Abstract
PURPOSE
The treatment of chronic lymphocytic leukemia (CLL) has been revolutionized by targeted therapies that either inhibit proliferation (ibrutinib) or reactivate apoptosis (venetoclax). Both significantly improve survival in CLL and replace chemoimmunotherapy for many patients. However, individually, they rarely lead to eradication of measurable residual disease (MRD) and usually are taken indefinitely or until progression. We present the CLARITY trial that combined ibrutinib with venetoclax to eradicate detectable CLL with the intention of stopping therapy.
PATIENTS AND METHODS
CLARITY is a phase II trial that combined ibrutinib with venetoclax in patients with relapsed or refractory CLL. The primary end point was eradication of MRD after 12 months of combined therapy. Key secondary end points were response by International Workshop on CLL criteria, safety, and progression-free and overall survival.
RESULTS
In 53 patients after 12 months of ibrutinib plus venetoclax, MRD negativity (fewer than one CLL cell in 10,000 leukocytes) was achieved in the blood of 28 (53%) and the marrow of 19 (36%). Forty-seven patients (89%) responded, and 27 (51%) achieved a complete remission. After a median follow-up of 21.1 months, one patient progressed, and all patients were alive. A single case of biochemical tumor lysis syndrome was observed. Other adverse effects were mild and/or manageable and most commonly were neutropenia or GI events.
CONCLUSION
The combination of ibrutinib plus venetoclax was well tolerated in patients with relapsed or refractory CLL. There was a high rate of MRD eradication that led to the cessation of therapy in some patients. The progression-free and overall survival rates are encouraging for relapsed and refractory CLL.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most common hematologic malignancy in the Western world, with an incidence of six per 100,000 per year.1 A proportion of patients have indolent disease and never require therapy.2 In contrast, other patients’ disease is more aggressive and results in significant ill health and reduced life expectancy. The median survival for the whole CLL population is approximately 10 years from diagnosis. Chemoimmunotherapy (CIT), such as fludarabine, cyclophosphamide, and rituximab (FCR) and bendamustine and rituximab (BR), result in remission in most patients. However, CIT is rarely curative, and the majority of patients experience relapse and eventually succumb to their disease. CIT is also associated with toxicity, which leads to significant immediate and late complications (including possible death) and limits its use across the whole patient population.3,4
In CLL, malignant B cells proliferate excessively through B-cell receptor (BCR)–dependent signaling and fail to undergo apoptosis efficiently as a result of overexpression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2). This dual pathophysiology leads to the accumulation of CLL cells and, thereby, progressive immune dysfunction and tissue infiltration. However, the outlook for patients with CLL has improved dramatically with therapies that directly target components of the BCR signaling pathway,5 particularly Bruton tyrosine kinase (Btk), or apoptosis through targeting the Bcl-2 protein.
Ibrutinib is an orally bioavailable irreversible inhibitor of Btk that blocks BCR signaling to prevent CLL cell proliferation and inhibit CLL cell migration and adhesion.6,7 Ibrutinib monotherapy is effective in CLL5 and leads to rapid reduction in lymphadenopathy and disease redistribution into the peripheral blood (PB).8-10 Ibrutinib has been approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) as a single agent for patients with previously untreated and relapsed CLL and in combination with BR in previously treated CLL because it leads to a prolongation of both progression-free and overall survival in both groups.9,11-15 Eradication of detectable CLL with single-agent ibrutinib is rare, and patients usually remain on ibrutinib indefinitely or until disease progression.12-15 In addition, ibrutinib leads to well-documented adverse effects in a proportion of patients, including diarrhea, fatigue, bruising and hemorrhage, hypertension, and atrial fibrillation, with approximately 10% of patients discontinuing the drug as a result.11-13,15
Venetoclax is an orally bioavailable small-molecule inhibitor of Bcl-2 that leads to CLL cell apoptosis.16,17 Venetoclax therefore would be expected to sensitize CLL cells to death by other discrete mechanisms. In early studies, venetoclax showed unexpected efficacy as monotherapy, with a proportion of patients with high-risk CLL achieving eradication of measurable residual disease (MRD) to either venetoclax monotherapy18 or the combination of venetoclax with rituximab.19 Venetoclax as a single agent is approved by the FDA for patients with CLL who have received at least one prior therapy and by the EMA for previously untreated patients with CLL in the presence of chromosome del(17p) or TP53 mutations and for patients with relapsed CLL with or without del(17p) or TP53 mutations who experience treatment failure with a BCR pathway inhibitor and CIT. Both the FDA and the EMA have approved venetoclax in combination with rituximab in patients with relapsed CLL who have received at least one prior treatment. Venetoclax is generally well tolerated but can lead to GI adverse effects (nausea and diarrhea) and neutropenia. The most common adverse events (AEs) experienced at grade 3 and higher are neutropenia, infection, and anemia.20 Tumor lysis syndrome (TLS) occasionally occurs in the first month of venetoclax, but with initial dosing commencing at 20 mg/d and ramping up each week to the full dose of 400 mg/d, biochemical TLS occurs in 5% of patients, and clinical TLS is rare.20,21 However, the observation that a proportion of patients, probably approximately 15% with monotherapy,22 achieve eradication of MRD is encouraging and suggests that patients may be able to stop venetoclax after a defined duration of therapy.
