The roles of the MYC transcription factor in transcriptional regulation have been studied intensively. However, the general mechanism underlying the recruitment of MYC to chromatin is less clear. Here, we found that the Krüppel-like transcription factor ZFP281 plays important roles in recruiting MYC to active promoters in mouse embryonic stem cells.
KEYWORDS: ZFP281, MYC, transcription, LIN28/Let-7
ABSTRACT
The roles of the MYC transcription factor in transcriptional regulation have been studied intensively. However, the general mechanism underlying the recruitment of MYC to chromatin is less clear. Here, we found that the Krüppel-like transcription factor ZFP281 plays important roles in recruiting MYC to active promoters in mouse embryonic stem cells. At the genome scale, ZFP281 is broadly associated with MYC, and the depletion of ZFP281 significantly reduces the levels of MYC and RNA polymerase II at the ZFP281- and MYC-cobound genes. Specially, we found that recruitment is required for the regulation of the Lin28a oncogene and pri-let-7 transcription. Our results therefore suggest a major role of ZFP281 in recruiting MYC to chromatin and the integration of ZFP281 and the MYC/LIN28A/Let-7 loop into a multilevel circuit.
INTRODUCTION
In eukaryotes, sequence-specific transcription factors regulate transcription by RNA polymerase II (Pol II) by controlling the recruitment of general transcription factors and RNA Pol II to target transcription start sites (TSS), allowing transcription initiation, and promoting the entry of RNA Pol II to elongation phase (1, 2). The basic helix-loop-helix (bHLH) transcription factor MYC is a major driver of stem cell pluripotency and cancer cell growth (3, 4). MYC is one of the most frequently amplified oncogenes and widely overexpressed in many different human cancers (5). MYC affects numerous physiological and pathological processes by stimulating transcription (6–9). Recent studies suggest that, when highly expressed, MYC invades active promoters and functions as a transcription amplifier at different stages of transcription, including Pol II loading and elongation (10–14).
Many studies have intensively focused on the recruitment of MYC to chromatin. MYC is able to form a heterodimer with MAX and bind to the canonical enhancer box (E-box) CACGTG in vitro with higher affinity (15). Nevertheless, the E-box and its variants are not the major determinants of MYC genomic binding (16). MYC occupancy is strongly correlated with that of RNA Pol II (16). It was reported that the WD40-repeat protein WDR5 can interact with MYC and that deletion of the WDR5 recognition motif in MYC compromised the chromatin binding capacity of MYC (17, 18). A recent study in Drosophila S2 cells has suggested the interdependence between MYC and the PAF1 complex in their genomic occupancies (19). However, earlier works in mammals demonstrated that the depletion of PAF1 enhanced the expression of MYC target genes (20, 21). These studies suggest that the regulation of MYC and its target genes by the PAF1 complex could be context dependent. Thus, the mechanism underlying the loading of MYC to the core promoter elements of actively transcribed genes remains largely unaddressed.
Previously, we showed that Lin28a is a major direct target gene of the Krüppel-like zinc finger transcription factor ZFP281 in mouse embryonic stem (ES) cells (22). ZFP281 recruits AFF3, the central component of superelongation complex-like 3 (SEC-L3), to enhancer sites and regulates the expression of a subset of genes, including Lin28a. The members of the highly conserved LIN28 family of proteins, including LIN28A and LIN28B, are RNA binding factors with a well-established function in inhibiting the biogenesis of Let-7 microRNAs (miRNAs) (23–25). It has been demonstrated that the expression of MYC is finely tuned by the LIN28/Let-7 axis. MYC is inhibited posttranscriptionally by Let-7 miRNAs (26), and LIN28 activates MYC expression by repressing Let-7 (27). In addition, MYC can induce LIN28 in certain cancer models, revealing a complicated regulatory circuit consisting of MYC, LIN28, and Let-7 in stem cell pluripotency and oncogenesis (28).
Here, we sought to fully understand how ZFP281 is involved in the working and wiring of the MYC/LIN28/Let-7 regulatory circuit. We demonstrate that ZFP281 facilitates the loading of MYC to both Lin28a and pri-let-7 and regulates their transcription, providing an additional layer to the multilayered MYC/LIN28/Let-7 circuit. In addition, our genome-wide studies indicate that ZFP281 occupies virtually all the MYC-bound promoters and is required for the loading of MYC to the genome, suggesting a concerted role of ZFP281 and MYC in transcriptional regulation.
RESULTS
ZFP281 recruits MYC to Lin28a/b.
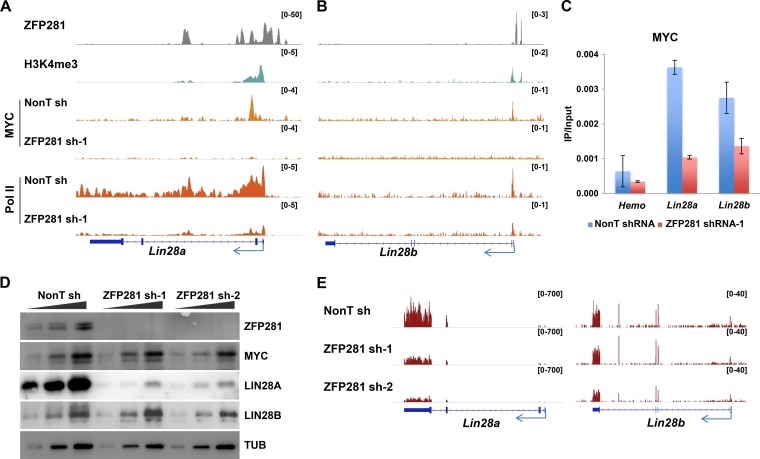
MYC is a key regulator of the LIN28/Let-7 feedback loop. The activation of LIN28A/B by MYC has been observed in human cancer cells (27, 29). We found that ZFP281 and MYC cooccupied the promoters of Lin28a and Lin28b in mouse ES cells (Fig. 1A and B). Next, in order to investigate the requirement of ZFP281 for MYC chromatin binding, we performed MYC chromatin immunoprecipitation (ChIP) after ZFP281 knockdown. Chromatin immunoprecipitation sequencing (ChIP-Seq) results indicated a significant reduction of MYC occupancies at both the Lin28a and Lin28b promoters after the depletion of ZFP281 in ES cells (Fig. 1A and B). Similar effects were also observed by MYC ChIP-quantitative PCR (qPCR) analysis after ZFP281 knockdown (Fig. 1C). Depletion of ZFP281 did not alter the MYC RNA or protein level in mouse ES cells, indicating that this effect is not due to the reduced expression of MYC (Fig. 1D). Therefore, ZFP281 is required for the recruitment of MYC to both Lin28a and Lin28b.
FIG 1.
