The U.S. Centers for Disease Control and Prevention (CDC) lists Neisseria gonorrhoeae as one of the most urgent antibiotic-resistant threats in the United States. This is due to the emergence of clinical isolates that have developed resistance to nearly every antibiotic used to treat gonorrhea and highlights the critical need to find new therapeutics.
KEYWORDS: IL-8, Neisseria gonorrhoeae, intracellular, repurposing
ABSTRACT
The U.S. Centers for Disease Control and Prevention (CDC) lists Neisseria gonorrhoeae as one of the most urgent antibiotic-resistant threats in the United States. This is due to the emergence of clinical isolates that have developed resistance to nearly every antibiotic used to treat gonorrhea and highlights the critical need to find new therapeutics. The present study discovered salicylamide, an analgesic and antipyretic drug, has antibacterial activity against 40 different antibiotic-resistant strains of N. gonorrhoeae (MIC, 8 to 32 μg/ml) with low frequency of resistance <2.4 × 10−9. Interestingly, salicylamide did not inhibit growth of bacterial species in the vaginal microflora involved in defense against gonococcal infections, such as Lactobacillus gasseri, Lactobacillus jensenii, Lactobacillus johnsonii, and Lactobacillus crispatus. A time-kill assay revealed that salicylamide is a rapidly bactericidal drug, as it eradicated a high inoculum of N. gonorrhoeae within 10 h. Salicylamide was superior to the drug of choice, ceftriaxone, in reducing the burden of intracellular N. gonorrhoeae by 97% in infected endocervical cells. Furthermore, salicylamide outperformed ceftriaxone in reducing expression of the proinflammatory cytokine interleukin 8 (IL-8) from endocervical cells infected with N. gonorrhoeae. A checkerboard assay revealed that salicylamide exhibited a synergistic interaction with tetracycline and additive relationships with azithromycin, ciprofloxacin, and ceftriaxone. A more in-depth investigation of the structure-activity relationship of derivatives of salicylamide revealed the amide and hydroxyl groups are important for antigonorrheal activity. In conclusion, this study identified salicylamide as a promising candidate for further investigation as a novel treatment option for multidrug-resistant gonorrhea.
INTRODUCTION
Neisseria gonorrhoeae is the causative agent of gonorrhea, a sexually transmitted infection that is the second most reported notifiable disease in the United States and one of the most important antimicrobial resistance threats worldwide (1, 2). If left untreated, gonorrhea can result in severe complications that include infertility, ectopic pregnancy, pelvic inflammatory disease, and increased susceptibility to HIV infections (3). The Centers for Disease Control and Prevention (CDC) estimate that over 820,000 new gonococcal infections occur annually in the United States, with an estimated total treatment cost of nearly $5 billion dollars (4). Few effective therapeutic options are available to treat gonorrhea. Current guidelines recommend treating gonorrhea with dual therapy involving azithromycin and ceftriaxone. However, increasing resistance to this treatment option has been reported, which has resulted in multidrug-resistant N. gonorrhoeae (i.e., super gonorrhea) becoming a critical public health concern. Compounding the problem further is that over the last 2 decades, almost no new classes of antibiotic drugs have been approved (5), raising a serious concern that super gonorrhea infections may be untreatable in the near future (6, 7).
The traditional process of drug discovery is complex, time consuming, and expensive (8–10). The approach of translating a new lead natural product or synthetic molecule from the bench to the clinic takes approximately 10 to 15 years and can cost more than $2 billion dollars (11, 12). However, due to the substantial financial investment and small window of therapeutic application, there has been a marked decrease in antibiotic drug discovery and development (13). Thus, alternative approaches to discovering and developing antibacterial agents are needed.
Drug repositioning is a promising field that investigates new therapeutic opportunities for existing approved drugs with readily available information on their pharmacokinetic profile, dosages, and toxicity to humans (14–19). This approach can significantly reduce the total cost, time, and effort to develop novel antibacterial agents. To this end, we screened FDA-approved drugs and clinical molecules present in the Johns Hopkins Clinical Compound Library in search of agents that possess antibacterial activity against multidrug-resistant N. gonorrhoeae. Salicylamide, an over-the-counter drug with analgesic and antipyretic properties, emerged as the lead candidate against N. gonorrhoeae. Salicylamide was evaluated against a diverse panel of N. gonorrhoeae clinical isolates, both alone and in combination with antibiotics used to treat gonorrhea. In addition, we investigated the ability of salicylamide to reduce intracellular N. gonorrhoeae and the effect on interleukin 8 (IL-8) expression in endocervical cells infected with N. gonorrhoeae. Furthermore, we investigated the structure-activity relationship of salicylamide by analyzing the antibacterial activity of other structurally related derivatives against N. gonorrhoeae. We also investigated the activity of salicylamide and representative analogues against vaginal microbiota that inhibit N. gonorrhoeae colonization, such as Lactobacillus gasseri and Lactobacillus crispatus.
RESULTS
Antibacterial susceptibility testing.
The antigonococcal activity of salicylamide was evaluated against a panel of 40 N. gonorrhoeae clinical isolates, including several multidrug-resistant strains. These strains exhibited resistance to azithromycin and ciprofloxacin. As presented in Table 1, salicylamide inhibited the growth of the 40 tested N. gonorrhoeae isolates at concentrations ranging from 8 to 32 μg/ml. The MIC50 of salicylamide was 16 μg/ml and MIC90 was 32 μg/ml. Moreover, salicylamide’s MIC was consistent against azithromycin-resistant strains, such as N. gonorrhoeae strains 167, 175, 179, 181, 193, 197, 199, and 202. Azithromycin inhibited growth of azithromycin-susceptible isolates at concentrations ranging from 0.5 to 4 μg/ml. The MIC of azithromycin ranged from 8 to 256 μg/ml against azithromycin-resistant N. gonorrhoeae isolates (strains 167, 175, 179, 181,193, 197,199, and 202). Five strains of N. gonorrhoeae (167, 175, 179, 181, and 194) were utilized in agar dilution method to confirm the MIC values of tested drugs. A 1-fold increase was observed in salicylamide’s MIC values in comparison with those from the microbroth method, whereas the control’s MICs remained constant. The results of these assays are presented in Table 2.
TABLE 1.
MIC of salicylamide and a control antibiotic against 40 N. gonorrhoeae isolates
| N. gonorrhoeae strain no. | MIC (μg/ml) |
|
|---|---|---|
| Salicylamide | Azithromycin | |
| 167 | 8 | 16 |
| 168 | 8 | 1 |
| 171 | 8 | 0.5 |
| 176 | 8 | 1 |
| 177 | 8 | 2 |
| 185 | 8 | 1 |
| 183 | 8 | 1 |
| 187 | 8 | 2 |
| 188 | 16 | 1 |
| 195 | 16 | 1 |
| 197 | 16 | 8 |
| 165 | 16 | 1 |
| 169 | 16 | 1 |
| 170 | 16 | 2 |
| 172 | 16 | 0.5 |
| 173 | 16 | 1 |
| 174 | 16 | 1 |
| 175 | 16 | 32 |
| 178 | 16 | 1 |
| 179 | 16 | 8 |
| 180 | 16 | 0.5 |
| 182 | 16 | 0.5 |
| 186 | 16 | 1 |
| 189 | 16 | 0.5 |
| 190 | 16 | 1 |
| 191 | 16 | 1 |
| 192 | 16 | 1 |
| 193 | 16 | 4 |
| 196 | 16 | 0.5 |
| 198 | 16 | 1 |
| 199 | 16 | 4 |
| 200 | 16 | 1 |
| 211 | 16 | 1 |
| 202 | 16 | 16 |
| 214 | 16 | 0.5 |
| 194 | 16 | 0.5 |
| 204 | 32 | 0.5 |
| 181 | 32 | 256 |
| 184 | 32 | 0.5 |
| 203 | 32 | 1 |
| MIC50 | 16 | 4 |
| MIC90 | 32 | 16 |
TABLE 2.
