Therapeutic drug monitoring (TDM) has been recommended in guidelines for patients receiving posaconazole oral suspension, but its utility in patients receiving posaconazole tablet, which has an improved bioavailability, remains unclear.
KEYWORDS: therapeutic drug monitoring, posaconazole, decision model, hematological malignancy
ABSTRACT
Therapeutic drug monitoring (TDM) has been recommended in guidelines for patients receiving posaconazole oral suspension, but its utility in patients receiving posaconazole tablet, which has an improved bioavailability, remains unclear. We used state transition models with first-order Monte Carlo microsimulation to re-examine the posaconazole exposure-response relationships reported in two phase III clinical trials (prophylaxis with posaconazole oral suspension, models 1 and 2) and a third multicenter observational TDM study (model 3). We simulated the impact of TDM-guided interventions to improve initial average posaconazole concentrations (Cavg) to reduce clinical failure (in models 1 and 2) and breakthrough invasive fungal disease (bIFD) in model 3. Simulations were then repeated using posaconazole tablet Cavg distributions in place of the oral suspension formulation. In all three models with posaconazole oral suspension, TDM interventions associated with maximal improvement in posaconazole Cavg reduced absolute rates of subtherapeutic exposures (Cavg < 700 ng/ml) by 25% to 49%. Predicted reductions in absolute clinical failure rates were 11% in model 1 and 6.5% in model 2 and a 12.6% reduction in bIFD in model 3. With the tablet formulation, maximally effective TDM interventions reduced subtherapeutic exposures by approximately 5% in all three models and absolute clinical failure rates by 3.9% in model 1 and 1.6% in model 2; there was a 1.6% reduction in bIFD in model 3. Our modeling suggests that routine TDM during prophylaxis with posaconazole tablets may have limited clinical utility unless populations with higher prevalence (>10%) of subtherapeutic exposures can be identified based on clinical risk factors.
INTRODUCTION
Posaconazole is a broad-spectrum triazole antifungal widely used for the prophylaxis or salvage treatment of invasive fungal disease (IFD). Posaconazole was originally licensed in 2005 as an oral suspension administered three or four times daily with food (preferably a high-fat meal) to ensure adequate absorption (1–3). However, these dosing requirements can be problematic in patients undergoing treatment for hematological malignancies. In particular, chemotherapy-associated gastrointestinal dysfunction, acid suppression therapy, and poor appetite may reduce the bioavailability of posaconazole oral suspension, resulting in substantial variability in systemic drug exposures (4, 5). Given the high mortality and the adverse impact of IFDs on subsequent chemotherapy or transplantation timing (6, 7), routine therapeutic drug monitoring (TDM) has been advocated to identify patients who require either dosage adjustment or a switch to an alternative systemic antifungal agent to reduce the risk of breakthrough IFD (bIFD).
A justification for routine TDM during posaconazole prophylaxis is supported by analysis of two pivotal posaconazole oral suspension prophylaxis trials that identified a relationship between posaconazole exposures (assessed by average plasma posaconazole concentrations [Cavg]) and the probability of clinical failure defined using a five-point composite endpoint (8). In these analyses, the probability of clinical failure increased when the posaconazole plasma Cavg fell below 700 ng/ml, a target that was not achieved in nearly 50% of patients. Accordingly, a TDM-guided dosing algorithm was proposed for patients with plasma concentrations less than 700 ng/ml, where patients below this target are administered higher posaconazole doses or are switched to an alternative antifungal.
These TDM recommendations were later challenged, because the five-point composite endpoint was not part of the original trial design and no correlation was evident between posaconazole serum Cavg and the low rates of bIFD observed in the studies (9). Nevertheless, a separate open-label study of posaconazole salvage therapy for invasive aspergillosis (10) and a multicenter TDM observational study during posaconazole (with oral suspension) prophylaxis and treatment (11) both supported evidence of an exposure-response relationship for posaconazole. As a result, TDM has been “marginally” recommended for patients receiving posaconazole prophylaxis in recent treatment guidelines (7, 12) due to the lack of clear and established evidence that TDM-guided interventions reduce the risk of bIFD.
Against this backdrop, a new extended release tablet formulation of posaconazole with improved bioavailability and pharmacokinetic exposure (and later an intravenous formulation in a number of countries) was introduced in 2014. In phase III studies, the tablet formulation achieved steady-state plasma trough concentrations (Cmin) of ≥700 ng/ml in 90% of patients, with 5% of patients at <500 ng/ml and 5% of patients between 500 and 700 ng/ml (14). These results were confirmed in subsequent retrospective observational studies (15, 16). However, evidence from other observational studies suggest that subsets of patients with risk factors for poor absorption (e.g., poor appetite, proton pump inhibitors, and diarrhea) and high body mass (>90 kg) may still be at risk for low systemic exposures (17–19). Given a lack of definitive evidence about the benefits of TDM-guided interventions, it is important to identify clinical scenarios where TDM for patients receiving posaconazole prophylaxis with the tablet formulation is most likely to be useful.
We sought to explore how differences among posaconazole formulations and the prevalence of subtherapeutic exposures may influence the effectiveness of TDM for patients receiving posaconazole prophylaxis. We developed state transition models with first-order Monte Carlo microsimulation to re-examine posaconazole exposure-response outcomes observed in the original pivotal phase III studies of posaconazole prophylaxis (8) as well as in a retrospective multicenter observational study of posaconazole TDM (11). We then incorporated theoretical TDM-guided interventions with varied effectiveness at improving posaconazole exposures (average concentrations [Cavg]) to explore how routine TDM might impact the probability of composite clinical failure or bIFD in patients receiving the oral suspension and the tablet formulations.
RESULTS
Impact of TDM on clinical failure of posaconazole for IFD prophylaxis during acute myelogenous leukemia or myelodysplastic syndrome induction chemotherapy.
