The eukaryotic cytosol contains multiple RNP granules, including P-bodies and stress granules. Three different methods have been used to describe the transcriptome of stress granules or P-bodies, but how these methods compare and how RNA partitioning occurs between P-bodies and stress granules have not been addressed. Here, we compare the analysis of the stress granule transcriptome based on differential centrifugation with and without subsequent stress granule immunopurification.
KEYWORDS: P-body, RNA, stress granule, transcriptome
ABSTRACT
The eukaryotic cytosol contains multiple RNP granules, including P-bodies and stress granules. Three different methods have been used to describe the transcriptome of stress granules or P-bodies, but how these methods compare and how RNA partitioning occurs between P-bodies and stress granules have not been addressed. Here, we compare the analysis of the stress granule transcriptome based on differential centrifugation with and without subsequent stress granule immunopurification. We find that while differential centrifugation alone gives a first approximation of the stress granule transcriptome, this methodology contains nonspecific transcripts that play a confounding role in the interpretation of results. We also immunopurify and compare the RNAs in stress granules and P-bodies under arsenite stress and compare those results to those for the P-body transcriptome described under nonstress conditions. We find that the P-body transcriptome is dominated by poorly translated mRNAs under nonstress conditions, but during arsenite stress, when translation is globally repressed, the P-body transcriptome is very similar to the stress granule transcriptome. This suggests that translation is a dominant factor in targeting mRNAs into both P-bodies and stress granules, and during stress, when most mRNAs are untranslated, the composition of P-bodies reflects this broader translation repression.
INTRODUCTION
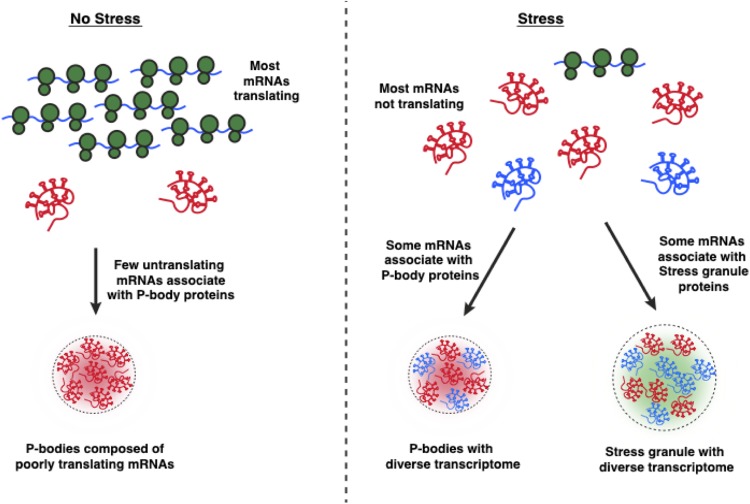
Eukaryotic cells contain a number of RNA-protein assemblies, collectively referred to as RNP granules. RNP granules occur in the nucleus, including Cajal bodies, paraspeckles, and the nucleolus, as well as the cytoplasm, including stress granules (SG) and P-bodies (PBs). PBs are constitutive assemblies of untranslating mRNA complexes with components of the RNA decay machinery (1), which can enlarge during stress (2, 3). SGs are also composed of nontranslating RNAs but only form, or become easily visible, during a stress response when a large number of mRNAs cease translation (4–6).
While SGs and PBs have long been known to contain RNA and protein, the exact RNA and protein composition of these assemblies has remained enigmatic due to lack of suitable purification techniques. Recently, three different methodologies were used to elucidate the transcriptomes of either stress granules or P-bodies to determine factors that affect mRNA partitioning to these RNP granules. Specifically, P-bodies were purified and sequenced through differential centrifugation and fluorescence-activated particle sorting from unstressed HEK293 cells, revealing the constitutive components of PBs (7). In contrast, stress granules were purified from stressed U-2 OS cells and sequenced through differential centrifugation followed by immunopurification (8, 9). Finally, another study has examined the composition of RNA granules through differential centrifugation alone (10). This study referred to the RNAs that pellet during differential centrifugation as the RNA granule (RG) pellet, which showed some similarity to the immunopurified SG transcriptome (8, 10).
Here, we address three issues of the purification and analysis of SG and PB transcriptomes. First, using a single cell type and consistent computational pipeline, we demonstrate that the RG transcriptome under stressed conditions is similar to the SG transcriptome but contains additional contaminating RNAs. Second, our analyses argue that the transcriptome of PBs shifts dramatically with stress, suggesting that targeting of mRNAs into PBs is primarily determined by their translation status. Finally, we demonstrate that the RNA compositions of PBs and SGs are similar during stress. These observations suggest that mRNAs from the same genes can partition into both SGs and PBs and that differences in mRNA composition and/or their associated proteins dictate the specificity of RNP granule association.
RESULTS
Characterization of the unstressed RNA granule pellet transcriptome.
The RNA granule (RG) transcriptome was recently characterized through differential centrifugation and transcriptome sequencing (RNA-Seq), and it was defined as the population of RNAs that pellet with and without stress (10). If a simple pelleting of RNP assemblies can give an accurate representation of the P-body or stress granule transcriptome, this would be less expensive, faster, and require fewer cells than more elaborate immunopurification methods (7, 8). While analyzing the RNA granule transcriptome solely by differential centrifugation has been performed in mouse fibroblast cells, we wanted to examine how a similar methodology performed in a human cell line (U-2 OS) and how well the pelleted RNP fraction correlated to the SG transcriptome defined by immunopurification from the same cell line (8). Thus, similar to previous methods (10), we isolated a heavy RNP fraction by differential centrifugation and analyzed the RNAs in these fractions, with and without stress, by RNA-Seq.
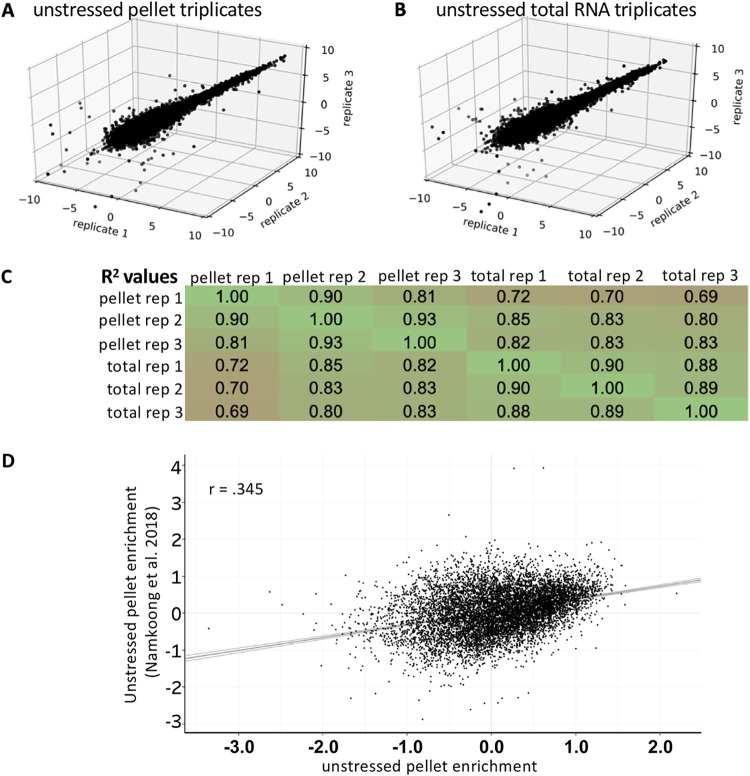
We first analyzed the RG pellet in the absence of stress. In this preparation, we first removed nuclei by centrifugation (1,000 × g). After this initial spin we isolated RNAs that pellet at 16,000 × g and compared this RNA population to nucleus-depleted total RNA. RNA-seq libraries from unstressed cells were reproducible for both the RG pellet and nucleus-depleted total RNA fractions (Fig. 1A and B). Total RNA triplicates tended to share more similarity to one another than to RG pellet RNA triplicates, suggesting the RG pellet contains a different subset of RNAs than total RNA (Fig. 1C). However, we note that the differences between total RNA and the RG pellet were small, suggesting that the unstressed RG pellet has a transcriptome similar to that of the cytosolic transcriptome. Consistent with the similar methodology, enrichment scores from the unstressed RG pellet positively correlated with the previously isolated unstressed RG pellet from mouse fibroblasts (R = 0.345) (Fig. 1D).
