1). Accordingly, other agents have been explored for bioconjugation to NPs, and these are described in the following sections.
2.2. Aptamer targeting agents
Aptamers are synthetic oligonucleotides selected by “systematic evolution of ligands by exponential enrichment” (SELEX) technology that have extremely high affinity for their targeted protein [28]. Compared to antibodies, aptamers are easier to synthesize, are lower in cost, have lower immunogenicity, and can more easily penetrate tumors because of their smaller size. These features, coupled with their high target affinity, make aptamers ideal agents for NP targeting. Aptamers are usually attached to NPs by electrostatic interactions or covalent conjugation methods, similar to antibody conjugation [29].
As synthetic molecules, aptamers can be easily made with site-specific modifications, and they can also be designed to target a variety of biomarkers. Notably, aptamers have been used not only to directly target markers on cancer cells, but also to target markers within the tumor microenvironment. For example, Hicke et al. showed that the TTA1 aptamer, which is specific to the extracellular matrix protein tenascin-C that is amplified in the tumor microenvironment, could be readily taken up by a variety of tumors, including glioblastoma, lung, colon, and breast cancers [30]. The ability to use a single type of aptamer to target various tumor types is an attractive strategy for developing new nanotherapeutics.
Much like antibodies, aptamers can be used not only as targeting agents but also as therapeutic entities [9, 29]. After being internalized by their target cells, aptamers (and aptamer–NP conjugates) can initialize apoptosis and alter oncogene expression [9]. Further, aptamer–NP conjugates are more effective than freely delivered aptamers because of the increased binding affinity afforded by the nanocarrier design. This was demonstrated by Odom and colleagues, who showed that gold nanostars decorated with AS1411 aptamers induce 1.5× higher caspase activity in cancer cells than ten times higher concentrations of free AS1411 aptamers [9]. The ability to use aptamers for both cell-specific targeting and therapeutic effects make them promising agents for targeted cancer nanotherapeutics.
2.3. Peptide targeting agents
Peptides, unlike aptamers, are naturally occurring ligands that have innate targeting capabilities, allowing them to specifically bind to cell surface receptors. They are frequently conjugated to NPs using aldehyde-amine reactions with a reducing agent or, alternatively, thiolated peptides are attached to maleimide-functionalized NPs [31]. Here, we describe some of the most common peptide targeting agents and briefly list their advantages and limitations.
One of the most frequently used targeting peptides is arginylglycylaspartic acid (RGD). This peptide motif is naturally occurring and especially important for cell adhesion to the extracellular matrix via the αVβ3 integrin. Many types of cancer, including skin, liver, and breast cancers, overexpress αVβ3 receptors, so targeting systems that use the RGD peptide show improved specificity for these tumors when compared to their non-targeted counterparts [32, 33]. In addition, NPs modified with RGD can efficiently deliver a variety of therapeutic molecules, including doxorubicin and siRNA, to tumors [33, 34]. Besides RGD, many other targeting peptides are in development for cancer therapies. As with antibody-based nanotherapeutics, EGFR is a common target for peptide-based therapies [35, 36], but peptides have also been used for less common targets. The F3 peptide, for example, is able to target tumor vasculature in glioma and successfully localize therapeutic agents to the diseased site [37]. In addition, a plectin-1 derived peptide has been shown to uniquely bind to a new biomarker useful for treating early stage pancreatic cancer [38].
As a whole, peptides are useful targeting agents for cancers that may require more specific selection of a chosen target. They can be easily identified using a combinatorial library method, a common practice in pharmaceuticals [39]. In addition, peptides have shown reduced levels of binding in the mononuclear phagocytic system (MPS) when compared to other targeting moieties, like antibodies [39]. However, peptides have not been as extensively studied in vivo as NP targeting agents, so their biodistribution and toxicology are relatively unknown [40].
2.4. Small molecule targeting agents
Small molecules are a slightly larger class of targeting agents that encompasses many ligands or ligand mimics specific to cell surface receptors. One of the most well-established examples is folic acid, which binds the folate receptor that is found on the surface of many different types of tumor cells [41]. These moieties and others are typically conjugated to NPs using similar mechanisms to peptides as they can undergo the same chemical modifications.
As mentioned above, the folate receptor is highly overexpressed in a number of cancers and is a promising target for nanotherapeutics [42]. Folic acid has therefore been used to target NPs to cervical cancer [43], liver cancer [44], and breast cancer [45, 46]. In addition to folic acid, small molecules like the transferrin protein [47], a ligand targeting prostate-specific membrane antigen [48], and hyaluronic acid [49, 50] have all been explored as promising targeting agents for nanotherapeutics in a wide variety of cancer types. There are many naturally occurring ligands that are specific to a single receptor, so small molecule targeting agents are versatile in their applications. However, small molecules do not have as high affinity as some of the other agents discussed here, and their production is more complex, so their application may be limited to specific cases where their use is desirable over alternative agents.
2.5. Design consideration for bioconjugated NPs: Position of targeting molecules
In the above examples, we described systems wherein targeting agents were tethered to NPs in a manner that left them exposed on the particle surface and extended beyond any other coating on the NP that was intended to increase stability and circulation time. Placing targeting agents at the outermost position on NPs was originally thought to be necessary in order to preserve the biomolecules’ function [51], but an alternative strategy is to partially embed the targeting agents in the stealthing layer. For example, Zhou et al. found that by placing hyaluronidase within the PEG shell of their NP formulation, the cell-specific binding could be maintained while also extending the NPs’ circulation time relative to NPs that had hyaluronidase positioned such that it extended past the PEG coating [52]. Maximizing circulation is imperative to increase the number of times NPs pass through tumors to potentially bind to their targeted receptor, so the position of targeting molecules on bioconjugated NPs is an important design parameter for researchers to consider. Based on the findings of Zhou et al., embedding targeting agents within stealth layers rather than leaving them exposed to the environment may be a promising strategy for future systems.
3. Recent transition in targeted nanomedicine: From bioconjugation to biomimicry
While bioconjugation is a promising and straightforward means of enhancing NPs’ retention in diseased tissue, its clinical use is met by several limitations. First, targeting agents may improve NP retention in tumors, but not overall accumulation, because targeting agents will not bind to their intended cellular receptor until the NPs have already reached the tumor. A recent meta-analysis emphasized this point, as it revealed that active targeting agents yield only modest improvements in the percentage of administered NPs that are efficiently delivered to tumors [53]. A further complication is that targeting agents may even reduce NP diffusion into tumors, as targeted NPs with a high binding affinity for their intended receptor will be captured by cells at the tumor periphery before they can penetrate deeply into the tumor [54]. Finally, the most common linker used to attach biomolecules to NPs is heterobifunctional PEG, and targeted NPs are often also passivated with additional methoxy-PEG molecules to enhance their stability. However, new studies have shown that the body can develop an anti-PEG immune response [55, 56], which may result in premature NP clearance. These findings have led researchers to begin exploring alternative strategies to enhance delivery of NPs to tumors. One particularly exciting approach involves designing NPs to “mimic” cells within the body, effectively hiding them from the immune system to prolong circulation time and enhance tumor delivery. For example, Discher and colleagues decorated NPs with CD47 “marker of self” peptides, which dramatically improved circulation and tumor retention [57]. Since then, an exciting new era of investigation has emerged in the realm of biomimetic NPs for targeted cargo delivery [58–65]. These biomimetic NPs are either partially or wholly disguised with naturally occurring cellular components, and because the body no longer perceives the NPs as “foreign” they can avoid early clearance to accumulate in tumors at unprecedented levels.