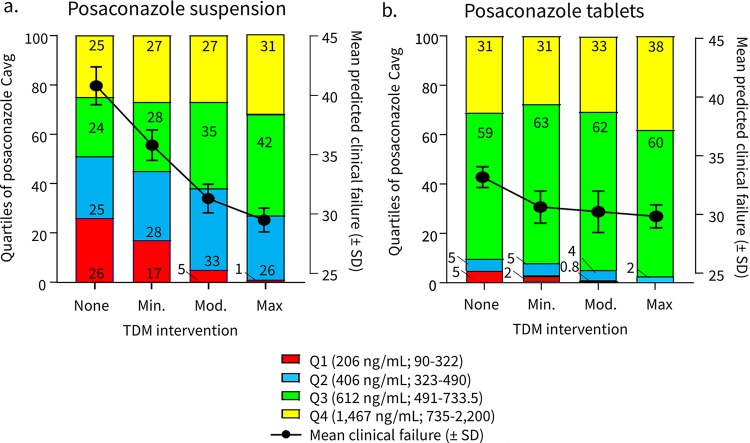
In model 1, a 10,000-patient simulation performed without TDM-guided interventions predicted that 50.3% of patients would remain in the bottom two quartiles of posaconazole exposure (Cavg < 490 ng/ml) with an overall mean clinical failure of 41.2% ± 1.6% (Fig. 1a). This is qualitatively similar to the 49.8% of patients reported with posaconazole Cavg of <490 ng/ml and the 41.4% composite failure rate reported in the prior analysis by Jang et al. (8). To explore the potential impact of TDM-guided interventions, we then simulated three levels of improvement in posaconazole Cavg following TDM screening in subsequent models (Table 1). Minimally, moderately, and maximally effective TDM-guided interventions were set to reduce the percentage of patients remaining in the lowest quartile of posaconazole exposure from 25% to 15%, 5%, and 0%, respectively. However, during modeling, we also assumed that the probability of an intervention triggered by TDM would decrease with increasing initial posaconazole Cavg, with 100% of patients having an intervention at the lowest exposures (quartile 1), 80% of patients at quartile 2, 50% of patients at quartile 3, and 0% undergoing a TDM-guided intervention at the highest posaconazole exposure (quartile 4). Based on these assumptions, simulation at the lowest level of TDM effectiveness reduced the number of patients in the lowest two quartiles of posaconazole exposure from 50.3% to 45% and the predicted mean clinical failure rate from 41.2% ± 1.6% to 36.5% ± 1.3% (Fig. 1a). Moderately and highly effective TDM interventions reduced the percentages of patients in the lowest two quartiles of posaconazole exposures (Cavg < 612 ng/ml) by 12% and 23.3%, respectively, with corresponding decreases in the mean clinical failure rates to 34.6% and 30.2%, respectively. Hence, a maximally effective TDM-triggered intervention was associated with an 11% absolute reduction in clinical failure rates among patients receiving posaconazole suspension in model 1.
FIG 1.
Model 1-predicted effect of TDM in patients undergoing remission-induction chemotherapy for AML/MDS. The effects of TDM-guided interventions with varied effectiveness are shown for quartiles of systemic posaconazole exposures (stacked bar graphs, left y axis) versus the average predicted probability ± standard deviation of clinical failure (plotted lines, right y axis) in patients receiving posaconazole suspension (a) or the extended-release tablet formulation (b).
TABLE 1.
Initial model assumptions on effectiveness of TDM interventions in AML/MDS population in model 1
| Quartile | Posaconazole exposure (ng/ml) (median [range]) | % of patients remaining in quartile after TDM intervention |
|||
|---|---|---|---|---|---|
| No TDM | Minimally effective | Moderately effective | Maximally effective | ||
| Oral suspension | |||||
| Quartile 1 | 206 (90–322) | 25 | 15 | 5 | 0 |
| Quartile 2 | 406 (323–90) | 25 | 30 | 25 | 15 |
| Quartile 3 | 612 (491–734) | 25 | 30 | 40 | 55 |
| Quartile 4 | 1,467 (735–2,200) | 25 | 25 | 30 | 30 |
| Tablet | |||||
| Quartile 1 | 206 (90–322) | 5 | 2.5 | 0 | 0 |
| Quartile 2 | 406 (323–490) | 5 | 7.5 | 5 | 0 |
| Quartile 3 | 612 (491–734) | 60 | 60 | 65 | 60 |
| Quartile 4 | 1,467 (735–2200) | 30 | 30 | 30 | 40 |
When posaconazole tablet Cavg distributions were substituted in the model for the oral suspension (Fig. 1b), the percentage of patients with Cavg of <612 ng/ml was reduced from 10% with no TDM to 2% with maximally effective TDM-guided interventions. Therefore, a maximally effective TDM-triggered intervention was associated with a 3.4% absolute reduction in clinical failure among patients receiving posaconazole tablets in model 1.
Impact of TDM on clinical failure of posaconazole for IFD prophylaxis during GVHD.
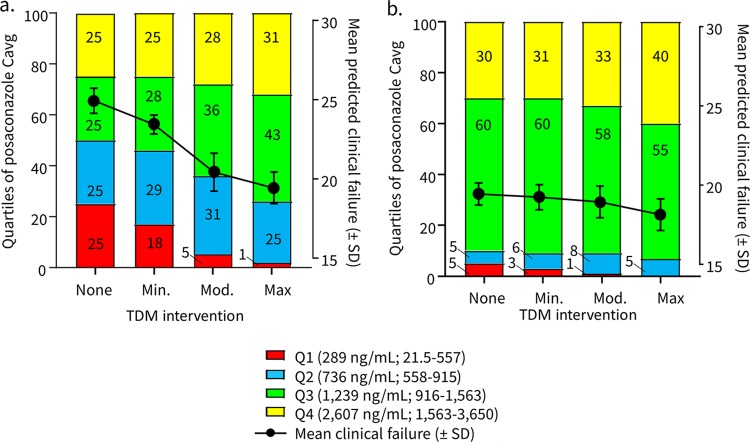
In model 2, a 10,000-patient simulation was performed without TDM of patients receiving posaconazole prophylaxis with the oral suspension formulation during immunosuppressive therapy for graft versus host disease (GVHD). For patients who did not undergo TDM, 50% of patients remained in the bottom two quartiles of posaconazole exposures with a predicted clinical failure rate of 25.0% ± 0.8% (Fig. 2b). Minimally effective TDM-guided interventions reduced the lowest two quartiles of posaconazole exposures (<915 ng/ml) from 50% to 46% and the mean clinical failure to 23.5% ± 0.6%. Highly effective TDM interventions reduced the percentage of patients in the lowest two quartiles of posaconazole Cavg to 26% with corresponding estimated clinical failure rate of 19.5% ± 1.3%. Hence, a maximally effective TDM-triggered intervention was associated with a 6.5% absolute reduction of clinical failure among patients receiving posaconazole suspension in model 2.