FIG 1.
Unstressed RNA granule pellet transcriptomes are reproducible. (A) Three-dimensional (3D) scatterplot depicting the normalized read counts from RNA-seq libraries from unstressed RNA granule pellet triplicates. (B) 3D scatterplot depicting the normalized read counts from RNA-seq libraries from unstressed total RNA triplicates. (C) Table depicting pairwise Pearson correlation coefficients between RNA granule pellets and total RNA in unstressed cells. (D) Scatterplot of unstressed RG enrichment obtained from mouse fibroblasts versus unstressed RG enrichment in U-2 OS cells.
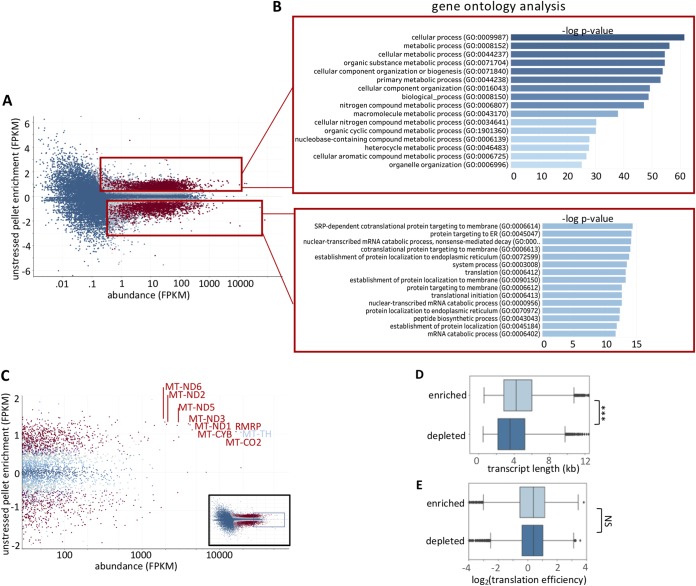
Since there were differences between the pellet RNA and total cytosolic RNA, we sought to identify which transcripts were differentially enriched within and depleted from the pellet. Differential enrichment analysis identified numerous transcripts that were significantly (P < 0.01) enriched in (n = 2,161) or depleted from (n = 1,304) the pellet (Fig. 2A). Gene ontology for enriched transcripts identified many transcripts involved in metabolic processes (Fig. 2B). Gene ontology for depleted transcripts identified many transcripts encoding proteins that are involved in endoplasmic reticulum (ER) membrane targeting (Fig. 2B). This is consistent with the ER remaining in the supernatant at 16,000 × g (11). Mitochondria should pellet at a spin of 16,000 × g (11). Indeed, we observe that mitochondrion-encoded transcripts represent some of the more highly expressed transcripts that are enriched by this methodology (Fig. 2C). Thus, the unstressed RNA pellet transcriptome is depleted of RNA associated with membranes and enriched in RNAs localizing to the mitochondria or encoding metabolic enzymes.
FIG 2.
Characterization of the unstressed RNA granule pellet. (A) MA plot depicting the log2 fold change values (unstressed RG pellet/unstressed total RNA) versus abundance (fragments per kilobase per million [FPKM]). Genes are color-coded by their significance. Significant genes (P < 0.01) are colored red, while nonsignificant (P > 0.01) genes are colored blue. (B) Gene ontology analysis for enriched and depleted transcripts. (C) Zoom image of scatterplot highlighting the position of mitochondrial transcripts. (D) Box plot depicting transcript length for RG-enriched and RG-depleted transcripts in both stressed and unstressed cells. (E) Box plot depicting translation efficiency values (18) for RG-enriched and RG-depleted transcripts in unstressed cells.
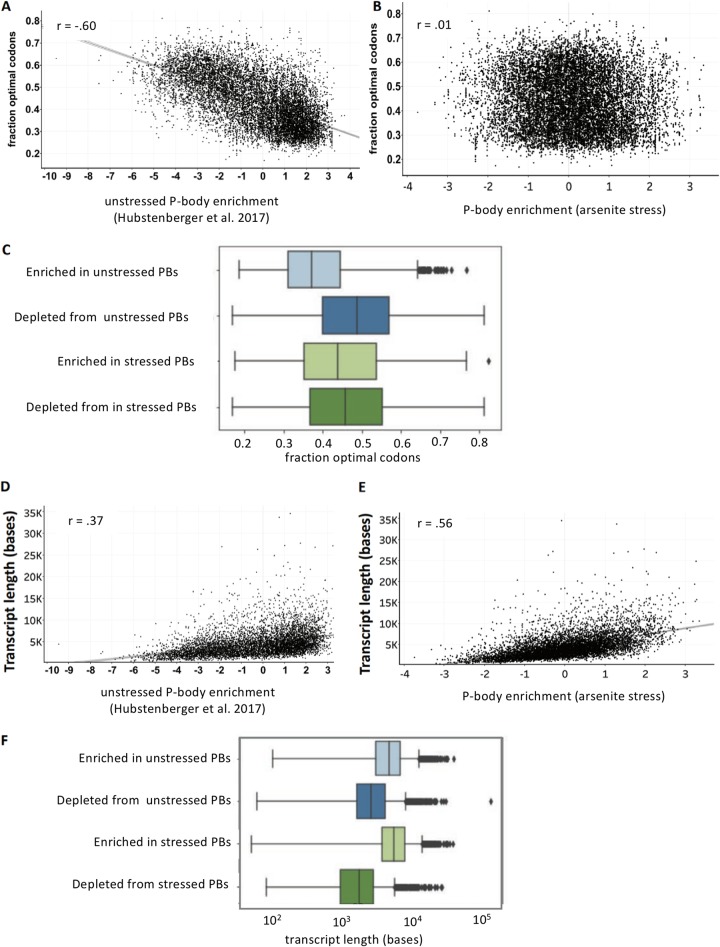
We sought to examine metrics that may play a role in determining whether an RNA is differentially enriched in the unstressed pellet. We and others (7, 8, 10, 12) have previously identified translation and transcript length as two predominant metrics that correlate with RNA localization to cytoplasmic assemblies such as P-bodies and stress granules. We first tested whether transcript length correlated with enrichment in the pellet. Consistent with observations in stress granules and P-bodies, long RNAs also tend to accumulate in the pellet in the absence of stress (Fig. 2D). However, the length bias is much less pronounced than the length bias observed in stress granules (8). Thus, length plays some role in determining the RNA composition of the RG pellet fraction even during unstressed conditions.