MICs of salicylamide and a control antibiotic against 5 N. gonorrhoeae isolates using agar dilution method
| N. gonorrhoeae strain no. | MIC (μg/ml) |
|
|---|---|---|
| Salicylamide | Azithromycin | |
| 167 | 16 | 16 |
| 178 | 32 | 1 |
| 198 | 32 | 1 |
| 181 | 64 | 256 |
| 194 | 32 | 1 |
Effect of salicylamide and control drugs on the vaginal microbiota.
The vaginal microbiota competes with N. gonorrhoeae for adhesion to the urinary tract in addition to creating an acidic environment that prevents gonococcal colonization. Thus, we investigated the activity of salicylamide against different species of Lactobacillus that comprise the female urogenital tract microbiota. Salicylamide exhibited a strong selectivity toward N. gonorrhoeae without inhibiting growth of different species of Lactobacillus up to a concentration of 256 μg/ml (Table 2). In contrast, both drugs of choice for gonorrhea infections, azithromycin and ceftriaxone, inhibited growth of the 11 species of Lactobacillus tested at concentrations as low as 1 μg/ml.
Time-kill assay of salicylamide against N. gonorrhoeae.
After confirming salicylamide’s ability to inhibit growth of a large panel of antibiotic-resistant N. gonorrhoeae isolates in vitro, we moved to examine if salicylamide is a bacteriostatic or bactericidal agent. Thus, a time-kill assay was used to evaluate the ability of salicylamide and azithromycin (both at 5× MIC) to reduce N. gonorrhoeae strain 194’s CFU over the course of 24 h. Salicylamide exhibited a strong bactericidal activity in vitro and reduced the bacterial inoculum to below the limit of detection (100 CFU/ml) after 10 h (Fig. 1). This was comparable to azithromycin, which reduced the bacterial inoculum to below the limit of detection after 8 h.
FIG 1.
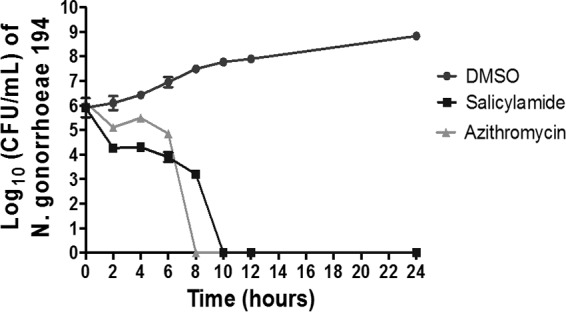
Time-kill assay of salicylamide and azithromycin (both at 5× MIC) against multidrug-resistant Neisseria gonorrhoeae strain 194 over a 24-h incubation period at 37°C. DMSO (solvent for the compounds) served as a negative control. The error bars represent standard deviation values obtained from triplicate samples used for each test agent.
Combination testing of salicylamide with azithromycin, ciprofloxacin, ceftriaxone, and tetracycline against N. gonorrhoeae.
Because N. gonorrhoeae exhibits high-level resistance to numerous antibiotics, monotherapy is not recommended for the treatment of gonorrhea (20). As dual therapy is the most effective strategy for treating gonococcal infections, this prompted us to investigate the combination of salicylamide with various antibiotics. Azithromycin and ceftriaxone were chosen because they are drug of choice for the treatment of gonorrhea. Tetracycline and ciprofloxacin were also investigated, as they were previously shown to possess a synergistic relationship with derivatives of salicylamide against vancomycin-resistant enterococci (21). As presented in Table 3, salicylamide displayed an additive relationship when combined with either azithromycin, ceftriaxone, or ciprofloxacin against four different N. gonorrhoeae isolates (fractional inhibitory concentration indices [FICIs] ranged from 1 to 1.5). Salicylamide possessed a synergistic relationship with tetracycline against one strain (181) with an FICI of 0.5 and showed additive activity with the remaining three N. gonorrhoeae strains tested (FICIs ranged from 0.625 to 1).
TABLE 3.
MICs of salicylamide, azithromycin, and ceftriaxone against a panel of vaginal microflora strains
| Strain designation | MIC (μg/ml) |
||
|---|---|---|---|
| Salicylamide | Azithromycin | Ceftriaxone | |
| L. gasseri HM-642 | >256 | ≤1 | ≤1 |
| L. gasseri HM-644 | >256 | ≤1 | ≤1 |
| L. gasseri HM-403 | >256 | ≤1 | ≤1 |
| L. gasseri HM-400 | >256 | ≤1 | ≤1 |
| L. crispatus HM-638 | >256 | ≤1 | ≤1 |
| L. crispatus HM-370 | >256 | ≤1 | 2 |
| L. jensenii HM-640 | 256 | ≤1 | 4 |
| L. jensenii HM-105 | >256 | ≤1 | ≤1 |
| L. jensenii HM-639 | >256 | ≤1 | ≤1 |
| L. johnsonii HM-643 | >256 | ≤1 | ≤1 |
Intracellular clearance of N. gonorrhoeae.
N. gonorrhoeae is able to invade mucosal epithelia and survive intracellularly. Ceftriaxone, because of its complex and bulky structure, high molecular weight (554.58 g/mol), high hydrophilicity, and low active transport, exhibits low cellular permeability and is unable to clear intracellular N. gonorrhoeae infections (22–24). Salicylamide, in contrast, is a much smaller molecule (molecular weight of 137.14 g/mol). To determine if salicylamide can clear intracellular N. gonorrhoeae, an intracellular clearance assay was conducted. As depicted in Fig. 2, after 24 h, salicylamide (at 5× MIC) successfully reduced intracellular N. gonorrhoeae from infected endocervical cells (End1/E6E7) by 1.4 log10 (equivalent to 97% reduction in the bacterial count), relative to cells treated with dimethyl sulfoxide (DMSO). Ceftriaxone, as expected, produced a nonsignificant 0.25-log10 reduction in intracellular N. gonorrhoeae. This result indicates that salicylamide is able to enter endocervical cells and effectively reduce the burden of intracellular N. gonorrhoeae.
FIG 2.
