Blood cultures are routinely collected in pairs of aerobic and anaerobic bottles. Artificial sterilization of Gram-negative bacteria in aerobic bottles containing clinically meaningful antibiotic concentrations has previously been observed. This study assessed recovery from anaerobic bottles with and without antibiotic binding resins.
KEYWORDS: Escherichia coli, Klebsiella pneumoniae, bacteremia, antimicrobial stewardship, carbapenem, cephalosporin, piperacillin, levofloxacin
ABSTRACT
Blood cultures are routinely collected in pairs of aerobic and anaerobic bottles. Artificial sterilization of Gram-negative bacteria in aerobic bottles containing clinically meaningful antibiotic concentrations has previously been observed. This study assessed recovery from anaerobic bottles with and without antibiotic binding resins. We studied the recovery of Escherichia coli and Klebsiella pneumoniae when exposed to meropenem, imipenem, cefepime, cefazolin, levofloxacin, and piperacillin-tazobactam in resin-containing BacT/Alert FN Plus and BD Bactec Plus anaerobic/F bottles as well as resin-free BacT/Alert SN and BD Bactec standard anaerobic bottles. Bottles were inoculated with bacteria and whole blood containing peak, midpoint, or trough concentrations and incubated for up to 120 hours in their respective detection systems. In E. coli resin-containing bottles, recovery was observed in 10/24 (42%), 17/24 (71%), and 18/24 (75%) (P = 0.034) of those exposed to peak, midpoint, and trough concentrations, respectively. In K. pneumoniae resin-containing bottles, recovery was observed in 8/16 (50%), 10/16 (63%), and 10/16 (63%) (P = 0.710), respectively. No growth was detected in bottles containing cefepime regardless of concentration, while recovery was observed in the presence of all concentrations of cefazolin and piperacillin-tazobactam. Recovery in bottles with meropenem and imipenem was more frequently observed in BacT/Alert FN Plus bottles compared with Bactec Plus bottles. Resin-free bottles demonstrated significantly lower recovery than bottles containing binding resin. Clinical concentrations of certain antibiotics can adversely affect detection of E. coli and K. pneumoniae in anaerobic blood culture bottles. Obtaining blood cultures immediately before a dose and utilizing resin-containing anaerobic bottles will maximize the likelihood of recovery.
INTRODUCTION
Optimal management of bloodstream infection relies on blood cultures to guide antibacterial therapy (1). The Surviving Sepsis Campaign guidelines recommend that two or more sets of blood cultures be drawn, each consisting of one aerobic and one anaerobic bottle (2). While aerobic bacteria are likely to grow in the aerobic bottle, the anaerobic bottle provides an ideal environment for anaerobes (3). As facultative anaerobes, certain Enterobacterales, such as Escherichia coli and Klebsiella pneumoniae, may grow in both environments. These Gram-negative pathogens have become increasingly prevalent in bloodstream infections over the last 2 decades (4–7).
Ideally, blood cultures are to be drawn before starting antibiotics, due to the dangers of artificial sterilization (2). However, more often than not, antibiotics have already been administered to inpatients by the time blood cultures are obtained (8). Blood culture bottles containing antibiotic binding resins have been developed to address this issue, though their effectiveness is not always guaranteed. Grupper and colleagues investigated the impact of clinically relevant antibiotic concentrations on Pseudomonas aeruginosa recovery from aerobic blood culture bottles containing antibiotic binding resins and found that growth was only consistently detected in bottles with trough concentrations of meropenem, ceftolozane-tazobactam, and ceftazidime-avibactam (9). Our lab has also previously investigated the recovery of E. coli and K. pneumoniae in aerobic bottles and observed recovery more frequently at lower antibiotic concentrations (10). Other studies have come to similar conclusions that resins may not completely eliminate artificial sterilization risks (11–13), thus supporting the practice of obtaining blood cultures when the lowest concentration of antibiotic remains in the patient’s system or right before an upcoming dose.
Unfortunately, few studies assessing the recovery of facultative anaerobes from resin-containing anaerobic bottles have been performed. Grohs and colleagues found that nearly 14% of the 994 patients with positive blood cultures had only their anaerobic blood culture bottle alarm positive, and of these, two-thirds of the recovered bacteria were not obligate anaerobes; thus, in vitro sterilization could have clinically relevant consequences between the two bottle types (3). We sought to determine the recovery and time to detection (TTD) of E. coli and K. pneumoniae from BacT/Alert and Bactec anaerobic bottles with and without antibiotic binding resins when exposed to clinically meaningful concentrations of antibiotics commonly used empirically in the hospital.
(These results were presented in part in an abstract at ASM Microbe 2019, San Francisco, CA, 20 to 25 June 2019 [14].)