Given the dual pathogenesis of CLL, we hypothesized that the combination of ibrutinib with venetoclax would be expected to be additive or possibly synergistic and would lead to a higher proportion of patients achieving an MRD-negative remission and therefore being able to stop therapy. The CLARITY trial is a phase II single-arm study to investigate the safety and efficacy of combining ibrutinib with venetoclax in patients with CLL who have either progressed during or after conventional CIT (FCR or BR) or for patients with chromosome del(17p) who have experienced treatment failure with at least one prior line of therapy.
PATIENTS AND METHODS
Study Conduct
CLARITY is a single-arm, phase II study in 54 patients with relapsed/refractory CLL. The study was approved by the National Research Ethics Committee and regulatory review bodies. The review boards of participating institutions approved the study protocol (Data Supplement), which was conducted according to the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent. An independent data monitoring committee reviewed safety data throughout the trial. The study was run independently through the National Cancer Research Institute CLL clinical study group and sponsored by the University of Birmingham. Data were collected by investigators and analyses conducted by the study statistician and investigators.
Patients, Investigations, and Treatment
Patients with CLL who required therapy according to the 2008 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines,23 who had been treated previously with CIT (FCR or BR), or who had chromosome del(17p) and experienced treatment failure with at least one line of therapy were recruited. Prior treatment with idelalisib was allowed. Patients previously treated with either ibrutinib (or an alternative Btk inhibitor) or venetoclax were excluded, as were patients with significant comorbidity, previous Richter transformation, CNS involvement, or active autoimmune complications. Creatinine clearance had to be greater than 50 mL/min. Full eligibility criteria are provided in the Data Supplement.
AEs were assessed at protocol-specified time points from day 1 of treatment until 30 days after the end of therapy according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4). AEs that met the definition of seriousness could be reported after the 30-day cutoff at investigator discretion. Dose modification guidelines are included in the Data Supplement.
Patients were initially treated with 8 weeks of ibrutinib monotherapy (420 mg/d). Four patients who discontinued ibrutinib as a result of toxicity did not start combination therapy with venetoclax and were replaced. Fifty patients started venetoclax in combination with ibrutinib on day 1 of week 9. In the first three patients, venetoclax was added at a starting dose of 10 mg/d, with a weekly dose ramp-up to 20 mg, 50 mg, 100 mg, 200 mg, and the maximum dose of 400 mg/d. No TLS was seen for these three patients, so according to the protocol, all subsequent patients began venetoclax at 20 mg/d. Before starting venetoclax, patients were assessed for risk of TLS (Data Supplement) and categorized as low, medium, or high risk. High-risk patients were admitted for the first two doses of venetoclax.
PB and bone marrow (BM) MRD assessments, clinical assessments, and computed tomography (CT) scans were performed at screening (before ibrutinib), week 8 (before venetoclax), month 8 (6 months of ibrutinib plus venetoclax), month 14 (12 months of ibrutinib plus venetoclax), and month 26 (24 months of ibrutinib plus venetoclax). PB and BM MRD were assessed by highly sensitive multiparameter flow cytometry using an assay capable of detecting one CLL cell in 100,000 leukocytes.24 The methods used are described in the Data Supplement.
Treatment and End Points
The primary end point of the trial was the eradication of MRD to fewer than one CLL cell in 10,000 leukocytes (MRD4) according to the 2008 iwCLL guidelines23 in both PB and BM after 12 months of ibrutinib plus venetoclax (month 14). Secondary end points were the eradication of MRD (below MRD4) in PB and BM after 6 months of ibrutinib plus venetoclax (month 8) and 24 months of ibrutinib plus venetoclax (month 26). MRD was assessed in a single central laboratory (Haematological Malignancy Diagnostic Service, Leeds, United Kingdom). Other secondary end points were investigator-assessed response by iwCLL criteria (including measurement of lymph node response by CT scan), progression-free and overall survival, and toxicity of ibrutinib plus venetoclax.
The duration of therapy was defined by the confirmed MRD response with the following three possibilities: MRD4 in both PB and BM at month 8 to stop ibrutinib and venetoclax at month 14, MRD detectable at month 8 but MRD4 in both PB and BM at month 14 and/or at month 26 to stop ibrutinib and venetoclax at month 26, and MRD detectable at month 26 to stop venetoclax but continue ibrutinib until progression. Confirmation of MRD response was clinically defined by three consecutive PB samples below MRD4, with the last concurrently confirmed by a BM below MRD4. In addition to confirmation of MRD response, patients were assessed by iwCLL 2008 criteria and confirmed to have a complete response (CR) before stopping therapy.
Statistical Analyses
The primary assumption was that after 12 months of ibrutinib plus venetoclax, at least 30% of patients would have MRD eradication (PB and BM) according to iwCLL criteria23 (MRD4). An A’Hern design25 was used with a one-sided statistical significance (α) of 2.5% and statistical power of 95.5% to test the null hypothesis that the rate of eradication is no greater than 10% against the alternative that it exceeds 30%. Thus, if at least 10 of 50 patients achieved MRD eradication in both the PB and the BM, then the combined treatment would be considered of interest for additional investigation. Data were frozen on November 5, 2018.
