WCK 5222 is a combination of cefepime and the novel β-lactam enhancer (BLE) zidebactam. Zidebactam has a dual mechanism of action involving high-affinity penicillin binding protein 2 (PBP2) binding as well as inhibition of Ambler class A and C enzymes.
KEYWORDS: WCK 5222, metallo-β-lactamase, Enterobacteriaceae, pharmacodynamics
ABSTRACT
WCK 5222 is a combination of cefepime and the novel β-lactam enhancer (BLE) zidebactam. Zidebactam has a dual mechanism of action involving high-affinity penicillin binding protein 2 (PBP2) binding as well as inhibition of Ambler class A and C enzymes. In the current study, we evaluated the effect of zidebactam on the cefepime pharmacodynamic parameter target time above MIC (T>MIC) exposure required for efficacy against a diverse group of carbapenem-resistant Enterobacteriaceae (CRE) strains secondary to metallo-β-lactamase (MBL) production. Plasma and epithelial lining fluid (ELF) pharmacokinetic (PK) studies were performed for both cefepime (6.25, 25, and 100 mg/kg of body weight) and zidebactam (3.125, 12.5, and 50 mg/kg) after subcutaneous administration to mice. Only total drug was considered protein binding is <10%. The two drugs exhibited similar PK exposures, including terminal elimination half-life (cefepime, ∼0.4 h; zidebactam, 0.3 to 0.5 h). The penetration into ELF was concentration dependent for both drugs, reaching 50% and 70% for cefepime and zidebactam, respectively. Dose ranging studies were performed in lung-infected mice with one of eight MBL-producing clinical strains. WCK 5222 was administered in regimens of every 4 h (q4h) and q8h to adjust exposures from 0% to 100% T>MIC. The results were modeled to evaluate the relationship between the cefepime T>MIC (when zidebactam was coadministered) and therapeutic effect. The results revealed a strong association between T>MIC and effect (R2 = 0.82). Net stasis in organism burden occurred at cefepime T>MIC exposures of only 18%. A 1-log kill endpoint was demonstrated for the group of organisms at approximately 31% T>MIC. These target exposures for stasis and 1-log kill are much lower than previously observed cephalosporin monotherapy PK/PD targets.
INTRODUCTION
Carbapenem-resistant Gram-negative infections are a global public health threat with limited effective treatment options and result in significant health care costs and mortality (1–5). Carbapenem-resistant Enterobacteriaceae (CRE) strains, whose resistance is commonly conferred by plasmid-mediated carbapenemase production, have significantly increased in prevalence in many areas of the world. However, the enzymatic mechanisms by which these bacteria are resistant are heterogeneous, challenging therapeutic efforts (6).
New β-lactamase inhibitors (BLI) have been developed in the past few years that are effective against Ambler class A, B, and some D enzymes, including carbapenemases within these classes. However, reports of resistance to these agents have been documented. Additionally, no β-lactam/BLI combination has been developed that is effective against all current classes of carbapenemases. Thus, novel therapies for CRE are urgently needed (7–13). Most notably, patients with infections with Gram-negative rods (GNR) that carry class B metallo-β-lactamases (MBL), such as NDM, VIM, and IMP, have extremely limited treatment options. Given this gap in therapy, we can expect ongoing increases in the incidence of MBL-producing GNR infections and subsequent poor outcomes. Indeed, a recent publication highlights the burgeoning issue of CRE in Southeast Asia, where carbapenem resistance was noted in >5% of Klebsiella and Escherichia coli isolates in almost 50% of nations with sufficient reporting, and in those, NDM-mediated resistance was most common (14). Increased world population mobility and antimicrobial pressure will undoubtedly disseminate these strains globally in a manner similar to previous epidemic shifts of extended-spectrum-β-lactamase (ESBL)-producing organisms.
WCK 5222 is an antibiotic in development that combines a currently approved cephalosporin, cefepime, with a novel β-lactam enhancer (BLE), zidebactam. The combination of cefepime with zidebactam has been shown to improve potency over the use of cefepime alone against drug-resistant GNR, including Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii (15–19). Against organisms that produce Ambler class A and C enzymes, the improved efficacy can, in part, be explained by the ability of zidebactam to inhibit the β-lactamase enzyme. However, zidebactam also exhibits an additional mode of action against GNR by selectively binding to penicillin binding protein 2 (PBP2) (16, 17, 20). The complementary binding of multiple PBPs by cefepime (potently for PBP3 and to a lesser extent for PBP1a and PBP2) and zidebactam (potently for PBP2) has been shown to be responsible for the enhanced spectrum of activity against GNR pathogens (16, 17, 20). This complementary binding profile also leads to demonstrable efficacy against MBL producers, despite zidebactam having no inherent inhibition activity against class B enzymes (18, 20). Given the relative lack of effective treatment options and limited studies examining pharmacodynamic (PD) optimization of therapy for MBL-producing Enterobacteriaceae, we evaluated the pharmacodynamic activity of WCK 5222 against a diverse group of MBL-producing CRE strains, including determining target cefepime exposures in the presence of the BLE zidebactam.