MATERIALS AND METHODS
This was a prospective in vitro analysis to assess recovery and TTD of E. coli or K. pneumoniae in BacT/Alert FN Plus anaerobic and Bactec Plus anaerobic/F resin-containing blood culture bottles inoculated with clinically meaningful peak, midpoint, or trough concentrations of cefepime, cefazolin, imipenem, levofloxacin, meropenem, and piperacillin/tazobactam. The institutional review board (IRB) at Hartford Hospital (Hartford, CT, USA) reviewed and approved the study. Written informed consent was obtained from healthy volunteers before donating whole blood for the study.
Antibiotics.
Commercially available formulations of meropenem (Fresenius Kabi USA, Inc., Lake Zurich, IL), imipenem (Fresenius Kabi USA, Inc., Lake Zurich, IL), cefazolin (Sagent Pharmaceuticals, Schaumburg, IL), cefepime (WG Critical Care, LLC, Paramus, NJ), levofloxacin (Aurobindo Pharma LTD, Telangana, India), and piperacillin-tazobactam (Fresenius Kabi USA, Inc., Lake Zurich, IL) were obtained through Cardinal Health (Dublin, OH) and reconstituted according to their package inserts.
Bacteria.
E. coli ATCC 25922 and K. pneumoniae ATCC 13883 were obtained from American Type Culture Collection (ATCC, Manassas, VA). Isolates were frozen on skim milk, stored at –80°C until use, subcultured two times on 5% blood Trypticase soy agar, and incubated at 37°C overnight before each experiment. MICs were determined in triplicate using Clinical and Laboratory Standards Institute (CLSI) broth microdilution, and susceptibility categorization was assigned based on published CLSI and European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints (15, 16).
Blood culture bottles.
BacT/Alert FN Plus anaerobic and SN standard bottles were provided by bioMérieux, Inc., (Durham, NC) and tested in the Virtuo BacT/Alert detection system in the Center for Anti-Infective Research and Development (CAIRD) laboratory at Hartford Hospital. Bactec Plus anaerobic/F and standard anaerobic bottles (Becton Dickinson and Company, Franklin Lakes, NJ) were purchased from the manufacturer and tested in the BD Bactec FX detection system located in the Hartford Hospital clinical microbiology department.
Blood culture bottle preparation.
Fresh whole blood was collected from healthy adult volunteers and pooled on the first day of each experiment. Antibiotics were then introduced into the pooled blood to achieve the mean peak, midpoint, or trough plasma concentrations observed in published studies concerning critically ill patients (10). Four bottles (2 BacT/Alert FN Plus anaerobic and 2 Bactec Plus anaerobic/F bottles) were prepared for each organism-antibiotic concentration combination. If growth was detected in the resin-containing bottles, two of the applicable manufacturers’ resin-free bottles (BacT/Alert SN and Bactec standard anaerobic bottles) were also prepared. Combinations that remained negative in resin-containing bottles were assumed to not produce detectable growth without the presence of resin and were therefore defined as “no growth.”
After incubation, bacteria were emulsified in sterile 0.9% sodium chloride to a McFarland density of 1 and diluted to a target of ∼7 to 30 CFU per 0.5 ml, which was then injected into each bottle to simulate the minimum CFU detectable by the Virtuo BacT/Alert and Bactec FX blood culture systems (11). Once inoculated, 10 ml of antibiotic-containing blood was added to each bottle. Each isolate also had a set of four control bottles that were injected with antibiotic-free whole blood. From each set, one bottle per manufacturer was loaded into its respective detection system within 1 hour of preparation and incubated until growth was detected up to a maximum of 120 hours. The duplicate bottles were incubated at 37°C in the CAIRD laboratory.
Blood culture sampling.
CAIRD bottles were sampled at 0, 4, and 12 hours after inoculation for antibiotic concentration and at 0, 4, 12, 24, 48, 72, 96, and 120 hours after inoculation for bacteria CFU. Antibiotic concentration samples were frozen at –80°C until assayed. The number of CFU was quantified via manual colony counts of serially diluted plates with a lower limit of detection of 1.7 log10 CFU/ml. Prior to loading into the detection systems, bottles were sampled at 0 h for antibiotic concentration and bacteria CFU. Upon alarming positive, bottles were resampled for CFU within 6 hours, and the TTD from the detection system’s log was noted. Bottles that remained negative at 120 hours were sampled at that time to confirm the absence of the study isolate.
Data analyses.
TTD was normalized to the loading time into the respective detection system. Bottles that remained negative at 120 hours were listed as “no growth.” Mean CFU/ml at each sampling time point for sister bottles was calculated. Chi-square or Fisher’s exact tests were used to compare the proportion of bottles that alarmed positive for each drug-organism combination at each concentration and for comparisons between BacT/Alert and Bactec bottles. The mean CFU per bottle and TTD between manufacturers were compared using Welch’s t test. All analyses were conducted using Sigma Plot version 14 (Systat Inc., San Jose, CA). An a priori P of <0.05 was considered statistically significant.