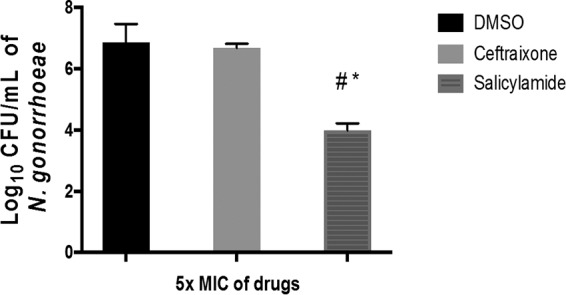
Effectiveness of salicylamide and ceftriaxone (both at 5× MIC) against intracellular N. gonorrhoeae in infected human endocervical cells (End1/E6E7). End1/E6E7 cells were infected with N. gonorrhoeae strain 194 for 6 h and then treated with either salicylamide or ceftriaxone for 24 h. End1/E6E7 cells were subsequently lysed and intracellular bacterial CFU was determined. Error bars represent standard deviation values from triplicate samples used for each test agent; test was conducted twice. *, P < 0.01 versus DMSO; #, P < 0.01 versus ceftriaxone by unpaired t test.
Effect of salicylamide on IL-8 expression.
We next investigated the anti-inflammatory effect of salicylamide against human endocervical cells (End1/E6E7) infected with N. gonorrhoeae. IL-8 is a major proinflammatory cytokine that is produced during gonococcal infection of epithelial cervical cells (16). IL-8 was measured in the End1/E6E7 cell supernatant after establishing infection with N. gonorrhoeae strain 194 in the presence or absence of salicylamide and control antibiotics (at 0.5× MIC). DMSO was used as a negative control. Treatment with salicylamide resulted in a significant reduction equivalent to 37.7% of IL-8 expression in comparison to that with ceftriaxone at 3.9% (minor reduction in IL-8 concentration was observed) (Fig. 3).
FIG 3.
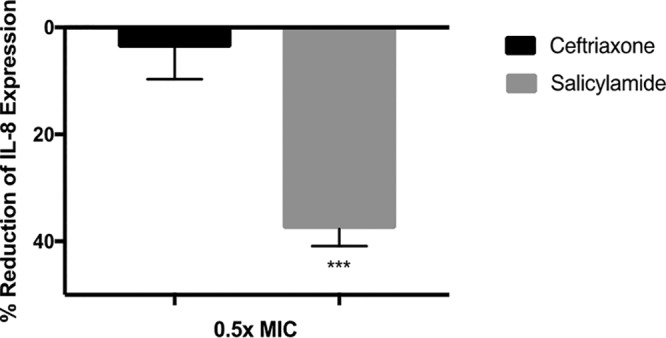
Effects of salicylamide and ceftriaxone on IL-8 expression in infected endocervical cells. IL-8 levels were assessed in End1/E6E7 endocervical cells infected with N. gonorrhoeae strain 194 in the presence and absence of 0.5× MIC of salicylamide or ceftriaxone. OD450 coincides with the level of IL-8 in the cell supernatant. Error bars represent standard deviation values from triplicate samples used for each test agent; test was conducted twice. ***, P < 0.01 versus ceftriaxone by unpaired t test.
Evaluation of structure-activity relationship of salicylamide.
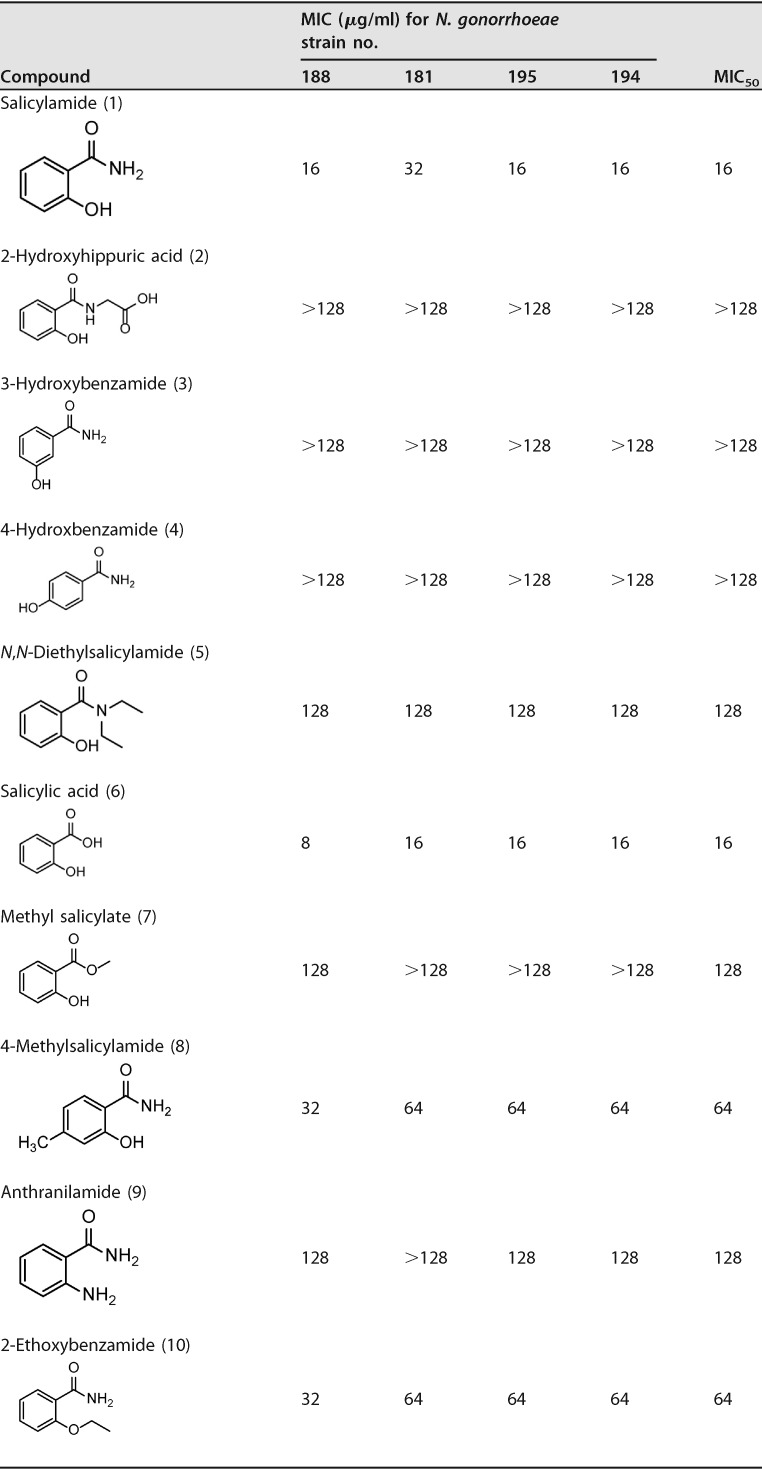
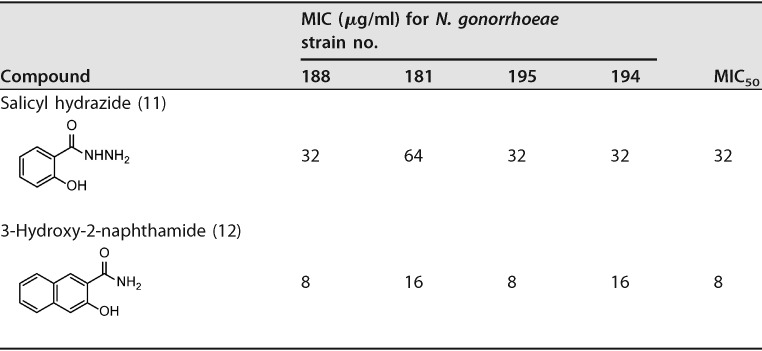
We investigated the antibacterial structure-activity relationship (SAR) of salicylamide by testing the antigonorrheal activity of 11 commercially available hydroxybenzamide derivatives and related analogues. Salicylamide’s MIC50 value against the 11 N. gonorrhoeae strains was 16 μg/ml (Table 4). On the other hand, the other 3- and 4-hydroxy regioisomers (compounds 3 and 4, 3-hydroxybenzamide and 4-hydroxybenzamide, respectively) were devoid of any antigonorrheal activity (MIC50 > 128 μg/ml). Alkylation of the amino group of benzamide yielded compound 5 (N,N-diethylsalicylamide) with weak activity against N. gonorrhoeae (MIC50 = 128 μg/ml). Similarly, alkylation of the hydroxyl group furnished an inactive derivative (compound 9, anthranilamide). para-Alkylation of the benzene skeleton also had a negative impact on antigonorrheal potency, as the 4-methyl derivative of salicylamide possessed a MIC50 value of 64 μg/ml. In contrast to the N-alkylation that caused the compounds to become inactive against N. gonorrhoeae, replacing the amide group with a carboxylate moiety maintained the drug’s antigonorrheal activity. For example, salicylic acid (compound 6) possessed the same MIC50 value as its corresponding amide derivative (compound 1). Again, alkylation of the carboxylate group of salicylic acid (compound 6) negatively impacted the compound’s antibacterial activity (compound 7, MIC50 = 128 μg/ml). Further expansion of the polar part at the amide group yielded either a completely inactive compound (compound 2, 2-hydroxyhippuric acid) or a less active one (compound 11, salicylhydrazide, MIC50 = 32 μg/ml). Finally, expanding the lipophilic bulkiness through substitution of the benzene ring with a naphthalene ring (compound 12, 3-hydroxy-2-naphthamide) enhanced the compound’s antigonorrheal activity by 1-fold (MIC50 of 8 μg/ml) compared to salicylamide.
