Abstract
[Purpose] The aim of this study was to evaluate the effect of pulsed high intensity neodymium-doped yttrium aluminum garnet (Nd: YAG) laser on staphylococcus aureus (S. aureus) and pseudomonas aeruginosa (P. aeruginosa) bacterial growth, which cause many health problems and establish which doses are effective in bacterial inhibition. [Materials and Methods] Five samples of S. aureus and five samples of P. aeruginosa were prepared in the microbiology lab, one used as control sample and the other 4 samples acted as experimental samples. The experimental samples received pulsed high intensity Nd: YAG laser with a total dose of 500, 600, 700 and 800 joules. The primary measures are colony count and the percentage decrease in colony count, the colony count was assessed at baseline and after 24 h of laser application. [Result] There was significant decrease in colony count and the percentage decrease in colony count after pulsed high intensity Nd: YAG laser application in all experimental samples of S. aureus and P. aeruginosa after 24 h of application for all doses (500, 600, 700 and 800 j) as compared with the control sample, with the most effect in higher doses of pulsed high intensity Nd: YAG laser than lower doses in both types of bacteria. [Conclusion] pulsed high intensity Nd: YAG laser was found to be an effective modality for inhibition of S. aureus and P. aeruginosa growth after a single application.
Keywords: Pulsed high intensity neodymium-doped yttrium aluminum garnet (Nd YAG) laser, Colony count, Bacterial growth
INTRODUCTION
Laser is a physical therapy modality that has the characteristics of being noninvasive, painless, very safe and easily applied for a variety of pathological conditions1,2,3). It has a significant analgesic effect in both acute and chronic cases such as carpal tunnel syndrome, chronic osteoarthritis, shoulder pain, and post-operative pain2,3,4).
Many studies have proven the effectiveness of low-intensity laser therapy (LILT) in the treatment of musculoskeletal disorders, soft tissue injuries and wound healing, which may be colonised by bacterial species5,6,7); also there is pre-clinical literature evidence that LILT has an inhibitory effect on bacterial growth through monochromaticity and a photobiomodulator effect which assess inactivation of proliferation of human and animal cells in vitro, which is important for healing8,9,10).
Monochromaticity is one of the main basic mechanisms of laser, which alters cellular and tissue function based on the characteristic of the light itself (e.g., wavelength, coherence)10). Which allows the efficient coupling to the peak absorption of chromophores, enabling maximal photoactivation and stimulation of biological processes11).
The pulsed neodymium-doped yttrium aluminum grant (ND: YAG) laser, is a type of high-intensity laser therapy which involves radiation, with high peak power (3 KW), and a wavelength of 1,064 nm 2). This causes minor and slow light absorption by chromophores; the absorption is obtained not with concentrated light, but with diffuse light in all directions (scattering phenomenon), increasing the mitochondrial oxidative reaction and adenosine triphosphate (ATP), ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) production (photochemistry effects), resulting in the phenomenon of tissue stimulation (photobiology effect)10).
Wounds are most commonly colonised by the most commonly found bacteria connected with wounds and skin infections such as S. aureus and P. aeruginosa, due to its ability to modify the integrity of the skin barrier12,13,14). They are the major cause of hospitalised infections in most health centres15, 16).
Staphylococcus aureus is found in the skin and nasal passages of healthy people and taking charge of many chronic and deteriorating infections like osteomyelitis, endocarditis, and the infections of the cystic fibrosis (CF) of the lung; also many penetrating trauma and burn infections, venous leg ulcers, pressure ulcers, and diabetic foot ulcers12,13,14,15,16,17).
Pseudomonas aeruginosa which is involved in respiratory infections, urinary tract infections, gastrointestinal infections, keratitis, otitis media, and bacteremia in patients with compromised host defences (e.g. due to a burn, HIV, cystic fibrosis (CF), and cancer which represent a significant role in morbidity and mortality16). Due to the development of antimicrobials-resistant variants of S. aureus and P. aeruginosa, it is increasingly difficult to treat, due to intrinsic antibiotic resistance that including decreased permeability, expression of efflux systems, production of antibiotic-inactivating enzymes and target modification. So healing by using new ways to eliminate the infection began to be studied in Europe in the 1970s, where positive results were gained with laser irradiation on cultured cells and in animals18, 19).