Concentrations of meropenem, cefazolin, cefepime, and piperacillin in whole blood and blood culture bottle samples were assayed using previously published validated high-performance liquid chromatography procedures (10). The concentrations were plotted graphically to compare the rates of antibiotic binding relative to the applicable MICs for the organism. Zero hour concentrations were calculated as the total concentration in whole blood divided by two to account for a normal hematocrit in a healthy adult and then further divided by the dilution of volume from blood culture media (5× for the BacT/Alert bottles and 4× for the Bactec bottles).
RESULTS
In vitro susceptibilities.
Antibiotic MICs for the selected E. coli and K. pneumoniae isolates are provided in Table 1. Both isolates were susceptible to the agents in this study, with the exception of the K. pneumoniae isolate against cefazolin (MIC 4 μg/ml, intermediate [15]).
TABLE 1.
Modal MICs and susceptibility categorization (CLSI/EUCAST) of study isolates and antibioticsa
| Isolate | Antibiotic MIC (μg/ml) (categorical interpretation via CLSI/EUCAST breakpoints)b,c |
|||||
|---|---|---|---|---|---|---|
| Meropenem | Imipenem | Levofloxacin | Cefazolin | Cefepime | Piperacillin-tazobactam | |
| E. coli ATCC 25922 | ≤0.063 (S/S) | 0.25 (S/S) | ≤0.063 (S/S) | 2 (S/–) | ≤0.063 (S/S) | 4/4 (S/S) |
| K. pneumoniae ATCC 13883 | 0.25 (S/S) | NT | NT | 4 (I/–) | 0.125 (S/S) | 4/4 (S/S) |
S, susceptible; I, intermediate; NT, not tested; –, unavailable.
CLSI susceptibility breakpoints for E. coli and K. pneumoniae are ≤1 μg/ml (meropenem and imipenem), ≤0.5 μg/ml (levofloxacin), ≤2 μg/ml (cefazolin), ≤2 μg/ml (cefepime), and ≤16/4 μg/ml (piperacillin-tazobactam) (15).
EUCAST susceptibility breakpoints for E. coli and K. pneumoniae are ≤2 μg/ml (meropenem and imipenem), ≤0.5 μg/ml (levofloxacin), ≤1 μg/ml (cefepime), and ≤8/4 μg/ml (piperacillin-tazobactam). EUCAST has not currently published a susceptibility breakpoint for cefazolin (16).
Antibiotic concentrations.
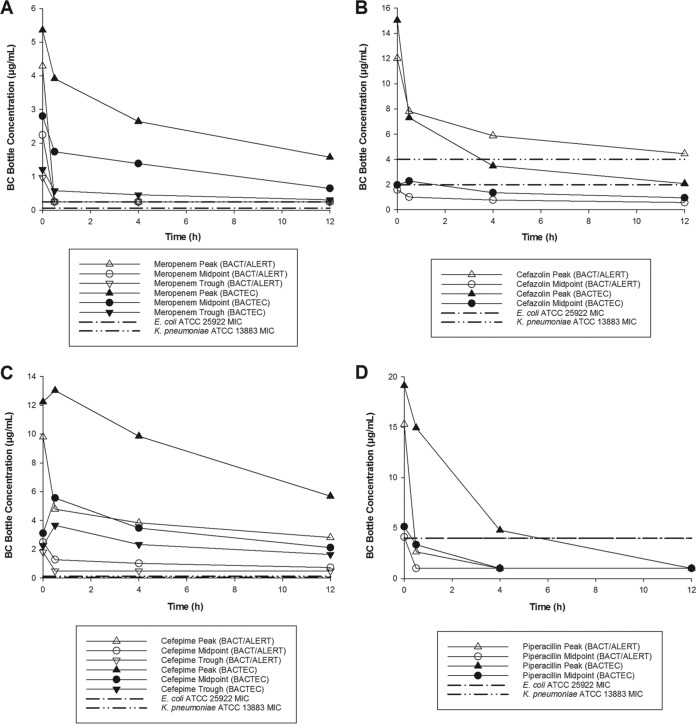
Observed whole-blood antibiotic concentrations are presented in Table 2 and were consistent with targeted peak, midpoint, and trough concentrations from critically ill patients (17–22). Observed antibiotic concentrations upon initiation of the experiment (0 hours) and then within 0.5, 4, and 12 hours of inoculation are displayed in Fig. 1 for meropenem, cefazolin, cefepime, and the piperacillin component of piperacillin-tazobactam. The mean percent concentration decrease at these times is provided in Table 3. All observed concentrations were sourced from the bottles that contained resin.
TABLE 2.
