Abstract
Arid and semiarid regions with rain shortage and scarce good quality water must make use of low-quality water for irrigation. Consequently, improved plant cultivars for use in these areas should show adaptation capacities to confer drought and salt resistance and allow the cultivation under limited water availabiltiy. The present study was conducted to determine the effect of deficit irrigation with saline water on two local barley landraces, “Karkeni” and “Bengardeni”. Plants were saline-irrigated with three watering regimes during tillering, heading, and grain filling stages. Biochemical traits, carbon isotope discrimination (Δ13C), mineral composition, grain yield (GY) and water use efficiency based on grain yield (WUEgy) were evaluated as performance indicators. Almost all of the studied traits (e.g. soluble carbohydrates, proline, ∆13C, Na concentration, and GY) were significantly affected by deficient saline-irrigation regimes at different growth stages. The hierarchical clustering analysis clearly showed that Δ13C placed very close to GY averaging two barley landraces, which was in accordance with the scatter plot result. Multiple linear regression performed between GY as the dependent variable and other traits studied as the independent variables indicated that WUEgy, Δ13C, and soluble carbohydrates significantly explained the variability in GY (R2=95.64%). A significant positive correlation that observed between ∆13C and GY at three growth stages, indicated that ∆13C may be an important proxy component for indirect selection of yield potential in barley under deficient irrigation regimes with saline water. According to our result, “Karkeni” seems to be more efficient in terms of higher GY, WUEgy, proline and carbohydrate contents, K, Mg and Zn concentrations, as well as lower Δ13C and lipid peroxidation as compared with “Bengardeni”, under low osmotic potential imposed by deficient irrigation treatments with saline water, “Karkeni” can thus be selected and used as a parent in order to obtain more tolerant plants against such stresses in future breeding programs.
Keywords: barley, carbon isotope discrimination, deficit irrigation with saline water, grain yield, multivariate analysis
Introduction
Drought and salinity are the two major abiotic stresses dramatically limiting crop growth and productivity (Wang et al. 2003). There are over 800 million hectares of land, which are affected by salinity worldwide (Farooq et al. 2015; Munns and Tester 2008), and about 30% of the arable lands in the world have experienced yield reduction as a result of the periodical and/or unpredictable drought (Chaves and Oliveira 2004). In Tunisia, the semi-arid climates cover large parts, where the crops do not get any kind of rainfall. The water contained in reservoirs and wells is marked by a saltconcentration extending from 3 to 8.5 dS m−1, and from 6 to 10 dS m−1, respectively (Slama 2004). In these regions, the agricultural production is frequently altered by salt accumulation in soil or water, which limits majorly the crop growth (Ashraf et al. 2008; Kausar et al. 2013). Therefore, improving drought and salinity tolerance of the species cultivated in these regions has been employed as a technology for exploitation of these areas and their saline water sources for crops like barley, which is one of the most cultivated plants in Tunisia.
Salt tolerance and further growth in a saline soil require a reduction in internal plant water potential below that of the soil in order to maintain water uptake and turgor (Colla et al. 2012). Synthesis and accumulation of osmolytes such as proline and soluble sugars to facilitate osmo-regulation under abiotic stress including drought and salinity has been widely observed in plants (Trovato et al. 2008). These osmolytes not only increase cellular osmotic pressure and maintain cell turgidity but also protect cell compartments from oxidative damage, act as signaling molecules and stimulate the activity of antioxidant enzymes (Rolland et al. 2002; Sekmen et al. 2014).
Malondialdehyde (MDA), a decomposition product of polyunsaturated fatty acids, is indeed regarded as a biochemical indicator for evaluation of lipid peroxidation or damage to plasmalemma and organelle membranes and has often been used as a tool to assess the severity of the oxidative stress and the degree of plant sensitivity (Perez-Lopez et al. 2009; Sarabi et al. 2017; Wang et al. 2012).
It is well known that high ion concentrations of Na and Cl in the soil or water may depress nutrient ion activities and enhance competitions of Na vs. K, Ca, and Mg (Khan et al. 2000) and impair assimilation of nitrates due to Cl (Mansour 2000). Therefore, the plant becomes susceptible to specific-ion injury (e.g., Na, Cl) as well as to nutritional disorders that may result in reduced growth and yield (Grattan and Grieve 1999). Othman et al. (2006) found that Na concentration in barley seeds significantly increased with increasing salinity level, while K concentration decreased. In salt tolerant plants, a selective distribution of Na, Cl and K with exclusion of Na from growing tissues and enrichment of K in meristematic cells and leaf mesophyll cells is evident (Wolf et al. 1991).
The measurement of stable isotope ratios has emerged as an approach that integrates physiological processes over time (Ehleringer et al. 1993). Farquhar and Richards (1984) showed that carbon isotope discrimination (Δ13C) is linearly related to the ratio Ci/Ca (Ci and Ca are the intercellular and atmospheric partial pressures of CO2, respectively) in C3 plants. The Ci/Ca ratio is determined by leaf stomatal conductance and photosynthetic capacity and, therefore, by genetic and environmental factors such as water availability (Meinzer et al. 1992), and salinity (Rivelli et al. 2002). The changes in stomatal conductance and/or photosynthetic capacity can affect Δ13C. This discrimination is thus used as an indicator of water use efficiency (WUE) and as a criterion for drought resistance in breeding programs (Rizza et al. 2012). In many plant species with the C3 photosynthetic pathway, WUE has been shown to be negatively correlated to the discrimination against 13C (Δ13C) that occurs during CO2 diffusion into leaf cells and during photosynthetic CO2 fixation by carboxylating enzymes (Farquhar and Richards 1984; Farquhar et al. 1989). For instance, low Δ has been proposed as an indicator of high WUE in several C3 plants including wheat (Farquhar and Richards 1984), rice (Dingkuhn et al. 1991), and various other crops (Knight et al. 1994).
Although plant responses to drought and salinity stress have much in common, few attempts have been made to quantify the combined effects of both salt and water stress on Δ13C, as well as the use of Δ13C as a breeding character to select for higher-yielding landraces under these unfavorable conditions. If it can be confirmed that Δ13C and salinity tolerance under deficient irrigation with saline water are correlated, a rapid advance in breeding barley for increasing salinity and drought tolerance may be expected. Δ13C in plant organic material has been successfully used for studying the genetic variability in salinity tolerance in several species including cotton and common bean (Brugnoli and Lauteri 1991), barley (Jiang et al. 2006), wheat (Kafi et al. 2007) and melon (Sarabi et al. 2017). Handley et al. (1994) have reported that the genes from chromosome 4 control Δ13C in barley. As genetic variability in Δ13C could be easily determined through molecular marker techniques these may offer a more efficient alternative to screening for Δ13C.
The relationship between Δ13C and yield in barley has often been found to be positive, indicating that high yield is associated with low WUE. Romagosa and Araus (1991) and Febrero et al. (1994) found a positive correlation between grain Δ13C and grain yield among a set of barley cultivars. Austin et al. (1990) and Craufurd et al. (1991) also found significant positive correlations between grain Δ13C and grain yield, and showed that the correlations were strongest under water stress conditions. Masle et al. (1992) and Mayland et al. (1993) proposed leaf ash content as a trait correlated with WUE since it was positively correlated with Δ13C. For barley, Febrero et al. (1994) and, for wheat, Araus and Nachit (1996) suggested the combined use of Δ13C and ash content of grain as indicator of grain yield in rain-fed conditions. Although the physiological relationship between ash content and WUE is not as consistent as that between WUE and Δ13C, further research on this trait could be of interest to cereal breeders due to its low cost and simplicity of measurement.
In this experiment two local barley landraces, “Karkeni” and “Bengardeni”, from Southern Tunisia with aridity and salinity problems were used. These landraces are known for their high tolerance/resistance to severe environmental conditions especially salinity and water scarcity (Ben Khaled et al. 2014). Nonetheless, studies on the tolerance of these landraces to salinity under water deficit are still limited and therefore the physiological and biochemical basis involved in stress tolerance are still unclear. Thus, further studies are needed to test the hypothesis that these two local barley are tolerant to deficit irrigation with saline water, and to gain a better understanding of how these plants adapt to adverse environment.
The main objectives of the present work were (i) to study the effects of deficit irrigation with saline water on grain yield, water use efficiency based on grain yield (WUEgy), mineral composition and some biochemical characteristics in two barley landraces at three growth stages (tillering, heading and grain filling), (ii) to quantify the effects of deficit irrigation regimes with saline water on Δ13C as well as its potential utility as a breeding character to obtain tolerant landraces under such stress and, (iii) to seek relationships between traits studied and Δ13C and grain yield.
