The optimal method to screen for gastrointestinal colonization with carbapenem-resistant organisms (CRO) has yet to be established. The direct MacConkey (direct MAC) plate method demonstrates high sensitivity for CRO detection, but established zone diameter (ZD) criteria for ertapenem (≤27 mm) and meropenem (≤32 mm) result in high rates of false positives upon confirmatory testing.
KEYWORDS: CRO, carbapenem-resistant organism, direct MacConkey Plate method, colonization, method
ABSTRACT
The optimal method to screen for gastrointestinal colonization with carbapenem-resistant organisms (CRO) has yet to be established. The direct MacConkey (direct MAC) plate method demonstrates high sensitivity for CRO detection, but established zone diameter (ZD) criteria for ertapenem (≤27 mm) and meropenem (≤32 mm) result in high rates of false positives upon confirmatory testing. To increase specificity, we screened for CRO in two high-risk wards using the direct MAC plate method, recorded ZDs for each sample, and generated receiver operating characteristic (ROC) curves to evaluate the optimal ZD cutoff criteria. Of 6,868 swabs obtained over an 18-month period, 4,766 (69%) had growth on MAC plates, and 2,500 (36%) met criteria for further evaluation based on previously established ZDs around the carbapenem disks. A total of 812 (12%) swabs were confirmed positive for at least one CRO and included 213 (3%) carbapenemase-producing organisms (CPO), resulting in a specificity of 78% for the direct MAC plate method. Reducing the ertapenem and meropenem ZDs to ≤25 mm improved specificity to 83%, decreasing the confirmatory testing workload by 32%. The sensitivities with the lower ZD criteria were 89% for CRO and 94% for CPO, respectively. The direct MAC plate method criteria for CRO testing can be modified to balance the sensitivity and specificity of CRO while reducing the burden on clinical microbiology laboratories. These modifications can be particularly helpful in regions with a low CRO prevalence.
INTRODUCTION
Carbapenem-resistant organisms (CRO) are well recognized as significant public health concerns. Several global health entities, including the Centers for Diseases Control and Prevention (CDC) and the World Health Organization, have designated these organisms as high threat and critical priority pathogens, respectively (1, 2). Rectal surveillance cultures for carbapenem-resistant Enterobacterales (CRE) is recommended by the CDC in limited scenarios (3). Although universal admission screening would be the optimal approach, allowing for rapid isolation of patients to limit the spread in the health care setting, both logistical and cost constraints prevent this approach from being widely adopted (4). Some institutions have opted to screen high-risk patients, such as intensive care unit (ICU) or oncology and transplant patients, where colonization and subsequent infection can cause the highest morbidity and mortality (5, 6).
The optimal method to screen for gastrointestinal colonization with CRO has yet to be established. Most methods focus on CRE detection or identification of the “big five” carbapenemase genes (blaKPC, blaNDM, blaVIM, blaIMP, and blaOXA-48-like) (7). These methods include broth enrichment, direct selective culture, chromogenic media, and detection of carbapenemase genes using molecular methods from fecal samples (8–14). Our group previously demonstrated that the direct MacConkey (direct MAC) plate method using ertapenem disks had the highest sensitivity for CRO detection compared to broth enrichment methods, the ChromID Carba chromogenic medium (research use only [RUO] version; bioMérieux, France), and the Check-Direct CPE molecular assay (RUO version; Check-Points, Wageningen, The Netherlands) (14). The direct MAC plate method is an affordable and user-friendly approach that utilizes standard laboratory materials. However, this method—as currently implemented—lacks specificity, leading to frequent isolation of carbapenem-susceptible organisms (8, 11). Previously, we processed 6,868 CRO surveillance rectal swabs among two high-risk wards applying the direct MAC plate method, using an ertapenem disk in the first quadrant and a meropenem disk in the second quadrant. Confirmatory testing was performed on isolated organisms. The objective of the current study was to reevaluate the zone diameter criteria for ertapenem and meropenem to determine whether the direct MAC plate method’s specificity can be increased while maintaining acceptable sensitivity. We also present the descriptive epidemiology of CRO among the study cohort.
MATERIALS AND METHODS
Specimens.
From January 2016 to June 2017, consecutive rectal ESwabs (Copan Diagnostics, Murrieta, CA) from patients in the medical intensive care unit (MICU) and solid organ transplant unit at The Johns Hopkins Hospital were collected at unit admission and weekly thereafter. The swabs were collected as part of a long-standing vancomycin-resistant Enterococcus (VRE) surveillance program. At the completion of standard-of-care testing, and prior to disposal, the swabs were deidentified and held at 4°C for up to 4 days prior to further testing. This study was approved by The Johns Hopkins University School of Medicine Institutional Review Board, with a waiver of informed consent.
Direct MacConkey plate method.