RESULTS
Organisms and in vitro susceptibility testing.
Eight (2 E. coli and 6 Klebsiella pneumoniae) MBL-producing CRE strains were utilized and are listed in Table 1. The genotypic mechanism of resistance and the phenotypic MIC results for cefepime, zidebactam, and WCK-5222 are also listed for each strain. The resistance mechanisms included 6 NDM, 2 VIM, and 1 IMP. The cefepime MIC ranged from 4 to 256 mg/liter, whereas the MIC values for WCK 5222 were 0.125 to 2 mg/liter, a decrease of ∼128-fold in the presence of zidebactam.
TABLE 1.
Strains utilized in the mouse pneumonia model, including MBL resistance genotype and susceptibility testing resultsa
| Organism | Genotype | MIC (mg/liter) |
Control growth at 24 h (log10 CFU/lungs) | ||
|---|---|---|---|---|---|
| WCK 5222 (FEP/ZID, 1:1) | Cefepime | Zidebactam | |||
| E. coli BAA-2452 | NDM-1 | 0.25 | 16 | 256 | 2.74 |
| E. coli 2671 | VIM-2 | 0.125 | 4 | 0.125 | 2.65 |
| K. pneumoniae NCTC 13443 | NDM-1 | 2 | 256 | 256 | 2.43 |
| K. pneumoniae BAA-2146 | NDM-1 | 1 | 128 | 1 | 2.08 |
| K. pneumoniae 53816 | NDM-1 | 0.25 | 32 | 0.125 | 2.27 |
| K. pneumoniae 1104866 | VIM-1 | 0.25 | 64 | 256 | 2.28 |
| K. pneumoniae 2693 | NDM-1 | 1 | 256 | 2 | 2.53 |
| K. pneumoniae 2697 | IMP-4 | 0.125 | 8 | 0.125 | 2.33 |
Also shown is the control growth in untreated animals, demonstrating equivalent levels of fitness in the model. FEP, cefepime; ZID, zidebactam.
Pharmacokinetics.
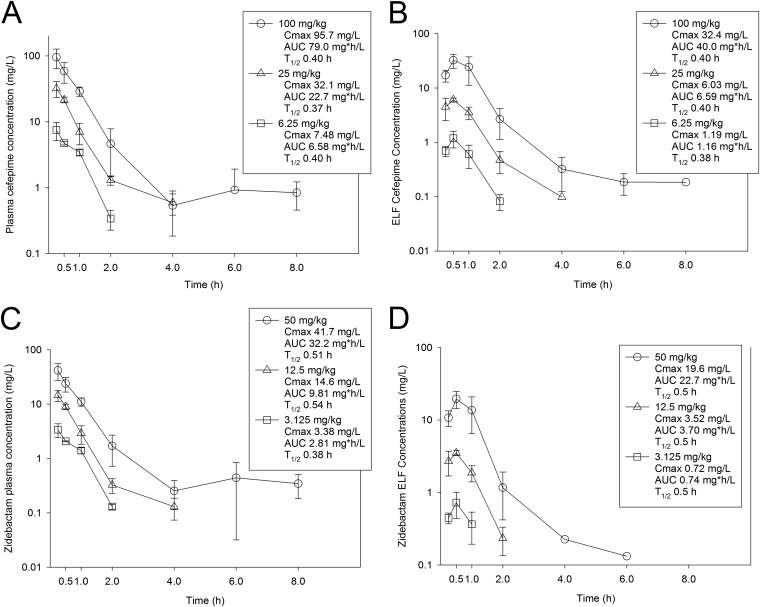
The time courses of plasma and epithelial lining fluid (ELF) pharmacokinetics (PK) for cefepime and zidebactam are shown in Fig. 1. After subcutaneous administration of cefepime at 100, 25, and 6.25 mg/kg of body weight, the Cmax values were 95.7, 32.1, and 7.48 mg/liter, respectively, and the values for the area under the concentration-time curve from time 0 to ∞ (AUC0-∞) were 79.0, 22.7, and 6.58 mg * h/liter, respectively. Both the Cmax values and the AUC0-∞ values observed over the dose range were linear (R2 = 0.999 and 0.993, respectively). Finally, the elimination half-life (t1/2) values for cefepime were also similar across the dose range (0.37 to 0.40 h). The values presented above were used to calculate time above MIC for all dosing regimens. Interestingly, very similar pharmacokinetics, calculated on a milligram-per-kilogram basis, were observed for zidebactam in mice. Because WCK 5222 is dosed at a cefepime-to-zidebactam ratio of 2:1, each of the three doses in the zidebactam pharmacokinetic studies was one-half that of cefepime on a milligram-per-kilogram basis. For both Cmax and AUC0-∞ (see Fig. 1C), the value for zidebactam was approximately 50% that of cefepime. The range of zidebactam half-life was also very similar to that of cefepime, i.e., 0.38 to 0.54 h. In the ELF, both drugs had concentration-time curves similar to those measured in plasma (see Fig. 1B and D), with concentration-dependent penetration into the ELF (see Table 2) and similar elimination half-lives. In sum, these two partner drugs exhibited similar and linear pharmacokinetics in the mouse model.