ZFP281 recruits MYC to Lin28a and Lin28b and differentially regulates Lin28a and Lin28b in mouse ES cells. (A and B) ChIP-Seq genome browser track showing the localization of ZFP281, H3K4me3, MYC, and Pol II in the Lin28a (A) and Lin28b (B) genes. ChIP-Seq of MYC in control and ZFP281-depleted cells revealed the recruitment of MYC to the promoters of both the Lin28a (A) and Lin28b (B) genes. ChIP-Seq of Pol II showed that ZFP281 knockdown leads to transcriptional inhibition of Lin28a (A) but not Lin28b (B). The ChIP-Seq enrichment is displayed as reads per million. (C) ChIP-qPCR showing that the occupancies of MYC at the promoters of Lin28a and Lin28b are reduced after ZFP281 knockdown in mouse ES cells. The Hemo gene served as a negative control for ChIP-qPCR. Error bars represent standard deviations (n = 3). (D) Western blot analyses of MYC, LIN28A, and LIN28B after depletion of ZFP281 using two independent shRNAs. No significant changes were observed for MYC following ZFP281 knockdown. β-Tubulin (TUB) served as a loading control. (E) RNA-Seq analysis showing reduced expression of Lin28a, but not Lin28b, upon ZFP281 knockdown. sh-1, shRNA-1; sh-2, shRNA-2; NonT sh, nonT shRNA; IP, immunoprecipitation. The RNA-Seq results are displayed as reads per kilobase million.
It is well established that ZFP281 activates the expression of Lin28a (22). We further examined the requirement of ZFP281 for Lin28b expression. However, only Lin28a and not Lin28b was activated by ZFP281 (Fig. 1D and E). We noticed that in mouse ES cells Lin28a is highly transcribed, with Pol II traveling throughout the gene, while Lin28b is poised with Pol II paused at its proximal promoter region (Fig. 1A and B). Thus, a possible explanation for the discrepancy between the regulation of Lin28a and Lin28b by ZFP281 is that the transcription of the highly transcribed Lin28a is more vulnerable to MYC dissociation, which occurs after ZFP281 depletion, than Lin28b. We have previously demonstrated that AFF3 is recruited to the enhancer of Lin28a by ZFP281 (22). Thus, ZFP281, MYC, and AFF3 could cooperatively regulate the expression of Lin28a.
ZFP281 directly regulates the transcription of the pri-let-7 gene in mouse ES cells.
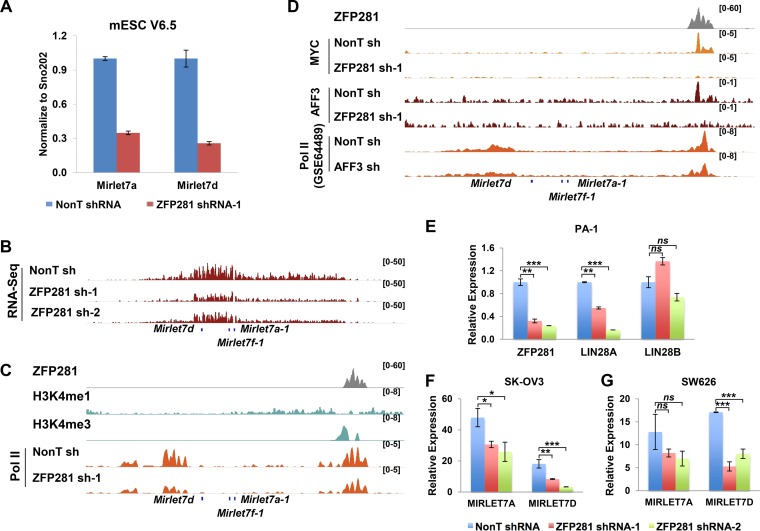
The levels of mature Let-7 miRNAs are reciprocal to the level of LIN28A/B (30). We further examined whether the mature Let-7 miRNAs Mirlet7a and Mirlet7d are upregulated after ZFP281 knockdown. Contrary to what was expected, the depletion of ZFP281 led to a significant reduction in the levels of the mature Let-7 miRNAs (Fig. 2A). Further inspection of the total transcriptome sequencing (RNA-Seq) data revealed that the expression of the Let-7 precursor was reduced after ZFP281 knockdown with two independent short hairpin RNAs (shRNAs) (Fig. 2B), suggesting that ZFP281 might directly regulate the transcription of pri-let-7. Pol II occupancy analysis demonstrated that the pri-let-7 gene is actively transcribed, although the mature let-7 miRNAs were hard to detect in mouse ES cells (Fig. 2C). In addition, we found that ZFP281, MYC, and AFF3 also cooccupied the pri-let-7 promoter. ZFP281 was essential for the association of both MYC and AFF3 with the pri-let-7 promoter (Fig. 2D). Depletion of either ZFP281 or AFF3 led to a reduction of Pol II occupancies over the pri-let-7 transcribing unit, suggesting the control of pri-let-7 gene transcription by ZFP281 via AFF3 (Fig. 2C and D). Thus, ZFP281 recruits both AFF3 and MYC to finely regulate the pri-let-7 gene, maintaining a balanced expression status in ES cells.
FIG 2.
ZFP281 directly regulates the transcription of the pri-let-7 gene in mouse ES cells. (A) RT-qPCR showing the reduction in the RNA levels of Mirlet7a and Mirlet7d by knockdown of ZFP281 in mouse ES cells (mESC). Mature miRNA levels were normalized using the level of SNO202 RNA. Error bars represent standard deviations (n = 3). (B) RNA-Seq analysis showing reduced expression of the pri-let-7 gene upon ZFP281 knockdown using two independent shRNAs (sh-1 and sh-2). The RNA-Seq results are displayed as reads per kilobase million. (C) ChIP-Seq genome browser track showing the localization of ZFP281, H3K4me1, H3K4me3, and Pol II at the pri-let-7 gene. ChIP-Seq of Pol II showed that ZFP281 activates pri-let-7 at the transcription level. The ChIP-Seq enrichment is displayed as reads per million (RPM). (D) ChIP-Seq genome browser track showing the localization of ZFP281, MYC, AFF3, and Pol II at the pri-let-7 gene. ChIP-Seq of MYC and AFF3 in control and ZFP281-depleted cells showed the requirement of ZFP281 for the binding of MYC and AFF3 to the pri-let-7 promoter, respectively. ChIP-Seq of Pol II showed that AFF3 knockdown leads to the transcriptional elongation of pri-let-7. ChIP-Seq data for Pol II after AFF3 knockdown were downloaded from the GEO database (GEO accession number GSE64489). (E) RT-qPCR showing the efficiency of ZFP281 knockdown by two independent shRNAs in PA-1 cells. The transcript levels of LIN28A, but not LIN28B, are reduced by knockdown of ZFP281 in PA-1 cells. (F and G) RT-qPCR showing that the RNA levels of MIRLET7A and MIRLET7D are reduced by knockdown of ZFP281 using two independent shRNAs in SK-OV3 (F) and SW626 (G) cells. (E to G) Significant differences (determined by a t test) are marked with asterisks. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars represent standard deviations (n = 3).
We also confirmed the regulation of LIN28A and PRI-LET-7 by ZFP281 in human cell lines. The expression levels of LIN28A, LIN28B, and LET-7 were examined in several ovarian cancer cell lines after ZFP281 knockdown. Consistently, depletion of ZFP281 led to a reduction of LIN28A expression in LIN28A- and LIN28B-expressing PA-1 cells and also reduced LET-7 expression in SK-OV3 and SW626 cells (Fig. 2E to G). In summary, our results revealed an activating function of ZFP281 in the transcription of LIN28A and PRI-LET-7 in human cells and lin28a and pri-let-7 in mouse cells, providing an additional regulatory layer to the LIN28/Let-7 feedback loop.