RESULTS
Study Population
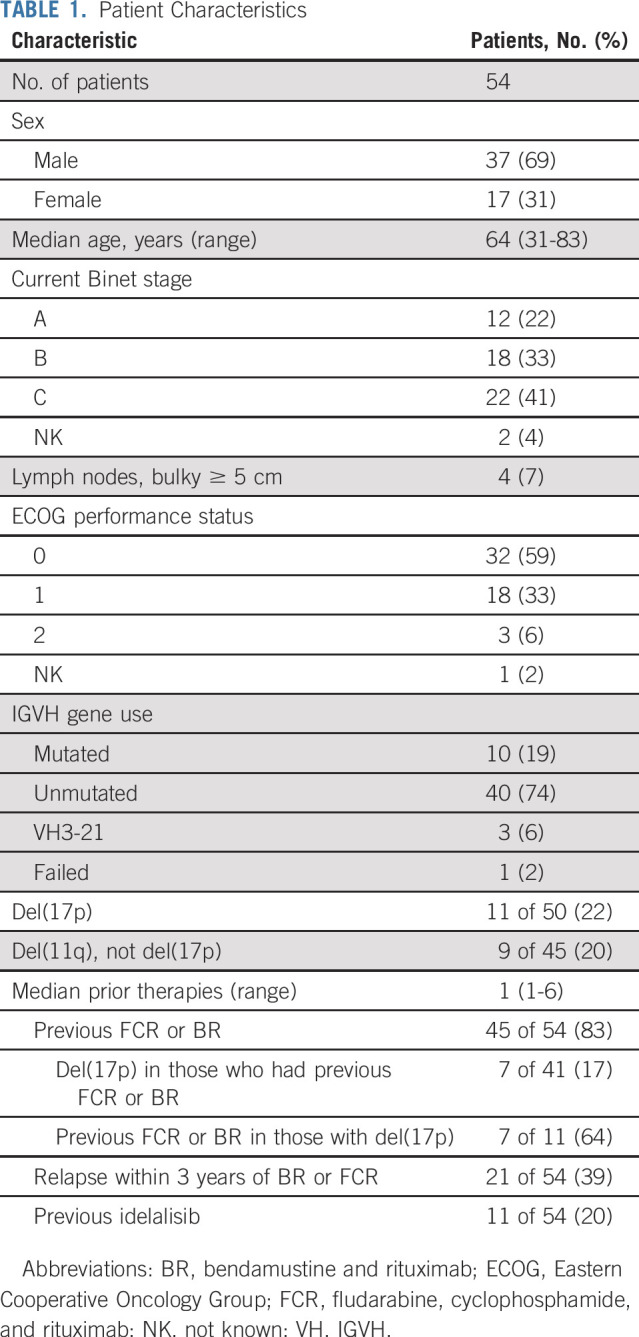
Fifty-four patients were recruited from May 2016 to November 2017 (Table 1). The median number of prior therapies was one (range, one to six therapies), including FCR or BR in 45 patients (83%) and idelalisib-containing treatments in 11 (20%). Eleven (22%) of 50 patients had del(17p), nine (20%) of 45 patients had del(11q) but not del(17p), and 40 (75%) of 53 patients had unmutated IGVH genes. Four patients stopped ibrutinib because of AEs in the first 8 weeks before starting venetoclax. The reasons for discontinuation were Aspergillus infection (brain abscess, grade 3), mucosal infection (grade 1), postural hypotension (grade 2), and multiple intolerances (grade 1 arthralgia, diarrhea, and dizziness). The remaining 50 patients completed the dose ramp-up of venetoclax combined with ibrutinib (Data Supplement).
TABLE 1.
Patient Characteristics
Safety and Tolerability
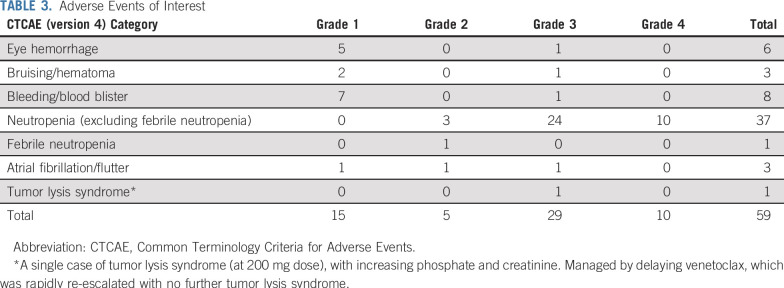
A single biochemical TLS event (grade 3) was reported with an increase in both creatinine and phosphate. Dosing of venetoclax was interrupted until the biochemical abnormalities resolved, and the patient subsequently ramped up to 400 mg/d of venetoclax with no additional TLS. At the data lock on November 5, 2018, two suspected unexpected serious adverse reactions, 36 serious AEs, 99 grade 3 or 4 AEs (Tables 2 and 3), and 1,049 AEs (all grades; Data Supplement) were reported. Of note, there were nine grade 3 or 4 infections and 34 episodes of grade 3 or 4 neutropenia. Thus far, all serious AEs have resolved with appropriate management, and all patients remain in the study after resolution. No AEs have been fatal.
TABLE 2.
