Abstract
PURPOSE
CheckMate 032 is an open-label, multicohort study that includes patients with unresectable locally advanced or metastatic urothelial carcinoma (mUC) treated with nivolumab 3 mg/kg monotherapy every 2 weeks (NIVO3), nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks (NIVO3+IPI1), or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks (NIVO1+IPI3). We report on the expanded NIVO1+IPI3 cohort and extended follow-up for the NIVO3 and NIVO3+IPI1 cohorts.
METHODS
Patients with platinum-pretreated mUC were enrolled in this phase I/II multicenter study to receive NIVO3, NIVO3+IPI1, or NIVO1+IPI3 until disease progression or unacceptable toxicity. Primary end point was investigator-assessed objective response rate per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, including duration of response.
RESULTS
Seventy-eight patients were treated with NIVO3 (minimum follow-up, 37.7 months), 104 with NIVO3+IPI1 (minimum follow-up, 38.8 months), and 92 with NIVO1+IPI3 (minimum follow-up, 7.9 months). Objective response rate was 25.6%, 26.9%, and 38.0% in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively. Median duration of response was more than 22 months in all arms. Grade 3 or 4 treatment-related adverse events occurred in 21 (26.9%), 32 (30.8%), and 36 (39.1%) patients treated with NIVO3, NIVO3+IPI1, and NIVO1+IPI3, respectively. Grade 5 treatment-related pneumonitis occurred in one patient each in the NIVO3 and NIVO3+IPI1 arms.
CONCLUSION
With longer follow-up, NIVO3 demonstrated sustained antitumor activity alone and in combination with ipilimumab. NIVO1+IPI3 provided the greatest antitumor activity of all regimens, with a manageable safety profile. This result not only supports additional study of NIVO1+IPI3 in mUC, but demonstrates the potential benefit of immunotherapy combinations in this disease.
INTRODUCTION
Immunotherapies have become a standard of care for previously treated metastatic urothelial carcinoma (mUC).1 Programmed death 1 (PD-1) immune checkpoint inhibitor nivolumab is approved as monotherapy for patients with locally advanced or mUC who experienced progression after platinum-containing chemotherapy.2 In the single-arm, phase II CheckMate 275 trial, nivolumab demonstrated a clinically meaningful objective response rate (ORR) of 20.4%, median overall survival (OS) of 8.6 months, 1-year OS rate of 40%, and a tolerable safety profile with median follow-up of 24.5 months.3
Other immunotherapy monotherapies for platinum-resistant mUC include pembrolizumab, atezolizumab, durvalumab, and avelumab,1 with reported median OS ranging from 6.5 months to 18.2 months and ORR ranging from 13.4% to 21.1% in programmed death ligand 1 (PD-L1) unselected patients.4-8 Of phase III trials reported in this setting, OS benefit was observed in one study of pembrolizumab versus investigator’s choice of chemotherapy.4,9 The clear benefits observed with immune checkpoint monotherapies demand investigation of how outcomes might be improved with combination therapies.
Combination treatments are under investigation in mUC to optimize the antitumor effects of immune checkpoint inhibition.10 Combined inhibition of PD-1 and cytotoxic T-lymphocyte antigen-4 with nivolumab and ipilimumab has demonstrated benefit in several tumor types.11-14 This treatment is approved for the treatment of patients with microsatellite instability–high or mismatch repair–deficient metastatic colorectal cancers that have progressed after combination therapy with fluoropyrimidine, oxaliplatin, and irinotecan, as well as intermediate- and poor-risk patients with previously untreated advanced renal cell carcinoma (RCC) and in patients with previously untreated metastatic melanoma.2,15
CheckMate 032 evaluates several advanced tumor types.16 Patients in the locally advanced or metastatic platinum-pretreated urothelial carcinoma (UC) cohort received nivolumab monotherapy (nivolumab 3 mg/kg every 2 weeks [NIVO3]) or one of two nivolumab plus ipilimumab combination regimens (nivolumab 3 mg/kg + ipilimumab 1 mg/kg [NIVO3+IPI1] every 3 weeks for four doses followed by nivolumab monotherapy maintenance or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg [NIVO1+IPI3] every 3 weeks for four doses followed by nivolumab monotherapy maintenance). Interim results for patients with mUC who received NIVO3 (minimum follow-up, 9 months),16 outcomes with NIVO3 after longer follow-up (minimum follow-up, 24 months),17 and initial results for the combination treatment arms (minimum follow-up, 3.9 months [NIVO1+IPI3] and 14.5 months [NIVO3+IPI1])18 have been reported. Here, we report the results from CheckMate 032 with extended follow-up data from all three treatment arms (minimum follow-up, 37.7 months, 38.8 months, and 7.9 months in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively).
METHODS
Study Design and Participants
CheckMate 032 is a multicenter, open-label, multiarm, phase I/II trial.16 Patients in the UC cohort were enrolled at 38 sites in eight countries. Eligible patients were age 18 years or older with histologically or cytologically confirmed UC of the renal pelvis, ureter, bladder, or urethra; had experienced disease progression after receiving one or more previous platinum-based chemotherapy for metastatic or locally advanced unresectable disease; had experienced recurrence within 1 year of completing platinum-based neoadjuvant or adjuvant treatment; or had refused standard treatment with chemotherapy for metastatic or locally advanced unresectable disease. Patients had Eastern Cooperative Oncology Group performance status of 0 or 1 and measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Key exclusion criteria were active brain metastases, history of or active autoimmune disease, conditions that required systemic corticosteroids (> 10 mg per day prednisone equivalent), and any prior treatment with experimental antitumor vaccines or a modulator of T-cell function or immune checkpoint pathway.
Patients in the UC cohort were assigned to treatment with either NIVO3, NIVO3+IPI1, or NIVO1+IPI3. Patients were randomly assigned among treatment arms that were open at the time of enrollment. The NIVO1+IPI3 arm was later expanded via protocol amendment. Additional patients were enrolled in this arm after enrollment was closed for the NIVO3 and NIVO3+IPI1 arms.
The protocol was approved by the institutional review board or independent ethics committee at each site and conducted in accordance with Good Clinical Practice guidelines according to International Conference on Harmonisation guidelines. All patients provided written informed consent to participate, per the Declaration of Helsinki.
Procedures
Patients received treatment with NIVO3, NIVO3+IPI1, or NIVO1+IPI3 until disease progression or unacceptable toxicity. Dose reductions were not permitted. Treatment beyond disease progression was permitted if a patient tolerated study treatment and experienced investigator-assessed clinical benefit. Patients in the NIVO3 arm could switch to NIVO3+IPI1 or NIVO1+IPI3 combination treatment after disease progression if they met protocol-specified criteria. Patients who achieved an initial objective response that lasted 3 or more months could hold treatment. In the case of subsequent progression, patients could undergo re-exposure with the combination treatment if they achieved an initial objective response or stable disease that lasted 3 or more months, had subsequent documented disease progression, and met other predefined criteria.
Tumor assessments were performed using computed tomography or magnetic resonance imaging at baseline, every 6 weeks (±1 week) from the first dose for the first 24 weeks, then every 12 weeks (±1 week) thereafter. Assessments were completed by the investigator, per RECIST v1.1. Safety assessments were completed continuously. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Tumor PD-L1 expression was assessed retrospectively from mandatory tumor biopsies using the Dako PD-L1 immunohistochemical 28-8 pharmDx kit (Dako, Santa Clara, CA).
Outcomes
Primary end point was ORR, per the investigator, which required confirmation per RECIST v1.1. ORR was further characterized by the duration of response (DOR). ORR was also evaluated by blinded independent central review (BICR) in the NIVO3 and NIVO1+IPI3 arms to evaluate efficacy in the NIVO1+IPI3 expansion cohort.
Secondary end points included progression-free survival (PFS), OS, and safety and tolerability. Treatment-related select AE categories were those with a potential inflammatory mechanism that required more frequent monitoring or unique intervention, such as immunosuppressants or endocrine replacement therapy. Efficacy by tumor PD-L1 expression was an exploratory end point.
