Even though antibiotic resistance in bacteria is a natural phenomenon, the alarming increase in pathogenic bacteria refractory to a wide range of antimicrobials is attracting attention worldwide. Indeed, the World Health Organization (WHO) has recently published a list of priority pathogens for which new antimicrobial alternatives are urgently needed. Among these pathogens, methicillin-resistant Staphylococcus aureus (MRSA) strains are perhaps the best known by the general public.
KEYWORDS: Staphylococcus aureus, HA-MRSA, CA-MRSA, LA-MRSA, antibiotic resistance, soft-tissue infections, osteomyelitis, endocarditis, phage therapy, endolysins, bacteriophage therapy, bacteriophages, endolysin
ABSTRACT
Even though antibiotic resistance in bacteria is a natural phenomenon, the alarming increase in pathogenic bacteria refractory to a wide range of antimicrobials is attracting attention worldwide. Indeed, the World Health Organization (WHO) has recently published a list of priority pathogens for which new antimicrobial alternatives are urgently needed. Among these pathogens, methicillin-resistant Staphylococcus aureus (MRSA) strains are perhaps the best known by the general public. In addition to its potential to acquire antibiotic resistance, S. aureus can produce a large number of virulence factors, such as hemolysins, enterotoxins, and proteases, and exhibits the ability to form biofilms as well as to evolve into different clones that can spread and colonize new environments. This review provides a brief overview of the latest options in antibacterial therapies, mainly focusing on phage therapy. In this regard, the current stage of research about antimicrobial compounds based on bacteriophages and endolysins against MRSA infections is shown and discussed.
INTRODUCTION
Antimicrobial resistance in pathogenic bacteria is an urgent health problem that must be addressed by governments and health authorities worldwide. Recent estimates based on clinical data indicate that approximately 25,000 deaths per year in the European Union and 700,000 globally are due to infections caused by antibiotic-resistant pathogens (1). This type of data has prompted the start of several programs intended for stopping this alarming trend. In particular, the World Health Organization (WHO) is making a considerable effort to implement a global action plan to tackle antimicrobial resistance by setting strategic goals, which include awareness, surveillance, and research of antimicrobial resistance. Additionally, this plan aims to promote the development of new medicines, diagnostic tools, and vaccines. Following these guidelines, the European authorities have established a common strategy, which has been laid out in different official documents (e.g., the Conclusions of the European Union Council of 29 May 2012). Importantly, all Member States have agreed to implement action plans to control the development of antimicrobial resistance from the perspective of both human and veterinary medicine (One Health Approach). As a result of this strategy, several countries have already created their own national plans and published reports about antibiotic consumption and how it relates with resistance development in human and animal health environments.
One of the most problematic bacterial pathogens at present is Staphylococcus aureus, the causative agent of skin and soft tissue infections (SSTIs), osteomyelitis, endocarditis, and pneumonia, among other serious infections, all of which can ultimately lead to bacteremia and sepsis. Strains of this bacterium have been gradually acquiring antimicrobial resistance determinants throughout the antibiotic era. Methicillin resistance in S. aureus is due to the presence of the mecA (or mecC) gene, which encodes the penicillin-binding protein PBP2a, which has low affinity for semisynthetic penicillins (2). These methicillin resistance determinants are part of a mobile genetic element termed the staphylococcal cassette chromosome mec (SCCmec), which is inserted into the S. aureus chromosome. The most recent data regarding methicillin-resistant Staphylococcus aureus (MRSA) incidence, obtained from 85 (44%) of the WHO member states, reported values exceeding 20% in all WHO regions, and even 80% in some countries. Interestingly, data from 2013 to 2016 have shown a decrease in the prevalence of MRSA in the United States and Europe, although the mortality rates are still very high. For most European countries, MRSA prevalence among invasive S. aureus isolates ranges between 1.2 and 50.5%, with higher percentages in the Mediterranean countries than in Northern Europe. However, the prevalence of methicillin resistance has increased in some countries (e.g., Spain), reaching values of 25.8%. To make the situation even worse, resistance to other antibiotics (macrolides, lincosamides, and type B streptogramins) and intermediate resistance to vancomycin are currently emerging (3). MRSA strains causing infections in humans can be divided into the following three categories according to their origin: health care-associated MRSA (HA-MRSA), community-associated MRSA (CA-MRSA), and livestock-associated MRSA (LA-MRSA). HA-MRSA strains are endemic in many hospitals worldwide, and consequently are one of the main causes of nosocomial infections. S. aureus can be a commensal bacterium frequently found on the skin and mucous membranes of healthy individuals. Indeed, about 20% of individuals in the general population are nasal carriers. This fact increases the risk of self-infection and/or of transmitting the pathogen to patients through the hands of health care workers or equipment. Routine decolonization of patients with mupirocin is only usually performed before high-risk surgery (cardiothoracic and orthopedic) due to its high cost and risk of bacterial resistance selection.