4. Biomimetic strategies
In this section, we describe biomimetic NP platforms as two distinct categories (Fig. 4): (1) NPs that present naturally occurring structures, and (2) NPs that are completely disguised by natural structures. The design features, benefits, and limitations of materials that fall into each of these categories are discussed in detail below.
Figure 4.
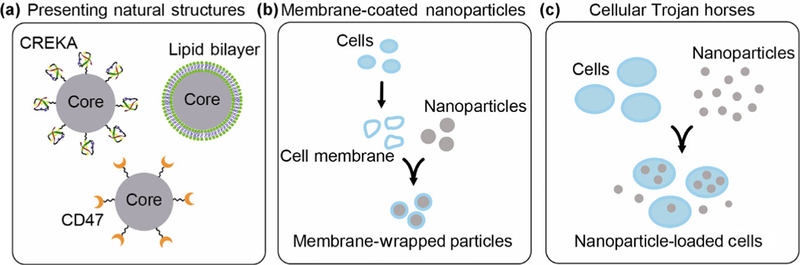
Biomimetic strategies for targeting nanoparticles to tumors include: (a) designing nanoparticles to present natural cellular structures, (b) coating nanoparticles with cell-derived membranes, and (c) loading nanoparticles within entire cells that can act as “Trojan horses”.
4.1. Nanoparticles that present naturally occurring structures
NPs that present naturally occurring structures often use a single type of critical molecule, or a combination of a few types of critical molecules, derived from endogenous vesicles (cells, exosomes, etc.) to coat the NP and provide a unique targeting capability (Fig. 4(a)). For example, as introduced above, NPs can be functionalized with CD47, a naturally occurring membrane protein considered a “marker of self”, to help the particles avoid macrophage-driven clearance and thereby enhance cargo delivery to the diseased site [57]. Besides CD47, various other molecules can be decorated on NPs to avoid immune recognition and promote tumor retention. Researchers have taken inspiration from many endogenous materials, including viruses (reviewed elsewhere [60]), high density lipoproteins (HDLs), and platelets, as described below.
HDLs are often referred to as one of “nature’s nanoparticles”, as they are endogenous NPs that are normally responsible for cholesterol transport within the body. Cancer cells have an increased need for cholesterol to maintain their membrane integrity and proliferate, so they overexpress scavenger receptor B1, which facilitates HDL binding, resulting in high accumulation of HDL at tumor sites [66]. Accordingly, NPs designed to mimic HDL can undergo HDL receptor-mediated uptake specifically in cancer cells [66–68]. This has been demonstrated using NPs coated with various combinations of apolipoproteins and phospholipids (which are the constituents of HDL), and these NPs have been used to mediate drug and nucleic acid delivery to tumors [66–68].
In addition to designing NPs to mimic HDLs, researchers have also developed NPs to present components of platelets. Platelets have innate properties of immune evasion, long circulation times, and strong cell adhesion that are ideal characteristics for drug delivery [63]. Accordingly, researchers have coated NPs with CREKA, a clot-binding peptide that also has tumor-homing abilities, to create platelet-mimicking NPs [69]. This design allows the NPs to accumulate in tumors, where they form a synthetic clot, thus reducing the tumor’s supply of nutrients [69]. Excitingly, the presence of these clots also attracts more synthetic platelets to the tumor site, resulting in enhanced therapy [69]. Moreover, if the NPs are loaded with drugs, they can provide drug delivery in addition to cutting off a tumor’s nutrient supply [69]. The ability to use platelet-mimicking NPs for multifaceted therapy makes them highly desirable for cancer nanomedicine.
In summary, NPs designed to present naturally occurring structures show substantial promise as targeted nanomedicines. However, NPs that utilize simple presentation as a biomimicry strategy cannot fully replicate the complexities of the structures they are attempting to imitate. Thus, techniques to fully disguise NPs with biological materials have been explored, as described in the following section.
4.2. Nanoparticles that are disguised by naturally occurring structures
Nanoparticles that are completely disguised by naturally occurring structures fall into two main subcategories. The first consists of individual NPs that are wrapped with cell-derived membranes (Fig. 4(b)), and the second consists of entire cells that are filled with NPs and used as “Trojan horses” to shuttle the NPs into tumors (Fig. 4(c)). Wrapping NPs with cell-derived membranes or camouflaging NPs within cells provides the benefit that no foreign material is “seen” by the body, as the outer layer is a biological membrane containing numerous “markers of self”. This effectively hides the NPs from the immune system and allows them to accumulate in tumors at previously unrealized levels, as discussed in detail for each type of system below.
4.2.1. Membrane-wrapped nanoparticles
One approach to disguise NPs with natural structures is to coat them with cell-derived membranes (Fig. 4(b)), and this unique targeting strategy has been met with great enthusiasm. In this method, the outer membranes of source cells are separated from their intracellular contents, and then the membranes are wrapped around synthetic NPs via electrostatic interactions [65, 70]. One of the first types of NPs made using this strategy was poly(lactic-co-glycolic acid) (PLGA) NPs coated with red blood cell (RBC) membranes, as RBCs are “nature’s long-circulating delivery vehicles” [64, 71]. Impressively, the half-life of RBC-wrapped NPs in blood is more than double that of PEG-coated NPs [64], and Luk et al. found that RBC membrane-coated NPs delivering doxorubicin as their cargo could double the survival time of mice bearing lymphoma tumors [71]. These exciting results demonstrate the immense potential of using membrane-wrapped NPs for tumor targeting.
Following the initial development of RBC-mimetic NPs, the membrane wrapping technique was rapidly extended to other membrane coatings such as those derived from platelets [61, 63], stem cells [72], leukocytes [73], and more [74]. The advantage of these systems, as compared to RBCs, is that they offer a level of tumor cell-specific binding in addition to long circulation. For example, as introduced above, platelets can effectively accumulate in tumors, and in fact, platelet membrane-wrapped NPs functionalized with tumor necrosis factor related apoptosis-inducing ligand (TRAIL) cytokines and loaded with doxorubicin cargo have been used to treat primary tumors in mice [75]. Additionally, platelet membrane-wrapped NPs have also been used to kill circulating tumor cells (CTCs) [59]. Since CTCs normally bind platelets as a mechanism of shielding themselves from the immune system, platelet membrane-wrapped NPs can bind CTCs to deliver their cargo, effectively killing the CTCs, and this can lead to reduced metastasis formation [59].
Similarly, membranes derived from mesenchymal stem cells (MSCs) [76, 77] and leukocytes (which includes monocytes and macrophages) [73, 78] have also been used to wrap NPs. The rationale for this was that these cells can naturally traffic to tumors via chemotaxis and strongly bind to cancer cells via unique cell–cell interactions [55, 77, 79]. Although it is unclear how well the extracted membranes retain these properties of the host cells, each of these membrane-wrapped NPs have been shown to display better tumor delivery than their non-wrapped counterparts, demonstrating the power of this technology. Notably, membranes for NP wrapping can be derived not only from entire cells, but also from extracellular vesicles such as microvesicles, exosomes, and outer membrane vesicles [80–85]. Additionally, researchers have shown that cell engineering approaches can be used to modify membrane coatings and impart wrapped NPs with capabilities beyond those native to the source cells [86]. Further, hybrid coatings can be created by simultaneously wrapping NPs with membranes derived from two different cell types. For example, NPs have been coated with both RBC and platelet membranes simultaneously to provide both long circulation and enhanced tumor cell binding [61]. To date, membrane-wrapped NPs have been utilized for applications including tumor-specific drug delivery [55], anticancer vaccination [55], and photothermal therapy [87, 88], and it is likely that the number of therapies being developed based upon this technique will grow rapidly in the coming decade.
