New antibiotics with activity against carbapenem-resistant Enterobacteriaceae (CRE) improve outcomes of CRE-infected patients. However, companies developing these drugs have faced financial difficulties. Sales of ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin in the United States totaled $101 million from February 2018 to January 2019. We estimate that the current annual U.S. market for new anti-CRE antibiotics is $289 million (range, $169 to $439 million).
KEYWORDS: carbapenem-resistant Enterobacteriaceae, ceftazidime-avibactam, meropenem-vaborbactam, plazomicin, Orphan Drug Act, Enterobacteriaceae, carbapenem, resistant, treatment
ABSTRACT
New antibiotics with activity against carbapenem-resistant Enterobacteriaceae (CRE) improve outcomes of CRE-infected patients. However, companies developing these drugs have faced financial difficulties. Sales of ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin in the United States totaled $101 million from February 2018 to January 2019. We estimate that the current annual U.S. market for new anti-CRE antibiotics is $289 million (range, $169 to $439 million). Without new antibiotic development models and/or reimbursement reform, the majority of anti-CRE drugs will be commercially inviable.
TEXT
Carbapenem-resistant Enterobacteriaceae (CRE) are estimated to cause 1.5 to 4.5 million infections requiring hospitalization worldwide each year, including 19,000 to 49,000 such infections in the United States (1). The development of antibiotics with activity against CRE is endorsed as an urgent medical priority by health care organizations globally (2). Since 2015, five such agents (ceftazidime-avibactam, meropenem-vaborbactam, plazomicin, eravacycline, and imipenem-cilastatin-relebactam) have been approved by the U.S. Food and Drug Administration (FDA) (Table 1). Observational studies and randomized trials have shown that ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin are significantly more effective and less toxic in treating CRE-infected patients than polymyxin-based (colistin or polymyxin B) or other previous salvage regimens (3–7).
TABLE 1.
Approved and late-pipeline antibiotics with activity against carbapenem-resistant Enterobacteriaceae
| Agent | Status | Spectrum of activity in vitro |
Salesa | |
|---|---|---|---|---|
| CREf | Other MDR Gram-negative bacteriag | |||
| Ceftazidime-avibactam | FDA approved, February 2015 | Active against KPC and OXA48 producers | Active against MDR Pseudomonas speciesb | $92.4 M |
| Meropenem-vaborbactam | FDA approved, August 2017 | Active against KPC producers | Activity against Pseudomonas species is equivalent that of to meropenem | $7.9 M |
| Plazomicin | FDA approved, June 2018 | Active against KPC, OXA48, and MBL producers | Active against MDR Pseudomonas speciesc | $0.7 M |
| Eravacycline | FDA approved, August 2018 | Active against KPC, OXA48, and MBL producersd | Inactive against Pseudomonas; active against MDR Acinetobacter and Stenotrophomonas species | NAd |
| Imipenem-cilastatin-relebactam | FDA approved, July 2019 | Active against KPC producers | Active against MDR Pseudomonas speciese | NAe |
| Cefiderocol | Phase 3 enrollment complete, data pending | Active against KPC, OXA48, and MBL producers | Active against MDR Pseudomonas, MDR-Acinetobacter, Stenotrophomonas, and Burkholderia species | NA |
U.S. sales for February 2018 through January 2019 (IQVIA, Durham, NC). M, million; NA, not applicable.
Ceftazidime-avibactam is positioned primarily as an anti-CRE agent since other agents are more active against many MDR and carbapenem-resistant Pseudomonas.
Plazomicin activity against Pseudomonas is generally insufficient for use as monotherapy against systemic infections.
Eravacycline is more potent than tigecycline, an earlier tetracycline with some anti-CRE activity. The role of eravacycline in treating CRE infections is unclear since clinical data remain limited. Based on activity against anaerobic bacteria, the agent may be positioned against intra-abdominal infections. There were limited sales data for eravacycline through January 2019.