CA-MRSA strains usually affect apparently healthy individuals and exhibit clear differences from HA-MRSA strains regarding resistance to other antibiotics and the presence of the virulence factor Panton-Valentine leucocidin (PVL) (4). However, CA-MRSA isolates have become an important cause of hospital outbreaks. Finally, LA-MRSA strains have been detected in both farm and companion animals, as well as in food from animal sources, including pigs, poultry, and cattle. For this reason, the European Food Safety Authority (EFSA) recommends monitoring food-producing animals in order to follow the evolution of zoonotically acquired MRSA in humans. Although LA-MRSA strains have been traditionally considered poor colonizers of humans, recent evidence has shown that the clone CC398, the most frequently isolated from animals, is transmissible between humans. Moreover, a recent report suggested that some LA-MRSA strains from poultry meat can colonize humans (5), which is indicative of the remarkable ability of this pathogen to evolve and adapt to different hosts and environmental conditions. In this review, we aim to show and discuss the possibilities offered by bacteriophages and endolysins, especially highlighting the properties that make these antimicrobials suitable for fighting against this pathogen.
NEW AND DEVELOPING TREATMENTS
The challenge of successfully treating MRSA infections has intensified research on novel prophylaxis and treatment strategies. Indeed, several new drugs are currently under different stages of development, including some which have already been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Some of the most promising antimicrobials are shown in Table 1 and include new antibiotics belonging to different families (reviewed in reference 6). Indeed, broad-spectrum lipoglycopeptides (dalbavancin, oritavancin, and telavancin), a glycopeptide-cephalosporin antibiotic (TD-1792), two cephalosporins (ceftaroline and ceftobiprole), iclaprim (analogue of trimethoprim), oxazolidinones (tedizolid and radezolid), tetracyclines (eravacycline and omadacycline), fluoroquinolones (delafloxacin, zabofloxacin, and nemonoxacin), and pleuromutilins (retapamulin and lefamulin) are under different stages of development or undergoing clinical trials, and some of them have already been approved. A remarkable step forward in the fight against bacterial pathogens has been the recent development of a compound active against persister cells, the main cause of chronic and recurrent infections. Persisters are nongrowing or dormant bacterial subpopulations with high tolerance to antibiotics, being therefore very difficult to eliminate. Two synthetic retinoids (CD437 and CD1530) have the ability to disrupt lipid bilayers and kill bacterial cells, even those in the persister state (7). Similarly, some research has focused on compounds with activity against small-colony variants (SCV), which are low-growth-rate cells with auxotrophy for key compounds and which have been associated with a poor response to antibiotic treatment. Four new drugs (daunorubicin, ketoconazole, rifapentine, and sitafloxacin) have been identified (8). Other strategies, such as vaccines, are also being explored. In this regard, a d-alanine auxotrophic live vaccine (9), which elicits a protective immune response in mice and generates cross-reactive antibodies, is a promising strategy to be used for prophylaxis purposes in both high-risk patients and health care workers to avoid S. aureus infection and transmission, respectively. It is also worth highlighting that standard S. aureus attenuated vaccines do not trigger an immune response, probably because this bacterium is a common colonizer of the human body. Several passive immunization strategies are currently in preclinical development phase. They aim to target and block several different staphylococcal virulence determinants involved in pathogenesis. For example, monoclonal antibodies (MAbs) targeting enterotoxin K (SEK) and alpha-toxin were evaluated for the prevention or treatment of S. aureus sepsis and pneumonia (10). Finally, some antimicrobial peptides (AMPs), such as aurein peptides formulated in a pegylated micelle, were found to have potential for treating MRSA skin infections (11).
TABLE 1.