TABLE 4.
Fractional inhibitory concentration index range of salicylamide in combination with azithromycin, ciprofloxacin, and tetracycline against N. gonorrhoeae isolates
| Drug(s) | Description | MIC (μg/ml) and FICI results for Neisseria gonorrhoeae strain no.: |
|||
|---|---|---|---|---|---|
| 175 | 178 | 181 | 194 | ||
| Salicylamide | Alone | 16 | 16 | 32 | 16 |
| Combination | 8 | 8 | 32 | 16 | |
| Azithromycin | Alone | 16 | 1 | 256 | 0.5 |
| Combination | 8 | 0.5 | 64 | 0.25 | |
| Salicylamide-azithromycin | FICI | 1 | 1 | 1.25 | 1.5 |
| Interpretationa | ADD | ||||
| Salicylamide | Alone | 16 | 16 | 32 | 16 |
| Combination | 4 | 8 | 8 | 4 | |
| Ciprofloxacin | Alone | 0.015 | 16 | 0.015 | 0.015 |
| Combination | 0.015 | 8 | 0.015 | 0.015 | |
| Salicylamide-ciprofloxacin | FICI | 1.25 | 1 | 1.25 | 1.25 |
| Interpretation | ADD | ||||
| Salicylamide | Alone | 16 | 16 | 32 | 16 |
| Combination | 4 | 8 | 16 | 4 | |
| Tetracycline | Alone | 1 | 8 | 1 | 1 |
| Combination | 0.25 | 4 | 0.125 | 0.5 | |
| Salicylamide-tetracycline | FICI | 0.5 | 1 | 0.625 | 0.75 |
| Interpretation | SYN | ADD | |||
| Salicylamide | Alone | 16 | 16 | 32 | 16 |
| Combination | 16 | 8 | 16 | 16 | |
| Ceftriaxone | Alone | 0.015 | 0.125 | 0.06 | 0.25 |
| Combination | 0.015 | 0.06 | 0.06 | 0.25 | |
| Salicylamide-ceftriaxone | FICI | 2 | 1 | 1.5 | 2 |
| Interpretation | ADD | ||||
FICI was interpreted as follows: an FICI of ≤0.5 is indicative of a synergistic relationship (SYN), an additive relationship (ADD) was defined as an FICI of >0.5 but ≤4, and antagonism was defined as an FICI of >4.
Neisseria gonorrhoeae spontaneous mutation frequency.
Salicylamide’s ability to develop resistance was tested using the single step resistance assay as described previously (25) to calculate the frequency of spontaneous mutation. Results are reported in Table 5. After 72 h, no colonies were observed. Salicylamide’s frequency of mutation was calculated as <2.4 × 10−9 (Table 6), a rate that is comparable to azithromycin, which is the drug of choice (26). Rifampin’s frequency of mutation was higher (1.8 × 10−6), as previously reported in other bacterial strains (27, 28).
TABLE 5.
Antibacterial activity of salicylamide and structurally related analogues against four Neisseria gonorrhoeae isolates
TABLE 6.
Spontaneous mutation frequencies of salicylamide and rifampin at 10× agar MIC against N. gonorrhoeae 197, 202, and 206
| N. gonorrhoeae strain no. | Drug | Frequency of mutation |
|---|---|---|
| 197 | Salicylamide | <2.4 × 10−9 |
| Rifampicin | 1.8 × 10−6 | |
| 202 | Salicylamide | <2.4 × 10−9 |
| Rifampicin | 4.17 × 10−6 | |
| 206 | Salicylamide | <2.4 × 10−9 |
| Rifampicin | 1.2 × 10−6 |
DISCUSSION
N. gonorrhoeae infections have evolved into a challenging disease, as resistance has rapidly emerged to all antibiotics used for treatment. With no effective vaccines available, the development of effective antibacterial agents is a critical priority (7). Using a drug repurposing approach, we identified salicylamide as a promising new candidate for the treatment of drug-resistant gonorrhea infections. Salicylamide is an FDA-approved drug that has been extensively used in humans. Therefore, its pharmacokinetic and safety profiles in humans are well established.
The antibacterial activity of salicylamide was investigated against a panel of 40 clinical isolates of N. gonorrhoeae utilizing the broth microdilution method. Current CLSI guidelines recommend the use of the agar dilution method for the susceptibility testing of N. gonorrhoeae (29). Nevertheless, several previous studies have shown great results using the broth microdilution method (30). Salicylamide successfully inhibited the growth of the 40 clinical isolates of N. gonorrhoeae tested (MIC values ranged from 8 to 32 μg/ml). Salicylamide (at 5× MIC) exhibited bactericidal activity against N. gonorrhoeae, successfully reducing the bacterial burden below the limit of detection after 10 h. Azithromycin, the current drug of choice for gonorrhea, achieved the same effect in 8 h, which is in agreement with previous reports (31, 32). Due to the nature of gonococcal infection, a bactericidal drug is preferred over bacteriostatic drugs in quelling infection and preventing transmission.