Materials and methods
Plant materials and growth conditions
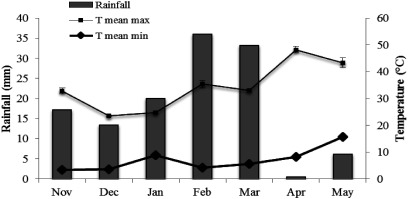
In this study, two local barley (Hordeum vulgare L.) landraces, “Karkeni” and “Bengardeni”, that are under cultivation for many years in arid regions in southern Tunisia were obtained from the Institute of Arid Regions of Medenine, Tunisia. The experiment was conducted from November 2014 to May 2015 in a field at the institute of arid regions, located at South-East of Medenine (33°29′N; 10°38′E; 106 m a.s.l.). The climate in this Mediterranean region is accompanied by hot summers, mild winters and average annual rainfall of 125 mm. The monthly average of the minimum and maximum temperatures recorded during the experiment were between 3.5–15.7°C and 16–39.8°C, respectively (Figure 1). The experimental sol was sandy. Before seed sowing, 175 kg ha−1 urea, 100 kg ha−1 phosphorous (P2O5) and 125 kg ha−1 potassium (K2O) were equally distributed for all rows. The crop evapotranspiration was calculated as:
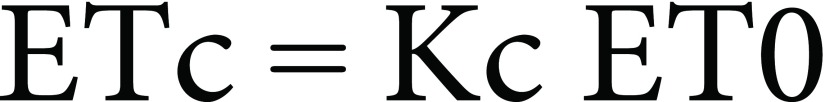 |
where, ETc is the crop evapotranspiration (mm d−1), Kc the crop coefficient (dimensionless) and ET0 the reference crop evapotranspiration (mm d−1) that was determined by the Penman–Monteith equation (Allen et al. 1998), using the climatic data recorded at Weather Station of the Institute of Arid Regions of Medenine, Tunisia.
Figure 1. Monthly rainfall (mm) and average temperatures (°C) in the region of Elfjè, Medenine from November 2014 to May 2015. Maximum and minimum temperatures for each month are also shown (source: Weather Station of the Institute of Arid Regions of Medenine, 2014–2015).
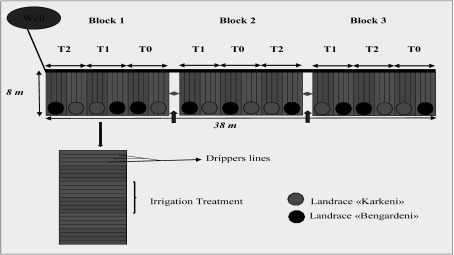
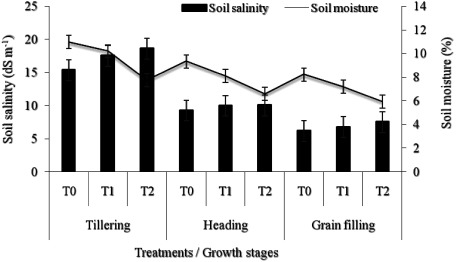
The experimental design consisted of three blocks with a total area of 304 m2 (i.e. 101.3 m2 block−1, Figure 2). Each block was divided into three equal plots for different irrigation treatments corresponding to the 100%, 75% and 50% of ETc, T0, T1 and T2, respectively. Each plot was divided into two subplot containing the two barley landraces (Karkeni and Bengardeni), each subplot including three rows spaced by 40 cm (in total there were 54 lines), with an approximately 230 plants per m2. All blocks were drip-irrigated with a water having an EC varying between 8 and 12 dS m−1. The application of irrigation regimes (T0, T1 and T2) was carried directly after sowing until maturation as a function of the ETc. Detailed information about soil moisture content and salt concentration for each irrigation treatment during growth stages at a depth of 30 cm from soil surface for two landraces studied is given in Figure 3. The youngest fully expanded leaves at tillering stage and also flag leaves at heading and grain filling stages were sampled with 18 replicates per treatment and landrace (six rows×three leaves in each row, Figure 2). These leaf samples were immediately frozen in liquid nitrogen and stored at −80°C for biochemical analysis.
Figure 2. Experimental design.
Figure 3. Variation in soil salinity (dS/m) and moisture content (%) among irrigation treatments (T0, T1 and T2) at different growth stages at a depth of 30 cm soil surface for two landraces studied. Each data point corresponds to the mean values±SE (n=6).
Measurement of biochemical parameters
Proline content in leaves was determined by following the method of Bates et al. (1973). Amount of proline was calculated from the calibration curve obtained with known concentrations of proline and expressed as µg g−1 Fresh Weight (FW). The amount of soluble carbohydrates were estimated by the anthrone reagent method (Dubois et al. 1956). Contents of sugars were calculated using glucose as a standard. Lipid peroxidation was determined in terms of thiobarbituric acid-reactive substances (TBARS) concentration according to the method of Heath and Packer (1968). The MDA concentration was quantified by its extinction coefficient of 155 mM−1 cm−1.
Mineral analysis
Dried leaves were ground into fine powder and ashed in a furnace at 550°C for 6 h. Then 2 N HCl was added to the cooled ash, and the solution was filtered after 15 min and analyzed. Concentrations of Na, K, Ca, Mg, Fe, Zn and Mn were determined by an Atomic Absorption Spectrometry (Thermo SCIENTIFIC iCE 3000 AA Spectrometer) and expressed as mg/100 g Dry Weight (DW).
Grain yield and water use efficiency for grain yield
At maturity, plants were harvested and the data regarding grain yield including spike number per square meter, grain number per spike, and 1000 grain weight were recorded. Water use efficiency based on grain yield (WUEgy) was calculated as the ratio of grain yield (GY) to the total amount of water applied (Irrigation+Rainfall) and expressed in t l−1. The total water used (Irrigation+Rainfall) was 360, 270 and 180 mm for T0 (100% ETc), T1 (75% ETc) and T2 (50% ETc), respectively.
Analyses of carbon isotope discrimination
The carbon isotope composition was determined on leaf dry matter. After freeze-drying of leaf samples (three replicates per treatment and landrace), they were finely ground with a Retsch MM200 mill ball (Bioblock Scientific, Illkirch, France) to ensure homogeneity. Then 600–800 µg per sample weighed in stain capsules (Courtage Analyse Service, Mont Saint-Aignan, France) and used for 13C analysis with an isotope ratio mass spectrometer, IRMS (VG Optima; Micromass, France) coupled to an elemental analyzer (Flash A, Thermo-Finnigan, Villebon-sur-Yvette, France) at the Institute of Plant Sciences Paris-Saclay (IPS2, Orsay, France). Carbon isotope composition (δ13C) was calculated as deviation of the carbon isotope ratio (13C/12C, called R) from the international standard (Vienna Pee Dee Belemnite):
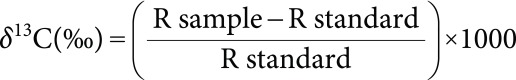 |
The carbon isotope discrimination (Δ13C) was calculated using the formula:
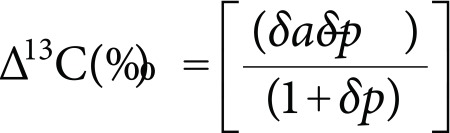 |
where, δp is the δ13C of the leaves and δa is the δ13C of the atmospheric CO2 (assumed to be −8‰). Two standards (glutamic acid and sucrose) from IAEA, and one laboratory standard (glutamic acid), were also measured in order to correct for the drift of the IRMS (1 capsule of laboratory glutamic acid every 6 samples, and 2 capsules of each AIEA standard every 24 samples).
Data analysis and statistics
The experiment was laid out in a randomized split plot design with three replications per deficient irrigation treatment with saline water and landrace, irrigation treatments (T0, T1 and T2) being the main plot and the landraces (“Karkeni” and “Bengardeni”) the subplot. The data obtained were subjected to statistical analysis using the computer program PASW statistics 20 (SPSS Inc., Chicago, IL, USA) and when significant differences were observed, Duncan’s Multiple Range Test was used to determine significance of differences between variables (p<0.05). The data collected from all treatments across two landraces were used for principal component analysis (PCA) using SPSS software as well as for obtaining Pearson’s correlation coefficients (r). The measured physiological and biochemical traits were also clustered using heatmap function with row wise scaling and Euclidean-based clustering. Moreover, stepwise multiple linear regression was performed on the data from grain filling stage, using grain yield as a dependent variable, and other traits studied as independent variables.
Results
Soluble carbohydrates and proline
The effect of deficient irrigation treatments with saline water on leaf proline content of barley plants at different growth stages was significantly different (p<0.001, Table 1), depending on the landraces. In general, proline content decreased constantly during growth stages (Table 2). “Karkeni” had the higher proline content than “Bengardeni” over all growing stages (p<0.05). Also interaction between landraces and treatments showed that proline content was higher in “Karkeni” than in “Bengardeni” at tillering stage under all treatments (T0, T1, and T2, p>0.05, Table 2).
Table 1. Summary ANOVA table for the parameters under assessment in two barley landraces, “Karkeni” and “Bengardeni”, in response to deficient irrigation regimes with saline water (T0, T1 and T2) at different growth stages (tillering, heading and grain filling).
| Proline | Soluble carbohydrates | MDA | ∆13C | Ash content | Na+ | K+ | Ca2+ | Mg2+ | Fe2+ | Zn2+ | Mn2+ | Grain yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments (T) | *** | *** | ns | *** | ** | ** | ns | * | ns | ns | ** | ns | * |
| Landraces (L) | * | *** | ns | *** | ns | ns | ns | * | * | ns | ** | ns | ** |
| Growth stages (G) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns |
| T×L | ns | ** | ns | * | ns | ns | ns | ** | ns | ns | ns | ns | ns |
| T×G | *** | *** | ns | ns | ns | *** | ns | * | * | ns | * | ns | — |
| L×G | ** | *** | ns | ** | ns | * | ns | ns | ns | ns | ** | ns | — |
ns, (*), (**), (***) are non-significant or significant at p<0.05, p<0.01 or p<0.001, respectively.