FIG 2.
Model 2-predicted effects of TDM in patients receiving posaconazole prophylaxis during GVHD. The effects of TDM-guided interventions with varied effectiveness are shown for quartiles of systemic posaconazole exposures (stacked bar graphs, left y axis) versus the average predicted probability ± standard deviation of clinical failure (plotted lines, right y axis) in patients receiving posaconazole suspension (a) or the extended-release tablet formulation (b).
When posaconazole tablet Cavg were substituted for the oral suspension in the model, the percentage of patients with Cavg of <915 ng/ml was reduced from 10% with no TDM to 5% with a maximally effective TDM-guided intervention. These pharmacokinetic changes were associated with a 1.3% absolute reduction in clinical failure rates among patients receiving posaconazole tablets.
Impact of TDM on bIFD during posaconazole prophylaxis.
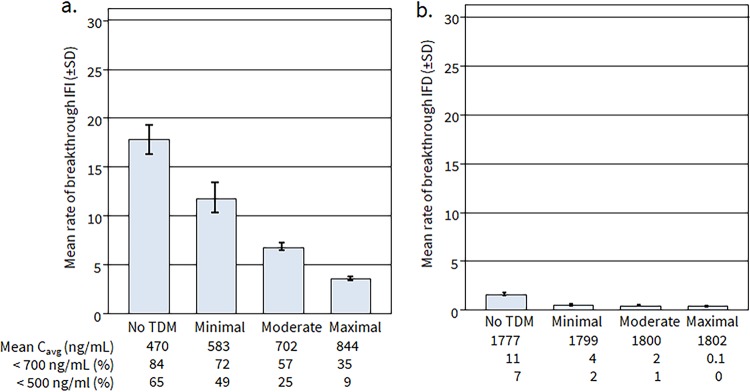
Without TDM-guided interventions, our simulations indicated that patients receiving posaconazole suspension would exhibit a mean rate of bIFD of 17.1% ± 1.3% and posaconazole Cavg of 470 ng/ml, with 84% and 65% of patients below the 700 ng/ml and 500 mg/ml targets, respectively, similar to the results of the TDM study used to parametrize the model (Fig. 3a). With minimally effective TDM-based intervention, the percentages of patients below 700 and 500 ng/ml targets were reduced to 72% and 49%, respectively, with associated rates of bIFD reduced to 12.6% ± 1.4%. With moderately and maximally effective TDM intervention, percentages of patients remaining below 700 and 500 ng/ml thresholds were reduced to 57% and 25%, respectively, and 35% and 9%, respectively, with a corresponding estimated rate of bIFD of 4.4% ± 0.02%. Hence, a maximally effective TDM-triggered intervention in model 3 resulted in 12.7% absolute reduction of bIFD among patients receiving posaconazole suspension.
FIG 3.
Model-predicted effects of TDM-guided interventions in patients receiving posaconazole prophylaxis with suspension (a) and tablet formulations (b) using real-life observational data.
When posaconazole tablet Cavg distributions were substituted in the model for the oral suspension (Fig. 3b), the percentages of patients below the target posaconazole Cavg thresholds of 700 and 500 ng/ml who did not undergo TDM decreased to 10.5% and 7%, respectively, with an estimated bIFD rate of 2.1% ± 0.2%. With minimally effective TDM-based intervention, the percentages of patients below 700 and 500 ng/ml targets were reduced to 3.6% and 2.1%, respectively, with predicted rates of bIFD reduced to 0.8% ± 0.05%. With maximally effective TDM intervention, the percentages of patients below 700 and 500 ng/ml were further reduced to 2% and 1%, respectively, with an estimated bIFD of 0.4% ± 0.04% or a 1.6% absolute decrease in bIFD among patients receiving posaconazole tablets.
Sensitivity analysis for models 1 and 2.
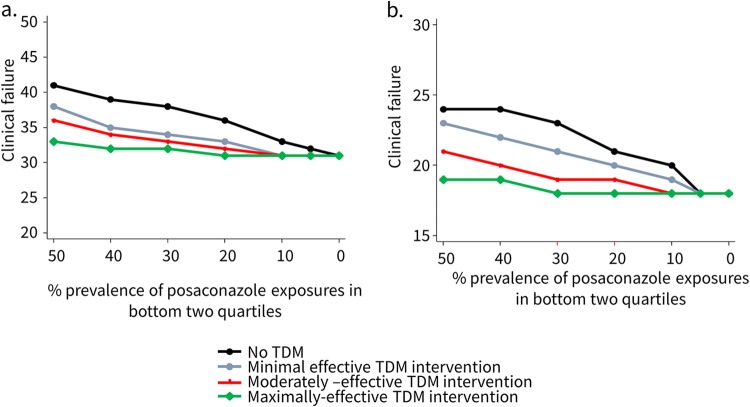
In a sensitivity analysis, performed for models 1 and 2, varying the rates of posaconazole Cavg in the bottom two quartiles of exposure from 0% to 50% for both acute myelogenous leukemia or myelodysplastic syndrome (AML/MDS) and acute GVHD (aGVHD)/allogeneic hematopoietic stem cell transplantation (allo-HSCT) populations (Fig. 4) resulted in a diminishing overall TDM effectiveness as the prevalence of posaconazole “subtherapeutic” exposures decreased, with clinical failure rates largely converging irrespective of TDM-guided intervention effectiveness at a prevalence of less than 10%. We also separately explored how changes in intervention rates (0% to 100%) following a TDM result could affect predicted rates of clinical failure at each quartile of posaconazole exposure (data not shown). Across all three models, higher intervention rates were associated with reduced rates of clinical failure in patients at the lowest posaconazole exposures (quartile 1), with less impact in patients in quartile 2, and minimal impact on model-predicted clinical failure for patients in quartiles 3 and 4.
