Abstract
Exhaled breath contains a large amount of biochemical and physiological information concerning one’s health and provides an alternative route to noninvasive medical diagnosis of diseases. In the case of lung diseases, hydrogen peroxide (H2O2) is an important biomarker associated with asthma, chronic obstructive pulmonary disease, and lung cancer and can be detected in exhaled breath. The current method of breath analysis involves condensation of exhaled breath, is not continuous or real time, and requires two separate and bulky devices, complicating the periodic or long-term monitoring of a patient. We report the first disposable paper-based electrochemical wearable sensor that can monitor exhaled H2O2 in artificial breath calibration-free and continuously, in real time, and can be integrated into a commercial respiratory mask for on-site testing of exhaled breath. To improve precision for sensing H2O2, we perform differential electrochemical measurement by amperometry in which screen-printed Prussian Blue-mediated and nonmediated carbon electrodes are used for differential analysis. We were able to measure H2O2 in simulated breath in a concentration-dependent manner in real time, confirming its functionality. This proposed system is versatile, and by modifying the chemistry of the sensing electrodes, our method of differential sensing can be extended to continuous monitoring of other analytes in exhaled breath.
Keywords: paper-based sensors, exhaled breath testing, electrochemical analysis, wearables, respiratory diseases
The global air pollution has been rising at an alarming rate due to the advancing industrialization and dependency on motorized vehicles. This leads increasingly to severe health problems.1 For example, disorders concerning lung and airways, such as asthma, lung cancer, and chronic obstructive pulmonary disease, are on the rise according to the World Health Organization.2 In total, 3.9 million deaths each year worldwide are caused by respiratory diseases.3,4 A large proportion of respiratory diseases are chronic and require frequent check-ups to monitor their progression. Inflammatory cells, such as macrophages and neutrophils, produce H2O2 in a reaction to respiratory diseases.5,6 Detection of H2O2 in exhaled breath as a biomarker of respiratory illnesses may provide a noninvasive route to detect these diseases quickly, noninvasively, and inexpensively. Unfortunately, H2O2 is difficult to measure in exhaled breath since it is easily oxidized in air and is not stable with increasing pH in aqueous solutions, including exhaled breath condensate (EBC) with a pH between 7.8 and 8.1.7−9
For analysis of exhaled breath, EBC is first collected and then measured in centralized laboratories.10,11 The sample collection is done by cooling the exhaled breath of a patient and breathing into a special device that consists of a mouthpiece and a cooled tube. The EBC is analyzed where the H2O2 concentration is determined using electrochemical (amperometric) and optical (fluorometric, colorimetric, chemiluminescence, and fluorescence) methods of detection.6,12 Devices for the EBC collection and analysis are already commercially available (ECoScreen and ECoCheck by FILT, Germany); however, due to their size and cost, they are not suitable for an on-site or wearable continuous monitoring. Furthermore, the current approaches for analyzing EBC are susceptible to errors since the sample collected is influenced by the duration of storage, temperature, amount of saliva, changing flow, and volume of exhaled air.13
Wearable sensors may provide a viable alternative to monitor constituents of exhaled breath, without reliance on EBC, for accelerated and inexpensive diagnosis of respiratory diseases. Wearable devices already exist in various forms including smart watches, fitness bracelets, and digital glasses.14−18 The concept of wearable sensors has already been accepted by the general public and their adoption has increased dramatically over the past few years.14 For biochemical analysis, however, the reusability of a medical device is often out of the question; therefore, a wearable sensor for monitoring exhaled breath must be low-cost and disposable. For biochemical analysis, paper as a substrate offers many advantages: it (i) is easy-to-handle, (ii) is permeable for gases, (iii) is capable of absorbing fluids and allowing their passive transport, (iv) can be folded, (v) has a good compatibility with chemicals and biomolecules, (vi) is cost-effective, (vii) is hygroscopic, and (viii) is disposable.19−21 Furthermore, the cellulose fibers can easily be functionalized to modify paper’s permeability, hydrophilicity, or reactivity, by patterning with waxes, inks, or polymers through thermal curing.22
A notable example for paper-based wearable devices is the electrical respiration sensor introduced by Güder and colleagues.23 In this approach, the respiratory activity (such as breathing rate, relative volume, etc.) of a person can be observed using a wearable mask with an integrated paper-based sensing device. This sensor comprises two printed carbon electrodes on an ordinary cellulose paper. The detecting principle is electrical and based on the change in moisture in the paper. This again results in a detectable change of the obtained signal between the carbon electrodes while breathing. The cellulose paper is able to absorb up to 10% of its own weight in water from humidity in air. This device can be used without calibration since only the changes between inhaled and exhaled air are measured. It is suitable for on-site monitoring as it is simple to use via a mobile app and does not require any additional equipment. This device, however, is limited to the determination of the physical quantities related to breathing and cannot provide any biochemical information that may be extracted from exhaled breath.