Grade 3/4 Adverse Events
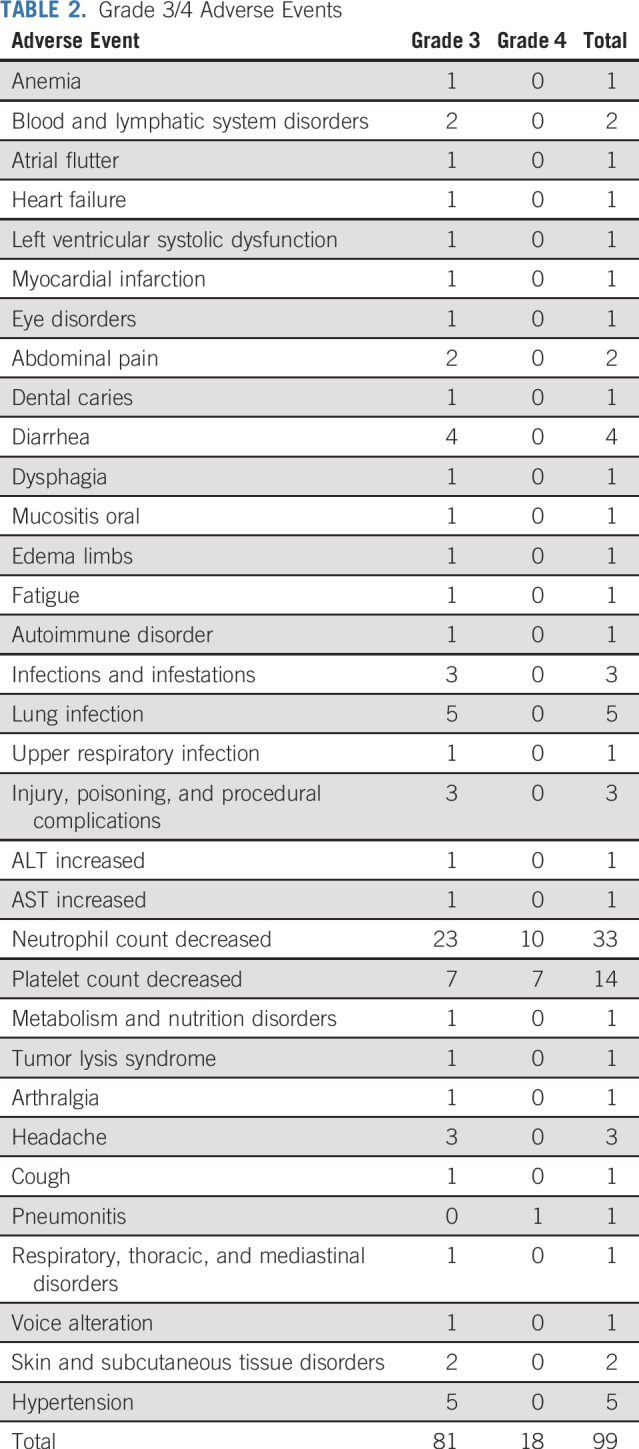
TABLE 3.
Adverse Events of Interest
Ibrutinib treatment was interrupted in 28 patients for a median of 9 days (range, 1 to 80 days) and reduced in eight patients for a median of 6 days (range, 5 to 121 days). Venetoclax was interrupted in 23 patients for a median of 8 days (range, 1 to 62 days) and reduced in 11 patients for a median of 15 days (range, 3 to 78 days). The majority of treatment modifications for each drug were associated with toxicity; diarrhea and decreased neutrophil count were the most frequently cited AEs.
Efficacy
As expected, there was an increase in the level of CLL in PB (Fig 1) during the first 8 weeks of ibrutinib therapy (median absolute increase, 27.3 × 109/L; range, 376 × 109/L decrease to 404 × 109/L increase). Figure 1 shows a rapid response to ibrutinib in all patients, with the level of CLL dropping rapidly again when venetoclax was added and a sustained response in all but one patient.
FIG 1.
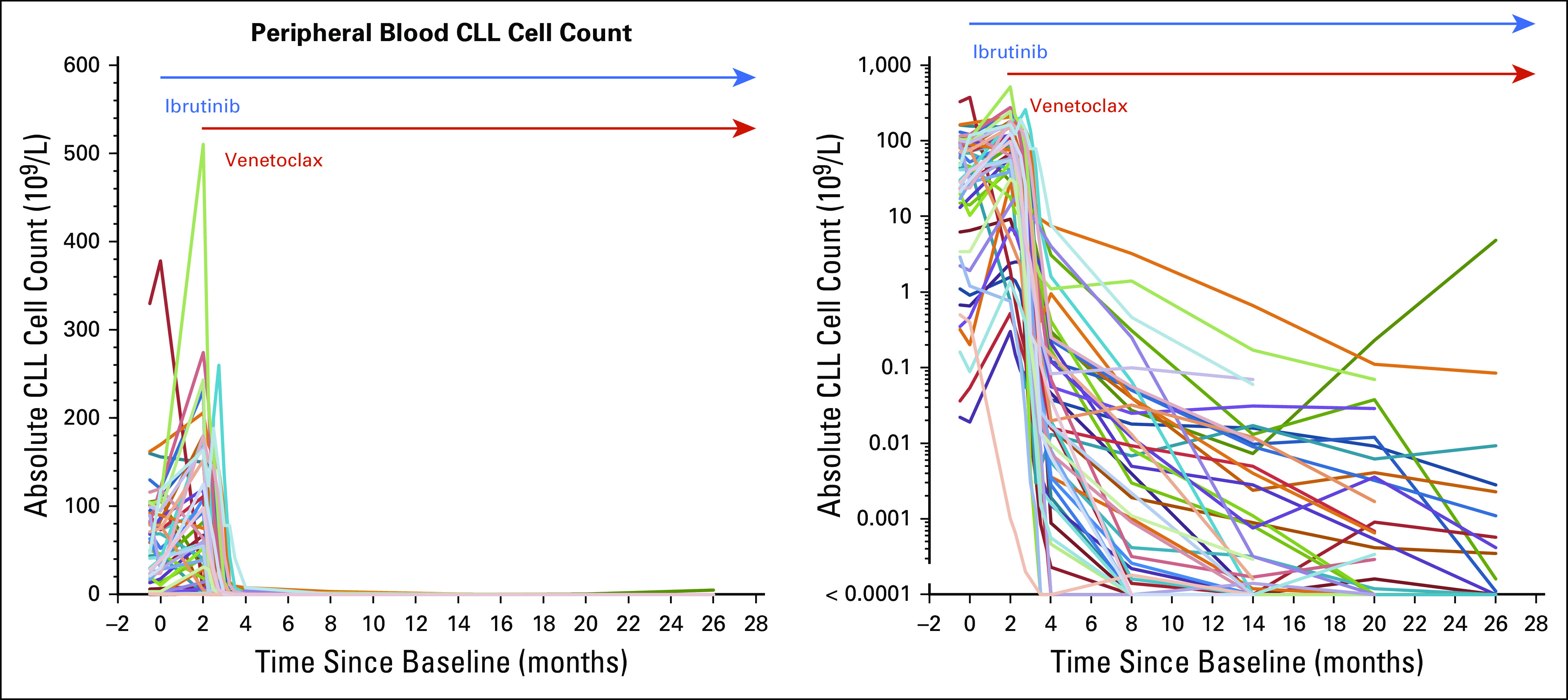
Dynamic of response. CLL, chronic lymphocytic leukemia.
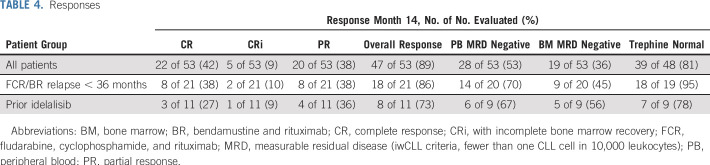