The ESwabs were vortexed for 10 s, and a 100 μl aliquot of the liquid Amies broth was inoculated to a MacConkey (MAC) plate and streaked for isolation. An ertapenem disk was placed in the first quadrant and a meropenem disk in the second quadrant, followed by incubation overnight (18 to 24 h) at 35°C. The zone diameters (ZDs) around the carbapenem disks were recorded. If growth did not reach the meropenem disk, only the ertapenem zone diameter was assessed. Any Gram-negative bacilli (GNB) growing within a ZD of ≤27 mm of the ertapenem disk or ≤32 mm of the meropenem disk were further subcultured (8, 11) and identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Inc., Billerica, MA). Carbapenem antimicrobial susceptibility testing (AST) of GNB growing within the predefined carbapenem ZD was performed by disk diffusion, including ertapenem, meropenem, and imipenem for the Enterobacterales and meropenem and imipenem for glucose-nonfermenting organisms. Clinical and Laboratory Standards Institute (CLSI) interpretive criteria were applied (15). Carbapenem disk diffusion testing was not performed for Stenotrophomonas maltophilia, as this organism is intrinsically resistant to carbapenems due to a chromosomally encoded metallo-β-lactamase.
Carbapenemase detection.
Carbapenemase production was determined using the modified carbapenem inactivation method (mCIM) for all GNB not susceptible (intermediate or resistant) to the carbapenems, as previously described (16–18). For isolates that were mCIM indeterminate or positive, genomic DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Inc., Valencia, California). Identification of β-lactamase genes was performed using the Check-MDR CT103XL microarray-based assay (Check-Points, Wageningen, The Netherlands).
A convenience sample of 60 randomly selected swabs, that were found to be negative by the direct MAC method and for which remnant ESwab broth (300 μl) was available, were tested by the CARBA-R assay (Cepheid, Sunnyvale, CA) using an in-house-validated method. The convenience sample was chosen to confirm that swabs near the ZD cutoff (ertapenem ZD = 28 mm) for further workup were indeed negative for the most common carbapenemase genes (blaKPC, blaNDM, blaOXA-48-like, blaVIM, and blaIMP).
Statistical analysis.
The sensitivity and specificity of alternative ZD cutoffs, individually and in combination, for detecting CRO, carbapenemase-producing organisms (CPO), CRE, and carbapenemase-producing Enterobacterales (CPE) using the cultured isolate phenotypic and/or genotypic results as the reference standard were determined. Any direct MAC plate with no growth or with carbapenem ZDs that did not qualify for further workup was considered negative. Receiver operating characteristic (ROC) curves were generated for ertapenem and meropenem ZDs to evaluate the optimal ZDs for detection of CRO, CPO, CRE, and CPE.
RESULTS
Direct MAC culture results.
A total of 6,868 rectal swabs from 2,968 patients (median of 2 swabs per patient; range, 1 to 35) were cultured for CRO by the direct MAC plate method. Of those, 4,766 (69.4%) had growth on MAC plates, with 2,500 (36%) meeting criteria for additional CRO evaluation based on the ZDs around the carbapenem disks. Of the 1,869 (27.2%) plates considered negative based on ertapenem ZD of >27 mm and meropenem ZD of >32 mm, a convenience sample of 60 swabs with direct MAC results near the ZD cutoff were selected and found to be negative for carbapenemase genes by the CARBA-R assay. Of the 2,500 requiring further workup, 1,542 (24.5%) qualified based on both carbapenem ZDs, whereas 557 (8.1%) qualified based solely on ertapenem ZD criteria and 401 (5.8%) qualified based solely on meropenem ZD criteria. Of note, as the meropenem disk was placed in the second quadrant of growth, there were some instances (n = 115) where meropenem ZDs could not be assessed due to no bacterial growth in the relevant quadrant. A total of 812 (11.8%) swabs were confirmed positive for at least one CRO based on the carbapenem AST (853 CRO isolates total) results, including 482 (7.1%) CRE, 362 (5.3%) carbapenem-resistant nonfermenters (CR-NF), and 9 (0.1%) carbapenem-resistant Aeromonas species (here grouped with the CR-NF). There were 41 (0.6%) swabs growing both CRE and CR-NF. Based on confirmed CRO by AST of recovered isolates, the specificity of the direct MAC plate method for detection of CRO using existing ZD criteria was 78.2% [6,056 (true negative)/6,056 + 1,688 (false positive) × 100] in this study.
Detection of carbapenemase producers.
Of the 853 CRO isolated, 213 (3.1%) CPO were identified by the phenotypic mCIM method, including 108 (1.6%) CPE and 105 (1.5%) CP-CR-NF. Three swabs had both a CPE and carbapenemase-producing CR-NF (CP-NF) isolated. The CPO isolated in this study are described in Table 1. The most common CPEs were Klebsiella pneumoniae (n = 45; 41.7%), followed by Enterobacter cloacae complex (n = 35; 32.7%), Citrobacter amalonaticus (n = 12; 11.2%), and Escherichia coli (n = 8; 7.5%). The most common carbapenemase genes identified among CPE were 53 (49.5%) blaKPC, 12 (11.2%) blaOXA-48-like and blaNDM, 9 (8.4%) blaNDM, 2 (1.9%) blaKPC and blaNDM, and 1 (0.9%) blaKPC and blaOXA-48-like. S. maltophilia (n = 74; 70.5%) was the most common CP-NF encountered and was presumably mediated by the chromosomally encoded L1 metallo-β-lactamase; it was followed by Acinetobacter baumannii (n = 19; 18.1%), with the great majority being OXA-24 producers. Of note, of 238 carbapenem-resistant Pseudomonas aeruginosa isolates, only 10 (4.2%) were identified as carbapenemase producers, with 3 VIM producers identified (1.3%).