Prussian Blue (PB) is known to be one of the most efficient electrocatalysts for the detection of H2O2, which is taken advantage of especially in the field of bio- and immunosensors.24−26 Here, oxidoreductase systems, most commonly glucose oxidase, alkaline phosphatase, and horseradish peroxidase, are employed either for the highly specific detection of an analyte or for the labeling of a bioassay. The reaction product in all of these cases is H2O2, which can be electrochemically detected with high sensitivity using PB-modified electrodes.27 Screen-printed carbon electrodes are well suited for depositing PB and transduction of the catalytic redox currents taking place on PB.12,28 Another advantage is that the potential for this reductive H2O2 detection lies at around 0.0 V versus Ag/AgCl (0.1 M KCl), which is low enough to avoid the oxidation of possible interfering substances.27 On the other hand, oxygen is not reduced yet at a considerable rate at this potential, which is an important prerequisite for the measurement of biological samples.
As mentioned, the interfering substances is an important issue for the monitoring of exhaled breath. Besides the main gaseous constituents (N2, O2, CO2, and H2O), a vast number of more or less complex compounds can be found in breath. Many of them are transferred from blood, although normally in a 100- to 1000-fold lower concentration, usually in the ppt to ppm range. These are mainly volatile organic compounds (VOCs), including acetone, isoprene, toluol, and limonene.29 However, even nonvolatile molecules, such as glucose and peptides, are present in exhaled breath.30 Furthermore, low-molecular-weight compounds such as ammonia, thiols, and carbon monoxide are found. However, none of these compounds can easily be reduced electrochemically. Only nitric oxide (NO), being a biomarker for inflammatory diseases as well, might be a potential interfering substance for H2O2 sensing. Yet, concentrations of NO in exhaled breath lie in the range of <20 ppb for healthy persons and its half-life is just a few seconds.31
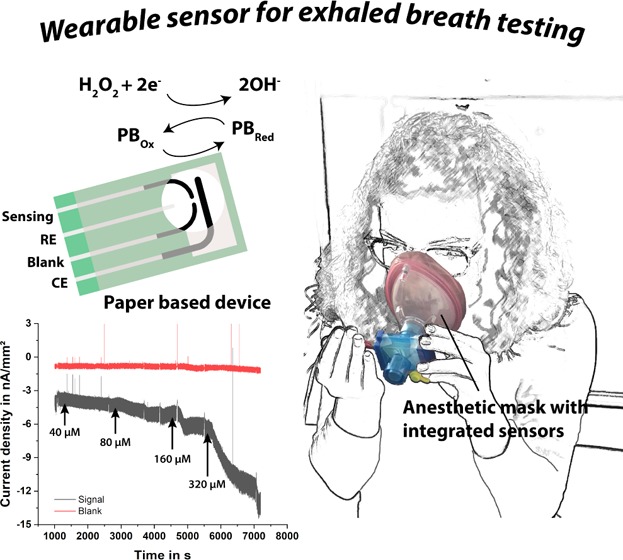
This article reports a low-cost, lightweight, and “calibration-free” approach for the continuous, real-time, and on-site surveillance of the concentration of H2O2 in simulated exhaled breath. The wearable system developed employs an easy-to-fabricate paper-based electrochemical sensor comprising a differential electrode design with a PB-mediated carbon electrode for H2O2 detection and carbon blank electrode for subtracting the background signals. A silver/silver chloride (Ag/AgCl) reference and a carbon counter electrode are used to complete the electrolytic cell. The signal detection is achieved as H2O2 oxidizes the PB, contained in the sensing electrode, which is subsequently reduced at the electrode and results in a detectable cathodic current signal. This decrease in the amperometric signal increases with increasing H2O2 concentration. Thanks to its differential design and the use of PB-mediated electrodes (highly selective for H2O2), any unspecific redox reaction can be canceled out. For compatibility with a standardized respiratory mask, the developed paper-based H2O2 sensor is integrated into the housing of a commercially available airway filter mainly used in anesthetic applications.
