Abstract
Objective
To investigate age-associated changes in airway microbiome composition and their relationships with lung function and arterial stiffness among genetically matched young and elderly pairs.
Methods
Twenty-four genetically linked family pairs comprised of younger (≤40 years) and older (≥60 years) healthy participants were recruited (Total n = 48). Lung function and arterial stiffness (carotid-femoral pulse wave velocity (PWV) and augmentation index (AIx)) were assessed. Sputum samples were collected for targeted 16S rRNA gene amplicon sequencing and correlations between microbiome composition, lung function and arterial stiffness were investigated.
Results
Elderly participants exhibited reductions in lung function (FEV1 (p<0.001), FVC (p<0.001) and percentage FEV1/FVC (p = 0.003)) and a 1.3–3.9-fold increase in arterial stiffness (p<0.001) relative to genetically related younger adults. Elderly adults had a higher relative abundance of Firmicutes (p = 0.035) and lower relative abundance of Proteobacteria (p = 0.014), including specific genera Haemophilus (p = 0.024) and Lautropia (p = 0.020) which were enriched in the younger adults. Alpha diversity was comparable between young and elderly pairs (p>0.05) but was inversely associated with lung function (FEV1%Predicted and FVC %Predicted) in the young (p = 0.006 and p = 0.003) though not the elderly (p = 0.481 and p = 0.696). Conversely, alpha diversity was negatively associated with PWV in the elderly (p = 0.01) but not the young (p = 0.569). Specifically, phylum Firmicutes including the genus Gemella were correlated with lung function (FVC %Predicted) in the young group (p = 0.047 and p = 0.040), while Fusobacteria and Leptotrichia were associated with arterial stiffness (PWV) in the elderly (both p = 0.004).
Conclusion
Ageing is associated with increased Firmicutes and decreased Proteobacteria representation in the airway microbiome among a healthy Asian cohort. The diversity and composition of the airway microbiome is independently associated with lung function and arterial stiffness in the young and elderly groups respectively. This suggests differential microbial associations with these phenotypes at specific stages of life with potential prognostic implications.
Introduction
Ageing is an identified risk factor for several chronic health conditions, including lung and cardiovascular disease (CVD), that exhibit disproportionate susceptibility, morbidity and mortality in older individuals [1, 2]. During ageing, subclinical lung function decline [3, 4], vascular endothelial dysfunction and arterial stiffening occur in the healthy population [5–7]. In older individuals above age 40 [8], parameters of lung function are inversely associated with arterial stiffness parameters, even after adjustment for cardiovascular risk factors, smoking history and lung disease [9, 10]. Reduced pulmonary function is independently associated with precursors for CVD including arterial stiffness, regardless of age, sex or anthropometry [8–12] and is an independent risk factor for cardiovascular morbidity and mortality [13]. Pulmonary function also predicts the development of atherosclerotic plaques and is a strong risk factor for arterial stiffness, suggesting a causal relationship between the decline in lung function and arterial stiffness [11, 12, 14].
While evidence supports the relationship between declining lung function, arterial stiffness and subsequent onset of CVD [13], the mechanisms underlying these associations are not known. Possible mechanisms include inflammation, as arterial stiffness is markedly increased in patients with chronic obstructive pulmonary disease (COPD), a condition characterized by airflow obstruction and inflammation [15–17]. While systemic inflammation, weight, smoking history, hypercholesterolemia, hypertension and diabetes have all been implicated, they do not fully account for the relationship between pulmonary function and arterial stiffness, suggesting that other factors could be involved [9, 13, 18].
The lung microbiome encompasses the collective genetic information of all microorganisms that colonize the lung and differs between healthy and diseased individuals [19]. Lower microbiome diversity and the presence of specific microbial taxa are associated with decreased lung function in pathogenic disease states [19, 20]. In healthy individuals, the lung microbiome comprises a community of low density and transiently present microorganisms, whereas pathogenic organisms predominate in disease [21–23]. While the effects of ageing on the lung microbiome remain to be established, the gut microbiome has been found to influence healthy ageing through alteration of inflammatory markers, diet and immune response [24–26]. Furthermore, age-associated alterations in immune function may affect the microbiome (or vice versa), generating low-grade inflammation, a key contributor to arterial stiffness and declining lung function [27]. An emerging question is whether the observed immunopathological associations between gut microbiota and disease also hold true at other anatomical sites such as the lung, where the microbiome may underpin age-associated changes in lung function with a consequent impact on arterial stiffness.
Here we investigated the effect of age on the airway microbiome in healthy individuals of Asian origin. Age-related changes in airway microbiota, between young and elderly family pairs, were assessed for their association with normative age-associated changes in lung function and arterial stiffness, as potential prognostic indicators of cardiorespiratory health.
Methods
Subjects
Twenty-four genetically linked family pairs (parent and child) were prospectively recruited. Younger participants were 22–39 years old and older participants were 60–71 years old. All participants were non-smokers, not on long-term inhaled medication, had no history of chronic respiratory diseases and resided in Singapore over the 10–12-month period preceding recruitment. None of the female participants were on oral contraceptives or hormone replacement therapy. The participants were recruited through posters, social media and community outreach events. All the participants were briefed on the nature and risks involved in the study and their rights to withdraw their participation without obligation before giving their written informed consent to participate in the study. The procedures in the study were approved by the institutional review board of Nanyang Technological University, Singapore (IRB-2017-12-010).
Study design and procedures
Participants abstained from caffeine and dietary supplementation for 24 h and kept to their regular diet and sleep routines, as well as refrained from strenuous physical activity for 48 h prior to their visit to the laboratory. Participants arrived at the laboratory between 0900 h and 1000 h after having consumed a light meal >2 h before the trial. They had their blood pressure (BP) measured and declared that they were well for participation. Nude body weight was measured using an electronic scale (SECA, Hamburg, Germany) and height was measured using a stadiometer (SECA). Body mass index (BMI) was calculated as body weight (kg) divided by height (m) squared. Waist circumference (WC) was recorded using a tape measure (SECA) placed snugly at the waistline, midway between the lowest ribs and iliac crest in a standing position [28], recorded to the nearest 0.1 cm in triplicate and averaged from the measurements.