N. gonorrhoeae is known to invade epithelial cells of the genital tract and cross the epithelial barrier into the subepithelial space (33). Previous studies have shown that N. gonorrhoeae can survive inside host cells and pass epithelial cell layers (a key step in causing disseminated infections). As salicylamide exhibited bactericidal activity in vitro, we examined if the drug could reduce the burden of intracellular N. gonorrhoeae present in infected endocervical cells. End1/E6E7 cells were infected with N. gonorrhoeae and subsequently treated with either salicylamide or ceftriaxone (both at 5× MIC) for 24 h. Salicylamide generated a 1.4-log10 reduction in N. gonorrhoeae inside infected endocervical cells while ceftriaxone, in contrast, was ineffective. The results collectively indicate that salicylamide has the ability to gain entry into endocervical cells at a concentration high enough to significantly reduce intracellular N. gonorrhoeae at a rate that is superior to that for ceftriaxone.
Dual therapy with ceftriaxone and azithromycin is the recommended approach to treat infections caused by N. gonorrhoeae. The use of two antibiotics in conjunction is thought to curb the rapid emergence of resistance to either antibiotic used alone. Thus, we investigated the potential of salicylamide to be used in combination with other antibiotics against N. gonorrhoeae. Using a standard checkerboard assay, salicylamide was found to possess a synergetic relationship with tetracycline against one strain of N. gonorrhoeae. Salicylamide exhibited an additive relationship with azithromycin, ceftriaxone, and ciprofloxacin against four N. gonorrhoeae strains. This suggests that dual therapy involving salicylamide and one of these antibiotics may be feasible, though further investigation is needed.
To assess the potential for rapid emergence of resistance of N. gonorrhoeae to salicylamide, we attempted to generate a N. gonorrhoeae mutant that is resistant to salicylamide using a single-step mutation assay. N. gonorrhoeae mutants exhibiting resistance to salicylamide could not be isolated, indicating a low likelihood of rapid resistance emerging to these drugs. The inability to generate a resistant mutant of N. gonorrhoeae to salicylamide suggests this drug may have multiple targets.
Two mechanisms that enhance gonococcal colonization and infection are disruption of the healthy microbiota present in the genitourinary tract and release of proinflammatory cytokines by epithelial cells in the reproductive tract. Most bacteria that reside in the female genital tract are from the genus Lactobacillus (34). Lactobacilli are Gram-positive bacteria, in contrast to N. gonorrhoeae. One of the disadvantages of the first-line drugs used to treat N. gonorrhoeae infections is that they are nonspecific and inhibit growth of both gonococci and commensal bacteria indiscriminately (35). In contrast to the current first-line treatments, which inhibited both N. gonorrhoeae and Lactobacillus species equally (MIC values ranged from 1 to 4 μg/ml), salicylamide did not inhibit growth of Lactobacillus strains tested at concentrations up to 256 μg/ml. Previous studies have demonstrated the role of a disturbed vaginal microbiota in establishing and maintaining gonococcal infections (36, 37). The commensal vaginal flora decreases the vaginal pH and can directly compete with pathogens for space and adherence to the epithelial cells lining the vaginal tract (38–40). Therefore, salicylamide’s specificity is a valuable characteristic in the treatment of N. gonorrhoeae and reducing gonococcal colonization (36).
Once N. gonorrhoeae has successfully colonized the genitourinary tract, infections can induce a severe inflammatory response resulting in the production of several proinflammatory cytokines that are considered a hallmark of gonococcal infections (37). Dampening this response is a potential approach to reduce the severity of the infection. As salicylamide is a nonsteroidal anti-inflammatory drug, we hypothesized it would be capable of reducing the expression of proinflammatory cytokines from host epithelial cells. Using preinfected endocervical cells, we confirmed that salicylamide significantly reduced IL-8 expression compared with ceftriaxone. Thus, salicylamide was found to possess three advantages over ceftriaxone as an alternative treatment for N. gonorrhoeae infections: (i) the ability to reduce the burden of intracellular N. gonorrhoeae in infected endocervical cells, (ii) the lack of activity against lactobacilli, and (iii) the ability to reduce expression of proinflammatory cytokines that contribute to gonococcal infections.
Salicylamide by its very simple chemical nature (low molecular weight, small number of hydrogen bond donors and acceptors, and low lipophilicity) represents a very attractive lead structure for medicinal chemists (41). In other words, after identifying a lead compound, the optimization step starts, mainly by adding substructures, to understand the SAR (Fig. 4). In this vein, the study of the antigonorrheal activity of eleven congruent benzamide derivatives provided insight into the essential functionalities. In brief, regioselectivity was found to be an essential issue, as only ortho-hydroxybenzamide was active and the other two meta and para isomers were inactive. The amino group can only be replaced with a hydroxyl group. Any N- or O-alkylation nullifies the antigonorrheal activity of the compounds. Finally, the naphthalene core exhibited the best antigonorrheal activity and provides a bulkier skeleton for further structural optimization.
FIG 4.
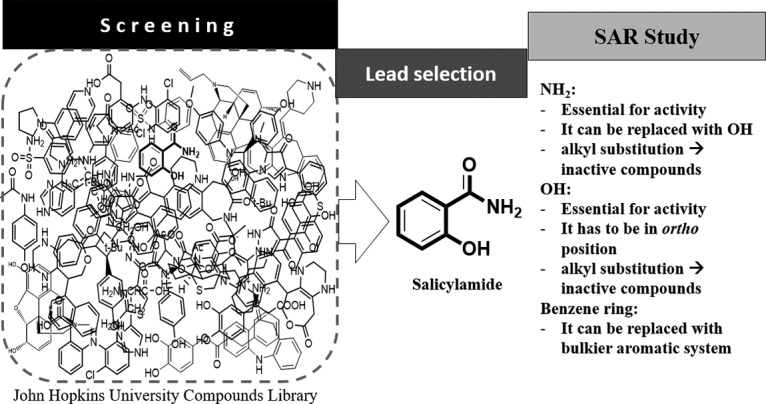
Diagrammatic representation of antibacterial SAR for optimization of salicylamide’s antigonococcal activity.
In conclusion, we report that salicylamide, an FDA-approved over-the-counter drug, has potent in vitro antibacterial activity against N. gonorrhoeae without inhibiting the vaginal Lactobacillus spp. N. gonorrhoeae mutants exhibiting resistance to salicylamide could not be isolated, indicating a low likelihood of rapid resistance emerging to this drug. Furthermore, salicylamide can gain access inside endocervical cells and reduce intracellular infection by N. gonorrhoeae as well as reduce IL-8 expression to alleviate inflammation caused by infection. Additionally, salicylamide possesses a synergetic or additive relationship with other antibiotics that could be used for dual therapy. Further modification to salicylamide’s chemical structure might be utilized to increase its potency and inhibitory activity against multidrug-resistant N. gonorrhoeae. The preliminary SAR analysis conducted with derivatives of salicylamide against N. gonorrhoeae will aid medicinal chemists in designing analogues that are more potent.
MATERIALS AND METHODS
Bacterial strains and reagents.
