Abstract
VAN3 is a plant ACAP-type ADP-ribosylation factor-GTPase activating protein (ARF-GAP) that regulates auxin transport-mediated plant morphogenesis such as continuous venation and lateral root development in Arabidopsis. Previous studies suggested that VAN3 localizes at the plasma membrane (PM) and intracellular structures. However, the role of PM localization in mediating the van3 mutant phenotype is not clear. Here we performed subcellular localization analysis of VAN3 and its regulators CVP2 and VAB to determine their endogenous functions. We found that GFP-tagged CVP2 and VAB preferentially localize at the PM in stably transformed plants. We determined that transgenic plants with lower expression levels of GFP- or mRFP-tagged VAN3 displayed PM localization, which was sufficient to rescue the van3 mutant. Functional VAN3-mRFP and VAB-GFP colocalized at PMs. The van3 mutant phenotype was suppressed by mutation of VAN7/GNOM, which encodes an ARF-GEF that localizes at the PM and Golgi apparatus. These combined results suggest that ARF-GTPase machinery at the PM regulates auxin transport-mediated plant growth and development.
Keywords: ARF, auxin, PIN, transport, VAN3, vesicle
In plants, cell polarity establishment, cell wall formation, and biotic and abiotic responses all depend on intracellular membrane trafficking. ADP-ribosylation factor (ARF) GTPase machinery is one of the most studied membrane trafficking regulators that mediates cargo loading and vesicle formation. ARF GTPases cycle between GDP-bound and GTP-bound forms via the activity of ARF-guanine nucleotide exchange factors (ARF-GEFs) and ARF GTPase-activating proteins (ARF-GAPs), which are essential for ARF GTPase function. ARF-GEFs exchange bound GDP for GTP, whereas ARF-GAPs promote GTP hydrolysis to GDP, which promotes cargo loading and vesicle formation. Spatiotemporal localization of distinct ARF-GEFs and ARF-GAPs regulate the timing and place of ARF GTPase action, which contributes to the transport of specific cargos to specific sites (D’Souza-Schorey and Chavrier 2006; Gillingham and Munro 2007; Vernoud et al. 2003).
Functional characterization of plant ARF-GEFs and ARF-GAPs identified several regulators involved in plant development (Geldner et al. 2003; Koizumi et al. 2000, 2005; Liu et al. 2013; Naramoto et al. 2014, 2016; Richter et al. 2010, 2011; Teh and Moore 2007; Yoo et al. 2008), including ARF-GAP VASCULAR NETWORK DEFECTIVE3 (VAN3)/SCARFACE (SFC) and ARF-GEF GNOM/VAN7 (Aihara et al. 2014; Geldner et al. 2003; Koizumi et al. 2005; Naramoto et al. 2009, 2010, 2014; Richter et al. 2007; Sieburth et al. 2006; Teh and Moore 2007). VAN3 encodes an ARF-GAP similar to ACAP (ARF-GAP with coiled-coil ankyrin repeat and PH domain), which is characterized by the presence of Bin1-amphiphysin-Rvs167p/Rvs161p (BAR) domains that sense or induce membrane curvature and also the pleckstrin homology (PH) domains that mediate phospholipid binding (Jackson et al. 2000; Koizumi et al. 2005; Peter et al. 2004). GNOM encodes an ARF-GEF similar to Golgi-specific Brefeldin A resistance factor (GBF), which is characterized by large molecular size and three different domains [dimerization and cyclophilin binding (DCB), homology upstream of Sec7 (HUS), and homology down stream of Sec7 (HDS) (Anders and Jurgens 2008; D’Souza-Schorey and Chavrier 2006; Geldner et al. 2003). Both ARF-GAP VAN3 and ARF-GEF GNOM localize at the plasma membranes (PM) and regulate auxin transport-mediated patterning processes such as vascular development and lateral root development (Geldner et al. 2004; Naramoto et al. 2010). These combined results suggest that VAN3 ARF-GAP and GNOM ARF-GEF act within the same pathway on common ARF substrates at the PM (Naramoto 2017).