Results and Discussion
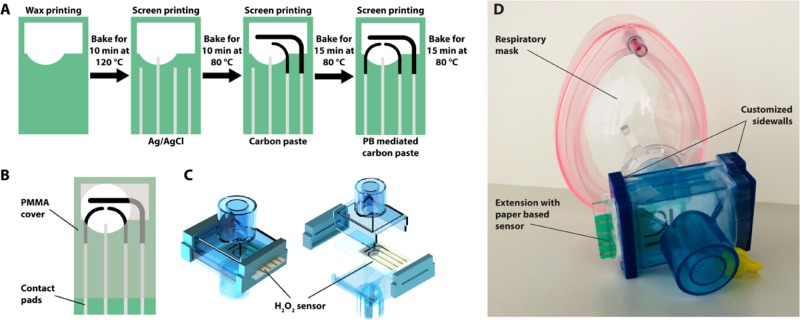
The fabrication procedure for the paper sensors is illustrated schematically in Figure 1A. First, wax patterns are printed on the chromatography paper (grade 1 CHR, 200 × 200 mm2, Whatman, U.K.) using a commercially available wax printer (ColorQube 8580, Xerox corporation, USA) and baked for 10 min at 120 °C in a conventional oven. When heated, the layer of wax printed on the surface of paper wicks through the bulk of the substrate and forms a hydrophobic barrier, defining the electrolytic cell. The wax barrier plays two important roles: (i) It prevents wicking of any droplets of water condensed during exhalation to the contact pads during operation. (ii) The wax pattern contains a solution of electrolyte before the water is evaporated from the substrate to form a solid electrolyte. Next, the reference electrode (RE) and conducting tracks are screen-printed with Ag/AgCl paste (C2040308P2, Gwent Group, U.K.) and baked for 10 min at 80 °C. Finally, the carbon counter (CE), blank, and PB-mediated sensing electrodes are screen-printed using the nonmediated and mediated carbon pastes (C2030519P4 and C2070424P2, Gwent Group, U.K.) and baked for 15 min at 80 °C.
Figure 1.
(A) Schematics of chip fabrication steps including wax isolation and screen printing of the Ag/AgCl, carbon, and PB-mediated electrodes, (B) CAD drawing of the electrochemical sensor with PMMA carrier, (C) SolidWorks model of a filter extension for respiratory mask, including the paper-based hydrogen peroxide sensor, and (D) image of respiratory mask with the commercial filter extension with customized sidewalls, containing the sensor chip.
We place the paper-based sensor chip inside a wearable respiratory mask, such that the patient is breathing directly onto the sensor. For integration into the ventilation mask, the paper chip is glued between two PMMA sheets with an opening for the electrodes, as depicted in Figure 1B. With this, the sensor is mechanically stabilized and, at the same time, the conducting tracks are isolated from potential shorts due to water droplets originating from exhaled breath. Furthermore, we modify the housing of a commercial filter (Figure 1C) by replacing the sidewalls with custom-made 3D printed parts to mount the paper-based sensor into the housing, which allows us to place it directly in the respiratory flow. With this approach, moisture from the breath is captured by the paper sensor to wet the electrodes, forming a liquid electrolytic cell, which is crucial for the operability of the sensor. The entire system is illustrated in Figure 1D.
Because paper itself is not ionically conductive, a droplet of electrolyte is placed on the paper and dried, before measuring analytes from exhaled breath. For the first measurements, 0.1 M phosphate-buffered saline (PBS) is used as the electrolyte, but during the measurement, the sensor dried quickly; thus, it is not possible to maintain constant conditions. To solve this problem, several electrolytes and humectants are tested (see Figure S9). We have captured cyclic voltammograms (CVs) in dry (after 1 day) and wet (DI water added) conditions and finally with a droplet of 160 μM H2O2. Most of the compounds tested either could not keep the paper wet or inhibited the detection of H2O2. Since 1 M potassium chloride (KCl) provides better characteristics for the CVs and better sensitivity for H2O2 in amperometric measurements than all other examined compounds, it is chosen as the electrolyte for further experiments.