The N. gonorrhoeae panel was obtained from the U.S. Centers for Disease Control and Prevention (CDC), and Lactobacillus strains were obtained from BEI Resources (Manassas, VA, USA) (Table 7). Salicylamide, 2-hydroxyhippuric acid, 3-hydroxybenzamide, 4-hydroxybenzamide, N,N-diethylsalicylamide, salicylic acid, methyl salicylate, 4-methylsalicylamide, anthranilamide, 2-ethoxybenzamide, salicylhydrazide, 3-hydroxy-2-naphthamide, ciprofloxacin, and tetracycline were obtained from Sigma-Aldrich (St. Louis, MO). Azithromycin (TCI America, Portland, OR), ceftriaxone, cefixime (Acros Organics, Morris Plains, NJ), and gentamicin (Thermo Fisher Scientific, Waltham, MA) were purchased from commercial vendors, as were yeast extract and dextrose (Fisher Bioreagents, Fairlawn, NJ), proteose-peptone, agarose, NAD, Triton X-100, and phosphate-buffered saline (PBS) (Sigma-Aldrich, St. Louis, MO), pyridoxal (Chem-Impex International, Wood Dale, IL), and calcium chloride (Gibco, Big Cabin, OK). Chocolate II agar (GC II Agar with hemoglobin and IsoVitaleX), Brucella supplemented broth base (BSB), gonococcal GC medium base (Thermo Fisher Scientific, Waltham, MA), and Difco lactobacilli MRS broth were purchased from Becton, Dickinson and Company (Cockeysville, MD). Keratinocyte medium (KSFM), bovine pituitary extract, and epidermal growth factor (Gibco, Big Cabin, OK) were purchased from commercial vendors.
TABLE 7.
Bacterial strains that were used in this study
| Strain | Description |
|---|---|
| Neisseria gonorrhoeae strains | |
| N. gonorrhoeae 214 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 213 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 212 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 211 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 210 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 209 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 208 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 207 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 206 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 205 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 204 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 203 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 202 | Resistant to azithromycin |
| N. gonorrhoeae 201 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 200 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 199 | Resistant to azithromycin, tetracycline, and penicillin |
| N. gonorrhoeae 198 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 197 | Resistant to azithromycin tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 196 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 195 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 194 | Resistant to penicillin, not susceptible to ceftriaxone, cefixime, and cefpodoxime |
| N. gonorrhoeae 193 | Resistant to azithromycin, tetracycline, and penicillin |
| N. gonorrhoeae 192 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 191 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 190 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 189 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 188 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 187 | Resistant to penicillin, less susceptible to azithromycin |
| N. gonorrhoeae 186 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 185 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 184 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 183 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 182 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 181 | Resistant to tetracycline and azithromycin |
| N. gonorrhoeae 180 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 179 | Resistant to azithromycin |
| N. gonorrhoeae 178 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 177 | Resistant to tetracycline, less susceptible to azithromycin |
| N. gonorrhoeae 176 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 175 | Resistant to azithromycin |
| N. gonorrhoeae 174 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 173 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 172 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 171 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 170 | Resistant to tetracycline, penicillin, and ciprofloxacin, less susceptible to azithromycin |
| N. gonorrhoeae 169 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 168 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 167 | Resistant to azithromycin |
| N. gonorrhoeae 166 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| N. gonorrhoeae 165 | Resistant to tetracycline, penicillin, and ciprofloxacin |
| Lactobacillus strains | |
| L. gasseri HM-642 | Vaginal isolate from a healthy U.S. woman, obtained in 2007 |
| L. gasseri HM-644 | Vaginal mucosal isolate of a healthy U.S. woman of child-bearing age, obtained in 2007 |
| L. gasseri HM-403 | Isolated from human patient’s mid-vaginal wall in March 2010 in Richmond, VA, USA |
| L. gasseri HM-400 | Isolated from human patient’s mid-vaginal wall in March 2010 in Richmond, VA, USA |
| L. crispatus HM-638 | Vaginal isolate from a healthy Chinese woman, obtained in 2007 |
| L. crispatus HM-370 | Isolated from human patient’s mid-vaginal wall in March 2010 in Richmond, VA, USA |
| L. jensenii HM-640 | Isolated in 2007 from the vaginal mucosa of a healthy Chinese woman |
| L. jensenii HM-105 | Human vaginal isolate obtained from Texas |
| L. jensenii HM-639 | Isolated in 2007 from the vaginal mucosa of a healthy U.S. woman |
| L. johnsonii HM-643 | Isolated in 2007 from the vaginal mucosa of a Chinese woman |
Antibacterial susceptibility testing of salicylamide against N. gonorrhoeae.
Salicylamide was tested against a panel of clinical isolates of multidrug-resistant N. gonorrhoeae using the broth microdilution assay as described previously (30), with slight modifications. Briefly, a 1.0 McFarland standard was prepared and diluted in BSB (Brucella broth was used instead of Colombia broth, and it was supplemented with yeast extract, dextrose, agarose, proteose-peptone, NAD, pyridoxal and hematin) to reach a bacterial count of 5 × 105 CFU/ml. Drugs were added and serially diluted. Plates were then incubated for 24 h at 37°C in the presence of 5% CO2. A confirmatory test was conducted on 5 strains of N. gonorrhoeae using the CLSI recommended agar dilution method as described before (29) to confirm MIC values obtained from the broth microdilution assay.
Salicylamide’s activity against vaginal microflora.
Antimicrobial susceptibility testing against Lactobacillus gasseri, Lactobacillus jensenii, Lactobacillus johnsonii, and L. crispatus was conducted using the broth microdilution assay in accordance with the CLSI guidelines (29) to test the activity of salicylamide against a panel of strains that are important members of the vaginal microbiome.
Time-kill assay.
To determine if salicylamide is a bacteriostatic or bactericidal antibacterial in vitro, a time-kill assay was utilized, as described previously (42, 43), against N. gonorrhoeae strain 194. Briefly, N. gonorrhoeae was grown in BSB to logarithmic phase and further diluted to reach an initial inoculum of 5 × 106 CFU/ml. Salicylamide and azithromycin (positive control) were then added (at 5× MIC in triplicates). Bacteria exposed to DMSO (solvent of drugs) alone served as a negative control. An aliquot from each sample was serially diluted and plated onto chocolate II agar plates. Plates were incubated for 24 h at 37°C in the presence of 5% CO2 to determine the CFU count. Test agents were categorized as exhibiting bactericidal activity if the bacterial CFU was reduced by at least 3 log10 over a 24-h period.
Synergetic activity of salicylamide with azithromycin, ciprofloxacin, ceftriaxone, and tetracycline.
The relationship between salicylamide and four antibiotics (azithromycin, ciprofloxacin, ceftriaxone, and tetracycline) against N. gonorrhoeae was assessed via a standard checkerboard assay, as described previously (43, 44). Briefly, a 1.0 McFarland standard solution of bacteria was prepared and then diluted in BSB to achieve a bacterial inoculum of 5 × 105 CFU/ml. Salicylamide and antibiotics were added at the corresponding concentrations, and media containing bacteria were subsequently added. The plates were then incubated for 24 h at 37°C in the presence of 5% CO2. Interactions where the fractional inhibitory concentration index (FICI) was ≤0.5 were categorized as synergistic. An FICI of >0.5 but ≤1.25 was categorized as additive. An FICI of >1.25 but ≤4 was considered indifferent, while an FICI >4 was categorized as antagonistic (45, 46).
Intracellular clearance assay.