Pulsed high intensity Nd: YAG laser has been used in many fields of physical therapy with excellent results20), but until now, there was little research explaining its effect on bacterial growth, and the efficient dose for killing bacteria is not yet quantified. Therefore, our study was done to evaluate the effect of pulsed high intensity Nd: YAG laser on bacterial growth, especially on S. aureus and P. aeruginosa, and to clarify which dose is more effective.
MATERIALS AND METHODS
To evaluate the effect of laser on selected cultures of S. aureus and P. aeruginosa, a quantitative culture technique indicating the number of colony-forming units (CFU/ml) was used. All tested and control samples were subjected to the same procedure of counting, and the counting was done by two persons twice and the mean was calculated for all samples.
The tested organisms were S. aureus and P. aeruginosa, both of which are aerobes. The bacterial strains were obtained from the Department of Medical Microbiology lab at the College of Medicine, Umm Al-Qura University. The organisms were cultured on nutrient agar plates (NAP) to get isolated single pure colonies, and then diluted in nutrient broth tubes (NB). The broth tubes were used to dilute the concentration of bacteria until reaching a suitable dilution, which later yields a countable number of bacteria when sub-cultured on agar plates. The dilution tubes were sub-cultured on NAP to count colony-forming units CFU.
The number of living bacterial cells in a given volume or area for a given sample or suspension is called a viable cell count. It is applicable to single-celled organisms such as bacteria, spores, yeasts, and certain protozoa. A colony-counting procedure was used for determining viable cell counts by counting the number of colonies that develop on a solid medium that has been inoculated with the sample or bacterial suspension. This may involve the technique of dilution plating. It is assumed that each colony that develops on the medium arises from a single cell in the original sample. Counts may then be expressed as colony-forming units (CFU) for a given volume or area of the sample, where a CFU is any entity that can give rise to a single colony. As a first step in the microbiology lab, we chose suitable dilution of bacteria suspension, which was in a NB tube. Ten microlitres of suspension were inoculated in the centre of solid growth medium NAP, using a sterile cotton swab. Then the plate was incubated for 24 h at 35–37°C. The next day, the colonies were counted to determine the amount of bacterial cell in test suspension tubes, the tubes were then kept at 4°C21).
The second step was the preparation for laser exposure. At the microbiology lab we took the chosen suspension tubes, one of which was S. aureus and the other was P. aeruginosa. After that, twelve new, clear and sterile NAPs were brought out from the fridge. Six plates were inoculated with 10 µL S. aureus suspension, in the centre of the plate without spreading. Two plates were labelled as control, which will not be exposed to any treatment or radiations. The remaining four inoculated plates were to be exposed to radiation. The same steps applied on the other six plates which were inoculated with 10 µL of the P. aeruginosa suspension tube.
The plates were brought back to the microbiology lab after being irradiated by pulsed high intensity Nd: YAG laser then with a sterilised cotton swab, we spread the primary inoculum to cover all the surface of the agar in order to facilitate the colonies count. Eventually, all plates were incubated at 37°C for 24 h.
After a 24 h incubation, the colonies were counted to determine the number of CFU. This is done manually using a colony counter instrument which is sensitive to pen pressure on plates and colonies were counted automatically on a digital screen.
All prepared samples of S. aureus and P. aeruginosa received a pulsed high intensity Nd: YAG laser produced by a HIRO 3 device (ASA Laser, Arcugnano, Italy). The apparatus provided pulsed emission (1,064 nm), very high peak power (3 kW), a high level of fluency/energy density (510–1,780 mJ/cm), brief duration (120–150 μs), a low frequency (10–40 Hz), a duty cycle of about 0.1%, a probe diameter of 0.5 cm, and a spot size of 0.2 cm 2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22).
