The laboratory diagnosis of latent tuberculosis infection (LTBI) is mainly performed with interferon gamma release assays (IGRAs). We compared the performance of a new enzyme-linked immunosorbent assay (ELISA)-based IGRA, the Standard E TB-Feron ELISA (TBF; SD Biosensor, Gyeonggi-do, Republic of Korea), with that of a widely used assay, the QuantiFERON-TB Gold In-Tube assay (QFT-GIT; Qiagen, Hilden, Germany), in a population of 425 health care workers (HCWs).
KEYWORDS: interferon gamma release assay, latent tuberculosis infection, Standard E TB-Feron ELISA kit, QuantiFERON-TB Gold In-Tube ELISA
ABSTRACT
The laboratory diagnosis of latent tuberculosis infection (LTBI) is mainly performed with interferon gamma release assays (IGRAs). We compared the performance of a new enzyme-linked immunosorbent assay (ELISA)-based IGRA, the Standard E TB-Feron ELISA (TBF; SD Biosensor, Gyeonggi-do, Republic of Korea), with that of a widely used assay, the QuantiFERON-TB Gold In-Tube assay (QFT-GIT; Qiagen, Hilden, Germany), in a population of 425 health care workers (HCWs). All HCWs were screened by both assays per the manufacturers’ protocols and in a cross-manner, where tube sets from one assay were used with the alternative ELISA. The results were compared both qualitatively and quantitatively. TBF and QFT-GIT identified 11.3% (48/425) and 12.9% (55/425) of the positive samples, respectively. TBF demonstrated 81.6% positive and 97.4% negative percent agreement with QFT-GIT, with a Cohen’s kappa value of 0.78 (strong agreement). Discordant results were detected in 20 subjects (4.3%): 13 samples (65.0%) were TBF negative and QFT-GIT positive, 6 samples (30.0%) were TBF positive and QFT-GIT negative, and 1 sample provided TBF and QFT-GIT indeterminate/negative results. We observed a statistically significant degree of correlation between the interferon gamma reactivity between the two assays (Spearman’s rho [rs] value = 0.551, P < 0.01) and between standard assays and cross-manner tests (rs value range, 0.449 to 0.816; P < 0.01 for all combinations). Cross-manner tests also revealed that the ELISA kit of TBF provided higher values for the tube containing the tuberculosis (TB) antigen and the negative-control tube than the ELISA of QFT-GIT under the same conditions (P < 0.01), although these differences disappeared when the value for the negative-control tube was subtracted from that for the TB antigen tube. TBF showed a comparable and acceptable clinical performance in detecting LTBI compared to QFT-GIT. TBF represents a useful alternative tool as an ELISA-based IGRA, especially for large-scale screening for LTBI in HCWs.
INTRODUCTION
Latent tuberculosis infection (LTBI) is characterized by infection with Mycobacterium tuberculosis without evidence of active tuberculosis (TB) disease, including the absence of clinical signs or symptoms and a normal chest radiograph (1). In 2018, the World Health Organization (WHO) reported that approximately 1.7 billion people, or 23% of the world’s population, were estimated to have LTBI (2), and in a lifetime, approximately 8 to 10% of cases of LTBI reactivate into active TB with its clinical manifestations and spread potential (1).
The risk of progression to active TB can be significantly reduced by preventive medication (3), and thus, the identification of a person with LTBI leading to adequate therapy is a fundamental component of TB elimination not only for individual clinical benefit but also for the public prevention of TB spread and for TB elimination (1, 4). The Republic of Korea has an intermediate M. tuberculosis burden, and the government operates a massive LTBI screening program for TB transmission in high-risk populations, such as health care workers (HCWs) and military service personnel (5).
Interferon gamma (IFN-γ) release assays (IGRAs) are widely used laboratory alternatives to the tuberculin skin test (TST) (6). In TST, the purified protein derivative (PPD) is used as the antigen, and cellular immunity to PPD antigens can reflect exposure to M. bovis bacillus Calmette-Guérin (BCG) vaccine strains or nontuberculous mycobacteria (6). IGRAs are ex vivo blood tests measuring the T-cell response after overnight stimulation with antigens specific for M. tuberculosis complex, such as early secreted antigenic target 6 (ESAT-6), culture filtrate protein 10 (CFP-10), and TB7.7 (7). These antigens are more specific for M. tuberculosis than PPD and are not produced by BCG strains, and thus, IGRA results are not impacted by prior BCG vaccination (8). In countries with a high TB prevalence, the majority of the population receives the BCG vaccine; therefore, in these clinical settings, IGRAs are a more useful LTBI diagnostic tool than TST (6, 8).
The QuantiFERON-TB Gold In-Tube assay (QFT-GIT; Qiagen, Germantown, MD) is one of the most widely used IGRAs and is an enzyme-linked immunosorbent assay (ELISA)-based, whole-blood test that uses three antigens (the ESAT-6, CFP-10, and TB7.7 peptides) to predominantly stimulate CD4+ T cells in an in-tube format (9). Three tubes are used in the assay, a TB antigen tube, a positive-control tube (mitogen), and a negative-control tube (nil), and quantitative values from each tube are used to generate a qualitative result.
Recently, a new ELISA-based IGRA, the Standard E TB-Feron ELISA (TBF; SD Biosensor, Gyeonggi-do, Republic of Korea), was approved by the Ministry of Food and Drug Safety of the Republic of Korea. The principles of this assay, including the antigens for stimulating T cells, are almost the same as those of QFT-GIT (10, 11). One distinctive difference between TBF and QFT-GIT is the structure of the TB-specific antigens; the antigens in TBF are recombinant whole proteins of ESAT-6, CFP-10, and TB7.7 (11), but those of QFT-GIT are TB-specific synthetic peptide antigens (10). In addition, the incubation temperature and the time of the incubation procedure in ELISA differ, and TBF offers advantages in terms of cost-effectiveness compared to QFT-GIT.
