This study investigated the in vitro activity of finafloxacin against bacterial strain panels of the biodefense pathogens. Broth microdilution assays were performed at neutral and acidic pH to determine the effectiveness of the antibiotics under conditions typical of an intracellular environment. In all instances, finafloxacin demonstrated superior activity at low pH.
Keywords: finafloxacin, in vitro activity, acidic pH, biothreat pathogens, acid environments, biodefense, in vitro
ABSTRACT
This study investigated the in vitro activity of finafloxacin against bacterial strain panels of the biodefense pathogens. Broth microdilution assays were performed at neutral and acidic pH to determine the effectiveness of the antibiotics under conditions typical of an intracellular environment. In all instances, finafloxacin demonstrated superior activity at low pH. These results highlight the importance of evaluating antimicrobial efficacy under conditions relevant to those encountered in vivo.
TEXT
Antimicrobial resistance is an evolving issue, and new therapeutics are needed to treat infections caused by the pathogens of biodefense interest and those that are considered to be of public health concern. It is important that new antimicrobials are evaluated under conditions that model those encountered within the environment of a host, including the low-pH environment of the cell (the phagolysosome) that is particularly relevant to intracellular pathogens and infected body sites. It has been shown previously that the activity of certain classes of antibiotics (including fluoroquinolones) can be affected by a reduction in pH (1–4). Finafloxacin is a fluoroquinolone derivative with an 8-cyano substituent and 7-pyrrolo-oxazinyl moiety that is being developed for the treatment of urinary tract infections in hospitalized patients (5, 6). This modification has conferred activity in low-pH environments, which has resulted in superior in vitro activity against a range of organisms, including Staphylococcus aureus and Acinetobacter baumannii (7, 8).
The availability of formulations of finafloxacin that can be delivered orally and systemically makes finafloxacin a worthy alternative for the treatment of a range of infections. In addition to good safety and efficacy data obtained in patients suffering from complicated urinary tract infections and pyelonephritis, previous studies have also demonstrated efficacy against the biothreat agents Burkholderia pseudomallei and Francisella tularensis in vitro and in vivo (6, 9–11). The aim of this study was to further evaluate the in vitro activity of finafloxacin against larger strain panels of biodefense pathogens.
Antibiotic susceptibility was determined at pH 5 and pH 7 for B. pseudomallei (n = 10), Burkholderia mallei (n = 10), F. tularensis (n = 10), Bacillus anthracis (n = 10), and Yersinia pestis (n = 10), held at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) (Table 1). In addition, a B. pseudomallei strain panel (n = 11) provided by the Biomedical Advanced Research and Development Authority (BARDA) was screened (Table 1) (12). These assays were performed under biosafety level 3 (BSL3) conditions. Antibiotic susceptibility was reported as the MIC50 or MIC90, defined as the lowest concentration of the antibiotic at which the growth of 50% or 90% of the isolates, respectively, were inhibited.
TABLE 1.
Panel of bacterial strains evaluated
| Organism | Straina | Origin | Source |
|---|---|---|---|
| B. pseudomallei | 316c | Thailand | Human |
| E203 | Thailand | Unknown | |
| NCTC4845 | Singapore | Monkey | |
| STW115-2 | Thailand | Water | |
| STW199-2 | Thailand | Water | |
| E8 | Thailand NE | Soil | |
| P52237 | Vietnam | Unknown | |
| WRAIR1188 | Malaysia | Human | |
| K96243 | Thailand | Human | |
| 1026b | Thailand | Human | |
| K96243* | Thailand | Human | |
| 1026b* | Thailand | Human | |
| HBPUB10134A* | Thailand | Human | |
| HBPUB10303A* | Thailand | Human | |
| 1106a* | Thailand | Human | |
| MSHR 305* | Australia | Human | |