TABLE 1.
Distribution of carbapenemase-producing organisms isolated from 6,868 rectal swabs using the direct MacConkey plate method
| Organism group or species | No. of organisms isolated | Carbapenemase (CP) gene(s) detectedb |
|---|---|---|
| CPE isolates (n = 108) | ||
| Citrobacter amalonaticus | 7 | blaKPC |
| 2 | blaKPC and blaNDM | |
| 1 | blaNDM | |
| 2 | No CP detected | |
| Citrobacter freundii | 1 | blaKPC |
| 2 | No CP detected (blaCMY-2) | |
| Enterobacter cloacae | 8 | blaKPC |
| 1 | blaKPC and blaOXA-48-like | |
| 26a | No CP detected (18 blaACT or blaMIR, 8 negative) | |
| Escherichia coli | 6 | blaKPC |
| 2 | No CP detected | |
| Hafnia alvei | 1 | blaNDM |
| Klebsiella (Enterobacter) aerogenes | 1 | blaKPC |
| Klebsiella pneumoniae | 26 | blaKPC |
| 13 | blaOXA-48-like and blaNDM | |
| 5 | blaNDM | |
| 1 | No CP detected | |
| Morganella morganii | 3 | No CP detected (1 blaACT or blaMIR, 1 blaDHA, and 1 negative) |
| CP-NF isolates (n = 105) | ||
| Acinetobacter baumannii complex | 12 | blaOXA-24 |
| 1 | blaOXA-23 | |
| 6 | No CP detected | |
| Acinetobacter radioresistans | 1 | blaOXA-23 |
| Pseudomonas aeruginosa | 3 | blaVIM |
| 7 | No CP detected | |
| Pseudomonas otitidis | 1 | No CP detected |
| Stenotrophomonas maltophilia | 74 | Not tested due to chromosomally encoded L1 metallo-β-lactamase |
A total of 14 isolates were indeterminate by the mCIM.
Where no CP genes were identified, other β-lactamase genes detected are listed in parentheses.
Direct MAC plate ZDs for detecting CRO.
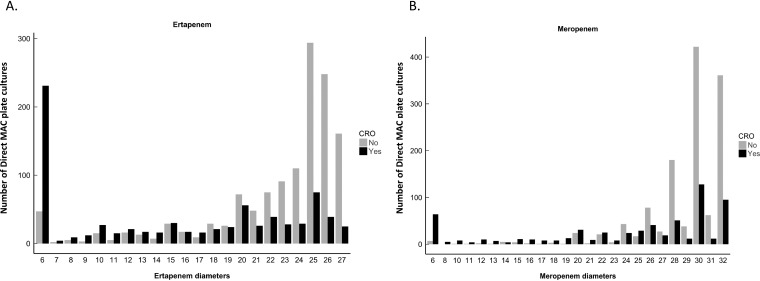
Analysis of the median ZD was limited to results for which both ertapenem and meropenem disk criteria were available, which included 2,500 swabs requiring further workup and 2,102 swabs that were negative by the direct MAC plate method based on the carbapenem ZDs (Fig. 1). Median ertapenem and meropenem ZDs were 16 mm (interquartile range [IQR], 6 to 23) and 26 mm (IQR, 20 to 30) for direct MAC plates positive for CRO, versus 25 mm (IQR, 21 to 26) and 30 mm (IQR, 28 to 32) for direct MAC plates negative for CRO, respectively (P values <0.001). For CPO, the median ertapenem and meropenem ZDs were 11 mm (IQR, 6 to 20) and 20 mm (IQR, 6 to 28) for positives versus 24 mm (IQR, 18 to 25) and 30 mm (IQR, 27 to 31) for negatives, respectively (P values <0.001). Further breakdown of the median ZDs based on carbapenem-resistant, carbapenemase-producing, and carbapenemase genotype status are summarized in Table 2. Histograms of the direct MAC plate ertapenem and meropenem ZDs that were positive or negative for CRO indicated a distribution of both CRO-positive and CRO-negative cultures across all ZDs, but with a tendency for CRO-positive cultures to cluster at lower ZDs and CRO-negative cultures to cluster at higher ZDs (Fig. 2).