Observed versus predicted antibiotic concentrations in whole-blood before inoculation into blood culture bottlesa
| Antibiotic | Mean observed peak ± SD (predicted) (μg/ml) | Mean observed midpoint ± SD (predicted) (μg/ml) | Mean observed trough ± SD (predicted) (μg/ml) |
|---|---|---|---|
| Meropenem | 21.5 ± 2.9 (20) | 11.2 ± 3.2 (10) | 4.9 (2.5) |
| Cefazolin | 60.2 ± 3.4 (54) | 7.9 ± 0.4 (6.5) | 1.8 ± 0.2 (1.5) |
| Cefepime | 49.0 ± 1.3 (52.5) | 12.4 ± 0.3 (16.5) | 9.0 ± 0.9 (10.5) |
| Piperacillin | 76.5 ± 2.75 (88.5) | 20.5 ± 2.2 (21) | 6.0 ± 0.9 (6) |
SD, standard deviation; NT, not tested. Data are presented as mean ± standard deviation for all antibiotic concentrations (μg/ml) except the meropenem trough, where a single observation tested. Target whole-blood concentration was determined as 1/2 the target plasma concentration based on the assumption of 50% hematocrit of blood from the healthy volunteers.
FIG 1.
Meropenem (A), cefazolin (B), cefepime (C), and piperacillin (D) concentrations over the initial 12 hours upon inoculating blood culture bottles with antibiotic and applicable study isolate MICs.
TABLE 3.
Percent reduction in meropenem, cefepime, cefazolin, and piperacillin concentrations over 12 h in BacT/Alert FN Plus anaerobic and Bactec Plus anaerobic/F blood culture bottlesa
| Antibiotic | Category (observed 0 h concentration in bottle [BacT Alert/Bactec Plus], μg/ml)b | Mean % reduction of observed zero concentration |
|||||
|---|---|---|---|---|---|---|---|
| 0.5 h |
4 h |
12 h |
|||||
| BacT/Alert | Bactec Plus | BacT/Alert | Bactec Plus | BacT/Alert | Bactec Plus | ||
| Meropenemc | P (4.29/5.36) | BDL | 27 | BDL | 51 | BDL | 71 |
| M (2.24/2.80) | BDL | 38 | BDL | 50 | BDL | 77 | |
| T (0.97/1.21) | BDL | 52 | BDL | 62 | BDL | 74 | |
| Cefazolind | P (12.03/15.04) | 35 | 51 | 51 | 77 | 63 | 86 |
| M (1.58/1.97) | 36 | +15e | 51 | 31 | 63 | 52 | |
| T (0.37/0.46) | BDL | BDL | BDL | BDL | BDL | BDL | |
| Cefepimef | P (9.79/12.24) | 51 | +6e | 61 | 20 | 71 | 54 |
| M (2.49/3.11) | 49 | +69e | 59 | +12e | 71 | 32 | |
| T (1.80/2.25) | BDL | +63e | BDL | +4e | BDL | 27 | |
| Piperacilling | P (15.31/19.13) | 83 | 22 | BDL | 75 | BDL | BDL |
| M (4.11/5.14) | BDL | 35 | BDL | BDL | BDL | BDL | |
| T (1.20/1.49) | BDL | BDL | BDL | BDL | BDL | BDL | |
P, peak; M, midpoint; T, trough; h, hour; BDL, below detectable limit.
Zero hour concentration in blood culture bottles was 50% of the observed concentration in whole blood, divided by 5 (BacT/Alert) or 4 (Bactec Plus) to account for dilution in each bottle (10 ml whole blood inoculated with antibiotic, 40 ml blood culture media in BacT/Alert bottles, 30 ml blood culture media in Bactec Plus bottles).
The lower limit of detection for meropenem was 0.25 μg/ml.
The lower limit of detection for cefazolin was 0.5 μg/ml.
Positive numbers denote that the observed concentration was higher than the 0 h, suggesting that no binding reductions were observed.
The lower limit of detection for cefepime was 0.5 μg/ml.
The lower limit of detection for piperacillin was 2 μg/ml.
Starting inoculums and controls.
The mean starting inoculums in bottles were 5.5 ± 1.8 and 6.0 ± 2.5 CFU/bottle for the E. coli and K. pneumoniae experiments, respectively, in resin-containing bottles. For the E. coli experiments, control bottles containing antibiotic-free blood grew to 8.87 ± 0.13 and 8.92 ± 0.14 log10 CFU/ml over 120 hours in the resin-containing BacT/Alert and Bactec bottles, respectively. The TTD was 9.9 ± 0.5 hours versus 11.9 ± 0.6 (P ≤ 0.001) hours in these bottles, respectively (Table 4). For the experiments with K. pneumoniae, control bottles demonstrated growth to 8.92 ± 0.14 log10 CFU/ml in the BacT/Alert bottles and 8.10 ± 0.71 log10 CFU/ml in the Bactec bottles at 120 hours. Similar to observations with E. coli, the Virtuo BacT/Alert system detected bacteria more rapidly than the BD Bactec FX system (10.5 ± 0.2 versus 12.2 ± 0.4 h, P ≤ 0.001) in the absence of antibiotics (Table 4).