FIG 4.
Sensitivity analysis of mean clinical response rates per prevalence of subtherapeutic exposures for both AML/MDS (model 1) (a) and GVHD (b).
DISCUSSION
No prospective randomized study has evaluated the effectiveness of TDM-guided interventions in patients receiving posaconazole prophylaxis (5, 20). We used state transition models based on individual patient-level Monte Carlo simulation to re-examine outcomes related to posaconazole exposures that were reported in pivotal clinical trials and a retrospective, multicenter observational study. We then modeled interventions of varied effectiveness based on hypothetical TDM-guided interventions and compared how changes in posaconazole exposures achieved with the oral suspension and tablet formulations influenced the TDM effectiveness with respect to clinical failure and bIFD.
The main finding from our modeling is that TDM-guided interventions demonstrated clinical benefit in reducing clinical failure rates or bIFD provided they were applied in a population with sufficiently high pretest probability of subtherapeutic exposures. In the two models based on the analysis of phase III trials of posaconazole oral suspension (8), this inflection point occurred when the prevalence of patients in the bottom two quartiles of posaconazole Cavg exceeded 10%, with greater impact on reducing clinical failure rates when the prevalence exceeded 30%. When the prevalence approaches 50%, as reported in patients with hematological malignancies receiving posaconazole oral suspension, maximally effective TDM-guided interventions reduced the absolute risk of clinical failure by 11% in patients with AML/MDS and 6.5% in patients with GVHD. Hence, averages of 18 (AML/MDS) and 36 (GVHD) patients receiving posaconazole oral suspension would need to be screened by TDM at this prevalence threshold to potentially detect/prevent one clinical failure. However, if the prevalence of subtherapeutic posaconazole exposures approaches 10% (as reported in the phase III studies with the tablet formulation), the numbers needed to screen would increase to 90 (AML/MDS) and 182 (GVHD) patients assuming maximally effective TDM-guided interventions.
Although model 3 was based on retrospective “real-life” data and was developed using different methodology and study endpoints, the findings were qualitatively similar to those of model 1 and model 2. At maximal TDM-guided intervention effectiveness, the percentage of patients below posaconazole Cavg of 700 ng/ml was reduced by 49% and the absolute rate of bIFD was reduced by 12.6%. However, when posaconazole Cavg distributions achieved with the tablet were substituted in the model, the absolute difference in the rates of bIFD between non-TDM versus maximally effective TDM-guided interventions was only 1.5%. Hence, if the prevalence of subtherapeutic posaconazole exposures in patients receiving prophylaxis with posaconazole tablets was less than 10%, nearly 667 patients would need to undergo TDM screening to potentially prevent 1 case of bIFD.
The prevalence of patients with posaconazole Cavg or trough concentrations (Cmin) of <700 ng/ml reported in observational TDM studies with posaconazole tablets varies widely. A number of early observational studies reported that between 0% and 10% of patients did not achieve Cavg or Cmin above 700 ng/ml (21–29), similar to the phase III posaconazole registration trials (14, 30). Other studies have reported rates of posaconazole Cmin below 700 ng/ml, ranging from 10% to 20% of patients (16, 19, 31, 32). One study reported that nearly one-third of patients with hematological malignancies receiving the tablet formulation did not achieve target exposures (17). Diarrhea and weight (>90 kg) were associated with a lower probability of achieving posaconazole Cmin of >700 ng/ml in one observational study (17). Notably, rates of bIFD, which were reported in six studies, ranged between 0% and 6.75%. However, these bIFD rates were not correlated with posaconazole plasma exposures. This is not surprising, as our models suggested that more than 600 patients may need to be monitored to identify a few patients with clinical failures linked to subtherapeutic posaconazole exposures. These findings indicate that rational selective TDM strategies (33), which target subpopulations at higher risk for subtherapeutic exposures (>30% risk), may be of benefit for such monitoring to be clinically useful in patients receiving posaconazole tablets.
As with almost all decision analytic modeling studies, there are potential limitations with our analysis. First, several components of the composite endpoint for clinical failure of the posaconazole suspension (i.e., deaths from all causes, discontinuation of study drugs during the primary time period, patients lost to follow-up) derived from the study by Jang et al. (8) have no direct link to posaconazole plasma concentrations and likely represent an overestimation of the negative impact of low posaconazole serum concentrations (9). Second, to simplify the modeling process, we assumed that patients who do not undergo TDM would not have improvements in posaconazole Cavg before the transition probability to clinical failure or success was calculated. Similarly, we assumed that patients undergoing a TDM-guided intervention would not transition to lower posaconazole Cavg following an intervention. These assumptions may potentially bias the model results in favor of showing a benefit with TDM. These decisions were undertaken to reduce model complexity or “transition state explosion” and aid model symmetry (34). Our modeling strategies and ability to introduce additional levels of uncertainty or variation in the posaconazole exposure-response relationships analyzed in the model were also limited by the available (published) data. Third, we made assumptions about the effectiveness of TDM-guided interventions in the absence of published data regarding TDM “efficacy” or typical TDM-triggered clinical intervention rates during posaconazole prophylaxis. To address some of the uncertainly, we compared various degrees of effectiveness and intervention rates over a range of prevalence of subtherapeutic posaconazole exposures. Therefore, we do not believe overall results will change if model assumptions are realistically altered.
Finally, it is important to highlight that our analysis specifically focused on the impact of TDM in patients receiving posaconazole for IFD prophylaxis with the oral suspension or tablet formulation. In settings which were not considered in our present analysis, such as documented fungal disease, suspected clinical failure, unexplained toxicity, or drug interactions, the rationale for TDM may differ.