Statistical Analysis
The study was conducted with a one-stage design with a sample size of 60 to 100 patients in the NIVO3 and NIVO3+IPI1 arms for 90% to 97% power to reject the null hypothesis of 10% response rate if the true response rate was 25% with a two-sided type I error rate of 5%. For the NIVO1+IPI3 arm, initial enrollment of 26 patients was planned, of which six patients were enrolled for safety evaluation while the NIVO3 arm was open, and 20 patients after the other two arms completed enrollment; there were 26 patients in the NIVO1+IPI3 arm at the initial disclosure of results with minimum follow-up of 3.9 months.18 The protocol was amended in October 2016 to expand the NIVO1+IPI3 arm to enroll 92 patients—an additional 66 patients. On the basis of a 19.6% ORR for nivolumab monotherapy,7 this would provide 93% power to reject the null hypothesis of a 19.6% ORR if the true ORR was 35% with a two-sided type I error rate of 5%. We analyzed ORR using the Clopper-Pearson method.19 DOR was analyzed using the Kaplan-Meier methodology, and median values along with two-sided 95% CI were calculated using the Brookmeyer and Crowley method.20 PFS and OS were summarized descriptively using the Kaplan-Meier method. Safety outcomes were tabulated using the worst grade according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, by system organ class and preferred term. All analyses were performed in all treated patients from each arm. Analyses reported here were completed when the primary end point of ORR could be evaluated in all treated patients.
RESULTS
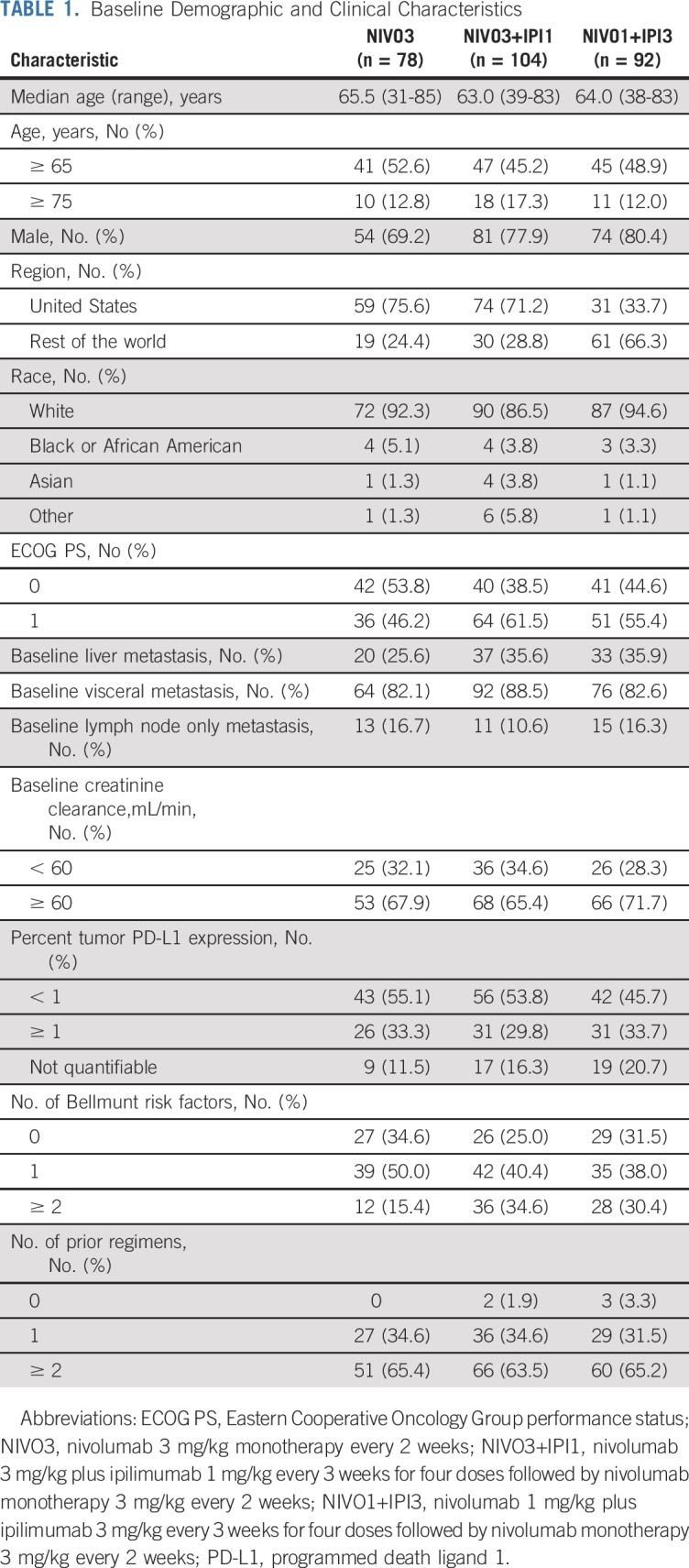
Patients in the CheckMate 032 UC cohort were enrolled from June 5, 2014, to September 28, 2017. There were 78, 104, and 92 treated patients in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively. Patients were randomly assigned between the NIVO3 and NIVO3+IPI1 arms, which were enrolling at the same time. In contrast, 86 of 92 treated patients in the NIVO1+IPI3 arm were enrolled after the other two arms completed enrollment, thus leading to the differing lengths of follow-up. Baseline demographic and clinical characteristics were generally similar across treatment arms; however, more patients in the NIVO3+IPI1 and NIVO1+IPI3 arms had two or more Bellmunt risk factors and liver metastasis compared with the NIVO3 arm (Table 1). Two patients (1.9%) in the NIVO3+IPI1 arm and three patients (3.3%) in the NIVO1+IPI3 arm did not receive prior chemotherapy. More than 60% of patients in each arm received two or more prior treatment regimens.
TABLE 1.
Baseline Demographic and Clinical Characteristics
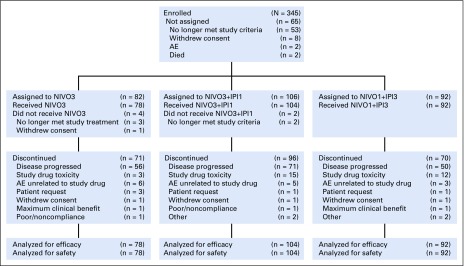
Patients received a median of 8.5 nivolumab doses (range, one to 93 doses) in the NIVO3 arm, 4.0 nivolumab doses (range, one to 87 doses), and 4.0 ipilimumab doses (range one to eight doses) in the NIVO3+IPI1 arm, and 4.0 nivolumab doses (range, one to 102 doses) and 3.0 ipilimumab doses (range, one to eight doses) in the NIVO1+IPI3 arm. In the NIVO3+IPI1 and NIVO1+IPI3 arms, 53 (50.9%) and 44 (47.8%) patients, respectively, received all four doses of the combination. Median duration of therapy was 3.5 months (95% CI, 2.3 to 5.1 months), 2.1 months (95% CI, 1.4 to 2.3 months), and 3.2 months (95% CI, 2.1 to 6.9 months) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively. At the data cutoff, seven (9.0%), eight (7.7%), and 22 patients (23.9%) were continuing treatment in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively. Disease progression was the most common reason for discontinuation (Fig 1). In the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, 26 (33.3%), 28 (26.9%), and 16 patients (17.4%), respectively, received subsequent systemic therapy.
FIG 1.
CONSORT diagram. AE, adverse event; NIVO3, nivolumab 3 mg/kg monotherapy every 2 weeks; NIVO3+IPI1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; NIVO1+IPI3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks.
