LETTER
Emerging novel resistance mechanisms pose a great public health concern. Recently, two studies reported the emergence of plasmid-mediated tigecycline resistance genes tet(X3) and tet(X4) among Enterobacteriaceae and Acinetobacter (1, 2), which suggested that the efficacy of tigecycline as a last-resort drug to treat multidrug-resistant (MDR) severe infections would be impaired. Despite low prevalence of tet(X) in clinical strains, the widespread presence of tet(X) in various bacteria is a great concern (1, 3). Here, we genetically and functionally investigated an Empedobacter brevis strain, SE1-3, harboring one novel plasmid-mediated tet(X3.2) variant of shrimp origin.
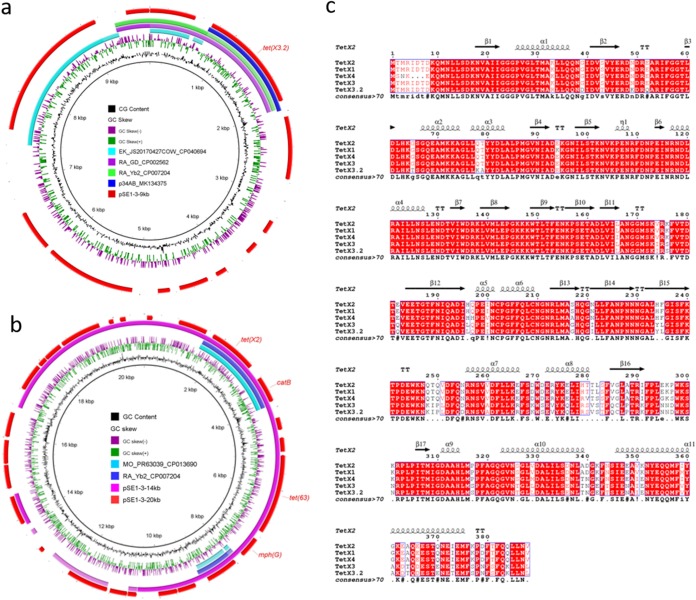
One strain SE1-3 isolate conferring resistance to tigecycline from a shrimp sample in Yangzhou, China, was isolated in June 2019. With 16S rRNA sequencing, this strain was identified as Empedobacter brevis, which can be involved in nosocomial infections (4). PCR with primers targeting tet(X) was performed (1), and two different tet(X) variants were identified after sequencing. MICs against different antibiotics were detected using the broth microdilution method with Escherichia coli ATCC 25922 as the control (5). A conjugation assay was performed with E. coli J53 AriZ as the receipt strain but failed after three repeats. An electrotransformation assay failed to obtain a positive transformant for extracted plasmids with E. coli EC600 and DH5α as receipt strains after three repeats. Whole-genome sequencing (WGS) combining short-read Illumina and long-read Nanopore MinION platforms was performed, followed by hybrid de novo assembly, long-read analysis, and annotation (6–8). Strain SE1-3 harbored one chromosome (3,591,742 bp, 32.7% GC content) and three plasmids, pSE1-3-20kb (20,734 bp, 31.4% GC content), pSE1-3-14kb (14,090 bp, 31.8% GC content), and pSE1-3-9kb (9,780 bp, 30.1% GC content). The GC content of the chromosome and plasmids in SE1-3 was consistent with that of four deposited Empedobacter brevis genomes (31.1% to 32.8%) in the NCBI database. One novel tet(X) variant, designated tet(X3.2), showing 97.26% and 84.29% identity to tet(X3) and tet(X4), respectively, was identified in pSE1-3-9kb (Fig. 1a). Tet(X3.2) displayed the most similarity (97.94%) to Tet(X3) with eight amino acid substitutions (Fig. 1c). No other resistance genes, replicons, or insertion sequences were found in pSE1-3-9kb, and no similar structure was retrieved from the NCBI nonredundant (nr) database, suggesting that this was a novel tet(X)-mediated plasmid. The surrounding genetic environment was different from that of tet(X3) and tet(X4), indicating that tet(X3.2) has not acquired the ability to transfer, which was proved by unsuccessful conjugation and electrotransformation assays. The cooccurrence of two instances of tet(X2) in two similar plasmids, pSE1-3-20kb and pSE1-3-14kb, of the strain was observed (Fig. 1b). Online BLASTn search of tet(X2) indicated that it mainly existed in non-Enterobacteriaceae bacteria such as Myroides odoratimimus. However, the occurrence of tet(X2) in Enterobacteriaceae should be considered. The tet(X3.2)-bearing plasmid pSE1-3-9kb was most similar to the chromosome of an Elizabethkingia species strain (CP040694, 99.90% identity at 26% coverage) and Riemerella anatipestifer (CP007204, 98.88% identity at 23% coverage) (Fig. 1a). This observation shed light on the origin of tet(X3.2).
