Abstract
Neuroinflammation, as defined by the activation of microglia and astrocytes, has emerged in the last years as a key element of the pathogenesis of neurodegenerative diseases based on genetic findings and preclinical and human studies. This has raised the need for new methodologies to assess and follow glial activation in patients, prompting the development of PET ligands for molecular imaging of glial cells and novel structural MRI and DTI tools leading to a multimodal approach. The present review describes the recent advancements in microglia and astrocyte biology in the context of health, ageing, and Alzheimer’s disease, the most common dementia worldwide. The review further delves in molecular imaging discussing the challenges associated with past and present targets, including conflicting findings, and finally, presenting novel methodologies currently explored to improve our in vivo knowledge of the neuroinflammatory patterns in Alzheimer’s disease. With glial cell activation as a potential therapeutic target in neurodegenerative diseases, the translational research between cell biologists, chemists, physicists, radiologists, and neurologists should be strengthened.
Electronic supplementary material
The online version of this article (10.1007/s00259-019-04462-w) contains supplementary material, which is available to authorized users.
Keywords: TSPO, Microglia, Astrocyte, Alzheimer’s disease, PET, MRI
Introduction
There is increasing evidence that inflammation plays a key role in the development and progression of neurodegenerative diseases such as Alzheimer’s disease (AD), likely being involved in its etiology and as a disease-promoting factor [1, 2]. Indeed, accumulating experimental, genetic, and epidemiological data implicate the innate immune system in AD, most prominently involving genes for immune receptors or immune-related functions such as APOE (apolipoprotein E), CR1 (complement receptor 1), B1N1 (bridging integrator 1), CD33, TREM2 (triggering receptor expressed on myeloid cells 2), and PICALM (phosphatidylinositol binding clathrin assembly protein). These new developments have raised the interest in investigating glial cells towards understanding the origin of AD and to design novel disease-modifying therapies.
Neuropathologically, AD is defined by the aggregation and deposition of amyloid-β (Aβ) plaques in the parenchyma and hyperphosphorylated tau in neurons, and therefore, AD has been mostly conceptualised as a proteinopathy. The majority of clinical trials have aimed at clearance of these protein forms, however, with very limited success clinically [3]. While longitudinal neuroimaging studies using PET (positron emission tomography) tracers for Aβ and tau suggest that Aβ precedes tau pathology [4], the mechanistic link between these two pathological features is not well known. Interestingly, new preclinical data suggest that neuroinflammation might be a possible link between Aβ and tau proteinopathies. In particular, Aβ aggregation may trigger activation of microglia and astrocytes, the cells of the brain immune system, leading to the release of neuroinflammatory markers that might be contributing to tau pathology and spreading [5, 6]. These studies have shown that microglia activation can lead to astrocyte activation and vice-versa, but the regional and temporal patterns of astrocytosis and microgliosis in relation to other pathophysiological changes are not well known. These recent findings stimulate further research on neuroimaging of microglia and astrocytes using PET tracers in combination with other imaging modalities and fluid biomarkers.
Microglia in the healthy brain
Microglia migrate from the yolk sac [7] and colonise the brain early during embryogenesis to form the resident immune cells of the brain. Their maintenance and expansion in the central nervous system (CNS) relies exclusively on their capacity of self-renewal without any further colonisation [8–10]. The population remains stable over the lifetime with a slow turnover [10], of an approximate lifespan of 4 years in humans [11], and they are the main identified proliferating cells in the human brain from the age of 3 years old [12]. Microglia are defined by a unique molecular signature, known as their homeostatic signature, which is driven by the expression of the transforming growth factor (TGF)-β1 cytokine, differentiating them from the other brain cells and myeloid populations [13]. This signature encompasses expression of a specific set of genes reported in Table 1.
Table 1.
Microglial markers identified from rodent models and human studies
| Phenotype | Mouse (gene, protein) | Human (gene, protein) | Morphology [142] |
|---|---|---|---|
| Homeostatic, physiological status [13–16, 143] | Tgfbr1, Smad3, C1qa, C1qb, Cst3, Csf1r, Ctsd, Ctss, Cx3cr1, Entpd1 Fcrls, Hexb, Olfml3, P2y12, Tmem119, Tmsb4x, Sparc |
TMEM119 P2Y12 CX3CR1 Fclrs, C1qa, Pros1, Mertk, Gas6 Iba1 |
Ramified with fine processes |
| Ageing [17, 18] |
MHC class II Complement CR3 (CD11b) CD68, CD11c TLR members CD86 |
FcγRs (CD64, CD32, CD16b) MHC class II (HLA subtypes, HLA-DR) Complement (C1qA, C1qB, C1qC, C1s, C3, C3AR1, C4, C5, C5AR1), C1QBP, CFH, CFHR1, clusterin TLR members (, TLR2, TLR4, TLR5, MYD88) Calprotectin (S10A8, S100A9), CD14 IRAK3, SOCS3 Inflammasome (Casp1, TXNIP, PANX1, PANX2) Chemokines (CCR1, CXCL5, CXCL16) CD68, CD11b |
Ramified Less ramified with shorter and thicker processes |
| Alzheimer’s disease [14–17, 19] |
MGnD profile Apoe,Trem2,Tyrobp,Axl,Clec7a, Ccl2,Csf1,Itgax,Lilrb4, Lgal3, Gpnmb, Fabp5, Spp1 DAM profile Apoe,Trem2,Tyrobp,Axl, B2m, Fth1, Lyz2, Ctsb, Ctsd, Ctsl, Cst7,Csf1, Ccl6, Lpl, Cd9,Itgax,Clec7a,Lilrb4, Timp2 |
FcγRs (CD64, CD32b, CD16b) MHC II (HLA-DR, DP, DQ) Complement (C4, C3AR1, C5AR1) S100A8 CSFR1 Chemokines (CXCL16, CCR2) TLR (TLR2, TLR4, TLR5, TLR7) CD68, CHI3L1, IL4R, MSR-A |
Ramified Less ramified with shorter and thicker processes Ameboid shape (round shape with no processes) |
Underlined, common genes identified in both mouse microglial profiles
Apoe, Apolipoprotein; Axl, Tyrosine-protein kinase receptor; B2m, beta-2 microglobulin; C1qA, Complement C1q subcomponent A; C1QBP, C1q binding protein; C3AR1, Complement component 3a receptor 1; C5AR1, Complement component 5a receptor 1; Casp1, caspase 1; Clec7, C-type lectin domain family 7; CCR, Chemokine (C-C motif) receptor type; CHI3L1, Chintinase-3 Like-1; CXCL, Chemokine (C-X-C motif) ligand; Cx3cr1, CX3C chemokine receptor 1 (fractalkine receptor); CFH, Complement Factor H; CFHR1, Complement Factor H Related 1; CR3, Complement receptor 3; Cst3, Cystatin; Ctsd, Cathepsin D; Ctsl, Cathepsin L; Ctss, Cathespin S; Csf1r, Colony-stimulating factor 1 receptor; Entpd1, Ectonucleoside triphosphate diphosphohydrolase 1; Fabp5, Fatty acid binding protein 5; FcγR, Fcγ receptor; Fclrs, Fc receptor-like S; Fth1, Ferritin heavy chain 1; GAS6, growth arrest-specific 6; Gpnmb, Glycoprotein Nmb; GPR34, G protein-coupled receptor 34; HLA, Human leucocyte antigen; Hexb, Beta-hexosaminidase subunit beta; Iba1, Ionised calcium-binding adaptor molecule 1; IRAK3, Interleukin 1 receptor associated kinase 3; Itgax, Integrin alpha X; IRAK3, Interleukin-1 receptor associated kinase 3; Lilrb4, Leukocyte Immunoglobulin Like Receptor B4; Lgal3, Galectin-3; Lpl, lipoprotein lipase; Lyz2, Lysozyme 2; MERTK, C-mer proto-oncogene tyrosine kinase; MHC, Major histocompatibility complex; MSR, Macrophage scavenger receptor; MYD88, myeloid differentiation primary response 88; PAX, Pannexin; Olfml3, Olfactomedin Like 3; P2y12, Purinergic receptor P2Y12; Pros1, Protein S; S100A8, S100 calcium-binding protein A8; Smad3, SMAD family member 3; SOCS3, Suppressor of cytokine signalling 3; Sparc, Secreted protein acidic and cysteine rich; Spp1, Secreted phosphoprotein 1; Timp2, Metallopeptidase inhibitor 2; Tgfbr1, Transforming Growth Factor Beta Receptor 1; TLR, Toll-like receptor; TMEM, Transmembrane protein; Tmsb4x, Thymosin Beta 4 X-Linked; Tyrobp, TYRO Protein Tyrosine Kinase Binding Protein; Trem2, triggering receptor expressed on myeloid cells 2; TXNIP, Thioredoxin-interacting protein
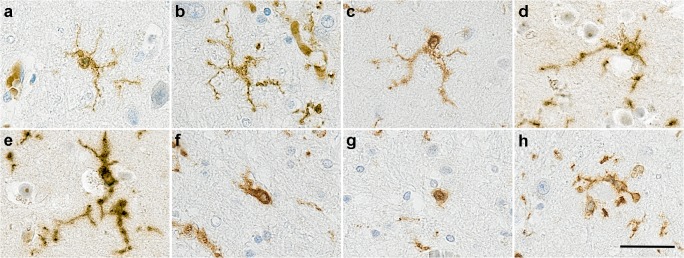
In humans, microglia represent 0.5 to 16% of the total brain cells [20], with significantly more cells in the white than in grey matter. They have a ramified morphology having multiple fine processes (Fig. 1a-d), and time-lapse imaging experiments in mice have demonstrated that microglia are extremely motile cells, screening constantly the parenchyma via their processes [21]. During brain development, microglia participate in the wiring and maturation of the brain [22] by pruning excessive synapses [23] and influencing neurogenesis [24]. In adult, microglia survey and sense any changes in order to respond rapidly to any insult or injury [21], and participate in synaptic communication [25, 26]. The properties of sensing and interacting with their micro-environment are performed via microglia-specific transcripts termed the microglial sensome [27] with several of the sensome genes identified later as components of the homeostatic signature such as P2Y12 [13, 27]. It is assumed that similar functions are performed by microglia in humans.