Imipenem-cilastatin-relebactam has excellent activity against MDR and carbapenem-resistant Pseudomonas. It may be positioned primarily as an anti-MDR Pseudomonas and anti-KPC-producing CRE agent. Imipenem-cilastatin-relebactam was not approved during the time period under study.
KPC, Klebsiella pneumoniae carbapenemase; OXA48, OXA48-like carbapenemase; MBL, metallo-beta-lactamase.
MDR, multidrug resistant.
We recently estimated that these three new agents were used to treat 23% (range, 16% to 42%) of CRE infections in the U.S. from February 2018 to January 2019, and that their monthly use against CRE infections surpassed that of intravenous polymyxins in December 2018 (3). In a survey of U.S. hospital-based pharmacists, the new drugs were positioned as first-line against 67.5% of CRE infections. Therefore, we estimated the drugs were employed against only 35% (range, 23% to 62%) of CRE infections in which their use was expected based on positioning (3). These data are noteworthy because companies developing ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin have struggled financially (8). Our objectives in this study were to report sales data and estimate the size of the U.S. market for new anti-CRE antibiotics. We anticipated that results would provide important insights into the financial viability of the marketplace, even as other agents targeting CRE receive FDA approval or move through early and late-stage development.
We obtained data on numbers of units sold and sales figures for ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin from IQVIA (Durham, NC). We used our previous estimates of CRE infections treated (n = 7,941, February 2018 to January 2019) or anticipated to be treated (n = 22,950; range, 12,825 to 34,055) with new anti-CRE agents to determine market size (U.S. dollars). Eravacycline and imipenem-cilastatin-relebactam were not included in our analysis, since sales of the former were negligible through January 2019 and the latter was not approved by FDA until July 2019.
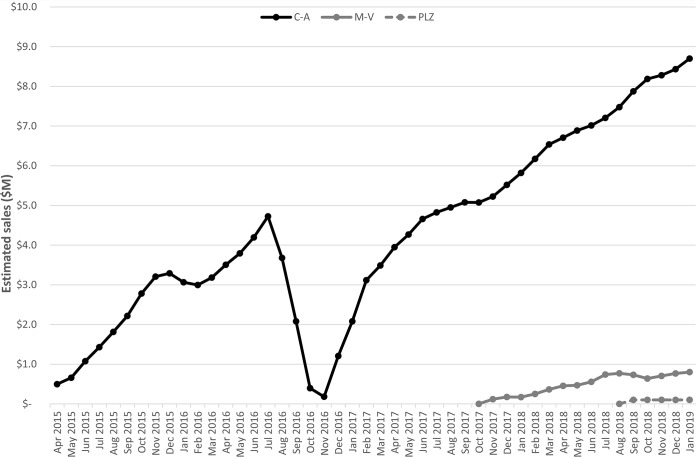
Monthly sales of ceftazidime-avibactam, meropenem-vaborbactam and plazomicin in the United States were calculated as 3-month moving averages (Fig. 1). Between February 2018 and January 2019, estimated sales of these agents were $92.4, $7.9, and $0.7 million, respectively (Table 1). Combined sales during this 12-month period were $101 million. Based on anticipated use of agents against CRE infections, we calculate that the current annual U.S. market for new anti-CRE antibiotics is $289 million (range, $169 to $439 million).
FIG 1.
Monthly sales of ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin in the United States. Sales data are presented as 3-month moving averages in U.S. dollars, beginning with the introduction of ceftazidime-avibactam to the market. The dip and subsequent rebound in sales of ceftazidime-avibactam in 2016 and 2017 corresponded to a nationwide supply shortage of the drug and its resolution.