An intracellular bacterial clearance experiment was utilized to investigate the ability of salicylamide to enter endocervical cells and reduce the burden of intracellular N. gonorrhoeae, as described previously (33, 47, 48). Briefly, human endocervical cells (ATCC CRL-2615, End1/E6E7) were seeded at a density of ∼1 × 105 cells per well in 96-well tissue-culture-treated plates for 24 h at 37°C with 5% CO2 before being infected with bacteria. The cells were routinely grown in serum-free keratinocyte medium (KSFM) supplemented with 0.05 mg/ml bovine pituitary extract, 0.1 ng/ml epidermal growth factor, and 44.1 mg/liter calcium chloride. Following incubation, the cells were washed once with PBS. Cells were subsequently infected with N. gonorrhoeae strain 194 (at a multiplicity of infection of 100:1) for 6 h at 37°C with 5% CO2. The cells were washed three times with PBS containing 320 μg/ml gentamicin and were further incubated for 1 h to kill and wash off nonphagocytized bacteria. End1/E6E7 cells were subsequently exposed to either salicylamide or ceftriaxone, at 5× MIC, and incubated for 24 h at 37°C with 5% CO2. DMSO (solvent for the drugs) served as a negative control. After incubation, the test agents were removed, and endocervical cells were washed with PBS and subsequently lysed using 0.01% Triton X-100 to collect intracellular bacteria. The lysate was serially diluted in PBS and plated on chocolate II agar plates. Plates were incubated at 37°C with 5% CO2 for 24 h. Experiments were performed using triplicate samples for each treatment group, and the experiment was repeated at least twice. Data were analyzed via unpaired t tests using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA).
IL-8 cytokine expression in infected endocervical cells.
To investigate the anti-inflammatory activity of salicylamide, the proinflammatory cytokine IL-8 was detected in supernatants of N. gonorrhoeae-infected human endocervical cells exposed to either salicylamide or ceftriaxone, as described in a previous study (35). Briefly, cells were infected with N. gonorrhoeae strain 194 for 2 h followed by treatment with 0.5× MIC of ceftriaxone or salicylamide (in triplicates) for 4 h. DMSO served as a negative control. Cell supernatants were collected and tested for IL-8 concentration using the Human IL-8 ELISA kit (Bosterbio, Pleasanton, CA), according to the manufacturer’s instructions. Data were analyzed via unpaired t tests using GraphPad Prism 6.0.
Evaluation of the structure-activity relationship of the benzamide scaffold.
Salicylamide is a simple benzamide derivative with only one ortho-hydroxyl group. To investigate the structure-antibacterial activity relationship (SAR) of salicylamide, we evaluated the antigonorrheal activity of 11 salicylamide analogs (2-hydroxyhippuric acid, 3-hydroxybenzamide, 4-hydroxybenzamide, N,N-diethylsalicylamide, salicylic acid, methyl salicylate, 4-methylsalicylamide, anthranilamide, 2-ethoxybenzamide, salicylhydrazide, and 3-hydroxy-2-naphthamide) using the broth dilution assay described above.
Neisseria gonorrhoeae spontaneous mutation frequency.
Three N. gonorrhoeae strains (197, 202, and 206) were tested against salicylamide or rifampin for a single-step mutation assay as previously described (25). Briefly, drugs were mixed with gonococcal GC medium agar supplemented with 5% horse blood to produce a 10× MIC mixture. Plates were then prepared in triplicates and allowed to dry at room temperature. A bacterial suspension was prepared and matched to a 7 McFarland standard solution; bacterial suspension was then diluted to achieve a bacterial concentration of 1010 CFU/ml. A 10-fold serial dilution of the inoculum was plated and incubated at 37°C (with 5% CO2) for 72 h. Plates were routinely inspected at 24 h and 48 h. The spontaneous mutation frequency was calculated as described previously (25).
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We thank Haroon Mohammad for editing our manuscript.
REFERENCES
- 1.World Health Organization. 2011. Emergence of multi-drug resistant Neisseria gonorrhoeae: threat of global rise in untreatable sexually transmitted infections. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Burnett AM, Anderson CP, Zwank MD. 2012. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med 30:1114–1117. doi: 10.1016/j.ajem.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Kirkcaldy RD, Harvey A, Papp JR, Del Rio C, Soge OO, Holmes KK, Hook EW, Kubin G, Riedel S, Zenilman J, Pettus K, Sanders T, Sharpe S, Torrone E. 2016. Neisseria gonorrhoeae antimicrobial susceptibility surveillance—the gonococcal isolate surveillance project, 27 sites, United States, 2014. MMWR Surveill Summ 65:1–19. doi: 10.15585/mmwr.ss6507a1. [DOI] [PubMed] [Google Scholar]
- 5.Wise R, BSAC Working Party on The Urgent Need: Regenerating Antibacterial Drug Discovery and Development. 2011. The urgent need for new antibacterial agents. J Antimicrob Chemother 66:1939–1940. doi: 10.1093/jac/dkr261. [DOI] [PubMed] [Google Scholar]
- 6.Barbee LA. 2014. Preparing for an era of untreatable gonorrhea. Curr Opin Infect Dis 27:282. doi: 10.1097/QCO.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomquist PB, Miari VF, Biddulph JP, Charalambous B. 2014. Is gonorrhea becoming untreatable? Future Microbiol 9:189–201. doi: 10.2217/fmb.13.155. [DOI] [PubMed] [Google Scholar]
- 8.Mohammad H, AbdelKhalek A, Abutaleb NS, Seleem MN. 2018. Repurposing niclosamide for intestinal decolonization of vancomycin-resistant enterococci. Int J Antimicrob Agents 51:897–904. doi: 10.1016/j.ijantimicag.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younis W, Thangamani S, Seleem MN. 2015. Repurposing non-antimicrobial drugs and clinical molecules to treat bacterial infections. Curr Pharm Des 21:4106–4111. doi: 10.2174/1381612821666150506154434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thangamani S, Younis W, Seleem MN. 2015. Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol 6:750. doi: 10.3389/fmicb.2015.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMasi JA, Hansen RW, Grabowski HG. 2003. The price of innovation: new estimates of drug development costs. J Health Econ 22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 12.Thangamani S, Mohammad H, Younis W, Seleem MN. 2015. Drug repurposing for the treatment of staphylococcal infections. Curr Pharm Des 21:2089–2100. doi: 10.2174/1381612821666150310104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silver LL. 2011. Challenges of antibacterial discovery. Clin Microbiol Rev 24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schäberle TF, Hack IM. 2014. Overcoming the current deadlock in antibiotic research. Trends Microbiol 22:165–167. doi: 10.1016/j.tim.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 15.AbdelKhalek A, Abutaleb NS, Mohammad H, Seleem MN. 2018. Repurposing ebselen for decolonization of vancomycin-resistant enterococci (VRE). PLoS One 13:e0199710. doi: 10.1371/journal.pone.0199710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AbdelKhalek A, Abutaleb NS, Elmagarmid KA, Seleem MN. 2018. Repurposing auranofin as an intestinal decolonizing agent for vancomycin-resistant enterococci. Sci Rep 8:8353. doi: 10.1038/s41598-018-26674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thangamani S, Maland M, Mohammad H, Pascuzzi PE, Avramova L, Koehler CM, Hazbun TR, Seleem MN. 2017. Repurposing approach identifies auranofin with broad spectrum antifungal activity that targets Mia40-Erv1 pathway. Front Cell Infect Microbiol 7:4. doi: 10.3389/fcimb.