Forty-nine patients had reached the 14-month (12 months of ibrutinib plus venetoclax) PB, BM, and CT scan assessments at the time of the data freeze. In the analysis of these patients, and the treatment of the four patients who did not commence venetoclax as nonresponders, 47 (89%) of the 53 had an overall response, and 27 (51%) achieved a CR or CR with incomplete BM recovery. Twenty patients (38%) achieved a partial response (11 had lymphadenopathy [generally small residual nodes on a CT scan], two had BM involvement, two had no trephine, three were MRD positive in PB and/or BM, and two were unconfirmed).
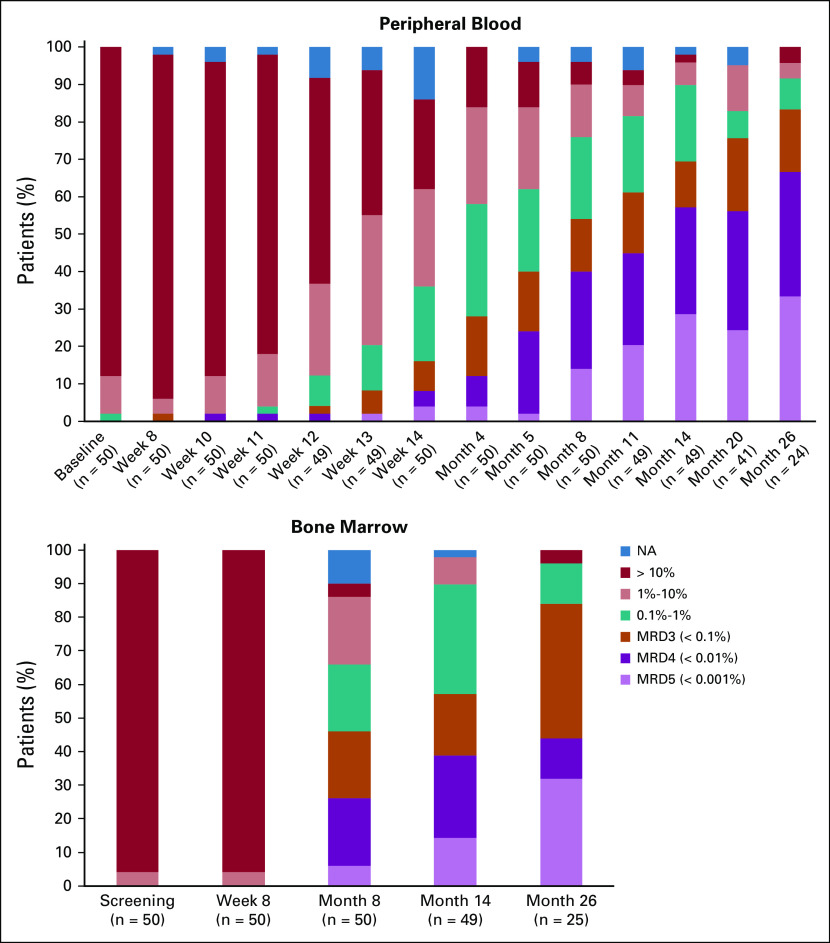
The primary end point was the proportion of patients with MRD-negative BM (defined according to iwCLL criteria as MRD4 in the BM at month 14 after 12 months of combined ibrutinib plus venetoclax). This was achieved in 19 (36%) of 53 patients, which thus exceeded the assumption that the combination would be of interest if an excess of 30% of patients became MRD negative. Twenty-eight (53%) of 53 patients were MRD negative in the PB at month 14. Thirty-nine (81%) of 48 patients had no morphologically evident CLL in the BM biopsy (Table 4). Continuous improvement was seen in the depth of MRD reduction, with 11 (44%) of 25 patients achieving MRD4 or below by flow cytometry at month 26 (Fig 2).