VAN3 and GNOM localize at intracellular organelles in addition to the PM. VAN3 is observed in close proximity to the trans-Golgi network/early endosome (TGN/EE) (Koizumi et al. 2005; Naramoto et al. 2009; Prabhakaran Mariyamma et al. 2017). GNOM ARF-GEF localizes at the Golgi apparatus besides PM (Naramoto et al. 2010, 2014). Furthermore, COTYLEDON VASCULAR PATTERN2 (CVP2)-5-phosphatase and VAN3-BINDING PROTEIN/FORKED1 (VAB/FKD1) (contains a PH domain), both of which function as VAN3 regulator colocalize with VAN3 at intracellular compartments when they are transiently expressed in Arabidopsis protoplasts (Carland and Nelson 2009; Carland and Nelson 2004; Hou et al. 2010; Koizumi et al. 2005; Naramoto et al. 2009). Importantly, the cvp2 and the vab mutant phenocopied the van3 mutant in terms of discontinuous venation, suggesting that VAN3, CVP2, and VAB localize and function at intracellular compartment to regulate vein continuity. These localization patterns obscure whether PM localization of VAN3 and GNOM contributes to auxin-mediated plant growth and development. To clarify the cellular function of VAN3 and GNOM and also to get insights into the cellular function of CVP2 and VAB, we performed subcellular localization analyses of VAN3, CVP2, and VAB. We also re-examined the relationships between VAN3 and GNOM by performing double-mutant analyses.
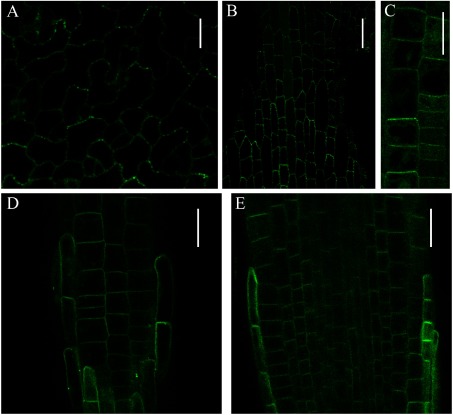
Previous work shows that CVP2 colocalizes with VAN3 at intracellular organelles when they are transiently co-expressed in tobacco leaf epidermal cells (Naramoto et al. 2009). To further characterize CVP2 localization, we generated transgenic Arabidopsis plants that stably express CVP2 as an eYFP fusion protein. We first expressed eYFP-CVP2 under the control of its authentic promoter. A total of 46 independent transgenic lines were established, but none of them emit sufficient fluorescence to observe by confocal microscopy. Next, we expressed eYFP-CVP2 under control of the 35S promoter and observed eYFP-CVP2 fluorescence in leaves and roots. eYFP-CVP2 specifically localized at the PM in pavement and petiole cells with minor cytosolic localization (Figure 1A, B). Similar localization was observed in root epidermal cells (Figure 1C). The eYFP-CVP2 fluorescence was not uniform along the PM, but displayed punctate localization. We also performed subcellular localization analysis of COTYLEDON VASCULAR PATTERN2-LIKE2 (CVL2), which encodes the second-closest CVP2 homologue and forms a subclade with CVP2 in Arabidopsis (Carland and Nelson 2009). Similar to CVP2, eYFP-CVL2 localizes at PMs in root tip cells. By contrast, CVL2 did not display a punctate localization pattern at the PM (Figure 1C, D). These observations suggested that both CVP2 and CVL2 preferentially localizes at the PMs, but also have distinct cellular functions despite the similarity of their amino acid sequences.
Figure 1. Subcellular localization analysis of eYFP-tagged CVP2 and CVL2 under control of the 35S promoter in Arabidopsis. (A–C) Subcellular localization of eYFP-CVP2 in pavement cells (A), petiole cells (B), and in root cells (C). (D and E) Subcellular localization of eYFP-CVL2 in root tips. Surface view (D) and median longitudinal section (E) of root tips are shown. eYFP-CVP2 and eYFP-CVL2 were excited with 515 nm laser at 30% of laser power and images are taken by Olympus FV1000 confocal laser microscope. Scale bars: 10 µm (A), 30 µm (B), 20 µm (C–E).