Fig. 1.
Illustration of different microglial morphologies in human brain identified with Iba1. (a-g) From ramified to ameboid microglia; (h) cluster of microglia around amyloid plaques as observed only in Alzheimer’s disease. Counterstaining Haematoxylin, scale bar = 30 μm
Ageing per se predisposes to peripheral inflammation, a concept known as “inflammaging” [28], and this concept is supported by the presence of altered mRNA expression and proteins of inflammation-related genes in the middle-aged human and mouse brain [29] (Table 1). In aged mice, the expression of the sensome genes is diminished [27]. Interestingly, the major changes of the expression profiles of immune- and inflammation-related genes occur during the course of cognitively normal ageing rather than disease conditions and involve upregulation of genes coding for inflammasome signalling, Fcγ receptors, and HLA [17]. Morphological microglial changes characterised by reduced branching and arborised area have been described in humans [30] (Fig. 1e-g) suggesting that microglia become dysfunctional or senescent with ageing. These molecular and morphological changes imply that with age, microglia lose their neuroprotective properties associated with chronic neurodegeneration [31]. A recent study investigating the phenotype of aged human microglia highlighted that microglial ageing manifests as loss of function as well as gain of function changes to give a unique aged-related microglia phenotype [32]. This profile was enriched in susceptibility genes for AD but interestingly, independent from APOE4, the main risk factor for AD.
A number of concepts related to the phenotypes that microglia can acquire have emerged from experimental models. Microglial priming is defined as a prolonged and exaggerated immune challenge resulting from an acute inflammatory event in an ongoing inflammatory environment [33]; whereas innate immune memory is associated to cell reprogramming following a primary immune stimulus that leads to increased (trained) or decreased (tolerant) responses to a secondary inflammatory stimulus [34]. Primed or reprogrammed microglia have an enhanced response to a second stimulus. Therefore, an integrated nomenclature under the term of microglial memory was recently proposed to encompass both phenotypes [34] (Table 2). These concepts are important and highly relevant to the lifetime of infections in humans and to the microglial lifespan with some of cells reported to be more than 20 years old [11], and thus capable of remembering the inflammatory events [19, 35]. Indeed, even in the absence of brain injury, insult, or infection, it is now acknowledged that systemic immunity will affect the brain immunity [18]. However, the relevance of the rodent experiments to humans is still unclear. To summarise, microglia are adaptive cells with the phenotype and morphology determined by their local environment (Table 1).
Table 2.
Definitions of the different microglial phenotypes
| Status | Definition |
|---|---|
| Physiological/homeostatic | Microglia in an immune stimulus-free environment. |
| Primed microglia (one immune stimulus) | Prolonged and exaggerated increased immune response due to microglia already activated by an initial event. |
| Trained microglia (repetitive immune stimuli) | Increased microglial responses following priming. |
| Immune memory | Long-term consequences of trained microglia inducing cell reprogramming. This leads to either increased (primed) or decreased (tolerant) immune responses. |
Microglia in Alzheimer’s disease
Genome-wide association studies have highlighted variation in genes of the innate immunity as risk factors for AD, emphasising the role of microglia, not only in responding to the neurodegeneration, but also in the onset and progression of the disease. Two microglial phenotypes have been determined from mouse AD models. The Disease-Associated Microglia (DAM) is characterised by low expression of homeostatic markers (e.g. CX3CR1, P2y12, Tgfb1) and elevated levels of lipid metabolism, phagocytosis, apoptosis, and AD-associated genes (e.g. APOE, Trem2) [15]. Similarly, the Microglial Neurodegenerative Phenotype (MGnD) is driven by a rise in APOE and apoptotic markers (e.g. Axl, Clec7a), and the fading of the microglial homeostatic signature [16] (Table 1). Both profiles are consistent with microglia responding to and participating in the ongoing neurodegeneration, and thus are found in any mouse models of neurodegenerative diseases characterised by neuronal loss [16]. Therefore, it is unclear to what extent microglia promote or respond to neurodegeneration, likely both, and we still do not have insight into how microglia participate in the onset of the disease.
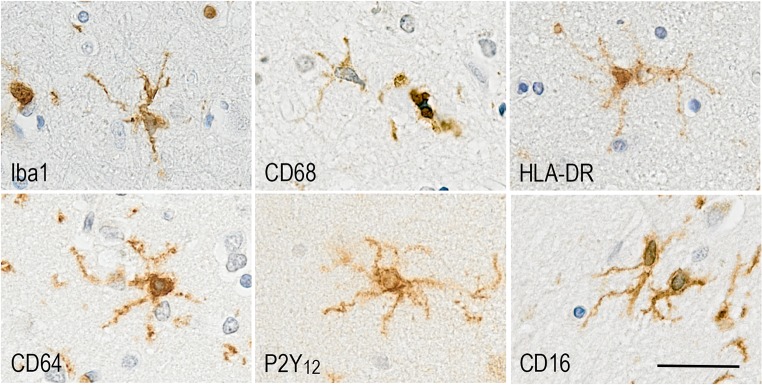
In humans, microglial activation is a neuropathological feature of the disease and microglia participate to the formation of the neuritic plaques, clustering around Aβ deposits (Fig. 1h). Using a mathematical model, activated microglia have been placed after Aβ plaques but before tau pathology [6]. However, longitudinal studies of PET imaging using microglia, Aβ, and tau ligands are necessary to confirm in vivo the dynamics of these events. In an unbiased elderly population, immunophenotyping of microglia using markers associated with specific microglial functions (Fig. 2) has revealed that in AD, microglia appear to lose motility-associated proteins (e.g. Iba1), a key function in synaptic support [36] and change towards a more phagocytic phenotype (CD68, macrophage scavenging receptor (MSR)-A), partly driven by the APOE genotype [14]. This study highlighted that microglia were responding differently to Aβ and tau in participants with or without dementia and were able to adopt different functions relatively independently, emphasising the likely coexistence of different microglial populations within the same brain. Interestingly, the brain environment reveals inflammatory heterogeneity, with a mixture of pro- and anti-inflammatory compounds, observed post-mortem in late stages of the disease [19, 37]. However, in the early stages of AD, a more polarised inflammatory environment towards either pro- or anti-inflammatory profile was reported to be associated with different pathological severity [37], possibly reflecting different stages of the disease or different microglial populations.
Fig. 2.
Microglial proteins expressed in human brain and associated with different functions: Iba1 (Motility); CD68 (phagocytosis); HLA-DR (antigen presentation); CD64 (FcγRI high-affinity activating receptor reflecting presence of immunoglobulins); P2Y12 (homeostasis); CD16 (FcγRIII low-affinity activating receptor for immune complexes). Counterstaining Haematoxylin, scale bar = 30 μm
The immune reactions are clearly complex in AD, with evidence of temporal changes of microglia. However, these studies lack information on the specific functions performed by microglia throughout the course of the disease. Microglia activation undergoes various stages, which have been traditionally classified into pro-inflammatory/detrimental (M1) or anti-inflammatory/protective (M2). However, there is increasing evidence that the M1/M2 division is somewhat artificial and even considered obsolete. Instead, microglia assume a continuum of activation states characterised by the expression of multitude of markers that overlap between M1 and M2 states [38], continuum which can evolve, potentially driving, the disease [37]. This complexity of microglial activation represents one of the main challenges to identify targets suitable for molecular imaging.
Molecular imaging targets for microglia in Alzheimer’s disease and mild cognitive impairment (MCI) patients
Molecular imaging studies in AD have largely focused on visualising activated microglia, most commonly measured by elevated expression of translocator protein 18 kDa (TSPO), a five transmembrane domain protein mainly located in the outer mitochondrial membrane of microglia [39]. While mostly considered as a specific marker of microglial activation, TSPO has been found to be overexpressed in activated astrocytes [40, 41]. One of the limitations associated with the use of TSPO is its inability to distinguish between the different phenotypes expressed by microglia, potentially lacking disease-specificity. These limitations have led to the search for alternative biological targets for imaging microglia. Some of the emerging tracers target P2X7 receptors, cannabinoid receptor type 2 (CB2), cyclooxygenase (COX)-2, colony-stimulating factor 1 receptor (CSF1R), and reactive oxygen species (reviewed in [39, 42], Fig. 3). Although progress has been made, most of the emerging tracers target only the pro-inflammatory (M1) phenotype of activated microglia, and several tracers have issues of limited specificity as they bind also to other cells such as astrocytes and endothelial cells. Among the most promising novel tracers are those that target purinergic receptors including P2X7, selective towards the M1 phenotype and overexpressed in AD brain, and P2Y12, selective towards the M2 phenotype and with reduced expression around Aβ plaques in AD brain [42]. Of note, P2Y12 PET tracers are yet the only ones identified to target the M2 anti-inflammatory phenotype, which has been associated with physiological/homeostatic microglia.