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thangamani S, Mohammad H, Abushahba MF, Sobreira TJ, Seleem MN. 2016. Repurposing auranofin for the treatment of cutaneous staphylococcal infections. Int J Antimicrob Agents 47:195–201. doi: 10.1016/j.ijantimicag.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thangamani S, Younis W, Seleem MN. 2015. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep 5:11596. doi: 10.1038/srep11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unemo M. 2015. Current and future antimicrobial treatment of gonorrhoea - the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis 15:364. doi: 10.1186/s12879-015-1029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pospisilova S, Michnova H, Kauerova T, Pauk K, Kollar P, Vinsova J, Imramovsky A, Cizek A, Jampilek J. 2018. In vitro activity of salicylamide derivatives against vancomycin-resistant enterococci. Bioorg Med Chem Lett 28:2184–2188. doi: 10.1016/j.bmcl.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Chiu C-H, Lin T-Y, Ou JT. 1999. In vitro evaluation of intracellular activity of antibiotics against non-typhoid Salmonella. Int J Antimicrob Agents 12:47–52. doi: 10.1016/s0924-8579(99)00038-2. [DOI] [PubMed] [Google Scholar]
- 23.Bretschneider B, Brandsch M, Neubert R. 1999. Intestinal transport of β-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm Res 16:55–61. doi: 10.1023/a:1018814627484. [DOI] [PubMed] [Google Scholar]
- 24.Zaki NM, Hafez MM. 2012. Enhanced antibacterial effect of ceftriaxone sodium-loaded chitosan nanoparticles against intracellular Salmonella typhimurium. JAP 13:411–421. doi: 10.1208/s12249-012-9758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler MM, Waidyarachchi SL, Connolly KL, Jerse AE, Chai W, Lee RE, Kohlhoff SA, Shinabarger DL, Bowlin TL. 2018. Aminomethyl spectinomycins as therapeutics for drug-resistant gonorrhea and chlamydia coinfections. Antimicrob Agents Chemother 62:e00325-18. doi: 10.1128/AAC.00325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binet R, Maurelli AT. 2007. Frequency of development and associated physiological cost of azithromycin resistance in Chlamydia psittaci 6BC and C. trachomatis L2. Antimicrob Agents Chemother 51:4267–4275. doi: 10.1128/AAC.00962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGrath M, Gey van Pittius N, Van Helden P, Warren R, Warner DJ. 2014. Mutation rate and the emergence of drug resistance in Mycobacterium tuberculosis. J Antimicrob Chemother 69:292–302. doi: 10.1093/jac/dkt364. [DOI] [PubMed] [Google Scholar]
- 28.Björkholm B, Sjölund M, Falk PG, Berg OG, Engstrand L, Andersson DI. 2001. Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori. Proc Natl Acad Sci U S A 98:14607–14612. doi: 10.1073/pnas.241517298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Takei M, Yamaguchi Y, Fukuda H, Yasuda M, Deguchi TJ. 2005. Cultivation of Neisseria gonorrhoeae in liquid media and determination of its in vitro susceptibilities to quinolones. J Clin Microbiol 43:4321–4327. doi: 10.1128/JCM.43.9.4321-4327.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nash EE, Henning TC, Pham CD, Pettus K, Sharpe S, Kersh EN. 2019. In vitro activity of EDTA and TOL-463 against Neisseria gonorrhoeae. Diagn Microbiol Infect Dis 93:369–371. doi: 10.1016/j.diagmicrobio.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foerster S, Unemo M, Hathaway LJ, Low N, Althaus CL. 2016. Time-kill curve analysis and pharmacodynamic modelling for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol 16:216. doi: 10.1186/s12866-016-0838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu P, Wang S, Lu Y, Neculai D, Sun Q, van der Veen SJT. 2018. A subpopulation of intracellular Neisseria gonorrhoeae escapes autophagy-mediated killing inside epithelial cells. J Infect Dis 219:133–144. doi: 10.1093/infdis/jiy237. [DOI] [PubMed] [Google Scholar]
- 34.Redondo-Lopez V, Cook RL, Sobel JD. 1990. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis 12:856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 35.Lenz JD, Shirk KA, Jolicoeur A, Dillard JP. 2018. Selective inhibition of Neisseria gonorrhoeae by a dithiazoline in mixed infections with Lactobacillus gasseri. Antimicrob Agents Chemother 62:e00826-18. doi: 10.1128/AAC.00826-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foschi C, Salvo M, Cevenini R, Parolin C, Vitali B, Marangoni AJ. 2017. Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: an in vitro study. Front Cell Infect Microbiol 7:502. doi: 10.3389/fcimb.2017.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spurbeck RR, Arvidson CG. 2011. Lactobacilli at the front line of defense against vaginally acquired infections. Future Microbiol 6:567–582. doi: 10.2217/fmb.11.36. [DOI] [PubMed] [Google Scholar]
- 38.Coman M, Verdenelli M, Cecchini C, Silvi S, Orpianesi C, Caspani M, Mondello F, Cresci AJ. 2015. In vitro evaluation on HeLa cells of protective mechanisms of probiotic lactobacilli against Candida clinical isolates. J Appl Microbiol 119:1383–1390. doi: 10.1111/jam.12947. [DOI] [PubMed] [Google Scholar]
- 39.Lewis FM, Bernstein KT, Aral SO. 2017. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol 129:643–654. doi: 10.1097/AOG.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarate G, Nader-Macias M. 2006. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett Appl Microbiol 43:174–180. doi: 10.1111/j.1472-765X.2006.01934.x. [DOI] [PubMed] [Google Scholar]
- 41.Michne WF. 2010. Lead generation approaches in drug discovery. John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- 42.Mohamed MF, Hamed MI, Panitch A, Seleem MN. 2014. Targeting methicillin-resistant Staphylococcus aureus with short salt-resistant synthetic peptides. Antimicrob Agents Chemother 58:4113–4122. doi: 10.1128/AAC.02578-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammad H, Mayhoub AS, Cushman M, Seleem MN. 2015. Anti-biofilm activity and synergism of novel thiazole compounds with glycopeptide antibiotics against multidrug-resistant staphylococci. J Antibiot (Tokyo) 68:259–266. doi: 10.1038/ja.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamed MF, Hammac GK, Guptill L, Seleem MN. 2014. Antibacterial activity of novel cationic peptides against clinical isolates of multi-drug resistant Staphylococcus pseudintermedius from infected dogs. PLoS One 9:e116259. doi: 10.1371/journal.pone.0116259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meletiadis J, Pournaras S, Roilides E, Walsh TJ. 2010. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob Agents Chemother 54:602–609. doi: 10.1128/AAC.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eldesouky HE, Li X, Abutaleb NS, Mohammad H, Seleem MN. 2018. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int J Antimicrob Agents 52:754–761. doi: 10.1016/j.ijantimicag.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Fichorova RN, Desai PJ, Gibson FC, Genco CA. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 69:5840–5848. doi: 10.1128/iai.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallegol J, Fernandes P, Seah C, Guyard C, Melano RG. 2013. Determination of in vitro activities of solithromycin at different pHs and its intracellular activity against clinical isolates of Neisseria gonorrhoeae from a laboratory collection. Antimicrob Agents Chemother 57:4322–4328. doi: 10.1128/AAC.00564-13. [DOI] [PMC free article] [PubMed] [Google Scholar]