FIG 1.
Plasma and ELF drug concentration-time curves for cefepime (A and B, respectively) and zidebactam (C and D, respectively). Groups of three mice per time point were administered single subcutaneous doses of cefepime or zidebactam. At 7 different time points, plasma and BAL fluid samples were collected and assayed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) techniques. ELF concentrations were determined using the urea correction method. Each symbol represents the mean ± standard deviation (SD) of results from three animals. The maximum concentration (Cmax), the area under the drug concentration from 0 h to infinity (AUC), and the elimination half-life (T1/2) are shown for each dose.
TABLE 2.
Plasma and ELF pharmacokinetic results for cefepime and zidebactam in the mouse model
| Drug | Dose (mg/kg) | AUC0-∞ (mg * h/liter) |
% plasma drug penetration into ELF | |
|---|---|---|---|---|
| Plasma | ELF | |||
| Cefepime | 6.25 | 6.58 | 1.16 | 17.7 |
| 25 | 22.71 | 6.59 | 29.0 | |
| 100 | 79.0 | 39.97 | 50.6 | |
| Zidebactam | 3.125 | 2.81 | 0.74 | 26.3 |
| 12.5 | 9.81 | 3.70 | 37.7 | |
| 50 | 32.23 | 22.73 | 70.5 | |
Relationship between cefepime PK/PD parameter percent time above MIC (%T>MIC) and efficacy.
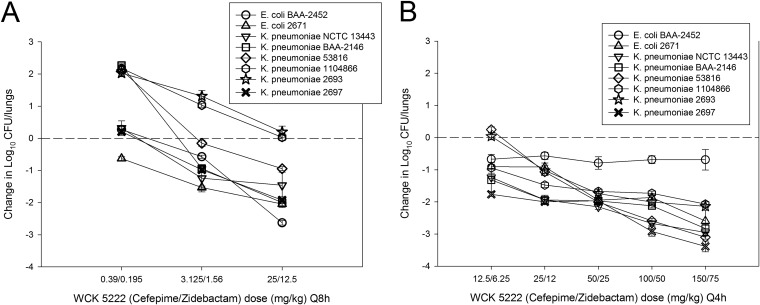
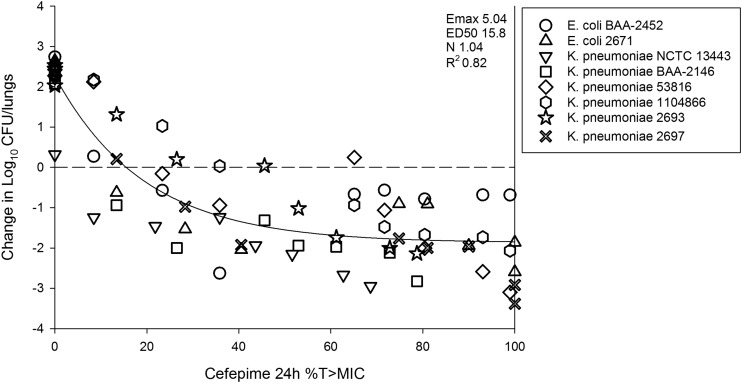
The in vivo dose-response curves are shown in Fig. 2. As mentioned in Materials and Methods, the WCK 5222 dosing studies were carried out using two different dosing intervals (with dose administration every 4 h [q4h] and q8h) over a wide dose range (0.39 to 150 mg/kg) in order to ensure cefepime %T>MIC exposures of 0% to 100% for all strains. The q8h regimens in general produced cefepime T>MIC exposures ranging from 0% to 35%, whereas the q4h regimens resulted in 35% to 100% T>MIC. Untreated control groups showed net bacterial growth of 2.07 to 2.74 log10 CFU/lungs over 24 h (see Table 1). There was, in general, net growth at very low WCK 5222 doses, and as doses were increased, therapeutic efficacy increased (see Fig. 2). It is interesting that for many strains there was a plateauing of effect at high doses in the q4h regimen, which would be expected given the time-dependent nature of β-lactam activity. Modeling all of the combination treatment data relative to cefepime T>MIC, there was a strong relationship between this pharmacokinetic/pharmacodynamic (PK/PD) index and treatment effect, with an R2 value of 0.82 (see Fig. 3). The individual PK/PD targets in terms of %T>MIC are shown in Table 3. The mean values corresponding to the cefepime T>MIC necessary for net stasis and 1-log kill, in the combination with zidebactam, were only 17.9% (standard error, 4.3) and 30.0% (standard error, 6.9), respectively.