Table 2. Effects of deficient irrigation regimes with saline water (T0, T1 and T2) on proline, soluble carbohydrates, MDA, ∆13C and ash content in Karkeni and Bengardeni landraces at different growth stages (tillering, heading and grain filling).
| Character | Treatments | Tillering | Heading | Grain filling | |||
|---|---|---|---|---|---|---|---|
| Karkeni | Bengardeni | Karkeni | Bengardeni | Karkeni | Bengardeni | ||
| Proline (µg g−1 FW) | T0 | 84.84±3.61b | 54.91±3.02b | 45.36±3.5a | 53.26±2.88a | 44.26±6.85a | 50.32±5.98a |
| T1 | 200.73±20a | 185.86±7.93a | 50.32±4.23a | 52.53±3.4a | 44.08±1.68a | 52.34±3.97a | |
| T2 | 217.81±4.09a | 188.61±11.29a | 61.16±11.69a | 50.87±7.05a | 43.16±2.7a | 38.55±2.31a | |
| Soluble carbohydrates (mg g−1 FW) | T0 | 2±0.12b | 2.17±0.06b | 11.49±0.52c | 6.34±0.02d | 8.69±0.28d | 6.77±0.62e |
| T1 | 2.35±0.08b | 2.47±0.28ab | 13.08±0.14b | 11.80±0.58c | 11.55±0.06c | 10.95±0.09c | |
| T2 | 3.5±0.67a | 3.57±0.41a | 15.20±0.15a | 13.64±0.47b | 16.35±0.7a | 13.68±0.38b | |
| MDA (µg g−1DW) | T0 | 28.68±0.62a | 26.60±0.81a | 46.36±4.51ab | 49.09±1.65ab | 48.72±0.27a | 49.72±0.22a |
| T1 | 26.69±2.2a | 25.68±2.56a | 42.36±0.98b | 53.09±1.95a | 50.37±1.29a | 50.73±2.15a | |
| T2 | 24.55±1.06a | 24.28±2.55a | 47.84±2.4ab | 44.45±0.53b | 51.04±0.88a | 53.05±2.6a | |
| ∆13C (‰) | T0 | 19.87±0.03b | 20.54±0.33ab | 21.86±0.04ab | 22.12±0.13a | 21.80±0.09bc | 22.18±0.39a |
| T1 | 19.77±0.12b | 20.33±0.12ab | 21.66±0.15ab | 21.85±0.04ab | 21.54±0.33c | 21.91±0.05ab | |
| T2 | 20±0.23ab | 20.29±0.27a | 21.66±0.21ab | 21.02±0.06b | 21.43±0.4c | 21.71±0.04c | |
| Ash content (%) | T0 | 12.61±0.4a | 12.39±0.2a | 11.56±0.45a | 12.17±0.35a | 13.51±0.09ac | 14.74±0.54a |
| T1 | 12.21±0.22a | 12.21±0.13a | 11.09±0.22a | 11.75±0.35a | 12.88±0.67bc | 14.03±0.16ab | |
| T2 | 12±0.22a | 12.01±0.46a | 11.35±0.76a | 11.20±0.6a | 12.38±0.42c | 12.38±0.5c | |
Values are means±SE of 3 replicates. Means followed by different letters in the same column are significantly different (p<0.05, Duncan’s multiple range tests). Values with different letters (a, b, c, and d) are significanlty different at p<0.05.
Table 2 shows the mean comparison for total soluble carbohydrates in barley plants. Generally, soluble carbohydrates increased under deficient irrigation treatments with saline water at different growth stages. The average of carbohydrate content varied among growth stages such that the highest (11.93 mg g FW−1) and the lowest (2.68 mg g FW−1) values were observed at heading and tillering stages, respectively. “Karkeni” exhibited higher carbohydrate contents at heading and grain filling stages as compared to “Bengardeni” at T0, T1, and T2 (p<0.001, Table 2).
Lipid peroxidation
With respect to MDA (as an index of lipid peroxidation), there was a significant difference between growth stages, when both landraces are considered (p<0.001, Table 1). Averaging two landraces, the MDA content (µg g−1 DW) was elevated to 45% and 48% at heading and grain filling stages, respectively, as compared to tillering stages (Table 2). Averaged all treatments, the maximum MDA content was observed in “Bengardeni” at heading and grain filling stages (48.88 and 51.17 µg g−1 DW, respectively, Table 2). Also, the overall trend observed in two landraces was to reduce the MDA content at the tillering stage and increase it at the grain filling stage.
Carbon isotope discrimination
Deficient irrigation treatments with saline water produced a continuous and significant (p<0.001, Table 1) decrease in Δ13C of “Karkeni” and “Bengardeni” at each growth stage, with the exception of the T2 at tillering stage in “Karkeni”. The maximum values of Δ13C were around 21.40‰ for the least stressed plants, and the minimum values were around 21.02‰ for the most stressed plants (p<0.001, Table 1 and Table 2). “Karkeni” showed lower Δ13C values than “Bengardeni” when exposed to deficient irrigation regimes with saline water at tillering and grain filling stages. In addition, averaged two landraces, there was a significant increase in Δ13C at grain filling stage compared with tillering and heading stages (1.56‰ and 1.63‰, respectively, p<0.001, Table 1).
Ash content
The ash cotent was used to determine the impact of saline soils on combustion. The average ash content of flag leaves in two landraces was significantly affected by deficit irrigation scheduling regimes with saline water at different growth stages (p<0.01 and p<0.001, respectively, Table 1). Averaged two barley landraces and three growth steps, ash made up about 13% of the total dry mass in control plants, and there was a significant decrease in ash content with increasing stress level, reaching to 12.4% and 11.9% of the total dry mass for T1 and T2, respectively (Table 2). Furthermore, the maximum ash content was observed at grain filling stage (13.32%), followed by tillering and heading stages, 12.2% and 11.5%, respectively.
Mineral composition
The elements concentrations in barley plants as a function of deficit irrigation regimes with saline water at three growth stages are displayed in Table 3. Na concentration was significantly affected by treatments, growth stage, and treatment×growth stage interaction (p<0.01, p<0.001, and p<0.001, respectively; Table 1). Concentration of Na reduced by 60% and 62% at heading and grain filling stages, when compared with tillering stage across the two studied landraces (Table 3). When averaged over all treatments at each growth stage, the lower Na concentrations were observed in “Bengardeni” at tillering and heading stages, 912 and 373 mg 100 g−1 DW, respectively.
Table 3. Effects of deficient irrigation regimes with saline water (T0, T1 and T2) on mineral composition in Karkeni and Bengardeni landraces at different growth stages (tillering, heading and grain filling).
| Character | Treatments | Tillering | Heading | Grain filling | |||
|---|---|---|---|---|---|---|---|
| Karkeni | Bengardeni | Karkeni | Bengardeni | Karkeni | Bengardeni | ||
| Na+ (mg 100 g−1 DW) | T0 | 897.55±31bc | 810.57±22.89c | 426.66±35.89a | 396.19±25.28ab | 330.16±24.26a | 404.24±8.96a |
| T1 | 936.44±21.87bc | 848.82±4.06bc | 321.50±15.08b | 361.42±35.85ab | 385.66±60.19a | 415.80±8.57a | |
| T2 | 1213.52±156.92a | 1077.15±60.64ab | 426.05±16.19a | 360.70±5.53ab | 325.33±6.66a | 326.41±4.89a | |
| K+ (mg 100 g−1 DW) | T0 | 2524.72±150.33ab | 2684.18±199.1ab | 1470.58±137.28a | 1346.43±67a | 813.48±51.11b | 834.02±61.33b |
| T1 | 3113.27±257a | 2486.72±230.39b | 1357.84±44.84a | 1335.28±15.59a | 947.48±63.15b | 918.69±33.71b | |
| T2 | 2901.84±37.88ab | 3034.75±83.49ab | 1307.44±58.67a | 1302.62±99.18a | 1008.87±101.31a | 926.37±25.66b | |
| Ca2+ (mg 100 g−1 DW) | T0 | 83.94±7.8a | 91.36±6.74a | 52.52±20.6d | 202±5.92a | 47±8.51c | 78.63±11.88b |
| T1 | 84.27±5.61a | 92.98±12.44a | 189.65±2.3ab | 139.24±28.94bc | 58.31±5.78c | 121.64±53.6a | |
| T2 | 96.56±13.29a | 94.27±2.62a | 103.94±14.55cd | 73.16±11.61d | 84.68±2.94b | 82.68±6.59b | |
| Mg2+ (mg 100 g−1 DW) | T0 | 78.11±4.01ab | 73.39±2.58b | 39.25±3a | 26.45±2.25b | 44.32±1.85c | 49.22±1.03ac |
| T1 | 84.64±6ab | 75.34±8.88ab | 24.73±3.53b | 28.06±2.34b | 55.42±1.43a | 48.92±3.73bc | |
| T2 | 95.86±7.16a | 80.83±7.4ab | 31.05±0.62b | 26.33±1.65b | 47.40±0.72bc | 50.93±1.14ab | |
| Fe2+ (mg 100 g−1 DW) | T0 | 39.19±3.97a | 41.61±3.54a | 6.27±0.22a | 5.88±0.13a | 26.25±5.83b | 31.15±5.89a |
| T1 | 36±2.12a | 35.32±4.13a | 5.60±0.38a | 5.73±0.3a | 21.88±3bc | 26.07±3.5b | |
| T2 | 44.75±5.2a | 40.03±3.23a | 5.66±0.91a | 5.52±0.26a | 19.96±1.51c | 18.86±1.7c | |
| Zn2+ (mg 100 g−1 DW) | T0 | 3.28±0.58ab | 2.71±0.04b | 0.98±0.24ac | 0.67±0.05c | 0.84±0.07a | 0.91±0.06a |
| T1 | 3.45±0.46ab | 2.17±0.46b | 1.15±0.13ab | 0.79±0.1bc | 0.84±0.05a | 0.93±0.12a | |
| T2 | 4.86±0.54a | 3.32±0.6ab | 1.29±0.12a | 1.03±0.01ac | 0.97±0.13a | 0.94±0.02a | |
| Mn2+ (mg 100 g−1 DW) | T0 | 4.46±0.54a | 4.54±0.51a | 2.39±0.26a | 2.55±0.09a | 3.66±0.31a | 3.83±0.34a |
| T1 | 3.93±0.07a | 4.30±0.35a | 2.40±0.14a | 2.93±0.11a | 3.63±0.21a | 3.68±0.31a | |
| T2 | 5.19±0.68a | 4.60±0.4a | 2.60±0.34a | 2.30±0.1a | 3.44±0.32a | 3.07±0.07a | |
Values are means±SE of 3 replicates. Means followed by different letters in the same column are significantly different (p<0.05, Duncan’s multiple range tests). Values with different letters (a, b, c, and d) are significanlty different at p<0.05.