The aim of this study was to compare the analytical performance of the Standard E TB-Feron assay with that of QFT-GIT for the detection of LTBI in HCWs. In addition, because both assays consist of two stages, blood incubation and ELISA-based IFN-γ measurement, we tested the two IGRAs in a cross-manner, in which the tubes from one assay were combined with the ELISA from the other, to investigate the performance of each stage separately.
MATERIALS AND METHODS
Study population.
The evaluation was performed from August 2018 to May 2019 at the Department of Laboratory Medicine, Chung‐Ang University Hospital, Seoul, Republic of Korea. In the Chung-Ang University Hospital, HCWs are screened for LTBI or TB infection with an IGRA and a chest X ray annually. Once an HCW shows positive results, the particular HCW is no longer screened for LTBI and is usually subjected to a prophylactic treatment. Within this framework, a total of 425 HCWs were involved in this study, and they were examined by chest X ray and two IGRAs, TBF and QFT-GIT. The subjects who were suspected of having active TB based on chest X-ray findings were excluded. None of the subjects was undergoing treatment for TB or had a medical history of LTBI, positive results from IGRAs, or active TB.
Standard E TB-Feron ELISA.
TBF procedures consisted of two stages, where the first stage included incubation of blood from each tube, and the second stage involved plasma IFN-γ analysis by ELISA. For analysis, 1 ml of whole blood was collected into each of the following three tubes: TB antigen, nil, and mitogen tubes. Immediately afterward, the tubes were gently shaken to coat the entire inner surface with blood. Then, the tubes were transferred to a 37°C incubator within 4 h after collection. After 16 h of incubation, the tubes were centrifuged for 15 min at 2,500 × g and the plasma was separated. Then, the ELISA kit was used to quantify the IFN-γ in the separated plasma from each tube. The ELISA kit of TBF required 60 min of incubation at 37°C for accurate reactions. The results were interpreted as positive when the IFN-γ value of the TB antigen tube (TB antigen value) minus that of the nil tube (nil value), referred to as the TB antigen-minus-nil value, was ≥0.35 IU/ml and at least 25% of the nil value. Where the nil value was >8.0 IU/ml or the value of the mitogen tube (mitogen value) minus the nil value was <0.50 IU/ml, the results were considered indeterminate. Any other results were determined to be negative.
QuantiFERON-TB Gold In-Tube assay.
The QFT-GIT procedure was almost analogous to that of TBF, with the exception that the incubation step during ELISA was conducted at room temperature for 120 min, whereas the ELISA kit of TBF required incubation at 37°C for 60 min. The interpretation criteria were identical to those for TBF, as described above.
Cross-manner IGRAs.
Plasma samples were additionally analyzed using the tubes from one assay and the ELISA from the other (referred to here as “cross-manner” IGRAs), where the QFT-GIT ELISA was used to measure IFN-γ from TBF tubes (referred to here as TBF tubes/QFT ELISA) and the ELISA of TBF was used to measure IFN-γ from QFT-GIT tubes (referred to here as QFT tubes/TBF ELISA). Cross-manner tests were conducted at the same time as the standard IGRAs.
Statistical analysis.
To determine the qualitative concordance between TBF and QFT-GIT, the positive percent agreement (PPA), the negative percent agreement (NPA), and Cohen’s κ value were assessed using 3-by-3 crosstab analysis. The κ values were interpreted according to the criteria proposed by Landis and Koch (12), as follows: bad for values of 0.01 to 0.20, fair for values of 0.21 to 0.40, moderate for values of 0.41 to 0.60, strong for values of 0.61 to 0.80, and almost perfect for values of 0.81 to 1.00. Confidence intervals (95%) were also calculated for PPA, NPA, and κ values.
The normality of the quantitative IGRA values was assessed using the Kolmogorov-Smirnov test. IFN-γ values, including those from cross-manner tests, were compared using an independent Student's t test or one-way analysis of variance when the values were normally distributed and the Mann-Whitney U test or the Kruskal-Wallis test when the distribution was not normal. Spearman’s rank correlation test was performed, and Spearman’s rho (rs) values were calculated to investigate the correlation between the nonparametric values from each IGRA. The rs values were interpreted as follows: a very high correlation for values of 0.90 to 1.00 (absolute values), a high correlation for values of 0.70 to 0.90, a moderate correlation for values of 0.50 to 0.70, a low correlation for values of 0.30 to 0.50, and a negligible correlation for values of 0.00 to 0.30 (13). In addition, Bland-Altman plots were drawn to evaluate the agreement among two IGRAs and cross-manner tests. Statistical analyses were performed using SPSS software (v.19; IBM, Armonk, NY, USA) or MedCalc software (v.18.9.1; MedCalc Software, Ostend, Belgium), and when the P value was ≤0.05, the observations were considered statistically significant.
Ethical approval.
The protocol was approved by the Institutional Review Board (IRB) of Chung-Ang University Hospital (Seoul, Republic of Korea; approval no. 1873-001-334), and informed consent was obtained from the study subjects according to the IRB’s policy. Study subjects with positive IGRA results were managed in accordance with the guidelines of the Korea Centers for Disease Control and Prevention (KCDC) (5), based on the QFT-GIT results.
RESULTS
Subject characteristics.
