Abstract
Sorghum is a recalcitrant crop for Agrobacterium-mediated genetic transformation. Several parameters related to Agrobacterium-mediated transformation were tested to optimize sorghum transformation frequencies. In this study, we evaluated pretreatment of sorghum variety Tx430 immature embryos using Agrobacterium strain GV2260. Pretreatment of immature embryos with heat (43°C) treatment for 15 or 21 min, and centrifugation resulted in a transformation efficiency of up to 1.9% of immature embryos treated. Although further optimization to enhance transformation efficiency is required, this study contributes to the genetic validation of genes of interest and molecular breeding in sorghum plants.
Keywords: centrifugation, cold pretreatment, genetic transformation, heat pretreatment, Sorghum bicolor
Sorghum is one of the most important cereal crops cultivated mainly in Asian and African countries. It adapts well to arid areas that are not suitable for growing other major cereals. Furthermore, sorghum has the capacity to produce high amounts of biomass, and is therefore also used as feedstuff for livestock. Sorghum has recently received a lot of attention as a source of renewable biofuel energy in many countries including Japan. Because sorghum is the first C4 crop with a full genome sequence available (Paterson et al. 2009), genetic transformation of sorghum has the potential to improve biomass yield and digestibility toward biofuel conversion.
Transgenic sorghum plants were first generated using a particle bombardment method (Casas et al. 1993), with a transformation efficiency of 0.286%. Another study reported successful Agrobacterium-mediated transformation with a transformation efficiency of 2.1% (Zhao et al. 2000). To date, multiple studies on optimizing Agrobacterium-mediated transformation methods have been carried out, focusing on factors such as Agrobacterium-strain selection, medium composition, and pretreatment of immature embryos (Carvalho et al. 2004; Gao et al. 2005; Gurel et al. 2009; Howe et al. 2006; Kumar et al. 2011; Wu et al. 2014). The highest transformation efficiency reached was 33% (Wu et al. 2014). Although several groups have reported protocols for the genetic transformation of sorghum, the production of transgenic sorghum is not yet reproducible, suggesting a need for optimization. In this study, we evaluated pretreatment of sorghum variety Tx430 immature embryos and found that the combination of heat treatment at 43°C for 15 or 21 min and centrifugation is effective for genetic transformation.
Seeds of the inbred line Tx430 were obtained from the genebank at the National Agriculture and Food Research Organization (Ibaraki, Japan). Immature embryos were isolated from sterilized immature seeds approximately 2 weeks after flowering. The immature embryos were inoculated with Agrobacterium tumefaciens GV2206 harboring binary vector pEKH2, which contains a hygromycin phosphotransferase (hpt) gene under the control of the 35S cauliflower mosaic virus promoter, an intron-containing β-glucuronidase (GUS) gene under the control of the maize ubiquitin promoter, and a neomycin phosphotransferase II gene (nptII) under the control of the nopaline synthase promoter in the T-DNA region. The A. tumefaciens strain GV2260 was grown for 20 h at 28°C in LB liquid medium containing 50 mg/l kanamycin and hygromycin. The bacterial solution was diluted to a final density of OD600 0.7 with an inoculation liquid medium containing 200 µM acetosyringone. The basic compositions of the culture media used in this study were as described by Wu et al. (2014). The immature embryos were placed on a co-cultivation medium containing 200 µM acetosyringone with the scutellar side facing upward, and incubated in the dark for 4–7 days at 23–25°C. The immature embryos were transferred to a callus induction medium containing 1 g/l polyvinylpyrrolidone and 25 mg/l meropenem (Supplemental Table) and cultured for about 1 week. Next, the embryos were transferred to a selection medium containing 1 g/l polyvinylpyrrolidone, 25 mg/l meropenem, and 15 mg/l hygromycin (Supplemental Table) and cultured for approximately 1.5 months. The obtained surviving calli were transferred onto a regeneration medium containing 25 mg/l meropenem and 5 mg/l hygromycin (Supplemental Table) and incubated in the dark. When the generated shoots had reached 2–3 cm in size, they were incubated for 16 h in the light and 8 h in the dark. The surviving shoots were subcultured on a rooting medium containing 25 mg/l meropenem and 10 mg/l hygromycin (Supplemental Table) for about 1 month.