The concentration of K in plant tissues was sharply declined during growth (p<0.001), with the highest values recorded at tirelling stage (2791 mg 100 g−1 DW), followed by heading and grain filling stages (1353 and 908 mg 100 g−1 DW, respectively) across two barley landraces. At three growth stages, “Karkeni” displayed higher K concentration compared to “Bengardeni”. In addition, the overall trend observed in the two landraces was to reduce K in the heading stage and increase it in the grain filling stage with increasing stress levels.
Concentration of Mg in two barley landraces was significantly affected by growth stages (p<0.001, Table 1). Also, the amount of Mg in leaves of “Karkeni” was 11% and 15% higher than of “Bengardeni” at tillering and heading stages, respectively (p<0.05, Table 3).
The concentration of Ca was significantly affected by treatments and growth stages (p<0.05 and p<0.001, respectively; Table 1). There was a significant difference between the two landraces, with the highest values observed in “Bengardeni” at three growth stages. Moreover, the general trend observed in the two landraces, with the exception of the heading stage in “Bengardeni”, was an increase in Ca concentrations with increasing stress levels at various stages of growth.
Growth stages had a significant effect on Zn concentration in landraces (p<0.001, Table 1), such that about 70% and 73% decline were recorded at heading and grain filling stages, respectively, as compared to the tillering stage, when averaged over the two barley landraces (Table 3). There was a steady increase in Zn concentration for two landraces over treatments at different growth stages as well. Further, “Karkeni” showed higher Zn content than “Bengardeni” at tillering and heading stages.
The concentration of Fe and Mn in leaves was not significantly affected by landraces and treatments (p>0.05, Table 1). In contrast, the effect of growth stages was highly significant with respect to concentrations of these micronutrients (p<0.001, Table 1), with the highest and lowest values recorded at tillering and heading stages, respectively (Table 3).
Grain yield and water use efficiency
Grain yield (GY) showed a significant decline under stress conditions imposed by deficient irrigation regimes with saline water in both landraces (p<0.05, Table 1). “Karkeni” had significantly higher GY under all treatments compared to “Bengardeni” (p<0.01). The decrease in GY was 30% and 53% in “Karkeni” and 31% and 54% in “Bengardeni” under T1 (75% ETc) and T2 (50% ETc), respectively, when compared with their respective controls (Table 4).
Table 4. Effect of deficient irrigation with saline water on grain yield component and WUEgy at grain filling stage in two barley landraces.
| Treatment | Landraces | Spike number/m2 | Grain number/spike | 1000 g weight (g) | GY (t ha−1) | WUEgy (t L−1) |
|---|---|---|---|---|---|---|
| T0 | Karkeni | 1146.67a | 53.33a | 46.94a | 2.87a | 0.082a |
| T0 | Bengardeni | 1053.33a | 48.67a | 43.55ab | 2.23b | 0.064b |
| T1 | Karkeni | 960.00b | 48.67a | 42.72ab | 2.01b | 0.077ab |
| T1 | Bengardeni | 900.00bc | 40.67b | 41.37b | 1.53c | 0.058c |
| T2 | Karkeni | 873.33c | 38.67b | 40.46b | 1.36c | 0.078a |
| T2 | Bengardeni | 760.00d | 36.67b | 37.12c | 1.03d | 0.059c |
| Treatments (T) | *** | *** | *** | *** | *** | |
| Landraces (L) | ** | ** | *** | ** | ** | |
| T×L | ns | ** | *** | ns | ns |
T0, T1 and T2 represent the 100%, 75% and 50% ETc, respectively. Values are means of seven replicates. Means followed by different letters in the same column are significantly different (p<0.05, Duncan’s multiple range tests). Values with different letters (a, b, c, and d) are significanlty different at p<0.05. ** mean highly significant (at p<0.01). *** mean very highly significant (at p<0.001).
The analysis of yield components, indicated that “Karkeni” had generally higher grain number per spike, spike number per square meter and thousand-grain weight than “Bengardeni” at three irrigation regimes with saline water (Table 4). The average of the two landraces revealed a decrease in grain number per spike and spike number per square meter by 26% and 25%, respectively, under 50% ETc, when compared with their controls. However, thousand-seed weight appeared to be less affected under stress conditions than the other yield components, so that there were only 13.8% decrease in “Karkeni” and 14.8% decrease in “Bengardeni” compared with their respective controls.
Deficit irrigation scheduling with saline water induced a slight decrease in WUEgy by 7% in T1 and 6% in T2, compared with those of controls (average of two landraces, Table 4). “Karkeni” showed higher WUEgy under all treatments, which was 22.2%, 23.8% and 24.2% more than that of “Bengardeni” at T0, T1 and T2, respectively.
Relationships among measured parameters
Correlation analysis was carried out to determine the strength of the relationship between traits. Interesting correlations were found between the studied variables at three growth stages (Supplementary Tables S1–S3). For example, Ca and Na concentrations as well as grain yield and ash content displayed the highest positive correlations (r=1, Supplementary Table S1), while, both grain yield and ash content were negatively correlated with K concentration at tillering stage (r=−0.999, r=−0.998, respectively, p<0.05, Supplementary Table S1).
The results obtained with two barley landraces at heading and stage indicated that soluble carbohydrates were highly and negatively correlated with K concentration and grain yield (r=−0.999, r=−0.998, p<0.05, Supplementary Table S2). Likewise, significant negative correlation was found between Δ13C and proline content r=−0.999, p<0.05, Supplementary Table S2). Also, a significant positive correlation was noted between grain yield and K concentration as well as ash content and Fe concentration (r=1, r=0.997, p<0.05, Supplementary Table S2).
At grain filling stage, the strong correlations between soluble carbohydrates, MDA and Fe concentrations were observed (r=1 and r=−1, respectively, Supplementary Table S3). Significant negative correlations were noted between some of the measured parameters including Fe concentration and MDA, Mn and Zn as well as WUEgy and Ca concentration (r=−0.999, r=−0.998, r=−0.998, p<0.05, respectively, Supplementary Table S3).
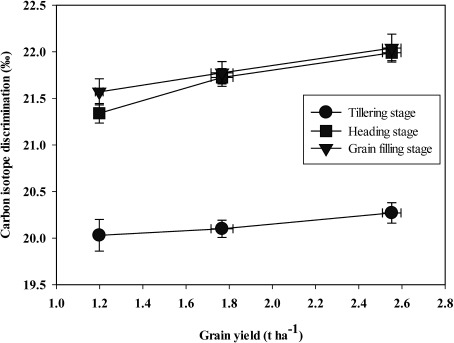
One interesting result obtained from correlation analysis was that the relationships between Δ13C and grain yield of landraces were significantly positive in tillering, heading and grain filling stages (r=0.999, r=0.998, r=0.999, respectively, p<0.05; Supplementary Table S1–S3; Figure 4). In addition, a weak positive correlation was observed between ∆13C and WUEgy (averaged all treatments) at three growth stages (r=0.872, r=0.662, r=0.856, p>0.05, at tillering, heading and grain filling stages, respectively, Supplementary Tables S1–S3). Also, the results showed that correlations among traits studied varied according to different growth stages, indicating the complex nature of stress conditions and plant performance.
Figure 4. Relationships between carbon isotope discrimination (‰) and grain yield (t ha−1) in two barley landraces studied across three growth stages (R2=0.98, 0.96 and 0.99 for tillering, heading and grain filling stages, respectively). For each growth stage, the data points from the left to the right correspond to the irrigation treatments (T0, T1 and T2, respectively). Data points are the mean values±SE (n=3).