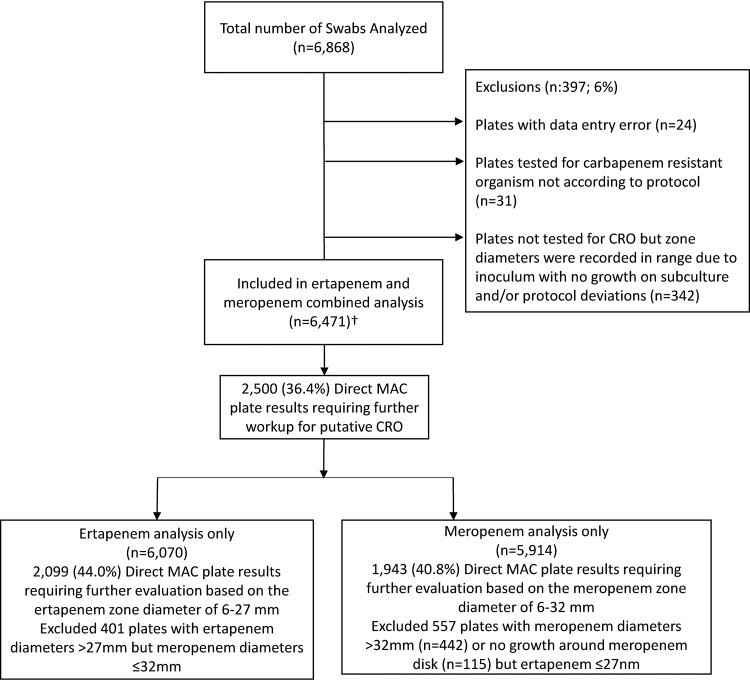
FIG 1.
Receiver operator curve study design. The analysis marked with † included 2,500 (36.4%) plates with ertapenem diameters of 6 to 27 mm or meropenem diameters of 6 to 32 mm, 2,102 (30.6%) plates without MAC growth, and 1,869 (27.2%) plates with ertapenem diameters of >27 mm and meropenem diameters of >32 mm.
TABLE 2.
Median ertapenem and meropenem zone diameter by carbapenem-resistant organism and carbapenemase-producing organism status
| Organism status | Ertapenem (ZD = 6–27 mm, n = 2,099) |
Meropenem (ZD = 6–32 mm, n = 1,943) |
||||
|---|---|---|---|---|---|---|
| No. positive (%) | Median (IQR) diams (mm) |
No. positive (%) | Median (IQR) diams (mm) |
|||
| Positive | Negative | Positive | Negative | |||
| CRO | 777 (37) | 16 (6–23) | 25 (21–26) | 636 (32.7) | 26 (20–30) | 30 (28–32) |
| Organisms | ||||||
| CRE | 454 (21.6) | 20 (11–24) | 24 (18–26) | 385 (19.8) | 28 (22–30) | 30 (27–32) |
| CR-NF | 363 (17.3) | 10 (6–20) | 24 (20–26) | 289 (14.9) | 24 (17–30) | 30 (28–32) |
| Mechanisms | ||||||
| CPO | 202 (9.6) | 11 (6–20) | 24 (18–25) | 174 (9) | 20 (6–28) | 30 (27–31) |
| CPE | 105 (5) | 12 (6–20) | 23 (17–25) | 94 (4.8) | 20 (6–28) | 30 (26–31) |
| CP-NF | 103 (4.9) | 10 (6–20) | 23 (17–25) | 86 (4.4) | 19 (6–28) | 30 (26–31) |
| KPC CP-CRE | 51 (2.4) | 6 (6–17) | 23 (16–25) | 46 (2.4) | 16 (6–25) | 30 (26–31) |
| NDM CP-CRE | 21 (1) | 6 (6–12) | 23 (16–25) | 19 (1) | 6 (6–18) | 30 (26–31) |
| OXA-48-like CP-CRE | 13 (0.6) | 6 (6–6) | 23 (16–25) | 12 (0.6) | 6 (6–12) | 30 (26–31) |
FIG 2.
Histograms demonstrating the distribution of the direct MacConkey plate method ertapenem and meropenem zone diameters that were determined to be positive or negative for CRO.
Receiver operating characteristic curves.