ZFP281 is enriched at the MYC-occupied promoters.
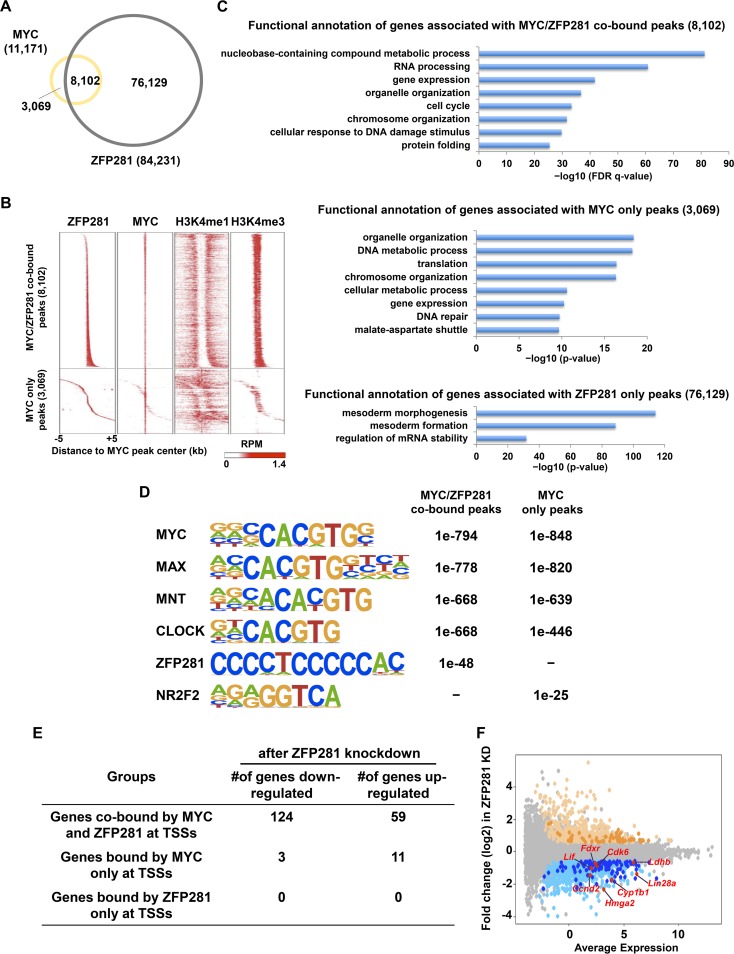
To determine whether ZFP281 is generally associated with MYC, we first examined whether these two factors colocalized genome wide in mouse ES cells. A total of 84,231 and 11,171 genomic sites were bound by ZFP281 and MYC, respectively (Fig. 3A). A total of 8,102 sites were cooccupied by both ZFP281 and MYC (Fig. 3A). In other words, ZFP281 was detected at more than 72% of the MYC-bound sites, suggesting that ZFP281 binding is a possible prerequisite for the loading of MYC to genome. Further analysis indicated that the MYC- and ZFP281-cooccupied peaks with high levels of H3K4me3 were located at active promoters, as exemplified by the Lin28a promoter (Fig. 1A and 3B). Functional annotation of the three groups of peaks using the Genomic Regions Enrichment of Annotations Tool (GREAT) indicated that genes associated with the MYC/ZFP281-cobound peaks and the MYC-only peaks are involved in housekeeping processes, such as metabolism, and that, in contrast, the genes nearest the ZFP281-only peaks are mainly involved in the mesoderm developmental process (31) (Fig. 3C).
FIG 3.
ZFP281 and MYC cooccupy a subset of promoters in mouse ES cells. (A) Venn diagram showing the overlap between ZFP281 and MYC binding sites in mouse ES cells. (B) Heat maps of the binding profiles in mouse ES cells for ZFP281, MYC, H3K4me1, and H3K4me3 within 5 kb of the center of the MYC peaks, which were partitioned into the MYC/ZFP281-cobound group and the MYC-only group, are shown. RPM, numbers of reads per million. (C) Functional annotation of the MYC/ZFP281-cobound peaks (top), the MYC-only peaks (middle), and the ZFP281-only peaks (bottom), as reported by GREAT. The P value was used to determine the significance of the enrichment of terms. (D) The overrepresented motifs in both the MYC/ZFP281-cobound peaks and the MYC-only peaks are shown. The statistical significance (P value) of the overrepresentation of the motifs in each group is shown. (E) Table showing the numbers of genes downregulated after ZFP281 knockdown in the different groups. (F) Gene expression MA plot showing the differential expression of genes after ZFP281 knockdown (KD) in mouse ES cells. Significantly upregulated genes are shown in light yellow; downregulated genes are shown in light blue. Genes that were cobound by MYC and ZFP281 at their promoters are highlighted by dark yellow and dark blue for upregulated and downregulated genes, respectively. The known MYC target genes Ccnd2, Cdk6, Lif, Lin28a, Fdxr, Cyp1b1, Ldhb, and Hmga2, which were cobound by both MYC and ZFP281 and downregulated after ZFP281 knockdown, are highlighted in red.
ZFP281 regulates the expression of MYC target genes.
It has been shown that MYC is able to dimerize with MAX to bind to the E-box (32). Indeed, motif analysis demonstrated that the E-box containing MYC, MAX, MNT, and CLOCK consensus sequences is highly enriched in both the MYC/ZFP281-cobound peaks and the MYC-only peaks (Fig. 3D). The GC-rich ZFP281 motif is specifically enriched in the MYC/ZFP281-cobound peaks. We also found that the NR2F2 binding motif is significantly enriched only in the MYC-only peaks (Fig. 3D).
MYC can function as a transcriptional activator or repressor at the target gene. We found that 124 significantly downregulated genes and 59 significantly upregulated genes (P < 0.05, fold change in expression > 1.5) after ZFP281 knockdown were cooccupied by ZFP281 and MYC at their promoters (Fig. 3E and F). In contrast, only a few or none of the promoters of the genes differentially expressed after ZFP281 knockdown were bound by either MYC or ZFP281 only at the promoters (Fig. 3E). For example, metabolic genes, such as Ldhb, Fdxr, and Cyp1b1, which were bound directly by both MYC and ZFP281, required ZFP281 for their expression. The cell cycle-related MYC target genes Ccnd2 and Cdk6, which were identified in other cellular contexts, were also bona fide target genes of ZFP281 (33, 34). It has been reported that mouse ES cell self-renewal can be maintained in the absence of LIF after the expression of a stable form of MYC (MYC T58A) (35). Interestingly, we found that ZFP281 occupied the Lif gene together with MYC and was essential for its proper expression (Fig. 3F). Thus, our data suggest that ZFP281 is associated with virtually all of the MYC-bound promoters and functions in regulating the expression of a subset of genes.
ZFP281 is generally required for the chromatin localization of MYC.