TABLE 4.
Time to detection (TTD) of E. coli and K. pneumoniae in BacT/Alert and Bactec anaerobic bottles containing antibiotic binding resins (BacT/Alert FN Plus and Bactec Plus) and without binding resins (BacT/Alert SN and Bactec standard) by antibiotic and concentration category
| Antibiotic | Category | Mean TTD ± SD (h)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BacT/Alert FN Plus |
BacT/Alert SNb |
Bactec Plus |
Bactec standardb |
||||||
| E. coli | K. pneumoniae | E. coli | K. pneumoniae | E. coli | K. pneumoniae | E. coli | K. pneumoniae | ||
| Control | NA | 9.9 ± 0.5 | 10.5 ± 0.2 | 9.6 | 10.0 ± 0.3 | 11.9 ± 0.6 | 12.2 ± 0.4 | 11.3 | 11.6 ± 0.3 |
| Meropenem | Peak | NG | NG | NG | NG | NG | NG | NG | NG |
| Midpoint | 16.0 | 12.6 | NG | NG | NG | NG | NG | NG | |
| Trough | 10.0 | 10.3 | NG | NG | NG | NG | NG | NG | |
| Imipenem | Peak | 9.5 | Not done | NG | Not done | NG | Not done | NG | Not done |
| Midpoint | 9.7 | Not done | NG | Not done | 14.5 | Not done | 12.5 | Not done | |
| Trough | 10.0 | Not done | NG | Not done | 16.0 | Not done | 12.0 | Not done | |
| Levofloxacin | Peak | NG | Not done | NG | Not done | NG | Not done | NG | Not done |
| Midpoint | 11.9 ± 0.6 | Not done | NG | Not done | 15.2 ± 0.8 | Not done | NG | Not done | |
| Trough | 11.2 ± 1.2 | Not done | NG | Not done | 14.3 ± 1.5 | Not done | NG | Not done | |
| Cefazolin | Peak | 10.6 ± 0.6 | 11.9 ± 0.1 | NG | NG | 12.9 ± 0.7 | 12.9 ± 0.3 | NG | NG |
| Midpoint | 9.5 | 10.5 ± 0.5 | 21.6 | 13.5 | 12.5 | 12.3 ± 0.4 | 15.3 | 24.8 | |
| Trough | 9.5 | 10.5 ± 0.1 | 9.7 | 10.1 | 12.2 | 11.9 ± 0.4 | 11.5 | 11.2 | |
| Cefepime | Peak | NG | NG | NG | NG | NG | NG | NG | NG |
| Midpoint | NG | NG | NG | NG | NG | NG | NG | NG | |
| Trough | NG | NG | NG | NG | NG | NG | NG | NG | |
| Piperacillin-tazobactam | Peak | 9.5 | 10.5 ± 0.1 | NG | NG | 12.0 | 13.6 ± 1.5 | NG | NG |
| Midpoint | 9.8 | 10.8 | NG | NG | 12.2 | 12.2 ± 0.1 | NG | NG | |
| Trough | 9.5 | 10.6 | NG | 25.5 | 12.0 | 12.3 | 20.8 | 11.7 | |
Data are provided as mean TTD (h) ± standard deviations (SD) for those with multiple observations; NG, no growth; NA, not applicable.
BacT/Alert SN and Bactec standard bottles containing no antibiotic binding resin were assumed to have no growth if the same concentration-organism combination demonstrated no growth in the antibiotic binding resin bottle. If growth was demonstrated in the binding resin bottle, SN and standard bottles were tested, and the observed results reported.
E. coli recovery.
During experiments containing antibiotics, growth was detected in 10/24 (42%), 17/24 (71%), and 18/24 (75%) of the E. coli-inoculated resin-containing bottles with blood exposed to clinically relevant peak, midpoint, and trough concentrations (P = 0.034 between drug concentration categories), respectively (Table 4). There was no statistically significant difference observed between the proportions of detected bottles at any concentration between the two systems, but the Virtuo detected E. coli in several bottles that the FX did not, including those containing midpoint and trough concentrations of meropenem and the peak of imipenem. Cefepime at all concentrations caused sterilization in both systems, while growth was detected in all cefazolin and piperacillin-tazobactam bottles. In resin-containing bottles exposed to antibiotics detected by both systems, the BacT/Alert bottles had a shorter TTD (10.1 ± 0.8 versus 13.7 ± 1.6 h, P ≤ 0.001) (Table 4).