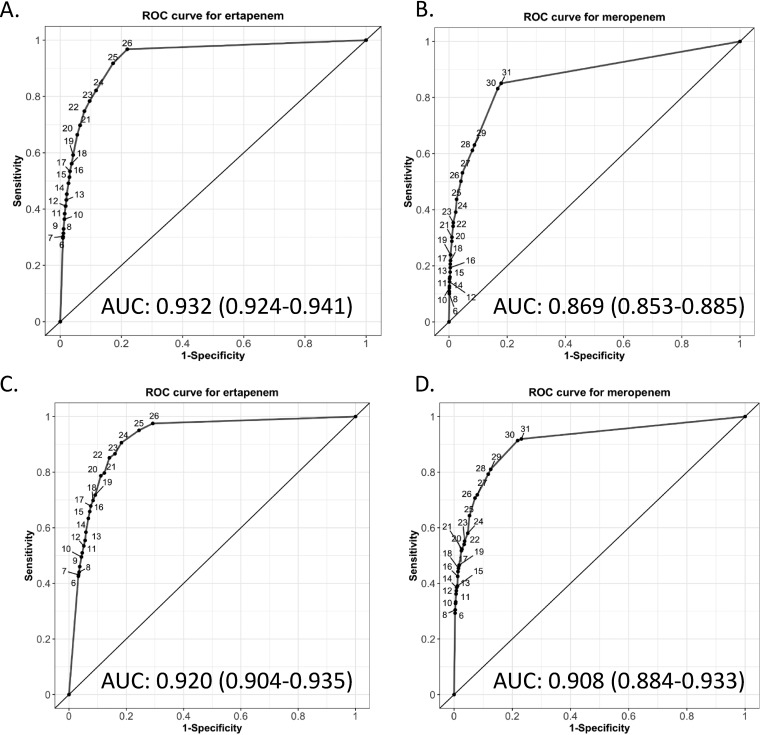
ROC curves were constructed to evaluate the optimal cutoff criteria to balance sensitivity and specificity for implementation of the direct MAC plate method. The ROC curves for CRO using the direct MAC plate method showed an area under the curve (AUC) of 0.93 (95% confidence interval [CI], 0.92 to 0.94) for ertapenem and an AUC of 0.87 (95% CI, 0.85 to 0.89) for meropenem (Fig. 3). The ROC curves for CPO using the direct MAC plate method indicated an AUC of 0.92 (95% CI, 0.90 to 0.94) for ertapenem and an AUC of 0.91 (95% CI, 0.88 to 0.93) for meropenem (Fig. 3). Table 3 summarizes the sensitivity and specificity of the combined ertapenem and meropenem ZD cutoffs for the various CRO groups when a ZD could be measured.
FIG 3.
Receiver operator curves for ertapenem and meropenem zone diameters to detect carbapenem-resistant organisms and carbapenemase-producing organisms by the direct MacConkey plate method. ROC curves for (A) ertapenem and (B) meropenem for detection of carbapenem-resistant organisms (CRO). ROC curves for (C) ertapenem and (D) meropenem for detection of carbapenemase-producing organisms (CPO). The numerical points on the ROC represent different zone diameter readings for the direct MAC cultures. AUC, area under the curve.
TABLE 3.
Performance characteristics of the combined ertapenem and meropenem zone diameter results for the direct MAC plate method
| Ertapenem zone diameter (mm) | Meropenem zone diameter (mm) | CRO (%) |
CPO (%) |
||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| 24 | 24 | 81 | 88 | 91 | 82 |
| 24 | 25 | 81 | 88 | 91 | 82 |
| 24 | 26 | 81 | 87 | 91 | 81 |
| 24 | 27 | 82 | 87 | 91 | 81 |
| 24 | 28 | 84 | 85 | 92 | 78 |
| 24 | 29 | 84 | 84 | 92 | 78 |
| 24 | 30 | 89 | 79 | 94 | 73 |
| 24 | 31 | 90 | 79 | 95 | 72 |
| 25 | 24 | 89 | 83 | 94 | 76 |
| 25 | 25 | 89 | 83 | 94 | 76 |
| 25 | 26 | 89 | 83 | 94 | 76 |
| 25 | 27 | 90 | 82 | 94 | 76 |
| 25 | 28 | 91 | 81 | 94 | 74 |
| 25 | 29 | 91 | 81 | 94 | 74 |
| 25 | 30 | 94 | 77 | 97 | 70 |
| 25 | 31 | 94 | 76 | 97 | 69 |
| 26 | 24 | 94 | 79 | 97 | 72 |
| 26 | 25 | 94 | 79 | 97 | 72 |
| 26 | 26 | 94 | 79 | 97 | 72 |
| 26 | 27 | 94 | 79 | 97 | 72 |
| 26 | 28 | 94 | 78 | 97 | 71 |
| 26 | 29 | 94 | 77 | 97 | 71 |
| 26 | 30 | 97 | 75 | 99 | 68 |
| 26 | 31 | 97 | 74 | 99 | 67 |
Establishing new zone diameter criteria—balancing sensitivity and specificity.
Due to the reduced specificity of the direct MAC plate method (36% of swabs required further workup, while only 12% were positive for CRO), we assessed whether we could establish new ZD criteria for when further testing would be necessary. We generated individual and combined ertapenem and meropenem ZD charts to assess the impact of narrowing the ZD on both sensitivity and specificity (Table 3; see also Tables S1 and S2). Using these charts, we found that an ertapenem and meropenem ZD of ≤25 mm provided sensitivities for CRO and CPO detection of 89% and 94%, respectively. The specificities for CRO and CPO detection were 83% and 76%, respectively. Of the 2,500 cultures requiring additional evaluation, the smaller ZDs would have reclassified 814 as negative, resulting in an increased specificity from 76% to 83% for CRO and a 32% reduction in workload. When narrowing the ZD size to 25 mm, 89 swabs positive for 92 CRO would have been missed, including 79 that were positive for one or multiple non-CP-CRO and 10 that were positive for CPO. The 10 positive CPO swabs included 6 S. maltophilia, 2 carbapenemase-producing K. pneumoniae (1 KPC and 1 NDM), 1 NDM-producing Hafnia alvei, and 1 A. baumannii isolate. The non-CP-CRO isolates were mostly CRE (n = 34) that were intermediate or resistant to ertapenem only, or the Morganella-Proteus-Providencia group (n = 19) that demonstrate intrinsically elevated MICs to imipenem.