The handpiece was positioned perpendicular to all samples, with the same manner of pulsed high intensity Nd: YAG laser application and the same position of agar plates. Slow manual scanning was performed transversely and longitudinally in the colony area to cover all areas of the colony. A total energy dose of 500, 600, 700 and 800 J was administered through the final phase of an application according to the machine manual. In the final phase, the laser fluency was set to three successive sub-phases of 610, 710, and 810 mJ/cm2 and the application time for all doses was measured approximately. The pulsed high intensity Nd: YAG laser device calculated the energy applied during each dose and the total energy delivered to the colony during the application session. Pulsed high intensity Nd: YAG laser was applied for one session for all doses and for all experimental samples and the application was done in accord with safety measures and an isolated environment.
The primary outcome measures were performed at baseline and after 24 h of laser application. The measured outcomes were colony count, and the % ↓in colony count was calculated for all samples using the following equation:
Statistical data analysis used SPSS for Windows (IBM, Inc.) version 22. The differences between control and experimental samples were analysed using an unpaired t-test. One-way analysis of variance (ANOVA) was used to analyse the data to detect the overall differences between the means of all laser doses (Bonferroni multiple comparison test). Means and standard deviations were reported, and the alpha level of significance was 0.05.
RESULTS
For this study, 4 samples of Staphylococcus aureus and Pseudomonas aeruginosa were included. The colony count was significantly decreased after pulsed high intensity Nd: YAG laser application for all samples of Staphylococcus aureus and Pseudomonas aeruginosa, as compared with the control samples after 24 h of application. No significant difference in colony count was observed between all samples of Staphylococcus aureus and Pseudomonas aeruginosa at baseline (p>0.001). The colony count after the application was significantly decreased in all samples as compared to the colony count at baseline.
There was a significant decrease in colony count in all experimental samples of S. aureus after 24 h of pulsed high intensity Nd: YAG laser application for all doses 500, 600,700, and 800 J (i.e. 135 ± 0.60, 92 ± 0.50,80 ± 0.90 and 62 ± 0.95, respectively) as compared with the control samples (i.e., 275.25 ± 0.75, 274.50 ± 0.577, 273.75 ± 0.957 and 275.25 ± 0.500, respectively). The % ↓ in colony count after the application was 50.90%, 66.42%, 70.69%, 77.45% for 500 J, 600 J, 700 J and 800 J respectively, with more effect at higher doses of pulsed high intensity Nd: YAG laser than lower doses. The differences between the colony count for control and experimental samples after pulsed high intensity Nd: YAG laser application and the % ↓ in colony count are shown in Table 1 and Fig. 1.
Table 1. The mean values of colony count for control and experimental samples and the % ↓ in colony count for Staphylococcus aureus.
| Time of HILT application |
Control (CFU/mL) |
Experimental (CFU/mL) |
p value | % ↓ in colony count |
|
|---|---|---|---|---|---|
| 500J | 3:21 min | 275.25 ± 0.75 | 135.60 ± 0.80 | <0.0001* | 50.90% |
| 600J | 4:01 min | 274.50 ± 0.577 | 92.50 ± 0.75 | <0.0001* | 66.42% |
| 700J | 4:42 min | 273.75 ± 0.957 | 80 ± 0.90 | <0.0001* | 70.69% |
| 800J | 5:22 min | 275.25 ± 0.500 | 62 ± 0.95 | <0.0001* | 77.45% |
CFU/ml: colony-forming units; *Significant.
Fig. 1.

Difference in colony count of Staphylococcus aureus for control and experimental samples.
There was a significant decrease in colony count in all experimental samples of P. aeruginosa after 24 h of pulsed high intensity Nd: YAG laser application for all doses 500, 600, 700 and 800 J (i.e. 550 ± 0.55, 520 ± 0.80, 360 ± 0.90 and 300 ± 0.95, respectively) as compared with the control samples (i.e. 1,274.80 ± 0.90, 1,275.75 ± 0.50, 1,274.50 ± 0.89 and 1,276.00 ± 0.70, respectively), with the most effect at higher doses than lower doses. The % ↓ in colony count after the application was 56.82%, 59.21%, 71.76%, 76.45% for 500 J, 600 J, 700 J, and 800 J respectively. The differences between the colony count for the control and experimental samples after pulsed high intensity Nd: YAG laser and the % ↓ in colony count are shown in Table 2 and Fig. 2.