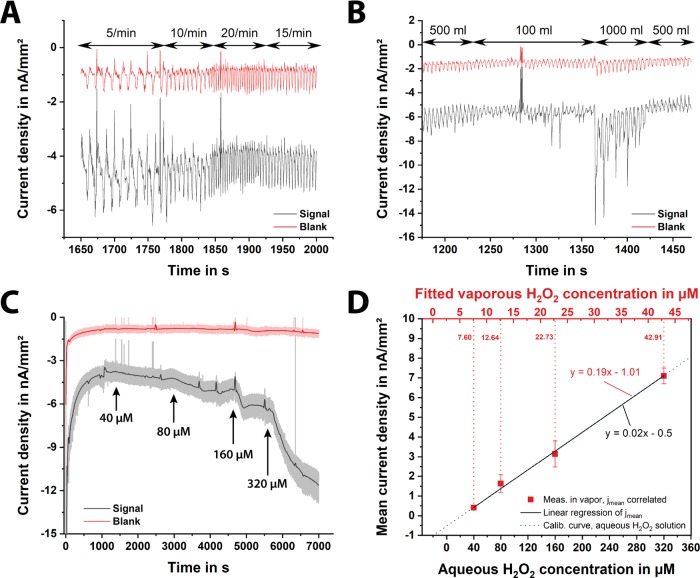
For the calibration of the paper-based H2O2 sensor, the current behavior over time is recorded for different hydrogen peroxide concentrations. Amperometry at a constant potential of 0.0 V versus Ag/AgCl (a screen-printed RE electrode) is carried out using different paper chips (n = 7). The frontside of the electrodes is isolated using an adhesive tape, as the paper-based sensors are positioned in the respiratory mask with the backside facing the user; hence, the frontside of the electrode structures has no direct contact with the exhaled breath. First, a droplet of 1 M KCl solution is placed on the electrolytic cell of the paper chip, and then, measurements with increasing the H2O2 concentration are performed. The obtained calibration curve is shown in Figure 2A. Here, a linear measurement range between 5 and 320 μM hydrogen peroxide is achieved with a sensitivity of 0.19 nA μM–1 mm–2 and a correlation coefficient of 0.99. Besides, the current values at a given H2O2 concentration, obtained from seven different sensors, prove a very small deviation. This means that a single prior calibration of the final sensor design delivers reliable absolute values and the H2O2 measurement in simulated breath can be performed calibration-free (without any calibration).
Figure 2.
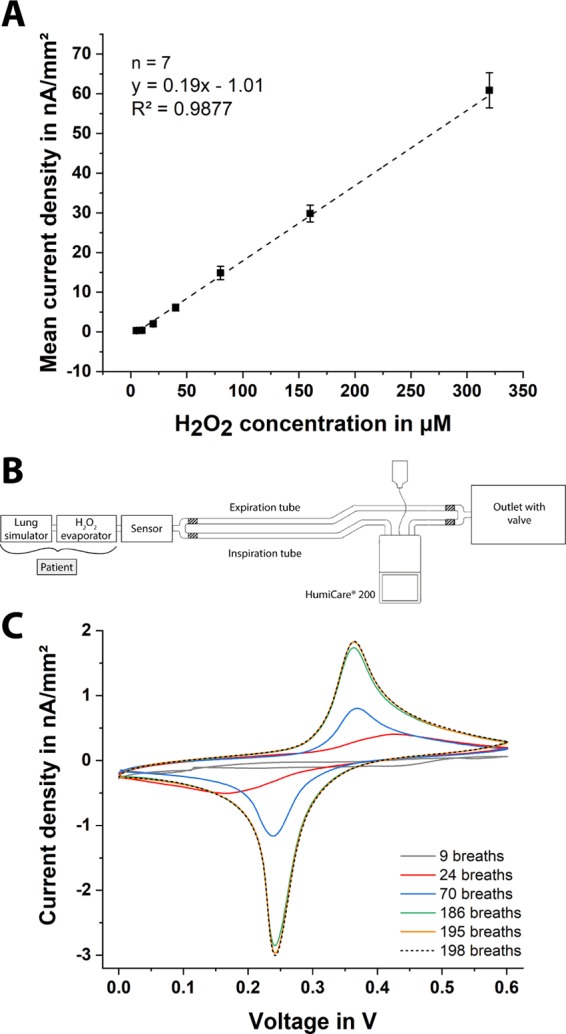
(A) Calibration curve of the paper-based H2O2 sensors with different hydrogen peroxide concentrations: 5–320 μM H2O2 in 1 M KCl solution. Herein, the frontside of the chip was insulated with an adhesive tape since the sensor is placed into the filter with the backside toward the patient, and thus, the frontside of the electrodes has no direct contact with the exhaled breath. Error bars represent ±standard deviation (SD) of n = 7 replicates. (B) Scheme of measurement setup for simulation of respiration, including a lung simulator, a humidifier, a H2O2 evaporator, and a filter housing with an integrated H2O2 sensor. (C) Cyclic voltammograms of a dry chip with a PB-coated working electrode, pretreated with 1 M KCl, in vapor after 9 (gray), 24 (red), 70 (blue), 185 (green), 195 (orange), and 198 (black, dashed) breaths at a scan rate of 100 mV s–1.