FIG 1.
Circular comparison of tet(X)-bearing sequences and protein alignment of Tet(X) variants. (a) Comparison between tet(X3.2)-bearing plasmid pSE1-3-9kb, tet(X3)-bearing plasmid p34AB, and other similar sequences in the nr database. The outermost circle denotes the reference plasmid pSE1-3-9kb, with arrows showing the predicted coding sequences. (b) Comparison between tet(X2)-bearing plasmids pSE1-3-20kb, pSE1-3-14kb, and other similar sequences in the nr database. (c) Alignment of Tet(X3.2) reported in this study and other Tet(X) variants generated using ESPript 3.0 (9). A secondary structure based on the Tet(X2) protein (PDB number 4A6N) served as the structure reference.
To probe the function of tet(X3.2), the full length of tet(X3.2) in SE1-3 was amplified using PCR with primers tet(X)-F cgagctcATGACAATGCGAATAGATAC and tet(X)-R cccaagcttTACTGTTTATAGATTCAA, cloned into the pET28a vector, and transformed chemically into E. coli BL21, and the MICs were tested against different tetracycline antibiotics. Under IPTG (isopropyl-β-d-thiogalactopyranoside) induction, E. coli BL21 harboring the pET28a-tet(X3.2) conferred resistance to tetracycline (64 mg/liter), tigecycline (8 mg/liter), oxytetracycline (16 mg/liter), doxycycline (64 mg/liter), and minocycline (32 mg/liter) with at least 16-fold increased MICs compared with BL21 without the vector (see Table S1 in the Supplemental Material). This demonstrated that tet(X3.2) encoded similar resistance phenotypes as tet(X3) and tet(X4) (1, 2).
To conclude, a novel plasmid-mediated tigecycline resistance gene, tet(X3.2), identified from a food sample, was characterized. The potential risk of tet(X3.2) occurrence in Enterobacteriaceae warrants intense surveillance. Considering the wide distribution of tet(X) genes in different sources, other novel tet(X) variants conferring resistance to tigecycline should be investigated.
Data availability.
The complete genome sequences of SE1-3 have been deposited in the NCBI database under BioProject accession number PRJNA563978 (CP043637.1, CP043636.1, CP043635.1, and CP043634.1).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grants 31872523 and 31871899), the Natural Science Foundation of Jiangsu Province (grant BK20180900), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01636-19.
REFERENCES
- 1.He T, Wang R, Liu D, Walsh TR, Zhang R, Lv Y, Ke Y, Ji Q, Wei R, Liu Z, Shen Y, Wang G, Sun L, Lei L, Lv Z, Li Y, Pang M, Wang L, Sun Q, Fu Y, Song H, Hao Y, Shen Z, Wang S, Chen G, Wu C, Shen J, Wang Y. 2019. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat Microbiol 4:1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Chen C, Cui CY, Zhang Y, Liu X, Cui ZH, Ma XY, Feng Y, Fang LX, Lian XL, Zhang RM, Tang YZ, Zhang KX, Liu HM, Zhuang ZH, Zhou SD, Lv JN, Du H, Huang B, Yu FY, Mathema B, Kreiswirth BN, Liao XP, Chen L, Liu YH. 2019. Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat Microbiol 4:1457–1464. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leski TA, Bangura U, Jimmy DH, Ansumana R, Lizewski SE, Stenger DA, Taitt CR, Vora GJ. 2013. Multidrug-resistant tet(X)-containing hospital isolates in Sierra Leone. Int J Antimicrob Agents 42:83–86. doi: 10.1016/j.ijantimicag.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Bellais S, Girlich D, Karim A, Nordmann P. 2002. EBR-1, a novel Ambler subclass B1 beta-lactamase from Empedobacter brevis. Antimicrob Agents Chemother 46:3223–3227. doi: 10.1128/aac.46.10.3223-3227.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing: twenty-fourth informational supplement M100-S24. CLSI, Wayne, PA. [Google Scholar]
- 6.Li R, Xie M, Dong N, Lin D, Yang X, Wong MHY, Chan EW, Chen S. 2018. Efficient generation of complete sequences of MDR-encoding plasmids by rapid assembly of MinION barcoding sequencing data. Gigascience 7:1–9. doi: 10.1093/gigascience/gix132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, Chen K, Chan EWC, Chen S. 2018. Resolution of dynamic MDR structures among the plasmidome of Salmonella using MinION single-molecule, long-read sequencing. J Antimicrob Chemother 73:2691–2695. doi: 10.1093/jac/dky243. [DOI] [PubMed] [Google Scholar]
- 8.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia FF, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete genome sequences of SE1-3 have been deposited in the NCBI database under BioProject accession number PRJNA563978 (CP043637.1, CP043636.1, CP043635.1, and CP043634.1).