A total 425 HCWs were involved in this study. The subjects were between 21 and 63 years old, with a median age of 31 years, and 80.5% of the subjects were female. The professions of the subjects were nurse (49.9%, 212/425), medical technician (25.9%, 110/425), clinician (8.9%, 38/425), clerk (7.3%, 31/425), or other professions (8.0%, 34/425), such as hospital cleaner. There were 20 subjects who showed discordant results between the two standard IGRAs. The median age of the subjects with discordant results was 32 years (range, 23 to 56 years), and 85% (17/20) of them were females. Moreover, 35% (7/20) were nurses, 20% (4/20) were medical technicians, 20% (4/20) were clerks, and 20% (4/20) had other professions; 1 of the 20 (5%) subjects with a discordant result was a clinician.
Qualitative comparison of QuantiFERON-TB Gold In-Tube and Standard E TB-Feron assays.
TBF showed 11.3% positivity, which was a value slightly lower than that for QFT-GIT, which showed 12.9% positivity. The absolute agreement between the QFT-GIT and TBF qualitative results was 95.3% (405/425), with the κ value being 0.78 (P < 0.001; 95% confidence interval, 0.69 to 0.87), which was interpreted as strong agreement. The PPA and NPA were 81.6% and 97.4%, respectively. A total of 4.7% (20/425) of the samples showed discordant results between the two IGRAs. Details of the qualitative comparison tests are shown in Table 1.
TABLE 1.
Qualitative comparisons between TBF and QFT-GIT in 425 HCWsa
| QFT-GIT result | No. (%) of HCWs with the following TBF result: |
|||
|---|---|---|---|---|
| Positive | Negative | Indeterminate | Total | |
| Positive | 42 (9.9) | 13 (3.1) | 0 (0.0) | 55 (12.9) |
| Negative | 6 (1.4) | 363 (85.4) | 1 (0.2) | 370 (87.1) |
| Indeterminate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 48 (11.3) | 376 (88.5) | 1 (0.2) | 425 (100.0) |
Abbreviations: TBF, Standard E TB-Feron ELISA; QFT-GIT, QuantiFERON-TB Gold In-Tube assay.
The 20 discordant results are summarized in Fig. 1. Among the samples with discordant results, 13 (65%) were TBF negative (TBF−) and QFT-GIT positive (QFT-GIT+), where the median IFN-γ value was 0.24 IU/ml (range, 0.09 to 0.34 IU/ml) using TBF and 0.78 IU/ml (range, 0.37 to 1.89 IU/ml) using QFT-GIT. Six samples (30.0%) were TBF positive (TBF+) and QFT-GIT negative (QFT-GIT−), where the median TBF and QFT-GIT IFN-γ values were 0.43 IU/ml (range, 0.35 to 0.63 IU/ml) and 0.16 IU/ml (range, 0.0 to 0.31 IU/ml), respectively. One sample (5.0%) was interpreted as indeterminate in the TBF assay, where the mitogen tube-minus-nil tube value was 0.43 IU/ml, which is <0.5 IU/ml, and the sample was negative by QFT-GIT.
FIG 1.
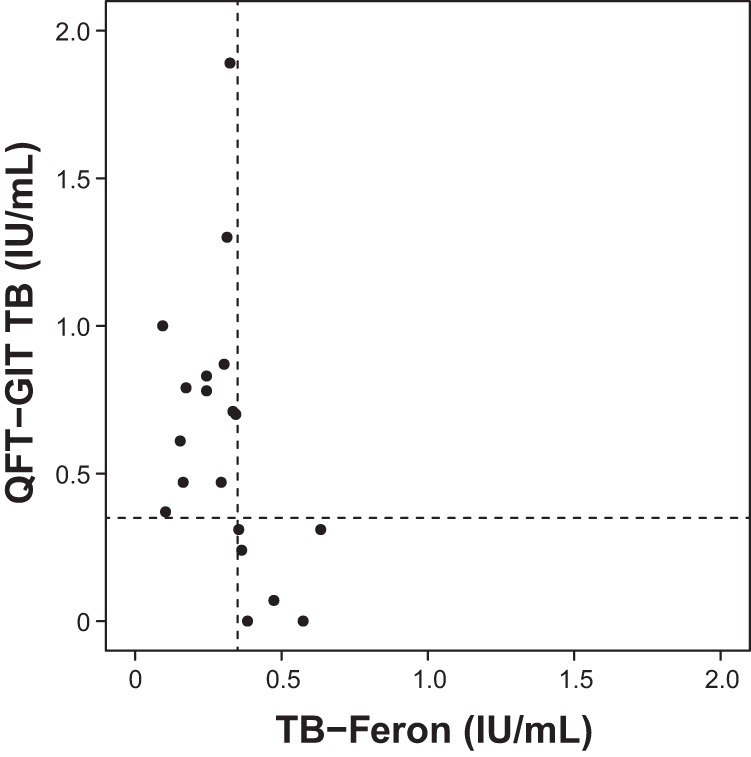
Scatter plot for the TB antigen-minus-nil values from the samples which showed discordant results between the Standard E TB-Feron ELISA (TBF) and the QuantiFERON-TB Gold In-Tube assay (QFT-GIT). Among the 20 samples with discordant results, 13 samples (65.0%) were negative with TBF and positive with QFT-GIT and 6 samples (30.0%) showed positive TBF and negative QFT-GIT results. One sample showed an indeterminate result with TBF and a negative result with QFT-GIT (these data were not plotted). The dashed lines designate the cutoff values for the determination of positive results from the IGRAs (0.35 IU/ml).
Quantitative comparison of QuantiFERON-TB Gold In-Tube and Standard E TB-Feron assays.
Spearman’s rank correlation test of quantitative IGRA results revealed a statistically significant correlation between the two assays for TB antigen and TB antigen-minus-nil values. For IFN-γ reactivity in TB antigen tubes, rs was 0.551 (P < 0.01) and the TB antigen-minus-nil value was 0.461 (P < 0.01); these results were interpreted as a moderate correlation. For all combinations of comparisons between the results of IGRAs, including cross-manner tests, rs ranged from 0.449 to 0.816 (P < 0.01). Details of the correlation analyses are listed in Table S1 in the supplemental material.