Table 2. The mean values of colony count for control and experimental samples and the % ↓ in colony count for pseudomonas aeruginosa.
| Time of HILT application |
Control (CFU/mL) |
Experimental (CFU/mL) |
p value | % ↓ in colony count |
|
|---|---|---|---|---|---|
| 500J | 3:21 min | 1,274.80 ± 0.90 | 550 ± 0.55 | <0.0001* | 56.82% |
| 600J | 4:01 min | 1,275.75 ± 0.50 | 520 ± 0.80 | <0.0001* | 59.21% |
| 700J | 4:42 min | 1,274.50 ± 0.89 | 360 ± 0.90 | <0.0001* | 71.76% |
| 800J | 5:22 min | 1,276.00 ± 0.70 | 300 ± 0.95 | <0.0001* | 76.45% |
CFU/ml: colony-forming units; *Significant.
Fig. 2.
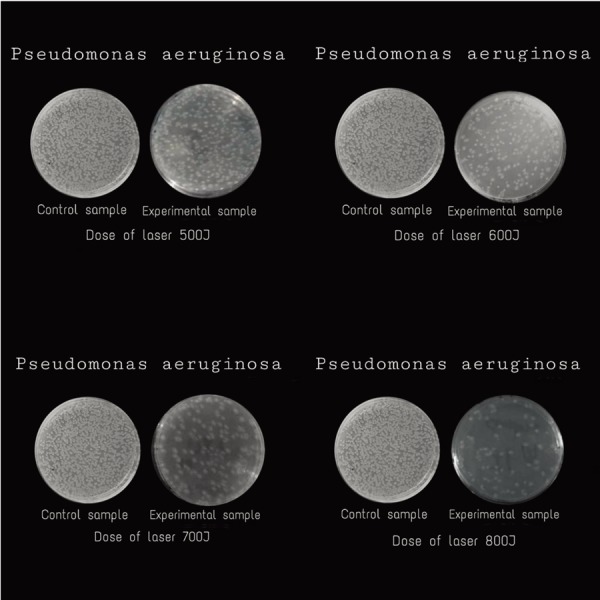
Difference in colony count of Pseudomonas aeruginosa for control and experimental sample.
There were significant differences between all doses of laser in both types of bacteria (p<0.001) with an extremely significant difference observed in higher doses.
DISCUSSION
The primary finding of this study is that pulsed high-intensity Nd: YAG laser therapy decreases the colony count of both types of bacteria and the benefit was clear after 24 h of laser application. The best effect of the laser was observed in the higher doses more than in the lower doses and our results were consistent with previous studies8, 23,24,25).
The development of laser medicine has provided a number of new therapy modalities capable of destructing pathogenic organisms and the influence of light on inhibition of bacterial growth. Some preliminary studies indicate that pulsed high-intensity Nd: YAG laser is more potent than low-level laser therapy (LLLT), because of its higher intensity and the greater depth reached by the laser. Pulsed high-intensity Nd: YAG laser works using a specific wavelength (1,046 nm) and high peak power (3 kW) with ordinary peaks of elevated amplitude values and low duty cycle26, 27).
The effect of antibiotic therapy on bacteria usually takes from 10 to 14 days or more, being considered to take a shorter time where there are adequate sources of infection control18). In contrast, laser therapy has gained credibility and followers throughout the world due to its significant effect in the reduction of the pathogenic bacteria8, 23,24,25).Over short periods of time, it would be unlikely to develop resistance in the target bacteria and damage to adjacent host tissues can be avoided by laser selectivity in removing the bacterial plaque and necrotic tissue without eliminating essential tissue28).
Laser irradiation is able to compromise the physiological function of bacterial cells, by the suppression of the DNA metabolism and the cell division, degenerative change cytomorphology up to cell pyknosis. The degree of destruction is dose dependent, ranging from decreased cell growth to the loss of metabolic activity and physical structural damage29). Increasing pulse energy, pulse rate, or an increased time of irradiation created an extended diameter of the pyknotic cell zone30).