TABLE 4.
Responses
FIG 2.
Peripheral blood and bone marrow responses with time. MRD3, fewer than one CLL cell in 1,000 leukocytes; MRD4, measurable residual disease to fewer than one chronic lymphocytic leukemia cell in 10,000 leukocytes; MRD5, residual disease undetectable below a threshold of one CLL cell in 100,000 leukocytes; NA, not available.
At the time of the data freeze, two patients were reported to have stopped ibrutinib plus venetoclax after confirmation of MRD-negative remission at month 14. These patients since have not experienced relapse and remain below MRD4 in PB and BM. The remaining patients are continuing therapy within the trial protocol, including those MRD-negative patients with residual lymphadenopathy.
After a median follow-up of 21.1 months, only one patient (adverse IGHV [subset #2/VH3-21], no TP53/ATM deletion, not MRD negative at any point) has had progressive CLL, and no patients developed transformed disease. All 50 patients were alive at the latest follow-up (Data Supplement).
DISCUSSION
In a study by Ahn et al26 with a 5-year follow-up, the overall response rate (ORR) for ibrutinib monotherapy in relapsed CLL was 83% early in the treatment but increased to 95% with prolonged treatment; this includes a minority of patients with persistent lymphocytosis, but no patients achieved an MRD-negative remission after 24 months of ibrutinib. In another study, the ORR for venetoclax monotherapy in relapsed poor-risk CLL was 82%, with 10% of patients achieving a CR.27 MRD response for venetoclax monotherapy in del(17p) CLL was reported by Stilgenbauer et al22 as 30% MRD negative in the PB at 12 months, but there were limited BM data for confirmation. The combination of ibrutinib plus venetoclax has been investigated in mantle cell lymphoma, with a 71% ORR at week 16 and 75% progression-free survival rate at 12 months, a much better outcome than reported for ibrutinib or venetoclax monotherapies in that indication.28
The current results of ibrutinib plus venetoclax after 12 months of combined therapy in a similar patient population are encouraging, with 89% of patients responding to treatment and 51% achieving a CR or CR with incomplete BM recovery. In addition, 36% of patients achieved an MRD-negative remission after 12 months of combination therapy, which is rarely seen with ibrutinib monotherapy and occurs in a small proportion of patients treated with venetoclax. In the MURANO (Venetoclax Plus Rituximab Compared With Bendamustine Plus Rituximab in Relapsed or Refractory CLL) study, 194 patients with relapsed CLL received venetoclax plus rituximab with an ORR of 93.3% and a CR rate of 26.8%; MRD-negative BM was observed in 27.3% at month 9.29
Previous reports have demonstrated that achieving MRD negativity in CLL with a variety of therapies, including chemotherapy, immunotherapy, and transplantation, is associated with improved progression-free and overall survival, regardless of the therapy used to achieve a response.30 In the CLARITY study, ibrutinib plus venetoclax seems to be effective in achieving MRD eradication in PB and BM by month 14 in patients with CLL who were refractory to CIT and/or had received prior treatment with idelalisib regardless of poor prognostic features, such as chromosome del(17p) or immunoglobulin mutation status. In CLARITY, only one patient progressed, and all patients were alive at the latest follow-up.
The combination of ibrutinib with venetoclax was well tolerated in relapsed and refractory CLL. There were no significant additional AEs with the combination compared with published data on either drug alone. In particular, the incidence of TLS with the combination was one (2%) of 50 patients, which compares favorably with 10 (18%) of 56 patients for venetoclax monotherapy before the dose ramp-up but similar to one (2%) of 60 patients when dose ramp-up was instituted.27 When venetoclax was combined with rituximab in a phase III trial, six (3.1%) of 194 patients experienced TLS.29 Therefore, with gradual venetoclax dose ramp-up and careful monitoring, TLS is manageable, even when venetoclax is combined with ibrutinib. The other AEs observed with the combination were as expected with either drug alone, including bruising and bleeding (seen with ibrutinib) and neutropenia (seen with venetoclax). There were two suspected unexpected serious adverse reactions: pemphigus (grade 3) and abdominal pain (grade 3). The majority of the other AEs were manageable without delaying or permanently stopping either therapy.
This is the initial description of the combination of two therapies that target the key pathophysiologic pathways seen in relapsed CLL, namely BCR-associated signaling by ibrutinib and apoptosis through venetoclax targeting of Bcl-2. Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression.26 We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects. The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission. This observation is critical before taking the MRD-guided approach into larger phase III trials. Whether the combination leads to permanent disease eradication in a subset of these patients remains to be seen.