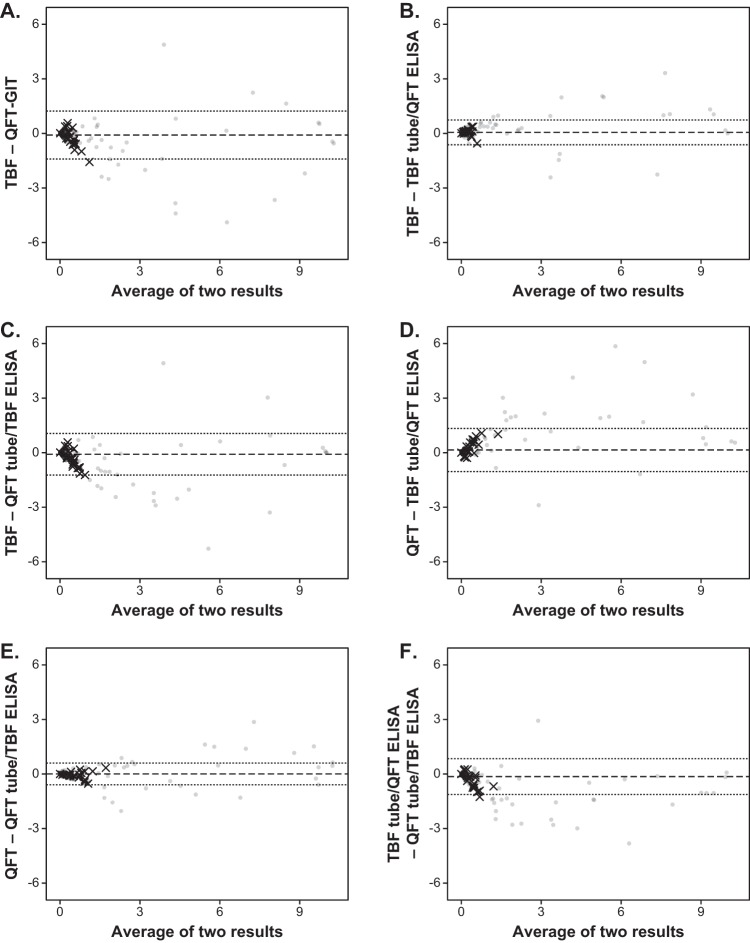
A Bland-Altman plot between two IGRAs for the TB antigen-minus-nil values is illustrated in Fig. 2A. It was revealed that 96.2% (409/425) of the values were within 2 standard deviations (2SD), and among the 20 samples with discordant results, only 1 sample showed differences higher than 2SD.
FIG 2.
Bland-Altman plots for the TB antigen-minus-nil values (in international units per milliliter) among the Standard E TB-Feron ELISA (TBF), the QuantiFERON-TB Gold In-Tube assay (QFT-GIT), and the two cross-manner tests (TBF tubes/QFT ELISA and QFT tubes/TBF ELISA). The dashed line and the dotted lines designate the mean value and two standard deviations of the difference between the results of the two assays, respectively. The samples with discordant results between TBF and QFT-GIT are plotted using multiplication signs, and the others are plotted using filled circles.
Cross-manner IGRAs.
Qualitative comparisons of the two standard IGRAs and the two cross-manner tests, TBF tubes/QFT ELISA and QFT tubes/TBF ELISA, are listed in Table 2. κ values ranged from 0.72 (TBF and QFT tubes/TBF ELISA, P < 0.01) to 0.88 (QFT-GIT and QFT tubes/TBF ELISA, P < 0.01). Noticeably, higher κ values were observed when comparing a standard IGRA to a cross-manner test using the same tubes used in the IGRA. The proportions of positive results for TBF tubes/QFT ELISA and QFT tubes/TBF ELISA were 8.9% and 14.8%, respectively. Two cases of indeterminate results were recorded for QFT tubes/TBF ELISA.
TABLE 2.
Qualitative results obtained by comparison of TBF and QFT-GIT results to the results from TBF tubes/QFT-GIT ELISA and QFT-GIT tubes/TBF ELISAa
| Assay and result | TBF tubes/QFT ELISA |
QFT tubes/TBF ELISA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) of HCWs with the following result: |
Kappa value (95% CI) | No. (%) of HCWs with the following result: |
Kappa value (95% CI) | |||||||
| Positive | Negative | Indeterminate | Total | Positive | Negative | Indeterminate | Total | |||
| TBF | ||||||||||
| Positive | 34 (8.0) | 14 (3.3) | 0 (0.0) | 48 (11.3) | 43 (10.1) | 5 (1.2) | 0 (0.0) | 48 (11.3) | ||
| Negative | 4 (0.9) | 372 (87.5) | 0 (0.0) | 376 (88.5) | 20 (4.7) | 354 (83.3) | 2 (0.5) | 376 (88.5) | ||
| Indeterminate | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | ||
| Total | 38 (8.9) | 387 (91.1) | 0 (0.0) | 425 (100.0) | 0.75b (0.65–0.86) | 63 (14.8) | 360 (84.7) | 2 (0.5) | 425 (100.0) | 0.72b (0.67–0.77) |
| QFT-GIT | ||||||||||
| Positive | 36 (86.8) | 19 (4.5) | 0 (0.0) | 55 (12.9) | 54 (12.7) | 1 (0.2) | 0 (0.0) | 55 (12.9) | ||
| Negative | 2 (0.5) | 368 (86.9) | 0 (0.0) | 370 (87.1) | 9 (2.1) | 359 (84.5) | 2 (0.5) | 370 (87.1) | ||
| Indeterminate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total | 38 (8.9) | 387 (91.1) | 0 (0.0) | 425 (100.0) | 0.75b (0.64–0.85) | 63 (14.8) | 360 (84.7) | 2 (0.5) | 425 (100.0) | 0.88b (0.82–0.95) |
Abbreviations: TBF, Standard E TB-Feron ELISA; QFT-GIT, QuantiFERON-TB Gold In-Tube assay; QFT ELISA, QuantiFERON-TB Gold In-Tube ELISA; CI, confidence interval.