Fig. 3.
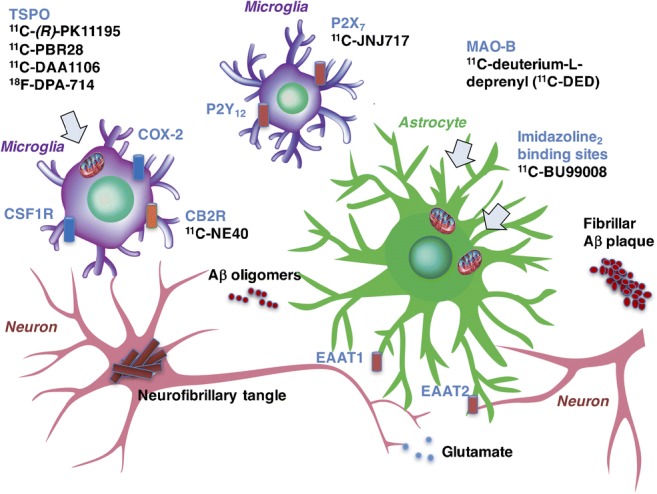
Cartoon illustrating emerging targets and PET tracers for the selective in vivo visualisation of activated microglia and astrocytes in Alzheimer’s disease
Below, we will review the human imaging studies aiming to characterise in vivo microglial activation in MCI and AD dementia patients, both with TSPO and non-TSPO tracers.
PET studies using TSPO tracers
When reviewing the work done on using PET tracers for TSPO, the literature shows divergent and sometimes conflicting results. This is partially due to different binding properties of the various PET tracers but also due to methodological issues when quantifying the PET signal.
The 11C-labelled (R)-isomer of PK11195 is the best-characterised PET ligand for imaging activated microglia in vivo. In a pilot PET study in eight AD patients (median age 63.5 years, median MMSE 19.5) vs. 15 control subjects, increased 11C-(R)-PK11195 binding potential (BP) was measured in the inferior and middle temporal gyri, fusiform gyri, left parahippocampal gyrus, amygdala, and posterior cingulate [43]. A subsequent study examined 11C-(R)-PK11195 and 11C-Pittsburgh compound B (11C-PiB, a ligand for Aβ) BP in 13 AD patients (mean age 65.6 years, mean MMSE 21.2) using both region-of-interest (ROI) and voxel-based analysis. Significant increases in 11C-(R)-PK11195 BP were found in frontal, temporal, parietal, and occipital association cortices, cingulate cortex, and striatum, in a pattern that corresponded with areas of increased 11C-PiB binding [44], connecting microglial activation to Aβ plaque deposition. Interestingly, 11C-(R)-PK11195 binding in the posterior cingulate, frontal, and parietal cortices, but not 11C-PiB uptake ratio, was negatively correlated with MMSE [44]. This implied that microglia rather than Aβ deposition was the culprit for the cognitive decline, before the confirmation by the genetics of a role of the innate immunity in AD pathogenesis [45]. These findings were replicated by others [46]. Another study using the alternative TSPO tracer 11C-DAA1106 [47] also detected increased signal in AD patients in all regions investigated but did not assess the link with the cognitive decline.
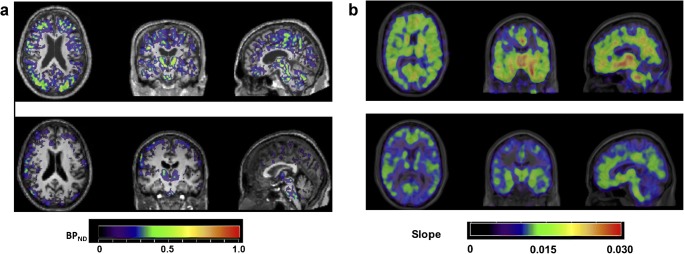
Assessment of microglial activation has also been of particular interest in patients with MCI to aim at exploring the kinetic and temporal association of the different neuropathological features. In our own group study of 12 MCI compared with 7 healthy volunteers, ROI analysis revealed increased 11C-(R)-PK11195 binding in the posterior cingulate gyrus, parietal, occipital, frontal, and temporal lobes as well as in the putamen (Fig. 4a) (A. Gerhard, unpublished results). Interestingly, in this series, the MCI patients that were either homo- or heterozygote for the ApoE ε4 allele seemed to show higher binding than the ones with an ApoE ε3/3. Our study links two risk factors for AD, immunity, and APOE genotype. Study of 11C-(R)-PK11195 and 11C-PiB PET in 14 patients with amnestic MCI (mean MMSE 27.7), 22 with AD (mean MMSE 21.5), and 10 controls (mean MMSE 29.9) identified increased 11C-(R)-PK11195 BP in frontal cortex of PiB-positive MCI patients [48], but also observed MCI patients with either increased PK11195 or PiB signal. The authors propose microglial activation and Aβ deposition as two independent processes in AD. This might be the case; however, we can argue that the discrepancy, especially in the PiB-negative MCI patients, might be the consequence of different processes with some leading to another dementia than AD. Nevertheless, a recent study confirmed the spatial association between the 11C-(R)-PK11195 binding and cortical Aβ load as measured with 11C-PiB in 42 MCI patients, with an overlapped distribution [49].
Fig. 4.
a Parametric 11C-(R)-PK11195 PET images of microglial activation in one MCI patient (upper row) and one healthy volunteer (lower row); PET images are displayed on each subject’s respective T1-weighted MRI scan normalised to the SPM5 T1 brain template and the colour bar indicates non-displaceable binding potential (BPND) values. b Parametric 11C-deuterium-L-deprenyl PET images of astrocyte activation in one Aβ-positive MCI patient (upper row) and one healthy volunteer (lower row); PET images are displayed on each subject’s respective T1-weighted MRI scan normalised to the SPM5 T1 brain template and the colour bar indicates modified-reference (cerebellar grey matter) Patlak slope values; the 11C-deuterium-L-deprenyl PET imaging data were kindly provided by Prof. Agneta Nordberg, Karolinska Institutet, Sweden
In contrast to the above-listed positive studies, a study of six MCI patients (mean MMSE 28), six AD patients (mean MMSE 19), and five controls failed to find any differences in 11C-(R)-PK11195 binding using ROI analysis, even when 11C-PiB uptake was taken into account [50]. A further study failed to identify any significant group differences in PK11195 binding between controls, AD, and MCI patients using both ROI-based and voxel-based analysis approaches [51]. Therefore, it is not yet clear whether the disparities in the findings between the studies are due to differences in methodology, or in the selection and clinical stage of patients.
As mentioned above, while PK11195 is thought to bind predominantly to activated microglia, increases in binding to activated astrocytes have been reported by some authors [52]. However, 3H-(R)-PK11195 binding in post-mortem tissue from patients with neuroinflammatory disorders correlated strongly with CD68-phagocytic microglia, but not GFAP-positive astrocytes, albeit with a lower binding affinity compared to the novel TSPO ligand 3H-DAA1106 [53]. A further potential limitation is that in vivo studies with 11C-(R)-PK11195 generally display a relatively low signal-to-noise ratio [54].
The most appropriate method of analysis for PET data in neurodegenerative disorders is also the subject of debate, as the selection of a reference region is difficult due to constitutive expression of TSPO within the brain and potentially very widespread pathology. Modified methods using kinetic analysis to identify a reference tissue cluster have been employed in the analysis of 11C-(R)-PK11195 PET data in neurodegenerative disease [55]. Lastly, as a 11C-labelled tracer, 11C-(R)-PK11195 has only a short half-life and necessitates a cyclotron on site for tracer production, limiting its use. These limitations have led to the development of novel TSPO ligands for PET imaging.
Over the last years, numerous novel radiotracers for activated microglia have been developed [56]. The compounds DAA1106 and PBR28 have been shown to have 10-fold higher in vitro affinity for TSPO compared to PK11195 [57, 58]. 11C-DAA1106 has been shown to have higher BP in prefrontal, parietal, anterior cingulate, and occipital cortex, striatum, and cerebellum in a PET study of 10 AD patients vs. 10 healthy controls, although partial volume effects could explain some of these changes [47]. A further study in seven MCI patients, 10 AD patients, and 10 healthy controls showed increased 11C-DAA1106 BP in temporoparietal cortex, anterior cingulate, and striatum in both MCI and AD patients compared to controls [59]. However, in contrast to the previous studies using PK11195, no difference in the DAA1106 binding was observed between the MCI and AD patients. This might be the consequence of the finding of populations of high- and low-affinity binders for TSPO ligands other than 11C-(R)-PK11195 complicating further the analysis of the data [58]. Thus, studies utilising such ligands require analysis of TSPO binding affinity to correct for this factor. Of note, early-onset (< 65 years) patients were detected to have greater binding of the 11C-PBR28 ligand than late-onset patients in parietal cortex and striatum, and 11C-PBR28 binding correlated with lower age of onset [60], adding to the complexity of the interpretation of the TSPO data and our understanding of the role of microglia in AD.