Investigating CPE missed using a 25-mm zone diameter cutoff for carbapenems.
The most concerning misses using the smaller ZD cutoff of 25 mm for both carbapenems were the 3 CPE-positive swabs. The one KPC-producing K. pneumoniae isolate (ertapenem ZD, 40 mm; meropenem ZD, 32 mm) was from a patient with multiple admissions that throughout the course of the study had 7 swabs with only a single positive. The NDM-producing K. pneumoniae (ertapenem ZD, 26 mm; meropenem ZD, 32 mm) was from a patient that had 32 swabs throughout multiple admissions. Both patients had swabs that were negative before and after the one that was found to be positive. The NDM-producing H. alvei isolate (ertapenem ZD, 26 mm; meropenem ZD, 32 mm) was from a patient who was admitted for a month for which 5 swabs were collected over the admission, and the last swab was the one that was found to be positive.
DISCUSSION
Gastrointestinal colonization with CRO is a mounting public health concern, regardless of the underlying mechanism of carbapenem resistance or the particular Gram-negative organism. Detection of carbapenemase producers has received considerable attention, as carbapenemase genes are often located on readily transmissible plasmids and colonization with these organisms has fueled notable outbreaks (19, 20). However, colonization with other CRO, such as CR P. aeruginosa or CR A. baumannii, can serve as a reservoir for transmission and lead to devastating infections in high-risk populations (5, 6). Currently, there are no clear recommendations on optimal approaches to screen for CRO gastrointestinal colonization (7). Furthermore, screening decisions (e.g., carbapenemase genes versus CRE versus all CRO) are prevalence- and setting-dependent and require a balance between costs and risk that are subject to differing interpretations (4). Reevaluating the carbapenem ZD cutoffs to improve the specificity of the direct MAC plate method by testing 6,968 rectal swabs, we found that 11.8% of swabs were positive for at least one CRO, including 7.1% positive for CRE and 5.4% positive for CR-NF. Among CRO isolates, 3.1% were positive for CPO, including 1.6% positive for CPE and 1.5% positive for CP-NF. Furthermore, by balancing sensitivity and specificity, we found that reducing the ertapenem and meropenem ZDs to ≤25 mm improved specificity to 83%, decreasing the confirmatory testing workload by 32%.
The CRO prevalence estimates from the current work are similar to those in our point-prevalence study; however, the distribution of organisms and mechanisms of carbapenem resistance was much more heterogeneous in the current study (14). Not surprisingly, KPC-producing K. pneumoniae was the most common CPE isolate identified; however, we were surprised to identify multiple other carbapenemase variants, including those less commonly encountered in the United States, such as NDM and OXA-48-like carbapenemases. Interestingly, C. amalonaticus was the third most common CPE encountered, with isolates harboring KPC, NDM, or KPC and NDM. A review of clinical culture results over the study period did not yield any positive cultures with CP C. amalonaticus, for which only a single positive bacteremia case reported from our hospital over the last 5 years (21). Thus, C. amalonaticus may play a role as a “silent” reservoir in the GI tract for carbapenemase genes, with the potential to share mobile genetic elements with other, more virulent Gram-negative colonizers. Moreover, we report a high rate of colonization with glucose-nonfermenting organisms, which is not surprising among the MICU and solid organ transplant populations being screened. Among the CR-NF isolates OXA-24-producing A. baumannii and non-CP-CRO P. aeruginosa isolates were the most commonly encountered. Rates of CP P. aeruginosa were low, and all were VIM producers (when a carbapenemase gene was identified). S. maltophilia accounted for the large majority of CP-NF isolates.
In the present work, we attempt to establish optimal ZD cutoffs for the direct MAC plate method as a screening method for identifying CRO from rectal swabs. Initially, Lolans and colleagues described the direct MAC plate method using an ertapenem disk with a ≤27 mm zone diameter cutoff for detection of KPC-producing K. pneumoniae and E. coli. They tested the method with 149 rectal swabs, for which 38 KPC producers were identified with a sensitivity of 97% and specificity of 90% (11). Other studies have evaluated the direct MAC plate method in comparison to alternative screening methods for identifying KPC-producing Enterobacterales from fecal specimens by various carbapenem disks (ertapenem and/or meropenem and/or imipenem) and zone diameter cutoffs (range, 21 to 27 mm) with sensitivities ranging from 75 to 97% and specificities from 78 to 96% (11, 22–24). These differences in performance characteristics are mostly explained by variations in the direct MAC plate method applied and the comparator used in the studies. Blackburn et al. then used spiked simulated specimens to assess the optimal zone diameters for screening CRO by ROC analysis and defined optimal zone diameters as ≤24 mm for ertapenem, ≤34 mm for meropenem, and ≤32 mm for imipenem (8). We evaluated sensitivity, specificity, and ROC curves for CRO and performed subanalyses for CRE, CR-NF, CPO, CPE, and CP-NF. The zone diameters for CRO-positive and CRO-negative cultures overlapped, but cultures positive for CRO tended to cluster with smaller zone diameters, whereas cultures negative for CRO clustered with larger zone diameters; differences in median ZDs between CRO-positive and CRO-negative organisms were statistically significant. The variability in zone diameters is not surprising, as specimen sampling, inoculum, and diversity of the microbiota all contribute to ZD variation. Overall, when evaluating the carbapenems separately for identification of CRO, ertapenem was found to provide increased sensitivity, whereas meropenem provided increased specificity. Intrinsic resistance among NF to ertapenem did contribute to the lower specificity of ertapenem in distinguishing CRO-positive from CRO-negative cultures (Fig. 2A).