P < 0.001.
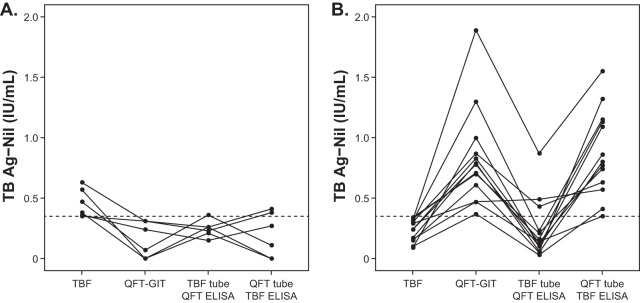
Among the 405 samples which showed concordant results between the two IGRAs, 19 (4.7%) showed discordant results in one or both of the cross-manner tests. Of the 42 samples positive by both IGRAs, 10 (23.8%) gave discordant results in cross-manner tests, of which the majority (90%, 9/10) was found with TBF tubes/QFT ELISA. Of the negative samples, 9 (2.4%) gave positive or indeterminate results with QFT tubes/TBF ELISA. Samples which showed discordant results between the two standard IGRAs provided a variety of results in the cross-manner studies. Of the 13 TBF− and QFT-GIT+ samples, the majority (10/13, 76.9%) were negative with TBF tubes/QFT ELISA and positive with QFT tubes/TBF ELISA. A schematic of the IFN-γ values (TB antigen-minus-nil values) from all assays, including cross-manner tests, for the samples with discordant results between the two standard IGRAs (except for the one TBF-indeterminate sample) is illustrated in Fig. 3, and the distribution of qualitative results for the standard IGRAs and the cross-manner tests is shown in Table S2.
FIG 3.
Distribution of interferon gamma levels (TB antigen-minus-nil values) in the samples measured in the Standard E TB-Feron ELISA (TBF), the QuantiFERON-TB Gold In-Tube assay (QFT-GIT), and the two cross-manner tests (TBF tubes/QFT ELISA and QFT tubes/TBF ELISA). The dashed lines designate the cutoff values for the determination of positive results from the IGRAs (0.35 IU/ml). (A) Results for samples which showed positivity by TBF but negativity by QFT-GIT (n = 6); (B) results for samples which had negative results by TBF but positive results by QFT-GIT (n = 13).
Quantitative comparisons are summarized in Table 3; three distinct findings were observed. First, TBF TB antigen and nil values were higher than those of QFT-GIT (P < 0.001), but no significant difference was observed for TB antigen-minus-nil values. Second, TB antigen-minus-nil, TB antigen, and nil values were statistically significantly higher in TBF than in TBF tubes/QFT ELISA (P < 0.001), and similarly, total TB antigen and nil values were higher in QFT tubes/TBF ELISA than in QFT-GIT (P < 0.001). In other words, the IFN-γ values measured using the ELISA kit of TBF were higher than those measured with the ELISA of QFT-GIT when the same tubes were used. Third, total TB antigen-minus-nil, TB antigen, and nil values were higher in QFT-GIT than in TBF tubes/QFT ELISA. This implies that the ELISA of QFT-GIT generates higher IFN-γ values in combination with the QFT-GIT tubes than in combination with the TBF tubes. On the other hand, there were no differences in the values between TBF and QFT tubes/TBF ELISA, except for the nil values for negative subgroups; therefore, the ELISA kit of TBF was not affected by the tubes used in the incubation stage.
TABLE 3.
IFN-γ values measured from TB antigen-tube-minus nil tube, TB antigen tube, and nil tube of TBF and QFT-GIT compared to those for TBF tubes/QFT-GIT ELISA and QFT-GIT tubes/TBF ELISAa
| Tube and result | Median (interquartile range) IFN-γ concn (IU/ml) |
P value | Kruskal-Wallis groupingb | |||
|---|---|---|---|---|---|---|
| TBF | QFT-GIT | TBF tubes/QFT-GIT | QFT tubes/TBF | |||
| TB antigen minus nil | ||||||
| Total | 0.00 (0.00–0.08) | 0.01 (0.00–0.09) | 0.00 (0.00–0.03) | 0.01 (0.00–0.09) | <0.001 | a, a, b, a |
| Positive | 1.02 (0.42–2.24) | 1.30 (0.71–4.53) | 1.06 (0.56–4.36) | 1.20 (0.59–3.31) | 0.089 | |
| Negative | 0.00 (0.00–0.04) | 0.00 (0.00–0.04) | 0.00 (0.00–0.02) | 0.00 (0.00–0.04) | <0.001 | a, a, b, a |
| TB antigen | ||||||
| Total | 0.23 (0.17–0.35) | 0.13 (0.07–0.31) | 0.07 (0.05–0.14) | 0.22 (0.16–0.39) | <0.001 | a, b, c, a |
| Positive | 1.28 (0.70–2.57) | 1.52 (0.86–6.16) | 1.15 (0.61–4.45) | 1.53 (0.86–2.92) | 0.298 | |
| Negative | 0.22 (0.17–0.29) | 0.11 (0.07–0.19) | 0.07 (0.04–0.11) | 0.20 (0.16–0.30) | <0.001 | a, b, c, a |
| Nil | ||||||
| Total | 0.21 (0.17–0.29) | 0.09 (0.06–0.14) | 0.06 (0.04–0.10) | 0.19 (0.15–0.27) | <0.001 | a, b, c, a |
| Positive | 0.25 (0.18–0.35) | 0.12 (0.06–0.24) | 0.07 (0.04–0.13) | 0.21 (0.18–0.40) | <0.001 | a, b, c, a |
| Negative | 0.21 (0.17–0.27) | 0.09 (0.06–0.15) | 0.06 (0.04–0.10) | 0.19 (0.15–0.26) | <0.001 | a, b, c, d |
Abbreviations: TBF, Standard E TB-Feron ELISA; QFT-GIT, QuantiFERON-TB Gold In-Tube assay; QFT ELISA, QuantiFERON-TB Gold In-Tube ELISA.