FIG 2.
Dose-response curves for WCK 5222 (cefepime/zidebactam) in the neutropenic mouse pneumonia model against 8 MBL-producing Enterobacteriaceae. Each point is the mean ± SD of the net change in bacterial burden from the start of therapy (0 h) to the end of therapy (24 h) for six mice. Two different studies dosing studies were performed, one with q8h dosing (A) and one with q4h dosing (B), to ensure a range of cefepime T>MIC exposures from 0% to 100% for all strains.
FIG 3.
Relationship between treatment effect and cefepime T>MIC under conditions of coadministration of cefepime and zidebactam in the neutropenic mouse pneumonia model against 8 MBL-producing Enterobacteriaceae strains. The y-axis data represent the change in bacterial burden from the start of therapy (0 h) to the end of therapy (24 h). Each point represents the mean burden from six mice. The x-axis data represent the PD parameter cefepime T>MIC. The curved line is the best-fit line based on the Hill equation. Also shown are the PD parameters maximal effect (Emax), 50% maximal effect (ED50), slope of the curve (N), and coefficient of determination (R2).
TABLE 3.
Cefepime time above MIC targets for each organism in the neutropenic mouse pneumonia model under conditions of coadministration of cefepime and zidebactam
| Organism | MIC (mg/liter) | Cefepime plasma concn (24-h %T>MIC) |
|
|---|---|---|---|
| Stasis | 1 log | ||
| E. coli BAA-2452 | 0.25 | 9.83 | 19.72 |
| E. coli 2671 | 0.125 | 10.84 | 16.22 |
| K. pneumoniae NCTC 13443 | 2 | 3.61 | 10.15 |
| K. pneumoniae BAA-2146 | 1 | 8.53 | 12.70 |
| K. pneumoniae 53816 | 0.25 | 27.75 | 49.10 |
| K. pneumoniae 1104866 | 0.25 | 37.49 | 61.81 |
| K. pneumoniae 2693 | 1 | 30.24 | 47.56 |
| K. pneumoniae 2697 | 0.125 | 15.17 | 26.66 |
| Mean | 17.93 | 30.49 | |
| Median | 13.0 | 23.19 | |
| SD | 12.23 | 19.58 | |
| SE of the mean | 4.3 | 6.9 | |
DISCUSSION
The relationship between pharmacokinetic exposures, MIC, and treatment outcome is the foundation of antimicrobial PK/PD studies. The association between the duration of time that free drug concentrations exceed the MIC and therapeutic effect for β-lactams was first observed in the 1950s by Harry Eagle and colleagues (21, 22). Over the next several decades, pharmacodynamic studies refined these early observations, culminating in the current PK/PD description of β-lactams as exhibiting time-dependent bactericidal activity that is best described by the PK/PD relationship time above MIC (T>MIC) (23–26). Multiple in vivo animal model studies have demonstrated that net bacterial stasis is typically observed when free drug concentrations of cephalosporins exceed the MIC for approximately 40% to 50% of the dosing interval for Enterobacteriaceae (23–27).
WCK 5222 is an antibiotic in development that combines a currently approved cephalosporin, cefepime, with a novel β-lactam enhancer (BLE), zidebactam. Zidebactam is the first member of the bicyclo-acyl hydrazide (BCH) group, the members of which are derived from diazabicyclooctanes (DBOs). DBOs are, in general, effective inhibitors of class A, C, and some D β-lactamases. However, BCHs, such as the aforementioned zidebactam, were developed because they not only exert β-lactamase inhibition but also have a direct antibacterial effect via high-affinity binding to PBP2 (16, 17, 20). The combination of agents that target PBP3 (cefepime) and PBP2 (zidebactam) has been shown to exhibit enhanced in vitro and in vivo activity compared to cefepime alone (15–20, 28–31). In fact, despite the fact that zidebactam possesses no direct inhibitory activity against MBL enzymes, potent activity against MBL-producing organisms has been demonstrated for cefepime with zidebactam, due to the complementary PBP binding activity (15, 16, 18, 20).
