LETTER
Recently, a novel plasmid-mediated tigecycline resistance mechanism, tet(X4), has raised a global antimicrobial resistance concern (1, 2), but our understanding of the plasmid vectors associated with tet(X4) dissemination remains limited. Until now, the tet(X4) gene has only been identified on IncQ1, IncF, and IncHI1 plasmids in Escherichia coli strains from pig samples in China (1–3). Here, we report the first identification of tet(X4) on a highly transferable IncX1 plasmid from E. coli of cow origin (Fig. 1a).
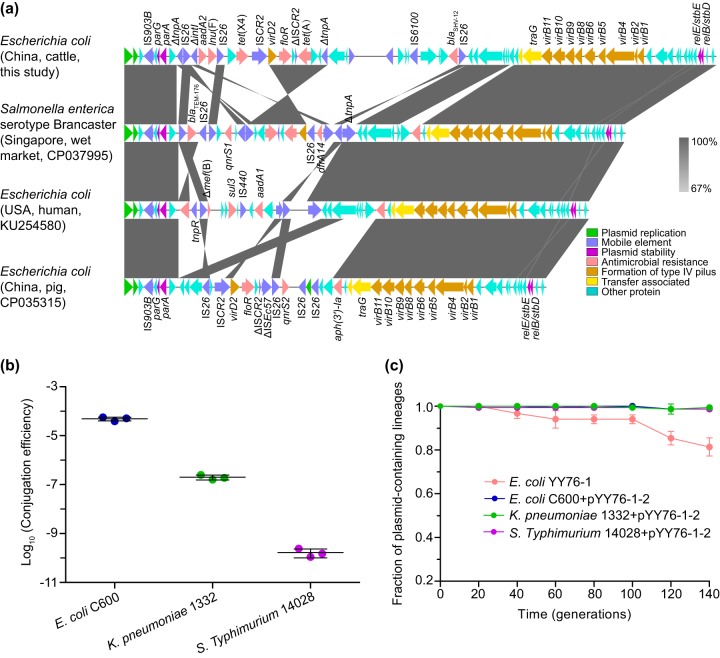
FIG 1.
(a) Comparative analysis of IncX1 plasmids. The arrows represent the positions and transcriptional directions of the open reading frames. Regions of homology ranging from 67% to 100% are marked by shading. The Δ symbol indicates that the gene is truncated. (b) Conjugation transfer efficiencies of the tet(X4)-harboring IncX1 plasmid pYY76-1-2 into E. coli, K. pneumoniae, and S. Typhimurium strains. The transfer efficiency (mean ± standard deviation) is calculated based on colony counts of the transconjugant and recipient cells in triplicate. (c) Plasmid stability experiment results. The fraction of plasmid-containing cells (mean ± standard deviation) is plotted against the passage generations in triplicate.
During a routine antimicrobial resistance surveillance study, a tet(X4)-positive strain, YY76-1, was isolated from the feces of a cow using a MacConkey agar plate containing tigecycline (2 μg/ml) in May 2018 in Hainan, China, which was further identified as E. coli using 16S rRNA sequencing analysis as previously reported (1). Antimicrobial susceptibility testing was conducted using broth microdilution and interpreted according to the CLSI guidelines (4, 5) with the EUCAST breakpoint (http://www.eucast.org/clinical_breakpoints/) for tigecycline. The tet(X4)-positive E. coli YY76-1 was resistant to tigecycline, tetracycline, kanamycin, cefotaxime, ciprofloxacin, florfenicol, and sulfamethoxazole-trimethoprim and also exhibited high MICs to eravacycline (4 μg/ml) and omadacycline (16 μg/ml) (see Table S1 in the supplemental material).
Conjugation experiments showed that the tigecycline resistance could be successfully transferred from E. coli YY76-1 into the recipient E. coli C600 (streptomycin-resistant) by filter mating at a frequency of (4.8 ± 0.7) × 10−5 per recipient cell (Fig. 1b). The resistance phenotypes for tetracycline, cefotaxime, and florfenicol were also cotransferred, as were those for eravacycline (2 μg/ml) and omadacycline (4 μg/ml) (Table S1). Additionally, the same phenotypes could be transferred with tigecycline resistance into the clinical KPC-2-producing sequence type 11 (ST11) Klebsiella pneumoniae 1332 (meropenem-resistant) and Salmonella enterica serovar Typhimurium ATCC 14028 (rifampin-resistant) at the frequency of (2.0 ± 0.4) × 10−7 and (1.7 ± 0.5) × 10−10, respectively (Fig. 1b; Table S1). After 14 days (approximately 140 generations) of passaging without antibiotic challenge, the plasmid was stable in both the parental strain YY76-1 and its transconjugants, with a >75% fraction of plasmid-containing populations (Fig. 1c).