Notwithstanding the caveats, we have demonstrated that state transition models based on individual patient-level Monte Carlo simulation can be used to re-examine the impact of TDM-guided interventions, with posaconazole oral suspension and tablet formulations, on clinical failure and bIFD reported in pivotal clinical trials and a retrospective observational study. Through our modeling, we also shed light on the effectiveness of TDM when the prevalence of subtherapeutic exposures varies. Our models suggest that at a high prevalence of subtherapeutic exposures (>30%), routine TDM could potentially reduce the absolute risk of clinical failure or bIFD by 5% to 12% in patients receiving posaconazole prophylaxis depending on the effectiveness of TDM-triggered interventions. However, at low prevalence of subtherapeutic exposures typically observed with posaconazole tablets (<10%), routine TDM during prophylaxis would only marginally reduce the absolute risk of clinical failure and bIFD unless such testing could be targeted to patients with clinical risk factors that predispose them to a higher prevalence of subtherapeutic exposures. We believe these findings can aid the design of future prospective studies and also inform the weighting of potential cost vis-à-vis benefits of routine TDM for patients receiving posaconazole prophylaxis.
MATERIALS AND METHODS
Overview of data sources.
Model 1 was based on exposure-response analysis performed by Jang et al. (8) for a phase III trial of posaconazole oral suspension prophylaxis in patients undergoing remission-induction chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome (AML/MDS) (35). Plasma posaconazole concentrations were measured 1 and 3 h after the first dose on day 8 and during the first episode of evaluation for a possible bIFD. An average posaconazole plasma concentration (Cavg) was then calculated for each patient based on the fact that plasma concentrations of posaconazole are relatively constant at steady state (achieved at 7 to 10 days) (36). The exposure-response relationship was analyzed by a logistic regression using Cavg as a continuous variable and the clinical failure as binary variable (yes or no) defined to meet one of any of the following five endpoints: (i) the occurrence of proven or probable IFDs, (ii) the need to administer >5 days of empirical treatment with a systemic antifungal drug other than the study drug during the primary time period, (iii) deaths from all causes, (iv) discontinuation of study drugs during the primary time period, i.e., the subjects were not followed for the period’s entire duration, or (v) patients lost to follow-up. The overall rate of clinical failure was 41.5%.
Model 2 was based on the analysis by Jang et al. (8) of a second phase III trial of posaconazole oral suspension prophylaxis for patients receiving immunosuppressive therapy for acute graft versus host disease (aGVHD) following allogeneic hematopoietic stem cell transplantation (HSCT) (37). In both studies, patient posaconazole Cavg were calculated from multiple sampling time points. In particular, plasma posaconazole concentrations were measured after dosing on day 2, at weeks 2, 4, 8, and 12, and either on the last day of treatment or at week 16 (often one sampling per visit), and patients were divided into four quartile ranges of drug exposure. Although the distribution of posaconazole Cavg and rates of clinical failure differed between the two trials (Table 2), the probability of composite clinical failure was estimated by logistic regression and reported for each quartile (Fig. 5a).
TABLE 2.
Relationship between quartiles of posaconazole Cavg and probability of clinical failure in models 1 and 2
| Quartile | Posaconazole exposure (ng/ml) (median [range])a | Probability of transition to clinical failure (%) |
|---|---|---|
| Model 1: Prophylaxis during AML/MDS induction chemotherapy (n = 215 patients) |
||
| Quartile 1 | 206 (90–322) | 55 |
| Quartile 2 | 736 (558–915) | 37b |
| Quartile 3 | 1,239 (915–1,563) | 33 |
| Quartile 4 | 2,607 (1,564–3,650) | 28 |
| Model 2: Prophylaxis during GVHD (n = 252 patients) |
||
| Quartile 1 | 289 (21.5–557) | 44 |
| Quartile 2 | 406 (323–490) | 21 |
| Quartile 3 | 612 (491–733) | 18 |
| Quartile 4 | 1,467 (734–2,200) | 18 |
Distributions of posaconazole plasma concentrations in both studies were derived from pharmacokinetic/pharmacodynamic analysis by Jang et al. (8).
Calculated from logistic regression results.
FIG 5.
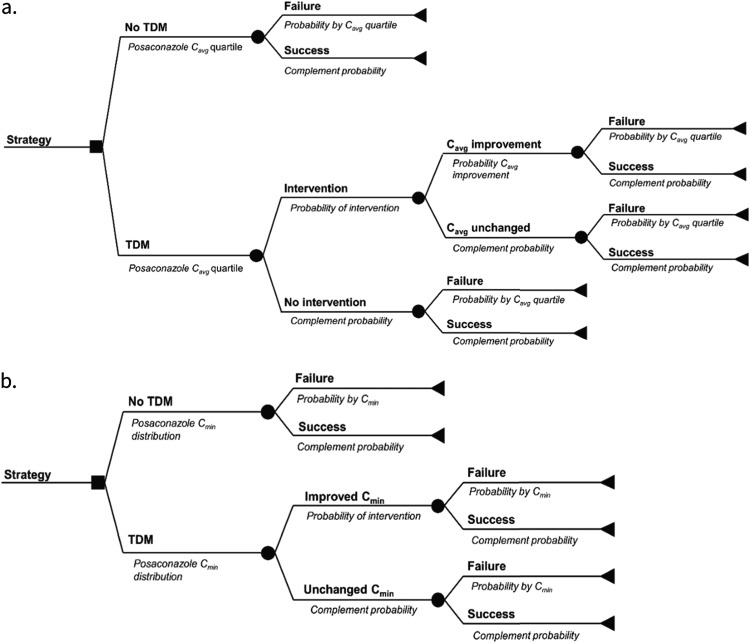
General model structure for TDM trial simulations. (a) Decision analytical model based on phase III clinical trials of posaconazole prophylaxis in patients with AML/MDS (model 1) (35) or GVHD (model 2) (37). (b) Decision analytical model based on a prospective multicenter observational study of posaconazole TDM (model 3) (11). Details on the models are provided in Materials and Methods.