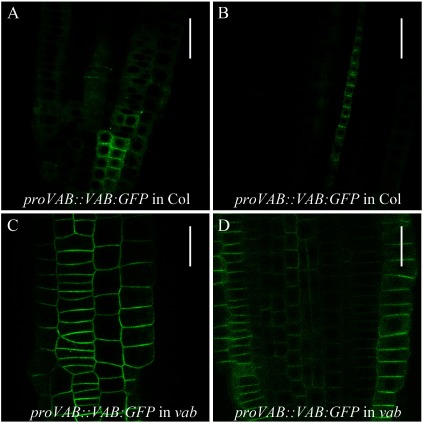
Previous transient expression analysis of tobacco epidermal cells detected VAB at intracellular organelles (Naramoto et al. 2009). Recent cell biological studies of stably transformed Arabidopsis expressing 35S::VAB/FKD1-GFP confirmed this result (Prabhakaran Mariyamma et al. 2017). To further define the subcellular localization of VAB in Arabidopsis, we expressed VAB-GFP in Columbia and observed GFP fluorescence around the cell periphery, where VAB-GFP occasionally displays a punctate localization pattern (Figure 2A, B). We also expressed the proVAB::VAB:GFP construct in the vab mutant. In contrast to the localization pattern in the Columbia background, VAB-GFP localized at the PM in the vab mutant and lacked the punctate localization pattern (Figure 2C, D). It is known that protein levels sometimes affect the subcellular localization of its protein. Therefore, the observation of punctate localization after expression of an additional copy of VAB suggests that it may be due to the higher abundance of VAB protein at the PM, where it is assumed to undergo self-assembly into clustered structures. Importantly, the vascular phenotype of the vab mutant is fully complemented by proVAB::VAB-GFP expression, demonstrating the functionality of VAB-GFP fusion proteins. These combined results suggested that VAB function at the PM is involved in establishing continuous venation.
Figure 2. Subcellular localization analysis of VAB under control of its authentic promoter in Arabidopsis. (A and B) Subcellular localization of VAB-GFP in Columbia root tips. Surface view (A) and median longitudinal section (B) of root tips are shown. VAB-GFP is expressed by its authentic promoter. (C and D) Subcellular localization of VAB-GFP in vab mutant root tips. Surface view (C) and median longitudinal section of (D) of root tips are shown. VAB-GFP is expressed by its authentic promoter. GFP was excited with 488 nm laser at 20% of laser power and images are taken by Olympus FV1000. Scale bars: 30 µm (A and B), 20 µm (C and D).
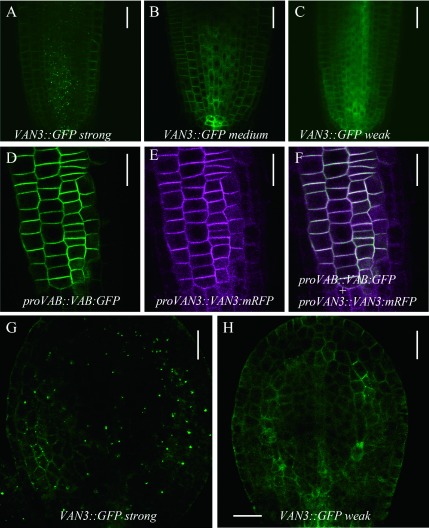
VAN3 and VAN3-like1 (VAL1)/ARF-GAP Domain1 (AGD1), the closest homolog of VAN3, localizes at intracellular organelles and the PM (Koizumi et al. 2005; Naramoto et al. 2009; Prabhakaran Mariyamma et al. 2017; Yoo et al. 2008). By contrast, our recent analysis generated VAL1/AGD1-GFP lines that did not display intracellular localization but displayed PM localization and complemented val1/agd1 mutants, which suggested the importance of PM localization (Yoo et al. 2017). To determine the subcellular localization involved in VAN3-mediated plant growth and development, we established complemented van3 mutant lines that express VAN3-GFP at different levels. Lines that express high levels of VAN3-GFP display strong GFP fluorescence at intracellular organelles and weak fluorescence at the PM (Figure 3A). Lines that express moderate levels of VAN3-GFP display weaker fluorescence at intracellular organelles (Figure 3B), and low-expression lines have barely detectable fluorescence at intracellular organelles. These results suggest that VAN3 localization at intracellular organelles is due to higher expression levels (Figure 3C). We established that functional proVAN3::VAN3-mRFP preferentially localized at the PM, and colocalized with functional VAB-GFP in van3vab mutants (Figure 3D–F). We finally analyzed VAN3-GFP localization in torpedo stage embryos at the start of continuous cotyledon venation development. In strong VAN3-GFP expression lines, we observed clear intracellular localization in procambium cells, which is consistent with previous observations (Naramoto et al. 2009). We also observed punctate localization at the PM in cells that are in the process of differentiating procambium. By contrast, weak VAN3-GFP expression lines displayed GFP fluorescence at the PM of cells throughout the cotyledon, and clear PM localization was detected in procambium cells, suggesting the importance of PM localization (Figure 3G). These combined results strongly suggested that VAN3 localizes at the PM and functions to regulate auxin-mediated growth, although we cannot exclude the possibility that VAN3 action at unknown intracellular organelles has additional critical roles in plant development.