Finally, another method of actively targeting both primary tumors and CTCs using biomimetic NP platforms is through wrapping NPs in cancer cell-derived membranes [62, 89, 90]. The key advantage of cancer cell membrane-wrapped nanoparticles (CCNPs) is that they can mediate “homotypic targeting”, the preferential delivery to homologous tumors in vivo (i.e., tumors that match the cell type used to create the membrane coating) [88, 90]. This homotypic targeting capability has been demonstrated in both in vitro and in vivo models of breast cancer [88, 90], hepatocellular carcinoma [62], and squamous carcinoma [62]. For example, Sun et al. showed that CCNPs coated with membranes derived from 4T1 breast cancer cells could increase the dose of paclitaxel delivered to 4T1 tumors in mice 4.3-fold relative to unwrapped NPs [89]. In an even more impressive finding, CCNPs were shown to preferentially accumulate in homologous tumors in vivo even in the competition of a heterologous tumor [62]. While the mechanisms of this homotypic targeting have not been fully elucidated, it is known that tumor cells strongly adhere to one another to promote the development of primary tumors and metastases [89]; therefore, CCNPs’ unprecedented “homing” capabilities are likely mediated by self-recognition proteins found on cancer cell membrane surfaces. Additionally, it is possible that CCNPs can avoid immune recognition to enhance tumor delivery since cancer cells elicit immune tolerance, immune-suppression, and immunosenescence [62]. However, it is unknown to what extent cell-derived membranes and membrane-wrapped NPs retain the immune evasion properties of their source cancer cells. More research needs to be done to examine the differences between CCNPs and cancer cells to fully understand the mechanisms of homotypic tumor targeting.
In summary, membrane-wrapped NPs show substantial promise as tools for tumor-specific therapy. They have been used for a variety of applications in pre-clinical testing, and given the rapid progress and excitement regarding these nanomaterials, it is expected that their development and implementation for human use will continue at an extraordinary pace. Next, we discuss a similar biomimetic targeting strategy, in which groups of NPs are carried by single cells that serve as “cellular Trojan horses” or “cellular backpacks”.
4.2.2. Cellular Trojan horses and cellular backpacks
In contrast to membrane-wrapped NPs, wherein a single NP is “cloaked” with a biological membrane, cellular Trojan horses and cellular backpacks consist of many nanoparticles carried within a single intact cell or on a single intact cell, respectively, with no change to its normal function (Fig. 4(c)) [91–93]. MSCs [92, 94], monocytes [93], macrophages [79, 95], and T cells [91, 96] have all been explored for this unique biomimetic targeting strategy.
The advantage of using cells such as MSCs, macrophages, and T cells as cellular Trojan horses is that they are naturally recruited to tumors [92, 93, 97]. Additionally, these cells readily endocytose NPs, so NP cargo can be easily loaded into these cells in vitro [92, 93]. The cellular carriers can then be systemically injected and will effectively shuttle their cargo into tumors without the NPs being detected by the body [92, 95]. This allows the NPs to remain non-toxic to the host until they have reached the tumor tissue [93].
Various types of NPs have been loaded into cellular Trojan horses for therapeutic applications. For example, biocompatible poly-lactic acid and lipid NPs have been introduced into MSCs and subsequently delivered by the MSC carriers to a variety of tumors, including glioma [94], lung adenocarcinoma [92], and ovarian cancer [92], showing the versatility of this targeting approach. Similarly, macrophages have been filled with doxorubicin-loaded NPs and gold nanoshells for delivery into glioma and breast cancer tumors, respectively [93, 95]. Likewise, T cells loaded with gold NPs have been shown to deliver four-fold more NPs into large cell lymphoma tumors than NPs not disguised by a cell [91]. One advantage of T cells as cellular Trojan horses is that they can be more easily expanded in culture than MSCs and macrophages while retaining their “homing” function, making their implementation more practical. Additionally, it has been shown that T cells loaded with NPs can continue to exhibit their normal anti-tumor functions, allowing them to provide multi-faceted therapy [91, 96]. Specifically, T cells’ ability to migrate to tumors via chemotaxis and produce IFN-γ were unaltered after loading with gold NPs [91].
Similar to cellular Trojan horses, cellular backpacks or cellular patches have also been explored for NP delivery using T cells [98] and MSCs [99] because of the innate properties of the host cells. In this approach, NPs are conjugated to the surface of the cells through functionalization of the cell surface and subsequent incubation with NPs modified with an appropriate linker. For example, human bone marrow-derived MSCs have been decorated with NeutrAvidin-coated NPs and shown to retain their tumoritropic properties in a three-dimensional (3D) extracellular matrix, demonstrating the utility of this strategy as a delivery mechanism [99]. However, cellular backpacks do not have the advantage of cloaking NPs as cellular Trojan horses do. Thus, the NPs and any cargo they carry may be more susceptible to degradation or clearance as they are recognized within the body.
Overall, the cellular Trojan horse and cellular backpack strategies are clever ways to effectively hide NPs from the body to promote tumor delivery, but will face similar regulatory hurdles as the membrane-wrapped NP approach. We discuss these hurdles in more detail in the following sections, while also summarizing translational considerations for all of the materials discussed in this review.
5. Considerations for the development of bioconjugated and biomimetic nanoparticles
Bioconjugation and biomimetic strategies have different advantages and limitations given how they interact with the body, and some of these have been elaborated upon in the preceding sections. Important considerations for designing targeted NPs that apply to both systems include the immunogenicity of the coatings, how these coatings impact the particles’ pharmacokinetics and biodistribution, the affinity of the targeting agents, the method and rate of particle internalization by cells, and the method of cargo release. Ideal targeted systems should have limited immunogenicity, long blood retention to maximize the number of times nanoparticles pass through a tumor in circulation, high affinity for their target, efficient and specific uptake by diseased cells, and a controlled release mechanism for the payload that ensures it is delivered at the right intra- or extra- cellular site. Below, we elaborate on these considerations in more detail.
One major design consideration for any cancer therapy is avoiding immune recognition. Intravenously injected NPs are often taken up by macrophages in the bloodstream or in the tumor microenvironment, and subsequently cleared to the liver and spleen. In comparison to targeted NPs that utilize bioconjugation methods, biomimetic strategies yield NPs that are less likely to be taken up by macrophage-like cells because they contain “markers of self” that reduce their recognition [63]. Further, cancer cell membrane-wrapped NPs can avoid immune recognition by retaining some of the immune evasion properties of their source cancer cells [62]. In general, targeted NP systems that are created from patient-specific cells will have a reduced likelihood of being taken up by macrophage-like cells and tagged for clearance [82]. However, the ability to obtain patient cells to synthesize these nanotherapeutics may be a substantial manufacturing challenge. Moving forward, researchers should investigate whether donor cells could be utilized to make membrane-wrapped NPs or cellular Trojan horses/backpacks, or they should develop methods to effectively and rapidly expand patient-specific cells.