Model 3 was based on retrospective multicenter study of posaconazole TDM performed in six Australian hospitals from 2008 to 2010 (11). In this study, 84% of patients exhibited median posaconazole concentrations lower than 700 ng/ml (Fig. 5b). A subset of adult patients 18 years or older receiving posaconazole prophylaxis (n = 72) with the oral suspension formulation (200 mg three to four times daily) who had at least one steady-state posaconazole concentration were analyzed for a risk of bIFD defined according to the 2008 guidelines from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group (38).
Modeling.
Three separate models were developed to correspond to the three sources of posaconazole exposure-response data (8). Specifically, state transition first-order Monte Carlo individual-based microsimulation models were developed to simulate the posaconazole exposure-response relationships in two phase III clinical trials and a third multicenter observational TDM study using Tree Age Pro Healthcare 2019, R1 software (Tree Age Software, Williamston, MA). We followed the principles outlines by the ISPOR-SMDM Modeling Task Force (39). The principal strategy compared in each model was the effect of TDM-guided intervention(s) on rates of clinical failure during posaconazole prophylaxis during induction chemotherapy for AML/MDS (model 1) or with immunosuppressive therapy for GVHD (model 2). In both models 1 and 2, simulated patients enter the model and are assigned to a TDM strategy (yes/no) (Fig. 5a). The patient is then randomly assigned to a quartile of posaconazole Cavg based on the probability distributions reported by Jang et al. (8) for their respective patient population. For patients who do not undergo TDM, the probability of transitioning to clinical failure was then calculated based on the relationship of posaconazole Cavg and clinical failure defined by a logistic regression analysis (Table 2). As each patient passes through the model, trackers are used after each branch to count the fraction of patients who remain or transition to a higher posaconazole Cavg quartile and ultimately transition to either clinical success or failure before exiting the model.
For patients assigned to the TDM arm, the initial quartile of posaconazole Cavg was assigned randomly as described above but TDM was assumed to trigger a nonspecified intervention (e.g., dose increase, optimization of gastrointestinal [GI] absorption parameters, discontinuation of interacting drug, etc.) that is associated with a given probability of transitioning to a higher Cavg quartile. We also assumed that TDM-triggered interventions would only improve posaconazole Cavg based on prespecified categories of intervention effectiveness and that the probability of an intervention to improve posaconazole Cavg would vary depending on the initial quartile of posaconazole Cavg identified through TDM. For purposes of mode development, we empirically specified that 100% of patients at the lowest quartile of posaconazole exposures progressed to TDM-triggered intervention versus 80% in patients in quartile 2, 50% in quartile 3, and 0% in quartile 4.
No prospective data describing the effectiveness of TDM-guided interventions for posaconazole are available. We therefore analyzed both models 1 and 2 using three different scenarios of TDM-triggered effectiveness (Tables 1 and 3): (i) a minimally effective intervention where the percentage of patients in the lowest posaconazole Cavg quartile is reduced from 25% to 15%; (ii) moderately effective intervention where the percentage of patients in the lowest posaconazole Cavg quartile is reduced from 25% to 5%; and (iii) a maximally effective intervention with no patients remaining in the lowest posaconazole Cavg quartile.
TABLE 3.
Initial assumptions on effectiveness of TDM interventions in GVHD population for model 2
| Quartile | Posaconazole exposure (ng/ml) (median [range]) | % of patients remaining in quartiles of posaconazole exposure after TDM intervention |
|||
|---|---|---|---|---|---|
| No TDMa | Minimally effective | Moderately effective | Maximally effective | ||
| Oral suspension | |||||
| Quartile 1 | 289 (21.5–557) | 25 | 15 | 5 | 0 |
| Quartile 2 | 736 (557–915) | 25 | 30 | 25 | 15 |
| Quartile 3 | 1,239 (915–1,563) | 25 | 30 | 40 | 55 |
| Quartile 4 | 2,607 (1,563–3,650) | 25 | 25 | 30 | 30 |
| Tablet | |||||
| Quartile 1 | 289 (21.5–557) | 5 | 2.5 | 0 | 0 |
| Quartile 2 | 736 (557–915) | 5 | 7.5 | 5 | 0 |
| Quartile 3 | 1,239 (915–1,563) | 60 | 60 | 65 | 60 |
| Quartile 4 | 2,607 (1,563–3,650) | 30 | 30 | 30 | 40 |
To explore how substitution of the delayed-release tablet formulation would influence the effectiveness of TDM-guided interventions defined above, microsimulations were repeated as described above except that the posaconazole Cavg distribution for the tablet formulation (14) was substituted for posaconazole oral suspension Cavg.
Model 3 was constructed using median posaconazole oral suspension pharmacokinetic data (11). A data set of posaconazole (here reported as Cavg for consistency) was generated by digitized analysis of the original manuscript figure (DigitizeIt, Braunschweig, Germany) and fit to a model with a log-normal distribution. As each simulated patient entered the model, they were assigned a specific TDM strategy (Fig. 5b) and initially randomly sampled based on a log normal distribution of posaconazole Cavg (11). In patients who do not undergo TDM, the transition probability of progressing to bIFD was defined by the logit function −0.005χ + 0.421, where χ is the initially assigned posaconazole concentration (ng/ml). Predicted probabilities are then calculated from the natural antilog of the logit.
In patients who undergo TDM, an intervention was triggered if the randomly selected posaconazole Cavg was less than 700 ng/ml. Minimally, moderately, and maximally effective TDM interventions were then simulated by changes in the log normal distribution of posaconazole Cavg after interventions that were randomly resampled to arrive at a new posaconazole Cavg (Table 4). As each patient passed through the model, trackers were similarly used to count the percentages of patients with posaconazole Cavg of <500 and <700 ng/ml who arrived at the state of bIFD. A 10,000-patient first-order Monte Carlo microsimulation in the non-TDM arm of the model resulted in posaconazole Cavg distribution and rate of bIFD (17%) analogous to that reported in 72 patients by Dolton et al. (11).
TABLE 4.