Figure 3. Subcellular localization analysis of different expression levels of VAN3. (A–C) Functional VAN3-GFP localization in root tips at different expression levels: strong expression lines (A), medium expression lines (B), and weak expression lines (C). (D–F) Colocalization analysis of VAN3-mRFP and VAB-GFP in van3vab mutants. GFP image (D), RFP image (E), and merged image (F). (G and H) Functional VAN3-GFP localization in cotyledons. Subcellular localization in strong expression lines (G) and weak expression lines (H). VAN3-GFP and VAB-GFP were excited with 488 nm laser at 15% and 20% of laser power, respectively. VAN3-mRFP was excited by 541 nm laser with 40% power. Images are taken by Olympus FV1000. Scale bars: 30 µm (A–C), 20 µm (D–H).
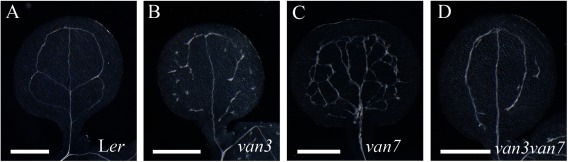
Our analysis determined that functional VAN3 localized at the PM in auxin-mediated plant growth and development. To further confirm this result, we performed double-mutant analysis of VAN3 and GNOM using the van3-1 and van7 mutant (a weak allele of gnom) mutants (Koizumi et al. 2000). VAN3 and GNOM encodes ARF-GAP and ARF-GEF, respectively, and have opposing biochemical functions. ARF-GEFs convert ARF-GDP to ARF-GTP, whereas ARF-GAPs promote the conversion of ARF-GTP to ARF-GDP. Therefore, if VAN3 and GNOM act on a common ARF substrate at the PM, the van3 and the van7 mutant phenotypes are expected to mutually suppress each other in the van3van7 double mutant. We observed that the van3van7 double mutant phenotype did show mutual suppression of each single mutant phenotype (Figure 4). The discontinuous venation in van3 mutants was largely repaired in van3van7 double mutants. The thick and highly interconnected venation in van7 mutants was less severe in van3van7 double mutants, consistent with previous reports (Figure 4D) (Koizumi et al. 2005; Sieburth et al. 2006). The frequency of fused cotyledons in van7 mutants was reduced in double mutants (Table 1). Besides shoots, VAN3 and VAN7 have genetic interactions in roots and lateral roots. The van3 and van7 mutants exhibit root meristem collapse, whereas the frequency of root meristem collapse was reduced in the van3van7 double mutant compared to the van3 single mutant (Table 2). The van3van7 double mutant developed lateral roots unlike the van7 single mutant, which does not develop lateral roots (Table 2). These double-mutant phenotypes consistently suggested that VAN3 and GNOM act on common ARF substrates at the PM to regulate auxin-mediated plant growth and development. In contrast to the weak PM localization of GNOM, GNOM primarily localizes at the Golgi apparatus where it regulates secretion (Naramoto et al. 2014; Richter et al. 2007; Teh and Moore 2007). A previous study also reports that GNOM regulates the recycling of PIN proteins to the PM through unknown mechanisms (Geldner et al. 2003). Therefore, we still cannot exclude the possibility that the mutual suppression phenotype of the van3van7 double mutant is due to opposing vesicle trafficking pathways (VAN3-mediated endocytosis versus GNOM-dependent recycling pathway). However, in any case, our combined results consistently suggest that VAN3 functions at the PM to regulate auxin-mediated plant growth.
Figure 4. Double-mutant analysis of van3 and van7 mutants. (A–D) Representative images of cotyledon vascular pattern in Ler (A), van3 (B), van7 (C) and van3van7 (D) mutants. Scale bars: 1 mm.