To mimic the human respiration, it is necessary to create a periodic air flow, generating a warm and humid gas flow using a lung simulator, as the human exhaled breath contains ∼100% RH at a temperature of around 34 °C. Using a customized LabVIEW software (National Instruments, USA), the lung simulator pumps a desired volume of air with a predefined frequency. The RH and temperature are adjusted using a commercially available humidifier (HumiCare 200, Gründler Medical, Germany) that contains heated tubing. To introduce different concentrations of H2O2 an evaporator with a heating element is placed in between the lung simulator and the paper sensor. A scheme of this setup is illustrated in Figure 2B, and a video describing the entire measurement setup can be found in the Supporting Information.
Since the moisture content of the paper is varying with the changing RH during inhalation and exhalation, to study the effect of RH on the redox characteristics of the PB-mediated carbon electrode, we performed CV measurements using a dry chip, pretreated with 1 M KCl, in H2O2-free simulated breath. In all experiments (except the tests of respiration frequency and volume), the lung simulator is set to generate a tidal volume of 500 mL and a frequency of 15 breaths per minute, which are realistic values for a healthy adult. As it can be observed in Figure 2C, the initially dry sensor can be wetted only by the respiratory stream itself, assuring a high and more reliable electrochemical signal. After 195 breaths (13 min), the measured current signals do not alter anymore and exhibit a typical PB–CV of a wet sensor in 1 M KCl.
In Figure 3A, the current signal of an amperometric measurement at different respiration frequencies is illustrated. It is noticeable that slower breathing results in a lower frequency of the signal measured and vice versa, while no significant signal change is observed by a volume change (see Figure 3B). During one breathing period, the water content in the paper changes periodically, as the air stream is drier during inhalation and reaches a RH of ∼100% during exhaling. Accordingly, the ionic conductivity of the paper fibers changes.21,23 Even though variations in conductivity are less important in an amperometric setup, the signal might decrease if the paper becomes too dry (see Figure 2C). However, as the blank electrodes without PB show a similar response, we conclude that the periodic variations must be mostly attributed to capacitive currents due to the humidity dependent changes of the dielectric properties of the paper.32 Probably, only fibers in direct contact with the electrode surface contribute to this effect. Hence, this capacitive part of the current is probably quite sensitive to the surface morphology of every individual electrode and is expected to reach its maximum at the reversal points of the respiratory movement.
Figure 3.
Signals of sensing (black) and blank (red) electrodes of an amperometric measurement at different respiration (A) frequencies and (B) volumes, (C) current density of a calibration measurement with 5–320 μM H2O2 in vapor, and (D) calibration curve of the aqueous and vaporous hydrogen peroxide in artificial breath. Error bars represent ±SD of n = 3 replicates.
To obtain a calibration curve for hydrogen peroxide in the vapor of the artificial breath, H2O2 solutions of different concentrations are evaporated and the current signal over time is recorded continuously. A typical measurement is shown in Figure 3C. As soon as a steady-state current is reached, the next higher concentration is added, as indicated with the arrows labeled with the corresponding concentration. In our measurement setup, there is an additional delay resulting from the time the analyte needs to be transported toward the sensor surface after the injection of the sample. Therefore, it is not possible to extract the real response time from this data. Nevertheless, the response time to obtain a steady-state current depends on the peroxide concentration in the vapor. The reason for this behavior is probably due to the time it takes for the concentration of H2O2 in vapor to equilibrate with its dissolved form in water (i.e., dissolved in the moisture within paper). At higher H2O2 concentrations in the vapor, the gradient between vapor and “paper electrolyte” is higher, and thus, a steeper current increase as well as a higher limiting current is expected, which is almost in line with our observations.
The behavior of the blank (background) electrode can be also observed in Figure 3A–C. The measured blank signals do not settle at the same baseline currents as the sensing electrode. However, these different baseline currents can be aligned for the evaluation by setting an offset value (see Figure S11).
For the construction of the calibration curve, the current densities of the blank curve are first subtracted from those of the sensor electrode. After averaging and baseline subtraction, a measurement value is taken for each hydrogen peroxide concentration at a point on the timeline shortly before the next higher concentration is injected. The baseline value of the sensor is taken right before addition of the first hydrogen peroxide concentration of 40 μM. The mean values for the calibration curve presented in Figure 3D are obtained from three independent measurements.