Posaconazole serum distributions utilized for model 3
| Assumed effectiveness | Posaconazole Cavg (ng/ml) |
|||
|---|---|---|---|---|
| Mean | Percentile |
|||
| 2.5 | 10 | 90 | ||
| Oral suspension | ||||
| No TDM | 467 | 136 | 196 | 812 |
| Minimally effective | 579 | 169 | 251 | 1,007 |
| Moderately effective | 698 | 304 | 398 | 1,059 |
| Maximally effective | 851 | 407 | 512 | 1,245 |
| Tablet | ||||
| No TDM | 1,773 | 507 | 732 | 3,110 |
| Minimally effective | 1,822 | 644 | 872 | 3,113 |
| Moderately effective | 1,777 | 766 | 986 | 3,296 |
| Maximally effective | 1,792 | 1,046 | 1,233 | 2,381 |
To explore how the use of the delayed-release tablet formulation would influence the effectiveness of TDM-guided interventions in model 2, we repeated the simulations as described above except that the log normal distribution of posaconazole Cavg observed with the oral suspension formulation was replaced with a Cavg distribution reported in phase III studies representative of the tablet formulation (Table 4) (14).
Sensitivity analysis.
Sensitivity analyses were performed over a range of AML/MDS (model 1) and GVHD (model 2) patients with various prevalences (0% to 50%) of posaconazole Cavg in the bottom two quartiles of exposure at each of the three levels of TDM-guided intervention effectiveness. Results were then plotted to identify a possible inflection point of subtherapeutic prevalence where TDM-guided interventions converge. A one-way sensitivity analysis was also performed separately at each quartile of posaconazole exposure to examine how changes in intervention rates (0% to 100%) following a TDM result could independently affect predicted rates of clinical failure. For each model, 10,000-patient first-order Monte Carlo microsimulations were repeated ten times to assess the stability of results. Aggregate results were then presented as mean rates of clinical failure or bIFD with standard deviations.
ACKNOWLEDGMENTS
Financial support for this study was provided by Merck & Co., Inc. (Kenilworth, NJ). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the report.
R.E.L. and P.V. are employees of the University of Bologna (Bologna, Italy); D.P.K. is an employee of the University of Texas M.D Anderson Cancer Center (Houston, TX, USA); and E.M.S. is an employee of Merck & Co. Inc. (Kenilworth, NJ, USA). The University of Bologna was paid by Merck & Co. for study design, execution, analysis, and manuscript development. E.M.S. reports no other conflicts of interest from Merck & Co., Inc. during the conduct of the study. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
- 1.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother 53:958–966. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoetic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627–1636. doi: 10.1592/phco.27.12.1627. [DOI] [PubMed] [Google Scholar]
- 3.Ezzet F, Wexler D, Courtney R, Krishna G, Lim J, Laughlin M. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin Pharmacokinet 44:211–220. doi: 10.2165/00003088-200544020-00006. [DOI] [PubMed] [Google Scholar]
- 4.Vanstraelen K, Prattes J, Maertens J, Lagrou K, Schoemans H, Peersman N, Vermeersch P, Theunissen K, Mols R, Augustijns P, Annaert P, Hoenigl M, Spriet I. 2016. Posaconazole plasma exposure correlated to intestinal mucositis in allogeneic stem cell transplant patients. Eur J Clin Pharmacol 72:953–963. doi: 10.1007/s00228-016-2057-6. [DOI] [PubMed] [Google Scholar]
- 5.Dekkers BGJ, Bakker M, van der Elst KCM, Sturkenboom MGG, Veringa A, Span LFR, Alffenaar JC. 2016. Therapeutic drug monitoring of posaconazole: an update. Curr Fungal Infect Rep 10:51–61. doi: 10.1007/s12281-016-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girmenia C, Micozzi A, Piciocchi A, Gentile G, Di Caprio L, Nasso D, Minotti C, Capria S, Cartoni C, Alimena G, Meloni G, Amadori S, Foa R, Venditti A. 2014. Invasive fungal diseases during first induction chemotherapy affect complete remission achievement and long-term survival of patients with acute myeloid leukemia. Leuk Res 38:469–474. doi: 10.1016/j.leukres.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang SH, Colangelo PM, Gobburu JV. 2010. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther 88:115–119. doi: 10.1038/clpt.2010.64. [DOI] [PubMed] [Google Scholar]
- 9.Cornely OA, Ullmann AJ. 2011. Lack of evidence for exposure-response relationship in the use of posaconazole as prophylaxis against invasive fungal infections. Clin Pharmacol Ther 89:351–352. doi: 10.1038/clpt.2010.261. [DOI] [PubMed] [Google Scholar]
- 10.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 11.Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. 2012. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother 56:5503–5510. doi: 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Brüggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux J-P, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Löffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinkó J, Skiada A, et al. 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24 Suppl 1:e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Cornely OA, Duarte RF, Haider S, Chandrasekar P, Helfgott D, Jimenez JL, Candoni A, Raad I, Laverdiere M, Langston A, Kartsonis N, Van Iersel M, Connelly N, Waskin H. 2016. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother 71:1747. doi: 10.1093/jac/dkw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung DS, Tverdek FP, Kontoyiannis DP. 2014. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother 58:6993–6995. doi: 10.1128/AAC.04035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tverdek FP, Heo ST, Aitken SL, Granwehr B, Kontoyiannis DP. 2017. Real-life assessment of the safety and effectiveness of the new tablet and intravenous formulations of posaconazole in the prophylaxis of invasive fungal infections via analysis of 343 courses. Antimicrob Agents Chemother 61:e00188-17. doi: 10.1128/AAC.00188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. 2015. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses 58:432–436. doi: 10.1111/myc.12339. [DOI] [PubMed] [Google Scholar]