Genomic DNA of E. coli YY76-1 was then completely sequenced using the combination of Nanopore GridION and Illumina HiSeq platforms (Nextomics, Wuhan, China), followed by assembling with Unicycler (6) and data mining via the Center for Genomic Epidemiology (CGE; https://cge.cbs.dtu.dk/services/). The results revealed that YY76-1 belonged to the sequence type 48 (ST48), which has been reported with the spread of NDM and MCR (7, 8). E. coli YY76-1 harbored one chromosome of 4595,444 bp (cYY76-1) and two plasmids of 163,111 bp (IncFIB-like, pYY76-1-1) and 57,104 bp (IncX1, pYY76-1-2), respectively. The tet(X4) gene was found to be on the IncX1 plasmid pYY76-1-2, coharboring aadA2, blaSHV-12, lnu(F), floR, and tet(A) genes (Fig. 1a), which was in accordance with the cotransfer of cefotaxime and florfenicol resistance as described above. Moreover, pYY76-1-1 harbored 6 classes of antimicrobial resistance genes for β-lactams (blaTEM-1B), aminoglycosides [aph(3ʺ)-Ib, aph(6)-Id, aadA2, and aph(3′)-Ia], macrolide-lincosamide-streptogramin B [mph(A) and lnu(F)], phenicols (floR), sulfonamide-trimethoprim (sul1, sul2, and dfrA12), and tetracyclines [tet(B)] (Fig. S1), while the mdf(A) gene was on the chromosome cYY76-1.
A further blastn search of pYY76-1-2 against the NCBI database showed that it had >60% coverage and >99% identity to a number of IncX1 plasmids described from different Enterobacteriaceae species in human (KU254580), pig (CP035315), environmental (CP037995), and other sources. The sequence comparison conducted with Easyfig (9) indicated that they shared highly similar IncX1 plasmid backbones, including the regions for plasmid replication, maintenance, and transfer, but were diverse at the accessory regions with multiple resistance genes (e.g., blaTEM-176 and floR) (Fig. 1a). In addition, the tet(X4) gene in E. coli YY76-1 was identified between an IS26 element upstream and a single copy of ISCR2 downstream (Fig. 1a), which was different from the tet(X4)-harboring structure between two ISCR2 described previously (1).
In summary, we report the first tet(X4)-harboring IncX1 plasmid in E. coli from cattle, highlighting the wide spread of tet(X4) genes in different animal sources. Worrisomely, our previous (1) and current studies suggest that this plasmid-mediated tigecycline resistance mechanism could be readily mobilized and stabilized in clinically important Enterobacteriaceae bacteria, posing a potential threat for the clinical usage of tigecycline (as well as eravacycline and omadacycline) as the last-resort antibiotic to treat multidrug-resistant bacterial infections.
Accession numbers.
The genomic sequences of E. coli YY76-1 have been deposited in GenBank under the accession numbers CP040927 (cYY76-1), CP040928 (pYY76-1-1), and CP040929 (pYY76-1-2). The raw data are also deposited in the SRA (SRR10008514).
Supplementary Material
ACKNOWLEDGMENTS
This study was jointly supported by grants from the National Key Research and Development Program of China (2016YFD0501300), the Program for Innovative Research Team in the University of Ministry of Education of China (IRT_17R39), and the Foundation for Innovation and Strengthening School Project of Guangdong, China (2016KCXTD010).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01528-19.
REFERENCES
- 1.Sun J, Chen C, Cui C-Y, Zhang Y, Liu X, Cui Z-H, Ma X-Y, Feng Y, Fang L-X, Lian X-L, Zhang R-M, Tang Y-Z, Zhang K-X, Liu H-M, Zhuang Z-H, Zhou S-D, Lv J-N, Du H, Huang B, Yu F-Y, Mathema B, Kreiswirth BN, Liao X-P, Chen L, Liu Y-H. 2019. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat Microbiol 4:1457. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He T, Wang R, Liu D, Walsh TR, Zhang R, Lv Y, Ke Y, Ji Q, Wei R, Liu Z, Shen Y, Wang G, Sun L, Lei L, Lv Z, Li Y, Pang M, Wang L, Sun Q, Fu Y, Song H, Hao Y, Shen Z, Wang S, Chen G, Wu C, Shen J, Wang Y. 2019. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat Microbiol 4:1450. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 3.Bai L, Du P, Du Y, Sun H, Zhang P, Wan Y, Lin Q, Fanning S, Cui S, Wu Y. 2019. Detection of plasmid-mediated tigecycline-resistant gene tet(X4) in Escherichia coli from pork, Sichuan and Shandong Provinces, China, February 2019. Euro Surveill 24:1900340 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2019.24.25.1900340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CLSI. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 5.CLSI. 2013. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard-4th edition VET01-A4 and supplement VET01-S2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Wang Y, Walsh TR, Liu D, Shen Z, Zhang R, Yin W, Yao H, Li J, Shen J. 2017. Plasmid-mediated novel blaNDM-17 gene encoding a carbapenemase with enhanced activity in a sequence type 48 Escherichia coli strain. Antimicrob Agents Chemother 61:e02233-16. doi: 10.1128/AAC.02467-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zurfluh K, Nüesch-Inderbinen M, Klumpp J, Poirel L, Nordmann P, Stephan R. 2017. Key features of mcr-1-bearing plasmids from Escherichia coli isolated from humans and food. Antimicrob Resist Infect Control 6:91. doi: 10.1186/s13756-017-0250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.