Ibrexafungerp (formerly SCY-078), a novel glucan synthase inhibitor with oral availability, was evaluated for activity against Candida glabrata. The susceptibility of clinical strains to ibrexafungerp was determined by microdilution and time-kill assays. The MIC range against wild-type strains was 1 to 2 μg/ml. Ibrexafungerp was also active against the majority of echinocandin-resistant strains.
KEYWORDS: Candida glabrata, fks mutation, ibrexafungerp
ABSTRACT
Ibrexafungerp (formerly SCY-078), a novel glucan synthase inhibitor with oral availability, was evaluated for activity against Candida glabrata. The susceptibility of clinical strains to ibrexafungerp was determined by microdilution and time-kill assays. The MIC range against wild-type strains was 1 to 2 μg/ml. Ibrexafungerp was also active against the majority of echinocandin-resistant strains. Time-kill studies showed 4- to 6-log-unit reductions in growth at 24 and 48 h with concentrations of 0.25 to 4 μg/ml.
TEXT
Echinocandins have been shown to be effective against various Candida species; however, studies have shown that resistance to these agents is increasing, particularly in Candida glabrata. In this regard, strains that have shown resistance to both azoles and echinocandins have been isolated, with C. glabrata being among the most commonly reported (1, 2). The poor outcomes of echinocandin-resistant C. glabrata infections and reports of breakthrough C. glabrata infections during echinocandin therapy illustrate the clinical relevance of this phenomenon (3, 4).
Ibrexafungerp (formerly SCY-078) is a member of a new class of glucan synthase inhibitors that inhibit the synthesis of the fungal cell wall polymer beta-(1,3)-d-glucan. Although its mechanism of action is similar to that of the echinocandins, it is structurally distinct and has the advantage of both oral and intravenous formulations. Additionally, ibrexafungerp has demonstrated in vitro activity against azole-resistant isolates and the majority of echinocandin-resistant strains of Candida species (1, 2). In this study, we evaluated the in vitro antifungal activity of ibrexafungerp against both echinocandin-susceptible and echinocandin-resistant strains of this species.
MIC testing was performed in duplicate, according to the CLSI standard for susceptibility testing of yeasts (5). MIC endpoints were determined by visual examination at 50% inhibition, compared to the growth control. (Our testing was performed in the absence of serum, which has been shown to influence MIC results; therefore, this is a limitation of this study.) Time-kill assays were carried out in duplicate as described by Klepser et al. (6), with samples taken at 1, 4, 8, 24, and 48 h.
MIC testing was performed against wild-type (WT) (defined for this study as lacking an fks mutation) (n = 11) and echinocandin-resistant (n = 22) C. glabrata strains taken from our culture collection. Resistance to micafungin and caspofungin was defined as having MICs of ≥0.25 and ≥0.5, respectively, while >2 μg/ml for ibrexafungerp was considered to be elevated (7). The ibrexafungerp MIC range was 1 to 2 μg/ml, while the MIC mode, MIC50, and MIC90 for ibrexafungerp against WT strains were all 1 μg/ml. The MIC range, MIC mode, MIC50, and MIC90 for caspofungin against the WT strains were 0.25 to 1, 0.25, 0.25, and 0.5 μg/ml, respectively; for micafungin, the MIC range was <0.016 to 0.125 μg/ml and the MIC mode, MIC50, and MIC90 were all <0.016 μg/ml. Three WT strains had caspofungin MICs of ≥0.5 μg/ml, indicating resistance, whereas none of the isolates was resistant to micafungin.