| MSHR 668* | Australia | Human | |
| MSHR 5855* | Australia | Human | |
| MSHR 5848* | Australia | Human | |
| MSHR 5858* | Thailand | Human | |
| 406e* | Thailand | Human | |
| F. tularensis | LVS | Former Soviet Union | Water rat |
| OR01-1807 | USA | Unknown | |
| FRAN003 | USA | Unknown | |
| FRAN005 | USA | Unknown | |
| FRAN006 | USA | Unknown | |
| FRAN007 | USA | Unknown | |
| FRAN012 | USA | Unknown | |
| FRAN013 | USA | Unknown | |
| FRAN016 | USA | Unknown | |
| SCHUS4-1 | USA | Human | |
| B. anthracis | Vollum1B | USA | Bovine |
| Sterne | South Africa | Bovine | |
| Ames | USA | Bovine | |
| K1938 | Indonesia | Unknown | |
| K5926 | India | Unknown | |
| K7038 | South Korea | Unknown | |
| SK57 | England | Unknown | |
| K7978 | Namibia | Unknown | |
| Africa33 | South Africa | Unknown | |
| K8091 | Norway | Unknown | |
| B. mallei | GB3 (ATCC 120) | UK | Unknown |
| GB4 | Turkey | Human | |
| GB5 | Hungary | Unknown | |
| GB6 | Turkey | Human | |
| GB7 | Turkey | Unknown | |
| GB8 (China7) | Burma | Human | |
| GB9 | India | Mule | |
| GB10 | India | Horse | |
| GB11 | China | Horse | |
| GB12 | Hungary | Unknown | |
| Y. pestis | CO92 | USA | Human |
| C12 | USA | Human | |
| antiqua | Congo | Human | |
| pestoidesB | Former Soviet Union | Human | |
| pestoides Fmp1 | Former Soviet Union | Human | |
| Yeo154 | Japan | Human | |
| Angola | Angola | Human | |
| Java9 | Indonesia | Human | |
| M111(74) | Madagascar | Human | |
| LaPaz | Bolivia | Human |
Strains with an asterisk belong to the BARDA strain panel. All other strains were obtained from the USAMRIID Unified Culture Collection (UCC), Frederick, MD, USA.
Finafloxacin was supplied by MerLion Pharmaceuticals GmbH, and all other antibiotics were sourced from the U.S. Pharmacopeia, Selleckchem, or Sigma. Broth microdilution assays were performed as detailed by the Clinical and Laboratory Standards Institute (CLSI) (13), with the exception of a medium supplement (2%), IsoVitaleX (Becton, Dickinson), used to support the growth of F. tularensis. The activity of finafloxacin was determined at pH 5 and pH 7 (if the bacterial species was able to be cultured) and the MICs determined.
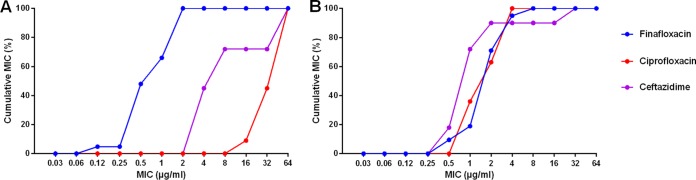
At pH 5, the MICs for B. pseudomallei ranged from 0.12 to 2 μg/ml, 16 to 64 μg/ml, and 4 to 64 μg/ml for finafloxacin, ciprofloxacin (CIP), and ceftazidime (CAZ), respectively, demonstrating the superior in vitro activity of finafloxacin at low pH. Although it is difficult to make comparisons between the efficacies of antibiotics simply by MIC, these values are lower than those determined for another fluoroquinolone, CIP, and a component of the treatment for B. pseudomallei infections in humans (CAZ) (Fig. 1A). At neutral pH, finafloxacin demonstrated a level of activity (0.5 to 8 μg/ml) similar to those observed with CIP (1 to 4 μg/ml) and CAZ (0.5 to 32 μg/ml) (Fig. 1B).
FIG 1.
Cumulative MICs determined for a panel of B. pseudomallei strains for finafloxacin (n = 21), ciprofloxacin (n = 11), and ceftazidime (n = 11) at pH 5 (A) and pH 7 (B).
A similar trend was observed with the other pathogens of biodefense interest. Finafloxacin had superior activity at pH 5 for B. anthracis, B. mallei, and Y. pestis compared to either CIP or azithromycin (AZM) (an antibiotic used for the treatment of B. mallei infection in humans and as a control in the in vitro assays) (Table 2). Unfortunately, only two strains of F. tularensis could be cultured in this low-pH environment; therefore, the MIC50 and MIC90 at pH 5 could not be determined. The most striking difference was observed for B. mallei. Finafloxacin had 9-fold and 7-fold improved activity over that of AZM against a panel of these strains (MIC50, 0.12 μg/ml compared to >64 μg/ml; MIC90, 0.5 μg/ml compared to >64 μg/ml) when performed at pH 5 (Table 2). At pH 7, finafloxacin demonstrated activity similar to those of the comparator antibiotics, with MIC50 and MIC90 of 0.5 μg/ml (at both pHs) for B. mallei and 0.06 μg/ml and 0.12 μg/ml for B. anthracis, respectively (Table 2).