In addition to avoiding immune recognition, maximizing binding and uptake by the desired cells is another critical design consideration for targeted nanotherapeutics. Table 1 summarizes the binding affinity of the biomolecular targeting agents used in bioconjugation strategies that were discussed in this review. In particular, aptamers have impressive affinity for their target. As they are isolated via an evolution-based method, their sequence, and thus specificity, can be tuned. For all bioconjugated NPs, perhaps the most tunable factors are the type of linker used and the orientation of the targeting molecule on the NP surface. These factors can both affect the availability of the functional region of the targeting agent, which will impact binding affinity. This has especially been demonstrated with antibodies, as was discussed previously. Notably, the type of targeting agent utilized may also impact the method and rate of cellular uptake. For example, scavenger receptor-mediated endocytosis is common for NP uptake, but with certain targeting moieties, cell uptake can be receptor-specific [100, 101]. The method of cellular uptake is an important consideration because it may ultimately dictate the final intracellular fate of the nanoparticle (i.e., within endolysosomal compartments, the cytosol, or the nucleus). If the type of cargo being carried by the NP needs to be delivered to a specific site within a cell, this is an important consideration that may ultimately impact the success of a targeted nanomedicine.
Finally, regardless of whether a bioconjugation or biomimetic strategy is utilized for targeting, NPs that incorporate a controlled release method for delivering their cargo are ideal for reducing off-target side effects. Typical payload release strategies include passive diffusion of drugs from the NP, but other NPs have been developed to be stimuli-responsive [102]. There are many biological and external stimuli that can be used for triggered cargo release [103], but two of the most commonly used stimuli are changes in pH and externally applied light. pH is a natural choice for a cargo release stimulus given the reduced pH in the tumor microenvironment and intracellular compartments [100]. As an external stimulus, light provides a significant level of control over the site and timing of payload release. In this approach, light of a specific wavelength is used to cleave a linker tethering a cargo to its carrier, and this causes the therapeutic molecule to be released and active only in areas that are irradiated [104]. As a result, the therapeutic ratio of these molecules is dramatically improved.
By taking into account the design considerations discussed here, future nanomedicines can be designed and implemented to dramatically improve patient outcomes. Below, we discuss the potential clinical impact of targeted nanomedicines and the future directions for optimal NP platform development.
6. Clinical impact of targeted nanoparticles
The potential clinical impact of targeting therapies specifically to cancer cells is widely recognized both by researchers and clinicians, and many cancers previously treated with chemotherapies have transitioned to treatments involving targeting agents. Monoclonal antibodies, for example, are often used for cancer treatment because they can specifically target receptors that are overexpressed on diseased cells compared to healthy cells. Currently, monoclonal antibodies are used to treat breast cancer (trastuzumab), chronic lymphocytic leukemia (alemtuzumab), and Hodgkins lymphoma (brentuximab vedotin), among others [105]. The widespread use of monoclonal antibodies in a variety of cancers is due to their high specificity towards their target and the subsequent anticancer effects. However, the clinical use of monoclonal antibodies and other targeting agents is met with limitations such as off-target side effects, high required treatment dosages, and low binding affinity to their targeted proteins. Antibody–NP conjugates overcome many of these limitations by increasing tumor-specific uptake [106], enhancing binding affinity between antibody and receptor [24, 26], and decreasing required treatment dosages [24]. Although the majority of NPs currently investigated in the clinic are not targeted, recently several targeted formulations have been introduced to enhance tumor-specific binding, uptake, and retention.
Although targeted nanosystems are yet to be approved by the FDA, there are a few currently in Phase I and II clinical trials (Fig. 5 and Table 2) for a variety of solid tumor cancers, some of which are showing great promise [107]. Of the actively targeted nanomedicines in clinical trials, antibody-based formulations are the most prevalent (Fig. 5). The common goal of these targeted systems is to improve upon tumor-specific delivery of the loaded cargo [106, 108]. For a thorough discussion of targeted NPs currently under investigation in clinical trials, readers should refer to recent reviews by Sonna et al. [106] and Anselmo et al. [107]. The majority of targeted NPs currently in clinical trials are either liposomal or polymeric because these materials are biocompatible and porous, thus enabling passive diffusion of the loaded cargo following delivery to the tumor site. Harder materials, such as gold, have been extensively studied for active targeting in preclinical applications [24, 109, 110], so it is expected that these types of materials will appear in clinical trials in the near future.
Figure 5.
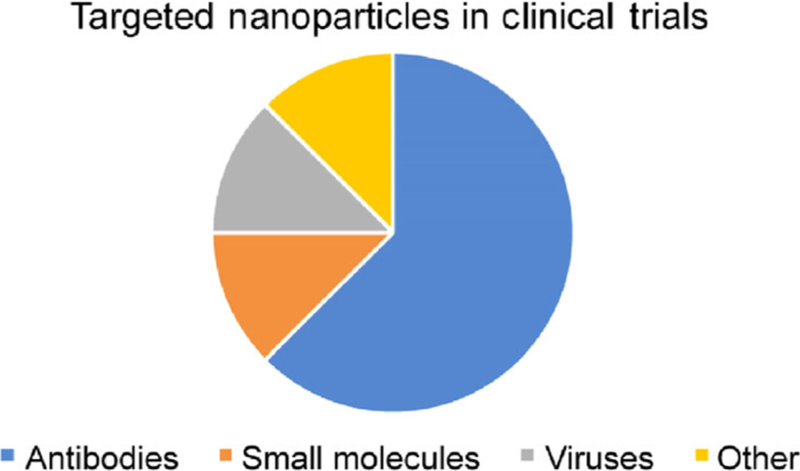
The distribution of the types of actively targeted nanoparticles in clinical trials from 2000 to present. Antibodies were the most prevalent targeting agents during this time. Clinical trials were found at https://clinicaltrials.gov/ and filtered with the following search terms: cancer, nanoparticles or nanomedicine, target or targeted, peptide or proteins, and membrane wrapped or coated. Nanoparticles found by the search that were only passively targeted were excluded from the analysis to create this chart.
Table 2.
Clinical trials found at https://clinicaltrials.gov/ with the terms: cancer, nanoparticles or nanomedicine, target or targeted, peptide or proteins, and membrane wrapped or coated. Passively targeted nanoparticles have been removed from the results so that only actively targeted systems are represented
| Identifier | Study title | Target | Phase | Phase Start date | Status |
|---|---|---|---|---|---|
| NCT00505713 | Safety and efficacy study using Rexin-G for sarcoma | Virus | I/II | 2007 | Completed |
| NCT00689065 | Safety study of CALAA-01 to treat solid tumor cancers | Virus | I | 2008 | Terminated |
| NCT01702129 | Anti-EGFR immunoliposomes in solid tumors | Antibody | I | 2007 | Completed |
| NCT02340156 | Phase II study of combined temozolomide and SGT-53 for treatment of recurrent glioblastoma | Antibody | II | 2014 | Recruiting |
| NCT02369198 | MesomiR 1: A phase I study of TargomiRs as 2nd or 3rd line treatment for patients with recurrent MPM and NSCLC | Antibody | I | 2015 | Completed |
| NCT02620865 | Bispecific antibody armed activated T-cells with aldesleukin and sargramostim in treating patients with locally advanced or metastatic pancreatic cancer | Antibody | I/II | 2015 | Active |
| NCT02766699 | A study to evaluate the safety, tolerability and immunogenicity of EGFR(V)-EDV-Dox in subjects with recurrent glioblastoma multiforme (GBM) (cerebral EDV) | Antibody | I | 2016 | Recruiting |
| NCT02979392 | Phase I study of TENPA in advanced solid cancer | Not specified | I | 2016 | Not yet recruiting |
There are several challenges to the clinical translatability of targeted NPs. First, targeting agents change the pharmacokinetics of NPs and their cargo. This requires researchers to thoroughly characterize and evaluate the biodistribution, release kinetics, and therapeutic efficacy of every unique NP formulation rather than only the naked therapeutic entity [108]. Second, solid tumors contain a very heterogeneous population of cells that express a wide variety of cell surface receptors that each influence tumor growth through different downstream signaling pathways. The anticancer effects of NPs that target only one cell surface receptor may therefore be limited. To overcome this challenge, NPs that target a variety of biomarkers should be clinically investigated. In addition to promoting tumor retention, these multi-targeted NPs may be able to inhibit cross-talk between several oncogenic signaling pathways, resulting in improved therapeutic outcomes. Note that biomimetic membrane- wrapped NPs will inherently target a variety of cell surface receptors, and this may be an advantage of these systems relative to NPs coated with a single type of biomolecular targeting agent. While a few platforms have been developed to target several biomarkers, they have not yet been introduced in the clinic. A final consideration for the clinical impact of targeted NPs is that the cost of production is often higher than non-targeted NPs or free ligands due to losses during NP synthesis and purification. However, this issue is counterbalanced by the fact that NPs containing targeting agents typically require lower treatment doses than their untargeted constituents, which can ultimately save costs for both pharmaceutical companies and patients.