ACKNOWLEDGMENT
We thank the patients who participated in this trial and their families. We acknowledge the study staff at the following sites: St James’s University Hospital, Leeds, United Kingdom; Hammersmith Hospital, London, United Kingdom; University Hospital of Wales, Cardiff, United Kingdom; Beatson West of Scotland Cancer Centre, Glasgow, United Kingdom; Churchill Hospital, Oxford, United Kingdom; Royal Liverpool University Hospital, Liverpool, United Kingdom; St Bartholomew’s Hospital, London, United Kingdom; King’s College Hospital, London, United Kingdom; Christie Hospital NHS Trust, Manchester, United Kingdom; Nottingham University Hospital, Nottingham, United Kingdom; and Southampton General Hospital, Southampton, United Kingdom. We also thank David Oscier, MD; Simon Rule, BM, BS; and Dena Howard for their work on the data monitoring committee.
Footnotes
Presented at the American Society of Hematology 60th Annual Meeting and Exposition, Atlanta, GA, December 1-4, 2018; American Society of Hematology 59th Annual Meeting and Exposition, Atlanta, GA, December 9-12, 2017; and the 22nd Congress of the European Hematology Association, Madrid, Spain, June 22-25, 2017.
Supported by Bloodwise under the Trials Acceleration Programme, and by the National Institute for Health Research (NIHR) Leeds Clinical Research Facility and NIHR Oxford Biomedical Research Centre. An unrestricted educational grant to support the trial and adjunctive science was provided by Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of Janssen-Cilag, AbbVie, Bloodwise, the NHS, the NIHR, or the Department of Health.
Processed as a Rapid Communication manuscript.
Clinical trial information: EudraCT 2015-003422-14 and ISCRTN13751862.
See accompanying Editorial on page 2711
AUTHOR CONTRIBUTIONS
Conception and design: Peter Hillmen, Andy C. Rawstron, Kristian Brock, Rebecca Bishop, Anna Schuh, John G. Gribben, Adrian Bloor, Talha Munir
Administrative support: Francesca J. Yates, Rebecca Bishop, Talha Munir
Provision of study material or patients: Peter Hillmen, Alison McCaig, Anna Schuh, Andrew Pettitt, John G. Gribben, Piers E.M. Patten, Stephen Devereux, Christopher P. Fox, Francesco Forconi, Talha Munir
Collection and assembly of data: Peter Hillmen, Andy C. Rawstron, Kristian Brock, Samuel Muñoz-Vicente, Francesca J. Yates, Donald MacDonald, Christopher Fegan, Alison McCaig, Anna Schuh, Andrew Pettitt, John G. Gribben,Piers E.M. Patten, Stephen Devereux, Christopher P. Fox, Francesco Forconi, Talha Munir
Data analysis and interpretation: Peter Hillmen, Andy C. Rawstron, Kristian Brock, Samuel Muñoz-Vicente, Rebecca Boucher, Christopher Fegan, Anna Schuh, Piers E.M. Patten, Christopher P. Fox, Talha Munir
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The CLARITY Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Peter Hillmen
Honoraria: Janssen Pharmaceuticals, AbbVie, Roche
Research Funding: Janssen Pharmaceuticals (Inst), Pharmacyclics (Inst), Roche (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Janssen Pharmaceuticals, AbbVie
Andy C. Rawstron
Consulting or Advisory Role: AbbVie, Janssen Pharmaceuticals, Celgene, Pharmacyclics, Roche
Speakers’ Bureau: AbbVie, BD Biosciences, Beckman Coulter
Research Funding: AbbVie (Inst), BD Biosciences (Inst), Beckman Coulter (Inst), Gilead Sciences (Inst), Janssen Pharmaceuticals (Inst), Roche (Inst), Pharmacyclics (Inst)
Patents, Royalties, Other Intellectual Property: Royalty payments from BD Biosciences for IntraSure kit
Travel, Accommodations, Expenses: Janssen Pharmaceuticals
Kristian Brock
Stock and Other Ownership Interests: AstraZeneca, GlaxoSmithKline
Honoraria: Eli Lilly
Travel, Accommodations, Expenses: Roche, Merck Sharp & Dohme
Samuel Muñoz-Vicente
Employment: GlaxoSmithKline
Research Funding: GlaxoSmithKline
Christopher Fegan
Honoraria: Roche, Janssen Pharmaceuticals
Consulting or Advisory Role: AbbVie
Travel, Accommodations, Expenses: AbbVie
Alison McCaig
Consulting or Advisory Role: AbbVie
Travel, Accommodations, Expenses: AbbVie
Anna Schuh
Consulting or Advisory Role: Gilead Sciences, AbbVie, Roche, Janssen Pharmaceuticals
Research Funding: Johnson & Johnson
Travel, Accommodations, Expenses: AbbVie, Gilead Sciences
Andrew Pettitt
Research Funding: Celgene (Inst), Gilead Sciences (Inst), Roche (Inst), NAPP Pharmaceuticals (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Kite Pharma, Celgene
John G. Gribben
Honoraria: AbbVie, Acerta Pharma, AstraZeneca, Celgene, Janssen Pharmaceuticals, Gilead Sciences, Roche, Genentech, TG Therapeutics
Consulting or Advisory Role: AbbVie, Acerta Pharma, AstraZeneca, Celgene, Janssen Pharmaceuticals
Research Funding: Acerta Pharma, AstraZeneca, Janssen Pharmaceuticals, Celgene
Piers E.M. Patten
Honoraria: AbbVie, Pharmacyclics, Gilead Sciences, Janssen-Cilag, Novartis, Roche
Consulting or Advisory Role: AbbVie, Novartis
Research Funding: Roche
Travel, Accommodations, Expenses: AbbVie, Janssen-Cilag, Gilead Sciences
Stephen Devereux
Honoraria: Gilead Sciences
Speakers’ Bureau: Gilead Sciences
Adrian Bloor
Honoraria: AbbVie, Janssen Pharmaceuticals, Novartis, Gilead Sciences
Consulting or Advisory Role: AbbVie
Speakers’ Bureau: Novartis, AbbVie
Travel, Accommodations, Expenses: AbbVie, Novartis, Gilead Sciences, Janssen Pharmaceuticals
Christopher P. Fox
Consulting or Advisory Role: AbbVie, Celgene, Gilead Sciences, Janssen Pharmaceuticals, Roche, Sunesis Pharmaceuticals, Takeda Pharmaceuticals, Atara Biotherapeutics
Travel, Accommodations, Expenses: Adienne
Francesco Forconi
Honoraria: AbbVie, Janssen-Cilag, Roche, Novartis
Consulting or Advisory Role: AbbVie, Janssen-Cilag, Menarini
Speakers’ Bureau: AbbVie, Janssen-Cilag
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: AbbVie, Janssen-Cilag
Talha Munir
Consulting or Advisory Role: Sunesis Pharmaceuticals, MorphoSys, Janssen-Cilag
Speakers’ Bureau: AbbVie, Janssen-Cilag, Gilead Sciences, Alexion Pharmaceuticals
Travel, Accommodations, Expenses: Janssen-Cilag, AbbVie, Gilead Sciences, Alexion Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1. Haematological Malignancy Research Network: Incidence, 2018. https://www.hmrn.org/statistics/incidence.