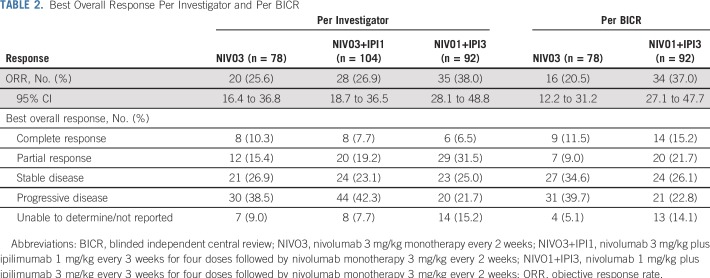
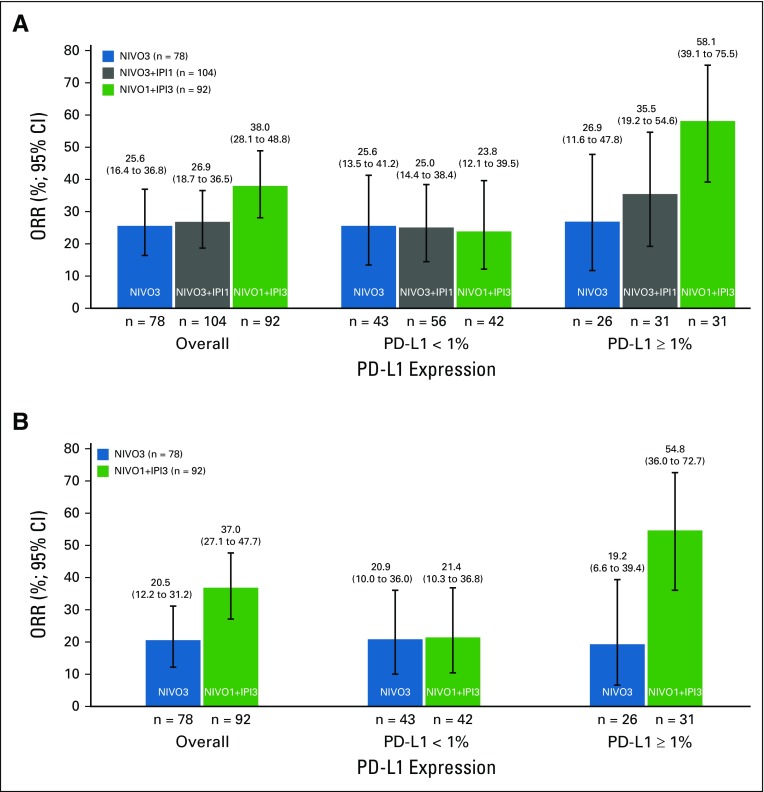
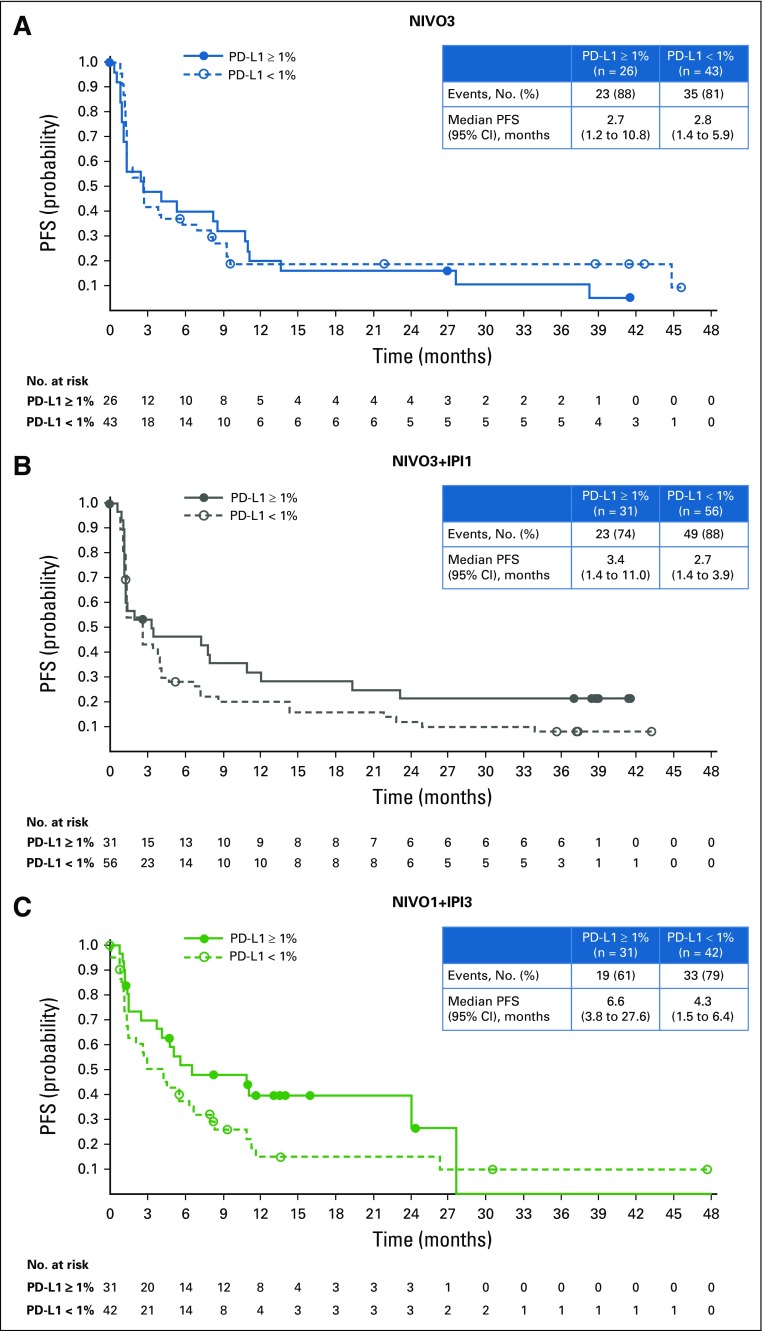
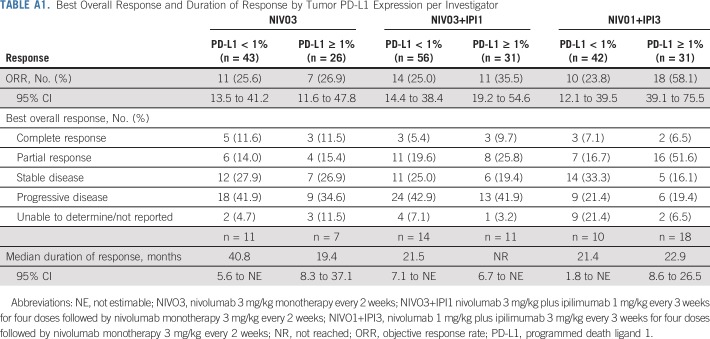
Confirmed ORR, per investigator, was 25.6% (95% CI, 16.4% to 36.8%) in the NIVO3 arm, 26.9% (95% CI, 18.7% to 36.5%) in the NIVO3+IPI1 arm, and 38.0% (95% CI, 28.1% to 48.8%) in the NIVO1+IPI3 arm (Table 2 and Appendix Fig A1A, online only). In the NIVO1+IPI3 arm, ORR was 23.8% (95% CI, 12.1% to 39.5%) in patients with tumor PD-L1 less than 1% at baseline and 58.1% (95% CI, 39.1% to 75.5%) in patients with tumor PD-L1 expression of 1% or greater at baseline (Appendix Fig A1A and Appendix Table A1, online only). ORR, per BICR, was concordant with the per-investigator assessment in the NIVO3 and NIVO1+IPI3 arms (Table 2 and Appendix Fig A1B). ORR by tumor PD-L1 expression level, per BICR, was also concordant with investigator review in the NIVO3 and NIVO1+IPI3 arms (Appendix Fig A1B).
TABLE 2.