Although biomimetic NPs have not yet appeared in the clinic, several particle designs for cancer treatment are currently in preclinical development [4, 111]. NP-carrying bacteria, for example, are being investigated for tumor-specific delivery of drugs, but their clinical implementation is limited by safety considerations such as gene transfer to healthy cells and environmental impacts associated with the production and generated waste of the bacterial carriers [112–114]. In addition to bacteria, other biomimetic NPs that were previously discussed in this review, such as HDL-mimicking particles, provide inherent benefits for drug delivery, particularly their natural affinity towards targets that are overexpressed on cancer cells. However, these NP carriers may also lead to off-target side effects, since they can specifically bind to their natural targets on healthy cells, thus delivering their loaded cargo to non-diseased tissues. As researchers are developing platforms that replicate natural structures for cancer treatment, it is critical to consider the off-target side effects due to their innate binding that can hinder therapy success.
Cell membrane-wrapped NPs are another class of biomimetic NPs currently being investigated in pre-clinical trials. These NPs are ideal for drug delivery to tumors because they imitate the natural presentation of native cells, and therefore are generally accepted by the host with minimal phagocytic uptake. They can also effectively deliver their cargo to diseased tissues by exploiting cells’ natural interactions with other cells and tissues [115]. The clinical translatability of membrane-wrapped NPs, however, is met with several supply and regulatory issues [116, 117]. First, cell membrane wrapping is innately heterogeneous because each cell may express different levels of biomarkers and present them differently. This heterogeneity is a key difference from the standard production and quality assurance procedures followed for other targeting agents (for example, monoclonal antibodies). Further, the heterogeneity in membrane-wrapped NPs makes it very challenging to control the batch-to-batch consistency of the particles [116]. Therefore, the supply chain of membrane-wrapped NPs is challenged by heterogeneous batches, mass production, and high testing costs [115]. In addition to supply and production issues, there are also regulatory hurdles that need to be accounted for in the clinical use of membrane-wrapped NPs. For example, the use of biological coatings raises safety concerns such as off-target effects and nonspecific toxicities to healthy tissues that are innate targets for the surface proteins wrapped onto the NPs, particularly since these platforms target numerous biomarkers. With these considerations in mind, we anticipate that the clinical translatability of biomimetic NPs will require new regulatory standards and toxicity analyses to ensure therapeutic efficacy and safety.
As the research and development of targeted NPs reaches clinical stages, we can expect the market value of nanomedicines to exponentially increase. According to the BBC, the Advanced and Targeted Drug Delivery Market, which was valued at $168 billion in 2016, is projected to grow to $319 billion by 2021 [118]. Further, the global nanomedicine market is expected to reach $350.8 billion by 2025, with a compound annual growth rate of 11.2% [119]. The substantial growth expected for the NP market, and targeted drug delivery in particular, truly demonstrates the market potential and the need for new therapies to be introduced into the clinic that are more cost effective and cause fewer side effects than currently available cancer therapies.
7. Future outlook
While it will take further experimentation and optimization to perfect the systems discussed in this review, there is substantial potential in the future outlook for both bioconjugation-based and biomimetic NP platforms. As mentioned in the preceding sections, researchers are actively investigating the use of targeting agents for both cell binding and signal cascade interference, and such multifaceted approaches have the potential to notably enhance the therapeutic effect of current NP systems. By optimizing the design considerations discussed in this review and exploring the realm of combination therapy, researchers can develop NPs that better address the heterogeneous needs of cancer therapy and allow nanomedicine to reach its full potential.
Beyond using targeted NPs to study and treat cancer, there are a wide range of other diseases that could benefit from targeted nanomedicines, including atherosclerosis; fibroproliferative lung, kidney, and joint diseases; and hemophilia [120]. Expanding the bioconjugation and biomimicry techniques already being used for cancer nanomedicine to create NPs to target different diseases may result in faster solutions. However, when developing any of these treatments, it is important to keep the scalability and reproducibility of the NPs in mind for manufacturing purposes. In summary, there has been great progress in developing targeted nanotherapeutics via bioconjugation or biomimetic strategies over the past several decades, but there is still much more to be done to reach the eventual goal of safe, specific, and effective cancer treatment through nanomedicine.
Acknowledgements
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM119659, and by a grant from the W.M. Keck Foundation. R. S. R. received support from an American Association of University Women Dissertation Fellowship. The content is solely the responsibility of the authors and does not necessarily reflect the views of the funding agencies.