Sputum collection and processing
Participants rinsed their mouth with water, before careful instructions were given on how to produce sputum from a deep cough, using the huff cough method [29]. Participants took three deep maximal breaths, before coughing as hard and as deeply as possible in a standing position. Sputum was collected by spontaneous expectorate from a deep cough (0.5–2 g) into sterile containers and were visually inspected before placing on ice immediately. Sputasol (ThermoFisher Scientific, Massachusetts, United States) was added into the sputum container in equal amounts. The sputum sample was then shaken at 200 rpm at 37 °C for 15 min. RNALater solution (ThermoFisher Scientific, Massachusetts, United States) was added and the sample stored in 1 mL aliquots at –80 °C. Saliva samples were assessed in 6 participants (3 matched pairs) to allow comparative assessments between oral and lung sampling. Prior to sputum collection, participants rinsed their mouth with water and saliva samples (5 mL) were collected into a 50 mL sterile tube by allowing saliva to accumulate before expectoration and the samples were placed on ice immediately [30]. Saliva samples were aliquoted (500 μL) and RNALater solution (1 mL) added and samples stored at –80 °C.
Spirometry
Lung function measurement was conducted using a portable spirometer (Spirolab, MIR Medical International Research SRL, Italy), according to international guidelines [31]. Lung function parameters were determined as the forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and percentage of FEV1/FVC. The FEV1 and FVC percentage predicted were calculated in the Spirolab software by the ATS/ERS standards, depending on ethnicity group [32].
Arterial stiffness measurement
An indirect and non-invasive measure of arterial stiffness was determined by the SphygmoCor XCEL device (AtCor Medical Pvt, Ltd, Sydney, NSW, Australia). The arterial stiffness parameters included carotid-femoral arterial pulse wave velocity (PWV) and augmentation index (AIx). The participants rested quietly in a supine position at room temperature for 15 min before measurements of arterial pulse pressure waveforms, by an inflated cuff at the brachial artery. SphygmoCor system calculates the central aortic augmentation pressure (AP) by subtracting the pressure at the first systole resulting from the return of the reflected wave from the systolic pressure. The AIx was calculated as the ratio of AP to pulse pressure [33]. PWV was measured simultaneously with pressure transducers, by acquiring a carotid pulse by applanation tonometry and a femoral pulse by volumetric displacement, within a cuff around the upper thigh (femoral artery) [34, 35]. The pulse waves were captured electronically on a computer using the SphygmoCor system and accepted by the system after consistent high-quality waveforms were measured. The average of approximately 3–5 measurements were taken.
DNA extraction and 16s rRNA gene sequencing
Sputum and saliva samples were thawed on ice and homogenised using glass beads (1mm, Sigma-Aldrich) using a bead mill homogeniser (VWR). DNA was purified using the Roche High-pure PCR Template Preparation Kit (Roche) as previously described [36]. Blank extractions from sterile PBS were also performed and served as negative extraction controls. All extracted samples were quantitated using the Qubit dsDNA High Sensitivity (HS) Assay Kit (Invitrogen) and visually assessed for integrity by electrophoresis on a 0.8% agarose gel.
Sequence data processing and taxonomic assignment
Using extracted sputum DNA samples, libraries for targeted amplicon sequencing were prepared following the “16S Metagenomic Sequencing Library Preparation” guide (Part# 15044223 rev. B, Illumina) [37]. This 300-bp paired-end sequencing protocol was performed on a MiSeq sequencing platform (Illumina) at the Lee Kong Chian School of Medicine (LKCMedicine), Singapore. Targeted amplicon sequences were analysed using the 16S metagenomics tool (version 1.0.1; Illumina), using as a taxonomic database the Illumina-curated version of the May 2013 Greengenes Consortium Database (greengenes.secondgenome.com) release and the Ribosomal Database Project (RDP) Classifier as the classification algorithm. Control samples from negative PCR and blank DNA extractions were also sequencing and assessed to detect potential contaminants using the decontam statistical package [38].
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences, version 23 (SPSS, Inc., Chicago, IL) and R version 3.3.3 (R Foundation for statistical computing, Vienna, Austria). Numerical variables are presented as mean (standard deviation, SD) in text and figures unless otherwise stated. The participant characteristics were analysed using paired t-test to assess potential differences between younger and older groups. Between group differences for relative abundance of both saliva and sputum microbiome phyla and genera, as well as alpha diversity (Shannon, Simpson and inverse Simpson index) were also assessed using paired t-test. Due to the skewed distribution of data, a square root transformation was performed for relative abundances of Firmicutes, Proteobacteria, Haemophilus and Lautropia, and a logarithmic transformation was performed for PWV to achieve a normal distribution for paired t-test. Pearson’s and Spearman’s correlation was used to evaluate associations between two variables that were either normally distributed or not normally distributed respectively, including any sub-group analyses. Principal coordinate analysis (PCoA) was used to assess the beta diversity and overall lung microbiome composition with age. PCoA plots for were generated using the first two principal coordinates according to age categories (young vs. elderly), as well as according to sample type (saliva vs. sputum). The ‘vegan’ R package (version 2.4–5) was used to calculate alpha diversity and implement ‘adonis’, which uses permutational multivariate analysis of variance, to test for statistical significance of association of overall beta diversity of lung microbiome composition with age. Bray Curtis distances between paired and unpaired young-elderly comparisons were assessed using the “dist_groups” function from the R package ‘udist’. All the results in the tables and figures are presented using the non-transformed data. A value of p < 0.05 was considered statistically significant.
Results
Age-associated changes in physiology of study cohort
Anthropometry and blood pressure
The demographics of healthy participants in our study are presented in Table 1. Despite no difference in weight (p = 0.734), older adults were shorter in stature (p < 0.001) and had higher BMI (p = 0.03) than the younger adults. Waist circumference was also greater in older than younger adults (p = 0.03). Compared with younger adults, older adults also had higher systolic BP (p = 0.002), although diastolic BP was similar between groups (p = 0.213) (Table 1).
Table 1. Mean and (standard deviation (SD)) of participant demographics.
| Young | Elderly | p value | |
|---|---|---|---|
| n | 24 | 24 | |
| Sex n (M/F) | 11/13 | 6/18 | |
| Age (years) | 29 (5) | 63 (2) | <0.001 |
| Weight (kg) | 60 (12) | 59 (13) | 0.734 |
| Height (cm) | 166 (9) | 157 (9) | <0.001 |
| BMI (kg/m2) | 21.7 (3.0) | 23.9 (3.5) | 0.03 |
| WC (cm) | 72.7 (9.4) | 79.5 (11.7) | 0.03 |
| Systolic BP (mmHg) | 112 (10) | 123 (16) | 0.002 |
| Diastolic BP (mmHg) | 71 (8) | 74 (9) | 0.213 |
| FEV1 (L) | 3.4 (0.7) | 2.0 (0.6) | <0.001 |
| FVC (L) | 3.9 (1.0) | 2.6 (0.6) | <0.001 |
| FEV1/FVC (%) | 86 (6) | 79 (8) | 0.003 |
| FEV1 (% Predicted) | 97 (14) | 88 (15) | 0.063 |
| FVC (% Predicted) | 96 (14) | 91 (17) | 0.361 |
| FEV1/FVC (% Predicted) | 100 (7) | 97 (10) | 0.157 |
| Pulse Wave Velocity (PWV) (m/s) | 6.5 (1.1) | 8.4 (1.7) | <0.001 |
| Augmentation Index (AIx) | 8.7 (9.9) | 24.6 (8.1) | <0.001 |
Paired t-test comparison between younger and older groups for anthropometry, blood pressure, lung function and arterial stiffness. Significant p values are indicated by bold typeface.