Best Overall Response Per Investigator and Per BICR
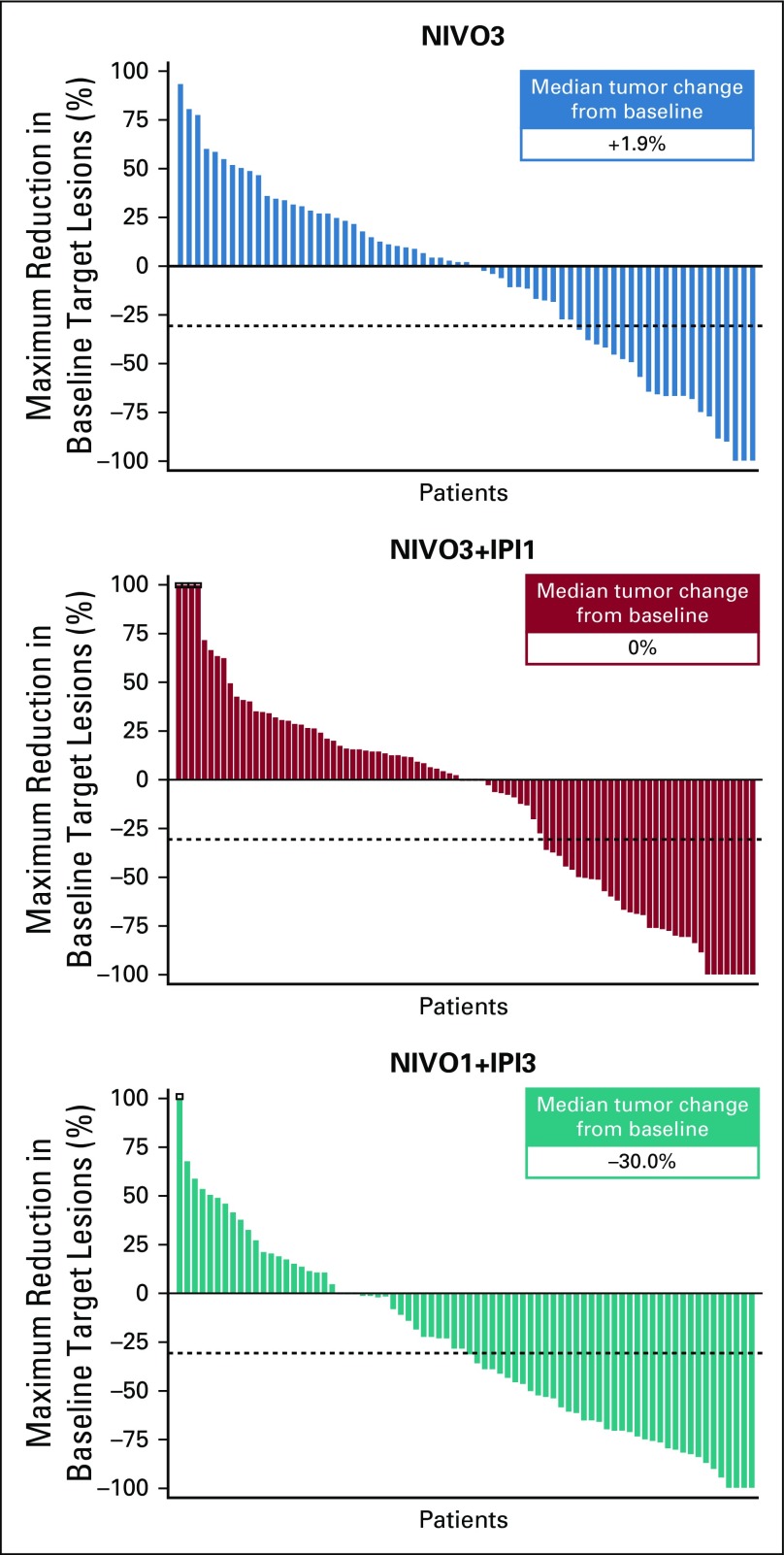
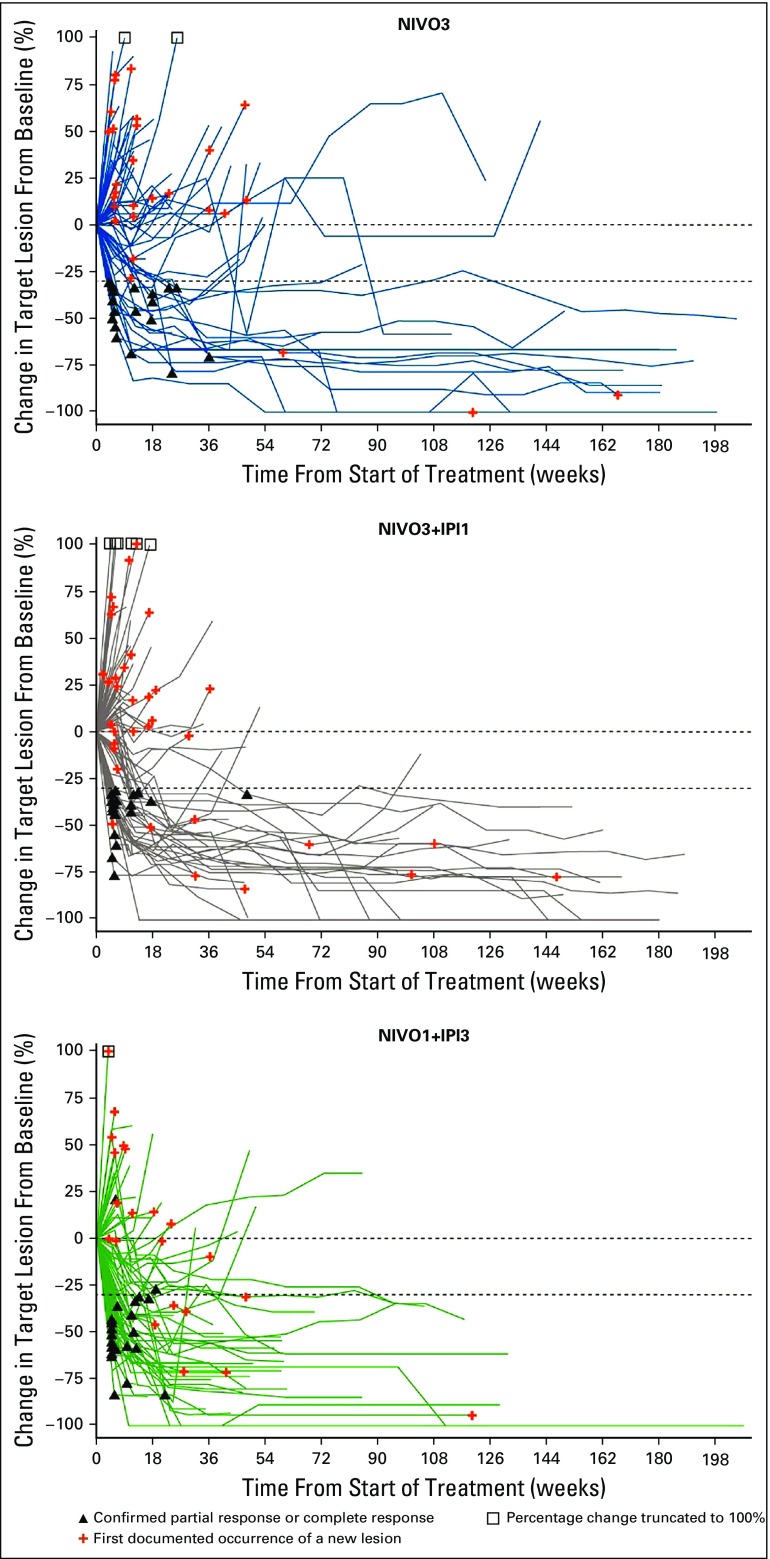
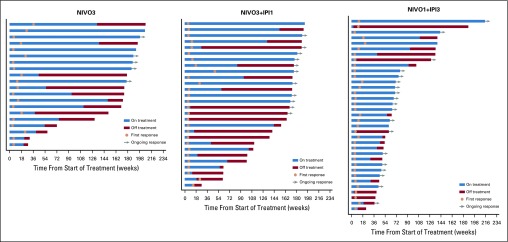
Median time to response, per investigator, was 2.0 months (range, 1.0 to 8.3 months), 1.4 months (range, 1.1 to 11.1 months), and 1.4 months (range, 1.1 to 5.1 months) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively. Median DOR was 30.5 months (95% CI, 8.3 months to not estimable [NE]) in the NIVO3 arm, 22.3 months (95% CI, 12.8 months to NE) in the NIVO3+IPI1 arm, and 22.9 months (95% CI, 9.8 months to NE) in the NIVO1+IPI3 arm. Median DOR in each arm was similar regardless of baseline tumor PD-L1 expression (Appendix Table A1). Median tumor change from baseline was +1.9%, 0%, and −30.0% in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively (Fig 2). Several patients in each arm demonstrated prolonged reduction in target lesions from baseline (Appendix Fig A2, online only) and durable responses (Appendix Fig A3, online only).
FIG 2.
Best tumor change from baseline in target lesion per investigator. NIVO3, nivolumab 3 mg/kg monotherapy every 2 weeks; NIVO3+IPI1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; NIVO1+IPI3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks.