The same letter (a) indicates nonsignificant differences between groups based on the Mann-Whitney U test or Scheffe’s multiple-comparison test.
Bland-Altman plots among the two IGRAs and the cross-manner tests between two IGRAs for the TB antigen-minus-nil values are illustrated in Fig. 2B to F. In all the plots except for those between the TBF tube/QFT ELISA and the QFT tube/TBF ELISA, more than 95.0% of the difference values were within 2SD; the percentages were 96.5% (410/425) for those between TBF and the TBF tube/QFT ELISA, 96.0% (408/425) for those between TBF and the QFT tube/TBF ELISA, 96.0% (408/425) for those between QFT-GIT and the TBF tube/QFT ELISA, 96.0% (408/425) for those between QFT-GIT and the QFT tube/TBF ELISA, and 94.1% (400/425) for those between the TBF tube/QFT ELISA and the QFT tube/TBF ELISA.
DISCUSSION
In this study, we revealed that the performance of TBF, a newly developed IGRA, was comparable to that of QFT-GIT, the most widely used assay in the same technical category, for the diagnosis of LTBI in HCWs. TBF showed a positive rate of LTBI detection comparable to that of QFT-GIT, and the κ value between the two IGRAs represented a strong degree of correlation. Recently, several ELISA-based IGRAs using three tubes were developed and released in the South Korean market, and accordingly, this has provided a range of choice to clinical laboratories wider than that in the past.
HCWs with LTBI are a potential risk group for the transmission of M. tuberculosis to other HCWs and patients. Thus, screening of HCWs for LTBI and treatment of HCWs are important components of TB infection control in hospitals (14–16). The Republic of Korea, where this study was performed, has an intermediate M. tuberculosis burden, with an annual notification rate of new TB cases of 60.4 per 100,000 population in 2016 (2, 5). The Korea Centers for Disease Control and Prevention (KCDC) recommends that HCWs working in departments with an increased risk of TB infection receive annual screening for LTBI (5). In these circumstances, the diagnostic performance of IGRAs detecting LTBI is fundamental for TB control.
Although the quantitative IFN-γ values from both IGRAs evaluated in this study were well correlated and the qualitative performance of TBF was comparable to that of QFT-GIT, discordant results were observed. In our quantitative comparison, we found that TB antigen and nil tube values were higher in TBF than in QFT-GIT, and these differences appear to originate from both the tube (incubation) and ELISA stages. By comparing these assays in a cross-manner, we revealed that the ELISA kit of TBF provided higher values for IFN-γ measurements than the ELISA of QFT-GIT. The incubation time and temperature during the ELISA stage were different between the two IGRAs, and this difference might contribute to the higher measurements observed in the ELISA kit of TBF.
TBF uses recombinant whole protein of TB-specific antigens, but the antigens of QFT-GIT are peptide forms. Whole recombinant proteins are degraded into diverse small peptides, and multiple epitopes can be presented onto the cell surface. The multiple epitopes could stimulate T cells more effectively and might, consequently, lead to better sensitivity or higher IFN-γ values. In addition, some HLA genotypes had a low affinity of binding to both the ESAT-6 and CFP10 epitopes (17, 18); hence, multiple epitopes might give advantages to the performance of the IGRA. However, in spite of those differences, the better performance or higher IFN-γ values of the TBF tubes than the QFT-GIT tubes were not found in the present study. Rather, QFT-GIT tubes appeared to generate higher IFN-γ values, as the use of TBF tubes with the QFT-GIT ELISA resulted in lower values. Although there were differences in TB antigen and nil values between the two IGRAs tested, those difference were removed when the TB antigen-minus-nil value calculation was performed, resulting in an acceptable concordance rate between the IGRAs.
Of the 425 HCW samples, 20 showed discordant results between the two IGRAs. The six TBF+ and QFT-GIT− samples may have resulted from the higher IFN-γ values generated by TBF. We speculate this because the majority of these samples were negative in TBF tubes/QFT ELISA and QFT tubes/TBF ELISA cross-manner tests (five and four out of six, respectively). The tendency toward higher IFN-γ values in the ELISA of TBF is also illustrated by the finding that two QFT-GIT-negative samples were positive with QFT tubes/TBF ELISA. Thirteen samples which showed TBF− and QFT-GIT+ results may have arisen due to a tube problem rather than an ELISA problem, wherein IFN-γ generation was low in TBF tubes and clearly high in QFT-GIT tubes. This finding implies that there would be a small group of individuals in whom LTBI is not detected by TBF. However, as we did not record or collect the subject characteristics extensively, we were not able to assess the possible factors contributing to these discordant results. Regarding the gray zone (TB antigen-minus-nil value, 0.2 to 0.7 IU/ml), three samples showed discordant TB antigen-minus-nil values for IGRAs outside of the gray zone (values lower than 0.2 and higher than 0.7 for each IGRA), and all of these samples showed TBF− and QFT-GIT+ results. Four samples showed discordant results, but both values from the two IGRAs were within the gray zone, and the remaining 13 samples showed values within the gray zone from only one of the IGRAs.