Main compounds under development or recently approved for the treatment of MRSA infections
| Antimicrobial groupd | Compound name(s)c | Disease(s) treatedb | Approved for MRSA treatmenta |
|---|---|---|---|
| New antibiotics | Dalbavancin | ABSSSIs | N |
| Oritavancin | SSTIs | N | |
| Telavancin | SSTIs and pneumonia | Y | |
| TD-1792 | SSTIs and ABSSSIs | N | |
| Ceftaroline | CAP and ABSSSIs | Y | |
| Ceftobiprole | CAP and HAP | Y | |
| Iclaprim | SSTIs and pneumonia | N | |
| Tedizolid | ABSSSIs | Y | |
| Radezolid | Acne vulgaris and bacterial vaginosis | N | |
| Eravacycline | Intraabdominal and urinary infections | Y | |
| Omadacycline | ABSSSIs and CAP | Y | |
| Delafloxacin | SSTIs | Y | |
| Zabofloxacin | CAP and chronic obstructive pulmonary disease | N | |
| Nemonoxacin | CAP | N | |
| Retapamulin | CAP and ABSSSIs | Y | |
| Lefamulin | CAP and ABSSSIs | N | |
| Anti-persister/SCV bacteria | CD437 and CD1530 | Chronic and recurrent infections | N |
| Daunorubicin, ketoconazole, rifapentine, and sitafloxacin | Chronic and recurrent infections | N | |
| Vaccines | d-Alanine auxotrophic live vaccine | Sepsis | N |
| Passive immunization | MAbs (alpha toxin) | Pneumonia | N |
| MAbs (enterotoxin K) | Sepsis | N | |
| AMPs | Aurein peptides | Skin infections | N |
Y, yes; N, no.
ABSSSI, acute bacterial skin and skin structure infection; SSTI, skin and soft tissue infections; CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia.
MAb, monoclonal antibody.
SCV, small-colony variant; AMP, antimicrobial peptide.
PHAGE THERAPY: PRECLINICAL AND CLINICAL STUDIES
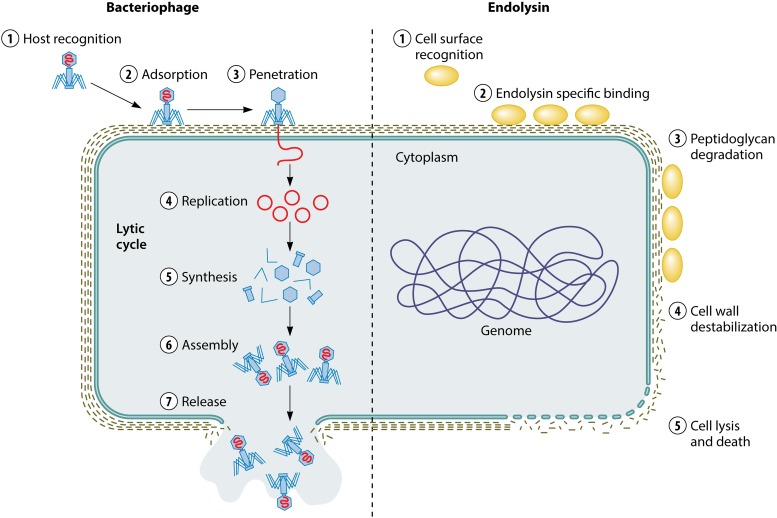
Rescued from the past, phage therapy is also a promising alternative for MRSA infection treatment. In fact, bacteriophages (in short, phages) have been used to fight infectious diseases from the early 20th century, mainly in Eastern European countries, whereas the arrival of antibiotics displaced their use in Western countries (12). In the last decade, we have witnessed the resurgence of phage research, mainly focusing on their use against multidrug-resistant bacteria. Moreover, phage lytic proteins (endolysins or enzybiotics) are also promising candidates as new antimicrobials (13) (Fig. 1).
FIG 1.
Lytic activity of bacteriophages and endolysins against bacterial cells.
In the case of S. aureus, several studies have proved the efficacy of phages and endolysins specific against this pathogen in different animal models and human clinical trials. For example, animal models of prosthetic joint infections have been used to assess the efficacy of phage φMR-5 in combination with linezolid to reduce bacterial adherence, which resulted in an earlier recovery of motion and leg function (14). Another interesting example is the treatment of diabetic wound infections with phages φMR-5 and φMR-10 (109 PFU) in a mouse model, which resulted in an improvement of the infection resolution rate and sped up wound healing (15). In this study, liposomes were used as delivery vehicles to increase the persistence of phages before reaching their target.