Lung function and arterial stiffness
Lung function parameters including FEV1 (p < 0.001), FVC (p < 0.001) and FEV1/FVC (p = 0.003) were all reduced in older adults compared with their younger family pairs (Table 1). Compared with younger adults, older adults had 41% or 1.4 L lower FEV1, 33% or 1.3 L lower FVC and 7% lower percentage of FEV1/FVC. However, adjusted FEV1 (%Predicted) and FVC (%Predicted) were comparable between younger and older adults (p = 0.063 and p = 0.361) suggesting lower lung function values were reflective of natural lung function decline in our healthy elderly cohort. The percentage of predicted value for FEV1/FVC percentage in younger and older family pairs was comparable (p = 0.157), and the values were all within healthy ranges [39]. Compared with younger adults, older adults had increased arterial stiffness, indicated by PWV and AIx (all p < 0.001). In both younger and older adults, arterial stiffness indicated by PWV and AIx were also within healthy ranges [35], and were 1.3-fold and 3.9-fold higher in older than in younger adults respectively (Table 1). Lung function was inversely associated with arterial stiffness. However, when age was adjusted for, in a partial correlation model, the associations between FEV1, FVC and percentage FEV1/FVC, with arterial stiffness parameters AIx and PWV were not significant (S1 Table).
Age-associated changes in lung microbiome composition
Composition of lung microbiome from sputum samples in healthy individuals
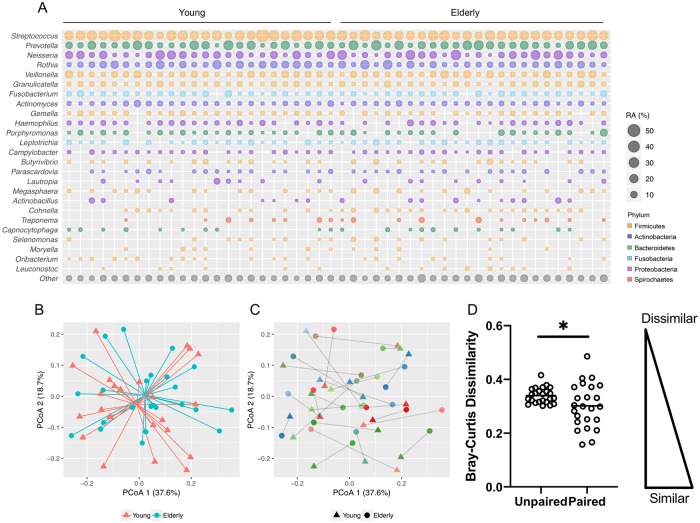
The main phyla and genera identified in the airway microbiome of younger and older adults are shown in Fig 1. Sputum samples from healthy individuals, both young and elderly, consisted mainly of microbes from phylum Firmicutes (46%), Proteobacteria (16%), Bacteroidetes (16%), Actinobacteria (14%) and Fusobacteria (6%). The main genera detected were Streptococcus (24%), Prevotella (12%), Neisseria (10%), Rothia (8%), Veillonella (7%), Granulicatella (4%), Fusobacterium (4%), Actinomyces (3%), Gemella (2%), Haemophilus (2%), Porphyromonas (2%), Leptotrichia (2%), Campylobacter (1%) and Lautropia (1%) (Fig 1A). Comparison of saliva samples (total n = 6) suggested a high degree of overlap between the microbiota of healthy sputum and saliva with no differences in alpha- (Shannon diversity index (SDI) p = 0.304) or beta-diversity (p = 0.582) (S1 Fig). However, sputum samples, which are commonly used to represent the lung microbiome, exhibited subtle differences in specific taxa including higher relative abundance of Granulicatella (4.3% vs. 3.0%, p = 0.031), lower relative abundance of Leptotrichia (0.8% vs. 1.4%, p = 0.013) and lower relative abundance of Corynebacterium (0.7% vs. 0.1%, p = 0.031) (S1 Fig).
Fig 1. Lung microbiome composition in genetically paired young and elderly subjects.
(A) Lung microbiome composition as detected by 16s rRNA gene profiling. Average relative abundance (RA) for the most representative taxa (present at >1%) is illustrated by circle size. Colour denotes phylum level membership. (B) Principle co-ordinate analysis (PCoA) of Bray-Curtis distance between microbiome profiles observed in young (red triangles) and elderly (turquoise circles) cohorts. (C) PCoA of microbiome profiles indicating linked young (triangles) and elderly (circles) participant pairs. Grey lines illustrate the distance between each paired sample which also share the same colour (D) Comparison of bray-curtis distance between PCoA points illustrating the reduction in dissimilarity observed between paired, genetically-related participants. * = p < 0.05.
Comparison of lung microbiota present in genetically related young and elderly family pairs
In order to allow some degree of control for genetic confounders, we assessed the airway microbiota in related young-elderly family pairs. PCoA analysis revealed that the sputum microbiome of younger and older adults was more similar when analysed as matched family pairs. Here, the measured Bray Curtis distance of matched young-elderly pairs was lower (equating to more similar microbiome profiles) when compared to the average distance between all other possible un-matched young-elderly comparisons (p = 0.036) (Fig 1C and 1D). Paired analysis showed that older adults generally had higher relative abundance of Firmicutes (47.4% vs 43.9%, p = 0.035) and lower Proteobacteria (13.9% vs. 19.0%, p = 0.014) with lower average relative abundance of Proteobacteria including Haemophilus (2.0% vs. 2.8%, p = 0.024) and Lautropia (0.5% vs. 1.1%, p = 0.020) (Fig 2). No significant differences in alpha diversity measures were observed between young and elderly groups (S2 Fig). Beta-diversity analysis also showed no difference between age groups in overall lung microbiome composition (p = 0.420, Fig 1B).
Fig 2. Differentially abundant taxa are observed in genetically paired young and elderly subjects.