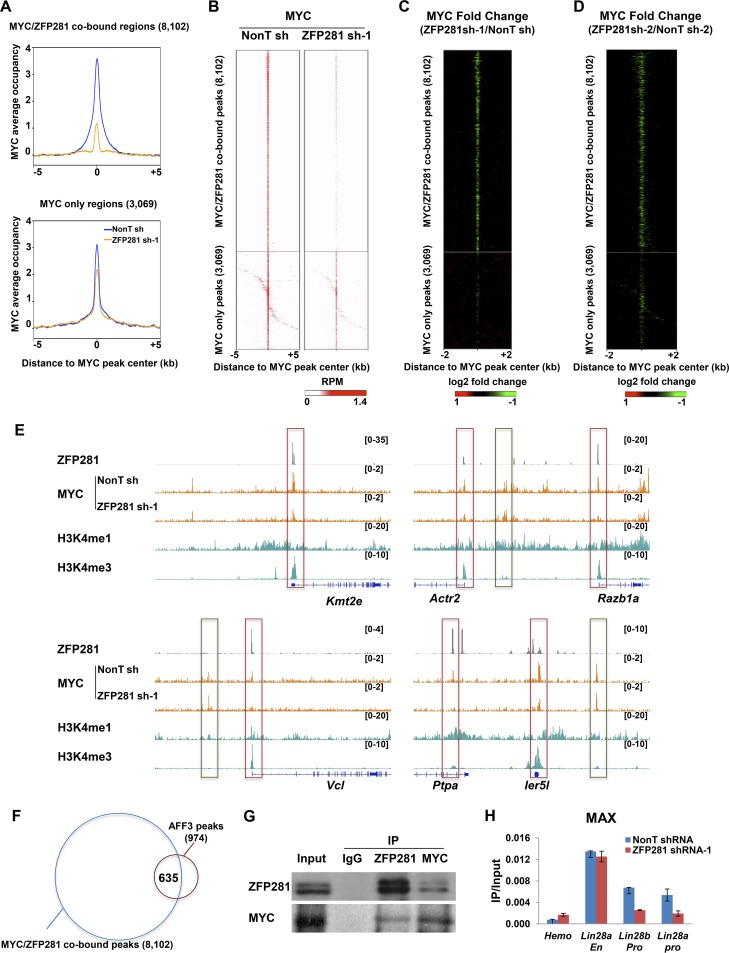
We then analyzed the genomic occupancy of MYC in control and ZFP281-depleted mouse ES cells and found that MYC occupancy was substantially reduced at the ZFP281- and MYC-cooccupied regions (Fig. 4A to C, top). In contrast, MYC occupancy at the MYC-only regions was only slightly affected by ZFP281 knockdown (Fig. 4A to C, bottom). The specific recruitment of MYC by ZFP281 to their cobound sites was confirmed by the knockdown of ZFP281 using an independent shRNA (Fig. 4D). We noticed that the occupancies of MYC at some of the MYC-only regions were slightly reduced after ZFP281 knockdown (Fig. 4A to E). It is likely that the MYC/ZFP281-cobound regions could interact with the MYC-only regions due to chromatin looping. Thus, once the occupancies of MYC at the MYC/ZFP281-cobound regions were reduced after ZFP281 knockdown, the MYC-only regions would also be affected. Indeed, about half of the genes nearest the MYC-only peaks were also cobound by both MYC and ZFP281 at their promoters.
FIG 4.
ZFP281 functions as a general recruiter of MYC at active promoters. (A) Average occupancy plots of MYC in control (nonT shRNA) and ZFP281 knockdown (ZFP281 shRNA-1) mouse ES cells. The MYC/ZFP281-cooccupied regions and the MYC-only region within 5 kb of the center of the MYC peaks are shown. (B) Heat maps of the binding profiles for MYC in control and ZFP281 knockdown mouse ES cells within 5 kb of the center of the MYC peaks are shown. (C) MYC occupancy log2 fold change after ZFP281 knockdown measured ±2 kb around the center of the MYC peaks. (D) MYC occupancy log2 fold change after knockdown of ZFP281 using ZFP281 shRNA-2 measured ±2 kb around the center of the MYC peaks. (E) ChIP-Seq genome browser track showing that ZFP281 and MYC are colocalized at the chromatin in mouse ES cells. The MYC- and ZFP281-cooccupied regions, to which the binding of MYC is dependent on ZFP281, are highlighted with red squares. The MYC-only regions, to which the binding of MYC is independent of ZFP281, are highlighted with green squares. The ChIP-Seq enrichment is displayed as reads per million. (F) Venn diagram showing the overlap between the AFF3 binding sites and the MYC/ZFP281-cobound sites in mouse ES cells. (G) Endogenous immunoprecipitations (IP) showing that MYC and ZFP281 can interact with each other. (H) ChIP-qPCR showing that MAX occupancy is reduced at the promoters of Lin28a and Lin28b after ZFP281 knockdown, while it remains unchanged at the Lin28a enhancer. The Hemo gene served as a negative control for ChIP-qPCR. Error bars represent standard deviations (n = 3).
We have previously shown that ZFP281 recruits AFF3 to enhancer sites in mouse ES cells (22). To evaluate whether the recruitment of MYC by ZFP281 is dependent on AFF3, we analyzed the AFF3 occupancy at the MYC/ZFP281-cobound regions. Only 635 (7.8%) of the 8,102 MYC/ZFP281-cobound peaks were also occupied by AFF3 (Fig. 4F). Thus, it is not likely that AFF3 plays a general role in facilitating the recruitment of MYC by ZFP281.
An interaction between ZFP281 and MYC has previously been reported in a large-scale proteomics analysis searching for potential MYC-interacting candidates (36). Here, we also performed a coimmunoprecipitation experiment and found that ZFP281 and MYC can interact with each other in mouse ES cells (Fig. 4G). Thus, the recruitment of MYC to chromatin by ZFP281 is mediated by the direct interaction between ZFP281 and MYC.
ZFP281 is also required for the chromatin localization of MAX.
We also measured the occupancy of MAX after ZFP281 knockdown. ChIP-qPCR results indicated that MAX was enriched not only at the MYC- and ZFP281-cobound Lin28a and Lin28b promoters but also at the Lin28a enhancer, which was occupied by ZFP281 only (Fig. 1A and 4H). The binding of MAX to the Lin28a and Lin28b promoters was impaired after ZFP281 knockdown, while it remained unchanged at the Lin28a enhancer region (Fig. 4H). The expression of MAX was not affected by ZFP281 knockdown (data not shown). Taken together, our data suggest that ZFP281 is essential for the binding of MYC and MAX to their target promoters in mouse ES cells.
ZFP281 specifically regulates Pol II occupancy at the highly transcribed ZFP281- and MYC-cooccupied genes.