We next tested whether there was a translation bias between pellet-enriched versus pellet-depleted RNA transcripts. We saw no significant translation efficiency bias when we compared pellet-enriched and pellet-depleted transcripts (Fig. 2E). This is in contrast to stress granules and P-bodies, which are both biased toward harboring poorly translated transcripts (7, 8, 12). This difference is, however, consistent with the gene ontology identification of metabolic genes in the RNA granule pellet, which are typically well-translated genes (Fig. 2B).
Taken together, our results indicate that a subpopulation of RNPs pellet during unstressed conditions. The transcripts that pellet tend to be long and/or tend to encode genes involved in metabolism or genes that encode proteins that are targeted to the mitochondria, while the transcripts that do not pellet tend to be shorter and/or encode genes that localize to the ER membrane.
Characterization of the stressed RNA granule pellet transcriptome.
The stressed RNA granule pellet has previously been shown to have an RNA composition similar to that of stress granules that were isolated by immunopurification (10). In this previous study the authors noted that some of the RNAs enriched in the stressed RG pellet were the same RNAs that pelleted under unstressed conditions. Thus, we wanted to examine how the stressed RG pellet relates to the unstressed RNA pellet. In order to isolate the stressed RG pellet, we treated U-2 OS cells with 0.5 mM sodium arsenite for 1 h and then sequenced the nucleus-depleted total cytoplasmic RNA and pelleted RNPs in the same manner.
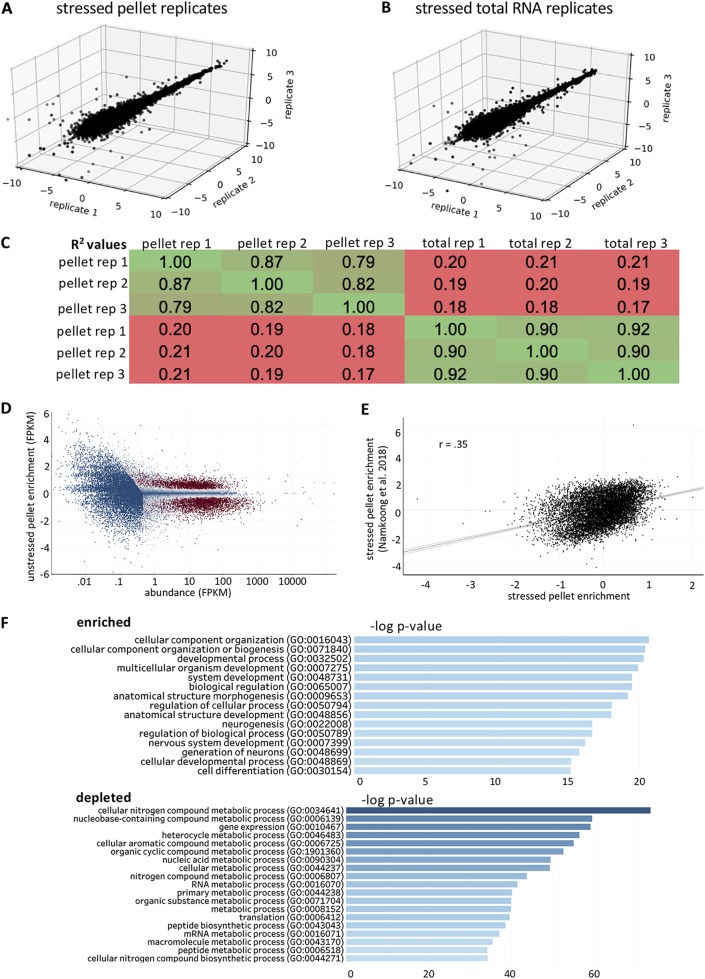
RNA-seq libraries were reproducible for both nuclear-subtracted total RNA triplicates and RG pellet triplicates (Fig. 3A and B). The RNAs that pelleted under stressed conditions differed substantially from stressed total cytosolic RNA (Fig. 3C). This finding is in contrast to unstressed conditions in which pellet RNA and total RNA showed only small differences. Thus, the stressed pellet shows an RNA composition distinct from that of total RNA during arsenite stress, likely due to the formation of stress granules during arsenite stress and the release of mRNAs from polysomes.
FIG 3.
Stressed RNA granule pellet transcriptomes are reproducible. (A) 3D scatterplot depicting the normalized read counts from RNA-seq libraries from stressed RNA granule pellet triplicates. (B) 3D scatterplot depicting the normalized read counts from RNA-seq libraries from stressed total RNA triplicates. (C) Table depicting pairwise Pearson correlation coefficients between RNA granule pellets and total RNA in stressed cells. (D) MA plot depicting the log2 fold change values (stressed RG pellet/stressed total RNA) versus abundance (FPKM). Genes are color-coded by their significance. Significant genes (P < 0.01) are colored red, while nonsignificant (P > 0.01) genes are colored blue. (E) Scatterplot of stressed RG enrichment obtained from mouse fibroblasts versus stressed RG enrichment in U-2 OS cells. (F) Gene ontology for enriched and depleted transcripts.
We performed a differential enrichment analysis in order to identify the transcripts that are enriched in and depleted from the stressed RNA pellet. Differential enrichment analysis identified numerous transcripts that were significantly (P < 0.01) enriched in (n = 1,053) and depleted from (n = 1,449) the stressed RNA granule pellet (Fig. 3D). Enrichment scores from our stressed RNA granule pellet positively correlated with the previous stressed RNA granule pellet isolated from mouse fibroblasts (R = 0.35) (Fig. 3E), which is consistent with these two experiments yielding similar results. Gene ontology for enriched transcripts revealed that RNAs that were enriched in the pellet typically encode transcripts generally involved in development or biogenesis (Fig. 3F). Specifically, genes involved in neurogenesis were differentially enriched in the pellet, which is consistent with the fact that SG-enriched transcripts tend to show large amounts of overlap long transcripts that are important for normal brain development (13).
We observed by gene ontology that transcripts involved in metabolism were significantly depleted from the stressed RG pellet (Fig. 3F). This is in direct contrast to unstressed conditions, in which the transcripts encoding metabolic transcripts were enriched in the pellet. We attribute this change to the fact that arsenite stress causes global translational shutoff, with only a minority of genes continuing translation. Since metabolic genes are likely to no longer be translating, they would not be associated with polysomes and therefore not be in an RNP state that is heavy enough to pellet at 16,000 × g. This may explain why metabolic genes shift to the cytosolic supernatant fraction during stressed conditions.
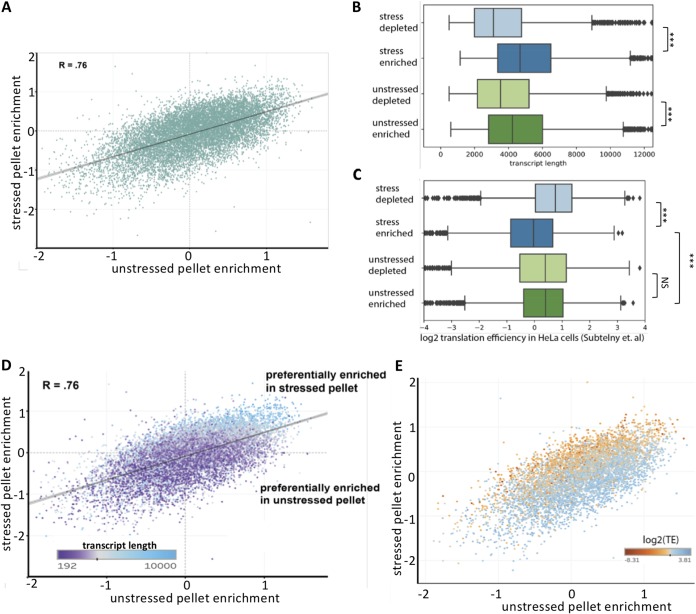
We also compared how closely the stressed RNA pellet resembled the unstressed RNA pellet. We observed a significant correlation between the stressed RNA pellet and the unstressed RNA pellet (R = 0.76) (Fig. 4A). However, there was substantial variance between the two conditions. To identify metrics that may explain the differences in enrichment, we examined how various RNA features correlated with differential enrichment.