At the phylum level, differences in Firmicutes (A) and Proteobacteria (B) are observed between young (white bars) and elderly (grey bars) paired groups, with elderly adults exhibiting higher relative abundance of Firmicutes, and lower relative abundance of Proteobacteria than young adults. At the genus level, Haemophilus (C) and Lautropia (D) are differentially abundant, with lower relative abundance in elderly than in younger adults. Black connecting lines indicate young-elderly pairs. * = p < 0.05.
Association of lung microbiome with lung function and arterial stiffness
Association of lung microbiome alpha diversity with lung function and arterial stiffness
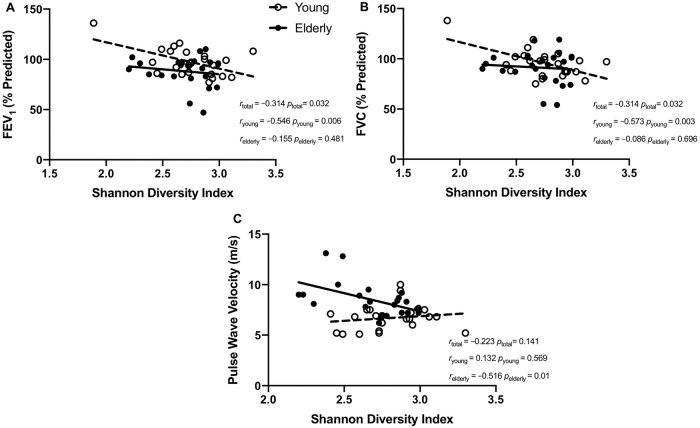
Alpha diversity of the lung microbiome was associated with lung function in an age-dependent manner. While SDI was associated with both FEV1 (%Predicted) and FVC (%Predicted) in the cohort as a whole (all r = –0.314, p = 0.032), the association was driven by the younger group (Fig 3A and 3B). In the younger group, FEV1 (%Predicted) and FVC (%Predicted) were both associated with SDI (r = –0.546, p = 0.006 and r = –0.573, p = 0.003) (Fig 3A and 3B). However, in the older group, the association between FEV1 (%Predicted) and FVC (%Predicted) with SDI were not significant (r = –0.155, p = 0.481 and r = –0.086, p = 0.696) (Fig 3A and 3B). In the young group, Shannon diversity was also associated with absolute FVC (r = –0.411, p = 0.031) (Table 2). Conversely, alpha diversity of the lung microbiome was correlated with arterial stiffness (PWV) in the older but not the younger group (r = –0.516, p = 0.01 vs r = 0.132, p = 0.569) (Fig 3C). Correlations with inverse Simpson index showed similar associations as SDI (Table 2).
Fig 3. Physiological correlates of microbiome alpha-diversity in young and elderly subjects.
Sub-group correlations between the Shannon diversity index of the lung microbiome and lung function parameters (A) FEV1 (% Predicted), (B) FVC (% Predicted) as well the arterial stiffness parameter (C) pulse wave velocity. Young and elderly groups are indicated by unfilled and filled circles respectively. The line of best fit is indicated with a broken line for the young population and with a continuous line for the elderly. Correlation coefficients and associated p values are indicated for the total population (rtotal), the young sub-group (ryoung) and the elderly sub-group (relderly).
Table 2. Correlation matrix comparing lung microbiome composition with lung function and arterial stiffness parameters among young and elderly subjects.
| FEV1 | FVC | FEV1 (%pred) |
FVC (%pred) |
AIx | PWV | ||
|---|---|---|---|---|---|---|---|
| Young | SDI | −0.362 (0.082) |
−0.411 (0.031)* |
−0.546 (0.006)** |
−0.573 (0.003)** |
−0.068 (0.769) |
0.132 (0.569) |
| ISI | −0.396 (0.055) |
−0.460 (0.024)* |
−0.440 (0.032)* |
−0.482 (0.017)* |
0.009 (0.967) |
0.169 (0.465) |
|
| Firmicutes | 0.318 (0.130) |
0.390 (0.060) |
0.363 (0.081) |
0.409 (0.047)* |
0.159 (0.492) |
0.160 (0.489) |
|
| Gemella | 0.082 (0.704) |
0.017 (0937) |
−0.348 (0.095) |
−0.422 (0.040)* |
−0.072 (0.756) |
−0.080 (0.732) |
|
| Actinobacteria | −0.055 (0.799) |
−0.070 (0.747) |
0.064 (0.767) |
0.023 (0.916) |
0.019 (0.935) |
−0.121 (0.603) |
|
| Actinomyces |
−0.408 (0.048)* |
−0.391 (0.059) |
−0.198 (0.354) |
−0.142 (0.508) |
−0.119 (0.608) |
0.205 (0.372) |
|
| Fusobacteria | −0.151 (0.480) |
−0.168 (0.432) |
−0.395 (0.056) |
−0.364 (0.080) |
0.014 (0.953) |
0.146 (0.528) |
|
| Leptotrichia | −0.132 (0.538) |
−0.112 (0.603) |
−0.353 (0.090) |
−0.216 (0.310) |
0.012 (0.960) |
0.170 (0.461) |
|
| Lautropia | 0.043 (0.843) |
−0.124 (0.563) |
0.015 (0.944) |
−0.209 (0.327) |
−0.492 (0.023)* |
−0.420 (0.058) |
|
| Elderly | SDI | −0.151 (0.493) |
−0.157 (0.475) |
−0.155 (0.481) |
−0.086 (0.696) |
0.035 (0.870) |
−0.516 (0.010)* |
| ISI | −0.216 (0.323) |
−0.206 (0.346) |
−0.112 (0.611) |
−0.017 (0.939) |
0.055 (0.789) |
−0.568 (0.004)** |
|
| Firmicutes | 0.190 (0.386) |
0.099 (0.653) |
−0.031 (0.888) |
−0.200 (0.360) |
0.193 (0.366) |
0.118 (0.582) |
|
| Gemella | −0.259 (0.232) |
−0.215 (0.324) |
−0.032 (0.887) |
0.082 (0.710) |
0.124 (0.564) |
0.280 (0.186) |
|
| Actinobacteria | 0.347 (0.105) |
0.447 (0.033)* |
0.382 (0.072) |
0.322 (0.134) |
0.137 (0.522) |
0.097 (0.651) |
|
| Actinomyces | 0.230 (0.290) |
0.238 (0.274) |
0.124 (0.572) |
0.073 (0.742) |
0.023 (0.915) |
−0.350 (0.094) |
|
| Fusobacteria | −0.361 (0.091) |
−0.344 (0.108) |
−0.286 (0.187) |
−0.114 (0.605) |
0.083 (0.701) |
−0.570 (0.004)** |
|
| Leptotrichia | −0.284 (0.189) |
−0.221 (0.310) |
−0.318 (0.139) |
−0.157 (0.473) |
0.207 (0.332) |
−0.569 (0.004)** |
|
| Lautropia | −0.088 (0.690) |
−0.112 (0.612) |
0.254 (0.242) |
0.080 (0.716) |
0.034 (0.873) |
−0.093 (0.665) |
Microbiome indices of alpha diversity including the Shannon diversity index (SDI) and inverse Simpson index (ISI) as well as specific phyla and genera showing significant correlation with lung function (FEV1, FVC, FEV1%predicted, FVC %predicted) and arterial stiffness (augmentation index (AIx) and pulse wave velocity (PWV)) are displayed for younger and elderly sub-groups. p values are shown in brackets. Significantly correlation scores are indicated by bold typeface.