References
- [1].Wang ZL; Qiao RR; Tang N; Lu ZW; Wang H; Zhang ZX; Xue XD; Huang ZY; Zhang SR; Zhang GX et al. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials 2017, 127, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].El-Sayed IH; Huang X; El-Sayed MA Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett 2006, 239, 129–135. [DOI] [PubMed] [Google Scholar]
- [3].Damodaran S; Olson EM Targeting the human epidermal growth factor receptor 2 pathway in breast cancer. Hosp. Pract 2012, 40, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dehaini D; Fang RH; Zhang LF Biomimetic strategies for targeted nanoparticle delivery. Bioeng. Transl. Med 2016, 1, 30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Byrne JD; Betancourt T; Brannon-Peppas L Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev 2008, 60, 1615–1626. [DOI] [PubMed] [Google Scholar]
- [6].Wang M; Thanou M Targeting nanoparticles to cancer. Pharmacol. Res 2010, 62, 90–99. [DOI] [PubMed] [Google Scholar]
- [7].Riley RS; Day ES Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. WIREs Nanomed. Nanobiotechnol 2017, 9, e1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumar A; Ma HL; Zhang X; Huang KY; Jin SB; Liu J; Wei T; Cao WP; Zou GZ; Liang X-J Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 2012, 33, 1180–1189. [DOI] [PubMed] [Google Scholar]
- [9].Dam DHM; Culver KSB; Odom TW Grafting aptamers onto gold nanostars increases in vitro efficacy in a wide range of cancer cell types. Mol. Pharm 2014, 11, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lowery AR; Gobin AM; Day ES; Halas NJ; West JL Immunonanoshells for targeted photothermal ablation of tumor cells. Int. J. Nanomedicine 2006, 1, 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Loo C; Lowery A; Halas N; West J; Drezek R Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett 2005, 5, 709–711. [DOI] [PubMed] [Google Scholar]
- [12].Brannon-Peppas L; Blanchette JO Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev 2012, 64, 206–212. [DOI] [PubMed] [Google Scholar]
- [13].Hou Y; Zhou J; Gao ZY; Sun XY; Liu CY; Shangguan DH; Yang WS; Gao MY Protease- activated ratiometric fluorescent probe for pH mapping of malignant tumors. ACS Nano 2015, 9, 3199–3205. [DOI] [PubMed] [Google Scholar]
- [14].Jeong S; Park JY; Cha MG; Chang HJ; Kim YI; Kim H-M; Jun B-H; Lee DS; Lee Y-S; Jeong JM et al. Highly robust and optimized conjugation of antibodies to nanoparticles using quantitatively validated protocols. Nanoscale 2017, 9, 2548–2555. [DOI] [PubMed] [Google Scholar]
- [15].Kumar S; Aaron J; Sokolov K Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat. Protoc 2008, 3, 314–320. [DOI] [PubMed] [Google Scholar]
- [16].Joshi PP; Yoon SJ; Hardin WG; Emelianov S; Sokolov KV Conjugation of antibodies to gold nanorods through Fc portion: Synthesis and molecular specific imaging. Bioconjug. Chem 2013, 24, 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Parolo C; de la Escosura-Muñiz A; Polo E; Grazú V; de la Fuente JM; Merkoçi A Design, preparation, and evaluation of a fixed-orientation antibody/gold-nanoparticle conjugate as an immunosensing label. ACS Appl. Mater. Interfaces 2013, 5, 10753–10759. [DOI] [PubMed] [Google Scholar]
- [18].Moynihan TJ HER2-positive breast cancer: What is it? https://www.mayoclinic.org/breast-cancer/expert-answers/faq-20058066 (accessed Mar 14, 2018).
- [19].Master AM; Sen Gupta A EGF receptor-targeted nanocarriers for enhanced cancer treatment. Nanomedicine 2012, 7, 1895–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Billingsley MM; Riley RS; Day ES Antibody- nanoparticle conjugates to enhance the sensitivity of ELISA- based detection methods. PLoS One 2017, 12, e0177592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bae KH; Lee K; Kim C; Park TG Surface functionalized hollow manganese oxide nanoparticles for cancer targeted siRNA delivery and magnetic resonance imaging. Biomaterials 2011, 32, 176–184. [DOI] [PubMed] [Google Scholar]
- [22].Palanca-Wessels MC; Booth GC; Convertine AJ; Lundy BB; Berguig GY; Press MF; Stayton PS; Press OW Antibody targeting facilitates effective intratumoral siRNA nanoparticle delivery to HER2-overexpressing cancer cells. Oncotarget 2016, 7, 9561–9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dilnawaz F; Singh A; Mohanty C; Sahoo SK Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials 2010, 31, 3694–3706. [DOI] [PubMed] [Google Scholar]
- [24].Riley RS; Day ES Frizzled7 antibody-functionalized nanoshells enable multivalent binding for Wnt signaling inhibition in triple negative breast cancer cells. Small 2017, 13, 1700544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scott AM; Wolchok JD; Old LJ Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [DOI] [PubMed] [Google Scholar]
- [26].Jiang W; Kim BYS; Rutka JT; Chan WCW Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol 2008, 3, 145–150. [DOI] [PubMed] [Google Scholar]
- [27].Schardt JS; Oubaid JM; Williams SC; Howard JL; Aloimonos CM; Bookstaver ML; Lamichhane TN; Sokic S; Liyasova MS; O’Neill M et al. Engineered multivalency enhances affibody-based HER3 inhibition and downregulation in cancer cells. Mol. Pharmacol 2017, 14, 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Prakash JS; Rajamanickam K Aptamers and their significant role in cancer therapy and diagnosis. Biomedicines 2015, 3, 248–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu X; Chen J; Wu M; Zhao JX Aptamers: Active targeting ligands for cancer diagnosis and therapy. Theranostics 2015, 5, 322–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hicke BJ; Stephens AW; Gould T; Chang Y-F; Lynott CK; Heil J; Borkowski S; Hilger C-S; Cook G; Warren S et al. Tumor targeting by an aptamer. J. Nucl. Med 2006, 47, 668–678. [PubMed] [Google Scholar]
- [31].Valetti S; Mura S; Noiray M; Arpicco S; Dosio F; Vergnaud J; Desmaële D; Stella B; Couvreur P Peptide conjugation: Before or after nanoparticle formation? Bioconjug. Chem 2014, 25, 1971–1983. [DOI] [PubMed] [Google Scholar]
- [32].Choi HS; Liu WH; Liu FB; Nasr K; Misra P; Bawendi MG; Frangioni JV Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol 2010, 5, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nasongkla N; Bey E; Ren JM; Ai H; Khemtong C; Guthi JS; Chin SF; Sherry AD; Boothman DA; Gao JM Multifunctional polymeric micelles as cancer- targeted, MRI-ultrasensitive drug delivery systems. Nano Lett 2006, 6, 2427–2430. [DOI] [PubMed] [Google Scholar]
- [34].Xiong XB; Lavasanifar A Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano 2011, 5, 5202–5213. [DOI] [PubMed] [Google Scholar]
- [35].Milane L; Duan ZF; Amiji M Therapeutic efficacy and safety of paclitaxel/lonidamine loaded EGFR-targeted nanoparticles for the treatment of multi-drug resistant cancer. PLoS One 2011, 6, e24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Milane L; Duan ZF; Amiji M Pharmacokinetics and biodistribution of lonidamine/paclitaxel loaded, EGFR-targeted nanoparticles in an orthotopic animal model of multi-drug resistant breast cancer. Nanomedicine 2011, 7, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Reddy GR; Bhojani MS; McConville P; Moody J; Moffat BA; Hall DE; Kim G; Koo YEL; Woolliscroft MJ; Sugai JV et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res 2006, 12, 6677–6686. [DOI] [PubMed] [Google Scholar]
- [38].Sanna V; Nurra S; Pala N; Marceddu S; Pathania D; Neamati N; Sechi M Targeted nanoparticles for the delivery of novel bioactive molecules to pancreatic cancer cells. J. Med. Chem 2016, 59, 5209–5220. [DOI] [PubMed] [Google Scholar]
- [39].Aina OH; Sroka TC; Chen ML; Lam KS Therapeutic cancer targeting peptides. Biopolymers 2002, 66, 184–199. [DOI] [PubMed] [Google Scholar]