Laser application leads to contracting or shrinking of the bacterial cell and deoxyribonucleic acid (DNA) which altered the gene expression that inhibits the bacterial growth and activity31).
The bacterial destruction and inhibition can be achieved in a different process by laser therapy where the irradiated photosensitiser produces the induction of reactive oxygen species (ROS) which have a high killing potential for bacteria32), by causing the acceleration of electron transfer in some areas of the respiratory chain. At higher doses, this excitation energy is transferred to oxygen to form singlet oxygen with resultant cyto-toxic effect at the cell membrane level in bacteria because their respiratory chain resides there. However, the respiratory chain within the mitochondrial membrane would still be affected, and free radical and singlet oxygen production would cause the death of bacteria33).
Photo-thermal effect acting on bacteria suggests that the laser light is absorbed by the bacteria leading to direct damage to the bacterial cell34). Due to the sensitivity of chromophores inside bacteria to laser light35, 36), the bacteria cannot defend against the extreme photodynamic of light, the dramatic rise in local tissue temperature and electromagnetic poisoning31) creating thermal resonance and causing protein denaturisation, tissue shrinkage, tissue disintegration, vaporisation, cutting, ablation, etc37). Furthermore, local heating inside the bacteria or light-induced modulation of enzymatic activity could be responsible for bacterial killing35, 36), and the absorption of pulsed high intensity Nd: YAG laser wavelength by the pigments inside the bacteria lead to vaporisation of water and cell lysis, and this is the basis of the selective bactericidal effect of pulsed high-intensity Nd: YAG laser38).
In terms of other processes acting on bacteria, the laser light is strongly absorbed in the substrate onto which the bacteria adhere, which causes a local rise in temperature high enough to result in the death of the attached organisms34).
Published studies showed that the inhibitory effect of the laser depends on wavelength, power, and dose. Using higher doses would have a greater destructive effect8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23). Exposure time to the laser represents a radiant energy dose; the longer the time of irradiation, the higher the dose. The dose of laser 800 j (5.22 minutes) was more effective in both the S. aureus and P. aeruginosa count than in all doses of the laser. Also, the dose of laser 700 j (4.42 minutes) clearly produced a decrease in cell count in both types of bacteria by more than 600 j (4.01minutes) and 500 j (3.21 minutes). Therefore, the time of exposure and the dose of laser were two factors determining the effect of laser on cell count and maybe more time and higher doses were needed for a better result33). Additional exposure might also have caused further heating and more photo-thermal effect of laser on bacteria.
Further research is needed to clarify the most effective dose and time. There were some limitations to our study: the study was conducted on small sample size, with a single laser application, so a more isolated suitable environment for application should be considered, using electronic counting rather than manual counting; and another alteration using higher doses than lower doses in both types of bacteria. In spite of having those limitations, we believe that this study served as a good report for the immediate effect of pulsed high-intensity Nd: YAG laser on bacterial inhibition and count. Finally, our results demonstrate that a higher dose of laser is more effective than lower doses which was important in the treatment of an infected wound.
Pulsed high-intensity Nd: YAG laser was found to be an effective modality for inhibiting the growth of S. aureus and P. aeruginosa after a single application. This study can be applied on rats to determine clinical suitability.
Conflict of interest
All authors declare no conflicts of interest.
Funding
This research received no specific grant from any funding agency, commercial enterprise, or not-for-profit institute.