*p < 0.05,
**p < 0.01.
Age-dependent association of lung microbiome composition and lung function
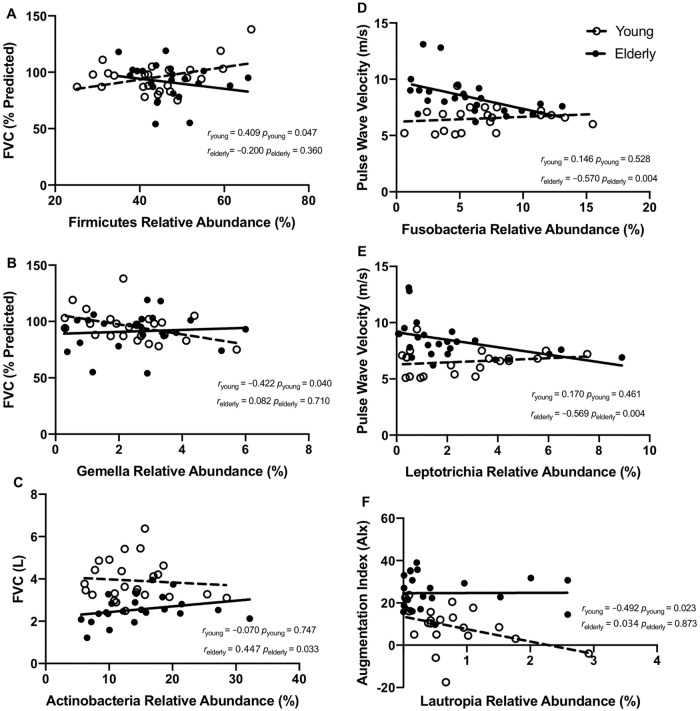
We found that within the younger cohort, the relative abundance of microbes from the Firmicutes phylum (r = 0.409, p = 0.047) and specifically the Gemella genus (r = –0.422, p = 0.04) was associated with FVC (%Predicted) (Fig 4A and 4B). However, similar to correlations with SDI, these associations between FVC (%Predicted) were seen in the younger but not the elderly cohort (r = –0.200, p = 0.360 and r = 0.422, p = 0.710) (Fig 4A and 4B). In younger subjects, the relative abundance of Actinomyces genus was also inversely associated with absolute FEV1 but this association was not observed among older individuals (r = –0.408, p = 0.048 vs. r = 0.230, p = 0.290) (Table 2). Within the older group, the only association with lung function parameters was the relative abundance of phylum Actinobacteria, which was positively associated with absolute FVC (r = 0.447, p = 0.033), an association that was not observed in the younger group (r = –0.070, p = 0.747) (Fig 4C).
Fig 4. Physiological correlates of the microbiome at phylum and genus level.
Sub-group correlations between specific lung microbiota, lung function and arterial stiffness parameters are indicated. Lung function—microbiome correlations were observed in the young but not the elderly group, between FVC (%Predicted) and relative abundance of (A) phylum Firmicutes and (B) genus Gemella. Correlations observed in the elderly but not the young group included (C) FVC and relative abundance of the genus Actinobacteria. Arterial stiffness and lung microbiome correlations were observed in the elderly group, between pulse wave velocity and relative abundance of (D) phylum Fusobacteria and (E) genus Leptotrichia while correlation between (F) augmentation index and the relative abundance of Lautropia was observed in young subjects. Young and elderly measures are indicated by unfilled and filled circles respectively. The line of best fit is indicated with a broken line for the young population and with a continuous line for the elderly. Correlation coefficients and associated p values are indicated for young sub-group (ryoung) and elderly sub-groups (relderly).
Age-dependent association of lung microbiome composition and arterial stiffness
In contrast to the associations between lung microbiome and lung function, which were more prevalent in the younger group, the associations between lung microbiome and arterial stiffness were more pronounced in the older group. Within the older group, the arterial stiffness parameter PWV exhibited a strong inverse association with relative abundances of the Fusobacteria (r = –0.570, p = 0.004), specifically the genus Leptotrichia (r = –0.569, p = 0.004) (Fig 4D and 4E). These associations of PWV with both Fusobacteria and Leptotrichia relative abundances were not significant in the younger group (r = 0.146, p = 0.528 and r = 0.170, p = 0.461) (Fig 4D and 4E). The only genus exhibiting correlation with arterial stiffness in the young was Lautropia, which was associated with AIx an observation that was not evident in the older cohort (r = –0.492, p = 0.023 vs r = 0.034, p = 0.873) (Fig 4F). These age-specific associations between airway microbiome composition, lung function and arterial stiffness are summarised in Fig 5.
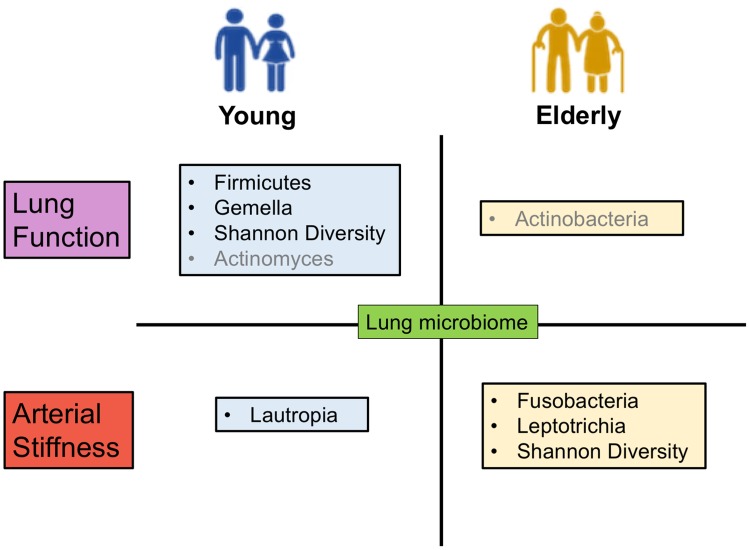
Fig 5. Summary schematic showing the significant associations between lung microbiome with lung function and arterial stiffness, within the young and elderly groups.