For the detection of CRO using the direct MAC plate method, we recommend implementing a cutoff ZD of 25 mm for both ertapenem and meropenem to maximize sensitivity and specificity. Furthermore, applying the same ZD cutoff for both carbapenems allows for ease of use and remembrance of the cutoff. At these ZDs, the sensitivity and specificity of the method for CRO are 89% and 83% and for CPO are 94% and 76%, respectively, for cultures with growth on MAC plates. Under these newly proposed ZDs, the number of cultures requiring further workup would decrease by 32% (814 cultures would be reclassified as negative), significantly reducing the laboratory workload. Although 3 CPE isolates among the 814 cultures were missed under the narrowed ZD criteria, there was no evidence of transmission from these patients to others, and in 2 cases subsequent follow-up swabs tested CRO negative. The larger zone diameters in these cases likely reflect a low burden of colonization with the CPE identified and perhaps reflect transient colonization. Although studies evaluating the burden of colonization and further transmission have yet to be completed for CRO, it is thought that a low burden of colonization presents a lower risk for spread, similarly to VRE (25). Thus, the additional work to find low-level colonization in these patients is likely outweighed by the presumably low potential for onward transmission. That said, each institution could weigh the risk with their individual procedures and populations based on the data that was generated in this study to determine the ZD criteria to apply (Table 3; see also Tables S1 and S2 in the supplemental material).
Limitations of this study include (i) some ESwabs were processed after the recommended 48-h period (up to 96 h after collection), (ii) the predefined ZD cutoffs may have underestimated the number of patients colonized with CRO due to organisms growing outside these ZDs not undergoing additional testing, (iii) the mCIM was used to detect CP among all nonfermenters and may not be the ideal method for organisms other than P. aeruginosa, and (iv) mCIM was indeterminate/positive among a large number of E. cloacae isolates in which no carbapenemase was detected by the Check-Direct multidrug resistance (MDR) assay. Whole-genome sequencing of these isolates is necessary to confirm the presence or absence of carbapenemase genes. Lastly, this study was limited to a single hospital setting and should be repeated in a broader population with different CRO prevalence to be generalizable to other settings.
In conclusion, we present the largest study to date evaluating the direct MAC plate method for detection of CRO colonization among high-risk inpatient wards over an 18-month period. We found similar rates of CRO (11.8%) and CPO (3.1%) colonization to our point-prevalence study, but with greater heterogeneity in carbapenemase production among various organisms. Our data indicate that the direct MAC plate method can be modified to balance the sensitivity and specificity of CRO detection, and we recommend reducing the zone diameter size for further workup to 25 mm for both ertapenem and meropenem. As CRO colonization screening assumes increasing importance, the data from this study can help institutions balance resource allocation and costs/benefits of using the direct MAC plate method.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the CDC Prevention Epicenters Program (grant CDC 1U54CK000447) and by funding from the National Institutes of Health (grant R21-AI130608, awarded to P.J.S.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01127-19.