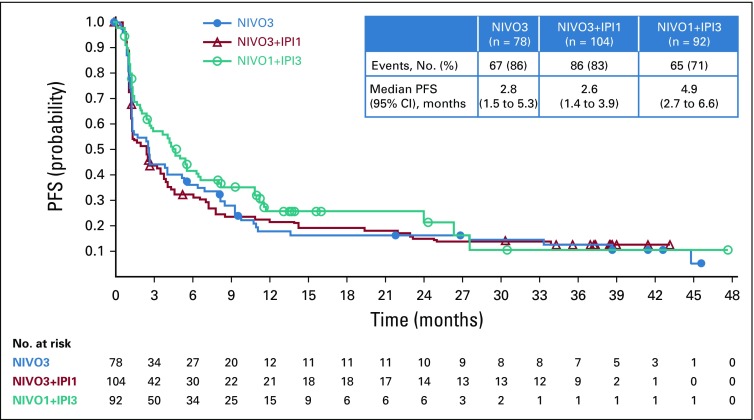
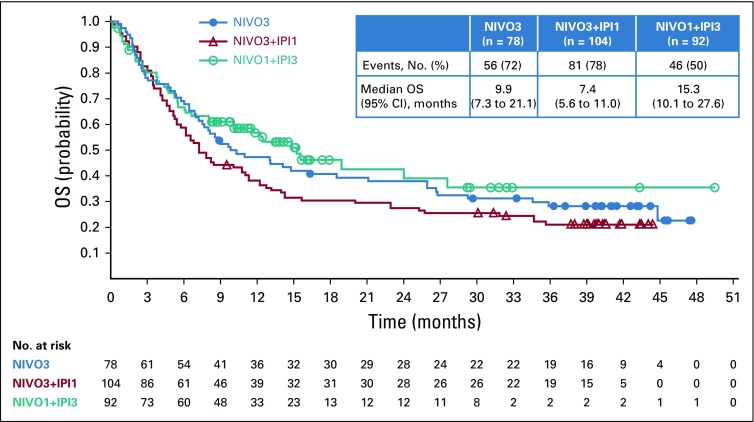
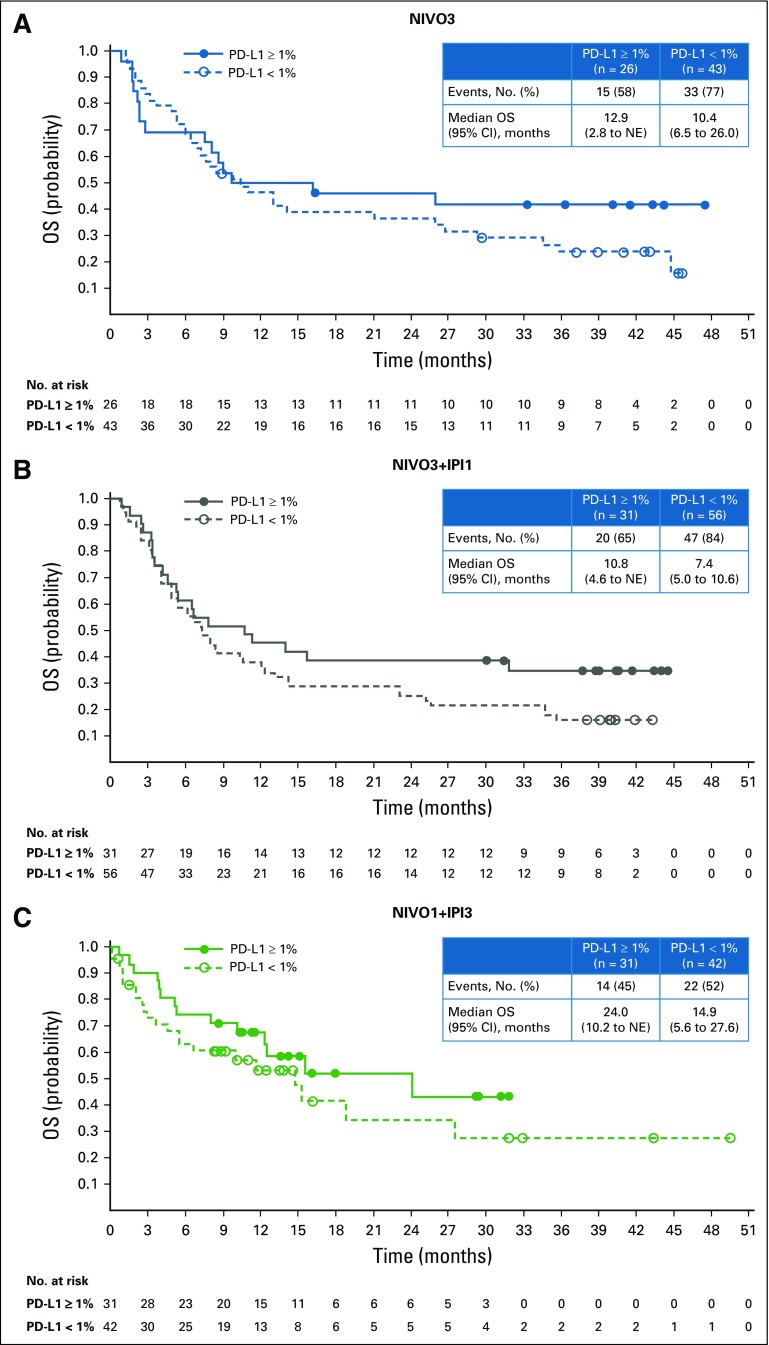
Median PFS was 2.8 months (95% CI, 1.5 to 5.3 months), 2.6 months (95% CI, 1.4 to 3.9 months), and 4.9 months (95% CI, 2.7 to 6.6 months) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms (Fig 3). Six-month PFS rates in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms were 36.3%, 32.3%, and 41.7%, respectively. Twelve-month PFS rates in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms were 17.9%, 22.6%, and 25.9%, respectively. In patients with baseline PD-L1 expression less than 1%, median PFS was 2.8 months (95% CI, 1.4 to 5.9 months), 2.7 months (95% CI, 1.4 to 3.9 months), and 4.3 months (95% CI, 1.5 to 6.4 months) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively. In patients with PD-L1 expression of 1% or greater, median PFS was 2.7 months (95% CI, 1.2 to 10.8 months), 3.4 months (95% CI, 1.4 to 11.0 months), and 6.6 months (95% CI, 3.8 to 27.6 months) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms (Appendix Fig A4, online only). Median OS was 9.9 months (95% CI, 7.3 to 21.1 months) in the NIVO3 arm, 7.4 months (95% CI, 5.6 to 11.0 months) in the NIVO3+IPI1 arm, and 15.3 months (95% CI, 10.1 to 27.6 months) in the NIVO1+IPI3 arm (Fig 4). Twelve-month OS rates were 47.3%, 38.3%, and 56.9% in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively. In patients with PD-L1 expression less than 1%, median OS was 10.4 months (95% CI, 6.5 to 26.0 months), 7.4 months (95% CI, 5.0 to 10.6 months), and 14.9 months (95% CI, 5.6 to 27.6 months) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively, and 12.9 months (95% CI, 2.8 months to NE), 10.8 months (95% CI, 4.6 months to NE), and 24.1 months (95% CI, 10.2 months to NE) in patients with PD-L1 expression of 1% or greater (Appendix Fig A5, online only).
FIG 3.
Progression-free survival per investigator. NIVO3, nivolumab 3 mg/kg monotherapy every 2 weeks; NIVO3+IPI1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; NIVO1+IPI3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; PFS, progression-free survival.
FIG 4.
Overall Survival. NIVO3, nivolumab 3 mg/kg monotherapy every 2 weeks; NIVO3+IPI1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; NIVO1+IPI3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; OS, overall survival.