Attempts at exploring the time course of microglial activation was performed in a large prospective study including 64 patients with AD (prodromal AD n = 38; AD dementia n = 26) and 32 controls. A simple ratio method was used with cerebellar grey matter as reference tissue to measure TSPO binding. The study showed that microglial activation appears at the prodromal and possibly at the preclinical stage of AD, and the authors concluded that microglia might play an early protective role in the clinical progression of the disease [61]. Subsequently the authors followed a proportion of the participants over 2 years and found that high initial 18F-DPA-714 binding was correlated with a low subsequent increase of microglial activation and favourable clinical evolution, whereas the opposite profile was observed when initial 18F-DPA-714 binding was low, independently of disease severity at baseline. They felt that their results support a pathophysiological model involving two distinct profiles of microglial activation signatures with different dynamics, which differentially impact on disease progression and may vary depending on patients rather than disease stages [62]. However, without the use of ligands associated with already identified profiles of microglia, as described previously, this remains speculative.
Only further clinical evaluations and direct comparisons with 11C-(R)-PK11195 will show whether any of the 2nd- and 3rd-generation TSPO tracers are indeed superior in the clinical context. As there are known to be species differences in TSPO binding, in vitro and preclinical animal data do not always directly translate into clinical applications in humans [56].
PET studies using non-TSPO tracers for neuroinflammatory changes in AD
It is important to realise that TSPO expression is only one phenomenon within the complex process of microglial activation and currently effort goes into the development of in vivo imaging markers that can measure other pathways of this process. Developing and evaluating PET ligands is a lengthy process and currently only very few novel non-TSPO ligands are being used in a clinical context.
The cannabinoid type 2 receptor (CB2R) is expressed by immune cells including monocytes and macrophages. In the brain, CB2R is primarily found on microglia. Its upregulation has been reported in animal models of AD, and 11C-NE40—a tracer for CB2R—has been evaluated in healthy controls and patients with AD. Surprisingly, AD patients showed overall significantly lower CB2R binding than healthy controls and no relationship was found between regional or global Aβ load and CB2R availability, possibly due to lower affinity or selectivity of 11C-NE40 to CB2R than to CB1R [63].
11C-JNJ717, a selective P2X7 receptor tracer, one of the ATP-gated ion channels expressed on activated microglia, was evaluated in healthy volunteers and Parkinson’s disease (PD) patients where microglia are known to become activated as part of the neuropathological changes. No difference was found in binding between healthy controls and PD patients [64]. So far, no clinical studies have been conducted using this tracer in AD.
The colony-stimulating factor 1 receptor (CSF1R) might also be a very interesting target for neuroinflammation imaging and very recently preclinical data in a murine model of AD have been acquired [65], but no clinical studies have been conducted as of yet.
Astrocytes in the healthy brain
Astrocytes derive from heterogeneous populations of progenitor cells in the neuroepithelium. They adopt a star-like morphology and in humans, form 20 to 40% of all glia [66]. Several populations of astrocytes cohabit in the adult brain [67] (Fig. 5). In the grey matter, we find (i) the protoplasmic astrocytes characterised by highly branched bushy processes and located in the cortical grey matter; (ii) the primate-specific interlaminar astrocytes located in the superficial cortical layers I and II of the cortex; and (iii) the varicose projection astrocytes in layers V and VI [67, 68]. The fibrous astrocytes are mainly present in the white matter along the myelinated axons and exhibit long and straight processes [67].
Fig. 5.
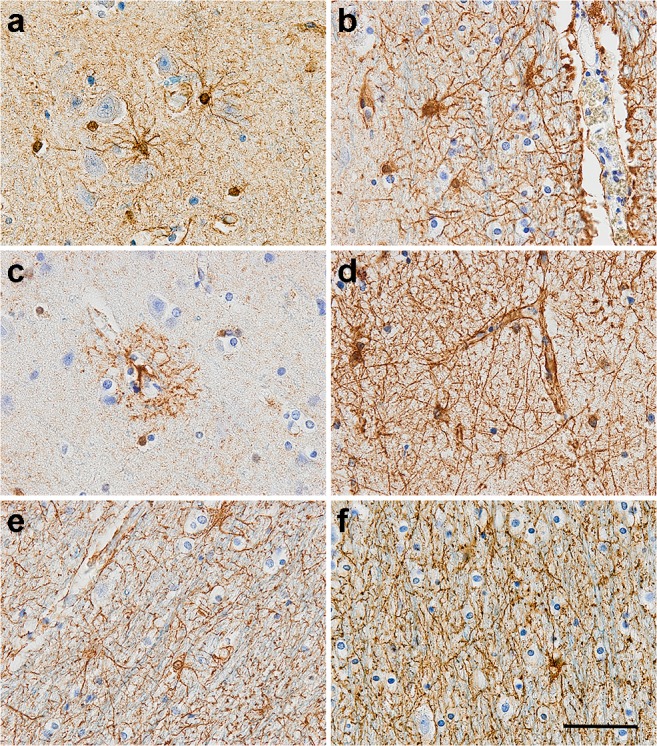
GFAP-positive astrocytes in human brain observed (a, b) in the grey matter; (c, d) with the endfeet of the processes forming a component of the blood-brain barrier; and (e, f) in the white matter. Counterstaining: Haematoxylin, scale bar = 50 μm
Astrocytes are key elements of brain homeostasis displaying numerous physiological functions. They are involved in (i) neurodevelopment [69]; (ii) synaptic function [70]; (iii) energy metabolism [71]; (iv) the neurovascular unit via their processes [72]; and recently, they were identified to be involved in (v) the circadian clock [73]. This large functional diversity is in keeping with the heterogeneous and pleomorphic astrocytes population throughout the brain. Glial fibrillary acidic protein (GFAP), an intermediate filament and major component of the astrocyte skeleton, is the most common marker to identify astrocytes, with its expression higher in fibrous than protoplasmic astrocytes [67]. However, it is now acknowledged that GFAP does not detect all astrocytes [74]. Other markers, based on the astrocyte functions, have been utilised sometimes in combination, to identify the different populations in humans including the enzyme aldehyde dehydrogenase 1 family member L1 (Aldh1l1), calcium-binding protein S100B, the glutamate transporters excitatory amino-acid transporter (EAAT) 1 and 2 for the synaptic transmission, the enzyme glutamine synthetase, and the Aquaporin 4 expressed at astrocyte endfoot processes to regulate water homeostasis [75]. Novel astrocyte-specific markers are emerging from rodent studies such as CD44 [76], CD51 (integrin alpha V protein), CD63 (LAMP-3), and CD71 (transferrin receptor 1) [77].
Ageing modifies astrocytes including their morphology with the processes becoming shorter and thicker, increasing cell density and GFAP expression [78], together considered as common features of activated astrocytes. Therefore, this suggests that, like microglia, astrocytes become reactive with age. The molecular profile of aged astrocytes, defined from rodent brain, encompasses upregulation of the complement system (C3 and C4B); major histocompatibility complex (H2-D1 and H2-K1); cytokines/chemokines (CXCL10 and CXCL5); peptidase inhibition (Serpina3n); alteration in lipid/cholesterol synthesis [79]; and potentially, the disruption of blood-brain barrier integrity and function [80].
To add to the complexity of the astrocyte population, subtypes of reactive astrocytes have been identified in mice giving rise to the A1 (neurotoxic) vs. A2 (neuroprotective) classification [81], with ageing driving the predominance of A1 astrocytes (characterised by the expression of C3) in rodent brain [79] (Table 3).
Table 3.