REFERENCES
- 1.World Health Organization. 2017. Global priority list of antibiotic resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. [Google Scholar]
- 4.Viau R, Frank KM, Jacobs MR, Wilson B, Kaye K, Donskey CJ, Perez F, Endimiani A, Bonomo RA. 2016. Intestinal carriage of carbapenemase-producing organisms: current status of surveillance methods. Clin Microbiol Rev 29:1–27. doi: 10.1128/CMR.00108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris AD, Jackson SS, Robinson G, Pineles L, Leekha S, Thom KA, Wang Y, Doll M, Pettigrew MM, Johnson JK. 2016. Pseudomonas aeruginosa colonization in the intensive care unit: prevalence, risk factors, and clinical outcomes. Infect Control Hosp Epidemiol 37:544–548. doi: 10.1017/ice.2015.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettigrew MM, Gent JF, Kong Y, Halpin AL, Pineles L, Harris AD, Johnson JK. 2018. Gastrointestinal microbiota disruption and risk of colonization with carbapenem-resistant Pseudomonas aeruginosa in ICU patients. Clin Infect Dis 69:604–613. doi: 10.1093/cid/ciy936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gniadek TJ, Carroll KC, Simner PJ. 2016. Carbapenem-resistant non-glucose-fermenting Gram-negative bacilli: the missing piece to the puzzle. J Clin Microbiol 54:1700–1710. doi: 10.1128/JCM.03264-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackburn J, Tsimiklis C, Lavergne V, Pilotte J, Grenier S, Gilbert A, Lefebvre B, Domingo MC, Tremblay C, Bourgault AM. 2013. Carbapenem disks on MacConkey agar in screening methods for detection of carbapenem-resistant Gram-negative rods in stools. J Clin Microbiol 51:331–333. doi: 10.1128/JCM.02878-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2008. Laboratory protocol for detection of carbapenem-resistant or carbapenemase-producing, Klebsiella spp. and E. coli from rectal swabs. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 10.Lau AF, Fahle GA, Kemp MA, Jassem AN, Dekker JP, Frank KM. 2015. Clinical performance of Check-Direct CPE, a multiplex PCR for direct detection of blaKPC, blaNDM and/or blaVIM, and blaOXA-48 from perirectal swabs. J Clin Microbiol 53:3729–3737. doi: 10.1128/JCM.01921-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lolans K, Calvert K, Won S, Clark J, Hayden MK. 2010. Direct ertapenem disk screening method for identification of KPC-producing Klebsiella pneumoniae and Escherichia coli in surveillance swab specimens. J Clin Microbiol 48:836–841. doi: 10.1128/JCM.01988-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore NM, Canton R, Carretto E, Peterson LR, Sautter RL, Traczewski MM, Carba R. 2017. Rapid Identification of five classes of carbapenem resistance genes directly from rectal swabs by use of the Xpert Carba-R assay. J Clin Microbiol 55:2268–2275. doi: 10.1128/JCM.00137-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simner PJ, Gilmour MW, DeGagne P, Nichol K, Karlowsky JA. 2015. Evaluation of five chromogenic agar media and the Rosco Rapid Carb screen kit for detection and confirmation of carbapenemase production in Gram-negative bacilli. J Clin Microbiol 53:105–112. doi: 10.1128/JCM.02068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simner PJ, Martin I, Opene B, Tamma PD, Carroll KC, Milstone AM. 2016. Evaluation of multiple methods for detection of gastrointestinal colonization of carbapenem-resistant organisms from rectal swabs. J Clin Microbiol 54:1664–1667. doi: 10.1128/JCM.00548-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing; twenty-ninth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Pierce VM, Simner PJ, Lonsway DR, Roe-Carpenter DE, Johnson JK, Brasso WB, Bobenchik AM, Lockett ZC, Charnot-Katsikas A, Ferraro MJ, Thomson RB Jr, Jenkins SG, Limbago BM, Das S. 2017. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol 55:2321–2333. doi: 10.1128/JCM.00193-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simner PJ, Johnson JK, Brasso WB, Anderson K, Lonsway DR, Pierce VM, Bobenchik AM, Lockett ZC, Charnot-Katsikas A, Westblade LF, Yoo BB, Jenkins SG, Limbago BM, Das S, Roe-Carpenter DE. 2018. Multicenter evaluation of the modified carbapenem inactivation method and the Carba NP for detection of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii. J Clin Microbiol 56:e01369-17. doi: 10.1128/JCM.01369-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock C, Curless MS, Cantara M, Mehta S, Marrone KA, Carroll KC, Simner P, Maragakis LL. 2017. Resolution of carbapenemase-producing Klebsiella pneumoniae outbreak in a tertiary cancer center; the role of active surveillance. Infect Control Hosp Epidemiol 38:1117–1119. doi: 10.1017/ice.2017.136:1-3. [DOI] [PubMed] [Google Scholar]
- 20.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, NISC Comparative Sequencing Program, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamma PD, Goodman KE, Harris AD, Tekle T, Roberts A, Taiwo A, Simner PJ. 2017. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 64:257–264. doi: 10.1093/cid/ciw741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler A, Navon-Venezia S, Moran-Gilad J, Marcos E, Schwartz D, Carmeli Y. 2011. Laboratory and clinical evaluation of screening agar plates for detection of carbapenem-resistant Enterobacteriaceae from surveillance rectal swabs. J Clin Microbiol 49:2239–2242. doi: 10.1128/JCM.02566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samra Z, Bahar J, Madar-Shapiro L, Aziz N, Israel S, Bishara J. 2008. Evaluation of CHROMagar KPC for rapid detection of carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 46:3110–3111. doi: 10.1128/JCM.00249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasoo S, Lolans K, Li H, Prabaker K, Hayden MK. 2014. Comparison of the CHROMagar KPC, Remel Spectra CRE, and a direct ertapenem disk method for the detection of KPC-producing Enterobacteriaceae from perirectal swabs. Diagn Microbiol Infect Dis 78:356–359. doi: 10.1016/j.diagmicrobio.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Zirakzadeh A, Patel R. 2006. Vancomycin-resistant enterococci: colonization, infection, detection, and treatment. Mayo Clin Proc 81:529–536. doi: 10.4065/81.4.529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.