- 2.Shanafelt TD, Byrd JC, Call TG, et al. Narrative review: Initial management of newly diagnosed, early-stage chronic lymphocytic leukemia. Ann Intern Med. 2006;145:435–447. doi: 10.7326/0003-4819-145-6-200609190-00007. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorst B, Fink A-M, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942. doi: 10.1016/S1470-2045(16)30051-1. [DOI] [PubMed] [Google Scholar]
- 4.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 5.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Rooij MFM, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 7.Herman SEM, Mustafa RZ, Jones J, et al. Treatment with ibrutinib inhibits BTK- and VLA-4-dependent adhesion of chronic lymphocytic leukemia cells in vivo. Clin Cancer Res. 2015;21:4642–4651. doi: 10.1158/1078-0432.CCR-15-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DD, Goldstein L, Cheng M, et al. Modeling absolute lymphocyte counts after treatment of chronic lymphocytic leukemia with ibrutinib. Ann Hematol. 2015;94:249–256. doi: 10.1007/s00277-014-2187-9. [DOI] [PubMed] [Google Scholar]
- 9.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman SEM, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: Correlative analyses from a phase II study. Leukemia. 2014;28:2188–2196. doi: 10.1038/leu.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanafelt TD, Wang V, Kay NE, et al. A randomized phase III study of ibrutinib (PCI-32765)-based therapy vs. standard fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy in untreated younger patients with chronic lymphocytic leukemia (CLL): A trial of the ECOG-ACRIN Cancer Research Group (E1912) Blood. 2018;132:LBA-4. [Google Scholar]
- 15.O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: A 5-year experience. Blood. 2018;131:1910–1919. doi: 10.1182/blood-2017-10-810044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson MA, Deng J, Seymour JF, et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood. 2016;127:3215–3224. doi: 10.1182/blood-2016-01-688796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts AW, Stilgenbauer S, Seymour JF, et al. Venetoclax in patients with previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2017;23:4527–4533. doi: 10.1158/1078-0432.CCR-16-0955. [DOI] [PubMed] [Google Scholar]
- 18.Wierda W, Chyla B, Eichhorst B, et al. Venetoclax in relapsed/refractory chronic lymphocytic leukemia (CLL) with 17p deletion: Outcome and minimal residual disease (MRD) from the full population of the pivotal M13-982 trial. Clin Lymphoma Myeloma Leuk. 2017;17:S303. [Google Scholar]
- 19.Seymour JF, Ma S, Brander DM, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: A phase 1b study. Lancet Oncol. 2017;18:230–240. doi: 10.1016/S1470-2045(17)30012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–778. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 21. Davids MS, von Keudell G, Portell CA, et al: Revised dose ramp-up to mitigate the risk of tumor lysis syndrome when initiating venetoclax in patients with mantle cell lymphoma. J Clin Oncol 36:3525-3527, 2018. [DOI] [PubMed]
- 22.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: Results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36:1973–1980. doi: 10.1200/JCO.2017.76.6840. [DOI] [PubMed] [Google Scholar]
- 23.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawstron AC, Fazi C, Agathangelidis A, et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: An European Research Initiative on CLL study. Leukemia. 2016;30:929–936. doi: 10.1038/leu.2015.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 26. Ahn IE, Farooqui MZH, Tian X, et al: Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood 131:2357-2366, 2018. [DOI] [PMC free article] [PubMed]
- 27.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211–1223. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 29. Seymour J, Kipps T, Eichhorst B, et al: Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 378:1107-1120, 2018. [DOI] [PubMed]
- 30.Kwok M, Rawstron AC, Varghese A, et al. Minimal residual disease is an independent predictor for 10-year survival in CLL. Blood. 2016;128:2770–2773. doi: 10.1182/blood-2016-05-714162. [DOI] [PubMed] [Google Scholar]