Principal component analysis, scatter plot and hierarchical clustering
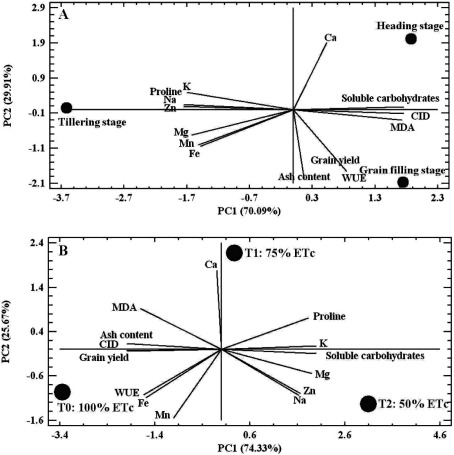
The aim of PCA is to determine the number of main factors that could be extracted to reduce the number of effective parameters. In this study, all of the observed variability at different growth stages (Figure 5A) and also across different irrigation treatments with saline water (Figure 5B) was explained by two components (PC1 and PC2) accounting for 100% of variation. The Eigenvalues and cumulative variance explained for the two principal components are presented in Table 5.
Figure 5. The scatter plot of PC1/PC2 plane showing the relationships between different growth stages (A) and different irrigation treatments (B) with the measured traits.
Table 5. Eigenvalue, percentage variation and cumulative variance derived from principal component analysis (PCA) for different growth stages and deficient irrigation treatments with saline water.
| Traits | Components of growth stages | Components of treatments | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| Proline | 0.981 | −0.194 | 0.577 | −0.817 |
| Soluble carbohydrates | −0.997 | 0.082 | 0.877 | −0.480 |
| MDA | −0.964 | 0.266 | −1 | 0.011 |
| Na+ | 0.986 | −0.165 | 0.999 | 0.035 |
| K+ | 0.933 | −0.36 | 0.827 | −0.562 |
| Ca2+ | −0.414 | −0.91 | −0.568 | −0.823 |
| Mg2+ | 0.969 | 0.249 | 0.970 | −0.244 |
| Fe2+ | 0.909 | 0.418 | −0.359 | 0.933 |
| Zn2+ | 0.986 | −0.169 | 0.998 | 0.060 |
| Mn2+ | 0.92 | 0.393 | 0.021 | 1 |
| Ash content | 0.026 | 1 | −0.887 | 0.462 |
| ∆13C | −0.985 | 0.172 | −0.841 | 0.541 |
| Grain yield | −0.374 | 0.928 | −0.842 | 0.539 |
| WUEgy | −0.374 | 0.928 | −0.399 | 0.917 |
| Eigenvalue | 9.81 | 4.19 | 10.41 | 3.59 |
| Variance (%) | 70.09 | 29.91 | 74.33 | 25.67 |
| Cumulative variance (%) | 70.09 | 100 | 74.33 | 100 |
PC1, the first principal component and PC2, the second principal component.
At different growth stages (Figure 5A), the two components explained about 70.09% and 29.91% of the variability, respectively. PC1 was mainly representingproline, soluble carbohydrates, MDA, Na, K, Mg, Fe, Zn, Mn and ∆13C, all of them with loading coefficient >0.90 (Table 5). PC2 explained Ca, ash content, GY and WUEgy (loading coefficient >0.91, Table 5).
For deficient irrigation treatments with saline water, the two PC explained 74.33% and 25.67% of the variation, respectively (Figure 5B). PC1 was represented by soluble carbohydrates, MDA, Na, K, Mg, Zn, ash content, ∆13C as well as GY, all them with loading coefficient >0.82 (Table 5). Also, proline, Ca, Fe, Mn and WUEgy (loading coefficient >0.81, Table 5) explained the PC2.
The PCA confirmed that the relations among the tested variables at different growth stages and irrigation regimes are different. Indeed, a correlation was observed in the Figure 5B between control treatment (100% ETc) and GY, WUEgy, ∆13C, ash content, Fe and Mn, while the Na, Zn, Mg, K and soluble carbohydrates were rather associated with T2 treatment (50% ETc). Additionally, a new group of interrelations was detected in Figure 5A tightly linking the proline and mineral composition (except Ca) with the tillering stage and the GY, WUEgy as well as ash content with grain filling stage.
In the Figures 5A and 5B high correlation were noticed between the ∆13C and GY, which this relationships was more evident in the Figure 5B and indicated that these parameters affected in a similar way.
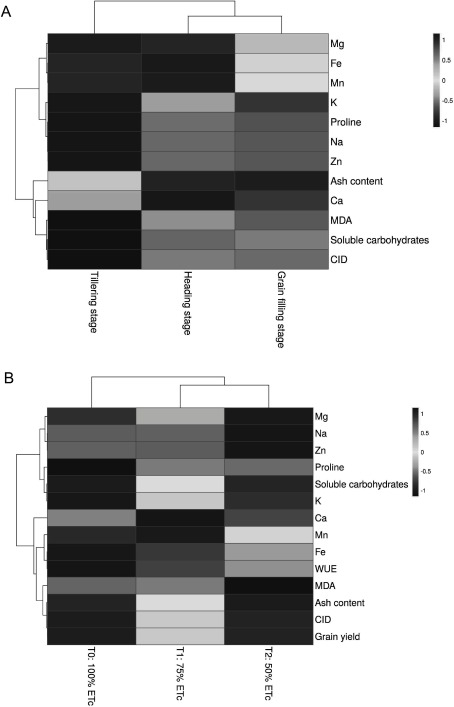
Also, the hierarchical clustering analysis (HCA) was performed on the dataset in order to show a better explaination how the measured traits are changed by the treatments. The obtained heatmaps from HCA are based on the data from different growth stages and also deficient irrigation treatments with saline water (Figure 6A and 6B, respectively). Moreover, HCA allowed the assessment of distantly and verified some of the relationships among studied traits. Accordingly, for different growth stages, a dendrogram was produced with two main groups (Figure 6A). The first sub-cluster mainly included Mg, Fe, Mn, K, proline, and Na that generally showed a decreasing trend as growth stages progressed. The second sub-cluster mainly consisted of ash content, Ca, MDA, ∆13C and soluble carbohydrates, which in general, exhibited an increasing trend during growth stages (with the exception of the Ca in grain filling stage). In addition, the Figure 6B showed that all the studied parameters at deficient irrigation regimes with saline water could be grouped into two major groups. The first main group was composed of Mg, Na, Zn, proline, soluble carbohydrates, and K. These parameters generally shown to be increased at 50% ETc as compared with T0 and T1 treatments. The other main group containing Ca, Mn, Fe, WUE, MDA, ash content, ∆13C, and GY, were generally characterized by decreasing at T2 compared with T0 and T1. Also, the HCA obviously evidenced that Δ13C placed very close to GY (Figure 6B) averaging two barley landraces, which is in accordance with PCA results (Figure 5B).
Figure 6. Hierarchical clustering analysis (HCA) for measured parameters of two barley landraces. The heatmap from HCA is based on the data from different growth stages (A) and deficient irrigation treatments with saline water (B), respectively.
Stepwise multiple linear regression
Stepwise multiple linear regression (MLR) was performed between GY as the dependent variable and other traits studied as the independent variables for two landraces at grain filling stage and averaging all treatments (T0, T1, and T2). The results indicated that WUEgy, Δ13C and soluble carbohydrates mainly explain the variability in GY (Table 6). Based on this analysis, GY is positively and significantly correlated with WUEgy (p<0.001), Δ13C (p<0.01) and negatively with soluble carbohydrates (p<0.001). The coefficient of determination of the MLR was 95.64% (significant at p<0.001). Also, the MLR analysis indicated that GY is not significantly correlated (p>0.05) with the other independent variables including proline, MDA, ash content and mineral composition.
Table 6. Summary of results of the stepwise multiple linear regression analysis for grain yield as dependent variable and other traits as independent variables at grain filling stage under deficient irrigation treatments with saline water.
| Independent Variablea | Parameter estimate |
|---|---|
| Constant | 0.762** |
| Soluble carbohydrates | −0.153*** |
| Δ13C | 0.311** |
| WUEgy | 40.294*** |
| R2 (%) | 95.64 |
| Adjusted R2 (%) | 95.06 |
| Standard error of the estimate | 0.14 |
a Only the variables with a significance of p<0.1 were included in the model. The variables were previously standardized. The equation of the fitted model is: Grain yield=0.762−0.153×Soluble carbohydrates+0.311×Δ13C +40.294×WUEgy Significance: **, p<0.01; ***, p<0.001.
Discussion
Plant responses to salinity and drought stresses have much in common. Salinity in root zone reduces the ability of plants to absorb water, and this results in growth rate decline, along with a suite of adaptive responses similar to those caused by water stress. The current study is based on the effects of deficient irrigation regimes with saline water on some physio-biochemical traits in two local barley landraces in southern Tunisia and ANOVA results showed that almost all of the measured traits (e.g., soluble carbohydrates, proline, ∆13C, and GY) were significantly affected by imposed stress condition. Accordingly, it could be stated that deficient saline-irrigation treatments seem to be sufficient to induce salt and osmotic related effects in barley studied.