Anti-CRE agents are case studies for difficulties facing companies developing new antibiotics. These drugs address a major, previously unmet medical need, and improve patient outcomes compared to previous treatments. Nevertheless, Allergan and the Medicines Company have sold off ceftazidime-avibactam and meropenem-vaborbactam, respectively, after realizing insufficient returns, and Achaogen declared bankruptcy after losing >$450 million developing plazomicin (8). Despite activity against some other multidrug-resistant (MDR) Gram-negative bacteria, these drugs are positioned at hospitals primarily as anti-CRE agents (Table 1) (3). The new anti-CRE antibiotic market we describe is likely too small to sustain multiple agents unless spectra of activity are expanded or clinicians demonstrate greater willingness to prescribe drugs empirically. To put our data in perspective, a billion dollars in incentives is believed to be necessary to ensure viability of a new antibiotic (9, 10). To be profitable and sustainable, new biopharmaceutical companies generally target about $300 million in annual revenue (https://www.quora.com/On-average-how-long-does-it-take-for-a-bio-pharmaceutical-company-to-become-profitable-after-inception/answer/Antoun-Nabhan). Market prospects will become even more daunting as more anti-CRE drugs are approved.
A recent paper endorsed a nonprofit antibiotic development model (9). Uncertainties of this intriguing proposal include whether nonprofits are more likely than for-profit companies to develop high-priority antibiotics that improve patient outcomes while limiting redundancy in coverage, to discover or select drug development “winners,” and/or to tolerate lack of early-stage revenue and subsequent low sales. Indeed, the Achaogen experience serves as a cautionary tale; the company received approximately $225 million in nondilutive, pre-FDA approval “push” funding to support its drug development program from government and public-private sources, including the U.S. Biomedical Advanced Research and Development Authority (BARDA) and the nonprofit Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) partnership (11; Alan Carr, personal communication).
Regardless of the entity developing antibiotics, we believe that financial “pull” incentives are crucial for sustainability (3, 10). Along these lines, multifaceted reforms to in-hospital reimbursement for antibiotics that target resistant infections by the Centers for Medicare and Medicaid Services (CMS), scheduled for implementation in or around October 2019 as part of the fiscal year 2020 Final Rule, are a major step forward (11, 12). CMS has waived the “substantial clinical improvement” criterion for qualified infectious diseases product (QIDP) antibiotics to be eligible for new technology add-on payments (NTAP), an important development, since antibiotics generally are approved by the FDA based on noninferiority data for treatment of predominantly non-MDR infections (13). In addition, NTAP payments to hospitals for QIDP antibiotics have been increased by 50%, which should encourage more rapid formulary decisions about new agents and offset at least some of the excess costs of their use. Finally, International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10) codes have been adjusted to increase the complexity of diagnosis-related group (DRG) classifications relevant to antibiotic-resistant infections, thereby increasing bundled reimbursements. As valuable as these steps are, NTAPs last for only 3 years, and increased reimbursements will not cover the full costs of QIDP antibiotics. Bipartisan legislation (the Developing an Innovative Strategy for Antimicrobial Resistant Microorganisms [DISARM] Act) has been introduced in the U.S. Congress that would codify and extend CMS reforms by carving out QIDP-designated antibiotics and antifungals from the DRG and by reimbursing hospitals for use of these drugs at 2% above cost (14, 15). Both the revised Final Rule and DISARM Act acknowledge the essential role of antimicrobial stewardship in reinforcing the effectiveness of payment reforms. CMS have established that the institution of stewardship and infection control programs will be a condition for hospital participation in Medicare (11).