Table 1. Frequency of fused cotyledons in van3, van7, and van3van7 mutant seedlings.
| Genotype | Frequency of cotyledon numbers | Total number of seedlings | |
|---|---|---|---|
| one/fused | two | ||
| Ler | 0 | 287 | 287 |
| van3 | 0 | 91 | 91 |
| van7 | 58 | 24 | 82 |
| van3van7 | 7 | 30 | 37 |
Table 2. Root phenotypes of two weeks old van3, van7, and van3van7 mutant seedlings.
| Genotype | frequency | total number | |
|---|---|---|---|
| Root meristem collapse | Ler | 0 (0%) | 87 |
| van3 | 45 (55.5%) | 81 | |
| van7 | 14 (15.7%) | 89 | |
| van3van7 | 7 (28%) | 25 | |
| Lateral root formation | Ler | 51 (100%) | 51 |
| van3 | 32 (74%) | 43 | |
| van7 | 0 (0%) | 68 | |
| van3van7 | 9 (75%) | 12 |
In summary, we identified that the VAN3 regulators CVP2 and VAB preferentially localize at the PM in stably transformed Arabidopsis lines. We also determined that VAN3 localization at the PM during vein formation is sufficient to complement the van3 mutant phenotype, which strongly suggests that VAN3 action at the PM has a critical role in continuous vein formation. These observations strongly suggest that ARF GTPase machinery functions at the PM to regulate auxin-mediated plant growth and development. Our results suggest that VAN3 and GNOM act together at the PM to control auxin-mediated plant growth and development. It may be conjectured that VAN3 and GNOM act on a common ARF substrate at the PM to properly localize PIN proteins (Naramoto 2017); however, the endogenous substrates of VAN3 and GNOM remain to be identified. Future work to identify ARF substrates for VAN3 and GNOM will further extend our knowledge of how ARF GTPase machinery at the PM regulates auxin-mediated plant growth.
Acknowledgements
This work was supported by Tomizawa Jun-ichi & Keiko Fund of Molecular Biology Society of Japan for Young Scientist, Toyota Physical and Chemical Research Institute, and the Japanese Society for Promotion of Science to SN (JSPS; 30612022).
References
- Aihara K, Naramoto S, Hara M, Mizoguchi T (2014) Increase in vascular pattern complexity caused by mutations in LHY and CCA1 in Arabidopsis thaliana under continuous light. Plant Biotechnol 31: 43–47 [Google Scholar]
- Anders N, Jurgens G (2008) Large ARF guanine nucleotide exchange factors in membrane trafficking. Cell Mol Life Sci 65: 3433–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland F, Nelson T (2009) CVP2- and CVL1-mediated phosphoinositide signaling as a regulator of the ARF GAP SFC/VAN3 in establishment of foliar vein patterns. Plant J 59: 895–907 [DOI] [PubMed] [Google Scholar]
- Carland FM, Nelson T (2004) Cotyledon vascular pattern2-mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs. Plant Cell 16: 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Chavrier P (2006) ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7: 347–358 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Richter S, Vieten A, Marquardt S, Torres-Ruiz RA, Mayer U, Jurgens G (2004) Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131: 389–400 [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Munro S (2007) The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol 23: 579–611 [DOI] [PubMed] [Google Scholar]
- Hou H, Erickson J, Meservy J, Schultz EA (2010) FORKED1 encodes a PH domain protein that is required for PIN1 localization in developing leaf veins. Plant J 63: 960–973 [DOI] [PubMed] [Google Scholar]
- Jackson TR, Brown FD, Nie Z, Miura K, Foroni L, Sun J, Hsu VW, Donaldson JG, Randazzo PA (2000) ACAPs are Arf6 GTPase-activating proteins that function in the cell periphery. J Cell Biol 151: 627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Sugiyama M, Fukuda H (2000) A series of novel mutants of Arabidopsis thaliana that are defective in the formation of continuous vascular network: Calling the auxin signal flow canalization hypothesis into question. Development 127: 3197–3204 [DOI] [PubMed] [Google Scholar]
- Koizumi K, Naramoto S, Sawa S, Yahara N, Ueda T, Nakano A, Sugiyama M, Fukuda H (2005) VAN3 ARF-GAP-mediated vesicle transport is involved in leaf vascular network formation. Development 132: 1699–1711 [DOI] [PubMed] [Google Scholar]