Impact of deficient irrigation treatments with saline water on compatible solute accumulation and lipid peroxidation
One of the physiological responses of plants to both drought and salinity is accumulation of compatible compounds like proline and soluble sugars in order to facilitate osmotic adjustment (Trovato et al. 2008). In this experiment, by intensifying salt stress under deficit irrigation, the accumulation of soluble sugars had increased in both landraces at heading and grain filling stages. Beside their role in osmoregulation process, sugars are the main source of energy and are building blocks for a variety of cell compartments and metabolites. Moreover, sugars have pleiotropic functions on growth and development, metabolism, signal transduction and gene expression (Rolland et al. 2002). However, upon growth stages, a dramatic decrease in the proline level was observed in two landraces, suggesting that studied plants may have used carbohydrates for sustained growth as osmo-compatible compounds rather than proline. This decrease may be a result of the inhibition of proline synthesis and the activation of its degradation at both transcriptional and post-transcriptional levels.
Salt and drought stresses could increase the production and accumulation of ROS, which causes oxidative stress (Malencic et al. 2010). The membrane lipid peroxidation under impoed stress, which is related to a higher production of ROS, is both a reflection and a scale of the stress-induced damage at the cellular level (Hao et al. 2011). Determining the MDA concentration and, hence, the extent of the membrane lipid peroxidation has often been used as a tool to assess the severity of the oxidative stress and the degree of plant sensitivity (Perez-Lopez et al. 2009). The results presented here indicated that the deficit irrigation regimes with saline water induced significant incremental increases in MDA content at heading and grain filling stages compared with tillering stage. The increased MDA concentrations in response to salt stress have been observed in different genotypes and cultivars of barley (Ahmed et al. 2013; Allel et al. 2018), tomato (Manaa et al. 2014) and also canola (Ahmadi et al. 2018). Our results indicated that “Karkeni” had a lower MDA content than “Bengardeni” at heading and grain filling stages, suggesting less induced oxidative damage in this landrace.
Effect of deficient irrigation regimes with saline water on mineral concentrations
The concentrations of Na in leaves were relatively lower for both “Karkeni” and “Bengardeni” at heading and grain filling stages, in comparison to those observed at tillering stage, indicating that transport of Na to leaves was inhibited, which is a typical response of many non halophytic plants to NaCl salinity (Graifenberg et al. 1996; Rouphael et al. 2006), suggesting that examined landraces more efficiently limited Na transport to leaves.
It has been suggested that in NaCl-stressed plants, potassium is one of the primary nutrients for normal water uptake and transpiration flow (Marschner 2011), cell expansion, osmoregulation, stomatal movement and photosynthesis (Munns and Tester 2008). It is also an energetically cheaper osmoticum than organic metabolites (Khademi Astaneh et al. 2018; Ortiz et al. 1994). In the present study, salinity stress decreased K concentration in leaves of barley plants at different stages of growth. Saqib et al. (2004) reported that in saline condition, high concentration of Na in root environment can depresses K uptake at root level due to the antagonism of Na and K at uptake sites of the roots.
In addition, decreased Na/K, Na/Ca and Na/Mg ratios were observed in both landraces at grain filling stage under salinity treatments compared with control (Data not shown). Na/K and Na/Ca ratios were reported to be associated with a relative salt tolerance in many species including barley (Ahmed et al. 2013), melon (Sarabi et al. 2017; Yasar et al. 2006) as well as eggplant (Akinci et al. 2004; Hannachi and Van Labeke 2018), tolerant genotypes having lower Na/K and Na/Ca ratios. In fact, the lower Na/K ratio in the cytosol is required for normal cellular functions of plants. While competing with K uptake, Na may act as an obstacle for the K specific transporters under salinity. This contributes to a toxic level of Na as well as insufficient K concentration for enzymatic reactions and osmotic adjustment (Yasar et al. 2006; Zhu 2003).
Although uptake of microelements have been studied in several plants (e.g., zucchini, cabbage palm and marigold) under salt stress (Koksal et al. 2016; Lao et al. 2013; Villora et al. 2000), the relation between microelement uptake and salinity is complicated (Koksal et al. 2016). Salinity could cause an increase and/or a decrease in microelement uptake or may have no effect on microelement accumulation in plants (Koksal et al. 2016). Grattan and Grieve (1999) stated that such contradictory results maybe be arising from several factor including plant species and tissue, severity and composition of salt stress, concentration of microelements in the culture medium and time exposure to stress. A significant decrease in Zn, Fe and Mn concentration was observed in our study at heading and grain filling stages comparing with tillering stage (p<0.001), while deficient irrigation treatments with saline water had no significant effect on Fe and Mn uptake (p>0.05) and there was no consistent pattern for accumulation of Fe and Mn in barley landraces. In agreement with our result, Eom et al. (2007) reported that salinity stress does not affect Fe or Zn uptake of six different types of ground cover plants and Valdez-Aguilar et al. (2009) indicated that increasing salinity in nutrient solution has little effect on the micronutrient uptake of marigold.
Carbon isotope discrimination, GY, WUEgy and ash content
Deficient irrigation with saline water resulted in a decrease in ∆13C in 50% ETc treatment in comparison to 100% ETc treatment, averaged two landraces, indicating that the imposition of such stress leads to less discrimination against the heavier isotope. One reason for lower ∆13C may be attributed to both diffusion limitations associated with reduced stomata conductance and reductions in photosynthetic efficiency apparently related to decreases in RuBP carboxylase activity (Jiang et al. 2006; Seemann and Critchley 1985). A decrease in ∆13C due to salinity has also been reported for wheat (Ansari et al. 1998; Shaheen and Hood-Nowotny 2005), barley (Isla et al. 1998; Jiang et al. 2006; Pakniyat et al. 1997) and melon (Sarabi et al. 2017) plants.
The observed positive relationships between ∆13C and GY in the present work accords with results of Romagosa and Araus (1991) as well as those of Isla et al. (1998) in barley and Kirda et al. (1992) on durum wheat, and shows that ∆13C may be a good indicator of yield potential in barley under deficient irrigation regimes with saline water. Higher ∆13C is caused by a higher Ci/Ca ratio mainly due to a larger stomatal conductance, which can leads to a higher photosynthetic rate and hence a higher yield, i.e., positive relationship between ∆13C and yield.
Stomatal closure and changes in plant water condition due to salinity stress could also affect the WUE (Barbieri et al. 2012). Although there are several definitions for WUE, they depend on the scale at which the relation is displayed (Sun et al. 2016). In the present study, WUE was described as the ratio of grain yield produced to the amount of water used during plant growth (i.e., WUEgy). WUEgy was slightly reduced under stress condition in both landraces in heading and grain filling stages. WUE is a complex indicator, which is affected by a range of mechanisms related to plant water uptake and use (Topbjerg et al. 2015). When plants are exposed to salinity or to hyper osmotic stresses, one of the first reactions is a reduction in their stomatal conductance (gs) that finally affects the transpiration rate (Orsini et al. 2012; Yordanov et al. 2000). In this regard, a significant difference was found in WUEgy between “Karkeni” and “Bengardeni” under different irrigation regimes with saline water. This may be explained by the different changes in gs and net photosynthetic rate under stress between landraces (leaf gas exchanges were not measured in the present work).
The lack of significant correlation between ∆13C and WUEgy at three growth stages supports the view of some authors that WUE has little to do with drought adaption and/or resistance and in certain cases selection for high WUE may even shift the population towards drought susceptibility (Read et al. 1993; Shaheen and Hood-Nowotny 2005). We also consider that a main reason for the lack of significant relationship between ∆13C and WUEgy under the influence of imposed stress may be due to the lack of landrace×treatment interaction for WUEgy.
Our results on the increase in Δ13C at grain filling stage compared with tillering and heading stages in two landraces differ from those reported under terminal water stress by Austin et al. (1990) and Craufurd et al. (1991) in barley, who observed that earlier cultivars had higher Δ13C because their growth accomplished when the vapour pressure deficit was less than that during the corresponding growth stages of the later ones. One reason for this inconsistent result may be that barley landraces used in the present study could not maintain their transpiration losses and as it was previously stated, the WUEgy of plants reduced by increasing stress levels at grain filling stage. This may resulted in a higher Ci/Ca leading to a higher discrimination against 13C, leading to a13C-depletion in plant organic matter compared with earlier growth stages.
In our study, highly significant (p<0.001, Table 1) between-landrace difference in ∆13C was observed at three growth stages. “Bengardeni” had a higher ∆13C (and probably higher transpiration losses) at tillering and grain filling stages, which resulted in lower WUE values in comparison with “Karkeni” (Table 4). Thus it may be concluded that “Bengardeni” was less efficient in terms of assimilate partitioning to the grains, which could explain its lower GY. In addition, ∆13C and grain ash content values were not significantly correlated at three growth stages (p>0.05). A similar result was obtained by Febrero et al. (1994) and Isla et al. (1998) in barley under rainfed and salinity conditions, respectively. This lack of correlation supports the opinion of these authors that mineral accumulation in kernel takes place actively through the phloem rather than passively through the xylem.
Effects on yield components
As it was stated above, the yield components of both barley landraces decreased to different values under stress condition. Accordingly, it may be concluded that adverse effect of irrigation regimes with saline water on GY resulted mainly from a reduction in grain number per spike and spike number per square meter rather than in 1000-seed weight. This indicated that plants were more sensitive to imposed stresses at seed setting (flowering) than at maturity. Lesser effects of salinity on 1000-seed weight than on other yield components have been reported in chickpea (Dua 1992), sorghum (Francois et al. 1984), pearl millet (Dua 1989) as well as broad bean and pea (Dua et al. 1989). These observations suggest that, in saline environments, barley landraces having high seed weight should be cultivated in order to take advantage of this finding and thus, indirectly, decrease the adverse effect of such stress on GY.