Bacterial recovery was significantly reduced in the resin-free bottles inoculated with E. coli (P ≤ 0.001 compared with bottles containing binding resin); recovery was detected in 0/24 (0%), 6/24 (25%), and 8/24 (33%) of the bottles with peak, midpoint, and trough concentrations, respectively (Table 4). BacT/Alert SN bottles only alarmed positive for E. coli in the presence of cefazolin midpoint and trough concentrations. In contrast, the Bactec standard bottles detected E. coli in cefazolin midpoint and trough concentrations, imipenem midpoint and trough concentrations, and piperacillin-tazobactam trough concentrations.
K. pneumoniae recovery.
K. pneumoniae was recovered from 8/16 (50%), 10/16 (63%), and 10/16 (63%) of the resin-containing bottles with antibiotic peak, midpoint, and trough concentrations, respectively (P = 0.710) (Table 4). BacT/Alert FN Plus bottles detected K. pneumoniae in the presence of meropenem midpoint and trough concentrations, whereas Bactec Plus bottles did not. Similar to observations with E. coli, none of the K. pneumoniae bottles exposed to cefepime alarmed positive, while all cefazolin and piperacillin-tazobactam containing bottles had detectable growth. Among the positive resin-containing bottles, the BacT/Alert bottles alarmed more quickly (10.8 ± 0.5 versus 12.5 ± 0.6 hours, P ≤ 0.001) (Table 4).
Significantly fewer anaerobic bottles alarmed positive for K. pneumoniae in the absence of antibiotic binding resins (P = 0.002). K. pneumoniae was detected in 0/16 (0%), 4/16 (25%), and 8/16 (50%) of the bottles (Table 4). Both systems recovered isolates in the same antibiotic conditions, cefazolin midpoint and trough, as well as piperacillin-tazobactam trough concentrations.
DISCUSSION
Certain common bloodstream pathogens, including, E. coli and K. pneumoniae, are facultative anaerobes and can grow in both aerobic and anaerobic bottles, but little research is available documenting their recovery from anaerobic bottles after exposure to antibiotics. In this study, recovery of these susceptible E. coli and K. pneumoniae isolates in anaerobic bottles was affected by clinically meaningful antibiotic concentrations, even in the presence of antibiotic binding resins.
For the E. coli experiments, antibiotic midpoint and trough concentrations permitted significantly greater recovery compared with peaks, whereas for K. pneumoniae, the rates of recovery were similar across concentrations but were still only observed in 50 to 63% of the bottles. These data suggest that the greatest likelihood of recovery overall will be in the presence of trough concentrations. Our observations are largely similar to past studies, with a few notable exceptions (9, 11–13, 23). In a recent study, Menchinelli and colleagues observed sterilization of all E. coli-inoculated anaerobic bottles exposed to meropenem (23), whereas we detected recovery at the midpoint and trough concentrations in the BacT/Alert bottles. The targeted plasma concentrations used in their study were higher than the ones used herein. In contrast, against K. pneumoniae, all meropenem bottles alarmed positive, whereas we observed growth in only the midpoint and trough concentrations of BacT/Alert bottles; we hypothesize this discordance to be due to the much higher starting inoculum used by Menchinelli and colleagues (50 to 100 versus 7 to 30 CFU per bottle). As such, recovery of bacteria depends not only on the actual plasma concentration at the time of culture collection relative to the MIC, but also the specific antibiotic itself, the inoculum in the bloodstream, the type of bottle used (i.e., media composition), and other factors such as blood volume collected, temperature stability, and length of time prior to incubation.
Our lab has also assessed recovery of these same E. coli and K. pneumoniae strains in BacT/Alert FA Plus and Bactec aerobic/F blood culture bottles, both aerobic resin-containing bottles (10). Certain antibiotic concentration-bacteria combinations, notably with the carbapenems, resulted in improved recovery in the anaerobic bottles. Meropenem midpoint and trough concentrations permitted recovery in BacT/Alert FN Plus anaerobic bottles, whereas no recovery was observed in meropenem-containing aerobic bottles. All of the BacT/Alert FN Plus anaerobic bottles inoculated with E. coli alarmed positive when exposed to all imipenem concentrations; their aerobic counterparts showed recovery only up to the midpoint concentration. This pattern also occurs between the two types of Bactec bottles. For levofloxacin, aerobic Bactec bottles inoculated with E. coli alarmed positive only at the trough concentration, whereas growth was detected in the anaerobic bottles injected with both the midpoint and trough concentration. In short, the combinations that alarmed positive in the anaerobic bottles include all of their positive aerobic bottle counterparts, as well as a few additional concentrations. These comparative observations support the practice of obtaining blood cultures with both aerobic and anaerobic blood culture bottles.