From these results, it can be concluded that H2O2 concentrations in the range between 40 and 320 μM give rise to a response with a sensitivity of 0.02 nA μM–1 mm–2 and a correlation coefficient of 0.99. It is crucial to note, however, that the resulting current signals for the respective H2O2 concentrations are significantly lower than those of the former calibration in aqueous solutions (Figure 2A). This may be due to the heating of the H2O2 in the evaporator and the poor stability of H2O2 in DI water (see Figure S10). By correlating the obtained current densities with those of the previous calibration in solution, the real H2O2 concentrations in the vapor of the artificial breath can be estimated to lie between 5 and 40 μM. This means that, after evaporation, the H2O2 concentration may be decreasing to approximately 1/8 of its original value while the same sensitivity as in solution is maintained, i.e., 0.19 nA μM–1 mm–2 (Figures 3D and S12). The humid air from the humidifier also dilutes the analyte, yielding a smaller concentration. Nevertheless, after accounting for all of these factors, a reliable quantification of different hydrogen peroxide concentrations in vapor is achieved and the proof-of-concept of the claimed approach for on-site H2O2 analysis in simulated exhaled breath is successfully demonstrated for the first time.
In summary, this work describes a differential electrochemical method using low-cost and lightweight porous materials (in our case cellulose paper) for on-site monitoring of hydrogen peroxide in exhaled breath. For compatibility with standardized ventilation masks, the sensor developed is integrated into the housing of a commercially available airway filter for anesthetic applications. Under realistic conditions by simulating human respiration with authentic lung volume and respiration rate, the proof-of-principle of the hydrogen peroxide measurements in artificial exhaled breath is successfully shown for the first time. The high reliability and reproducibility of our sensing approach allow us to measure calibration-free, i.e., it is unnecessary to perform a calibration before or after each measurement. With further modifications and improvements, this sensor model can be employed in a large variety of applications, including clinical or wearable monitoring of exhaled breath.
Our method has the following five advantages: (i) because of differential measurements, the influence of various interfering substances and/or environmental conditions (e.g., temperature, humidity) is eliminated; hence, the system always produces reliable results. (ii) By changing, modifying, and/or coating the material of the sensing electrode (for instance, with metals, metal oxides, semiconducting micro- and nanoparticles, enzymes, selective membranes, or conducting polymers), the sensor model presented can be extended for the analysis of other compounds of exhaled breath. (iii) A flexible and hygroscopic porous support, like paper, acts as a “solid electrolyte”, eliminating the need for additional membranes (containing the electrolyte) and at the same time as a substrate for the electrodes. (iv) Flexible and porous substrates can be shaped and patterned in a way that the sensing surface as well as the collection volume can be considerably increased. (v) The orientation and porosity of the sensing surface can be tuned to minimize breathing resistance and to improve signal quality.
The system proposed has the following disadvantages: (i) the sensor’s sensitivity achieved with the commercially available PB-mediated carbon paste does not allow us to measure within the clinically relevant concentration range of H2O2 in exhaled breath (0.1–1.5 μM in the case of EBC6) and (ii) the electrolyte employed (1 M potassium chloride) gets dried quickly under ambient conditions and thus the paper chip needs some running time (less than 15 min) to be moistened after its exposure to the respiration. Furthermore, as the humidity in paper changes while inhaling and exhaling, the conductivity and ion concentration of the “solid paper electrolyte” and thus the measured signals vary periodically.
These, however, can be overcome by a few improvements: (i) the search for the optimum humectant as a possible electrolyte should be continued since it would facilitate the handling and signal processing, if the sensor stayed wet and does not adsorb any humidity from the breath. (ii) PB-mediated carbon paste with different PB contents and modification procedures must be tested to further increase the H2O2 sensitivity. Alternatively, hydrophilic metal electrodes (especially Pt), realized by metallization of fabrics, can be examined for their applicability.33 Besides, the implemented system should be extended with a compact and low-power wearable signal readout unit along with a smartphone app to enable on-site monitoring. Overcoming these issues, mainly with their sensitivity and humectants, electrochemical wearable sensors using porous materials (such as paper) would pave the way for calibration-free, continuous monitoring of exhaled breath.
Acknowledgments
This research has been partially funded by the German Research Foundation (DFG) under grant numbers UR 70/18-01, SCHU 2499/7-1, and DI 2345/3-1. F.G. thanks the Wellcome Trust (207687/Z/17/Z) and EPSRC (EP/R010242/1) for their generous support. We would like to thank Richard Bruch for providing the lasered PMMA sensor carriers and helping with the 3D printer. Furthermore, we would also like to thank Dr Jochen Kieninger for discussions about the electrode design.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssensors.9b01403.