FIG 4.
Characterization of the stressed RNA granule pellet. (A) Scatterplot depicting the correlation between stressed pellet enrichment versus unstressed pellet enrichment. (B) Box plots showing transcript lengths for pellet-enriched and pellet-depleted transcripts during arsenite stress and unstressed conditions. (C) Same as panel B but for translation efficiency (TE) values (obtained from Subtelny et al. [18]). (D) Same as panel B but color-coded by transcript length. (E) Same as panel B but color-coded by TE values.
Transcript length was correlated with pellet enrichment under both stressed and unstressed conditions (Fig. 4B). However, we note that during stressed conditions, the length dependence of enrichment was more pronounced than that under unstressed conditions. This finding is consistent with the previous observation that length plays a significant role in defining which RNAs are enriched in stress granules (8, 14) and is consistent with stress granules being enriched in the pellet at 16,000 × g (8, 10, 15–17).
We also examined how translation efficiency correlated with RNA localization. During arsenite stress, the RNAs that pelleted had significantly lower TE values (18) than RNAs that were depleted from the pellet (Fig. 4C). This finding is in contrast to unstressed conditions, during which transcripts with higher TE values pelleted. Thus, poorly translated transcripts tend to pellet during arsenite stress, while transcripts with higher TE values tend to pellet during unstressed conditions.
Since length and translation correlate with RNA pellet enrichment during arsenite stress, we examined whether these two parameters account for the variance between unstressed pellet enrichment and stressed pellet enrichment. Color-coding the scatterplot of stressed pellet enrichment versus unstressed pellet enrichment by transcript length revealed that the pellet-enriched transcripts during stress tend to be longer than those of pellet-enriched transcripts during unstressed conditions (Fig. 4D). We also observed a similar dependency for translation efficiency, whereby poorly translated transcripts were enriched in the stressed pellet, while efficiently translated transcripts were enriched in the unstressed pellet (Fig. 4E). Consistent with these results, by multivariate linear regression analysis, length and translation efficiency account for an additional 20% of the variance between unstressed and stressed pellet enrichment (data not shown).
Taken together, these results indicate that there is a general correlation between the RNAs that pellet under stressed and unstressed conditions. Further, the variance in the correlation between pellet enrichment under stressed and unstressed conditions can largely be accounted for by considering the additional factors of transcript length and translation efficiency. During stress conditions, longer poorly translated transcripts tend to accumulate in the pellet, similar to what would be expected in stress granules (8), while under unstressed conditions relatively shorter, more efficiently translated transcripts also accumulate in the RG pellet.
Comparison of the RG transcriptomes to the stress granule transcriptome.
A key unresolved question is how well the RNA granule pellet transcriptomes compare to the stress granule transcriptome based on more selective immunopurification, and whether simple pelleting can be used as a suitable proxy for the full stress granule purification protocol. While Namkoong et al. compared the stressed RNA granule pellet transcriptome from mouse fibroblasts to our previous stress granule transcriptome from U-2 OS cells (R2 = 0.49), we wanted to utilize a similar approach in the same U-2 OS cell line. Thus, we reanalyzed the data in our previous stress granule transcriptome (8), using the same computational pipeline we used for the RNA granule pellet transcriptomes (Fig. 5A).
FIG 5.
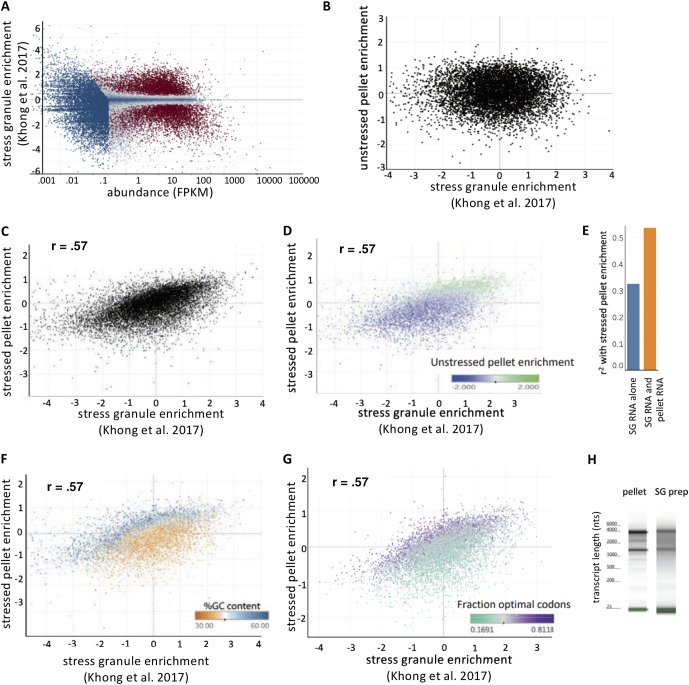
Polysomes likely account for the differences in the stressed RG transcriptome and the stress granule transcriptome. (A) MA plot depicting the log2 fold change values (stress granule RNA/stressed total RNA) versus abundance (FPKM). Genes are color-coded by their significance. Significant genes are colored red (P < 0.01), while nonsignificant genes are colored blue (P > 0.01). (B) Scatterplot of unstressed pellet enrichment versus stress granule enrichment. (C) Scatterplot of stressed pellet enrichment versus stress granule enrichment. (D) Same as panel C but color coded by pellet enrichment under unstressed conditions. (E) R2 values from multiple linear regression analysis for stressed pellet enrichment versus SG enrichment or stressed pellet enrichment versus SG enrichment and unstressed pellet enrichment. (F) Same as panel C but color-coded by percent GC content. (G) Same as panel C but color-coded by the fraction of optimal codons per transcript. (H) TapeStation analysis of the RG pellet during stress and of SG RNA using additional immunopurification steps.
We first compared the stress granule transcriptome to the unstressed RNA granule pellet transcriptome. We observed that stress granule enrichment scores and unstressed pellet enrichment scores showed no correlation (Fig. 5B). Thus, the stress granule transcriptome does not simply represent the RNAs that tend to pellet in the absence of stress.
In contrast, the stress granule enrichment scores and the stressed RNA granule pellet enrichment scores showed a significant positive correlation (Fig. 5C). This demonstrates that the stressed RNA granule pellet recapitulates the stress granule transcriptome to a certain degree, consistent with the idea that RNA pellets are a reasonable proxy for the full stress granule preparation.
Polysomes may contaminate the RG transcriptome.
It was unclear why the stressed RG transcriptome was not a more accurate reflection of the SG transcriptome determined by immunopurification. With an R2 value of only 0.32, this means that two-thirds of the variance in stress granule enrichment scores versus stressed RG enrichment scores remains unexplained. Some of this variability may lie in inconsistencies between sequencing runs and batch effects. We estimate that as much as 20% of the variance in enrichment scores lie in sequencing artifacts based on the variation between sequencing replicates (see R2 values in Fig. 1A and 2A). However, this means that ∼40% of the variance remains unexplained.