The different phenotypes acquired by the astrocytes
| Phenotype | Protein (gene) | Function |
|---|---|---|
|
Homeostatic/physiological status Human brain |
GFAP Aldh1l1 S100B EAAT1, EEAT2 AQP4 |
Structural protein (intermediate filament) Formate oxidation Calcium-binding protein Glutamate transporters Water transporter |
| A1 (neuroinflammation “neurotoxic”) from rodent brain [79, 82] |
Complement C3 (C3) Complement C1-inhibitor (Serping) GGTA1 (GGTA1) Interferon-inducible GTPase1 (Ligp1) Glycerol-1-phosphate phosphohydrolase 2 (Gpp2) Fibulin-5 (Fbln5) FKBP5 (Fkbp5) PSMB8 (Psmb8) Serglycin (Srgn) Amigo2 (Amigo2) |
Central role in complement activation Inhibition of the complement system (belongs to serpin superfamily) Glycosphingolipid biosynthesis Resistance to intracellular pathogens Glycerol biosynthesis Maintenance of the vessel wall after injury Role in immunoregulation and cellular processes involved in protein folding and trafficking Participate in the immunoproteasomes Mediator of granule-mediated apoptosis May contribute to signal transduction |
| A2 (ischemia “neuroprotective”) from rodent brain [79, 82] |
Cardiotrophin-like cytokine factor 1 (Clcf1) Keratinocyte tranglutaminase (Tgm1) Pentraxin 3 (Ptx3, TNF-inducible gene 14 protein) S100A10 (S100a10, p11) Sphingosine kinase 1 (Sphk1) CD109 (Cd109) Cyclooxygenase-2 (Ptgs2, COX2) Epithelial membrane protein 1 (Emp1) Solute carrier family 10 member 6 (Slc10a6) Transmembrane 4 L6 family member 1 (Tm4sf1) B3GNT5 (B3gnt5) CD14 (Cd14) STAT3 (Stat3) |
B cell activation Transglutaminase enzyme Activate the classical pathway of complement and facilitate pathogen recognition Transport of neurotransmitters (serotonin), receptor for tissue-type plasminogen activator Regulates proliferation and survival Involved in degradation of TGFβ receptor 1 Promote inflammation Cell migration and proliferation Sodium-dependent transporter Role in cell development, activation, growth, and motility Enzyme Co-receptor for the detection of bacterial lipopolysaccharide Essential for the differentiation of the TH17 Helper T cells |
Aldh1l1, Aldehyde dehydrogenase 1 family member L1; Amigo, Amphoterin-induced protein 2; AQP4, Aquaporin 4; B3GNT5, Lactosylceramide 1,3-N-acetyl-beta-D-glucosaminyltransferase; EEAT, Excitatory amino-acid transporter; GFAP, Glial fibrillary acidic protein; GGTA1, Alpha-1,3-galactosyltransferase; GPP2, Glycerol-1-phosphate phosphohydrolase 2; FKBP5, FK506 binding protein 5; PSMB8, Proteasome subunit beta type-8 (20S proteasome subunit beta-5i); STAT3, Signal transducer and activator of transcription 3
Astrocytes in Alzheimer’s disease
Astrocytes participate in the neuroinflammatory processes by their properties to react to a range of pro- and anti-inflammatory components [83]. Both microglia and astrocytes have roles in the phagocytosis of cell debris and Aβ, and in responding to damage [84]. In addition, astrocytes participate in neuronal metabolic support as evidenced by preclinical studies [85]. In primary cultures of astrocytes, Aβ induces increased glucose uptake [86], altering their metabolic phenotype. This resulted in increased astrocytic MAO-B expression [87], one of the molecular targets to image astrocytes. In a transgenic mouse model of AD, astrocytic MAO-B overexpression caused excess gamma-aminobutyric acid (GABA) and excitotoxic glutamate, modifying homeostasis and leading to cognitive deficits [88].
In humans, clusters of reactive astrocytes around Aβ plaques are a hallmark of AD [89, 90]. However, the spatial distribution of astrocytes is independent of plaque size, different from microglia whose number around plaques correlates to plaque size [90]. Neuropathological studies have demonstrated increased reactive astrocytes in the vicinity of plaques as the disease progresses, but are unclear in determining whether astrocytes reaction is an early event [91, 92] or whether the cells play a role in the late stage of disease when dementia develops [93]. Nevertheless, imaging and post-mortem human studies have started to provide some clues on the dynamics of the astrocytic response in AD. GFAP expression is upregulated in AD [78], with A1 astrocytes forming the main population of the reactive cells in humans [82], consistent with a previous study highlighting variation in the pattern of GFAP, EAAT1, EAAT2, and S100B in relation to AD pathology [94]. Overall, evidence in the human brain supports astrocyte changes in AD as phenotypic rather than proliferative [95, 96], which has implications in terms of imaging.
Molecular imaging targets for astrocytes
Multiple molecular imaging markers are needed to visualise astrocytosis as illustrated in Fig. 3, given that it is a highly dynamic process undergoing sequential protective (A2) and detrimental (A1) stages. There is increasing evidence that morphology and function may be inter-related in astrocyte activation, with an initial hypertrophic phase characterised by the overexpression of different markers involving MAO-B, intermediate filaments including nestin, vimentin, and GFAP [97] followed by an astrodegeneration phase characterised by atrophy, reduced branching and synaptic dysfunction, with reduced expression of markers such as Aquaporin-4 and glutamine synthetase.
Enzymes
Among few astrocyte PET tracers available, 11C-deuterium-L-deprenyl (11C-DED) binds to MAO-B, an enzyme overexpressed in activated astrocytes [98]. Autoradiography studies have demonstrated that 3H-L-deprenyl binding partly overlaps with GFAP in AD and other neurodegenerative diseases [98–101], indicating a good level of specificity of MAO-B to activated astrocytes. Autoradiography data using 11C-L-deprenyl in AD brain tissue showed the highest tracer uptake at the earliest Braak stages, suggesting early involvement of astrocytosis in AD [102]. 3H-L-deprenyl had a different laminar pattern than that of Aβ deposition as measured by 3H-PiB [103], but partly co-located with tau as measured by 3H-THK5117 [104].
11C-DED PET imaging has been used to investigate astrocytosis in neurodegenerative diseases including AD [105, 106], amyotrophic lateral sclerosis, [107] and Creutzfeldt-Jakob disease [108]. Multitracer PET studies using 11C-DED, 11C-PiB, and 18F-fluorodeoxyglucose (18F-FDG) have allowed investigating the spatio-temporal patterns of in vivo brain astrocytosis, fibrillar Aβ deposition, and glucose metabolism at different stages of disease progression. In these studies, significantly increased 11C-DED binding was found in prodromal AD in comparison to healthy controls or AD dementia patients [106]. In autosomal-dominant AD, astrocytosis was observed at early presymptomatic stages using 11C-DED PET [109]; longitudinally, Aβ plaque deposition (11C-PiB) increased while astrocytosis (11C-DED) declined [110]. Early astrocytosis preceding Aβ plaque deposition was observed in transgenic AD mice [111].
Receptors
The imidazoline-2 binding sites (I2BS) are located on the mitochondrial membranes of astrocytes [112] and are involved in the regulation of GFAP expression [113]. An increase in post-mortem density of I2BS has been observed in AD brain [114]. The novel PET tracer 11C-BU99008 has been characterised in preclinical species and demonstrated to be suitable to quantify cerebral I2BS density, as determined in rat [115], pig [116], and rhesus monkey [117]. More recently, 11C-BU99008 was found to have good kinetic properties in healthy human brain, with low affinity to MAO-A/MAO-B [118]. Studies in MCI and AD patients are underway to compare the regional distribution of 11C-BU99008 with that of Aβ and 18F-FDG-PET retention [119].
Metabolic markers
18F-FDG-PET hypometabolism has been traditionally considered a biomarker for neuronal injury and neurodegeneration. However, a recent preclinical study showed evidence that astrocytes can contribute to 18F-FDG-PET as measured in healthy rat brain [120]. This study adds support to the astrocyte-neuron lactate shuttle hypothesis initiated 20 years ago, that suggests that neuronal energy demands are mostly met by lactate, originated in astrocytes and shuttled to neurons [121, 122]. Consistent with these preclinical findings, it was recently shown that longitudinal decline in astrocytosis, as measured by MAO-B expression, was correlated to progressive hypometabolism in autosomal-dominant AD mutation carriers [123], indicating that astrocytes may in part reflect metabolic activity in AD. The observed decline in MAO-B, potentially reflecting reduced glucose demand by astrocytes, might represent astrodegeneration, a glial phenotype characteristic of late stages of AD [97]. This link remains to be confirmed in a study associating 18F-FDG imaging of sporadic AD patients with human post-mortem examination.
Other potential targets have been explored in preclinical studies, but in vivo studies in human are still rare. For example, the astrocyte-specific glutamate transporters GLT1 (in rodents) and EAAT2 (in humans) were reduced in post-mortem tissue [124–126], suggesting loss of function of astrocytes at late disease stages. Similarly, glutamine synthetase was observed to decline with age in a transgenic mouse model of AD [127]. Another potential marker for astrocyte-related metabolic dysfunction is the impairment in GLUT1 (glucose transporter 1) protein expression, a glucose transporter predominantly expressed in astrocytes [128]. Interestingly, aerobic glycolysis, known to take place mostly in astrocytes, was observed to decline as tau accumulated in preclinical AD individuals [129], supporting astrocyte dysfunction as an early event in AD. These studies motivate the current research on PET imaging tracers that could target astrocyte-specific glutamate transporters in human brain including EAAT1/EAAT2 and GLAST (glutamate aspartate transporter). These studies will greatly contribute to understanding the contribution of astrocytes to the metabolic changes observed in AD.
Emerging structural MRI and DTI imaging tools: Tracking morphological changes of activated astrocytes and microglia?
Microglia and astrocytes possess ramified morphologies, which under pathological conditions are modified towards a more ameboid-like (microglia) or hypertrophic-like (astrocytes) as described above. These morphological changes have motivated the development of structural magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) tools to incorporate into multimodal MRI/PET approaches that would better investigate the complex neuroinflammatory changes in AD. Using MRI and DTI, a recent study proposed a model of grey matter changes in AD [130], in which an early presymptomatic phase of decreased cortical mean diffusivity (MD) and increased cortical thickness (CTh), hypothesised to reflect hypertrophy or glial cell swelling due to neuroinflammation, is followed by increased cortical MD and decreased CTh reflecting neurodegeneration. More recently, the direct relationship between structural vs. inflammatory changes were tested in autosomal-dominant AD (Vilaplana et al., under review). In this study, 11C-DED binding had a negative association with cortical MD and a positive association with CTh in autosomal-dominant AD mutation carriers, suggesting that astrocyte activation and associated hypertrophy may explain the observed reduction in cortical MD and increases in CTh. Another recent study has found a positive correlation between microgliosis as measured by TSPO PET and grey matter volume at the early MCI stage of AD [131], suggesting that neuroinflammation is accompanied by cortical swelling from early stages. In the same lines, new analysis techniques for DTI imaging allow extracting a measure of cell body size called “cellular diffusivity” that is interpreted to reflect activated microglia or astrocytes [132]. These initial reports motivate further in vivo multimodal studies combining MRI, DTI, and PET imaging to fully characterise the complex molecular and morphological dynamics of neuroinflammation across disease progression in AD.