Experimental procedures describing the resolution of screen printing, resistance measurements, evaluation of different electrode designs, differential electrode design, system integration, electrochemically active electrode area, study of different electrolytes, stability of hydrogen peroxide solution, offset correction of measured current signals, correlation of vaporous and aqueous H2O2 measurement, and measurement setup for simulated exhaled breath analysis (PDF)
Measurement setup illustrating the simulated breath experiments (MP4)
The authors declare no competing financial interest.
Supplementary Material
References
- World Health Organization. WHO releases country estimates on air pollution exposure and health impact. https://www.who.int/news-room/detail/27-09-2016-who-releases-country-estimates-on-air-pollution-exposure-and-health-impact (accessed Aug 23, 2018).
- World Health Organization . The Global Impact of Respiratory Disease, 2nd ed.; World Health Organization: Sheffield, 2017. [Google Scholar]
- World Health Organization. Chronic respiratory diseases. https://www.who.int/respiratory/en/ (accessed Aug 23, 2018).
- World Health Organization. Fact sheet - Noncommunicable diseases. www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed Aug 23, 2018).
- Zhou M.; Liu Y.; Duan Y. Breath Biomarkers in Diagnosis of Pulmonary Diseases. Clin. Chim. Acta 2012, 413, 1770–1780. 10.1016/j.cca.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Svensson S.; Olin A.; Larstad M.; Ljungkvist G.; Toren K. Determination of Hydrogen Peroxide in Exhaled Breath Condensate by Flow Injection Analysis with Fluorescence Detection. J. Chromatogr. B 2004, 809, 199–203. 10.1016/S1570-0232(04)00513-6. [DOI] [PubMed] [Google Scholar]
- Paget-Brown A. O.; Ngamtrakulpanit L.; Smith A.; Bunyan D.; Hom S.; Nguyen A.; Hunt J. F. Normative Data for PH of Exhaled Breath Condensate. Chest 2006, 129, 426–430. 10.1378/chest.129.2.426. [DOI] [PubMed] [Google Scholar]
- Solvay Chemicals Inc. Hydrogen peroxide safety and handling, technical datasheet. https://www.solvay.us/en/binaries/HH-2323-236798.pdf (accessed Aug 21, 2018).
- Horváth I.; Hunt J.; Barnes P. J. Exhaled Breath Condensate: Methodological Recommendations and Unresolved Questions. Eur. Respir. J. 2005, 26, 523–548. 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- Gerritsen W. B. M.; Asin J.; Zanen P.; van den Bosch J. M. M.; Haas F. J. L. M. Markers of Inflammation and Oxidative Stress in Exacerbated Chronic Obstructive Pulmonary Disease Patients. Respir. Med. 2005, 99, 84–90. 10.1016/j.rmed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Dohlman A. W.; Black H. R.; Royall J. A. Expired Breath Hydrogen Peroxide Is a Marker of Acute Airway Inflammation in Pediatric Patients with Asthma. Am. Rev. Respir. Dis. 1993, 148, 955–960. 10.1164/ajrccm/148.4_Pt_1.955. [DOI] [PubMed] [Google Scholar]
- Komkova M. A.; Karyakina E. E.; Marken F.; Karyakin A. A. Hydrogen Peroxide Detection in Wet Air with a Prussian Blue Based Solid Salt Bridged Three Electrode System. Anal. Chem. 2013, 85, 2574–2577. 10.1021/ac303761h. [DOI] [PubMed] [Google Scholar]
- Mutlu G. M.; Garey K. W.; Robbins R. A.; Danziger L. H.; Rubinstein I. Collection and Analysis of Exhaled Breath Condensate in Humans. Am. J. Respir. Crit. Care Med. 2001, 164, 731–737. 10.1164/ajrccm.164.5.2101032. [DOI] [PubMed] [Google Scholar]
- Dincer C.; Bruch R.; Costa-Rama E.; Fernández-Abedul M. T.; Merkoçi A.; Manz A.; Urban G. A.; Güder F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739 10.1002/adma.201806739. [DOI] [PubMed] [Google Scholar]
- Bandodkar A. J.; Jeerapan I.; Wang J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. 10.1021/acssensors.6b00250. [DOI] [Google Scholar]