Multivariate analyses
Morphological and physiological characterization continues to be the first step for the description and classification of local germplasm and statistical methods like principal component analysis (PCA) are useful tools for screening the landraces in a collection. Also, PCA is a way of identifying patterns in data and expressing the data in such a way as to highlight their similarities and differences. The associations between traits emphasized by this method may correspond to genetic linkage between loci controlling traits or to a pleiotropic effect (Rakonjac et al. 2010).
In this experiment, the two principal component scores (PC1 and PC2) were plotted to aid visualization of growth stages and deficient irrigation treatments differences which were distributed to three sides of the plot (Figure 5A, B). Moreover, this plot showed the relation between physiological and biochemical traits with the tendency of different growth stages as well as irrigation treatments. In addition, the present study proved that the application of hierarchical clustering analysis (HCA) could be used to cluster measured traits at different growth stages and also under deficient saline-irrigation regimes.
The combination of ∆13C, soluble carbohydrates and WUEgy as statistically independent variables regressed on GY as the dependent variable gave a highly significant coefficient of determination, indicating that GY could be estimated with high precision from the combination of these three independent variables (standard error of the estimation was 0.14 t ha−1, Table 6). We suggest that the selection of high yielding barley landraces for saline environments under deficient irrigation may be performed by using ∆13C, soluble carbohydrates and WUEgy as screening criteria.
Conclusions
The present study was done to obtain preliminary information on the effects of deficit irrigation scheduling regimes with saline water on the physiological and biochemical characteristics in two local barley landraces in southern Tunisia. This experiment is the first survey to evaluate the use of ∆13C as an important proxy component for indirect selection of barley landraces with tolerance to deficient saline-irrigation regimes in these arid and semi-arid regions. The reported results showed considerable stress effects on most of the measured parameters e.g., soluble carbohydrate, proline, ∆13C, ash content, Na, Ca and Zn concentrations, as well as GY. Also, the significant correlations were observed between ∆13C and GY at different growth stages suggesting that ∆13C can be used as a powerful criterion in barley breeding programs aimed at selection of tolerant landraces. In addition, due to better performance of the “Karkeni” in terms of the tested parameters under imposed stresses, we deduced that this landrace could be considered as a valuable local landrace and possible source of relevant traits for future barley breeding programs. Another interesting finding in the current work was that PCA, HCA, and correlation analysis combined with the MLR could be used as a reliable and useful method to study the relationships between traits studied at different growth stages and treatments.
Acknowledgements
The authors are grateful to the Laboratory of Dryland and Oasis Cropping of Medenine, for having financed the cultures and experiments, and the “Metabolism-Metabolome” platform of the Institute of Plant Sciences of Paris-Saclay (IPS2), at the University of Paris-Sud (Orsay, France), for technical assistance in carbon isotope analysis.
Supplementary Data
References
- Ahmadi FI, Karimi K, Struik PC (2018) Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S Afr J Bot 115: 5–11 [Google Scholar]
- Ahmed IM, Dai H, Zheng W, Cao F, Zhang G, Sun D, Wu F (2013) Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol Biochem 63: 49–60 [DOI] [PubMed] [Google Scholar]
- Akinci IE, Akinci S, Yilmaz K, Dikici H (2004) Response of eggplant varieties (Solanum melongena) to salinity in germination and seedling stages. N Z J Crop Hortic Sci 32: 193–200 [Google Scholar]
- Allel D, Ben-Amar A, Abdelly C (2018) Leaf photosynthesis, chlorophyll fluorescence and ion content of barley (Hordeum vulgare) in response to salinity. J Plant Nutr 41: 497–508 [Google Scholar]
- Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration: Guidelines for computing crop water requirements. Irrigation and Drainage Paper No. 56, Food and Agriculture Organization of United Nations, Rome, Italy
- Ansari R, Naqvi MS, Khanzada NA, Hubick KT (1998) Carbon isotope discrimination in wheat cultivars under saline conditions. Pak J Bot 30: 87–93 [Google Scholar]
- Araus JL, Nachit M (1996) Evaluation of morphological and physiological traits related with yield on durum wheat under Mediterranean conditions. ICARDA Annual Report 1995. ICARDA, Aleppo, Syria
- Ashraf M, Athar HR, Harris PJC, Kwon TR (2008) Some prospective strategies for improving crop salt tolerance. Adv Agron 97: 45–110 [Google Scholar]
- Austin RB, Craufurd PQ, Hall MA, Acevedo E, Siveira Pinheiro B, Ngugi ECK (1990) Carbon isotope discrimination as a means of evaluating drought resistance in barley, rice and cowpeas. Bulletin De La Société Botanique De France 137: 21–30 [Google Scholar]
- Barbieri G, Vallone S, Orsini F, Paradiso R, De Pascale S, Negre-Zakharov F, Maggio A (2012) Stomatal density and metabolic determinants mediate salt stress adaptation and water use efficiency in basil (Ocimum basilicum L.). J Plant Physiol 169: 1737–1746 [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39: 205–207 [Google Scholar]
- Ben Khaled A, Hayek T, Mansour E, Ferchichi A (2014) Assessment of salt tolerance of some Tunisian barley accessions using gas exchange attributes and Na+ content. Int J Curr Microbiol Appl Sci 3: 647–661 [Google Scholar]
- Brugnoli E, Lauteri M (1991) Effects of salinity on stomatal conductance, photosynthesis capacity and carbon isotope discrimination of salt-tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non halophytes. Plant Physiol 95: 628–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J Exp Bot 55: 2365–2384 [DOI] [PubMed] [Google Scholar]
- Colla G, Rouphael Y, Cardarelli M (2012) Vegetable Crops: Improvement of tolerance to adverse chemical soil conditions by grafting. In: Tuteja N, Gill SS, Tiburcio AF, Tuteja R (eds) Improving Crop Resistance to Abiotic Stress. Wiley-Blackwell, pp 977–992
- Craufurd PQ, Austin RB, Acevedo E, Hall MA (1991) Carbon isotope discrimination and grain-yield in barley. Field Crops Res 27: 301–313 [Google Scholar]
- Dingkuhn M, Farquhar GD, De-Datta SK, O’Toole JC (1991) Discrimination of 13C among upland rices having different water-use efficiencies. Aust J Agric Res 42: 1123–1131 [Google Scholar]
- Dua RP (1989) Salinity tolerance in pearl millet (Pennisetum americanum). Indian J Agric Res 23: 9–14 [Google Scholar]
- Dua RP (1992) Differential response of chickpea (Cicer arietinum) genotypes to salinity. Journal of Agricultural Science, Cambridge 119: 367–371 [Google Scholar]
- Dua RP, Sharma SK, Mishra B (1989) Response of broad bean (Vicia faba) and pea (Pisum sativum) varieties to salinity. Indian J Agric Sci 59: 729–731 [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Calorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356 [Google Scholar]
- Ehleringer JR, Hall AE, Farquhar GD (1993) Stable Isotopes and Plant Carbon-water Relations. Academic Press, Inc, p 547
- Eom SH, Setter TL, Di Tommaso A, Weston LA (2007) Differential growth response to salt stress among selected ornamentals. J Plant Nutr 30: 1109–1126 [Google Scholar]
- Farooq M, Hussain M, Wakeel A, Siddique KHM (2015) Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron Sustain Dev 35: 461–481 [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11: 539–552 [Google Scholar]
- Febrero A, Bort J, Catala J, Marzabal P, Voltas J, Araus JL (1994) Grain yield, carbon isotope discrimination and mineral content in mature kernels of barley under irrigated and rainfed conditions. Agronomie 14: 127–132 [Google Scholar]
- Francois LE, Donovan T, Maas EV (1984) Salinity effects on seed yield, growth and germination of grain sorghum. Agron J 76: 741–744 [Google Scholar]
- Graifenberg A, Botrini L, Giustiniani L, Lipucci Di Paola M (1996) Yield, growth and element content of zucchini squash grown under saline-sodic conditions. J Hortic Sci 71: 305–311 [Google Scholar]
- Grattan SR, Grieve CM (1999) Salinity-mineral nutrient relations in horticultural crops. Sci Hortic (Amsterdam) 78: 127–157 [Google Scholar]
- Handley LL, Nevo E, Raven JA, Martlnez-Carrasco R, Scrimgeour CM, Pakniyat H, Forster BP (1994) Chromosome 4 controls potential water-use efficiency (δ13C) in barley. J Exp Bot 45: 1661–1663 [Google Scholar]
- Hannachi S, Van Labeke MC (2018) Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongena L.). Sci Hortic (Amsterdam) 228: 56–65 [Google Scholar]