Relationship between PET imaging of neuroinflammation vs. Aβ and tau proteinopathies
Recent preclinical evidence implies that glial activity could lead to tau deposition [133, 134]. It is still unclear whether the oligomeric forms of Aβ can cause the glial activation, which leads to tau formation and propagation throughout the cortex. Very few studies have imaged and compared inflammation and tau in human AD, and they support that inflammation is more strongly correlated to Aβ, likely preceding tau deposition [135–137]. However, longitudinal studies are needed to confirm these results.
CSF and plasma biomarkers of neuroinflammation
One of the most promising and more extensively investigated CSF glial biomarker is YKL-40, a secreted glycoprotein expressed by microglia and astrocytes [138]. Recently, an increasing number of CSF biomarkers are being developed to track microglia and astrocyte activation, neuroinflammation, and cerebrovascular dysfunction including YKL-40, sTREM2, IL-6, IL-7, IL-8, IL-15, IP-10, monocyte chemoattractant protein (MCP)-1, intercellular adhesion molecule (ICAM)-1, vascular adhesion molecule (VCAM)-1, placental growth factor, and fms-related tyrosine kinase 1 (Flt-1) [139, 140]. CSF levels of YKL-40, ICAM-1, VCAM-1, IL-15, and Flt-1 were increased in AD already from the preclinical and prodromal stages and were associated with CSF tau especially in Aβ-positive individuals [140]. Interestingly, their increase was associated to cortical thinning. Studies comparing neuroinflammation CSF and PET biomarkers are to our knowledge still lacking, and thus, these studies will add very valuable knowledge in the near future.
Neuroinflammation biomarkers and clinical trials
There is an urgent need to develop biomarkers for characterising multiple pathophysiological mechanisms including neuroinflammation [141]. Inflammation biomarkers are of interest in clinical trial design, due to their utility for patient stratification and to track biological effects of drugs. In this respect, the studies reviewed here motivate further research on the possible utility of neuroinflammation PET as well as structural MRI/DTI imaging measures, used alone or in combination, for their possible application as biomarkers in clinical trials. Consequently, it is essential to develop novel PET tracers that are more specific to astrocytes and to microglia, and that can selectively target their different inflammatory stages. Thus, translational studies in post-mortem human brain are necessary to characterise these profiles and to develop a solid knowledge about what these tracers are binding to.
Conclusion
To date, astrocytes have been investigated to a lesser extent than microglia for their role in AD; however, recent papers have emphasised the importance of a bidirectional communication between microglia and astrocytes via physical contacts and secreted molecules. Microglia, the sensors of changes in homeostasis, are the primary immune cells of the brain, which also regulate the immune functions of astrocytes. Experimental studies in diverse neurodegenerative diseases imply that microglia define the functions of astrocytes, ranging from neuroprotective to neurotoxic. Conversely, astrocytes appear to regulate microglial phenotypes and functions including motility and phagocytosis [92]. Of note, with this review we also emphasise the gaps between the markers identified in preclinical and human studies, the importance of phenotypes, and the choice of the imaging targets, highlighting the need for better translational research between these two areas.
Nevertheless, whether modulation of microglia/astrocyte cross-talk can ameliorate neurodegeneration in human AD remains to be demonstrated. Therefore, it is essential to increase our knowledge of the glial biology and of their interactions with the environment in a physiological/healthy context. This will identify targets to image glia which, whether in multimodal imaging studies or in combination with other parameters (fluid biomarkers), will clarify the involvement of glial cells in the course of the disease.
Electronic supplementary material
(DOCX 124 kb)
Funding
Prof. Boche is supported by grants from the Alzheimer’s Research UK, the British Neuropathological Society and the University of Southampton. Dr. Gerhard is supported by a grant from the Medical Research Council (MRC) UK, and the University of Manchester. Dr. Rodriguez-Vieitez is supported by grants from the Swedish Alzheimer Foundation (Alzheimerfonden), the Swedish Dementia Association (Demensfonden), Åke Wiberg Foundation, Gun and Bertil Stohne’s Foundation, Gamla Tjänarinnor’s Foundation, and the Karolinska Institutet foundations.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local research and ethics committee (National Research Ethics Committee North West – Greater Manchester North) and permission to administer radiotracers was obtained from the Administration of Radioactive Substances Advisory Committee of the Department of Health (ARSAC), UK (Fig. 4a). The PET imaging data presented in Fig. 4b were collected as part of a previously published study [106]. In that study, ethical approval was obtained from the regional Human Ethics Committee of Stockholm and the Faculty of Medicine and Radiation Hazard Ethics Committee of Uppsala University Hospital, Sweden. The study was conducted in accordance with the principles of the Declaration of Helsinki and its subsequent revisions. For all PET imaging data presented in Fig. 4b, all participants provided written informed consent to participate in the study, as previously published [106].
Footnotes
This article is part of the Topical Collection on Neurology
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16(3):229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 3.Vellas B, Bain LJ, Touchon J, Aisen PS. Advancing Alzheimer’s disease treatment: lessons from CTAD 2018. J Prev Alzheimers Dis. 2019;6(3):198–203. doi: 10.14283/jpad.2019.11. [DOI] [PubMed] [Google Scholar]
- 4.Hanseeuw Bernard J., Betensky Rebecca A., Jacobs Heidi I. L., Schultz Aaron P., Sepulcre Jorge, Becker J. Alex, Cosio Danielle M. Orozco, Farrell Michelle, Quiroz Yakeel T., Mormino Elizabeth C., Buckley Rachel F., Papp Kathryn V., Amariglio Rebecca A., Dewachter Ilse, Ivanoiu Adrian, Huijbers Willem, Hedden Trey, Marshall Gad A., Chhatwal Jasmeer P., Rentz Dorene M., Sperling Reisa A., Johnson Keith. Association of Amyloid and Tau With Cognition in Preclinical Alzheimer Disease. JAMA Neurology. 2019;76(8):915. doi: 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyns CEG, Holtzman DM. Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener. 2017;12(1):50. doi: 10.1186/s13024-017-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felsky D, Roostaei T, Nho K, Risacher SL, Bradshaw EM, Petyuk V, et al. Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun. 2019;10(1):409. doi: 10.1038/s41467-018-08279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43(1):92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 2017;18(2):391–405. doi: 10.1016/j.celrep.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 2017;20(4):779–784. doi: 10.1016/j.celrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT. Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol Appl Neurobiol. 2016;42(7):621–638. doi: 10.1111/nan.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minett T, Classey J, Matthews FE, Fahrenhold M, Taga M, Brayne C, et al. Microglial immunophenotype in dementia with Alzheimer’s pathology. J Neuroinflammation. 2016;13(1):135–145. doi: 10.1186/s12974-016-0601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169(7):1276–90.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47(3):566–81.e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179–197. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norden DM, Godbout JP. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39(1):19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakic S, Hung YMA, Smith M, So D, Tayler HM, Varney W, et al. Systemic infection modifies the neuroinflammatory response in late stage Alzheimer’s disease. Acta Neuropathol Commun. 2018;6(1):88. doi: 10.1186/s40478-018-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101(3):249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- 21.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 22.Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8(5):1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 23.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33(10):4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16(12):1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Gonzalez I, Schluter A, Aso E, Garcia-Esparcia P, Ansoleaga B, LLorens F, et al. Neuroinflammatory signals in Alzheimer disease and APP/PS1 transgenic mice: correlations with plaques, tangles, and oligomeric species. J Neuropathol Exp Neurol. 2015;74(4):319–344. doi: 10.1097/NEN.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 30.Davies Danielle S., Ma Jolande, Jegathees Thuvarahan, Goldsbury Claire. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer's disease. Brain Pathology. 2016;27(6):795–808. doi: 10.1111/bpa.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29(9):506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Olah M, Patrick E, Villani AC, Xu J, White CC, Ryan KJ, et al. A transcriptomic atlas of aged human microglia. Nat Commun. 2018;9(1):539. doi: 10.1038/s41467-018-02926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 34.Neher Jonas J., Cunningham Colm. Priming Microglia for Innate Immune Memory in the Brain. Trends in Immunology. 2019;40(4):358–374. doi: 10.1016/j.it.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556(7701):332–338. doi: 10.1038/s41586-018-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiyoshi Ryohei, Wake Hiroaki, Kato Daisuke, Horiuchi Hiroshi, Ono Riho, Ikegami Ako, Haruwaka Koichiro, Omori Toshiaki, Tachibana Yoshihisa, Moorhouse Andrew J., Nabekura Junichi. Microglia Enhance Synapse Activity to Promote Local Network Synchronization. eneuro. 