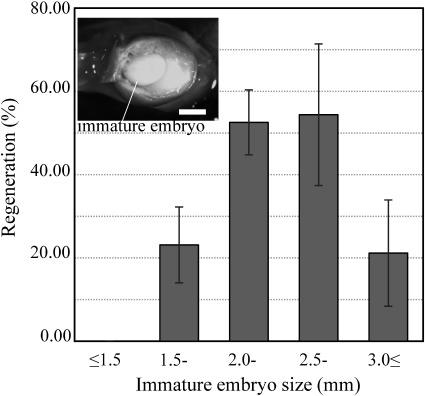
To determine the appropriate stage for explanting, regeneration efficiency was evaluated —among five size classes of immature embryos. The immature embryos were inoculated with A. tumefaciens strain GV2260 and cultured on callus induction and regeneration media without hygromycin. The regeneration ratio largely depended on the size of the immature embryos (Figure 1). The regeneration ratios of small-sized (less than 2.0 mm) and large-sized (more than 3.0 mm) immature embryos were low (0–23.1%). However, the intermediate-sized (2.0–3.0 mm) immature embryos at the milk stage of endosperm development showed a high regeneration ratio (52.6–54.4%). Thus, we used immature embryos of this size in subsequent experiments.
Figure 1. Effect of size of immature embryos on regeneration after Agrobacterium inoculation. Intermediate size (2–3 mm) of immature embryos showed the highest regeneration ratio. Inset shows an intermediate-sized immature embryo at the milk stage of endosperm development. Bar=2 mm.
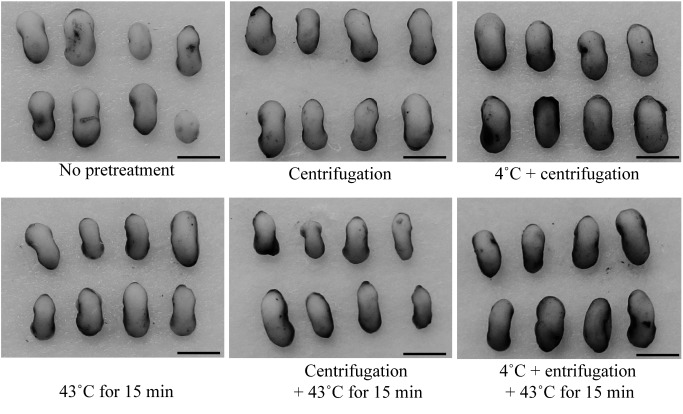
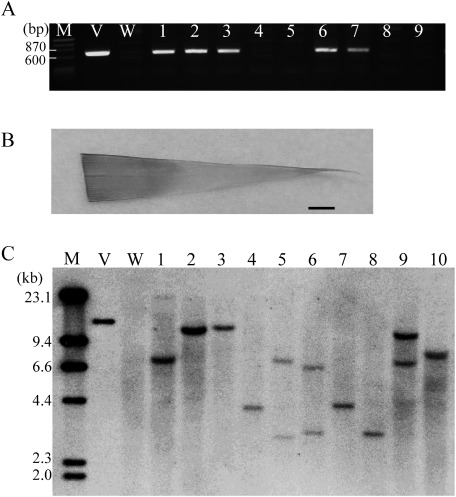
First, we performed genetic transformation following a previously reported method (Wu et al. 2014) using a standard binary vector and Agrobacterium strain GV2260. Although 198 immature embryos were inoculated, no transgenic plants were obtained (Table 1, without any pretreatments). Histochemical detection of GUS expression (Jefferson et al. 1987) in immature embryos showed that GUS staining was faint without any pretreatment (Figure 2). To enhance gene transformation efficiency, pretreatment conditions of immature embryos were compared. First, pretreatment combining centrifugation (20,000×g for 10 min) with heat (43°C) was tested (Table 1). Both centrifugation and heat treatments showed slightly increased GUS staining in immature embryos (Figure 2). Without centrifugation, no transgenic plants were obtained, regardless of the heat treatment duration. However, immature embryos pretreated with heat (43°C) for 15 or 21 min and centrifugation regenerated and rooted on medium containing hygromycin (Figures 3A and B). Because many escaped (non-transgenic) plants were observed among the regenerated plants, polymerase chain reaction (PCR) analysis was carried out to confirm the presence of the hpt gene. PCR was performed using KOD FX Neo polymerase (Toyobo, Osaka, Japan). DNA was extracted using an alkaline lysis method. Leaves about 0.5–1 cm in length were lightly homogenized using a 1,000-µl disposable tip in alkaline solution (80 mM Tris-HCl [pH 8.0], 80 mM NaOH). The solution was then centrifuged at 20,000 g for 1 min, and 1–2 µl of supernatant was used as a template. PCR amplification was carried out using the following thermal cycles: 35 cycles at 94°C for 2 min, at 56°C for 30 s, and at 68°C for 1 min. The PCR primers used for amplifying a 650-bp fragment inside the hpt gene were 5′-AGA TCG TTA TGT TTA TCG GCA CTT T-3′ and 5′-CAA GCT CTG ATA GAG TTG GTC AAG A-3′. The PCR-based selection successfully distinguished between transgenic from non-transgenic plants (Figure 4A). The average transformation efficiency of centrifugation and heat treatment for 15 min and 21 min was 1.4% and 1.3%, respectively (Table 1). We found that almost all the plants that regenerated early in culture were escaped plants. Therefore it may be advisable to cultivate the calli on regeneration medium for longer than 2 months. The obtained PCR-positive transgenic plants were grown in soil (Figure 3C).