- Hao Z, Wang L, He Y, Liang J, Tao R (2011) Expression of defense genes and activities of antioxidant enzymes in rice resistance to rice stripe virus and small brown plant hopper. Plant Physiol Biochem 49: 744–751 [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stiochiometry of fatty acid peroxidation. Arch Biochem Biophys 1251: 189–198 [DOI] [PubMed] [Google Scholar]
- Isla R, Aragues R, Royo A (1998) Validity of various physiological traits as screening criteria for salt tolerance in barley. Field Crops Res 58: 97–107 [Google Scholar]
- Jiang Q, Roche D, Monaco TA, Durham S (2006) Gas exchange, chlorophyll fluorescence parameters and carbon isotope discrimination of 14 barley genetic lines in response to salinity. Field Crops Res 96: 269–278 [Google Scholar]
- Kafi M, Griffiths H, Nazemi A, Kazaie HR, Sharif A (2007) Effects of salinity on carbon isotope discrimination of shoot and grain of salt-tolerant and salt-sensitive wheat cultivars. Asian J Plant Sci 6: 1137–1166 [Google Scholar]
- Kausar F, Shahbaz M, Ashraf M (2013) Protective role of foliar applied nitric oxide in Triticum aestivum under saline stress. Turk J Bot 37: 1155–1165 [Google Scholar]
- Khademi Astaneh R, Bolandnazar S, Zaare Nahandi F, Oustan S (2018) The effects of selenium on some physiological traitsand K, Na concentration of garlic (Allium sativum L.) under NaCl stress. Inf Process Agric 5: 156–161 [Google Scholar]
- Khan MA, Ungar IA, Showalter AM (2000) Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Ann Bot (Lond) 85: 225–232 [Google Scholar]
- Kirda C, Mohamed ARAG, Kumarasinghe KS, Montenegro A, Zapata F (1992) Carbon isotope discrimination at vegetative stage as an indicator of yield and water-use efficiency of spring wheat (Triticum turgidum L. var durum). Plant Soil 147: 217–223 [Google Scholar]
- Knight JD, Livingston NJ, Van Kessel C (1994) Carbon isotope discrimination and water-use efficiency of six crops under wet and dryland conditions. Plant Cell Environ 17: 173–179 [Google Scholar]
- Koksal N, Alkan-Torun A, Kulahlioglu I, Ertargin E, Karalar E (2016) Ion uptake of marigold under saline growth conditions. Springerplus 5: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao MT, Plaza BM, Jiménez S (2013) Impact of salt stress on micronutrients in Cordyline fruticosa var. ‘Red Edge’. J Plant Nutr 36: 990–1000 [Google Scholar]
- Malencic D, Kiprovski B, Popovic M, Prvulovic D, Miladinovic J, Djordjevic V (2010) Changes in antioxidant systems in soybean as affected by Sclerotinia sclerotiorum (Lib.) de Bary. Plant Physiol Biochem 48: 903–908 [DOI] [PubMed] [Google Scholar]
- Manaa A, Gharbi E, Mimouni H, Wasti S, Aschi-Smiti S, Lutts S, Ben Ahmed H (2014) Simultaneous application of salicylic acid and calcium improves salt tolerance in two contrasting tomato (Solanum lycopersicum) cultivars. S Afr J Bot 95: 32–39 [Google Scholar]
- Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43: 491–500 [Google Scholar]
- Marschner P (2011) Marschner’s Mineral Nutrition of Higher Plants. 3rd ed. Academic Press, London, UK
- Masle J, Farquhar GD, Wong SC (1992) Transpiration ratio and plant mineral content are related among genotypes of a range of species. Aust J Plant Physiol 19: 709–721 [Google Scholar]
- Mayland HF, Johnson DA, Asay KH, Read JJ (1993) Ash, carbon isotope discrimination, and silicon as estimators of transpiration efficiency in crested wheatgrass. Aust J Plant Physiol 20: 361–369 [Google Scholar]
- Meinzer FC, Saliendra NZ, Crisosto CH (1992) Carbon isotope discrimination and gas exchange in Coffee arabica during adjustment to different soil moisture regimes. Aust J Plant Physiol 19: 171–184 [Google Scholar]
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Orsini F, Alnayef M, Bona S, Maggio A, Gianquinto G (2012) Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environ Exp Bot 81: 1–10 [Google Scholar]
- Ortiz A, Martınez V, Cerda A (1994) Effects of osmotic shock and calcium on growth and solute composition of Phaseolus vulgaris plants. Physiol Plant 91: 468–476 [Google Scholar]
- Othman Y, Al-Karakt G, Al-Tawaha AR, Al-Horani A (2006) Variation in germination and Ion uptake in barley genotypes under salinity conditions. World J Agric Sci 2: 11–15 [Google Scholar]
- Pakniyat H, Handley LL, Thomas WTB, Connolly T, Macaulay M, Caligari PDS, Forster BP (1997) Comparison of shoot dry weight, Na content and δ13C values of ari-e and other semi-dwarf barley mutants under salt-stress. Euphytica 94: 7–14 [Google Scholar]
- Perez-Lopez U, Robredo A, Lacuesta M, Sgherri C, Munoz-Rueda A, Navari-Izzo F, Mena-Petite A (2009) The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol Plant 135: 29–42 [DOI] [PubMed] [Google Scholar]
- Rakonjac V, Akšić MF, Nikolić D, Milatović D, Čolić S (2010) Morphological characterization of ‘Oblacinska’ sour cherry by multivariate analysis. Sci Hortic (Amsterdam) 125: 679–684 [Google Scholar]
- Read JJ, Asay KH, Johnson DA (1993) Divergent selection for carbon isotope discrimination in crested wheatgrass. Can J Plant Sci 73: 1027–1035 [Google Scholar]
- Richardson PJ (2013) Positive plant interactions and community dynamics. Ann Bot (Lond) 111: vi–vii [Google Scholar]
- Rivelli AR, James RA, Muns R, Condon AG (2002) Effect of salinity on water relations and growth of wheat genotypes with contrasting sodium uptake. Funct Plant Biol 29: 1065–1074 [DOI] [PubMed] [Google Scholar]
- Rizza F, Ghashghaie J, Meyer S, Matteu L, Mastrangelo AM, Badeck FW (2012) Constitutive differences in water use efficiency between two durum wheat cultivars. Field Crops Res 125: 49–60 [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14 (Suppl 1): S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagosa I, Araus JL (1991) Genotype-environment interaction for grain yield and 13C discrimination in barley. Barley Genetics VI: 563–567 [Google Scholar]
- Rouphael Y, Cardarelli M, Rea E, Battistelli A, Colla G (2006) Comparison of the subirrigation and drip-irrigation systems for greenhouse zucchini squash production using a saline and non saline nutrient solutions. Agric Water Manage 82: 99–117 [Google Scholar]
- Saqib M, Akhtar J, Qureshi RH (2004) Pot study on wheat growth in saline and waterlogged compacted soil: I. grain yield and yield components. Soil Tillage Res 77: 169–177 [Google Scholar]
- Sarabi B, Bolandnazar S, Ghaderi N, Ghashghaie J (2017) Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol Biochem 119: 294–311 [DOI] [PubMed] [Google Scholar]
- Seemann JR, Critchley C (1985) Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 164: 151–162 [DOI] [PubMed] [Google Scholar]
- Sekmen AH, Ozgur R, Uzilday B, Turkan I (2014) Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ Exp Bot 99: 141–149 [Google Scholar]
- Shaheen R, Hood-Nowotny RC (2005) Effect of drought and salinity on carbon isotope discrimination in wheat cultivars. Plant Sci 168: 901–909 [Google Scholar]
- Slama F (2004) La Salinité et La Production Végétale. Centre de Publication Universitaire Tunis, p 163
- Sun ZW, Ren LK, Fan JW, Li Q, Wang KJ, Guo MM, Wang L, Li J, Zhang GX, Yang ZY, et al. (2016) Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant Soil Environ 11: 515–521 [Google Scholar]
- Topbjerg HB, Kaminski KP, Korup K, Nielsen KL, Kirk HG, Andersen MN, Liu F (2015) Screening for intrinsic water use efficiency in a potato dihaploid mapping population under progressive drought conditions. Acta Agriculturae Scandinavica Section B—Soil and Plant Science 6: 400–411 [Google Scholar]
- Trovato M, Mattioli R, Costantino P (2008) Multiple roles of proline in plant stress tolerance and development. Rend Lincei Sci Fis Nat 19: 325–346 [Google Scholar]
- Valdez-Aguilar LA, Grieve CM, Poss J, Layield DA (2009) Salinity and alkaline pH in irrigation water affect marigold plants: II. Mineral ion relations. HortScience 44: 1726–1735 [Google Scholar]
- Villora G, Moreno A, Pulgar G, Romero L (2000) Yield improvement in zucchini under salt stress: Determining micronutrient balance. Sci Hortic (Amsterdam) 86: 175–183 [Google Scholar]
- Wang Q, Wu C, Xie B, Liu Y, Cui J, Chen G, Zhang Y (2012) Model analysing the antioxidant responses of leaves and roots of switchgrass to NaCl-salinity stress. Plant Physiol Biochem 58: 288–296 [DOI] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 218: 1–14 [DOI] [PubMed] [Google Scholar]
- Wolf O, Munns R, Tonnet ML, Jeschke WD (1991) The role of the stem in the partitioning of Na+ and K+ in salt-treated barley. J Exp Bot 42: 697–704 [Google Scholar]
- Yasar F, Kusvuran S, Ellialtioglu S (2006) Determination of antioxidant activities in some melon (Cucumis melo L.) varieties and cultivars under salt stress. J Hortic Sci Biotechnol 81: 627–630 [Google Scholar]
- Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 38: 171–186 [Google Scholar]
- Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6: 441–445 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.