- Liu B, Butenko MA, Shi CL, Bolivar JL, Winge P, Stenvik GE, Vie AK, Leslie ME, Brembu T, Kristiansen W, et al. (2013) NEVERSHED and INFLORESCENCE DEFICIENT IN ABSCISSION are differentially required for cell expansion and cell separation during floral organ abscission in Arabidopsis thaliana. J Exp Bot 64: 5345–5357 [DOI] [PubMed] [Google Scholar]
- Naramoto S (2017) Polar transport in plants mediated by membrane transporters: Focus on mechanisms of polar auxin transport. Curr Opin Plant Biol 40: 8–14 [DOI] [PubMed] [Google Scholar]
- Naramoto S, Dainobu T, Tokunaga H, Kyozuka J, Fukuda H (2016) Cellular and developmental function of ACAP type ARF-GAP proteins are diverged in plant cells. Plant Biotechnol 33: 309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramoto S, Kleine-Vehn J, Robert S, Fujimoto M, Dainobu T, Paciorek T, Ueda T, Nakano A, Van Montagu M, Fukuda H, et al. (2010) ADP-ribosylation factor machinery mediates endocytosis in plant cells. Proc Natl Acad Sci USA 107: 21890–21895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramoto S, Otegui MS, Kutsuna N, de Rycke R, Dainobu T, Karampelias M, Fujimoto M, Feraru E, Miki D, Fukuda H, et al. (2014) Insights into the localization and function of the membrane trafficking regulator GNOM ARF-GEF at the Golgi apparatus in Arabidopsis. Plant Cell 26: 3062–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramoto S, Sawa S, Koizumi K, Uemura T, Ueda T, Friml J, Nakano A, Fukuda H (2009) Phosphoinositide-dependent regulation of VAN3 ARF-GAP localization and activity essential for vascular tissue continuity in plants. Development 136: 1529–1538 [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Millus IG, Vallis Y, Butler PJG, Evans PR, McMahon HT (2004) BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science 303: 495–499 [DOI] [PubMed] [Google Scholar]
- Prabhakaran Mariyamma N, Hou H, Carland FM, Nelson T, Schultz EA (2017) Localization of Arabidopsis FORKED1 to a RABA-positive compartment suggests a role in secretion. J Exp Bot 68: 3375–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Anders N, Wolters H, Beckmann H, Thomann A, Heinrich R, Schrader J, Singh MK, Geldner N, Mayer U, et al. (2010) Role of the GNOM gene in Arabidopsis apical-basal patterning: From mutant phenotype to cellular mechanism of protein action. Eur J Cell Biol 89: 138–144 [DOI] [PubMed] [Google Scholar]
- Richter S, Geldner N, Schrader J, Wolters H, Stierhof YD, Rios G, Koncz C, Robinson DG, Jurgens G (2007) Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature 448: 488–492 [DOI] [PubMed] [Google Scholar]
- Richter S, Muller LM, Stierhof YD, Mayer U, Takada N, Kost B, Vieten A, Geldner N, Koncz C, Jurgens G (2011) Polarized cell growth in Arabidopsis requires endosomal recycling mediated by GBF1-related ARF exchange factors. Nat Cell Biol 14: 80–86 [DOI] [PubMed] [Google Scholar]
- Sieburth LE, Muday GK, King EJ, Benton G, Kim S, Metcalf KE, Meyers L, Seamen E, Van Norman JM (2006) SCARFACE encodes an ARF-GAP that is required for normal auxin efflux and vein patterning in Arabidopsis. Plant Cell 18: 1396–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh OK, Moore I (2007) An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature 448: 493–496 [DOI] [PubMed] [Google Scholar]
- Vernoud V, Horton AC, Yang Z, Nielsen E (2003) Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol 131: 1191–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo CM, Naramoto S, Sparks JA, Khan BR, Nakashima J, Fukuda H, Blancaflor EB (2017) Deletion analysis of AGD1 reveals domains crucial for plasma membrane recruitment and function in root hair polarity. J Cell Sci 131: jcs203828. [DOI] [PubMed] [Google Scholar]
- Yoo CM, Wen J, Motes CM, Sparks JA, Blancaflor EB (2008) A class I ADP-ribosylation factor GTPase-activating protein is critical for maintaining directional root hair growth in Arabidopsis. Plant Physiol 147: 1659–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]