Lung microbiome is associated with lung function mostly in the young group, as Shannon diversity, Firmicutes phylum and its genus Gemella are associated with predicted lung function, while absolute FEV1 is associated with Actinomyces (in grey). In the elderly group, absolute FVC is associated with Actinobacteria (in grey). Lung microbiome is associated with arterial stiffness mostly in the elderly group, with Shannon diversity, Fusobacteria phylum and its genus Leptotrichia associated with pulse wave velocity. In the young group, Lautropia is associated with arterial stiffness parameter augmentation index.
Discussion
While a growing number of studies detail the ageing gut microbiome and its consequence for health and disease, comparable analyses of the respiratory microbiome are lacking [19, 40, 41]. Although several studies have investigated the airway microbiome in the context of respiratory disease, the present study is the first to explore age-associated differences in lung microbiome composition, with specific focus on healthy individuals of Asian origin. Our findings demonstrate remarkable consistency between young and elderly healthy microbiome profiles while identifying subtle taxonomic differences related to lung function and arterial stiffness in an age-dependent manner. These include associations between the lung microbiome and lung function (FEV1%Predicted and FVC %Predicted) in the younger adults, and arterial stiffness (PWV) in the elderly, suggesting that these relationships have greater relevance at different stages of life.
Given the paucity of evidence, the role of the airway microbiome in ageing is still unknown though long-speculated to be of clinical significance [42, 43]. Here, we have explored the effects of ageing on microbial constituents of the healthy airway microbiome by employing a paired genetic matching strategy, which controls the confounding influence of host genetics. Among patients with chronic respiratory disease such as COPD, overrepresentation of the Proteobacteria and reduction of Firmicute abundance is observed [44–47]. Enrichment of specific Proteobacteria including Haemophilus in the sputum microbiome of patients with COPD [48], and Lautropia in cystic fibrosis patients represent examples of disease-associated dysbiosis [46, 49]. In our healthy participants, however, the overall representation of phylum Proteobacteria (16%) was much lower than in disease states (~44%) [44]. Likewise, the average Firmicutes representation in our study (46%) was higher compared to diseased states (~16%) [44]. It is plausible that ‘healthy’ ageing of the lungs promotes enrichment of the Firmicutes and reduced relative abundance of Proteobacteria such as Haemophilus and Lautropia, which contrasted with the dysbiosis associated with chronic respiratory diseases.
We observed correlation between microbiome composition and lung function among younger subjects, noting an inverse relationship between lung microbiome diversity (SDI) and lung function (FEV1% predicted and FVC % predicted). Microbial diversity thus negatively correlated with lung function in our study, which contrasted with respiratory disease states where positive correlation is generally observed [50, 51]. At the phylum level, overall Firmicutes abundance positively associated with lung function (FVC %Predicted) in the younger adults in this study. This suggests enrichment of the Firmicutes has a beneficial effect on lung function and might explain the negative correlation between lung function and SDI. The increased Firmicute abundance, or that of other beneficial microbiome, could drive a corresponding reduction in SDI. Observed association between Gemella (a Firmicute linked to pulmonary exacerbation in cystic fibrosis patients) is also associated with reduced lung function in our young cohort, further suggesting its potentially negative implications for respiratory health [20, 52]. The positive influence of Firmicute abundance on lung function might be contingent on the presence and abundance of specific genera within this phylum, such as Gemella. The Actinobacteria (in the elderly) the genus Actinomyces (in the young) were associated with increased absolute FVC and decreased FEV1 respectively (Fig 5). However, absolute values of FEV1 and FVC are less robust lung function parameters that do not take into consideration normative physiological lung function decline with age. The association between lung microbiome and lung function parameters (FEV1 and FVC %Predicted) may thus be more relevant and pronounced in the younger group (Fig 5).
In contrast to lung function, we found that associations between airway microbiome composition and arterial stiffness were largely confined to older adults. Here, lung microbiome diversity was inversely associated with arterial stiffness parameter PWV (a negative clinical indicator), in the older adults. Reduced microbial diversity in lower airways is linked to inflammatory phenotypes of the airways [53–55], which could increase both systemic inflammation and arterial stiffness that occur during ageing [23, 48]. Our findings highlight the relative abundance of Fusobacteria phylum and its genus Leptotrichia in inverse association with arterial stiffness in older adults. The relevance of the Fusobacteria in lung respiratory disease has been noted, where they are associated with beneficial effects [56–58]. While associations between particular organisms and lung function occur mainly in the younger group, distinct organisms in the lung are linked to arterial stiffness in the elderly. These results highlight the complexity of the microbial interactions that may underpin age-dependent relationships between lung function and arterial stiffness across different stages of life (Fig 5).
Our study has several limitations. Although associations can be drawn from the study results, the cross-sectional design does not conclusively prove causality. This study is also unable to directly assess temporal dynamics of the lung microbiome during ageing given the lack of a longitudinal component. While the younger and older participants in this study are family pairs, which minimize genetic influences, we did not control for other confounders such as environmental factors, lifestyle or living conditions, that could have influenced the lung microbiome and represent significant confounders. As targeted bacterial 16s rRNA gene amplicon sequencing was employed, the resolution does not support species-level characterization of the microbiome, which could be important in understanding the ecological and functional interaction with ageing. Though our study was explorative in nature, it provides a first insight into age-dependant variability in the airway microbiome and its potential implications for respiratory and cardiovascular health. We have identified several potential microbial taxa whose clinical relevance should be further explicated in larger studies incorporating longitudinal experimental design to fully delineate the core healthy airway microbiome and the temporal dynamics of beneficial and deleterious taxa that may serve as early prognostic indicators of lung health status.
Supporting information
(A) Comparison of lung microbiome composition as detected by 16s rRNA gene profiling in DNA derived from saliva and sputum. Average relative abundance (RA) for the most representative taxa (present at >1%) is illustrated by circle size. Colour denotes phylum level membership. The saliva (V) and sputum (S) of three young (Y01-Y03) and three elderly matched family pairs (S01-S03) were analysed. (B) Principle co-ordinate analysis (PCoA) of Bray-Curtis distance between microbiome profiles observed in saliva samples (red open triangles) and sputum samples (black open circles) with indicated centroids (filled circles). Assessment of (C) Shannon diversity index and differences in relative abundance of (D) genus Granulicatella, (E) genus Leptotrichia and (F) genus Corynebacterium in saliva vs sputum samples among genetically paired young and elderly subjects. * = p < 0.05.