- 18.Pettit NN, Miceli MH, Rivera CG, Narayanan PP, Perissinotti AJ, Hsu M, Delacruz J, Gedrimaite Z, Han Z, Steinbeck J, Pisano J, Seo SK, Paskovaty A. 2017. Multicentre study of posaconazole delayed-release tablet serum level and association with hepatotoxicity and QTc prolongation. J Antimicrob Chemother 72:2355–2358. doi: 10.1093/jac/dkx122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang LA, Marini BL, Benitez L, Nagel JL, Miceli M, Berglund C, Perissinotti AJ. 2017. Risk factors for subtherapeutic levels of posaconazole tablet. J Antimicrob Chemother 72:2902–2905. doi: 10.1093/jac/dkx228. [DOI] [PubMed] [Google Scholar]
- 20.Dolton MJ, Ray JE, Marriott D, McLachlan AJ. 2012. Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob Agents Chemother 56:2806–2813. doi: 10.1128/AAC.05900-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cumpston A, Caddell R, Shillingburg A, Lu X, Wen S, Hamadani M, Craig M, Kanate AS. 2015. Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies. Antimicrob Agents Chemother 59:4424–4428. doi: 10.1128/AAC.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durani U, Tosh PK, Barreto JN, Estes LL, Jannetto PJ, Tande AJ. 2015. Retrospective comparison of posaconazole levels in patients taking the delayed-release tablet versus the oral suspension. Antimicrob Agents Chemother 59:4914–4918. doi: 10.1128/AAC.00496-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong W, Haywood P, Shanmuganathan N, Lindsay J, Urbancic K, Ananda-Rajah MR, Chen SC, Bajel A, Ritchie D, Grigg A, Seymour JF, Peleg AY, Kong DC, Slavin MA. 2016. Safety, clinical effectiveness and trough plasma concentrations of intravenous posaconazole in patients with haematological malignancies and/or undergoing allogeneic haematopoietic stem cell transplantation: off-trial experience. J Antimicrob Chemother 71:3540–3547. doi: 10.1093/jac/dkw322. [DOI] [PubMed] [Google Scholar]
- 24.Welch S, Pallotta A, Weber C, Siebenaller C, Cober E, Neuner E. 2017. Comparison of serum concentrations between different dosing strategies of posaconazole delayed-release tablet at a large academic medical centre. Mycoses 60:241–243. doi: 10.1111/myc.12587. [DOI] [PubMed] [Google Scholar]
- 25.Pham AN, Bubalo JS, Lewis JS II.. 2016. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses 59:226–233. doi: 10.1111/myc.12452. [DOI] [PubMed] [Google Scholar]
- 26.Chin A, Pergam SA, Fredricks DN, Hoofnagle AN, Baker KK, Jain R. 2017. Evaluation of posaconazole serum concentrations from delayed-release tablets in patients at high risk for fungal infections. Antimicrob Agents Chemother 61:e00569-17. doi: 10.1128/AAC.00569-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh HJ, Kim I, Cho JY, Park SI, Yoon SH, Lee JO, Koh Y, Song KH, Choe PG, Yu KS, Kim ES, Kim HB, Bang SM, Kim NJ, Song SH, Park WB, Oh MD. 2017. Comparison of plasma concentrations of posaconazole with the oral suspension and tablet in Korean patients with hematologic malignancies. Infect Chemother 49:135–139. doi: 10.3947/ic.2017.49.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belling M, Kanate AS, Shillingburg A, Lu X, Wen S, Shah N, Craig M, Cumpston A. 2017. Evaluation of serum posaconazole concentrations in patients with hematological malignancies receiving posaconazole suspension compared to the delayed-release tablet formulation. Leuk Res Treatment 2017:3460892. doi: 10.1155/2017/3460892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebenstein TK, Widmer KM, Fallon MJ. 2018. Retrospective analysis of goal drug level attainment of posaconazole for invasive fungal infection prophylaxis in patients with acute myeloid leukemia pre- and post-switch to tablet formulation. J Oncol Pharm Pract 24:599–603. doi: 10.1177/1078155217722405. [DOI] [PubMed] [Google Scholar]
- 30.Cornely OA, Robertson MN, Haider S, Grigg A, Geddes M, Aoun M, Heinz WJ, Raad I, Schanz U, Meyer RG, Hammond SP, Mullane KM, Ostermann H, Ullmann AJ, Zimmerli S, Van Iersel M, Hepler DA, Waskin H, Kartsonis NA, Maertens J. 2017. Pharmacokinetics and safety results from the phase 3 randomized, open-label, study of intravenous posaconazole in patients at risk of invasive fungal disease. J Antimicrob Chemother 72:3406–3413. doi: 10.1093/jac/dkx263. [DOI] [PubMed] [Google Scholar]
- 31.Stelzer D, Weber A, Ihle F, Matthes S, Ceelen F, Zimmermann G, Kneidinger N, Schramm R, Winter H, Zoller M, Vogeser M, Behr J, Neurohr C. 2018. Posaconazole liquid vs tablet formulation in lung transplant recipients. Mycoses 61:186–194. doi: 10.1111/myc.12724. [DOI] [PubMed] [Google Scholar]
- 32.Boglione-Kerrien C, Picard S, Tron C, Nimubona S, Gangneux JP, Lalanne S, Lemaitre F, Bellissant E, Verdier MC, Petitcollin A. 2018. Safety study and therapeutic drug monitoring of the oral tablet formulation of posaconazole in patients with haematological malignancies. J Cancer Res Clin Oncol 144:127–134. doi: 10.1007/s00432-017-2523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ensom MH, Davis GA, Cropp CD, Ensom RJ. 1998. Clinical pharmacokinetics in the 21st century. Does the evidence support definitive outcomes? Clin Pharmacokinet 34:265–279. doi: 10.2165/00003088-199834040-00001. [DOI] [PubMed] [Google Scholar]
- 34.Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, Kuntz KM, ISPOR-SMDM Modeling Good Research Practices Task Force. 2012. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–3. Value Health 15:812–820. doi: 10.1016/j.jval.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 36.Courtney R, Pai S, Laughlin M, Lim J, Batra V. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother 47:2788–2795. doi: 10.1128/aac.47.9.2788-2795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 38.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. , European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caro JJ, Briggs AH, Siebert U, Kuntz KM, Force I-SMGRPT. 2012. Modeling good research practices–overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–1. Value Health 15:796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]