One possibility is that RNAs that have a higher propensity to pellet under both stress and nonstress conditions are a source of nonspecific contamination in the RG pelleting purification method. If this prediction were true, we would anticipate that these contaminating RNAs would also have a high propensity to pellet even under unstressed conditions. Therefore, we color-coded our scatterplot of stress granule enrichment versus stressed pellet enrichment by the RNA pellet enrichment during unstressed conditions. Strikingly, we observed that transcripts that tend to enrich in stressed RG pellets relative to stress granules are the same transcripts that tend to pellet under unstressed conditions (Fig. 5D). Taken together, these data further suggest that the stressed RNA granule pellet contains additional RNAs that enrich even in the absence of stress.
To examine how much of the variance between stressed pellet enrichment and stress granule enrichment could be explained by contamination by RNAs that pellet regardless of the stress conditions, we performed a multivariate least-squares regression analysis. This analysis allows one to quantitatively examine how much of the variance of a given distribution is explained by the combination of multiple variables. When we added the unstressed RG pellet enrichment scores as an additional independent variable, we observed a greatly improved R2 metric of 0.536, as opposed to our original R2 metric of 0.32 (Fig. 5E). This suggests that the stressed RNA granule pellet contains some contaminating RNAs that enrich even in the absence of stress.
We further analyzed the data to determine why some RNAs pellet under both stressed and unstressed conditions. We observed that transcripts that preferentially enrich in the stressed RG pellet relative to stress granules tended to have higher GC content, while the transcripts that preferentially enriched in stress granules relative to the stressed RG pellet tended to have lower GC content (Fig. 5F).
Since GC content bias can affect codon usage, we hypothesized that codon usage plays a role in distinguishing a transcript’s relative enrichment in the stressed RG pellet versus stress granules. To test this hypothesis, we compiled a list of nonoptimal and optimal codons and compared the percentage of optimal codons in each transcript. We observed a clear bias, with transcripts containing higher fractions of optimal codons preferentially enriching in the stressed RG fraction relative to stress granules (Fig. 5G). We interpret these results to potentially represent polysome contamination in the stressed RG pellet. Codon composition is correlated with translation: transcripts with large percentages of optimal codons tend to show higher rates of translation. Consistent with polysomes contaminating the RG pellet, by TapeStation analysis, we observed strong rRNA bands in the pelleting approach, while in the SG purification we see a fainter ribosomal banding accompanied by a darker smear at sizes where long RNAs run (1.5 to 6 kb) (Fig. 5H). Thus, we suggest that transcripts that continue to be translated during stress contaminate the stressed RG pellet to some extent, since very large polysomes and stress granules tend to have similar sedimentation properties.
An interesting observation is that there is a population of RNAs that is enriched in both the stressed RG transcriptome and the SG transcriptome and also is enriched in the unstressed RG transcriptome (Fig. 5D). Thus, this set of RNAs is enriched in SG but is also prone to pelleting even in the absence of stress. One possibility is that this represents a population of RNAs that can be assembled into smaller stress granules even in the absence of stress and, thus, would be prone to pellet and would still be enriched in stress granules.
Analysis of P-body transcriptomes and their relationship to stress granules and RNA granule pellets.
Eukaryotic cells also contain P-bodies, which should also pellet under conditions that generate the RG transcriptomes (3, 16). Thus, we analyzed how closely the unstressed or stressed pellet resembled the P-body transcriptome, which was previously determined by collecting P-bodies on a cell sorter under no stress conditions from HEK293 cells (7).
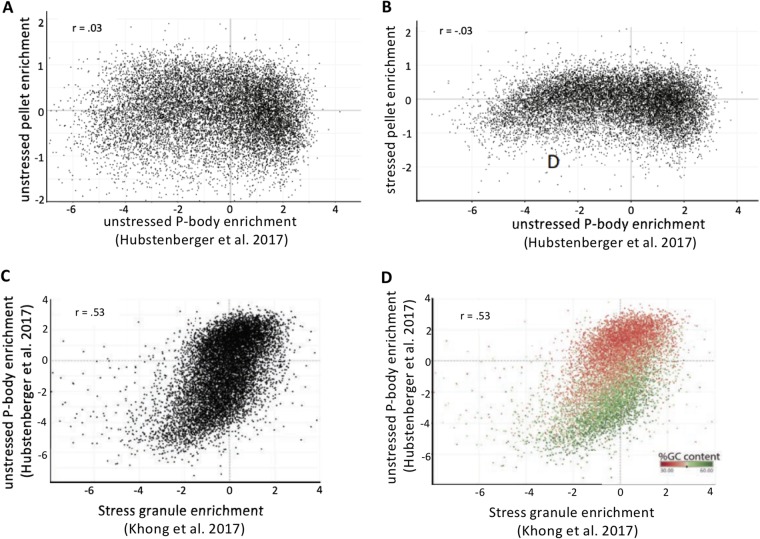
We observed that the unstressed and stressed RNA pellet transcriptomes showed virtually no correlation with the P-body transcriptome (Fig. 6A and B). This is consistent with our analysis described above, suggesting that the unstressed RG pellet represents genes encoding metabolic enzymes and mitochondrion-targeted transcripts, and the stressed RG pellet is a mix of stress granule RNPs and contaminating transcripts that pellet regardless of stress. This suggests that RNA pelleting is insufficient to purify the RNAs that associate with P-bodies and that additional purification is needed.
FIG 6.
Comparison of the SG and P-body transcriptome. (A) Scatterplot depicting the enrichment of RNAs in the unstressed RG pellet versus P-bodies. (B) Scatterplot depicting the enrichment of RNAs in the stressed RG pellet versus P-bodies. (C) Scatterplot depicting the enrichment of RNAs in P-bodies versus stress granules. (D) Same as panel C but color-coded by GC content.
Comparison of the stress granule and P-body transcriptome.
We wanted to examine how P-body enrichment correlated with the stress granule enrichment scores we previously reported (8). Thus, we reanalyzed the original P-body transcriptome (7) with the same computational pipeline in which we analyzed the stress granule transcriptome. We observed that comparison of the P-body transcriptome to the stress granule transcriptome yielded a relatively strong positive correlation (R = 0.53) (Fig. 6C). This correlation is noteworthy, particularly since we are comparing a P-body transcriptome from an unstressed condition with stress-induced stress granules in different cell types. This suggests that some of the same rules that play a role in RNA partitioning into stress granules also affect RNA partitioning into P-bodies. Consistent with such a similarity, poor translation and increased length correlates with accumulation of mRNAs in both P-bodies and SG (see below) (7, 8).
RNAs that enrich in P-bodies have strong AU bias (12). Given this bias, we wanted to examine the degree to which nucleotide composition correlates with the degree to which transcripts preferentially enrich in PB versus stress granules. Transcripts that were strongly enriched within PBs but not enriched within SGs tended to have higher levels of AU composition, while transcripts that were strongly enriched within SGs but not enriched within PBs tended to have higher levels of GC content (Fig. 6D). Examining AU content through multivariate linear regression analysis accounted for an additional 11% of the variance (data not shown). Thus, AU content may play a role in specifying which transcripts preferentially partition to P-bodies versus stress granules.
The P-body transcriptome shifts during stress.