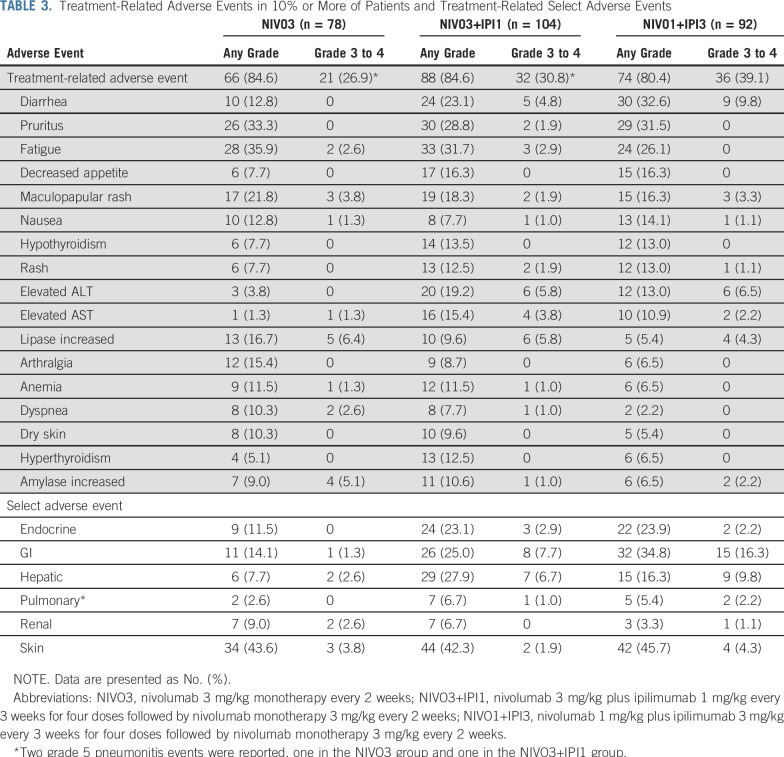
Any-grade treatment-related AEs (TRAEs) occurred in 66 (84.6%), 88 (84.6%), and 74 patients (80.4%) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively (Table 3). Grade 3 to 4 TRAEs occurred in 21 (26.9%), 32 (30.8%), and 36 patients (39.1%) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms. Grade 5 treatment-related pneumonitis occurred in one patient each in the NIVO3 and NIVO3+IPI1 arms. No grade 5 TRAEs occurred in the NIVO1+IPI3 arm. Any-grade treatment-related serious AEs occurred in nine (11.5%), 26 (25.0%), and 25 patients (27.2%) in the NIVO3, NIVO3+IPI1, and NIVO1+IPI3 arms, respectively, whereas grade 3 to 4 treatment-related serious AEs occurred in six (7.7%), 21 (20.2%), and 20 patients (21.7%). In the NIVO3 arm, three patients (3.8%) discontinued treatment as a result of grade 3 or greater TRAEs. In the NIVO3+IPI1 and NIVO1+IPI3 arms, 12 (11.5%) and 10 patients (10.9%), respectively, discontinued treatment because of grade 3 or greater TRAEs.
TABLE 3.
Treatment-Related Adverse Events in 10% or More of Patients and Treatment-Related Select Adverse Events
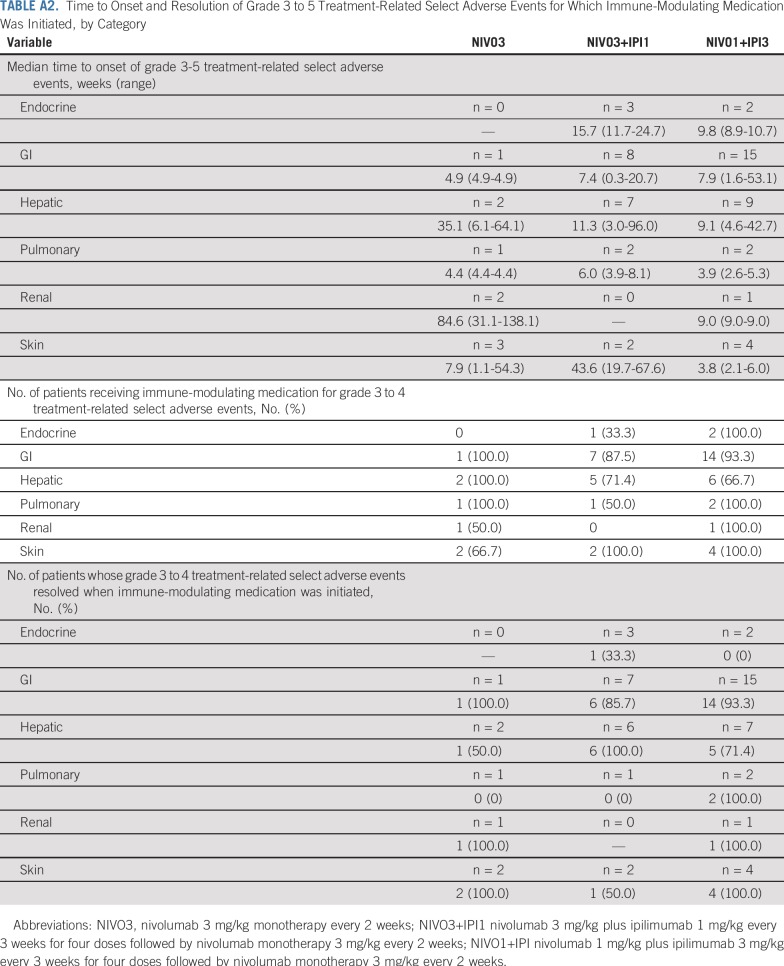
Treatment-related select AEs included endocrine, GI, hepatic, pulmonary, renal, and skin events (Table 3). Anygrade treatment-related select AEs that occurred in 10% or more of patients were endocrine (n = 9; 11.5%), GI (n = 11; 14.1%), and skin (n = 34; 43.6%) in the NIVO3 arm; endocrine (n = 24; 23.1%), GI (n = 26; 25.0%), hepatic (n = 29; 27.9%), and skin (n = 44; 42.3%) in the NIVO3+IPI1 arm; and endocrine (n = 22; 23.9%), GI (n = 32; 34.8%), hepatic (n = 15; 16.3%), and skin (n = 42; 45.7%) in the NIVO1+IPI3 arm (Table 3). Most patients received immune-modulating medication for grade 3 to 4 treatment-related select AEs (Appendix Table A2, online only). In all arms, the majority of grade 3 to 4 treatment-related select AEs resolved when immune-modulating medication was initiated (Appendix Table A2).
DISCUSSION
In CheckMate 032, NIVO3 continues to provide antitumor benefit with durable responses and prolonged OS with longer follow-up. Our results show evidence of clinical activity with combined PD-1 and cytotoxic T-lymphocyte antigen-4 inhibition in mUC, as previously observed with nivolumab plus ipilimumab across several tumor types.11-14 Especially promising efficacy was observed with the NIVO1+IPI3 combination, which resulted in the highest ORRs relative to the NIVO3 and NIVO3+IPI1 arms, although the study design precludes direct comparison. Furthermore, ORR with the NIVO1+IPI3 combination is higher than has been previously reported with other currently approved anti–PD-1 and anti–PD-L1 monotherapy agents, although this should be interpreted with caution.4-8 The promising efficacy observed with the NIVO1+IPI3 combination was also reflected in the prolonged OS and the greater proportion of patients with increased reduction in target lesions from baseline relative to the NIVO3 and NIVO3+IPI1 arms. However, additional follow-up will help fully characterize the clinical activity of the NIVO1+IPI3 combination regimen.
Responses were observed regardless of PD-L1 expression levels in all treatment arms. In this initial analysis of the NIVO1+IPI3 expansion cohort, ORR was highest in patients with baseline tumor PD-L1 expression of 1% or greater. This finding was consistent with ORR as assessed by BICR. Responses per investigator or per BICR in patients with baseline tumor PD-L1 expression less than 1% were similar across regimens; however, analysis of ORR by PD-L1 expression levels was exploratory and limited by the small numbers of patients in these subgroups.
The ORR, per investigator, was 38.0% (37.0% by BICR) with NIVO1+IPI3. In previous reports of PD-1– and PD-L1–targeted monotherapies, including pembrolizumab, nivolumab, atezolizumab, avelumab, and durvalumab, in a PD-L1–unselected population of patients with mUC, ORR ranged from 13.4% to 21.1%.4-8 Previously, the highest ORR reported with immunotherapy in the platinum-pretreated population was 21.1% at median follow-up of 27.7 months in the KEYNOTE-045 trial of pembrolizumab versus investigator’s choice of chemotherapy.4
Median OS of 15.3 months and the 1-year OS rate in this study of 56.9% were also promising. In studies of other immune checkpoint inhibitor monotherapies in this setting, median OS ranged from 6.5 to 10.5 months and 1-year OS rate ranged from 39% to 55%.4-9 Durable responses with nivolumab plus ipilimumab treatments have been observed in patients with melanoma and RCC.11,14 Longer follow-up with NIVO1+IPI3 in patients with mUC will indicate whether durability of response holds true in this malignancy.