(TIF)
No significant differences are observed in (A) Shannon diversity index (B) Simpson index and (C) Inverse Simpson index between young (white bars) and elderly (grey bars) paired groups. Black connecting lines indicate young-elderly pairs. ns = not significant.
(TIF)
Correlation coefficients and corresponding p values are shown in brackets. *p < 0.05, **p < 0.01, ***p < 0.001. Significant correlation scores are indicated by bold typeface. AIx = Augmentation index, PWV = Pulse wave velocity.
(DOCX)
Data Availability
All microbiome sequencing data files are available from the SRA database (accession number PRJNA559069).
Funding Statement
This work received a grant award by Ageing Research Institute for Society and Education (ARISE), Nanyang Technological University, Singapore, ARISE/2017/6 (http://arise.ntu.edu.sg) to SHC. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Budinger GRS, Kohanski RA, Gan W, Kobor MS, Amaral LA, Armanios M, et al. The Intersection of Aging Biology and the Pathobiology of Lung Diseases: A Joint NHLBI/NIA Workshop. J Gerontol A Biol Sci Med Sci. 2017;72(11):1492–500. 10.1093/gerona/glx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13(1):197–205. 10.1034/j.1399-3003.1999.13a36.x [DOI] [PubMed] [Google Scholar]
- 5.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107(1):139–46. 10.1161/01.cir.0000048892.83521.58 [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–45. 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- 7.Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values'. Eur Heart J. 2010;31(19):2338–50. 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowich MD, Taveira T, Wu WC. Decreased lung function is associated with increased arterial stiffness as measured by peripheral pulse pressure: data from NHANES III. Am J Hypertens. 2010;23(6):614–9. 10.1038/ajh.2010.37 [DOI] [PubMed] [Google Scholar]
- 9.Zureik M, Benetos A, Neukirch C, Courbon D, Bean K, Thomas F, et al. Reduced pulmonary function is associated with central arterial stiffness in men. Am J Respir Crit Care Med. 2001;164(12):2181–5. 10.1164/ajrccm.164.12.2107137 [DOI] [PubMed] [Google Scholar]
- 10.Barr RG, Ahmed FS, Carr JJ, Hoffman EA, Jiang R, Kawut SM, et al. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J. 2012;39(4):846–54. 10.1183/09031936.00165410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs DR Jr., Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, et al. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59(2):219–25. 10.1161/HYPERTENSIONAHA.111.184101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton CE, Cockcroft JR, Sabit R, Munnery M, McEniery CM, Wilkinson IB, et al. Lung function in mid-life compared with later life is a stronger predictor of arterial stiffness in men: the Caerphilly Prospective Study. Int J Epidemiol. 2009;38(3):867–76. 10.1093/ije/dyn374 [DOI] [PubMed] [Google Scholar]
- 13.Engstrom G, Lind P, Hedblad B, Wollmer P, Stavenow L, Janzon L, et al. Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation. 2002;106(20):2555–60. 10.1161/01.cir.0000037220.00065.0d [DOI] [PubMed] [Google Scholar]
- 14.Zureik M, Kauffmann F, Touboul PJ, Courbon D, Ducimetiere P. Association between peak expiratory flow and the development of carotid atherosclerotic plaques. Arch Intern Med. 2001;161(13):1669–76. 10.1001/archinte.161.13.1669 [DOI] [PubMed] [Google Scholar]
- 15.Polverino F, Celli BR, Owen CA. COPD as an endothelial disorder: endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series). Pulm Circ. 2018;8(1):2045894018758528 10.1177/2045894018758528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills NL, Miller JJ, Anand A, Robinson SD, Frazer GA, Anderson D, et al. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular risk. Thorax. 2008;63(4):306–11. 10.1136/thx.2007.083493 [DOI] [PubMed] [Google Scholar]
- 17.Qvist L, Nilsson U, Johansson V, Larsson K, Ronmark E, Langrish J, et al. Central arterial stiffness is increased among subjects with severe and very severe COPD: report from a population-based cohort study. Eur Clin Respir J. 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rooyen Y, Schutte AE, Huisman HW, Eloff FC, Du Plessis JL, Kruger A, et al. Inflammation as Possible Mediator for the Relationship Between Lung and Arterial Function. Lung. 2016;194(1):107–15. 10.1007/s00408-015-9804-9 [DOI] [PubMed] [Google Scholar]
- 19.Dickson RP, Huffnagle GB. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog. 2015;11(7):e1004923 10.1371/journal.ppat.1004923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep. 2015;5:10241 10.1038/srep10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, et al. Spatial Variation in the Healthy Human Lung Microbiome and the Adapted Island Model of Lung Biogeography. Ann Am Thorac Soc. 2015;12(6):821–30. 10.1513/AnnalsATS.201501-029OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. 2015;6(2):e00037 10.1128/mBio.00037-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, et al. Analysis of the lung microbiome in the "healthy" smoker and in COPD. PLoS One. 2011;6(2):e16384 10.1371/journal.pone.0016384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667 10.1371/journal.pone.0010667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. 10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- 26.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–73. 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Assar M, Angulo J, Vallejo S, Peiro C, Sanchez-Ferrer CF, Rodriguez-Manas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol. 2012;3:132 10.3389/fphys.2012.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell GF, DeStefano AL, Larson MG, Benjamin EJ, Chen MH, Vasan RS, et al. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation. 2005;112(2):194–9. 10.1161/CIRCULATIONAHA.104.530675 [DOI] [PubMed] [Google Scholar]
- 29.Gursli S, Sandvik L, Bakkeheim E, Skrede B, Stuge B. Evaluation of a novel technique in airway clearance therapy—Specific Cough Technique (SCT) in cystic fibrosis: A pilot study of a series of N-of-1 randomised controlled trials. SAGE Open Med. 2017;5:2050312117697505 10.1177/2050312117697505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan X, Peters BA, Min D, Ahn J, Hayes RB. Comparison of the oral microbiome in mouthwash and whole saliva samples. PLoS One. 2018;13(4):e0194729 10.1371/journal.pone.0194729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma WY, Yang CY, Shih SR, Hsieh HJ, Hung CS, Chiu FC, et al. Measurement of Waist Circumference: midabdominal or iliac crest? Diabetes Care. 2013;36(6):1660–6. 10.2337/dc12-1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 33.Nakagomi A, Shoji T, Okada S, Ohno Y, Kobayashi Y. Validity of the augmentation index and pulse pressure amplification as determined by the SphygmoCor XCEL device: a comparison with invasive measurements. Hypertens Res. 2018;41(1):27–32. 10.1038/hr.2017.81 [DOI] [PubMed] [Google Scholar]
- 34.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–90. 10.1161/CIRCULATIONAHA.104.483628 [DOI] [PubMed] [Google Scholar]
- 35.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–8. 10.1097/HJH.0b013e32834fa8b0 [DOI] [PubMed] [Google Scholar]
- 36.Mac Aogain M, Chandrasekaran R, Lim AYH, Low TB, Tan GL, Hassan T, et al. Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur Respir J. 2018;52(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amplicon PCR, Clean-up, P.C.R. & Index, P.C.R. 16S Metagenomic Sequencing Library Preparation. https://www.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf.