There are at least two possibilities as to why AU content modulates RNA localization to P-bodies. First, P-body-enriched transcripts are generally poorly translated and have high levels of nonoptimal codons (12). Thus, AU composition may determine RNA enrichment in P-bodies due to poor translation. Second, P-body proteins may have a stronger affinity for AU-rich transcripts than GC-rich transcripts, leading to the preferential enrichment of AU-rich transcripts in PBs.
To discern between these two models, we examined the P-body transcriptome during arsenite stress, in which translation is limited. If P-body-enriched transcripts partition into P-bodies through their association with P-body RNA binding proteins, one would anticipate that the unstressed P-body transcriptome would be expected to show high degrees of correlation with the arsenite-stressed P-body transcriptome. However, if transcripts primarily localize to P-bodies due to their nontranslating status, one would anticipate that the P-body transcriptome would change during stressed conditions. The rationale underlying of this hypothesis is that during stress translation is greatly reduced; thus, the available pool of RNA substrates should greatly increase and include more GC-rich transcripts.
In order to identify which RNAs localize to P-bodies during arsenite stress, we purified P-bodies from arsenite-treated U-2 OS cells. We adapted our stress granule purification scheme in order to purify P-bodies. In brief, P-bodies were isolated through successive rounds of centrifugation, after which P-bodies were further purified via immunoprecipitation with an anti-EDC3 antibody. In addition, we also immunopurified P-bodies by this method from G3BP1Δ/G3BP2Δ cells, which fail to make stress granules during arsenite treatment (19, 20). We added this control since SGs can dock to P-bodies (2), and their docking raised the possibility that immunopurified P-body preparations would have some contamination by stress granules.
We observed that libraries made from wild-type (WT) and G3BP1Δ/G3BP2Δ cells following P-body purification yielded reproducible results that were notably different from those for the total cytoplasmic transcriptome (Fig. 7A and B). The PB transcriptomes were dependent on the Edc3 antibody, since no RNA was recovered when an IgG control antibody was used for the immunopurification step (Fig. 7C and D). Moreover, the WT and G3BP1Δ/G3BP2Δ P-body transcriptomes were highly similar, suggesting that stress granules are not a substantial contaminant in our P-body preparations (Fig. 7E). Analysis of the P-body transcriptome from wild-type cells during stress revealed the following points.
FIG 7.
Comparison of the stressed and unstressed P-body transcriptome. (A) Table depicting pairwise r2 values between PB RNA and total RNA in arsenite-stressed cells. (B) Same as panel A but for G3BP1/G3BP2 knockout (ko) cells. (C) TapeStation analysis for IgG pulldown control. (D) TapeStation analysis for EDC3 pulldown of P-body RNA. (E) Scatterplot depicting the correlation between wild-type P-body enrichment and G3BP1Δ/G3BP2Δ P-body enrichment during stress. (F) P-body enrichment during unstressed conditions versus P-body enrichment during arsenite stress. (G) Stress granule enrichment versus P-body enrichment during arsenite stress.
First, when we compared the stressed P-body transcriptome to that of the original unstressed P-body transcriptome, we observed a fairly strong positive correlation (R = 0.55) (Fig. 7F). However, the stressed P-body transcriptome showed more similarity to the stress granule transcriptome (R = 0.85) (Fig. 7G) than the unstressed P-body transcriptome during arsenite-induced stress conditions (R = 0.55) (Fig. 4F). This suggests that the P-body transcriptome shifts during stress due to a broader available pool of nontranslating mRNAs.
When we examined parameters that correlate with P-body enrichment, we see a notable shift from nonstress to stress conditions. Specifically, the unstressed enriched P-body transcripts tended to show higher levels of nonoptimal codons than stressed enriched P-body transcripts (Fig. 8A to C). This finding is consistent with our hypothesis that translation defines which substrates are available for P-body sequestration. Moreover, during stressed conditions, the P-body transcriptome shows a significantly stronger correlation with transcript length (Fig. 8D to F) and less of a dependence on translation efficiency. This suggests that length plays a larger role in defining P-body composition during arsenite stress conditions when the majority of mRNAs are strongly translationally repressed.
FIG 8.
Interplay between translation and length define the P-body transcriptome. (A) Scatterplot showing the correlation between the fraction of optimal codons and P-body enrichment during unstressed conditions. (B) Same as panel A but during arsenite stress. (C) Box plot showing fraction of optimal codons for enriched and depleted transcripts in stressed and unstressed P-bodies. (D) Scatterplot showing the correlation between length and P-body enrichment during unstressed conditions. (E) Scatterplot showing the correlation between length and P-body enrichment during stressed conditions. (F) Box plot showing transcript length for enriched and depleted transcripts in stressed and unstressed P-bodies.
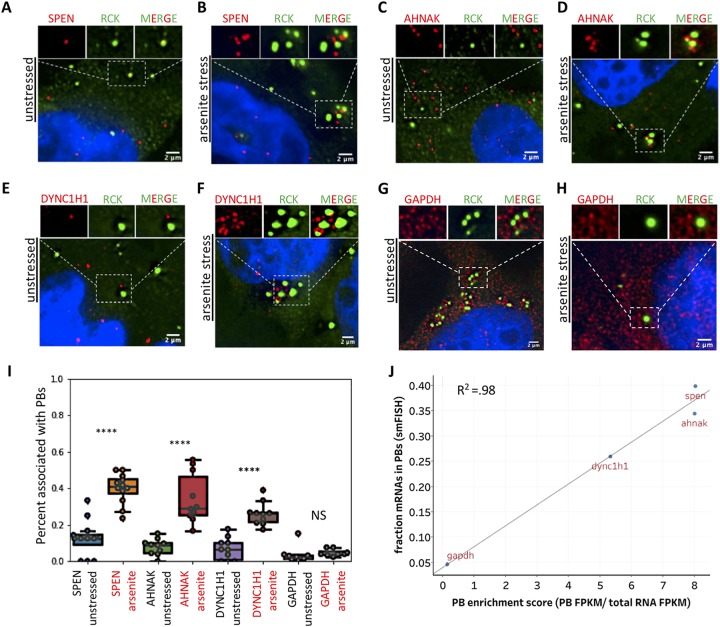
The analyses described above suggested specific mRNAs that would be preferentially enriched in P-bodies, and that those mRNAs should increase their partitioning into P-bodies during stress. To test this prediction, we examined how enriched transcripts from our RNA-seq data correlated with P-body enrichment via single-molecule fluorescent in situ hybridization (smFISH). We first examined the localization of SPEN, which was previously shown to be enriched in P-bodies in unstressed cells (7). Consistent with this observation, we found that ∼12.5% of SPEN molecules showed association with P-bodies during unstressed conditions (Fig. 9A). We also observed that SPEN was highly enriched in the stressed P-body transcriptome. Consistent with this observation, ∼40% of SPEN transcripts showed association with P-bodies in arsenite-treated cells (Fig. 9B). Thus, SPEN associates with P-bodies under unstressed conditions but further increases its association with PBs during arsenite stress.
FIG 9.
smFISH validation of the P-body transcriptome. (A) smFISH of SPEN and IF of RCK, a P-body marker, during unstressed conditions. (B) Same as panel A but during arsenite stress. (C) smFISH of AHNAK and IF of RCK, a P-body marker, during unstressed conditions. (D) Same as panel C but during arsenite stress. (E) smFISH of DYNC1H1and IF of RCK, a P-body marker, during unstressed conditions. (F) Same as panel E but during arsenite stress. (G) smFISH of GAPDH and IF of RCK, a P-body marker, during unstressed conditions. (H) Same as panel G but during arsenite stress. (I) Quantification of percentage of transcripts associated with PBs by smFISH for SPEN, AHNAK, DYNC1H1, and GAPDH during no stress and arsenite stress. (J) Scatterplot depicting the correlation between P-body enrichment assessed by smFISH versus P-body enrichment assessed by RNA-seq.