- [40].Juliano RL; Alam R; Dixit V; Kang HM Cell- targeting and cell-penetrating peptides for delivery of therapeutic and imaging agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2009, 1, 324–336. [DOI] [PubMed] [Google Scholar]
- [41].Zwicke GL; Mansoori GA; Jeffery CJ Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev 2012, 3, 18496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bazak R; Houri M; El Achy S; Kamel S; Refaat T Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol 2015, 141, 769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang C; Cheng L; Liu Z Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 2011, 32, 1110–1120. [DOI] [PubMed] [Google Scholar]
- [44].Liu T; Zeng LL; Jiang WT; Fu YT; Zheng WJ; Chen TF Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomedicine 2015, 11, 947–958. [DOI] [PubMed] [Google Scholar]
- [45].Yu B; Li XL; Zheng WJ; Feng YX; Wong Y-S; Chen TF PH-responsive cancer-targeted selenium nanoparticles: A transformable drug carrier with enhanced theranostic effects. J. Mater. Chem. B 2014, 2, 5409–5418. [DOI] [PubMed] [Google Scholar]
- [46].Zhang Q; Wang XL; Li PZ; Nguyen KT; Wang XJ; Luo Z; Zhang HC; Tan NS; Zhao YL Biocompatible, uniform, and redispersible mesoporous silica nanoparticles for cancer-targeted drug delivery in vivo. Adv. Funct. Mater 2014, 24, 2450–2461. [Google Scholar]
- [47].Huang YY; He LZ; Liu W; Fan CD; Zheng WJ; Wong YS; Chen TF Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials 2013, 34, 7106–7116. [DOI] [PubMed] [Google Scholar]
- [48].Valencia PM; Pridgen EM; Rhee M; Langer R; Farokhzad OC; Karnik R Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano 2013, 7, 10671–10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Assanhou AG; Li WY; Zhang L; Xue LJ; Kong LY; Sun HB; Mo R; Zhang C Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a tpgs and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials 2015, 73, 284–295. [DOI] [PubMed] [Google Scholar]
- [50].Xiao B; Han MK; Viennois E; Wang LX; Zhang MZ; Si XY; Merlin D Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy. Nanoscale 2015, 7, 17745–17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dai Q; Walkey C; Chan WCW Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angew. Chem., Int. Ed 2014, 53, 5093–5096. [DOI] [PubMed] [Google Scholar]
- [52].Zhou H; Fan ZY; Deng JJ; Lemons PK; Arhontoulis DC; Bowne WB; Cheng H Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett 2016, 16, 3268–3277. [DOI] [PubMed] [Google Scholar]
- [53].Wilhelm S; Tavares AJ; Dai Q; Ohta S; Audet J; Dvorak HF; Chan WCW Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater 2016, 1, 16014. [Google Scholar]
- [54].Hauert S; Berman S; Nagpal R; Bhatia SN A computational framework for identifying design guidelines to increase the penetration of targeted nanoparticles into tumors. Nano Today 2013, 8, 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kroll AV; Fang RH; Zhang LF Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjug. Chem 2017, 28, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Verhoef JJ; Anchordoquy TJ Questioning the use of pegylation for drug delivery. Drug Deliv. Transl. Res 2013, 3, 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rodriguez PL; Harada T; Christian DA; Pantano DA; Tsai RK; Discher DE Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339, 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang ZH; Chen J; Ding LL; Jin H; Lovell JF; Corbin IR; Cao WG; Lo PC; Yang M; Tsao MS et al. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small 2010, 6, 430–437. [DOI] [PubMed] [Google Scholar]
- [59].Li JH; Ai YW; Wang LH; Bu PC; Sharkey CC; Wu QH; Wun B; Roy S; Shen XL; King MR Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials 2016, 76, 52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rohovie MJ; Nagasawa M; Swartz JR Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med 2017, 2, 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dehaini D; Wei X; Fang RH; Masson S; Angsantikul P; Luk BT; Zhang Y; Ying M; Jiang Y; Kroll AV et al. Erythrocyte–platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv. Mater 2017, 29, 1606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhu JY; Zheng DW; Zhang MK; Yu WY; Qiu WX; Hu JJ; Feng J; Zhang XZ Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett 2016, 16, 5895–5901. [DOI] [PubMed] [Google Scholar]
- [63].Hu CMJ; Fang RH; Wang KC; Luk BT; Thamphiwatana S; Dehaini D; Nguyen P; Angsantikul P; Wen CH; Kroll AV et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hu C-MJ; Zhang L; Aryal S; Cheung C; Fang RH; Zhang LF Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl.Acad. Sci. USA 2011, 108, 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Luk BT; Zhang LF Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 2015, 220, 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McMahon KM; Foi L; Angeloni NL; Giles FJ; Gordon LI; Thaxton CS Synthetic high-density lipoprotein-like nanoparticles as cancer therapy. In Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer; Mirkin CA; Meade TJ; Petrosko SH; Stegh AH, Eds.; Springer: Switzerland, 2015; Vol. 166, pp 129–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Marrache S; Dhar S Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc. Natl. Acad. Sci. USA 2013, 110, 9445–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McMahon KM; Mutharasan RK; Tripathy S; Veliceasa D; Bobeica M; Shumaker DK; Luthi AJ; Helfand BT; Ardehali H; Mirkin CA et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett 2011, 11, 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Simberg D; Duza T; Park JH; Essler M; Pilch J; Zhang LL; Derfus AM; Yang M; Hoffman RM; Bhatia S et al. Biomimetic amplification of nanoparticle homing to tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wei XL; Gao J; Fang RH; Luk BT; Kroll AV; Dehaini D; Zhou JR; Kim HW; Gao WW; Lu WX et al. Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials 2016, 111, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Luk BT; Fang RH; Hu CMJ; Copp JA; Thamphiwatana S; Dehaini D; Gao WW; Zhang K; Li SL; Zhang LF Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics 2016, 6, 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lai P-Y; Huang R-Y; Lin S-Y; Lin Y-H; Chang C-W Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv 2015, 5, 98222–98230. [Google Scholar]
- [73].Parodi A; Quattrocchi N; Van De Ven AL; Chiappini C; Evangelopoulos M; Martinez JO; Brown BS; Khaled SZ; Yazdi IK; Enzo MV et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol 2013, 8, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhai YH; Su JH; Ran W; Zhang PC; Yin Q; Zhang ZW; Yu HJ; Li YP Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics 2017, 7, 2575–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hu QY; Sun WJ; Qian CG; Wang C; Bomba HN; Gu Z Anticancer platelet-mimicking nanovehicles. Adv. Mater 2015, 27, 7043–7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Toledano Furman NE; Lupu-Haber Y; Bronshtein T; Kaneti L; Letko N; Weinstein E; Baruch L; Machluf M Reconstructed stem cell nanoghosts: A natural tumor targeting platform. Nano Lett 2013, 13, 3248–3255. [DOI] [PubMed] [Google Scholar]
- [77].Gao CY; Lin ZH; Jurado-Sánchez B; Lin XK; Wu ZG; He Q Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small 2016, 12, 4056–4062. [DOI] [PubMed] [Google Scholar]
- [78].Xuan MJ; Shao JX; Dai LR; He Q; Li JB Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv. Healthc. Mater 2015, 4, 1645–1652. [DOI] [PubMed] [Google Scholar]
- [79].Beduneau A; Ma Z; Grotepas CB; Kabanov A; Rabinow BE; Gong N; Mosley RL; Dou H; Boska MD; Gendelman HE Facilitated monocyte-macrophage uptake and tissue distribution of superparmagnetic iron-oxide nanoparticles. PLoS One 2009, 4, e4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pascucci L; Coccè V; Bonomi A; Ami D; Ceccarelli P; Ciusani E; Viganò L; Locatelli A; Sisto F; Doglia SM et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [DOI] [PubMed] [Google Scholar]