TABLE 2.
MIC50, MIC90, and MIC range values determined for panels of the biothreat pathogens
| Species | MIC (μg/ml) by pHa
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 |
MIC90 |
Range |
||||||||||||||||
| pH 5 |
pH 7 |
pH 5 |
pH 7 |
pH 5 |
pH 7 |
|||||||||||||
| FIN | CIP | AZM | FIN | CIP | AZM | FIN | CIP | AZM | FIN | CIP | AZM | FIN | CIP | AZM | FIN | CIP | AZM | |
| B. anthracis | ≤0.03 | 0.06 | ND | 0.06 | 0.03 | ND | ≤0.03 | 0.06 | ND | 0.12 | 0.03 | ND | ≤0.03 to 0.06 | 0.03 to 0.06 | ND | 0.06 to 0.12 | 0.03 to 0.06 | ND |
| B. mallei | 0.12 | ND | >64 | 0.5 | ND | 0.25 | 0.5 | ND | >64 | 0.5 | ND | 0.5 | ≤0.03 to 0.5 | ND | 4 to >64 | ≤0.03 to 0.5 | ND | 0.06 to 0.5 |
| Y. pestis | ≤0.03 | 0.5 | ND | ≤0.03 | 0.015 | ND | ≤0.03 | 1 | ND | 0.06 | 0.03 | ND | ≤0.03 | 0.12 to 1 | ND | ≤0.03 to 0.12 | 0.008 to 0.03 | ND |
| F. tularensis | ND | ND | ND | ≤0.03 | 0.015 | ND | ND | ND | ND | ≤0.03 | 0.03 | ND | ND | ND | ND | ≤0.03 | 0.008 to 0.25 | ND |
ND, not determined.
The data set detailed in these studies demonstrates that finafloxacin has activity under both acidic and neutral conditions, with enhanced activity of finafloxacin in low-pH environments, where other antibiotics (including ciprofloxacin) have reduced activity. This has been demonstrated for all of the biodefense pathogens of interest and is in agreement with data generated by other groups (7, 8, 10, 11). The improved activity of finafloxacin compared to that of ciprofloxacin (a typical treatment for infections caused by B. anthracis, Y. pestis, and F. tularensis) further highlights the importance of evaluating therapies under conditions considered to be more like those encountered within a host and identifies finafloxacin as a novel broad-spectrum fluoroquinolone that could be used for prophylaxis or treatment following exposure to a range of pathogens.
Of particular interest is the activity of finafloxacin against the Burkholderia species evaluated. It has been demonstrated previously that fluoroquinolones are not effective as treatment for melioidosis in humans mainly due to B. pseudomallei possessing resistance mechanisms, including efflux pumps (14–17). The results detailed in this communication suggest that finafloxacin is not affected by the efflux pumps in B. pseudomallei that confer resistance to other fluoroquinolones, possibly due to the effect of the modified chemical structure (7, 10, 18). The promising data generated for B. mallei suggest that finafloxacin is a potential alternative for the treatment of infection caused by this organism.
Finafloxacin appears to have a wider spectrum of activity than the other fluoroquinolones and has the potential to be used to treat infections caused by all of the biothreat pathogens evaluated (19). It has also been shown to be safe and well tolerated in clinical trials (6). These encouraging in vitro findings warrant further investigation of finafloxacin which would determine whether this activity translates into comparable protection against all of these pathogens in vivo.
ACKNOWLEDGMENTS
This work was funded by the Defense Threat Reduction Agency (DTRA).
We acknowledge the Biomedical Advanced Research and Development Authority (BARDA) for use of their B. pseudomallei strain panel. These strains were developed with federal funds from the Office of the Assistant Secretary for Preparedness and Response, BARDA, under contract number HHSO100201100008I, task order HHSO10033001T, contract number HHSO100201100005I, and task order HHSO10033001T.
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
We declare no conflicts of interest.