The results by specific antimicrobials are also informational. Cefepime, for example, resulted in in vitro sterilization of all bottles at all concentrations. Its concentration reductions were insignificant, and concentrations remained above the MICs of these isolates throughout the initial 12 hours (Fig. 1C). Similar observations have been made for cefepime in aerobic bottles containing proprietary resins with other organisms as well (10, 11). In the aerobic bottles, these same isolates were not recovered in the presence of any clinically relevant cefepime concentrations (10). In contrast, the antibiotic binding resins were able to bind and reduce concentrations of cefazolin and piperacillin/tazobactam sufficiently (Fig. 1B and D, Table 4) to result in complete recovery at all tested concentrations. This also held true for their aerobic counterparts (10). Finally, Bactec Plus anaerobic bottles had difficulty recovering these isolates in the presence of any meropenem concentrations, whereas the BacT/Alert FN Plus bottles successfully identified bacteria after exposure to meropenem midpoint and trough concentrations.
Anaerobic bottles without binding resins were also included in this study. Not unexpectedly, recovery was significantly reduced compared with resin-containing bottles. Only cefazolin midpoint, cefazolin trough, and piperacillin-tazobactam concentrations reliably produced recovery of these organisms. These observations stress the importance of using binding resin bottles.
While the most important difference between manufacturer bottles was the ability for BacT/Alert FN Plus bottles to recover E. coli and K. pneumoniae in the presence of certain meropenem and imipenem concentrations, these bottles also had significantly shorter TTD. This was observed in both drug-free control isolates and bottles containing antibiotics. For E. coli, TTD was 10.1 ± 0.8 versus 13.7 ± 1.6 hours (P ≤ 0.001) for BacT/Alert FN Plus and Bactec Plus anaerobic bottles, respectively. For K. pneumoniae, TTD was 10.8 ± 0.5 versus 12.5 ± 0.6 hours (P ≤ 0.001), respectively. Reduced TTD may lead to more rapid identification of bloodstream pathogens.
The strengths of this study include the use of clinically relevant antibiotic concentrations across the dosing interval, which allows interpretation of when blood cultures might best be collected while patients are on antibiotics. Limitations of this study include its use of two ATCC organisms that were susceptible to the majority of antibiotic tested; therefore, results may not be applicable to other Enterobacterales or bacteria with higher MICs. Indeed, meropenem-resistant P. aeruginosa has been recovered in antibiotic binding resin bottles when exposed to meropenem peak concentrations (9).
In summary, clinically relevant antibiotic concentrations may decrease bacterial recovery in resin-containing blood culture bottles inoculated with susceptible strains of E. coli or K. pneumoniae, particularly at simulated peak concentrations. When clinically feasible, bacterial recovery should be maximized by collecting blood cultures just prior to the next scheduled antibiotic dose. The absence of antibiotic binding resins further decreases the likelihood of detection. The improved recovery of facultative anaerobes demonstrates the clinical benefit of using both aerobic and anaerobic bottles that contain antibiotic binding resins.
ACKNOWLEDGMENTS
This study was funded by bioMérieux, Inc., Durham, North Carolina, USA.
We acknowledge Lee Steere, Elizabeth Cyr, Debora Santini, Michelle Insignares, Courtney Bouchard, Kimelyn Greenwood, Elias Mullane, Sara Giovagnoli, Janice Cunningham, Nicole DeRosa, Lauren McLellan, Alissa Padgett, Christina Sutherland, Jennifer Tabor-Rennie, Tomefa Asempa, James Kidd, Lindsay Avery, and Safa Abuhussain from the Center for Anti-Infective Research and Development and Yanice Maldonado from the Department of Microbiology, Hartford Hospital, for their assistance with conducting this study.