There is no “gold standard” for IGRAs; it is therefore impossible to declare which IGRA was more accurate. Although our study did not assess the repeatability or variability of the two IGRAs, previous investigators reported that QFT-GIT had considerable variability in qualitative results, from ±0.24 IU/ml for the samples with responses in the borderline range of 0.25 to 0.8 IU/ml to ±0.6 IU/ml for all samples (9, 19, 20). The differences between the laboratory or research setting of the previous studies and our study thus make the findings of the previous study difficult to apply to our study. Indeed, applying these findings to our study could potentially allow reclassification of most of the discordant samples (19/20), with the exception of one sample (TB antigen-minus-nil values, 1.0 IU/ml in QFT-GIT and 0.09 IU/ml in TBF). The high variability of IGRA results arises from the fact that IGRAs have various potential sources of variability (9, 20–22). For example, preanalytical sources of variability include the time of blood collection, inadequate disinfection of the skin, the order of tube filling, the volume of blood drawn, vigorous shaking, a delay in sample incubation, and the transport of blood specimens at a lower ambient temperature. Analytical sources of variability or those in the ELISA stage include the matrix effect, imprecision of pipetting, errors in centrifugation, problems during washing steps, imprecision in final signal measurement, and the use of automated or manual methods. Furthermore, postanalytical errors can occur during manual data entry. As might be expected, manufacturing defects, such as faulty antigen tubes, can also occur, and this problem has been documented in QFT-GIT (23, 24). Additional research focusing on these possible sources of error and the repeatability of TBF is therefore warranted because these questions were not addressed in our study.
Recently, the QuantiFERON-TB Gold Plus assay (QFT-Plus; Qiagen), which is the next generation of QFT-GIT and which is a four-tube-based IGRA, was released (25–27). This assay contains tubes with peptides from only the ESAT-6 and CFP-10 antigens and two tubes with TB antigens (TB1 and TB2). Additionally, each tube is designed to elicit IFN-γ release from CD4+ and CD8+ T cells for accurate LTBI detection in individuals with low CD4+ T cell counts (25–28). However, in the screening programs with HCWs or military service personnel, most subjects have a normal immune status, and thus, several reports that evaluated the performance of QFT-Plus in HCWs reported that it showed a high correlation with QFT-GIT and results comparable to those of QFT-GIT (26, 29). In addition, QFT-Plus is more expensive and requires more resources for analysis; therefore, three-tube IGRAs remain a cost-effective way for screening of HCWs. Also, it is predicted that QFT-GIT reagents will be discontinued; thus, another three-tube IGRA, such as TBF, offers an alternative choice based on the fact that its performance is comparable to that of QFT-GIT.
This study had several limitations. First, we did not assess reproducibility, as described above. Second, we did not evaluate disease status affecting immune status; clinical characteristics, including smoking status; drugs taken other than anti-TB medication; or the medical histories of the study subjects. These factors may contribute to indeterminate or inaccurate IGRA results. However, we assumed that few HCWs in our study were impacted by these conditions or factors, because among the 425 subjects, only 1 subject had indeterminate results. The third limitation concerns our focus on HCWs rather than immunocompromised patients, who are a group at high risk of TB infection or LTBI. Further evaluation of TBF should be performed with this patient population. Finally, the exact TB exposure history was not collected individually for each subject, and because the subjects worked in different wards or departments, the degree of TB exposure could differ.
In conclusion, TBF showed a performance comparable to that of QFT-GIT and acceptable clinical performance for detecting LTBI; thus, TBF represents a useful, alternative ELISA-based IGRA, especially for large-scale screening of HCWs for LTBI. Both the tubes and the ELISAs of the IGRAs appeared to affect the assay performance, and although TBF showed higher TB antigen and nil values than QFT-GIT, the differences were eliminated by calculating TB antigen-minus-nil values. As some discordant results between the IGRAs were also present, caution is needed while interpreting the data, especially for results near the cutoff value.
Supplementary Material
ACKNOWLEDGMENTS
We thank SD Biosensor for providing the Standard E TB-Feron ELISA kits for this evaluation.
SD Biosensor provided technical support only and had no role in the study design, data collection, and interpretation.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01347-19.