Table 1. Transformation efficiency of independent experiments with pretreatment consisting of centrifugation, heat and 4°C.
| Replicate | hpt+ events/embryos inoculated (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | ||||||||||
| Cen* | − | − | − | + | + | + | + | + | + | |
| Heat† | 0 min | 15 min | 21 min | 0 min | 15 min | 21 min | 0 min | 15 min | 21 min | |
| 4°C | − | − | − | − | − | − | + | + | + | |
| 1 | 0/38 (0.0) | 0/56 (0.0) | 0/20 (0.0) | 0/61 (0.0) | 1/32 (3.1) | 0/51 (0.0) | 1/72 (1.4) | 0/33 (0.0) | 3/68 (4.4) | |
| 2 | 0/50 (0.0) | 0/50 (0.0) | 0/34 (0.0) | 0/21 (0.0) | 0/64 (0.0) | 0/53 (0.0) | 0/37 (0.0) | 3/48 (6.3) | 0/48 (0.0) | |
| 3 | 0/65 (0.0) | 0/35 (0.0) | 0/62 (0.0) | 0/28 (0.0) | 1/56 (1.8) | 1/20 (5.0) | 0/48 (0.0) | 0/38 (0.0) | 0/44 (0.0) | |
| 4 | 0/45 (0.0) | 0/9 (0.0) | 0/87 (0.0) | 0/32 (0.0) | 0/41 (0.0) | 0/40 (0.0) | 0/50 (0.0) | |||
| 5 | 0/49 (0.0) | 1/50 (2.0) | 0/40 (0.0) | 0/35 (0.0) | 2/40 (5.0) | |||||
| 6 | 0/107 (0.0) | |||||||||
| 7 | 0/50 (0.0) | |||||||||
*Cen=Centrifugation pretreatment. † Heat=43°C pretreatment
Figure 2. Effect of pretreatment (cold [4°C] for 12–16 h, centrifugation, and heat [43°C] for 15 min) on transient β-glucuronidase (GUS) expression in immature embryos 3 days after Agrobacterium inoculation. Each pretreatment enhanced transient GUS expression. Bars=2 mm.
Figure 3. Regeneration of hygromycin-resistant sorghum plants. A: Putative transgenic shoots on regeneration medium at 3 weeks (bar=2 cm). B: Putative transgenic plants on rooting medium at 2 weeks (bar=2 cm). C: Transgenic plants grown in pots in the plant incubator (bar=10 cm).
Figure 4. Detection of transgene in putative transgenic T0 plants. A: Polymerase chain reaction (PCR) analysis of hygromycin-resistant plants. Lanes 1–9: Nine regenerated hygromycin-resistant plants obtained from pretreatment of centrifugation and heating for 15 min (lanes 1–2); centrifugation and heating for 21 min (lanes 3–5); and centrifugation, heating for 15 min and 4°C (lanes 6–9). Lane M: Molecular size marker; Lane V: binary vector pEKH2 (positive control); Lane W: wild-type sorghum; Lanes 1–3, 6, and 7: T0 plants showing positive results of transgenic plants; Lanes 4, 5, 8, and 9: T0 plants showing negative results of non-transgenic plants. B: Histochemical GUS assay of leaf from PCR-positive transgenic sorghum (bar=2 mm). C: Southern blot hybridization of 10 PCR-positive transgenic T0 lines with hpt probe. Lane M: Molecular size marker; Lane V: binary vector pEKH2 (positive control); Lane W: untransformed plants (negative control); Lanes 1–10: transgenic plant obtained from pretreatment of centrifugation and heating for 15 min (lanes 1 and 2); centrifugation and heating for 21 min (lane 3); centrifugation and 4°C (lane 4), centrifugation, heating for 15 min and 4°C (lanes 5–7), and centrifugation, heating for 21 min and 4°C (lanes 8–10).