We also examined some transcripts that were not previously shown to be enriched in the unstressed P-body transcriptome but were highly enriched in the stressed P-body transcriptome, such as AHNAK and DYNC1H1. Consistent with RNA-seq analysis, these transcripts showed limited association with P-bodies in the absence of stress (Fig. 9C and E) but showed increased levels of association during arsenite stress (Fig. 9D and F). This observation confirmed that the RNA composition of PBs can shift during stressed conditions.
Finally, we examined glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control, since it was depleted from both unstressed and stressed P-bodies by RNA-seq. Consistent with this observation, GAPDH showed limited association with P-bodies in the presence or absence of stress (Fig. 9G to I). Thus, P-bodies do not simply increase association with all RNAs during stress but likely follow rules similar to those for SGs (Fig. 9I).
Quantitation of the mRNA localization to PBs revealed a strong correlation between smFISH and RNA-seq, suggesting that our sequencing is valid and reflects the in vivo reality of the RNAs associating with P-bodies (Fig. 9J). This observation confirmed that RNA association with P-bodies can greatly shift during stress and that during stress, PB RNA composition is similar to that of SGs.
DISCUSSION
Two different methods for the purification and analysis of SG transcriptomes have been described. In one method, RNAs enriched in a heavy pellet during stress conditions are designated the RG transcriptome and are proposed to correlate with the SG transcriptome (10). Alternatively, SGs can be enriched by differential centrifugation and then immunopurified in a subsequent step, a method which gives an SG transcriptome strongly validated by smFISH. Here, we have directly compared these two methods.
Our comparison indicates that while the stressed RG transcriptome shows some similarity to the SG transcriptome, it is more similar to the RG transcriptome from unstressed cells (Fig. 4 and 5). We observe that the RNAs enriched in the heavy pellet also include mitochondrial transcripts and some well-translated RNAs, which are not enriched in SGs. Moreover, a number of RNAs enrich in the pellet under both unstressed and stress conditions, revealing a background population of contaminating RNAs when using the RG pellet to define the SG transcriptome. Thus, the RG pellet under stress is an approximation of the SG transcriptome, but a more accurate representation is determined by immunopurification of stress granules (8).
Our results argue that the RNA composition of P-bodies is dramatically different under unstressed and stress conditions, when P-bodies increase in size and number (2, 3). This is based on our analysis of the P-body transcriptome during arsenite stress in U-2 OS cells compared to the P-body transcriptome from unstressed HEK293 cells (7). Our analysis showed a shift in the properties of RNAs that enrich in P-bodies under stress and unstressed conditions. In the absence of stress, mRNAs enriched in P-bodies strongly correlate with metrics of poorly translating mRNAs (Fig. 8). This is consistent with P-bodies accumulating untranslating mRNAs, which argues that the composition of P-bodies in the absence of stress is primarily dictated by the RNAs that are poorly translated (12). In contrast, during stress, where translation of most mRNAs is strongly inhibited, we observed the enrichment of mRNAs in P-bodies is now no longer dominated by metrics associated with poor translation and instead is biased toward longer RNAs (Fig. 8).
One limitation of this analysis is that we are comparing the PB transcriptome between two different cell lines. We made this comparison because the PB population in unstressed U-2 OS cells is quite small, and it was impractical to purify PB from this cell line under an unstressed condition. Nevertheless, since our smFISH experiments (Fig. 9) show a clear enrichment of specific mRNAs in PB with stress, we believe that this comparison is at least a reasonable approximation.
Based on this shift in P-body composition, we suggest that the composition of P-bodies is determined by different features under stress and nonstress conditions (Fig. 10), where poor translation efficiency is the primary determinant of P-body partitioning under unstressed conditions, but during stress conditions, where most mRNAs are no longer translating, mRNAs are differentially partitioned into PB based on length and perhaps other metrics, such as the ability of mRNAs to associate with key P-body components, such as Ddx6, Pat1l, Edc3, and Edc4.
FIG 10.
Translation determines the RNA composition of P-bodies. Model depicting the critical role of translation in defining the RNA composition of PBs. (Left) During unstressed conditions, only transcripts with poor translation metrics are available for P-body sequestration. (Right) During conditions of stress, in which translation is limited, the RNA composition of PBs shifts to include more RNAs and more closely resemble the SG transcriptome.
We also observed that the composition of P-bodies and stress granules was quite similar during stress (Fig. 7). This similarity is not due to SG contaminating our P-body preparations, since we observe a similar PB transcriptome even in G3BP1Δ/G3BP2Δ cells, which do not assemble to make SGs (Fig. 7). This is consistent with a situation during stress where most mRNAs have exited translation and are now available to assemble into either SGs or PBs. A similarity of mRNAs within PBs and SGs during stress is consistent with previous FISH experiments showing that the mRNAs from the same gene can be detected in both SGs and PBs (2), our smFISH experiments (Fig. 9), and single-molecule experiments showing that individual mRNAs can transit between both PB and SG (21). This suggests that specific features of individual mRNAs encoded by the same gene determine their association with PBs or SGs. For example, one possibility is that mRNAs with a shorter poly(A) tail, which can promote the binding of the PB complex, Pat1/Lsm1-7 (22), would be the molecules enriched in PBs, while molecules with longer poly(A) tails would preferentially accumulate in SG. Alternatively, RNA modifications might alter the binding of RNA binding proteins to affect their partitioning (23). Future work determining how individual mRNA molecules partition into PB and SG will be important to address these issues.
MATERIALS AND METHODS
U-2 OS growth conditions and reagents.
Human osteosarcoma U-2 OS cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, 10% fetal bovine serum, and 1% penicillin-streptomycin at 37°C and 5% CO2 and were used in all experiments.
Isolation of RG pellet and P-bodies.
U-2 OS cells were grown to ∼75% confluence in three 15-cm culture dishes. One hour prior to stress, cell culture medium was exchanged with fresh medium. Cells were then treated with 0.5 mM NaAsO2 for 1 h at 37°C and 5% CO2. After stress, cells were washed once with medium, transferred to Falcon tubes, and pelleted at 1,500 × g for 3 min. Upon aspirating the media, the pellets were immediately flash-frozen in liquid N2 and stored at –80°C until isolation of the RNA granule pellet was performed.
The RNA granule pellet isolation protocol was based on our original stress granule purification scheme, except without immunoprecipitation steps (8, 9). Briefly, the pellet was thawed on ice for 5 min, resuspended in 1 ml SG lysis buffer (50 mM Tris-HCl [pH 7.4], 100 mM potassium acetate, 2 mM magnesium acetate, 0.5 mM dithiothreitol, 50 μg/ml heparin, 0.5% NP-40, complete mini EDTA-free protease inhibitor [11836170001; Sigma-Aldrich], 1 U/μl of RNasin plus RNase inhibitor [N2615; Promega]), and passed 7 times through a 25-gauge 5/8 needle attached to a 1-ml syringe. Lysates were spun at 1,000 × g for 5 min at 4°C to pellet cell debris. Fifty microliters of the supernatants was transferred to new microcentrifuge tubes for isolating total RNA, and the remainder was used for the rest of the protocol.