The current report adds to the understanding of cefepime T>MIC targets for combinations of cefepime with the BLE zidebactam, and the data have important implications for dosing regimen design. We observed activity of the combination of cefepime and zidebactam (WCK 5222) against a diverse group of MBL-producing Enterobacteriaceae isolates. Given that zidebactam is unable to inhibit MBL enzyme activity and that cefepime is otherwise ineffective with respect to MBL-producing organisms, we speculate that the activity is likely attributable to dual PBP binding. In combination with zidebactam, the cefepime T>MIC for net stasis was only ∼18% and for 1-log kill was ∼30% T>MIC. These are 50% lower than the targets observed in previous studies of cephalosporins (23, 24). The significant aspects of our findings are severalfold. First, WCK 5222 may be the first agent to have been developed with activity against all current Ambler class enzymes. Second, a lower T>MIC target exposure could lead to the dose of cefepime necessary to meet threshold exposures for a given MIC value being reduced. Third, if the level of cefepime dosing with WCK 5222 stays the same as that used in current cefepime dosing regimens, a lower T>MIC target would mean that a higher MIC breakpoint could be met. Finally, lower T>MIC thresholds could be conducive to widening the dosing interval, for example, by utilizing a q12h regimen instead of a q6h or q8h regimen. The latter factor could lead to decreased complications (i.e., less line intervention), decreased line time, and/or a logistically improved outpatient antibiotic therapy (OPAT) option in comparison to many current β-lactams.
Our report also verifies earlier in vitro and in vivo work with WCK 5222. For example, Moya and colleagues demonstrated, both in vitro and with an in vivo murine thigh infection model, that the administration of cefepime or zidebactam alone against MBL-producing Enterobacteriaceae resulted in net increases in bacterial burden of at least 2 log10 CFU compared to start of therapy (20). However, administered in combination at the same doses as those used in the monotherapy experiments, the two drugs showed significant synergy, with 1 to 3 log10 CFU kill compared to the starting burden. We observed a similar net killing effect, as WCK 5222 achieved 1 to 3 log kill against all MBL-producing Enterobacteriaceae isolates. Additional studies have demonstrated comparable responses in mouse models utilizing the thigh and lung infection models with other GNR, including A. baumannii and P. aeruginosa (28–30). Similarly to the present study involving strains expressing enzymes that zidebactam do not inhibit (MBL), Almarzoky et al. and Avery et al. demonstrated a potent zidebactam-mediated enhancing effect on cefepime efficacy against MDR A. baumannii expressing zidebactam noninhibitable carbapenem-hydrolyzing class D enzymes (29, 30).
However, each of the aforementioned in vivo studies, while useful to demonstrate the enhancing effect of zidebactam via its complementary PBP binding, did not evaluate efficacy from a pharmacodynamic standpoint. In addition to our current study, a single previous study quantified the pharmacodynamic effect of zidebactam coadministration on cefepime T>MIC targets (31). Given the complementary PBP binding noted for WCK 5222 and PK/PD studies that have demonstrated lower targets of ∼20% T>MIC for the carbapenem class (32, 33), which bind multiple PBPs (34), it was hypothesized that the cefepime T>MIC target, under conditions of coadministration with zidebactam, would be similar to that observed with carbapenems. This was first shown by Bhagwat et al. using 5 strains of A. baumannii in the neutropenic mouse pneumonia model (31). After pharmacodynamic modeling of the exposure-response data, the cefepime T>MIC associated with net stasis was approximately 31% for cefepime monotherapy experiments, whereas in the presence of zidebactam, the cefepime T>MIC for stasis was less than 10%. In the current study, we similarly demonstrated lower T>MIC targets for net stasis at ∼18%, which, interestingly, does correlate well with previous studies in this model with carbapenems that exhibit similar dual PBP binding characteristics (32). Note a few differences between these two pharmacodynamic studies of WCK 5222. First, the previous study used A. baumannii strains, which are certainly quite different in many respects from Enterobacteriaceae, for example, in their cellular and growth characteristics, pathogenicity, and antibiotic efficacy. Second, the strains used in the Acinetobacter study were non-carbapenemase producing strains. Third, the exposure of zidebactam was fixed between cefepime dosing groups in the previous study, whereas the ratio of cefepime to zidebactam was held constant at 2:1, which is the planned dosing ratio in patients, in the current study. Finally, given that this has been demonstrated with only a single BLE with cefepime, it will be important to investigate this effect with future BLEs and other β-lactam partners to verify these results and determine the optimal T>MIC target for partner drugs.