Next, we evaluated the effect of cold treatment before centrifugation and heat treatment (Table 1). Although cold-treated immature embryos showed strong GUS staining (Figure 2), the transformation efficiency with 15 or 21-min heat treatment was 1.3% and 1.9%, respectively, which was almost same as those without cold (4°C) treatment (Table 1). Our results suggest that the combination of centrifugation and heat treatment of immature embryos may facilitate gene transformation. In rice and maize, combined heat and centrifugation pretreatment enhanced stable transformation (Hiei et al. 2006). In wheat, centrifugation treatment was a critical step before infection, but heat treatment was not effective (Hiei et al. 2014; Ishida et al. 2014). In sorghum, it has been reported that heat (43°C) treatment for 3 min increased transformation efficiency, whereas centrifugation decreased efficiency; however, a combination of heat and centrifugation enhanced explant survival and callus formation frequencies (Gurel et al. 2009). In another study, cold pretreatment at 4°C cold for 1 day improved explant survival and callus formation (Nguyen et al. 2007). Though the effects of pretreatment vary depending on species and genotype, an optimal combination of pretreatments would allow production of transgenic sorghum in other genotypes.
To confirm the expression of the GUS gene in the hygromycin-resistant plants, histochemical GUS staining (Jefferson et al. 1987) was performed. The leaf tissue of PCR-positive hygromycin-resistant plants showed intense staining (Figure 4B). Next, Southern hybridization analysis was carried out for the hpt gene to confirm the presence of transgenes in the genome of hygromycin-resistant plants. Genomic DNA was isolated from fresh leaves of PCR-positive plants using the cetyltrimethylammonium bromide (CTAB) method described by Murray and Thompson (1980). DNA (15 µg) was digested with HindIII, separated by electrophoresis in a 0.8% agarose gel, and transferred to a nylon membrane (Hybond-XL, GE Healthcare, Buckinghamshire, UK) using an alkaline method. The 650-bp hpt probe was labelled with digoxigenin (DIG) using the PCR DIG Probe synthesis kit (Roche Diagnostics, Mannheim, Germany). Hybridization, washing, and detection were carried out according to the instruction manual of the DIG labeling and detection system (Roche Diagnostics). Chemiluminescent signal was visualized using LAS4000mini (Fuji Film, Tokyo, Japan). All PCR-positive hygromycin-resistant plants showed hybridization signals with the integration of one or two copies of T-DNA in the transgenic plant genome (Figure 4C). To confirm that the transgene was transmitted to the next generation, four T0 plants representing independent events were self-pollinated and their T1 seedlings were analyzed for the hpt gene using PCR. These T1 progenies harboring one or two copies of T-DNA showed the expected Mendelian segregation ratio (Table 2).
Table 2. Integration and segregation pattern of the hpt gene in T1 progeny of transgenic sorghum.
| Event No. | Copy No.* | HPT gene | Segregation ratio (Chi-square value, p) | |
|---|---|---|---|---|
| Positive | Negative | |||
| 1 | 1 | 15 | 2 | 3 : 1 (1.59, 0.21) |
| 2 | 1 | 13 | 5 | 3 : 1 (0.07, 0.79) |
| 9 | 2 | 19 | 1 | 15 : 1 (0.05, 0.82) |
| 10 | 1 | 16 | 2 | 3 : 1 (1.85, 0.17) |
*Copy number was estimated using Southern blot analysis.
In conclusion, we established a protocol for reproducible genetic transformation of Sorghum bicolor Tx430. In this protocol, pretreatment of immature embryos with cold (4°C) for 12–16 h, heat (43°C) for 15–21 min, and centrifugation was effective in generating stable transgenic plants. We also succeeded in generating transgenic sorghum expressing Arabidopsis thaliana BOR4, which could confer high boron tolerance to the plants (unpublished result). Future studies should focus on further optimization of transformation efficiency and application to other sorghum lines. Such studies may contribute to the genetic validation of genes of interest, as well as to methods for molecular breeding of sorghum as a renewable biofuel energy source, and as feedstuff for livestock.