In addition to animal studies, the compassionate use of phages to treat patients with MRSA infections has also been quite successful. For instance, a recent publication reported the administration of a highly purified staphylococcal bacteriophage from the Eliava Institute (Tbilisi, Georgia) to treat osteomyelitis in an ulcerated foot from a diabetic female patient who refused amputation and/or long-term antibiotics. Injections (0.7 ml) containing the phage suspension were administered once a week for 7 weeks, until the ulcer healed (9). Similarly, local injection of a selected cocktail of 6 bacteriophages infecting Pseudomonas aeruginosa and S. aureus was applied locally (6 vials, 1010 PFU/ml of each bacteriophage) to a female patient with relapsing S. aureus prosthetic joint infection. In this example, treatment was safe and no clinical signs of persistent infection were observed (16).
Regarding clinical trials in humans, a phase I trial has been completed for the polyvalent phage preparation WPP-201 containing 8 bacteriophages against P. aeruginosa, S. aureus, and Escherichia coli causing venous leg ulcers but no results have been released yet (17). AmpliPhi Biosciences Corporation is also running an expanded access program for the anti-S. aureus bacteriophage product AB-SA01, which is intended for the treatment of serious infections. Their results indicate that bacteriophage treatment is well tolerated, with no detectable side effects, and successful in 83% of treated patients. A topical anti-staphylococcal bacteriophage cocktail is being evaluated for diabetic foot ulcers monoinfected by MRSA or methicillin-susceptible Staphylococcus aureus (MSSA). So far, phase I and II clinical trials are currently being carried out (https://clinicaltrials.gov/ct2/home; identifier, NCT02664740). Finally, a phage cocktail active against P. aeruginosa and S. aureus was applied to nine patients with infected burn wounds without any adverse effects or clinical abnormalities (18).
There are also several examples of phage-derived enzymes (endolysins) successfully used to treat staphylococcal infections. For example, in a septicemia mouse model, the synergistic action of lysin ClyS and oxacillin protected the animals from infection by MRSA. The mice were injected (5 × 105 CFU) intraperitoneally, and 3 h later, a single dose (1 mg) of ClyS was administered. All control mice died after 24 h, while 89% of the treated mice survived (19).
Regarding pneumonia treatment, a combined therapy of lysin LysGH15 (60 μg) and apigenin (500 μg) was evaluated in a S. aureus mouse model. The bacterial load in the lungs of treated mice was 1.5 log units within 24 h after challenged with 5 × 107 CFU, whereas the loads in unprotected mice or mice treated with apigenin or LysGH15 alone were 10.2, 4.7, and 2.6 log units, respectively. Of note, animals treated with both antimicrobials were healthier than those treated with LysGH15 or apigenin alone (20).
In this context, several clinical trials using endolysins as therapy are currently ongoing. More specifically, three lysins are currently in different stages of human clinical trials. For example, protein SAL200 has already successfully completed a phase I study and is currently in phase II (21). Similarly, protein CF-301, specifically formulated for the treatment of S. aureus bloodstream infections and endocarditis, showed no adverse effects in healthy volunteers, and phase II has been recently completed. Likewise, protein P128 (StaphTAME), intended for clearing nasal contamination of S. aureus in humans, is currently in phase II clinical trials. At this time, a recombinant endolysin (Staphefekt SA.100) is commercially available for topical skin application and has been registered in Europe as a class 1 medical device. Recently, there have been three reported cases of successful use of this compound in patients with chronic and recurrent dermatoses caused by S. aureus (22).
WHY BACTERIOPHAGE-BASED ANTIMICROBIALS COULD PALLIATE THE MRSA CRISIS
Both phages and endolysins have unique characteristics that make some of them particularly suitable to face S. aureus infections as an alternative or complement to antibiotic therapy. The pathogenicity of this microbe is partly due to its ability to evade host defenses and survive antimicrobial challenges; for instance, by forming biofilms on body tissues and medical devices. In addition, this bacterium can produce a number of important toxins and virulence factors, including enzymes that facilitate invasion and destruction of host tissue. The ability to bind endothelial cells and remain viable in phagocytes protects S. aureus from antimicrobials and the host immune system. Finally, the formation of SCVs and persister cells also contributes to persistence and the occurrence of recurrent infections.