In sum, WCK 5222 is a novel therapeutic agent that provides the dual activity of zidebactam as a BLI of many β-lactamases and BLE via complementary binding of PBPs when paired with cefepime. From a BLI perspective, it contains inhibitory activity against many of the class A and C enzymes (15, 18, 19). This is important with respect to its coformulated cephalosporin, cefepime, as the extended-spectrum β-lactamase (ESBL) CTX-M, which hydrolyzes cefepime effectively, has spread globally and now represents the most common ESBL mechanism worldwide (35–37). Thus, zidebactam would protect the activity of cefepime against organisms containing this and/or many of the other class A and C enzymes. Additionally, we have demonstrated that, even in the absence of inhibitory action against β-lactamases, the combination is highly effective in vivo against these organisms and zidebactam enhances the activity of cefepime, resulting in lower T>MIC target exposures for Enterobacteriaceae. Certainly, the dynamics of drug resistance do not ensure that this combination would provide adequate coverage for all possible β-lactamases, nor do they imply that other resistance mechanisms such as combinations of porin mutations (efflux pumps) would not emerge that, alone or in combination, would lead to resistance to this novel agent as well. However, in the current landscape of CRE, WCK 5222 is a promising agent. Further clinical studies, incorporating the pharmacodynamic targets observed in this study, are warranted, including optimizing clinical dosing regimens and setting preliminary clinical breakpoints.
MATERIALS AND METHODS
Organisms, media, and antibiotic.
Eight MBL-producing CRE strains were used, including 2 E. coli and 6 K. pneumoniae strains (see Table 1). Strains were chosen that differed in their patterns of genotypic and phenotypic resistance to cefepime and that were similarly pathogenic in the animal model based on log10 CFU growth in untreated controls. All organisms were grown, subcultured, and quantified using Mueller-Hinton broth (MHB) and agar (Difco Laboratories, Detroit, MI). The drug compounds used for in vitro and in vivo studies were supplied by Wockhardt Bio AG.
In vitro susceptibility studies.
The MICs for cefepime, zidebactam, and cefepime-zidebactam (1:1) against each strain were determined using Clinical and Laboratory Standards Institute microdilution methods (38). All MIC assays were performed in duplicate on three separate occasions. The median MIC of replicate assays is reported and was utilized in PK/PD analysis.
Murine model.
Animals were maintained in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International (39). All animal studies were approved by the Animal Research Committees of the William S. Middleton Memorial VA Hospital and the University of Wisconsin—Madison. Six-week-old, specific-pathogen-free, female ICR/Swiss mice (Harlan Sprague-Dawley, Indianapolis, IN) weighing 24 to 27 g were used for all studies. Mice were rendered neutropenic (<100 neutrophils/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) subcutaneously at 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before lung infection. Broth cultures of freshly plated bacteria were grown to the logarithmic phase overnight to an absorbance of 0.3 at 580 nm (Spectronic 88; Bausch and Lomb, Rochester, NY). After a 1:10 dilution into fresh Mueller-Hinton broth, 50 μl of inoculum (7.96 ± 0.27 log10 CFU/ml) was administered into the nares of isoflurane-anesthetized mice. Mice were simultaneously held upright to allow for aspiration into the lungs. Therapy with WCK 5222 was initiated 2 h after induction of infection, and therapy continued for 24 h, at which point the treatment groups and controls were sacrificed for CFU enumeration. Organism burden was quantified by CFU counts from dilutions of whole-lung homogenates.
Drug pharmacokinetics.
Single-dose plasma pharmacokinetics of cefepime and zidebactam were determined in mice. Dose levels of 6.25, 25, and 100 mg/kg of cefepime were administered subcutaneously. Groups of three mice were sampled for drug concentration determination at 0.25, 0.5, 1, 2, 4, 6, and 8 h. The same schema was used for zidebactam; however, doses were half those used for cefepime (i.e., 3.125, 12.5, and 50 mg/kg). In both experiments, plasma and bronchoalveolar lavage (BAL) fluid were obtained for pharmacokinetic analysis. Plasma was obtained from each animal by centrifugation of anticoagulated blood obtained by cardiac puncture. BAL fluid was obtained by instillation of 1 ml of sterile saline solution into the lungs of each animal, followed by immediate removal. The BAL fluid was centrifuged to remove blood and cellular debris, and the supernatant ELF was collected. Plasma and BAL fluid supernatant were stored at –80°C. All drug concentrations were determined using liquid chromatography-tandem mass spectrometry methods by Wockhardt Bio AG, Switzerland. ELF concentrations were calculated from BAL fluid concentrations by urea correction methodology according to the following formula: [drug]ELF = [drug]BAL fluid × ([urea]plasma/[urea]BAL fluid). Pharmacokinetic parameters (means ± standard deviations), including the elimination half-life (t1/2), AUC0–∞, and Cmax, were calculated using a noncompartmental model with mean concentration values from each group of mice. The half-life was determined by linear least-squares regression. The AUC was calculated from the mean concentrations using the trapezoidal rule. Pharmacokinetic estimates for dose levels that were not directly measured were calculated using linear interpolation for dose levels between those with measured kinetics and linear extrapolation for dose levels above or below the highest and lowest dose levels with kinetic measurements. The levels of protein binding of both cefepime and zidebactam (Wockhardt Bio AG, Switzerland; data on file) are <10% in the mouse; thus, only total drug concentrations were considered in the analysis.