In CheckMate 032, patients in the mUC cohort were heavily pretreated. The proportion of patients who received two or more prior regimens (65.2%) was greater than that reported in several other trials of immunotherapy agents in the previously treated mUC setting.5,6,21 The results in this heavily pretreated population are encouraging as it is known that these patients have limited subsequent treatment options. Patients also had a high rate of visceral metastases at baseline, which is associated with poor prognosis.22 In addition, more than one third of patients had baseline liver metastases and approximately one third of patients had two or more Bellmunt risk factors in both combination arms, which further highlights the notable efficacy of NIVO1+IPI3, even in patients with poor prognoses and high unmet need.
The safety profiles of the three nivolumab-containing regimens were similar and consistent with the previously reported safety profile of nivolumab monotherapy in CheckMate 032,16 and the incidence of certain AEs was consistent with the safety profiles of nivolumab plus ipilimumab in other cancer types.11,14 However, the incidence of high-grade TRAEs or high-grade treatment-related select AEs and the use of immune-modulating medication for high-grade treatment-related select AE resolution was highest in the NIVO1+IPI3 arm and may have arisen because of ipilimumab dose-related toxicity. Grade 3 to 4 treatment-related select AEs largely resolved with the use of immune-modulating medication. No new safety signals were observed with NIVO1+IPI3 in patients with mUC, and the incidence of both any-grade TRAEs and grade 3 or greater TRAEs was lower than has been reported in patients with unresectable stage III or IV melanoma.11 Similarly, the safety profile of NIVO3+IPI1 in the current study was consistent with that in patients with previously untreated advanced RCC who received the same dosing schedule of the NIVO plus IPI combination.14
This study is limited by the fact that it was not designed to directly compare outcomes among treatment arms, which each have a different length of follow-up, or with a standard current practice comparator. However, the ongoing phase III CheckMate 901 trial will further evaluate the NIVO1+IPI3 combination versus chemotherapy in patients with previously untreated mUC (ClinicalTrials.gov identifier: NCT03036098). The relatively small sample size in each arm of the current study could also be a limitation, especially in evaluating the effects of tumor PD-L1 expression on efficacy. Longer-term follow-up is needed to further characterize the efficacy and safety of the NIVO1+IPI3 combination.
In summary, these results show the continued clinical benefit with NIVO3 monotherapy and highlight the especially promising efficacy of NIVO1+IPI3 combination therapy in patients with platinum-pretreated locally advanced or mUC from CheckMate 032. The safety profile of this combination was manageable and similar to that of the NIVO3 and NIVO3+IPI1 regimens. These results provide a strong rationale by which to evaluate NIVO1+IPI3 in the first-line setting for mUC.
ACKNOWLEDGMENT
The authors thank the patients and their families who are making this study possible; Wen Hong Lin, who served as medical monitor; Ana Moreno and Candice Mayne, who served as protocol managers; and Dako, an Agilent Technologies, Inc. company, for collaborative development of the programmed death ligand 1 immunohistochemistry 28-8 pharmDx assay. Professional medical writing and editorial assistance were provided by Nicolette Belletier, PhD, and Lawrence Hargett of PAREXEL, funded by Bristol-Myers Squibb.
APPENDIX
FIG A1.
Objective response rate by tumor programmed death ligand 1 (PD-L1) expression (A) per investigator and (B) per blinded independent central review. NIVO3, nivolumab 3 mg/kg monotherapy every 2 weeks; NIVO3+IPI1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; NIVO1+IPI3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; ORR, objective response rate.
FIG A2.
Percent reduction from baseline in target lesions per investigator. Horizontal reference line indicates the 30% reduction consistent with a protocol-defined criteria response. Assessments are per investigator assessment using protocol-defined criteria. Crossover patients are truncated at the crossover date. NIVO3, nivolumab 3 mg/kg monotherapy every 2 weeks; NIVO3+IPI1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; NIVO1+IPI3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks.
FIG A3.
Time to and duration of response per investigator. NIVO3, nivolumab 3 mg/kg monotherapy every 2 weeks; NIVO3+IPI1, nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks; NIVO1+IPI3, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks.
FIG A4.
Progression-free survival (PFS) by tumor programmed death ligand 1 (PD-L1) expression per investigator. (A) NIVO3 (nivolumab 3 mg/kg monotherapy every 2 weeks). (B) NIVO3+IPI1 (nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks). (C) NIVO1+IPI3 (nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks).
FIG A5.
Overall survival (OS) by tumor programmed death ligand 1 (PD-L1) expression. (A) NIVO3 (nivolumab 3 mg/kg monotherapy every 2 weeks). (B) NIVO3+IPI1 (nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks). (C) NIVO1+IPI3 (nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for four doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks). NE, not estimable.
List of Study Sites and Investigators
TABLE A1.
Best Overall Response and Duration of Response by Tumor PD-L1 Expression per Investigator
TABLE A2.
Time to Onset and Resolution of Grade 3 to 5 Treatment-Related Select Adverse Events for Which Immune-Modulating Medication Was Initiated, by Category
Footnotes
Presented at the European Society for Medical Oncology 2018 Congress, Munich, Germany, October 19-23, 2018.
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT01928394.
Sponsored by Bristol-Myers Squibb and funded in part through National Cancer Institute Cancer Center Support Grant No. P30-CA008748.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study material or patients: Padmanee Sharma, Arlene Siefker-Radtke, Fillippo de Braud, Umberto Basso, Emiliano Calvo, Petri Bono, Michael A. Morse, Paulo A. Ascierto, Jose Lopez-Martin, Peter Brossart, Kristoffer Rohrberg, Begoña Mellado, Margaret K. Callahan, Jonathan Rosenberg
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Padmanee Sharma
Stock and Other Ownership Interests: Jounce Therapeutics, Neon Therapeutics, Jounce Therapeutics (I), Neon Therapeutics (I), Constellation Pharmaceuticals, Oncolytics, BioAtla, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak Biosciences, ImaginAb, BioAtla (I), Forty-Seven (I), Apricity (I), Polaris (I), Marker Therapeutics (I), Codiak Biosciences (I), ImaginAb (I), Tvardi Therapeutics (I), Hummingbird, Hummingbird (I), Optera, Optera (I), Dragonfly Therapeutics, Dragonfly Therapeutics (I)
Consulting or Advisory Role: Constellation Pharmaceuticals, Jounce Therapeutics, Neon Therapeutics, Amgen (I), Jounce Therapeutics (I), Neon Therapeutics (I), BioAtla, Pieris Pharmaceuticals, Oncolytics, Merck, Forty-Seven, Polaris, Apricity, Marker Therapeutics, Codiak Biosciences, ImaginAb, Forty-Seven (I), Apricity (I), Polaris (I), Marker Therapeutics (I), Codiak Biosciences (I), ImaginAb (I), Tvardi Therapeutics (I), Hummingbird, BioAtla (I), Hummingbird (I), Bristol-Myers Squibb (I), Merck (I), Optera, Optera (I), Dragonfly Therapeutics, Dragonfly Therapeutics (I)
Patents, Royalties, Other Intellectual Property: Patent licensed to Jounce, patents licensed to Bristol-Myers Squibb, Jounce, and Merck (I)
Arlene Siefker-Radtke
Consulting or Advisory Role: Janssen Pharmaceuticals, Merck, National Comprehensive Cancer Network, Eli Lilly, Bristol-Myers Squibb, AstraZeneca, BioClin Therapeutics, Bavarian Nordic, Seattle Genetics, Nektar, Genentech, Inovio Pharmaceuticals, EMD Serono
Research Funding: National Institutes of Health, Michael and Sherry Sutton Fund for Urothelial Cancer, Janssen Pharmaceuticals, Takeda, Bristol-Myers Squibb, BioClin Therapeutics, Nektar
Patents, Royalties, Other Intellectual Property: Methods of characterizing and treating molecular subsets of muscle-invasive bladder cancer