Throughout evolution, bacteriophages have adapted to penetrate biofilms and infect the bacterial cells sheltered inside these complex structures (23), as this is the most common lifestyle of bacteria in nature. Moreover, it has been shown that phages can infect persisters (24) and that phage-derived endolysins can effectively kill cells in the persister state (25). Bacteriophages also have the property of being self-replicating, which favors the increase in phage particle concentration at the infection site, in contrast to antibiotics, which exhibit a gradual decrease in concentration after the time of application. Furthermore, bacteriophages are able to infect their host regardless of their antibiotic resistance phenotype. This constitutes a valuable trait that should not be underestimated. For therapeutic purposes, it is desirable that phages have a broad host range. Nonetheless, a phagogram (a test that determines the susceptibility of a given strain to a battery of phages) using the bacteria causing infection in a particular patient should be carried out before phage treatment. A question of concern is whether bacterial cells may become resistant to phages, although the frequency of phage resistance acquisition is fairly low and goes down to almost zero when a mixture of different phages is used (26). No data are available yet regarding resistance development in clinical settings, but it would also be expected to occur at a low frequency, especially if personalized phage mixtures are designed for each patient. A desirable consequence of the ability of phages to specifically target multiresistant bacteria is that their use would help decrease the general levels of multidrug resistance in bacterial populations. Antibiotics have a broad spectrum of action and exert a selective pressure not only on the target pathogen but also on the whole bacterial community exposed. In contrast, phage application only exerts selective pressure on the target bacteria. This characteristic is also very advantageous when taking into account the health benefits of keeping the beneficial microbiota intact during antimicrobial therapy. Finally, since bacteriophages are highly diverse and abundant in nature, the chance of finding the most appropriate ones for each target increases.
In the case of endolysins, a major advantage is that they do not generally select resistant bacteria (13). Endolysins seem to attack chemical bonds in peptidoglycans that are essential to the cell wall. Moreover, endolysins from Gram-positive phages are endowed with two or more catalytic domains with different activities, a fact that diminishes the likelihood of resistance development. This aspect of endolysins makes them very well suited for prophylactic applications. Another advantage of these compounds is a consequence of their modular structure, which allows the design of new chimeric proteins with improved and/or tailored properties. As mentioned above, an important pathogenicity factor of S. aureus is its ability to penetrate and survive inside endothelial cells, making the pathogen less accessible to antimicrobials. This issue has already been solved in an outstanding work from Fischetti’s laboratory, in which lysins were modified to increase complement fixation to the bacterial surface and promote phagocytosis by macrophages and neutrophils (27). The authors synthesized lysibodies, engineered molecules that combine a high-affinity carbohydrate-binding domain of bacteriophage origin and an Fc effector portion of a human IgG antibody. A recent study showed that lysin V12CBD can attenuate S. aureus virulence and enhance host immune defenses by binding to the bacterial cells. Hence, this leads to a reduction in the pathogen’s ability to penetrate epithelial cells and an increase in its susceptibility to macrophages (28). In addition, a very important aspect regarding the use of these new antimicrobials is that they do not appear to affect the immune system. Several studies about safety and toxicity have been performed in human cell lines and animal models, but no changes in gene expression or proinflammatory cytokine levels were observed (29).
CONCLUSIONS
The high pathogenicity and antibiotic resistance of many S. aureus strains is severely affecting health care systems worldwide. An active research program has been proposed to tackle this issue through the development of novel antimicrobial molecules. The complex biology of this bacterium, which includes the ability to form biofilms, penetrate endothelial cells, and produce small-colony variants and persister cells, contributes to the difficulty of developing a comprehensive strategy applicable to the treatment of all S. aureus infections. This pathogen is undoubtedly responsible for a wide range of pathologies, ranging from skin infections to bacteremia, making it necessary to have a variety of therapeutic options, suitable for each scenario. In this regard, a remarkable number of novel compounds are in the pipeline for approval. Among this arsenal of antimicrobials, phage therapy provides a unique strategy rescued from the past and renewed with the latest technologies to develop target-specific therapeutics against staphylococcal infections. In addition to the experience gathered in Eastern European countries supporting the effectiveness of phage therapy, we also now have quite a few successful results obtained in animal models and clinical trials. Nonetheless, additional research is still necessary to provide the ultimate evidence to support their approval by the Health Authorities. Several clinical trials are currently ongoing that will hopefully demonstrate that bacteriophages are not only safe, but also effective, antimicrobials.