An unexplored reform is to classify antibiotics against high-priority, MDR bacteria as orphan drugs. The Orphan Drug Act (ODA), passed by U.S. Congress in 1983, offers research and development (R&D) tax credits, fee waivers, and extended market exclusivity to drugs against rare diseases (16). Historically, antibiotics against resistant pathogens have not been considered eligible because they can be used to treat common, susceptible infections. The ODA originally stipulated a commercial viability-based definition of a rare disease as one occurring so infrequently in the United States that there was “no reasonable expectation” that R&D costs would be recovered (17). In 1984, however, a prevalence-based modification was added to the definition that also allowed for diseases affecting fewer than 200,000 people in the United States regardless of prospects for commercial viability (16). In 2018, over half of new FDA-approved drugs had orphan designations, and orphan drugs comprised some of the most expensive and profitable products in the world (16–18). Companies increasingly “salami slice” drugs against relatively common diseases into orphan agents that target “rare” subsets of these diseases, such as biomarker-defined cancers or cystic fibrosis defined by specific mutations (17–19). Often, clinical trials employ surrogate endpoints that lower treatment success thresholds (e.g., slowing tumor growth rather than improving survival) and shorten time to approval (16, 19). Moreover, many orphan drugs have gained much broader, lucrative markets after approval (18). These developments invite the question of why life-saving, anti-CRE antibiotics should not be granted orphan status. In the bigger picture, there is growing debate about whether the intent of the ODA has been subverted and if the legislation should be revised so that resources are better allocated to drugs marginalized by market forces (16–19). Anti-CRE antibiotics are prototypes of such drugs.
Our market size calculations were based on estimates of numbers of CRE infections and positioning of new agents nationally, and current drug prices. It is plausible that market size will change as epidemiology or drug prices evolve. Moving forward, use of the recently approved antibiotic imipenem-cilastatin-relebactam and the late-pipeline agent cefiderocol (if FDA approved) may prove telling for the field. Imipenem-cilastatin-relebactam has excellent activity against MDR Pseudomonas species (20), and Merck may be able to leverage resources and experience that were unavailable to smaller companies introducing previous agents. Cefiderocol, a siderophore cephalosporin from Shionogi, has a novel mechanism of action and spectrum of activity that includes CRE expressing metallo-beta-lactamases and MDR, nonfermenting Gram-negative bacteria (21). Economic inviability of these agents, coupled with the earlier commercial failures mentioned above, would likely have a chilling effect on biopharmaceutical companies and investors contemplating whether to pursue antibiotic development. The steady but slow uptake of new anti-CRE agents and continued use of polymyxins attest to the need for research into economic and behavioral factors that influence antibiotic prescribing and stewardship practices (3). It is also imperative that infectious diseases clinicians and professional societies face their responsibilities in shaping public policy, educating other health care professionals, and developing and disseminating timely guidelines for the most appropriate use of new and older antibiotics. As an immediate priority, education and guidelines must state clearly that polymyxins or other salvage drugs are no longer justifiable therapeutic choices against the vast majority of CRE infections (3).
ACKNOWLEDGMENTS
We thank Alan Carr for his assistance with obtaining and interpreting IQVIA data.
There was no funding for the study.
Cornelius J. Clancy has been awarded investigator-initiated research grants from Astellas, Merck, Melinta, and Cidara for projects unrelated to this project, served on advisory boards or consulted for Astellas, Merck, the Medicines Company, Cidara, Scynexis, Shionogi, Qpex, and Needham & Company, and spoken at symposia sponsored by Merck and T2Biosystems. M. Hong Nguyen has been awarded investigator-initiated research grants from Astellas, Merck, Melinta, and Cidara for projects unrelated to this study, and served on advisory boards for Astellas, Merck, the Medicines Company, Scynexis, and Shionogi.