2018;5(5):ENEURO.0088-18.2018. doi: 10.1523/ENEURO.0088-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol Aging. 2013;34(4):1051–1059. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boche D, Perry VH, Nicoll JA. Activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39(1):3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 39.Albrecht DS, Granziera C, Hooker JM, Loggia ML. In Vivo imaging of human neuroinflammation. ACS Chem Neurosci. 2016;7(4):470–483. doi: 10.1021/acschemneuro.6b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306–328. doi: 10.1111/j.1365-2990.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavisse S, Guillermier M, Herard AS, Petit F, Delahaye M, Van Camp N, et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci. 2012;32(32):10809–10818. doi: 10.1523/JNEUROSCI.1487-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayanaswami V, Dahl K, Bernard-Gauthier V, Josephson L, Cumming P, Vasdev N. Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: outlook beyond TSPO. Mol Imaging. 2018;17:1536012118792317. doi: 10.1177/1536012118792317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, et al. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358(9280):461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 44.Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, et al. Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32(3):412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One. 2010;5(11):e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokokura M, Mori N, Yagi S, Yoshikawa E, Kikuchi M, Yoshihara Y, et al. In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2011;38(2):343–351. doi: 10.1007/s00259-010-1612-0. [DOI] [PubMed] [Google Scholar]
- 47.Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK, et al. Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiatry. 2008;64(10):835–841. doi: 10.1016/j.biopsych.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72(1):56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parbo P, Ismail R, Hansen KV, Amidi A, Marup FH, Gottrup H, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain. 2017;140(7):2002–2011. doi: 10.1093/brain/awx120. [DOI] [PubMed] [Google Scholar]
- 50.Wiley CA, Lopresti BJ, Venneti S, Price J, Klunk WE, DeKosky ST, et al. Carbon 11-labeled Pittsburgh compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol. 2009;66(1):60–67. doi: 10.1001/archneurol.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuitemaker Alie, Kropholler Marc A., Boellaard Ronald, van der Flier Wiesje M., Kloet Reina W., van der Doef Thalia F., Knol Dirk L., Windhorst Albert D., Luurtsema Gert, Barkhof Frederik, Jonker Cees, Lammertsma Adriaan A., Scheltens Philip, van Berckel Bart N.M. Microglial activation in Alzheimer's disease: an (R)-[11C]PK11195 positron emission tomography study. Neurobiology of Aging. 2013;34(1):128–136. doi: 10.1016/j.neurobiolaging.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Hazell AS, Desjardins P, Butterworth RF. Chronic exposure of rat primary astrocyte cultures to manganese results in increased binding sites for the ‘peripheral-type’ benzodiazepine receptor ligand 3H-PK 11195. Neurosci Lett. 1999;271(1):5–8. doi: 10.1016/S0304-3940(99)00489-9. [DOI] [PubMed] [Google Scholar]
- 53.Venneti S, Wang G, Nguyen J, Wiley CA. The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropathol Exp Neurol. 2008;67(10):1001–10. doi: 10.1097/NEN.0b013e318188b204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venneti Sriram, Lopresti Brian J., Wiley Clayton A. Molecular imaging of microglia/macrophages in the brain. Glia. 2012;61(1):10–23. doi: 10.1002/glia.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turkheimer FE, Edison P, Pavese N, Roncaroli F, Anderson AN, Hammers A, et al. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J Nuclear Med : official publication, Society of Nuclear Medicine. 2007;48(1):158–167. [PubMed] [Google Scholar]
- 56.Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35(12):2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- 57.Owen DR, Howell OW, Tang SP, Wells LA, Bennacef I, Bergstrom M, et al. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab. 2010;30(9):1608–1618. doi: 10.1038/jcbfm.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nuclear Med : official publication, Society of Nuclear Medicine. 2011;52(1):24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasuno Fumihiko, Kosaka Jun, Ota Miho, Higuchi Makoto, Ito Hiroshi, Fujimura Yota, Nozaki Shoko, Takahashi Sho, Mizukami Katsuyoshi, Asada Takashi, Suhara Tetsuya. Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment–dementia converters measured by positron emission tomography with [11C]DAA1106. Psychiatry Research: Neuroimaging. 2012;203(1):67–74. doi: 10.1016/j.pscychresns.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 60.Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136(Pt 7):2228–2238. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamelin L, Lagarde J, Dorothee G, Leroy C, Labit M, Comley RA, et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain. 2016;139(Pt 4):1252–1264. doi: 10.1093/brain/aww017. [DOI] [PubMed] [Google Scholar]
- 62.Hamelin L, Lagarde J, Dorothee G, Potier MC, Corlier F, Kuhnast B, et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain. 2018;141(6):1855–1870. doi: 10.1093/brain/awy079. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad Rawaha, Postnov Andrey, Bormans Guy, Versijpt Jan, Vandenbulcke Mathieu, Van Laere Koen. Decreased in vivo availability of the cannabinoid type 2 receptor in Alzheimer’s disease. European Journal of Nuclear Medicine and Molecular Imaging. 2016;43(12):2219–2227. doi: 10.1007/s00259-016-3457-7. [DOI] [PubMed] [Google Scholar]
- 64.Van Weehaeghe Donatienne, Koole Michel, Schmidt Mark E., Deman Stephanie, Jacobs Andreas H., Souche Erika, Serdons Kim, Sunaert Stefan, Bormans Guy, Vandenberghe Wim, Van Laere Koen. [11C]JNJ54173717, a novel P2X7 receptor radioligand as marker for neuroinflammation: human biodistribution, dosimetry, brain kinetic modelling and quantification of brain P2X7 receptors in patients with Parkinson’s disease and healthy volunteers. European Journal of Nuclear Medicine and Molecular Imaging. 2019;46(10):2051–2064. doi: 10.1007/s00259-019-04369-6. [DOI] [PubMed] [Google Scholar]
- 65.Horti AG, Naik R, Foss CA, Minn I, Misheneva V, Du Y, et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R) Proc Natl Acad Sci U S A. 2019;116(5):1686–1691. doi: 10.1073/pnas.1812155116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol. 2016;524(18):3865–3895. doi: 10.1002/cne.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29(10):3276–3287. doi: 10.1523/jneurosci.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vasile F, Dossi E, Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct. 2017;222(5):2017–2029. doi: 10.1007/s00429-017-1383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farhy-Tselnicker I, Allen NJ. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev. 2018;13(1):7. doi: 10.1186/s13064-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22(2):154–166. doi: 10.1038/s41593-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 71.Prebil M, Jensen J, Zorec R, Kreft M. Astrocytes and energy metabolism. Arch Physiol Biochem. 2011;117(2):64–69. doi: 10.3109/13813455.2010.539616. [DOI] [PubMed] [Google Scholar]
- 72.Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci. 2016;19(12):1619–1627. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brancaccio M, Edwards MD, Patton AP, Smyllie NJ, Chesham JE, Maywood ES, et al. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science. 2019;363(6423):187–192. doi: 10.1126/science.aat4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szu JI, Binder DK. The role of astrocytic aquaporin-4 in synaptic plasticity and learning and memory. Front Integr Neurosci. 2016;10:8. doi: 10.3389/fnint.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sosunov AA, Wu X, Tsankova NM, Guilfoyle E, McKhann GM, 2nd, Goldman JE. Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J Neurosci. 2014;34(6):2285–2298. doi: 10.1523/jneurosci.4037-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017;20(3):396–405. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jyothi HJ, Vidyadhara DJ, Mahadevan A, Philip M, Parmar SK, Manohari SG, et al. Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol Aging. 2015;36(12):3321–3333. doi: 10.1016/j.neurobiolaging.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 79.Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115(8):E1896–Ee905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goodall EF, Wang C, Simpson JE, Baker DJ, Drew DR, Heath PR, et al. Age-associated changes in the blood-brain barrier: comparative studies in human and mouse. Neuropathol Appl Neurobiol. 2018;44(3):328–340. doi: 10.1111/nan.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–6410. doi: 10.1523/jneurosci.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2014;20(2):160–172. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- 84.Thal DR. The role of astrocytes in amyloid beta-protein toxicity and clearance. Exp Neurol. 2012;236(1):1–5. doi: 10.1016/j.expneurol.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 85.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9(4):453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 86.Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA, et al. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci. 2010;30(9):3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song W, Zhou LJ, Zheng SX, Zhu XZ. Amyloid-beta 25-35 peptide induces expression of monoamine oxidase B in cultured rat astrocytes. Acta Pharmacol Sin. 2000;21(6):557–563. [PubMed] [Google Scholar]