ACKNOWLEDGMENTS
This study was funded by grants AGL2015-65673-R (MINECO/FEDER/EU, Spain), EU ANIWHA ERA-NET (BLAAT ID: 67)/PCIN-2017-001 (AEI/FEDER/EU, Spain), Proyecto Intramural CSIC201670E040, Proyecto Intramural CSIC 201770E016, IDI/2018/000119 (Asturias Innovation 2018 to 2020, Principado de Asturias, Spain) and FEDER/EU.
P.G. and A.R. are members of the bacteriophage network FAGOMA II and the FWO Vlaanderen-funded “Phagebiotics” research community (WO.016.14).
REFERENCES
- 1.Cecchini M, Langer J, Slawomirski L. 2015. Antimicrobial resistance in G7 countries and beyond: economic issues, policies and options for action. Organisation for Economic Co-operation and Development, Paris, France. [Google Scholar]
- 2.Hartman BJ, Tomasz A. 1984. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol 158:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control. 2017. Antimicrobial resistance surveillance in Europe 2016. European Centre for Disease Prevention and Control, Solna, Sweden. [Google Scholar]
- 4.Figueiredo A. 2017. What is behind the epidemiological difference between community-acquired and health-care associated methicillin-resistant Staphylococcus aureus? Virulence 8:640–642. doi: 10.1080/21505594.2017.1335847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen J, Stegger M, Andersen PS, Petersen A, Larsen AR, Westh H, Agersø Y, Fetsch A, Kraushaar B, Käsbohrer A, Feβler AT, Schwarz S, Cuny C, Witte W, Butaye P, Denis O, Haenni M, Madec J-Y, Jouy E, Laurent F, Battisti A, Franco A, Alba P, Mammina C, Pantosti A, Monaco M, Wagenaar JA, de Boer E, van Duijkeren E, Heck M, Domínguez L, Torres C, Zarazaga M, Price LB, Skov RL. 2016. Evidence for human adaptation and foodborne transmission of livestock-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 63:1349–1352. doi: 10.1093/cid/ciw532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon RJ, Lowy FD. 2008. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim W, Zhu W, Hendricks GL, Van Tyne D, Steele AD, Keohane CE, Fricke N, Conery AL, Shen S, Pan W, Lee K, Rajamuthiah R, Fuchs BB, Vlahovska PM, Wuest WM, Gilmore MS, Gao H, Ausubel FM, Mylonakis E. 2018. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 556:103–107. doi: 10.1038/nature26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trombetta RP, Dunman PM, Schwarz EM, Kates SL, Awad HA. 2018. A high-throughput screening approach to repurpose FDA-approved drugs for bactericidal applications against Staphylococcus aureus small-colony variants. mSphere 3:e00422-18. doi: 10.1128/mSphere.00422-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fish R, Kutter E, Bryan D, Wheat G, Kuhl S. 2018. Resolving digital staphylococcal osteomyelitis using bacteriophage—a case report. Antibiotics (Basel) 7:E87. doi: 10.3390/antibiotics7040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diep BA, Hilliard JJ, Le VT, Tkaczyk C, Le HN, Tran VG, Rao RL, Dip EC, Pereira-Franchi EP, Cha P, Jacobson S, Broome R, Cheng LI, Weiss W, Prokai L, Nguyen V, Stover CK, Sellman BR. 2017. Targeting alpha toxin to mitigate its lethal toxicity in ferret and rabbit models of Staphylococcus aureus necrotizing pneumonia. Antimicrob Agents Chemother 61:e02456-16. doi: 10.1128/AAC.02456-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P, Pletzer D, Haney E, Rahanjam N, Cheng JJT, Yue M, Aljehani W, Hancock REW, Kizhakkedathu JN, Straus SK. 2019. Aurein-derived antimicrobial peptides formulated with pegylated phospholipid micelles to target methicillin-resistant Staphylococcus aureus skin infections. ACS Infect Dis 5:443–453. doi: 10.1021/acsinfecdis.8b00319. [DOI] [PubMed] [Google Scholar]