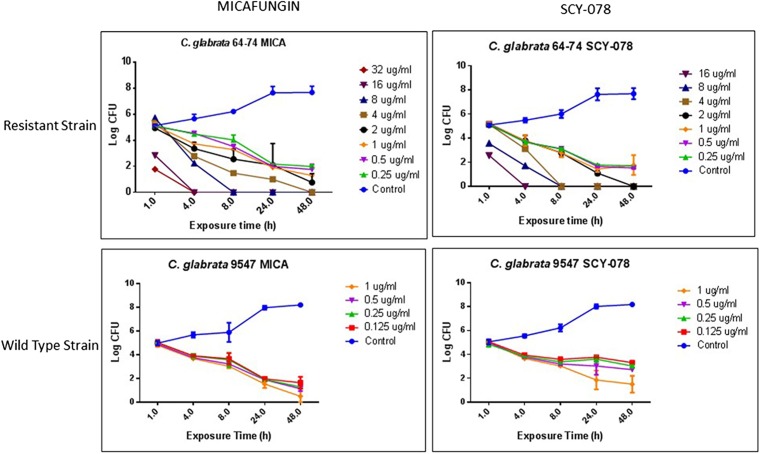
Against echinocandin-resistant isolates, ibrexafungerp demonstrated a MIC range of 0.5 to 4 μg/ml, while the MIC mode, MIC50, and MIC90 were 1, 1, and 4 μg/ml, respectively. The MIC range, MIC mode, MIC50, and MIC90 for caspofungin were 0.5 to 2, 1, 1, and 2 μg/ml, respectively, and those for micafungin were <0.016 to 2, 0.125, 0.125, and 1 μg/ml, respectively. Time-kill studies were conducted with 2 C. glabrata isolates, with micafungin MICs of 0.008 μg/ml (WT strain 9547) and 2 μg/ml (resistant strain 64-74) and ibrexafungerp MICs of 1 μg/ml for both (Fig. 1). Unlike the WT strain, which had no detected mutation, the elevated-MIC strain was known to have a mutation in fks2, namely, S663P. Exposure of the WT strain to ibrexafungerp at concentrations of 0.25 to 1 μg/ml resulted in an ∼6-log-unit reduction in growth at 48 h (Fig. 1). Furthermore, at higher drug concentrations (4 to 16 μg/ml), ibrexafungerp completely inhibited growth of the resistant strain, showing no growth from 4 to 48 h (Fig. 1). Importantly, the resistant strain exposed to ibrexafungerp showed an ∼6-log-unit reduction in growth at 48 h, compared to the untreated control, when exposed to a drug concentration of 0.25 μg/ml (Fig. 1). Micafungin had activity similar to that of ibrexafungerp against both susceptible and resistant isolates.
FIG 1.
Time-kill curves for ibrexafungerp (formerly SCY-078) and micafungin (MICA) against caspofungin-susceptible and caspofungin-resistant C. glabrata strains (strains 9547 and 64-74, respectively).
Our data showed that 21 of the echinocandin-resistant isolates with known fks mutations were resistant to caspofungin (MICs of ≥0.5 μg/ml), while 10 (45.5%) of 22 isolates were resistant to micafungin (MICs of ≥0.25 μg/ml). In contrast, only 3 (13.6%) of 22 isolates had elevated ibrexafungerp MICs. Isolates investigated in this study had a number of different fks mutations. Five of the isolates had a S663P mutation (1 of the strains had a R631G mutation in addition to S663P), which is the most frequently encountered mutation in echinocandin-resistant strains. All 5 strains with this mutation were resistant to caspofungin, while 3 were resistant to micafungin. In contrast, all of these isolates were susceptible to ibrexafungerp. Our data agree with the findings of Schell et al. (8), who demonstrated good antifungal activity of ibrexafungerp in vitro against C. glabrata strains with a S663P mutation (MICs 1 to 3 dilutions lower than those for the other echinocandins tested). Similarly, Pfaller et al. (7) reported the same observations in isolates with this mutation.
Three of the echinocandin-resistant strains had elevated MIC values for ibrexafungerp (4 μg/ml) and fks2 mutations, 1 each with fks2p F658del (strain CD-0320), F659del (strain 03-1498), and fks1p F625S (strain 04-2997). Cross-resistance to caspofungin was observed for all 3 strains, while cross-resistance to micafungin was observed only for the isolate with F659del (strain 03-1498). Deletions at positions F658 and F659 in fks2 were previously reported in association with ibrexafungerp (7, 9).