- [81].Ohno SI; Takanashi M; Sudo K; Ueda S; Ishikawa A; Matsuyama N; Fujita K; Mizutani T; Ohgi T; Ochiya T et al. Systemically injected exosomes targeted to EGFR deliver antitumor microrna to breast cancer cells. Mol. Ther 2013, 21, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tian YH; Li SP; Song J; Ji TJ; Zhu MT; Anderson GJ; Wei JY; Nie GJ A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [DOI] [PubMed] [Google Scholar]
- [83].Alhasan AH; Patel PC; Choi CHJ; Mirkin CA Exosome encased spherical nucleic acid gold nanoparticle conjugates as potent microRNA regulation agents. Small 2014, 10, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Berleman J; Auer M The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ. Microbiol 2013, 15, 347–354. [DOI] [PubMed] [Google Scholar]
- [85].Gujrati V; Kim S; Kim SH; Min JJ; Choy HE; Kim SC; Jon S Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 2014, 8, 1525–1537. [DOI] [PubMed] [Google Scholar]
- [86].Zhou H; Fan ZY; Lemons PK; Cheng H A facile approach to functionalize cell membrane-coated nanoparticles. Theranostics 2016, 6, 1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Xuan MJ; Shao JX; Dai LR; Li JB; He Q Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 9610–9618. [DOI] [PubMed] [Google Scholar]
- [88].Chen Z; Zhao PF; Luo ZY; Zheng MB; Tian H; Gong P; Gao GH; Pan H; Liu LL; Ma AQ et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano 2016, 10, 10049–10057. [DOI] [PubMed] [Google Scholar]
- [89].Sun HP; Su JH; Meng QS; Yin Q; Chen LL; Gu WW; Zhang PC; Zhang ZW; Yu HJ; Wang SL et al. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv. Mater 2016, 28, 9581–9588. [DOI] [PubMed] [Google Scholar]
- [90].Fang RH; Hu CMJ; Luk BT; Gao WW; Copp JA; Tai YY; O’Connor DE; Zhang LF Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 2014, 14, 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kennedy LC; Bear AS; Young JK; Lewinski NA; Kim J; Foster AE; Drezek RA T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale Res. Lett 2011, 6, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sadhukha T; O’Brien TD; Prabha S Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J. Control. Release 2014, 196, 243–251. [DOI] [PubMed] [Google Scholar]
- [93].Choi M-R; Stanton-Maxey KJ; Stanley JK; Levin CS; Bardhan R; Akin D; Badve S; Sturgis J; Robinson JP; Bashir R et al. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett 2007, 7, 3759–3765. [DOI] [PubMed] [Google Scholar]
- [94].Roger M; Clavreul A; Venier-Julienne MC; Passirani C; Sindji L; Schiller P; Montero-Menei C; Menei P Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials 2010, 31, 8393–8401. [DOI] [PubMed] [Google Scholar]
- [95].Pang L; Qin J; Han LM; Zhao WJ; Liang JM; Xie ZY; Yang P; Wang JX Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget 2016, 7, 37081–37091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Steinfeld U; Pauli C; Kaltz N; Bergemann C; Lee HH T lymphocytes as potential therapeutic drug carrier for cancer treatment. Int. J. Pharm 2006, 311, 229–236. [DOI] [PubMed] [Google Scholar]
- [97].Tan SW; Wu TT; Zhang D; Zhang ZP Cell or cell membrane-based drug delivery systems. Theranostics 2015, 5, 863–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Stephan MT; Moon JJ; Um SH; Bershteyn A; Irvine DJ Therapeutic cell engineering with surface- conjugated synthetic nanoparticles. Nat. Med 2010, 16, 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cheng H; Kastrup CJ; Ramanathan R; Siegwart DJ; Ma ML; Bogatyrev SR; Xu QB; Whitehead KA; Langer R; Anderson DG Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS Nano 2010, 4, 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].de Almeida CEB; Nascimento Alves L; Rocha HF; Cabral-Neto JB; Missailidis S Aptamer delivery of siRNA, radiopharmaceutics and chemotherapy agents in cancer. Int. J. Pharm 2017, 525, 334–342. [DOI] [PubMed] [Google Scholar]
- [101].Chen SY; Zhao XR; Chen JY; Chen J; Kuznetsova L; Wong SS; Ojima I Mechanism-based tumor-targeting drug delivery system. validation of efficient vitamin receptor-mediated endocytosis and drug release. Bioconjug. Chem 2010, 21, 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Biabanikhankahdani R; Alitheen NBM; Ho KL; Tan WS PH-responsive virus-like nanoparticles with enhanced tumour-targeting ligands for cancer drug delivery. Sci. Rep 2016, 6, 37891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kelley EG; Albert JNL; Sullivan MO; Epps TH III. Stimuli-responsive copolymer solution and surface assemblies for biomedical applications. Chem. Soc. Rev 2013, 42, 7057–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Greco CT; Epps TH; Sullivan MO Mechanistic design of polymer nanocarriers to spatiotemporally control gene silencing. ACS Biomater. Sci. Eng 2016, 2, 1582–1594. [DOI] [PubMed] [Google Scholar]
- [105].Scott AM; Allison JP; Wolchok JD Monoclonal antibodies in cancer therapy. Cancer Immun 2012, 12, 14. [PMC free article] [PubMed] [Google Scholar]
- [106].Sanna V; Pala N; Sechi M Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomedicine 2014, 9, 467–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Anselmo AC; Mitragotri S Nanoparticles in the clinic. Bioeng. Transl. Med 2016, 1, 10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kennedy PJ; Oliveira C; Granja PL; Sarmento B Antibodies and associates: Partners in targeted drug delivery. Pharmacol. Ther 2017, 177, 129–145. [DOI] [PubMed] [Google Scholar]
- [109].Bernardi RJ; Lowery AR; Thompson PA; Blaney SM; West JL Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: An in vitro evaluation using human cell lines. J. Neurooncol 2008, 86, 165–172. [DOI] [PubMed] [Google Scholar]
- [110].Park J-H; von Maltzahn G; Xu MJ; Fogal V; Kotamraju VR; Ruoslahti E; Bhatia SN; Sailor MJ Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Rink JS; Plebanek MP; Tripathy S; Thaxton CS Update on current and potential nanoparticle cancer therapies. Curr. Opin. Oncol 2013, 25, 646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Akin D; Sturgis J; Ragheb K; Sherman D; Burkholder K; Robinson JP; Bhunia AK; Mohammed S; Bashir R Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat. Nanotechnol 2007, 2, 441–449. [DOI] [PubMed] [Google Scholar]
- [113].Luo CH; Huang CT; Su CH; Yeh CS Bacteria- mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett 2016, 16, 3493–3499. [DOI] [PubMed] [Google Scholar]
- [114].Wegmann U; Carvalho AL; Stocks M; Carding SR Use of genetically modified bacteria for drug delivery in humans: Revisiting the safety aspect. Sci. Rep 2017, 7, 2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Blanco E; Shen HF; Ferrari M Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol 2015, 33, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Balmert SC; Little SR Biomimetic delivery with micro- and nanoparticles. Adv. Mater 2012, 24, 3757–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Meyer RA; Sunshine JC; Green JJ Biomimetic particles as therapeutics. Trends Biotechnol 2015, 33, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kelly Scientific Publications. Advanced and Targeted Drug Delivery Market Segmentation, Analysis, & Forecast to 2021 https://www.researchandmarkets.com/research/m3gm88/advanced_and (accessed Mar 14, 2018).
- [119].Grand View Research. Nanomedicine Market Size Worth $350.8 Billion by 2025 https://www.grandviewresearch.com/press-release/global-nanomedicine-market (accessed Mar 14, 2018).
- [120].Huang CC; Chiang CK; Lin ZH; Lee KH; Chang HT Bioconjugated gold nanodots and nanoparticles for protein assays based on photoluminescence quenching. Anal. Chem 2008, 80, 1497–1504. [DOI] [PubMed] [Google Scholar]