- 12.Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST. 2010. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 13.Fischetti VA. 2006. Using phage lytic enzymes to control pathogenic bacteria. BMC Oral Health 6:S16. doi: 10.1186/1472-6831-6-S1-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur S, Harjai K, Chhibber S. 2016. In vivo assessment of phage and linezolid based implant coatings for treatment of methicillin resistant S. aureus (MRSA) mediated orthopaedic device related infections. PLoS One 11:e0157626. doi: 10.1371/journal.pone.0157626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhibber S, Kaur J, Kaur S. 2018. Liposome entrapment of bacteriophages improves wound healing in a diabetic mouse MRSA infection. Front Microbiol 9:561. doi: 10.3389/fmicb.2018.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferry T, Leboucher G, Fevre C, Herry Y, Conrad A, Josse J, Batailler C, Chidiac C, Medina M, Lustig S, Laurent F, Lyon B. 2018. Salvage debridement, antibiotics and implant retention (“DAIR”) with local injection of a selected cocktail of bacteriophages: is it an option for an elderly patient with relapsing Staphylococcus aureus prosthetic-joint infection? Open Forum Infect Dis 5:ofy269. doi: 10.1093/ofid/ofy269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A. 2009. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 18:237–238, 240–243. doi: 10.12968/jowc.2009.18.6.42801. [DOI] [PubMed] [Google Scholar]
- 18.Rose T, Verbeken G, Vos DD, Merabishvili M, Vaneechoutte M, Lavigne R, Jennes S, Zizi M, Pirnay JP. 2014. Experimental phage therapy of burn wound infection: difficult first steps. Int J Burns Trauma 4:66–73. [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia F, Li X, Wang B, Gong P, Xiao F, Yang M, Zhang L, Song J, Hu L, Cheng M, Sun C, Feng X, Lei L, Ouyang S, Liu ZJ, Li X, Gu J, Han W. 2016. Combination therapy of LysGH15 and apigenin as a new strategy for treating pneumonia caused by Staphylococcus aureus. Appl Environ Microbiol 82:87–94. doi: 10.1128/AEM.02581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jun SY, Jang IJ, Yoon S, Jang K, Yu KS, Cho JY, Seong MW, Jung GM, Yoon SJ, Kang SH. 2017. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob Agents Chemother 61:e02629-16. doi: 10.1128/AAC.02629-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Totté JEE, van Doorn MB, Pasmans S. 2017. Successful treatment of chronic Staphylococcus aureus-related dermatoses with the topical endolysin Staphefekt SA.100: a report of 3 cases. Case Rep Dermatol 9:19–25. doi: 10.1159/000473872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González S, Fernández L, Gutiérrez D, Campelo AB, Rodríguez A, García P. 2018. Analysis of different parameters affecting diffusion, propagation and survival of staphylophages in bacterial biofilms. Front Microbiol 9:2348. doi: 10.3389/fmicb.2018.02348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tkhilaishvili T, Di Luca M, Abbandonato G, Maiolo EM, Klatt AB, Reuter M, Moncke-Buchner E, Trampuz A. 2018. Real-time assessment of bacteriophage T3-derived antimicrobial activity against planktonic and biofilm-embedded Escherichia coli by isothermal microcalorimetry. Res Microbiol 169:515–521. doi: 10.1016/j.resmic.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez D, Ruas-Madiedo P, Martínez B, Rodríguez A, García P. 2014. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS One 9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García P, Madera C, Martínez B, Rodríguez A. 2007. Biocontrol of Staphylococcus aureus in curd manufacturing processes using bacteriophages. Int Dairy J 17:1232. doi: 10.1016/j.idairyj.2007.03.014. [DOI] [Google Scholar]
- 27.Raz A, Serrano A, Lawson C, Thaker M, Alston T, Bournazos S, Ravetch JV, Fischetti VA. 2017. Lysibodies are IgG Fc fusions with lysin binding domains targeting Staphylococcus aureus wall carbohydrates for effective phagocytosis. Proc Natl Acad Sci U S A 114:4781–4786. doi: 10.1073/pnas.1619249114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Xu J, Li W, Wang S, Li J, Yu J, Li Y, Wei H. 2018. Staphylococcus aureus virulence attenuation and immune clearance mediated by a phage lysin-derived protein. EMBO J 37: e98045. doi: 10.15252/embj.201798045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harhala M, Nelson D, Miernikiewicz P, Heselpoth R, Brzezicka B, Majewska J, Linden S, Shang X, Szymczak A, Lecion D, Marek-Bukowiec K, Kłak M, Wojciechowicz B, Lahutta K, Konieczny A, Dąbrowska K. 2018. Safety studies of pneumococcal endolysins Cpl-1 and Pal. Viruses 10:638. doi: 10.3390/v10110638. [DOI] [PMC free article] [PubMed] [Google Scholar]