REFERENCES
- 1.Ebid AA, Ibrahim AR, Omar MT, et al. : Long-term effects of pulsed high-intensity laser therapy in the treatment of post-burn pruritus: a double-blind, placebo-controlled, randomized study. Lasers Med Sci, 2017, 32: 693–701. [DOI] [PubMed] [Google Scholar]
- 2.Alayat MS, Atya AM, Ali MM, et al. : Long-term effect of high-intensity laser therapy in the treatment of patients with chronic low back pain: a randomized blinded placebo-controlled trial. Lasers Med Sci, 2014, 29: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 3.Dundar U, Turkmen U, Toktas H, et al. : Effect of high-intensity laser therapy in the management of myofascial pain syndrome of the trapezius: a double-blind, placebo-controlled study. Lasers Med Sci, 2015, 30: 325–332. [DOI] [PubMed] [Google Scholar]
- 4.Ebid AA, El-Sodany AM: Long-term effect of pulsed high-intensity laser therapy in the treatment of post-mastectomy pain syndrome: a double blind, placebo-control, randomized study. Lasers Med Sci, 2015, 30: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 5.Chung W, Petrofsky J, Laymon M, et al. : The effects of low level laser radiation on bacterial growth. Phys Ther Rehabil Sci, 2014, 3: 20–26. [Google Scholar]
- 6.Gamaleya NF: Laser applications in medicine and biology. Laser biomedical research. Kiev: Springer, 1977, pp 1–173. [Google Scholar]
- 7.de Sousa NT, Gomes RC, Santos MF, et al. : Red and infrared laser therapy inhibits in vitro growth of major bacterial species that commonly colonize skin ulcers. Lasers Med Sci, 2016, 31: 549–556. [DOI] [PubMed] [Google Scholar]
- 8.Peplow PV, Chung TY, Baxter GD: Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg, 2010, 28: S3–S40. [DOI] [PubMed] [Google Scholar]
- 9.Nussbaum EL, Mazzulli T, Pritzker KP, et al. : Effects of low intensity laser irradiation during healing of skin lesions in the rat. Lasers Surg Med, 2009, 41: 372–381. [DOI] [PubMed] [Google Scholar]
- 10.Santamato A, Solfrizzi V, Panza F, et al. : Short-term effects of high-intensity laser therapy versus ultrasound therapy in the treatment of people with subacromial impingement syndrome: a randomized clinical trial. Phys Ther, 2009, 89: 643–652. [DOI] [PubMed] [Google Scholar]
- 11.Conlan MJ, Rapley JW, Cobb CM: Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol, 1996, 23: 492–496. [DOI] [PubMed] [Google Scholar]
- 12.Wietzikoski Lovato EC, Gurgel Velasquez PA, Dos Santos Oliveira C, et al. : High frequency equipment promotes antibacterial effects dependent on intensity and exposure time. Clin Cosmet Investig Dermatol, 2018, 11: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brook I: Anaerobic and aerobic bacteriology of decubitus ulcers in children. Am Surg, 1980, 46: 624–626. [PubMed] [Google Scholar]
- 14.Kontiainen S, Rinne E: Bacteria isolated from skin and soft tissue lesions. Eur J Clin Microbiol, 1987, 6: 420–422. [DOI] [PubMed] [Google Scholar]
- 15.Khan HA, Ahmad A, Mehboob R: Nosocomial infections and their control strategies. Asian Pac J Trop Biomed, 2015, 5: 509–514. [Google Scholar]
- 16.Morita Y, Tomida J, Kawamura Y: Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol, 2014, 4: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conlon BP: Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells: an investigation of persister cells, their formation and their role in S. aureus disease. BioEssays, 2014, 36: 991–996. [DOI] [PubMed] [Google Scholar]
- 18.Bassetti M, Vena A, Croxatto A, et al. : How to manage Pseudomonas aeruginosa infections. Drugs Context, 2018, 7: 212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jawhara S, Mordon S: Monitoring of bactericidal action of laser by in vivo imaging of bioluminescent E. coli in a cutaneous wound infection. Lasers Med Sci, 2006, 21: 153–159. [DOI] [PubMed] [Google Scholar]
- 20.Parra PF, Ghinassi S, Ciuti F: The neodymium-YAG laser in defocused for its evolution increasingly effective treatment of the injured athlete. Laser Technol, 1992, 2: 13–16. [Google Scholar]