Relationship between cefepime PK/PD parameter percent time above MIC (%T>MIC) and efficacy.
In vivo treatment studies were performed in the murine pneumonia model for each strain. Groups of six mice per dosing regimen and control group were utilized. All WCK 5222 doses were administered by the subcutaneous route, and the cefepime-to-zidebactam ratio for each dose was held constant at 2:1. The specific dosing regimens utilized for each organism were designed on the basis of the pharmacokinetic studies described above and the susceptibility results to adjust the cefepime exposure from 0% to 100% T>MIC for each organism. This scheme resulted in the utilization of at least 8 different WCK 5222 dosing groups per organism with cefepime dosages that ranged from 0.39 to 150 mg/kg (zidebactam dosage range, 0.19 to 75 mg/kg) and intervals that were either q4h or q8h. The dose-response effect was determined as described above by measurement of CFU in lung homogenates. The correlation between efficacy and the PK/PD parameter %T>MIC was determined by nonlinear least-squares multivariate regression (SigmaPlot version 13; Systat Software, San Jose, CA). The mathematical model used was derived from the Hill equation, i.e., E = (Emax × DN)/(ED50N – DN), where E is the effector, in this case, the log change in CFU per lung between treated mice and untreated controls after the 24 h period of study, Emax is the maximum effect, D is the 24 h %T>, ED50 is the %T>MIC required to achieve 50% of the Emax, and N is the slope of the dose-effect curve. The values for the indices Emax, ED50, and N were calculated using nonlinear least-squares regression. The coefficient of determination (R2) was used to estimate the variance that might be due to regression with the PK/PD parameter %T>MIC.
Target %T>MIC exposures for cefepime in combination with zidebactam resulting in net stasis and kill endpoints.
Using the sigmoid Emax model described above, the doses required to produce a net static effect (static dose) and 1 log kill compared to the start of therapy were calculated for each drug-organism combination. The plasma and ELF pharmacokinetic results were then used to estimate the %T>MIC exposure associated with each of the endpoints for each organism. The associated 24-h %T>MIC target exposures for plasma and ELF were calculated for each organism, and summary statistics (i.e., mean, median, and standard deviation) were determined for the group.
ACKNOWLEDGMENT
This study was funded by Wockhardt Bio AG, Switzerland.
REFERENCES
- 1.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 2.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. 2012. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 18:54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartsch SM, McKinnell JA, Mueller LE, Miller LG, Gohil SK, Huang SS, Lee BY. 2017. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect 23:48.e9–48.e16. doi: 10.1016/j.cmi.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States. 2013. http://www.cdc.gov/drugresistance/threat-report-2013/. Accessed 14 July 2019.
- 5.Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. 2018. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 66:1290–1297. doi: 10.1093/cid/cix893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. 24 April 2017, posting date Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum beta-lactamases. Antimicrob Agents Chemother doi: 10.1128/AAC.02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haidar G, Philips NJ, Shields RK, Snyder D, Cheng S, Potoski BA, Doi Y, Hao B, Press EG, Cooper VS, Clancy CJ, Nguyen MH. 2017. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 65:110–120. doi: 10.1093/cid/cix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemarajata P, Humphries RM. 1 May 2019, posting date Ceftazidime/avibactam resistance associated with L169P mutation in the omega loop of KPC-2. J Antimicrob Chemother doi: 10.1093/jac/dkz026. [DOI] [PubMed] [Google Scholar]
- 10.Munoz-Price LS, Reeme AE, Buchan BW, Mettus RT, Mustapha MM, Van Tyne D, Shields RK, Doi Y. 23 September 2019, posting date Patient-to-patient transmission of KPC variants with reduced ceftazidime-avibactam susceptibility. Antimicrob Agents Chemother doi: 10.1128/AAC.00955-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Kreiswirth BN, Nguyen MH, Clancy CJ. 23 February 2017, posting date Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K pneumoniae: a case report and review of literature. Open Forum Infect Dis 4:ofx101. doi: 10.1093/ofid/ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson WR, Kline EG, Jones CE, Morder KT, Mettus RT, Doi Y, Nguyen MH, Clancy CJ, Shields RK. 26 February 2019, posting date Effects of KPC variant and porin genotype on the in vitro activity of meropenem-vaborbactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother doi: 10.1128/AAC.02048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malchione MD, Torres LM, Hartley DM, Koch M, Goodman J. 29 July 2019, posting date Carbapenem and colistin resistance in Enterobacteriaceae in Southeast Asia: review and mapping of emerging and overlapping challenges. Int J Antimicrob Agents doi: 10.1016/j.ijantimicag.