Acknowledgements
We thank Dr. Mii and Dr. Nakamura for providing the binary vector pEKH2, and Dr. Fujiwara for providing the binary vector expressing the Arabidopsis BOR4 gene. This work was supported by the Network of Centers of Carbon Dioxide Resource Studies in Plants (NC-CARP) of the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT); the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15K18631; and the Advanced Low Carbon Technology Research and Development Program (ALCA) of the Japan Science and Technology Agency (JST).
Abbreviations
- CTAB
cetyltrimethylammonium bromide
- GUS
β-glucuronidase
- PCR
polymerase chain reaction
Supplementary Data
References
- Carvalho CHS, Zehr UB, Gunaratna N, Anderson J, Kononowicz HH, Hodges TK, Axtell JD (2004) Agrobacterium-mediated transformation of sorghum: Factors that affect transformation efficiency. Genet Mol Biol 27: 259–269 [Google Scholar]
- Casas AM, Kononowicz AK, Zehr UB, Tomes DT, Axtell JD, Butler LG, Bressan RA, Hasegawa PM (1993) Transgenic sorghum plants via microprojectile bombardment. Proc Natl Acad Sci USA 90: 11212–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Xie X, Ling Y, Muthukrishnan S, Liang GH (2005) Agrobacterium tumefaciens-mediated sorghum transformation using a mannose selection system. Plant Biotechnol J 3: 591–599 [DOI] [PubMed] [Google Scholar]
- Gurel S, Gurel E, Kaur R, Wong J, Meng L, Tan HQ, Lemaux PG (2009) Efficient, reproducible Agrobacterium-mediated transformation of sorghum using heat treatment of immature embryos. Plant Cell Rep 28: 429–444 [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ishida Y, Kasaoka K, Komari T (2006) Improved frequency of transformation in rice and maize by treatment of immature embryos with centrifugation and heat prior to infection with Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 87: 233–243 [Google Scholar]
- Hiei Y, Ishida Y, Komari T (2014) Progress of cereal transformation technology mediated by Agrobacterium tumefaciens. Front Plant Sci 5: 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe A, Sato S, Dweikat I, Fromm M, Clemente T (2006) Rapid and reproducible Agrobacterium-mediated transformation of sorghum. Plant Cell Rep 25: 784–791 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Tsunashima M, Hiei Y, Komari T (2014) Wheat (Triticum aestivum L.) transformation using immature embryos. In: Wang K (ed) Agrobacterium Protocols. Vol 1. Springer, New York, pp 189–198 [DOI] [PubMed]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Campbell LM, Rathore KS (2011) Rapid recovery- and characterization of transformants following Agrobacterium-mediated T-DNA transfer to sorghum. Plant Cell Tissue Organ Cult 104: 137–146 [Google Scholar]
- Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8: 4321–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Thu TT, Claeys M, Angenon G (2007) Agrobacterium-mediated transformation of sorghum (Sorghum bicolor (L.) Moench) using an improved in vitro regeneration system. Plant Cell Tissue Organ Cult 91: 155–164 [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556 [DOI] [PubMed] [Google Scholar]
- Wu E, Lenderts B, Glassman K, Berezowska-Kaniewska M, Christensen H, Asmus T, Zhen S, Chu U, Cho MJ, Zhao ZY (2014) Optimized Agrobacterium-mediated sorghum transformation protocol and molecular data of transgenic sorghum plants. In Vitro Cell Dev Biol Plant 50: 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZY, Cai T, Tagliani L, Miller M, Wang N, Pang H, Rudert M, Schroeder S, Hondred D, Seltzer J, et al. (2000) Agrobacterium-mediated sorghum transformation. Plant Mol Biol 44: 789–798 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Figure 2. Effect of pretreatment (cold [4°C] for 12–16 h, centrifugation, and heat [43°C] for 15 min) on transient β-glucuronidase (GUS) expression in immature embryos 3 days after Agrobacterium inoculation. Each pretreatment enhanced transient GUS expression. Bars=2 mm](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6194/6879392/22183f4cdfc1/plantbiotechnology-35-2-18.0424a-figure02.jpg)