REFERENCES
- 1.Akova M, Gür D, Livermore DM, Kocagöz T, Akalin HE. 1999. In vitro activities of antibiotics alone and in combination against Brucella melitensis at neutral and acidic pHs. Antimicrob Agents Chemother 43:1298–1300. doi: 10.1128/AAC.43.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cyphert EL, Wallat JD, Pokorski JK, von Recum HA. 2017. Erythromycin modification that improves its acidic stability while optimizing it for local drug delivery. Antibiotics 6:11–15. doi: 10.3390/antibiotics6020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, Wang K, Li H, Denstedt JD, Cadieux PA. 2014. The influence of urinary pH on antibiotic efficacy against bacterial uropathogens. Urology 84:731.e1–731.e7. doi: 10.1016/j.urology.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 4.Badoux P, Bles N, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, van Bambeke F. 2007. Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamics models of extracellular and intracellular infections. J Antimicrob Chemother 59:246–253. doi: 10.1093/jac/dkl489. [DOI] [PubMed] [Google Scholar]
- 5.Kocsis B, Domokos J, Szabo D. 2016. Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann Clin Microb Antimicrob 15:34. doi: 10.1186/s12941-016-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagenlehner F, Nowicki M, Bentley C, Lückermann M, Wohlert S, Fischer C, Vente A, Naber K, Dalhoff A. 2018. Explorative randomized phase II clinical study of the efficacy and safety of finafloxacin versus ciprofloxacin for treatment of complicated urinary tract infections. Antimicrob Agents Chemother 62:e02317-17. doi: 10.1128/AAC.02317-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemaire S, Van Bambeke F, Tulkens PM. 2011. Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila. Int J Antimicrob Agents 38:52–59. doi: 10.1016/j.ijantimicag.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Higgins PG, Stubbings W, Wisplinghoff H, Seifert H. 2010. Activity of the investigational fluoroquinolone finafloxacin against ciprofloxacin-sensitive and resistant Acinetobacter baumannii isolates. Antimicrob Agents Chemother 54:1613–1615. doi: 10.1128/AAC.01637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vente A, Bentley C, Lückermann M, Tambyah P, Dalhoff A. 2018. Early clinical assessment of the antimicrobial activity of finafloxacin compared to ciprofloxacin in subsets of microbiologically characterized isolates. Antimicrob Agents Chemother 62:e02325-17. doi: 10.1128/AAC.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes KB, Hamblin KA, Richards MI, Laws TR, Vente A, Atkins HS, Harding SV. 2017. Demonstrating the protective efficacy of the novel fluoroquinolone finafloxacin against an inhalational exposure to Burkholderia pseudomallei. Antimicrob Agents Chemother 61:e00082-17. doi: 10.1128/AAC.00082-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes KB, Hamblin KA, Richards MI, Laws TR, Vente A, Atkins HS, Harding SV. 2019. The fluoroquinolone finafloxacin protects BALB/c mice against an intranasal infection with Francisella tularensis strain SchuS4. Front Microbiol 2:904. doi: 10.3389/fmicb.2019.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Zandt K, Tuanyok A, Keim PS, Warren RL, Gelhaus HC. 2012. An objective approach for Burkholderia pseudomallei strain selection as challenge material for medical countermeasures efficacy testing. Front Cell Infect Microbiol 2:120. doi: 10.3389/fcimb.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Schweizer HP. 2012. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 7:1389–1399. doi: 10.2217/fmb.12.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore RA, Deshazer D, Reckseidler S, Weissman A, Woods DE. 1999. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob Agents Chemother 43:465–470. doi: 10.1128/AAC.43.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan YY, Tan TMC, Ong YM, Chua KL. 2004. BpeAB-OprB, a multidrug efflux pump in Burkholderia pseudomallei. Antimicrob Agents Chemother 48:1128–1135. doi: 10.1128/aac.48.4.1128-1135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mima T, Schweizer HP. 2010. The BpeAB–OprB efflux pump of Burkholderia pseudomallei 1026b does not play a role in quorum sensing, virulence factor production, or extrusion of aminoglycosides but is a broad-spectrum drug efflux system. Antimicrob Agents Chemother 54:3113–3120. doi: 10.1128/AAC.01803-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randall LB, Georgi E, Genzel GH, Schweizer HP. 2017. Finafloxacin overcomes Burkholderia pseudomallei efflux-mediated fluoroquinolone resistance. J Antimicrob Chemother 72:1258–1260. doi: 10.1093/jac/dkw529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stubbings W, Leow P, Yong GC, Goh F, Körber-Irrgang B, Kresken M, Endermann R, Labischinski H. 2011. In vitro spectrum of activity of finafloxacin, a novel, pH-activated fluoroquinolone, under standard and acidic conditions. Antimicrob Agents Chemother 55:4394–4397. doi: 10.1128/AAC.00833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]