The fact that significantly fewer echinocandin-resistant strains with fks mutations were resistant to ibrexafungerp, compared to the other two echinocandins tested, suggests that fks mutations in the target enzyme beta-(1,3)-glucan synthase tend to have less influence on the in vitro antifungal activity of ibrexafungerp. Supporting data for this possibility were provided by Pfaller et al., who noted that 84% of C. glabrata strains with fks mutations were resistant to clinically available echinocandins, compared to only 24% that were resistant to ibrexafungerp (7).
Time-kill studies showed that both ibrexafungerp and micafungin possessed potent fungicidal activity against the susceptible and resistant isolates, with up to 6-log-unit growth inhibition being observed for both. Ibrexafungerp was highly effective against the resistant strain, with exposure to a low concentration of ibrexafungerp (0.25 μg/ml) leading to a dramatic fungicidal effect at 48 h. Moreover, high drug concentrations (4 to 16 μg/ml) led to faster fungicidal effects. These data suggest a time- and concentration-dependent effect against C. glabrata.
The underlying reason for the effectiveness of ibrexafungerp against echinocandin-resistant C. glabrata isolates is unknown. Although ibrexafungerp has the same fungal target as caspofungin and micafungin, it is structurally different, which may present a basis for the difference in antifungal activity, perhaps through a difference in target engagement. Taken together, our data show that ibrexafungerp has potent fungicidal in vitro activity against echinocandin-susceptible and echinocandinresistant isolates, which differentiates it from currently available members of the echinocandin class.
ACKNOWLEDGMENTS
This work was supported by Scynexis, Inc. M.G. acts as a consultant for, receives contracts from, and participates on advisory boards for the following companies: Scynexis, Amplyx Pharmaceuticals, Cidara Therapeutics, and F2G.
REFERENCES
- 1.Pfaller MA, Messer SA, Motyl MR, Jones RN, Castanheira M. 2013. Activity of MK-3118, a new oral glucan synthase inhibitor, tested against Candida spp. by two international methods (CLSI and EUCAST). J Antimicrob Chemother 68:858–863. doi: 10.1093/jac/dks466. [DOI] [PubMed] [Google Scholar]
- 2.Jiménez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. 2014. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida species and Aspergillus species isolates. Antimicrob Agents Chemother 58:1248–1251. doi: 10.1128/AAC.02145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD. 2010. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol 48:2373–2380. doi: 10.1128/JCM.02390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Effron G, Chua DJ, Tomada JR, DiPersio J, Perlin DS, Ghannoum M, Bonilla H. 2010. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob Agents Chemother 54:2225–2257. doi: 10.1128/AAC.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. CLSI document M27-S4 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Klepser ME, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA. 1998. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother 42:1207–1212. doi: 10.1128/AAC.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. 2017. Differential activity of the oral glucan synthase inhibitor SCY-078 against wild-type and echinocandin-resistant strains of Candida species. Antimicrob Agents Chemother 61:e00161-17. doi: 10.1128/AAC.00161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schell WA, Jones AM, Borroto-Esoda K, Alexander BD. 2017. Antifungal activity of SCY-078 and standard antifungal agents against 178 clinical isolates of resistant and susceptible Candida species. Antimicrob Agents Chemother 61:e01102-17. doi: 10.1128/AAC.01102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcos-Zambrano LJ, Gómez-Perosanz M, Escribano P, Bouza E, Guinea J. 2017. The novel oral glucan synthase inhibitor SCY-078 shows in vitro activity against sessile and planktonic Candida spp. J Antimicrob Chemother 72:1969–1976. doi: 10.1093/jac/dkx010. [DOI] [PubMed] [Google Scholar]