Filippo de Braud
Consulting or Advisory Role: Ignyta, Pfizer, Amgen, Novartis, Daiichi Sankyo, Bristol-Myers Squibb, Servier, Dompè, Pierre Fabre, Roche, Octimet, Incyte
Speakers' Bureau: MSD Oncology, Novartis, Bristol-Myers Squibb, Roche, Menarini, Pfizer
Research Funding: Novartis (Inst), Roche (Inst), MSD Oncology (Inst), Ignyta (Inst), MedImmune (Inst), Nektar (Inst), Bristol-Myers Squibb (Inst), Merck Serono (Inst), Bayer (Inst), Celgene (Inst), GlaxoSmithKline (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), Pfizer (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Bristol-Myers Squibb, Celgene, Daiichi Sankyo
Umberto Basso
Consulting or Advisory Role: Roche, Janssen-Cilag, Pierre Fabre, Pfizer, Novartis, Incyte
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb, Pfizer, Sanofi, Astellas Pharma, Janssen-Cilag
Other Relationship: Ipsen
Emiliano Calvo
Employment: START, HM Hospitales Group
Leadership: START
Stock and Other Ownership Interests: START, Oncoart Associated, International Cancer Consultants
Honoraria: HM Hospitales Group
Consulting or Advisory Role: Novartis, Nanobiotix, Janssen-Cilag, PsiOxus Therapeutics, Seattle Genetics, EUSA Pharma, AbbVie, Celgene, AstraZeneca, Guidepoint Global, Genentech, GLG Pharma, Pfizer, Servier, Amcure
Speakers' Bureau: Novartis
Research Funding: AstraZeneca, Novartis, BeiGene, START
Travel, Accommodations, Expenses: Genentech
Other Relationship: President and founder of Foundation INTHEOS (Investigational Therapeutics in Oncological Sciences)
Petri Bono
Employment: Terveystalo
Stock and Other Ownership Interests: TILT Biotherapeutics, Faron Pharmaceuticals (I)
Consulting or Advisory Role: Pfizer, Novartis, MSD Oncology, Bristol-Myers Squibb, Orion Pharma, Ipsen, Faron Pharmaceuticals, Oncorena
Travel, Accommodations, Expenses: Faron Pharmaceuticals, MSD Oncology
Michael A. Morse
Stock and Other Ownership Interests: PhytoChem
Honoraria: Genentech, Novartis, Sanofi, Regeneron, Lexicon, Ipsen, Onyx Pharmaceuticals, Bayer, Taiho Pharmaceutical, Etubics, EMD Serono, Boehringer Ingelheim, Merrimack Pharmaceuticals, Eisai, Eli Lilly, Merck, Exelixis
Speakers' Bureau: Genentech, Novartis, Celgene, Taiho Pharmaceutical, Lexicon, Ipsen, Merck, Exelixis, Advanced Accelerator Applications, Eisai
Research Funding: Precision Biologics (Inst), Bristol-Myers Squibb (Inst), Onyx Pharmaceuticals (Inst), Eisai (Inst), Lexicon (Inst), MedImmune (Inst), Advanced Accelerator Applications (Inst), AlphaVax (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Vaccines against antigens involved in therapy resistance and methods of using same patent number: 9956276, pharmaceutical product, medical food or dietary supplement for preventing cancer and inflammatory disease: publication number: 20170246136, compositions and methods for modulating and redirecting immune responses: publication number: 20170015758
Paolo A. Ascierto
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Merck Sharp & Dohme, Novartis, Amgen, Array BioPharma, Merck Serono, Pierre Fabre, Newlink Genetics, Genmab, Incyte, MedImmune, AstraZeneca, Syndax, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), Array BioPharma (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Jose Lopez-Martin
Stock and Other Ownership Interests: PharmaMar
Consulting or Advisory Role: Novartis, Eli Lilly, Roche, GlaxoSmithKline, MSD Oncology, Celgene, Caris Life Sciences, Bayer, Pfizer, Pierre Fabre, Bristol-Myers Squibb, Bristol-Myers Squibb (Inst), Roche (Inst), Amgen, PharmaMar
Patents, Royalties, Other Intellectual Property: PharmaMar
Travel, Accommodations, Expenses: Roche, MSD Oncology, Roche, Bristol-Myers Squibb
Peter Brossart
Honoraria: Bristol-Myers Squibb, MSD
Consulting or Advisory Role: Bristol-Myers Squibb, MSD, Amgen, Roche, AstraZeneca
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD, AstraZeneca
Kristoffer Rohrberg
Research Funding: Eli Lilly (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), Symphogen (Inst), Pfizer (Inst), Novartis (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Roche, Roche, Sanofi
Begoña Mellado
Consulting or Advisory Role: Pfizer, Roche, AstraZeneca, Bayer, Astellas Pharma, Janssen Pharmaceuticals, Roche (I), Pfizer (I), Amgen (I)
Research Funding: Roche, Janssen Pharmaceuticals, Bayer
Travel, Accommodations, Expenses: Janssen-Cilag, Roche (I)
Bruce S. Fischer
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Stephanie Meadows-Shropshire
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Abdel Saci
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Margaret K. Callahan
Employment: Bristol-Myers Squibb (I), Celgene (I), Kleo Pharmaceuticals (I), Bristol-Myers Squibb (I)
Consulting or Advisory Role: AstraZeneca, Moderna Therapeutics, Merck
Research Funding: Bristol-Myers Squibb (Inst)
Other Relationship: Clinical Care Options, Potomac Center for Medical Education
Jonathan Rosenberg
Stock and Other Ownership Interests: Merck, Illumina
Honoraria: UpToDate, Bristol-Myers Squibb, AstraZeneca, Medscape, Vindico, Peerview, Chugai Pharma
Consulting or Advisory Role: Eli Lilly, Merck, Agensys, Genentech, Sanofi, AstraZeneca, MedImmune, Bristol-Myers Squibb, EMD Serono, Seattle Genetics, Bayer, Inovio Pharmaceuticals, BioClin Therapeutics, QED Therapeutics, Adicet Bio, Sensei Biotherapeutics, Fortress Biotech, Pharmacyclics, Western Oncolytics
Research Funding: Genentech (Inst), Oncogenex (Inst), Agensys (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Viralytics (Inst), Genentech (Inst), Incyte (Inst), Seattle Genetics (Inst), Bayer (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
Travel, Accommodations, Expenses: Genentech, Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Comprehensive Cancer Network Bladder cancer (version 5.2018) https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
- 2. Bristol-Myers Squibb: Opdivo (nivolumab) [package insert]. Princeton, NJ, Bristol-Myers Squibb, 2018.
- 3.Sharma P, Baron A, Necchi A, et al. Nivolumab monotherapy in patients with advanced platinum-resistant urothelial carcinoma: Efficacy and safety update and association between biomarkers and overall survival in CheckMate 275. Cancer Res. 2018;78(abstr CT178) doi: 10.1158/1078-0432.CCR-19-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmunt J, de Wit R, Vaughn DJ, et al. Two-year follow-up from the phase 3 KEYNOTE-045 trial of pembrolizumab (pembro) vs investigator’s choice (paclitaxel, docetaxel, or vinflunine) in recurrent, advanced urothelial cancer (UC) J Clin Oncol. 2018;36(abstr 410) [Google Scholar]
- 5.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell P MC, Keam B, Kim S-W, et al. Updated efficacy and safety profile of durvalumab monotherapy in urothelial carcinoma. Cancer Res. 2018;78(abstr CT301) [Google Scholar]
- 9.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalil DN, Smith EL, Brentjens RJ, et al. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. Erratum: Nat Rev Clin Oncol 13:394, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bristol-Myers Squibb . Yervoy (ipilimumab) [package insert] Princeton, NJ: Bristol-Myers Squibb; 2018. [Google Scholar]
- 16.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–1598. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in metastatic urothelial carcinoma: Longer-term efficacy and safety results from the CheckMate 032 study. J Clin Oncol. 2018;36(abstr 414) [Google Scholar]
- 18.Sharma P, Callahan MK, Calvo E, et al. Efficacy and safety of nivolumab plus ipilimumab in metastatic urothelial carcinoma: First results from the phase I/II CheckMate 032 study. J Immunother Cancer. 2016;4(abstr O3) [Google Scholar]
- 19.Clopper PJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 20.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 21.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3:e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito K, Urakami S, Komai Y, et al. Impact of C-reactive protein kinetics on survival of patients with advanced urothelial carcinoma treated by second-line chemotherapy with gemcitabine, etoposide and cisplatin. BJU Int. 2012;110:1478–1484. doi: 10.1111/j.1464-410X.2012.11153.x. [DOI] [PubMed] [Google Scholar]