The following steps were performed to isolate mammalian RG pellet and extract its RNA. (i) The 950 μl supernatant was spun at 16,000 × g for 20 min at 4°C to pellet SG cores. (ii) The resulting supernatant was discarded, and the pellet was resuspended in 1 ml SG lysis buffer. (iii) Steps i and ii were repeated. (iv) The resulting pellet then was resuspended in 300 μl of SG lysis buffer and spun at 850 × g for 2 min at 4°C. (v) The supernatant, which represents the RNA pellet, was transferred to a new tube.
For isolating total RNA and pellet RNA, RTrizolLS reagent (10296-028; Thermo Fisher Scientific) was added and RNA was extracted by following the manufacturer’s protocol. Following isopropanol precipitation, the RNA pellet was resuspended in 50 μl RNase-free H2O.
P-bodies were purified as described for SGs (8), except 20 μl of an anti-Edc3 antibody (ab168851) was used to isolate P-bodies.
Stress conditions.
To examine mRNA localization during stress, we performed the following stress conditions. For arsenite stress experiments, cells were treated with 0.5 mM sodium arsenite (S7400; Sigma-Aldrich) for 1 h. Cells were fixed after stress with 4% paraformaldehyde (NC0179595; Fisher Scientific).
Library construction and RNA sequencing.
RNA quality was assessed by TapeStation analysis. Paired-end cDNA libraries were prepared at the Biofrontiers Institute Sequencing Facility using the KAPA HyperPrep with RiboErase. cDNA libraries were sequenced on a NextSeq high-output device (150 cycles [2 × 75]).
Sequencing data analysis.
Read quality was assessed using FastQC. Illumina adaptors were trimmed using Trimmomatic 0.32 in paired and PE mode (24). An index genome was acquired from GENCODE (release 19 GRCh37.p13). Reads were aligned using TopHat (version 2.0.6) and Bowtie2 (version 2.0.2) and the following parameters: −b2 −fast −microexon-search (25, 26). Differential expression analysis was performed using Cuffdiff (version 2.2.1) with the default parameters (27). Gene ontology analysis was performed using the tools from the Gene Ontology Consortium (http://www.geneontology.org/). Transcript lengths were acquired from Ensembl’s Biomart Tool.
smFISH analysis.
smFISH was performed as previously described (8). For P-body staining we utilized immunofluorescence (IF) against RCK, a PB marker (MDL PD009).
Data availability.
Sequencing is available through NCBI GEO (GSE138988).
ACKNOWLEDGMENTS
We thank Anne Webb for assistance with figure preparations, as well as the Parker lab and Olke Uhlenbeck for helpful discussions. The data analysis and visualization work were performed at the BioFrontiers Institute Advanced Light Microscopy Core. We thank the University of Colorado BioFrontiers Institute Next-Gen Sequencing Core Facility, which performed the Illumina sequencing and library construction.
The Analysis Workstation and the software package Imaris were supported by NIH 1S10RR026680-01A1. This work was supported by NIH GM045443 to R.P. and funds from HHMI.
REFERENCES
- 1.Standart N, Weil D. 2018. P-bodies: cytosolic droplets for coordinated mRNA storage. Trends Genet 34:612–626. doi: 10.1016/j.tig.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. 2005. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol 169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. 2005. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panas MD, Ivanov P, Anderson P. 2016. Mechanistic insights into mammalian stress granule dynamics. J Cell Biol 215:313–323. doi: 10.1083/jcb.201609081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Protter DSW, Parker R. 2016. Principles and Properties of Stress Granules. Trends Cell Biol 26:668–679. doi: 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchan JR. 2014. mRNP granules. Assembly, function, and connections with disease. RNA Biol 11:1019–1030. doi: 10.4161/15476286.2014.972208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubstenberger A, Courel M, Bénard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot J-B, Munier A, Fradet M, Daunesse M, Bertrand E, Pierron G, Mozziconacci J, Kress M, Weil D. 2017. P-body purification reveals the condensation of repressed mRNA regulons. Mol Cell 68:144–157. doi: 10.1016/j.molcel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, Parker R, Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, Parker R. 2017. The stress granule transcriptome reveals principles of mRNA accumulation in stress granules resource the stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol Cell 68:808–820. doi: 10.1016/j.molcel.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khong A, Jain S, Matheny T, Wheeler JR, Parker R. 2018. Isolation of mammalian stress granule cores for RNA-Seq analysis. Methods 137:49–54. doi: 10.1016/j.ymeth.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namkoong S, Ho A, Woo YM, Kwak H, Lee JH, Namkoong S, Ho A, Woo YM, Kwak H, Lee JH. 2018. Granulation resource systematic characterization of stress-induced RNA granulation. Mol Cell 70:175–187. doi: 10.1016/j.molcel.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson CD, Wong DS, Bozidis P, Zhang A, Colberg-Poley AM. 2015. Isolation of endoplasmic reticulum, mitochondria, and mitochondria-associated membrane and detergent resistant membrane fractions from transfected cells and from human cytomegalovirus-infected primary fibroblasts. Curr Protoc Cell Biol 68:3.27.1–3.27.33. doi: 10.1002/0471143030.cb0327s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courel M, Clement Y, Foretek D, Vidal O, Yi Z, Kress M, Vindry C, Benard M, Bossevain C, Antoniewski C, Morillon A, Brest P, Hubstenberger A, Crollius HR, Standart N, Weil D. 2018. GC content shapes mRNA decay and storage in human cells. bioRxiv 10.1101/373498. [DOI] [PMC free article] [PubMed]
- 13.Greenblatt EJ, Spradling AC. 2018. Fragile X mental retardation 1 gene enhances the translation of large autism-related proteins. Science 361:709–712. doi: 10.1126/science.aas9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Treeck B, Protter DSW, Matheny T, Khong A, Link CD, Parker R. 2018. RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc Natl Acad Sci U S A 115:2734–2739. doi: 10.1073/pnas.1800038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler JR, Jain S, Khong A, Parker R. 2017. Isolation of yeast and mammalian stress granule cores. Methods 126:12–17. doi: 10.1016/j.ymeth.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. 2016. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164:487–498. doi: 10.1016/j.cell.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R. 2016. Distinct stages in stress granule assembly and disassembly. Elife 5:1–25. doi: 10.7554/eLife.18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subtelny AO, Eichhorn SW, Chen GR, Sive H, Bartel DP. 2014. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature 508:66–71. doi: 10.1038/nature13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kedersha N, Panas MD, Achorn CA, Lyons S, Tisdale S, Hickman T, Thomas M, Lieberman J, McInerney GM, Ivanov P, Anderson P. 2016. G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J Cell Biol 212:845–860. doi: 10.1083/jcb.201508028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tourrière H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J. 2003. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol 160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Moon SL, Morisaki T, Khong A, Lyon K, Parker R, Stasevich TJ. 2018. Imaging of single mRNA translation repression reveals diverse interactions with mRNP granules. bioRxiv 10.1101/332692. [DOI]
- 22.Chowdhury A, Tharun S. 2009. Activation of decapping involves binding of the mRNA and facilitation of the post-binding steps by the Lsm1-7-Pat1 complex. RNA 15:1837–1848. doi: 10.1261/rna.1650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anders M, Chelysheva I, Goebel I, Trenkner T, Zhou J, Mao Y, Verzini S, Qian S-B, Ignatova Z. 2018. Dynamic m(6)A methylation facilitates mRNA triaging to stress granules. Life Sci Alliance 1:e201800113. doi: 10.26508/lsa.201800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25-10. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. 2013. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing is available through NCBI GEO (GSE138988).