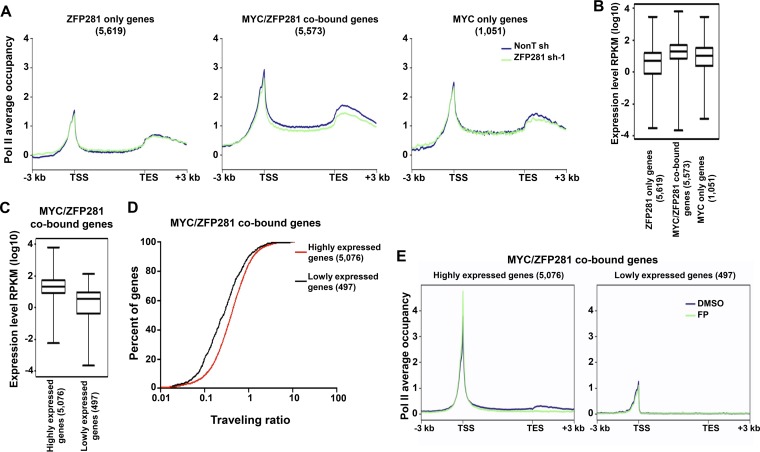
Since ZFP281 can facilitate the loading of MYC to active promoters, we then sought to determine how ZFP281 regulates Pol II occupancy by Pol II ChIP-Seq analyses in mouse ES cells following ZFP281 depletion. At genes associated with the MYC- and ZFP281-cooccupied peaks, to which the recruitment of MYC requires ZFP281, ZFP281 depletion resulted in reduced Pol II occupancy (Fig. 5A). In contrast, ZFP281 depletion had no obvious effect on the Pol II occupancy at the genes nearest either the ZFP281-only or the MYC-only peaks (Fig. 5A).
FIG 5.
ZFP281 regulates transcription of the highly expressed MYC/ZFP281-cooccupied genes. (A) Average Pol II binding plots for the ZFP281-only genes (left), the MYC/ZFP281-cobound genes (middle), and the MYC-only genes (right) in control (nonT shRNA) and ZFP281 knockdown (ZFP281 shRNA-1) mouse ES cells. Plots for from 3 kb upstream of the TSS to 3 kb downstream of the transcriptional end site (TES) are shown. (B) Gene expression (in number of reads per kilobase per million [RPKM]) analyses of the ZFP281-only genes, the MYC/ZFP281-cobound genes, and the MYC-only genes. (C) Gene expression (in number of reads per kilobase per million) analyses of the MYC/ZFP281-cobound highly expressed and lowly expressed genes. (B and C) Only genes with statistically sufficient coverage by RNA-Seq are shown. (D) Distribution of the percentage of the MYC/ZFP281-cobound highly expressed and lowly expressed genes with a given traveling ratio. (E) Average Pol II binding plots for the MYC/ZFP281-cobound highly expressed genes (left) and the MYC/ZFP281-cobound lowly expressed genes (right) in DMSO- and flavopiridol (FP)-treated mouse ES cells. Plots for the regions from 3 kb upstream of the TSS to 3 kb downstream of the transcriptional end site are shown.
Among the three groups of genes, on average, the MYC- and ZFP281-cobound genes had the highest expression level (Fig. 5B). Further analysis indicated that 91% of the MYC- and ZFP281-cobound genes (highly expressed genes) contained elongating Pol II at gene bodies and showed higher expression levels with a lower traveling ratio than the rest of the MYC- and ZFP281-cobound genes (lowly expressed genes) (Fig. 5C and D). It has been shown that actively transcribed genes are more sensitive to flavopiridol treatment (8). Indeed, we found that Pol II was more stalled at the proximal promoter regions after flavopiridol treatment in the highly expressed but not in the lowly expressed MYC/ZFP281-cobound group of genes (Fig. 5E).
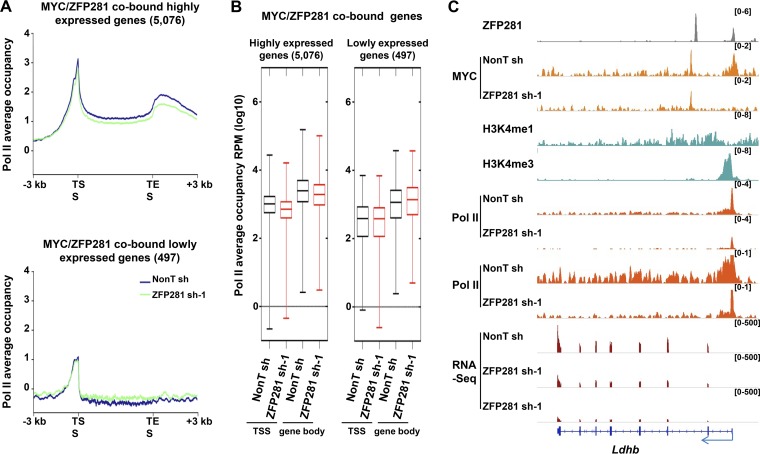
MYC plays broad roles in regulating RNA Pol II behavior at different stages, including Pol II promoter loading and pause release (8, 12–14). Consistently, our analyses also showed that a more significant reduction in the levels of both promoter- and gene body-associated Pol II was generally observed in the highly expressed MYC/ZFP281-cobound group of genes than in the lowly expressed MYC/ZFP281-cobound group of genes (Fig. 6A and B). For example, ZFP281 and MYC cooccupied the promoter of the highly transcribed Lin28a, Ldhb, and other genes (Fig. 1A and 6C). ZFP281 depletion led to the complete loss of MYC at their promoters and a clear reduction of Pol II across the whole transcribed units (Fig. 1A and 6C). Therefore, our results suggest that ZFP281 specifically stimulates transcription at the initiation and elongation stages at those highly transcribed ZFP281- and MYC-cooccupied genes.
FIG 6.
ZFP281 specifically regulates the transcription elongation of the highly transcribed ZFP281- and MYC-cooccupied genes. (A) Average Pol II binding plots for the MYC/ZFP281-cobound highly expressed genes (top) and lowly expressed genes (bottom) in control (nonT shRNA) and ZFP281 knockdown (ZFP281 shRNA-1) mouse ES cells. Plots are shown from 3 kb upstream of TSS to 3 kb downstream of the transcriptional end site (TES). (B) Box plots showing the changes in occupancy of TSS- and gene body-associated Pol II at the MYC/ZFP281-cobound highly expressed genes (left) and lowly expressed genes (right) in control (nonT sh) and ZFP281 knockdown (ZFP281 sh-1) mouse ES cells. (C) ChIP-Seq genome browser track showing the localization of ZFP281, MYC, H3K4me1, H3K4me3, and Pol II at the highly transcribed Ldhb. ChIP-Seq of MYC in control and ZFP281-depleted cells showing the requirement of ZFP281 for the binding of MYC to the promoters of Ldhb. ChIP-Seq of Pol II shows that ZFP281 knockdown leads to reduced Pol II occupancies at both the TSS and the gene body of Ldhb. RNA-Seq analysis shows the reduced expression of Ldhb upon ZFP281 knockdown. The ChIP-Seq enrichment is displayed as reads per million.
DISCUSSION
MYC affects numerous physiological and pathological processes via its function as a global transcription regulator. Although MYC and its partner, MAX, bind to the E-box or its variants with a high affinity in vitro, the specific DNA sequences are inadequate to interpret the genomic binding of MYC. Here, we found that (i) ZFP281 occupies the vast majority of the MYC-enriched promoters, including the promoters of the two antagonistic genes Lin28a and pri-let-7; (ii) ZFP281 is generally required for MYC’s association with promoters; and (iii) the loss of ZFP281 results in reduced Pol II occupancy at the highly transcribed MYC- and ZFP281-cooccupied genes. Therefore, our results support a critical role of ZFP281 in transcriptional control through its regulatory interplay with MYC at promoters.