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 16.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 24 October 2017, posting date Potent beta-lactam enhancer activity of zidebactam and WCK 5153 against Acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother doi: 10.1128/AAC.01238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 24 May 2017, posting date WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “beta-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-beta-lactamase-producing high-risk clones. Antimicrob Agents Chemother doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant beta-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 19.Thomson KS, AbdelGhani S, Snyder JW, Thomson GK. 23 March 2019, posting date Activity of cefepime-zidebactam against multidrug-resistant (MDR) Gram-negative pathogens. Antibiotics (Basel) doi: 10.3390/antibiotics8010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moya B, Barcelo IM, Cabot G, Torrens G, Palwe S, Joshi P, Umarkar K, Takalkar S, Periasamy H, Bhagwat S, Patel M, Bou G, Oliver A. 25 April 2019, posting date In vitro and in vivo activities of beta-lactams in combination with the novel beta-lactam enhancers zidebactam and WCK 5153 against multidrug-resistant metallo-beta-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother doi: 10.1128/AAC.00128-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eagle H, Fleischman R, Musselman AD. 1950. Effect of schedule of administration on the therapeutic efficacy of penicillin; importance of the aggregate time penicillin remains at effectively bactericidal levels. Am J Med 9:280–299. doi: 10.1016/0002-9343(50)90425-6. [DOI] [PubMed] [Google Scholar]
- 22.Eagle H, Fleischman R, Levy M. 1953. “Continuous” vs. “discontinuous” therapy with penicillin; the effect of the interval between injections on therapeutic efficacy. N Engl J Med 248:481–488. doi: 10.1056/NEJM195303192481201. [DOI] [PubMed] [Google Scholar]
- 23.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 22:89–96. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 24.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 25.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 17:479–501. doi: 10.1016/S0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 26.Drusano GL. 2007. Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis 45(Suppl 1):S89–S95. doi: 10.1086/518137. [DOI] [PubMed] [Google Scholar]
- 27.Andes D, Craig WA. 2005. Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin Microbiol Infect 11(Suppl 6):10–17. doi: 10.1111/j.1469-0691.2005.01265.x. [DOI] [PubMed] [Google Scholar]
- 28.Monogue ML, Tabor-Rennie J, Abdelraouf K, Nicolau DP. 24 June 2019, posting date In vivo efficacy of WCK 5222 (cefepime-zidebactam) against multidrug-resistant Pseudomonas aeruginosa in the neutropenic murine thigh infection model. Antimicrob Agents Chemother doi: 10.1128/AAC.00233-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almarzoky Abuhussain SS, Avery LM, Abdelraouf K, Nicolau DP. 21 December 2019, posting date In vivo efficacy of humanized WCK 5222 (cefepime-zidebactam) exposures against carbapenem-resistant Acinetobacter baumannii in the neutropenic thigh model. Antimicrob Agents Chemother doi: 10.1128/AAC.01931-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avery LM, Abdelraouf K, Nicolau DP. 24 October 2018, posting date Assessment of the in vivo efficacy of WCK 5222 (cefepime-zidebactam) against carbapenem-resistant Acinetobacter baumannii in the neutropenic murine lung infection model. Antimicrob Agents Chemother doi: 10.1128/AAC.00948-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhagwat SS, Periasamy H, Takalkar SS, Palwe SR, Khande HN, Patel MV. 27 March 2019, posting date The novel beta-lactam enhancer zidebactam augments the in vivo pharmacodynamic activity of cefepime in a neutropenic mouse lung Acinetobacter baumannii infection model. Antimicrob Agents Chemother doi: 10.1128/AAC.02146-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19:261–268. doi: 10.1016/S0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 33.Maglio D, Banevicius MA, Sutherland C, Babalola C, Nightingale CH, Nicolau DP. 2005. Pharmacodynamic profile of ertapenem against Klebsiella pneumoniae and Escherichia coli in a murine thigh model. Antimicrob Agents Chemother 49:276–280. doi: 10.1128/AAC.49.1.276-280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type beta-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Bevan ER, Jones AM, Hawkey PM. 2017. Global epidemiology of CTX-M beta-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother 72:2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 37.Mathers AJ, Peirano G, Pitout JD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CLSI. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed CLSI standard M07 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]