REFERENCES
- 1.Haley CA. 2017. Treatment of latent tuberculosis infection. Microbiol Spectr 5:TNMI7-0039-2016. doi: 10.1128/microbiolspec.TNMI7-0039-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2018. Global tuberculosis report 2018. World Health Organization Geneva, Geneva, Switzerland. [Google Scholar]
- 3.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, Kerrigan A, Weis SE, Weiner M, Wing D, Conde MB, Bozeman L, Horsburgh CR Jr, Chaisson RE. 2011. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 4.Fox GJ, Dobler CC, Marais BJ, Denholm JT. 2017. Preventive therapy for latent tuberculosis infection—the promise and the challenges. Int J Infect Dis 56:68–76. doi: 10.1016/j.ijid.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Korea Centers for Disease Control and Prevention. 2018. Korean guidelines for tuberculosis. Korea Centers for Disease Control and Prevention, Cheongju, Republic of Korea. [Google Scholar]
- 6.Pai M, Behr M. 2016. Latent Mycobacterium tuberculosis infection and interferon-gamma release assays. Microbiol Spectr 4:TBTB2-0023-2016. doi: 10.1128/microbiolspec.TBTB2-0023-2016. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho AC, Pezzoli MC, El-Hamad I, Arce P, Bigoni S, Scarcella C, Indelicato AM, Scolari C, Carosi G, Matteelli A. 2007. QuantiFERON-TB Gold test in the identification of latent tuberculosis infection in immigrants. J Infect 55:164–168. doi: 10.1016/j.jinf.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Andersen P, Munk ME, Pollock JM, Doherty TM. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 9.Banaei N, Gaur RL, Pai M. 2016. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? J Clin Microbiol 54:845–850. doi: 10.1128/JCM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiagen. 2016. QuantiFERON-TB Gold (QFT) ELISA package insert. Qiagen, Germantown, MD. [Google Scholar]
- 11.SD Biosensor. 2019. Standard E TB-Feron ELISA package insert. SD Biosensor, Gyeonggi-do, Republic of Korea. [Google Scholar]
- 12.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 13.Mukaka MM. 2012. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. 2005. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recommend Rep 54(RR-17):1–141. [PubMed] [Google Scholar]
- 15.Sosa LE, Njie GJ, Lobato MN, Bamrah Morris S, Buchta W, Casey ML, Goswami ND, Gruden M, Hurst BJ, Khan AR, Kuhar DT, Lewinsohn DM, Mathew TA, Mazurek GH, Reves R, Paulos L, Thanassi W, Will L, Belknap R. 2019. Tuberculosis screening, testing, and treatment of U.S. health care personnel: recommendations from the National Tuberculosis Controllers Association and CDC, 2019. MMWR Morb Mortal Wkly Rep 68:439–443. doi: 10.15585/mmwr.mm6819a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ringshausen FC, Schablon A, Nienhaus A. 2012. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: a systematic review. J Occup Med Toxicol 7:6. doi: 10.1186/1745-6673-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruhwald M, Aabye MG, Ravn P. 2012. IP-10 release assays in the diagnosis of tuberculosis infection: current status and future directions. Expert Rev Mol Diagn 12:175–187. doi: 10.1586/erm.11.97. [DOI] [PubMed] [Google Scholar]
- 18.Hang NT, Lien LT, Kobayashi N, Shimbo T, Sakurada S, Thuong PH, Hong LT, Tam DB, Hijikata M, Matsushita I, Hung NV, Higuchi K, Harada N, Keicho N. 2011. Analysis of factors lowering sensitivity of interferon-gamma release assay for tuberculosis. PLoS One 6:e23806. doi: 10.1371/journal.pone.0023806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. 2013. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med 187:206–211. doi: 10.1164/rccm.201203-0430OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagmouti S, Slater M, Benedetti A, Kik SV, Banaei N, Cattamanchi A, Metcalfe J, Dowdy D, van Zyl Smit R, Dendukuri N, Pai M, Denkinger C. 2014. Reproducibility of interferon gamma (IFN-gamma) release assays. A systematic review. Ann Am Thorac Soc 11:1267–1276. doi: 10.1513/AnnalsATS.201405-188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitworth WC, Goodwin DJ, Racster L, West KB, Chuke SO, Daniels LJ, Campbell BH, Bohanon J, Jaffar AT, Drane W, Sjoberg PA, Mazurek GH. 2014. Variability of the QuantiFERON(R)-TB gold in-tube test using automated and manual methods. PLoS One 9:e86721. doi: 10.1371/journal.pone.0086721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaur RL, Pai M, Banaei N. 2013. Impact of blood volume, tube shaking, and incubation time on reproducibility of QuantiFERON-TB gold in-tube assay. J Clin Microbiol 51:3521–3526. doi: 10.1128/JCM.01627-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slater M, Parsonnet J, Banaei N. 2012. Investigation of false-positive results given by the QuantiFERON-TB Gold In-Tube assay. J Clin Microbiol 50:3105–3107. doi: 10.1128/JCM.00730-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couturier MR, Myatt R, Dorn D, Yang DT, Pitstick N. 2014. Defective antigen tubes generate false-positive QuantiFERON tuberculosis test results. Clin Infect Dis 59:1649–1650. doi: 10.1093/cid/ciu644. [DOI] [PubMed] [Google Scholar]
- 25.Takasaki J, Manabe T, Morino E, Muto Y, Hashimoto M, Iikura M, Izumi S, Sugiyama H, Kudo K. 2018. Sensitivity and specificity of QuantiFERON-TB Gold Plus compared with QuantiFERON-TB Gold In-Tube and T-SPOT.TB on active tuberculosis in Japan. J Infect Chemother 24:188–192. doi: 10.1016/j.jiac.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Theel ES, Hilgart H, Breen-Lyles M, McCoy K, Flury R, Breeher LE, Wilson J, Sia IG, Whitaker JA, Clain J, Aksamit TR, Escalante P. 2018. Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube Interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol 56:e00614-18. doi: 10.1128/JCM.00614-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryu MR, Park MS, Cho EH, Jung CW, Kim K, Kim SJ, Oh HY, Huh W, Jang HR, Koh WJ, Park HY, Kim YH, Sinn DH, Choi JO, Joh JW, Kim JM, Kim SJ, Park JB, Kang ES. 2018. Comparative evaluation of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus in diagnosis of latent tuberculosis infection in immunocompromised patients. J Clin Microbiol 56:e00438-18. doi: 10.1128/JCM.00438-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho K, Cho E, Kwon S, Im S, Sohn I, Song S, Kim H, Kim S. 2012. Factors associated with indeterminate and false negative results of QuantiFERON-TB Gold In-Tube test in active tuberculosis. Tuberc Respir Dis (Seoul) 72:416–425. doi: 10.4046/trd.2012.72.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon HW, Gaur RL, Tien SS, Spangler M, Pai M, Banaei N. 2017. Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 55:1650–1657. doi: 10.1128/JCM.02498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.