Although it has been well proven that MYC is able to bind the E-box in vitro with a high affinity, the sequence-specific interaction is not sufficient for recruiting MYC to its chromatin target sites (16). Recently, WDR5 and the PAF1 complex subunit LEO1 were shown to interact with MYC and facilitate its recruitment to target genes in various systems (17–19). Here, we found that the DNA binding factor ZFP281 directly recruits MYC to active gene promoters in mouse ES cells. Future studies in a unified system will clarify whether different MYC recruiters are able to direct MYC to different genomic sites or coordinately load MYC to the same regions in a stepwise manner. In addition, we also found that the NR2F2 binding motif is significantly enriched in the MYC-only peaks. Whether NR2F2 or additional factors are involved in recruiting MYC to chromatin is worth further investigation.
Previous studies have suggested that the differentiation capability of mouse ES cells is impaired after MYC loss, though MYC-null mouse ES cells can be maintained in LIF-containing medium (37, 38). For example, embryoid bodies (EBs) derived from MYC-null ES cells were defective (37). Consistently, ZFP281 is dispensable for the self-renewal of ES cells but is required for their proper differentiation. EBs differentiated from Zfp281 knockout ES cells were much smaller and less well developed than their wild-type counterparts (39, 40). Thus, the loss of either ZFP281 or MYC impairs the ability of ES cells to differentiate properly.
The MYC/LIN28/Let-7 regulatory circuit operates in stem cells and cancer cells, coordinating stem cell pluripotency and tumorigenesis (30). It has been demonstrated previously that ZFP281 expression is regulated by MYC (41). In this study, we found that ZFP281 is central to MYC’s chromatin binding, suggesting a feed-forward regulation between ZFP281 and MYC. On the other hand, we demonstrated here that ZFP281 lies upstream of the LIN28/Let-7 axis via promoting the transcription of the two antagonistic partners LIN28A and PRI-LET-7 in both stem cells and cancer cells. Although mature Let-7 miRNAs are poorly detected in undifferentiated stem cells, the pri-let-7 gene is transcribed. The inhibition of Let-7 miRNA maturation by LIN28 and the activation of pri-let-7 transcription by ZFP281 maintain the mature Let-7 miRNAs at a low level in undifferentiated cells, but ready for accumulation and function upon differentiation. In addition, due to its activating function on the transcription of the two antagonistic partners LIN28 and PRI-LET-7, ZFP281 functions as a double-edged sword in the ZFP281/MYC/LIN28/Let-7 circuit, possibly endowing cells with the plasticity to override the fluctuation of the circuit component, which is often observed during tumorigenesis. Thus, it would be interesting to further investigate the role of ZFP281 in MYC-overexpressing cancers in the future.
It has been reported that the ectopic expression of ZFP281 in colorectal cancer cells can induce epithelial-mesenchymal transition (EMT), endowing cancer cells with metastatic competence (41). Recent large-scale cancer data set analysis also observed a positive correlation between enhanced ZFP281 expression and tumor recurrence (42). Thus, our observation on the requirement of ZFP281 for the general recruitment of MYC to chromatin might pinpoint ZFP281 as a potential critical target for cancer therapeutics.
MATERIALS AND METHODS
Cell culture.
Mouse V6.5 ES cells were grown on irradiated mouse embryonic fibroblast (iMEF) feeder layers in a 0.1% gelatin-coated tissue culture plate. The cells were cultured in Dulbecco modified Eagle medium (catalog number D6546; Sigma) supplemented with 15% ES cell-certified fetal bovine serum (HyClone), 0.1 mM nonessential amino acids, 2 mM l-glutamine, 0.1 mM β-mercaptoethanol, and recombinant LIF (Millipore). The cells were maintained under 5% CO2 at 37°C. The ovary cancer cell lines PA-1, SK-OV3, and SW626 were cultured according to ATCC’s instructions.
Lentivirus-mediated RNA interference.
Mouse ZFP281 shRNA constructs and the nontargeting (nonT) shRNA construct (SHC002) were described previously (22). Human ZFP281 shRNAs were cloned into the pLKO.1 vector (catalog number 10878; Addgene). Packaging to get lentivirus particles and infection were performed as described previously (43). Briefly, HEK293T cells were plated in a 150-mm culture plate and cotransfected with 8 μg of the shRNA construct or the nontargeting shRNA construct, 6 μg of psPAX2 packaging plasmids, and 2 μg of pMD2.G envelope plasmids using the Lipofectamine 2000 reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. Lentiviral supernatants were collected at 48 and 72 h after the transfection and filtered through 0.45-μm-pore-size filters. Cells were infected with filtered lentiviral supernatants together with Polybrene (Sigma) at a concentration of 8 μg/ml. After 24 h, the infected cells were subjected to selection with 2 μg/ml of puromycin for an additional 72 h. Mouse V6.5 ES cells were grown for one passage off feeders on tissue culture plates for 30 min, before they were harvested for experimental analyses.
Antibodies, immunoprecipitations, and Western blotting.
The antibodies to MYC (antibodies sc-42 and sc-40), MAX (antibody sc-8011), and RNA Pol II (antibody sc-899) were obtained from Santa Cruz. The antibody to ZFP281 was described previously (22). For immunoprecipitations, cells were lysed in high-salt lysis buffer containing 420 mM NaCl in the presence of protease inhibitor cocktail (Sigma) for 30 min at 4°C with gentle rotation. After centrifugation, the balance buffer (20 mM HEPES [pH 7.4], 1 mM MgCl2, 10 mM KCl) was added to the supernatant to make the final NaCl concentration 300 mM. The lysate was then incubated with antibodies and protein A beads overnight at 4°C. The beads were spun down and washed three times with wash buffer before boiling in SDS loading buffer. The proteins were resolved in SDS-PAGE gels and transferred to a polyvinylidene difluoride (PVDF) membrane.
Real-time quantitative PCR (RT-qPCR).
For measuring the levels of the mature miRNAs MIRLET7A and MIRLET7D, total RNA was isolated with an miRNeasy kit (Qiagen) according to the manufacturer’s instructions. Mature miRNA levels were determined by qPCR using miRNA TaqMan probe kits (Applied Biosystems) and normalized using SNO202 RNA levels. For measuring the RNA levels of LIN28A, LIN28B, and ZFP281, total RNA was isolated with an RNeasy kit (Qiagen), treated with RNase-free DNase I (New England Biolabs), and repurified with an RNeasy column. cDNAs were synthesized with the PrimeScript reverse transcriptase master mix (TaKaRa). The expression levels were measured with iTa Universal SYBR green supermix (Bio-Rad) on a CFX96 system (Bio-Rad). The relative expression levels of the genes of interest were normalized to the expression of the housekeeping gene Actb or GAPDH (glyceraldehyde-3-phosphate dehydrogenase). The relative fold changes in gene expression were calculated using the ΔΔCT (threshold cycle) method.
ChIP and ChIP-Seq library preparation.