- 21.Brugger SD, Baumberger C, Jost M, et al. : Automated counting of bacterial colony forming units on agar plates. PLoS One, 2012, 7: e33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zati A, Valent A: Physical therapy: new technologies in rehabilitation medicine (translated to English). Edizioni Minerva Medica, 2006, 2006: 162–185. [Google Scholar]
- 23.Dadras S, Mohajerani E, Eftekhar F, et al. : Different photoresponses of Staphylococcus aureus and Pseudomonas aeruginosa to 514, 532, and 633 nm low level lasers in vitro. Curr Microbiol, 2006, 53: 282–286. [DOI] [PubMed] [Google Scholar]
- 24.Risović D, Maver-Bišćanin M, Mravak-Stipetić M, et al. : Quantitative investigation of efficiency of ultraviolet and visible light in eradication of Candida albicans in vitro. Photomed Laser Surg, 2014, 32: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maver-Biscanin M, Mravak-Stipetic M, Jerolimov V: Effect of low-level laser therapy on Candida albicans growth in patients with denture stomatitis. Photomed Laser Surg, 2005, 23: 328–332. [DOI] [PubMed] [Google Scholar]
- 26.Ahuja RB, Gupta GK: A four arm, double blind, randomized and placebo controlled study of pregabalin in the management of post-burn pruritus. Burns, 2013, 39: 24–29. [DOI] [PubMed] [Google Scholar]
- 27.Zati A, Fortuna D, Benedetti E, et al. : HILT Therapy in the treatment of knee osteoarthritis: the first clinical cases and protocol for a multicenter double-blind randomized trial. Proceedings 1st National Conference Dominate Energy, Scientific Report, 2006.
- 28.Wilson M, Pratten J: Sensitization of Staphylococcus aureus to killing by low-power laser light. J Antimicrob Chemother, 1994, 33: 619–624. [DOI] [PubMed] [Google Scholar]
- 29.Yuan X, Song Y, Song Y, et al. : Effect of laser irradiation on cell function and its implications in Raman spectroscopy. Appl Environ Microbiol, 2018, 84: e02508–e02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutknecht N, Kanehl S, Moritz A, et al. : Effects of Nd:YAG-laser irradiation on monolayer cell cultures. Lasers Surg Med, 1998, 22: 30–36. [DOI] [PubMed] [Google Scholar]
- 31.Cabrera JE, Cagliero C, Quan S, et al. : Active transcription of rRNA operons condenses the nucleoid in Escherichia coli: examining the effect of transcription on nucleoid structure in the absence of transertion. J Bacteriol, 2009, 191: 4180–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seyedmousavi S, Hashemi SJ, Rezaie S, et al. : Effects of low-level laser irradiation on the pathogenicity of Candida albicans: in vitro and in vivo study. Photomed Laser Surg, 2014, 32: 322–329. [DOI] [PubMed] [Google Scholar]
- 33.DeSimone NA, Christiansen C, Dore D: Bactericidal effect of 0.95-mW helium-neon and 5-mW indium-gallium-aluminum-phosphate laser irradiation at exposure times of 30, 60, and 120 seconds on photosensitized Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Phys Ther, 1999, 79: 839–846. [PubMed] [Google Scholar]
- 34.Schoop U, Kluger W, Moritz A, et al. : Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg Med, 2004, 35: 111–116. [DOI] [PubMed] [Google Scholar]
- 35.Hellingwerf KJ, Hoff WD, Crielaard W: Photobiology of microorganisms: how photosensors catch a photon to initialize signalling. Mol Microbiol, 1996, 21: 683–693. [DOI] [PubMed] [Google Scholar]
- 36.Esteban B, Carrascal M, Abian J, et al. : Light-induced conformational changes of cyanobacterial phytochrome Cph1 probed by limited proteolysis and autophosphorylation. Biochemistry, 2005, 44: 450–461. [DOI] [PubMed] [Google Scholar]
- 37.Cortes M, ed.: The elimination of bacteria and biofilms in periodontal disease via the thermal laser. International Congress Series. New York, 2003. [Google Scholar]
- 38.Gokhale S, Padhye A, Sumanth S: Bactericidal effect of Nd: YAG laser in an in vitro tissue model—a light microscopic evaluation. J Oral Laser Appl, 2010, 10: 17. [Google Scholar]