REFERENCES
- 1.Goto M, Al-Hasan MN. 2013. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. . 2017. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 3.Grohs P, Mainardi JL, Podglajen I, Hanras X, Eckert C, Buu-Hoi A, Varon E, Gutmann L. 2007. Relevance of routine use of the anaerobic blood culture bottle. J Clin Microbiol 45:2711–2715. doi: 10.1128/JCM.00059-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcos M, Soriano A, Iñurrieta A, Martínez JA, Romero A, Cobos N, Hernández C, Almela M, Marco F, Mensa J. 2011. Changing epidemiology of central venous catheter-related bloodstream infections: increasing prevalence of Gram-negative pathogens. J Antimicrob Chemother 66:2119–2125. doi: 10.1093/jac/dkr231. [DOI] [PubMed] [Google Scholar]
- 5.Braun E, Hussein K, Geffen Y, Rabino G, Bar-Lavie Y, Paul M. 2014. Predominance of Gram-negative bacilli among patients with catheter-related bloodstream infections. Clin Microbiol Infect 20:O627–O629. doi: 10.1111/1469-0691.12565. [DOI] [PubMed] [Google Scholar]
- 6.de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H. 2013. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect 19:860–868. doi: 10.1111/1469-0691.12028. [DOI] [PubMed] [Google Scholar]
- 7.Luzzaro F, Vigano EF, Fossati D, Grossi A, Sala A, Sturla C, Saudelli M, Toniolo A, AMCLI Lombardia Hospital Infectious Study Group. 2002. Prevalence and drug susceptibility of pathogens causing bloodstream infections in northern Italy: a two-year study in 16 hospitals. Eur J Clin Microbiol Infect Dis 21:849–855. [DOI] [PubMed] [Google Scholar]
- 8.Zadroga R, Williams DN, Gottschall R, Hanson K, Nordberg V, Deike M, Kuskowski M, Carlson L, Nicolau DP, Sutherland C, Hansen GT. 2013. Comparison of 2 blood culture media shows significant differences in bacterial recovery for patients on antimicrobial therapy. Clin Infect Dis 56:790–797. doi: 10.1093/cid/cis1021. [DOI] [PubMed] [Google Scholar]
- 9.Grupper M, Nicolau DP, Aslanzadeh J, Tanner LK, Kuti JL. 2017. Effects of clinically meaningful concentrations of antipseudomonal beta-lactams on time to detection and organism growth in blood culture bottles. J Clin Microbiol 55:3502–3512. doi: 10.1128/JCM.01241-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen IH, Nicolau DP, Kuti JL. 2019. Recovery of Gram-negative bacteria from aerobic blood culture bottles containing antibiotic binding resins after exposure to β-lactam and fluoroquinolone concentrations. J Clin Microbiol doi: 10.1128/JCM.00849-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovern D, Katzin B, Johnson K, Broadwell D, Miller E, Gates A, Deol P, Doing K, van Belkum A, Marshall C, Mathias E, Dunne WM Jr. 2016. Antimicrobial binding and growth kinetics in BacT/ALERT(R) FA Plus and BACTEC(R) Aerobic/F Plus blood culture media. Eur J Clin Microbiol Infect Dis 35:2033–2036. doi: 10.1007/s10096-016-2759-9. [DOI] [PubMed] [Google Scholar]
- 12.Smith SM, Eng RH. 1983. In vitro evaluation of the BACTEC resin-containing blood culture bottle. J Clin Microbiol 17:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flayhart D, Borek AP, Wakefield T, Dick J, Carroll KC. 2007. Comparison of BACTEC PLUS blood culture media to BacT/Alert FA blood culture media for detection of bacterial pathogens in samples containing therapeutic levels of antibiotics. J Clin Microbiol 45:816–821. doi: 10.1128/JCM.02064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen IH, Nicolau DP, Kuti JL. 2019. Effect of clinically meaningful antibiotic concentrations on the recovery of Enterobacterales from anaerobic blood culture bottles with and without antibiotic binding resins (2019), abstr CPHM-852 ASM Microbe 2019, San Francisco, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI. 2019. M100 performance standards for antimicrobial susceptibility testing, 29th ed CLSI supplement M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.The European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. http://www.eucast.org.
- 17.Crandon JL, Ariano RE, Zelenitsky SA, Nicasio AM, Kuti JL, Nicolau DP. 2011. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med 37:632–638. doi: 10.1007/s00134-010-2105-0. [DOI] [PubMed] [Google Scholar]
- 18.Sakka SG, Glauner AK, Bulitta JB, Kinzig-Schippers M, Pfister W, Drusano GL, Sorgel F. 2007. Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob Agents Chemother 51:3304–3310. doi: 10.1128/AAC.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drusano GL, Preston SL, Fowler C, Corrado M, Weisinger B, Kahn J. 2004. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J Infect Dis 189:1590–1597. doi: 10.1086/383320. [DOI] [PubMed] [Google Scholar]
- 20.Smyth RD, Pfeffer M, Glick A, Van Harken DR, Hottendorf GH. 1979. Clinical pharmacokinetics and safety of high doses of ceforanide (BL-S786R) and cefazolin. Antimicrob Agents Chemother 16:615–621. doi: 10.1128/aac.16.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicasio AM, Ariano RE, Zelenitsky SA, Kim A, Crandon JL, Kuti JL, Nicolau DP. 2009. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 53:1476–1481. doi: 10.1128/AAC.01141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felton TW, Roberts JA, Lodise TP, Van Guilder M, Boselli E, Neely MN, Hope WW. 2014. Individualization of piperacillin dosing for critically ill patients: dosing software to optimize antimicrobial therapy. Antimicrob Agents Chemother 58:4094–4102. doi: 10.1128/AAC.02664-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menchinelli G, Liotti FM, Giordano L, De Angelis G, Sanguinetti M, Spanu T, Posteraro B. 2019. Efficient inactivation of clinically relevant antimicrobial drug concentrations by BacT/Alert or Bactec resin-containing media in simulated adult blood cultures. Antimicrob Agents Chemother 63:e00420-19. doi: 10.1128/AAC.00420-19. [DOI] [PMC free article] [PubMed] [Google Scholar]