ChIP assays were performed according to a previously described protocol (44). Briefly, a total of 5 × 107 control or ZFP281 knockdown mouse ES cells were used per ChIP that cross-linked with 1% paraformaldehyde for 10 min at room temperature. The cross-linking was quenched by the addition of glycine. The fixed chromatin was sonicated into the above-described 200-bp fragments. One percent of the starting chromatin was saved for use as the input sample. Immunoprecipitation with the MYC antibody was performed at 4°C overnight. The immune complex was collected by the use of protein A-agarose beads and washed with radioimmunoprecipitation assay buffer 5 times. The chromatin was eluted, purified, and used as a template for qPCR or for ChIP-Seq library preparation. ChIP-Seq of the MYC in control and ZFP281 knockdown mouse ES cells was repeated, with the knockdown of ZFP281 being achieved using two independent shRNAs. Libraries were prepared with a NEBNext sample preparation kit for further next-generation sequencing.
Total RNA-Seq analysis.
Total RNA-Seq data sets in control and ZFP281 knockdown mouse ES cells were downloaded from the Gene Expression Omnibus (GEO) database (GEO accession number GSE77115). RNA-Seq of control and ZFP281 knockdown mouse ES cells was performed in duplicate, with the knockdown of ZFP281 being achieved using two independent shRNAs. The clean reads were subjected to analysis with the TopHat and Cufflinks pipeline (45) based on the Mus musculus genome (UCSC genome mm9). The Cuffdiff program within Cufflinks software was used to test for statistically significant differences in gene expression between control and ZFP281 knockdown ES cells. The downstream analyses were performed in the R package CummeRbund. The MA plot (log fold change versus mean expression) was performed with the R package ggplot2 (46). The RNA-Seq results are displayed as reads per kilobase million (RPKM).
ChIP-Seq analysis.
MYC ChIP sequencing data for control and ZFP281 knockdown mouse ES cells were generated in this study and acquired through the default Illumina pipeline using the Casava (v1.8) program. Other data sets came from previously published studies. H3K4me1 ChIP-Seq data were from GEO accession number GSE24164 (47), H3K4me3 ChIP-Seq data were from GEO accession number GSE12241 (48), ZFP281 ChIP-Seq data were from GEO accession number GSE77115 (22), AFF3 and Pol II ChIP-Seq data for control and ZFP281 knockdown mouse ES cells were from GEO accession number GSE77115 (22), Pol II ChIP-Seq data for control and AFF3 knockdown ES cells were from GEO accession number GSE64489 (49), and Pol II ChIP-Seq data for cells treated with dimethyl sulfoxide (DMSO) and flavopiridol were from GEO accession number GSE20530 (8). Clean reads were aligned to the Mus musculus genome (UCSC genome mm9) using the Bowtie2 (v2.2.5) program, allowing uniquely mapping reads only (50).
Peak calling was performed with the MACS2 (v2.1.1) program (51). For ZFP281 peaks, associated control samples were used to determine statistical enrichment at a P value of <1e−8 and a false discovery rate (FDR) of <0.05. For MYC and the external data, the enrichments were determined at a P value of <1e−5 and an FDR of <0.05. The ZFP281- and MYC-cobound peaks were determined if the peak regions of two peaks overlapped each other. The ChIP-Seq enrichment is displayed as reads per million (RPM).
For the heat maps of the ChIP-Seq enrichment profiles, regions were divided into two groups, which were the ZFP281- and MYC-cobound regions and the MYC-only regions. Each row showed one peak, and regions were centered on the peak center. Regions were shown oriented from 5′ to 3′, corresponding to the orientation of the nearest annotated gene. Rows were sorted by the shortest distance of the peak center to the nearest annotated TSS. The regions spanned 5 kb on both sides.
For Pol II traveling ratio calculation, the ratio between the Pol II density in the promoter region and that in the gene region was calculated as previously described (8).
Data availability.
The raw and processed sequencing data generated for this study are available in the GEO database (GEO accession number GSE125435). Extra data, including all the scripts used in this study, are available on request.
ACKNOWLEDGMENTS
The studies described in this report were supported by funds provided by the National Natural Science Foundation of China (31671343 and 3197040262 to C.L.; 31970626 to Z.L.), National Key R&D Program of China to C.L. (2018YFA0800100), the Thousand Young Talents Plan of China to Z.L. (grant 6231000011), the Natural Science Foundation of Jiangsu Province of China to C.L. (grant BK20160026) and Z.L. (grants BK20160666 and BK20170020), Fundamental Research Funds for the Central Universities to C.L. (grant 3231008409) and Z.L. (grant 3231008201), and the Scientific Research Foundation of the Graduate School of Southeast University to Y.W. (grant YBPY1888).
We are grateful to Haitong Fang for critical discussion for this study. We also thank Zhuanzhuan Che, Siyan Meng, and Qian Dai for technical assistance.
C.L. and Z.L. conceived of the idea; Z.L., Y.W., and C.L. performed the experiments; Z.L., X.L., H.X., and C.L. analyzed the genome-wide sequencing data; Z.L. and C.L. wrote the manuscript. All authors discussed the results and commented on the manuscript.
We declare that we have no competing interests.