- Kenry; Yeo J. C.; Lim C. T. Emerging Flexible and Wearable Physical Sensing Platforms for Healthcare and Biomedical Applications. Microsyst. Nanoeng. 2016, 2, 16043 10.1038/micronano.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koydemir H. C.; Ozcan A. Wearable and Implantable Sensors for Biomedical Applications. Annu. Rev. Anal. Chem. 2018, 11, 127–146. 10.1146/annurev-anchem-061417-125956. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Gao W. Wearable and Flexible Electronics for Continuous Molecular Monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. 10.1039/C7CS00730B. [DOI] [PubMed] [Google Scholar]
- Rolland J. P.; Mourey D. A. Paper as a Novel Material Platform for Devices. MRS Bull. 2013, 38, 299–305. 10.1557/mrs.2013.58. [DOI] [Google Scholar]
- Dincer C.; Bruch R.; Kling A.; Dittrich P. S.; Urban G. A. Multiplexed Point-of-Care Testing – XPOCT. Trends Biotechnol. 2017, 35, 728–742. 10.1016/j.tibtech.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barandun G.; Soprani M.; Naficy S.; Grell M.; Kasimatis M.; Chiu K. L.; Ponzoni A.; Güder F. Cellulose Fibers Enable Near-Zero-Cost Electrical Sensing of Water-Soluble Gases. ACS Sens. 2019, 4, 1662–1669. 10.1021/acssensors.9b00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher P. J.; Gupta M.; Whitesides G. M. Patterning Precipitates of Reactions in Paper. J. Mater. Chem. 2010, 20, 5117–5122. 10.1039/c000358a. [DOI] [Google Scholar]
- Güder F.; Ainla A.; Redston J.; Mosadegh B.; Glavan A.; Martin T. J.; Whitesides G. M. Paper-Based Electrical Respiration Sensor. Angew. Chem., Int. Ed. 2016, 55, 5727–5732. 10.1002/anie.201511805. [DOI] [PubMed] [Google Scholar]
- Feldman B. J.; Melroy O. R. Ion Flux during Electrochemical Charging of Prussian Blue Films. J. Electroanal. Chem. Interfacial Electrochem. 1987, 234, 213–227. 10.1016/0022-0728(87)80173-0. [DOI] [Google Scholar]
- Itaya K.; Ataka T.; Toshima S. Spectroelectrochemistry and Electrochemical Preparation Method of Prussian Blue Modified Electrodes. J. Am. Chem. Soc. 1982, 104, 4767–4772. 10.1021/ja00382a006. [DOI] [Google Scholar]
- Neff V. D. Electrochemical Oxidation and Reduction of Thin Films of Prussian Blue. J. Electrochem. Soc. 1978, 125, 886–887. 10.1149/1.2131575. [DOI] [Google Scholar]
- Karyakin A.; Karyakina E. E.; Gorton L. On the Mechanism of H2O2 Reduction at Prussian Blue Modified Electrodes. Electrochem. Commun. 1999, 1, 78–82. 10.1016/S1388-2481(99)00010-7. [DOI] [Google Scholar]
- Cinti S.; Arduini F.; Moscone D.; Palleschi G.; Killard A. Development of a Hydrogen Peroxide Sensor Based on Screen-Printed Electrodes Modified with Inkjet-Printed Prussian Blue Nanoparticles. Sensors 2014, 14, 14222–14234. 10.3390/s140814222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakumura Y.; Koyama Y.; Tokutake H.; Hida T.; Sato K.; Itoh T.; Akamatsu T.; Shin W. Diagnosis by Volatile Organic Compounds in Exhaled Breath from Lung Cancer Patients Using Support Vector Machine Algorithm. Sensors 2017, 17, 287. 10.3390/s17020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankasala D.; Linnes J. C. Noninvasive Glucose Detection in Exhaled Breath Condensate. Transl. Res. 2019, 213, 1–22. 10.1016/j.trsl.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T. A. Human Exhaled Breath Analysis. Ann. Allergy, Asthma, Immunol. 2011, 106, 451–456. 10.1016/j.anai.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Gaspar C.; Olkkonen J.; Passoja S.; Smolander M. Paper as Active Layer in Inkjet-Printed Capacitive Humidity Sensors. Sensors 2017, 17, 1464. 10.3390/s17071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell M.; Dincer C.; Le T.; Lauri A.; Nunez Bajo E.; Kasimatis M.; Barandun G.; Maier S. A.; Cass A. E. G.; Güder F. Autocatalytic Metallization of Fabrics Using Si Ink, for Biosensors, Batteries and Energy Harvesting. Adv. Funct. Mater. 2019, 29, 1804798 10.1002/adfm.201804798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.