REFERENCES
- 1.Temkin E, Fallach N, Almagor J, Gladstone BP, Tacconelli E, Carmeli Y, Consortium D-A. 2018. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: a modelling study. Lancet Glob Health 6:e969–e979. doi: 10.1016/S2214-109X(18)30278-X. [DOI] [PubMed] [Google Scholar]
- 2.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Clancy CJ, Potoski BA, Buehrle D, Nguyen MH. 2019. Estimating the treatment of carbapenem-resistant Enterobacteriaceae infections in the United States using antibiotic prescription data. Open Forum Infect Dis 6:ofz344. doi: 10.1093/ofid/ofz344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinnell JA, Dwyer JP, Talbot GH, Connolly LE, Friedland I, Smith A, Jubb AM, Serio AW, Krause KM, Daikos GL, Group CS. 2019. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med 380:791–793. doi: 10.1056/NEJMc1807634. [DOI] [PubMed] [Google Scholar]
- 5.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, Cornely OA, Solomkin J, Bhowmick T, Bishara J, Daikos GL, Felton T, Furst MJL, Kwak EJ, Menichetti F, Oren I, Alexander EL, Griffith D, Lomovskaya O, Loutit J, Zhang S, Dudley MN, Kaye KS. 2018. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 7:439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae Infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr A. 2019. Achaogen, Inc. (AKAO). Dropping coverage. Needham and Company Newsletter. Needham and Company, New York, NY. [Google Scholar]
- 9.Nielsen TB, Brass EP, Gilbert DN, Bartlett JG, Spellberg B. 2019. Sustainable discovery and development of antibiotics—is a nonprofit approach the future? N Engl J Med 381:503. doi: 10.1056/NEJMp1905589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sciarretta K, Rottingen JA, Opalska A, Van Hengel AJ, Larsen J. 2016. Economic incentives for antibacterial drug development: literature review and considerations from the Transatlantic Task Force on Antimicrobial Resistance. Clin Infect Dis 63:1470–1474. doi: 10.1093/cid/ciw593. [DOI] [PubMed] [Google Scholar]
- 11.Verma S. 2 August 2019. Aligning payment and prevention to drive antibiotic innovation for Medicare beneficiaries. Health Care Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20190802.505113/full/.
- 12.Rex J. 4 August 2019. New mechanisms for antibiotic reimbursement in the united states: CMS’s IPPS FY2020 Final Rule. Solutions for Antimicrobial Resistance Blog. http://amr.solutions/blog/new-mechanisms-for-antibiotic-reimbursement-in-the-united-states-cmss-ipps-fy2020-final-rule.
- 13.Rex JH, Talbot GH, Goldberger MJ, Eisenstein BI, Echols RM, Tomayko JF, Dudley MN, Dane A. 2017. Progress in the fight against multidrug-resistant bacteria 2005–2016: modern noninferiority trial designs enable antibiotic development in advance of epidemic bacterial resistance. Clin Infect Dis 65:141–146. doi: 10.1093/cid/cix246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy CJ. 8 August 2019. Senator Casey’s bill to fight superbugs will save lives. Philadelphia Inquirer, Philadelphia, PA: https://www.inquirer.com/opinion/commentary/antibiotics-resistance-disarm-bill-bob-casey-20190808.html. [Google Scholar]
- 15.Carr A. 2019. Antibiotic and antifungal update September 2019. Needham & Company Newsletter. Needham & Company, New York, NY. [Google Scholar]
- 16.Bagley N, Chandra A, Garthwaite C, Stern AD. 19 December 2018. It’s time to reform the Orphan Drug Act. NEJM Catalyst; https://catalyst.nejm.org/time-reform-orphan-drug-act/. [Google Scholar]
- 17.Herder M. 2017. What is the purpose of the orphan drug act? PLoS Med 14:e1002191. doi: 10.1371/journal.pmed.1002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarpatwari A, Kesselheim AS. 2019. Reforming the Orphan Drug Act for the 21st century. N Engl J Med 381:106–108. doi: 10.1056/NEJMp1902943. [DOI] [PubMed] [Google Scholar]
- 19.Kesselheim AS, Treasure CL, Joffe S. 2017. Biomarker-defined subsets of common diseases: policy and economic implications of Orphan Drug Act Coverage. PLoS Med 14:e1002190. doi: 10.1371/journal.pmed.1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haidar G, Clancy CJ, Chen L, Samanta P, Shields RK, Kreiswirth BN, Nguyen MH. 2017. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 61:e00642-17. doi: 10.1128/AAC.00642-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean SS, Hsueh SC, Lee WS, Hsueh PR. 2019. Cefiderocol: a promising antibiotic against multidrug-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther 17:307–309. doi: 10.1080/14787210.2019.1612240. [DOI] [PubMed] [Google Scholar]