REFERENCES
- 1.Selth LA, Sigurdsson S, Svejstrup JQ. 2010. Transcript elongation by RNA polymerase II. Annu Rev Biochem 79:271–293. doi: 10.1146/annurev.biochem.78.062807.091425. [DOI] [PubMed] [Google Scholar]
- 2.Roeder RG. 2005. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett 579:909–915. doi: 10.1016/j.febslet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Meyer N, Penn LZ. 2008. Reflecting on 25 years with MYC. Nat Rev Cancer 8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 4.Eilers M, Eisenman RN. 2008. Myc’s broad reach. Genes Dev 22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabay M, Li Y, Felsher DW. 2014. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med 4:a014241. doi: 10.1101/cshperspect.a014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walz S, Lorenzin F, Morton J, Wiese KE, von Eyss B, Herold S, Rycak L, Dumay-Odelot H, Karim S, Bartkuhn M, Roels F, Wustefeld T, Fischer M, Teichmann M, Zender L, Wei CL, Sansom O, Wolf E, Eilers M. 2014. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature 511:483–487. doi: 10.1038/nature13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabo A, Kress TR, Pelizzola M, de Pretis S, Gorski MM, Tesi A, Morelli MJ, Bora P, Doni M, Verrecchia A, Tonelli C, Faga G, Bianchi V, Ronchi A, Low D, Muller H, Guccione E, Campaner S, Amati B. 2014. Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature 511:488–492. doi: 10.1038/nature13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. 2010. c-Myc regulates transcriptional pause release. Cell 141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberhardy SR, Farnham PJ. 2002. Myc recruits P-TEFb to mediate the final step in the transcriptional activation of the cad promoter. J Biol Chem 277:40156–40162. doi: 10.1074/jbc.M207441200. [DOI] [PubMed] [Google Scholar]
- 10.Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. 2012. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, Zhao K, Levens D. 2012. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kress TR, Pellanda P, Pellegrinet L, Bianchi V, Nicoli P, Doni M, Recordati C, Bianchi S, Rotta L, Capra T, Rava M, Verrecchia A, Radaelli E, Littlewood TD, Evan GI, Amati B. 2016. Identification of MYC-dependent transcriptional programs in oncogene-addicted liver tumors. Cancer Res 76:3463–3472. doi: 10.1158/0008-5472.CAN-16-0316. [DOI] [PubMed] [Google Scholar]
- 13.Kress TR, Sabo A, Amati B. 2015. MYC: connecting selective transcriptional control to global RNA production. Nat Rev Cancer 15:593–607. doi: 10.1038/nrc3984. [DOI] [PubMed] [Google Scholar]
- 14.de Pretis S, Kress TR, Morelli MJ, Sabo A, Locarno C, Verrecchia A, Doni M, Campaner S, Amati B, Pelizzola M. 2017. Integrative analysis of RNA polymerase II and transcriptional dynamics upon MYC activation. Genome Res 27:1658–1664. doi: 10.1101/gr.226035.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackwood EM, Eisenman RN. 1991. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Li T, Schipper J, Nilson KA, Fordjour FK, Cooper JJ, Gordan R, Price DH. 2014. Sequence specificity incompletely defines the genome-wide occupancy of Myc. Genome Biol 15:482. doi: 10.1186/s13059-014-0482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas LR, Wang Q, Grieb BC, Phan J, Foshage AM, Sun Q, Olejniczak ET, Clark T, Dey S, Lorey S, Alicie B, Howard GC, Cawthon B, Ess KC, Eischen CM, Zhao Z, Fesik SW, Tansey WP. 2015. Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC. Mol Cell 58:440–452. doi: 10.1016/j.molcel.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzin F, Benary U, Baluapuri A, Walz S, Jung LA, von Eyss B, Kisker C, Wolf J, Eilers M, Wolf E. 2016. Different promoter affinities account for specificity in MYC-dependent gene regulation. Elife 5:e15161. doi: 10.7554/eLife.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerlach JM, Furrer M, Gallant M, Birkel D, Baluapuri A, Wolf E, Gallant P. 2017. PAF1 complex component Leo1 helps recruit Drosophila Myc to promoters. Proc Natl Acad Sci U S A 114:E9224–E9232. doi: 10.1073/pnas.1705816114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaenicke LA, von Eyss B, Carstensen A, Wolf E, Xu W, Greifenberg AK, Geyer M, Eilers M, Popov N. 2016. Ubiquitin-dependent turnover of MYC antagonizes MYC/PAF1C complex accumulation to drive transcriptional elongation. Mol Cell 61:54–67. doi: 10.1016/j.molcel.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Lin L, Zhang JH, Panicker LM, Simonds WF. 2008. The parafibromin tumor suppressor protein inhibits cell proliferation by repression of the c-myc proto-oncogene. Proc Natl Acad Sci U S A 105:17420–17425. doi: 10.1073/pnas.0710725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Shen Y, Dai Q, Yang Q, Zhang Y, Wang X, Xie W, Luo Z, Lin C. 2017. A permissive chromatin state regulated by ZFP281-AFF3 in controlling the imprinted Meg3 polycistron. Nucleic Acids Res 45:1177–1185. doi: 10.1093/nar/gkw1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viswanathan SR, Daley GQ, Gregory RI. 2008. Selective blockade of microRNA processing by Lin28. Science 320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG. 2008. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol 10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 25.Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. 2008. Lin28 mediates the terminal uridylation of let-7 precursor microRNA. Mol Cell 32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. 2007. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 27.Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, Jung J, Gao P, Dang CV, Beer MA, Thomas-Tikhonenko A, Mendell JT. 2009. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A 106:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shyh-Chang N, Daley GQ. 2013. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM, Minn AJ, Rosner MR. 2009. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J 28:347–358. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viswanathan SR, Daley GQ. 2010. Lin28: a microRNA regulator with a macro role. Cell 140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 31.McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G. 2010. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackwood EM, Luscher B, Eisenman RN. 1992. Myc and Max associate in vivo. Genes Dev 6:71–80. doi: 10.1101/gad.6.1.71. [DOI] [PubMed] [Google Scholar]
- 33.Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, Luscher B. 2001. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev 15:2042–2047. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawasaki Y, Komiya M, Matsumura K, Negishi L, Suda S, Okuno M, Yokota N, Osada T, Nagashima T, Hiyoshi M, Okada-Hatakeyama M, Kitayama J, Shirahige K, Akiyama T. 2016. MYU, a target lncRNA for Wnt/c-Myc signaling, mediates induction of CDK6 to promote cell cycle progression. Cell Rep 16:2554–2564. doi: 10.1016/j.celrep.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. 2005. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 36.Koch HB, Zhang R, Verdoodt B, Bailey A, Zhang CD, Yates JR III, Menssen A, Hermeking H. 2007. Large-scale identification of c-MYC-associated proteins using a combined TAP/MudPIT approach. Cell Cycle 6:205–217. doi: 10.4161/cc.6.2.3742. [DOI] [PubMed] [Google Scholar]
- 37.Baudino TA, McKay C, Pendeville-Samain H, Nilsson JA, Maclean KH, White EL, Davis AC, Ihle JN, Cleveland JL. 2002. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev 16:2530–2543. doi: 10.1101/gad.1024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. 1993. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev 7:671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- 39.Fidalgo M, Shekar PC, Ang YS, Fujiwara Y, Orkin SH, Wang J. 2011. Zfp281 functions as a transcriptional repressor for pluripotency of mouse embryonic stem cells. Stem Cells 29:1705–1716. doi: 10.1002/stem.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fidalgo M, Huang X, Guallar D, Sanchez-Priego C, Valdes VJ, Saunders A, Ding J, Wu WS, Clavel C, Wang J. 2016. Zfp281 coordinates opposing functions of Tet1 and Tet2 in pluripotent states. Cell Stem Cell 19:355–369. doi: 10.1016/j.stem.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn S, Jackstadt R, Siemens H, Hunten S, Hermeking H. 2013. SNAIL and miR-34a feed-forward regulation of ZNF281/ZBP99 promotes epithelial-mesenchymal transition. EMBO J 32:3079–3095. doi: 10.1038/emboj.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hahn S, Hermeking H. 2014. ZNF281/ZBP-99: a new player in epithelial-mesenchymal transition, stemness, and cancer. J Mol Med (Berl) 92:571–581. doi: 10.1007/s00109-014-1160-3. [DOI] [PubMed] [Google Scholar]
- 43.Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. 2010. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell 37:429–437. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin C, Garruss AS, Luo Z, Guo F, Shilatifard A. 2013. The RNA Pol II elongation factor Ell3 marks enhancers in ES cells and primes future gene activation. Cell 152:144–156. doi: 10.1016/j.cell.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, NY. [Google Scholar]
- 47.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. 2010. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A 107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim T-K, Koche RP, Lee W, Mendenhall E, O'Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. 2007. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Z, Lin C, Woodfin AR, Bartom ET, Gao X, Smith ER, Shilatifard A. 2016. Regulation of the imprinted Dlk1-Dio3 locus by allele-specific enhancer activity. Genes Dev 30:92–101. doi: 10.1101/gad.270413.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw and processed sequencing data generated for this study are available in the GEO database (GEO accession number GSE125435). Extra data, including all the scripts used in this study, are available on request.