- 38.Davis NM, Proctor DM, Holmes SP, Relman DA, Callahan BJ. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 2018;6(1):226 10.1186/s40168-018-0605-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–91. 10.7326/0003-4819-155-3-201108020-00008 [DOI] [PubMed] [Google Scholar]
- 40.Seidel J, Valenzano DR. The role of the gut microbiome during host ageing. F1000Res. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214–5. 10.1126/science.aac8469 [DOI] [PubMed] [Google Scholar]
- 42.Murray MA, Chotirmall SH. The Impact of Immunosenescence on Pulmonary Disease. Mediators Inflamm. 2015;2015:692546 10.1155/2015/692546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chotirmall SH, Burke CM. Aging and the microbiome: implications for asthma in the elderly? Expert Rev Respir Med. 2015;9(2):125–8. 10.1586/17476348.2015.1002473 [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Nunez M, Millares L, Pomares X, Ferrari R, Perez-Brocal V, Gallego M, et al. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52(12):4217–23. 10.1128/JCM.01967-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu HY, Zhang SY, Yang WY, Su XF, He Y, Zhou HW, et al. Oropharyngeal and Sputum Microbiomes Are Similar Following Exacerbation of Chronic Obstructive Pulmonary Disease. Front Microbiol. 2017;8:1163 10.3389/fmicb.2017.01163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Bafadhel M, Haldar K, Spivak A, Mayhew D, Miller BE, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082–92. 10.1183/13993003.01406-2015 [DOI] [PubMed] [Google Scholar]
- 47.Leung JM, Tiew PY, Mac Aogain M, Budden KF, Yong VF, Thomas SS, et al. The role of acute and chronic respiratory colonization and infections in the pathogenesis of COPD. Respirology. 2017;22(4):634–50. 10.1111/resp.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sze MA, Dimitriu PA, Suzuki M, McDonough JE, Campbell JD, Brothers JF, et al. Host Response to the Lung Microbiome in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;192(4):438–45. 10.1164/rccm.201502-0223OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang YJ, Boushey HA. The Sputum Microbiome in Chronic Obstructive Pulmonary Disease Exacerbations. Ann Am Thorac Soc. 2015;12 Suppl 2:S176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Dwyer DN, Dickson RP, Moore BB. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J Immunol. 2016;196(12):4839–47. 10.4049/jimmunol.1600279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Budden KF, Shukla SD, Rehman SF, Bowerman KL, Keely S, Hugenholtz P, et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med. 2019. [DOI] [PubMed] [Google Scholar]
- 52.Carmody LA, Zhao J, Schloss PD, Petrosino JF, Murray S, Young VB, et al. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann Am Thorac Soc. 2013;10(3):179–87. 10.1513/AnnalsATS.201211-107OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi Y, Saito A, Chiba H, Kuronuma K, Ikeda K, Kobayashi T, et al. Impaired diversity of the lung microbiome predicts progression of idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):34 10.1186/s12931-018-0736-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flight WG, Smith A, Paisey C, Marchesi JR, Bull MJ, Norville PJ, et al. Rapid Detection of Emerging Pathogens and Loss of Microbial Diversity Associated with Severe Lung Disease in Cystic Fibrosis. J Clin Microbiol. 2015;53(7):2022–9. 10.1128/JCM.00432-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jorth P, Ehsan Z, Rezayat A, Caldwell E, Pope C, Brewington JJ, et al. Direct Lung Sampling Indicates That Established Pathogens Dominate Early Infections in Children with Cystic Fibrosis. Cell Rep. 2019;27(4):1190–204.e3. 10.1016/j.celrep.2019.03.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuleta I, Skowasch D, Aurich F, Eckstein N, Schueler R, Pizarro C, et al. Asthma is associated with atherosclerotic artery changes. PLoS One. 2017;12(10):e0186820 10.1371/journal.pone.0186820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinmann M, Abbas C, Singer F, Casaulta C, Regamey N, Haffner D, et al. Arterial stiffness is increased in asthmatic children. Eur J Pediatr. 2015;174(4):519–23. 10.1007/s00431-014-2423-2 [DOI] [PubMed] [Google Scholar]
- 58.Hosgood HD 3rd, Mongodin EF, Wan Y, Hua X, Rothman N, Hu W, et al. The respiratory tract microbiome and its relationship to lung cancer and environmental exposures found in rural China. Environ Mol Mutagen. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Comparison of lung microbiome composition as detected by 16s rRNA gene profiling in DNA derived from saliva and sputum. Average relative abundance (RA) for the most representative taxa (present at >1%) is illustrated by circle size. Colour denotes phylum level membership. The saliva (V) and sputum (S) of three young (Y01-Y03) and three elderly matched family pairs (S01-S03) were analysed. (B) Principle co-ordinate analysis (PCoA) of Bray-Curtis distance between microbiome profiles observed in saliva samples (red open triangles) and sputum samples (black open circles) with indicated centroids (filled circles). Assessment of (C) Shannon diversity index and differences in relative abundance of (D) genus Granulicatella, (E) genus Leptotrichia and (F) genus Corynebacterium in saliva vs sputum samples among genetically paired young and elderly subjects. * = p < 0.05.
(TIF)
No significant differences are observed in (A) Shannon diversity index (B) Simpson index and (C) Inverse Simpson index between young (white bars) and elderly (grey bars) paired groups. Black connecting lines indicate young-elderly pairs. ns = not significant.
(TIF)
Correlation coefficients and corresponding p values are shown in brackets. *p < 0.05, **p < 0.01, ***p < 0.001. Significant correlation scores are indicated by bold typeface. AIx = Augmentation index, PWV = Pulse wave velocity.
(DOCX)
Data Availability Statement
All microbiome sequencing data files are available from the SRA database (accession number PRJNA559069).