- 88.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20(8):886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24(3):173–182. doi: 10.1016/0165-5728(89)90115-X. [DOI] [PubMed] [Google Scholar]
- 90.Serrano-Pozo A, Muzikansky A, Gomez-Isla T, Growdon JH, Betensky RA, Frosch MP, et al. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in Alzheimer disease. J Neuropathol Exp Neurol. 2013;72(6):462–471. doi: 10.1097/NEN.0b013e3182933788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pike CJ, Cummings BJ, Cotman CW. Early association of reactive astrocytes with senile plaques in Alzheimer’s disease. Exp Neurol. 1995;132(2):172–179. doi: 10.1016/0014-4886(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 92.Jha MK, Kim JH, Song GJ, Lee WH, Lee IK, Lee HW, et al. Functional dissection of astrocyte-secreted proteins: implications in brain health and diseases. Prog Neurobiol. 2018;162:37–69. doi: 10.1016/j.pneurobio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Vehmas AK, Kawas CH, Stewart WF, Troncoso JC. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging. 2003;24(2):321–331. doi: 10.1016/S0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 94.Simpson JE, Ince PG, Haynes LJ, Theaker R, Gelsthorpe C, Baxter L, et al. Population variation in oxidative stress and astrocyte DNA damage in relation to Alzheimer-type pathology in the ageing brain. Neuropathol Appl Neurobiol. 2010;36(1):25–40. doi: 10.1111/j.1365-2990.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 95.Serrano-Pozo A, Gomez-Isla T, Growdon JH, Frosch MP, Hyman BT. A phenotypic change but not proliferation underlies glial responses in Alzheimer disease. Am J Pathol. 2013 [DOI] [PMC free article] [PubMed]
- 96.Marlatt MW, Bauer J, Aronica E, van Haastert ES, Hoozemans JJ, Joels M, et al. Proliferation in the Alzheimer hippocampus is due to microglia, not astroglia, and occurs at sites of amyloid deposition. Neural Plast. 2014;2014:693851. doi: 10.1155/2014/693851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164(4):603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 98.Ekblom J, Jossan SS, Bergstrom M, Oreland L, Walum E, Aquilonius SM. Monoamine oxidase-B in astrocytes. Glia. 1993;8(2):122–132. doi: 10.1002/glia.440080208. [DOI] [PubMed] [Google Scholar]
- 99.Ekblom J, Jossan SS, Oreland L, Walum E, Aquilonius SM. Reactive gliosis and monoamine oxidase B. J Neural Transm Suppl. 1994;41:253–258. doi: 10.1007/978-3-7091-9324-2_33. [DOI] [PubMed] [Google Scholar]
- 100.Jossan SS, Ekblom J, Aquilonius SM, Oreland L. Monoamine oxidase-B in motor cortex and spinal cord in amyotrophic lateral sclerosis studied by quantitative autoradiography. J Neural Transm Suppl. 1994;41:243–248. doi: 10.1007/978-3-7091-9324-2_31. [DOI] [PubMed] [Google Scholar]
- 101.Tong J, Rathitharan G, Meyer JH, Furukawa Y, Ang LC, Boileau I, et al. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain. 2017;140(9):2460–2474. doi: 10.1093/brain/awx172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gulyas B, Pavlova E, Kasa P, Gulya K, Bakota L, Varszegi S, et al. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int. 2011;58(1):60–68. doi: 10.1016/j.neuint.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Marutle A, Gillberg PG, Bergfors A, Yu W, Ni R, Nennesmo I, et al. (3)H-deprenyl and (3)H-PIB autoradiography show different laminar distributions of astroglia and fibrillar beta-amyloid in Alzheimer brain. J Neuroinflammation. 2013;10:90. doi: 10.1186/1742-2094-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lemoine L, Saint-Aubert L, Nennesmo I, Gillberg PG, Nordberg A. Cortical laminar tau deposits and activated astrocytes in Alzheimer’s disease visualised by 3H-THK5117 and 3H-deprenyl autoradiography. Sci Rep. 2017;7:45496. doi: 10.1038/srep45496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santillo AF, Gambini JP, Lannfelt L, Langstrom B, Ulla-Marja L, Kilander L, et al. In vivo imaging of astrocytosis in Alzheimer’s disease: an (1)(1)C-L-deuteriodeprenyl and PIB PET study. Eur J Nucl Med Mol Imaging. 2011;38(12):2202–2208. doi: 10.1007/s00259-011-1895-9. [DOI] [PubMed] [Google Scholar]
- 106.Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nuclear Med : official publication, Society of Nuclear Medicine. 2012;53(1):37–46. doi: 10.2967/jnumed.110.087031. [DOI] [PubMed] [Google Scholar]
- 107.Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, et al. Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci. 2007;255(1–2):17–22. doi: 10.1016/j.jns.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 108.Engler H, Nennesmo I, Kumlien E, Gambini JP, Lundberg P, Savitcheva I, et al. Imaging astrocytosis with PET in Creutzfeldt-Jakob disease: case report with histopathological findings. Int J Clin Exp Med. 2012;5(2):201–207. [PMC free article] [PubMed] [Google Scholar]
- 109.Scholl M, Carter SF, Westman E, Rodriguez-Vieitez E, Almkvist O, Thordardottir S, et al. Early astrocytosis in autosomal dominant Alzheimer’s disease measured in vivo by multi-tracer positron emission tomography. Sci Rep. 2015;5:16404. doi: 10.1038/srep16404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodriguez-Vieitez E, Saint-Aubert L, Carter SF, Almkvist O, Farid K, Scholl M, et al. Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer’s disease. Brain J Neurol. 2016;139(Pt 3):922–936. doi: 10.1093/brain/awv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodriguez-Vieitez E, Ni R, Gulyas B, Toth M, Haggkvist J, Halldin C, et al. Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: a correlative positron emission tomography and in vitro imaging study. Eur J Nucl Med Mol Imaging. 2015;42(7):1119–1132. doi: 10.1007/s00259-015-3047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Regunathan S, Feinstein DL, Reis DJ. Expression of non-adrenergic imidazoline sites in rat cerebral cortical astrocytes. J Neurosci Res. 1993;34(6):681–688. doi: 10.1002/jnr.490340611. [DOI] [PubMed] [Google Scholar]
- 113.Olmos G, Alemany R, Escriba PV, Garcia-Sevilla JA. The effects of chronic imidazoline drug treatment on glial fibrillary acidic protein concentrations in rat brain. Br J Pharmacol. 1994;111(4):997–1002. doi: 10.1111/j.1476-5381.1994.tb14842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garcia-Sevilla JA, Escriba PV, Guimon J. Imidazoline receptors and human brain disorders. Ann N Y Acad Sci. 1999;881:392–409. doi: 10.1111/j.1749-6632.1999.tb09388.x. [DOI] [PubMed] [Google Scholar]
- 115.Tyacke RJ, Fisher A, Robinson ES, Grundt P, Turner EM, Husbands SM, et al. Evaluation and initial in vitro and ex vivo characterization of the potential positron emission tomography ligand, BU99008 (2-(4,5-dihydro-1H-imidazol-2-yl)-1- methyl-1H-indole), for the imidazoline(2) binding site. Synapse. 2012;66(6):542–551. doi: 10.1002/syn.21541. [DOI] [PubMed] [Google Scholar]
- 116.Kealey S, Turner EM, Husbands SM, Salinas CA, Jakobsen S, Tyacke RJ, et al. Imaging imidazoline-I2 binding sites in porcine brain using 11C-BU99008. J Nuclear Med : official publication, Society of Nuclear Medicine. 2013;54(1):139–144. doi: 10.2967/jnumed.112.108258. [DOI] [PubMed] [Google Scholar]
- 117.Parker CA, Nabulsi N, Holden D, Lin SF, Cass T, Labaree D, et al. Evaluation of 11C-BU99008, a PET ligand for the imidazoline2 binding sites in rhesus brain. J Nuclear Med: official publication, Society of Nuclear Medicine. 2014;55(5):838–844. doi: 10.2967/jnumed.113.131854. [DOI] [PubMed] [Google Scholar]
- 118.Tyacke Robin J., Myers Jim F.M., Venkataraman Ashwin, Mick Inge, Turton Samuel, Passchier Jan, Husbands Stephen M., Rabiner Eugenii A., Gunn Roger N., Murphy Philip S., Parker Christine A., Nutt David J. Evaluation of 11C-BU99008, a PET Ligand for the Imidazoline2 Binding Site in Human Brain. Journal of Nuclear Medicine. 2018;59(10):1597–1602. doi: 10.2967/jnumed.118.208009. [DOI] [PubMed] [Google Scholar]
- 119.Fan Z, Calsolaro V, Mayers J, Tyacke R, Venkataraman A, Femminella GD, et al. Relationship between astrocyte activation using [11C]BU99008 PET, glucose metabolism and amyloid in Alzheimer’s disease: a dementia platform UK experimental medicine study. Alzheimers Dement. 2018;14(7):P1640. doi: 10.1016/j.jalz.2018.06.2999. [DOI] [Google Scholar]
- 120.Zimmer ER, Parent MJ, Souza DG, Leuzy A, Lecrux C, Kim HI, et al. [18F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci. 2017;20(3):393–395. doi: 10.1038/nn.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Magistretti PJ, Pellerin L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol Psychiatry. 1996;1(6):445–452. [PubMed] [Google Scholar]
- 122.Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20(4–5):291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 123.Carter SF, Chiotis K, Nordberg A, Rodriguez-Vieitez E. Longitudinal association between astrocyte function and glucose metabolism in autosomal dominant Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2019;46(2):348–356. doi: 10.1007/s00259-018-4217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Acosta Crystal, Anderson Hope D., Anderson Christopher M. Astrocyte dysfunction in Alzheimer disease. Journal of Neuroscience Research. 2017;95(12):2430–2447. doi: 10.1002/jnr.24075. [DOI] [PubMed] [Google Scholar]
- 125.Hefendehl JK, LeDue J, Ko RW, Mahler J, Murphy TH, MacVicar BA. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Abeta plaques by iGluSnFR two-photon imaging. Nat Commun. 2016;7:13441. doi: 10.1038/ncomms13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol. 1996;40(5):759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 127.Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: mechanism for deficient glutamatergic transmission? Mol Neurodegener. 2011;6:55. doi: 10.1186/1750-1326-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pascual JM, Van Heertum RL, Wang D, Engelstad K, De Vivo DC. Imaging the metabolic footprint of Glut1 deficiency on the brain. Ann Neurol. 2002;52(4):458–464. doi: 10.1002/ana.10311. [DOI] [PubMed] [Google Scholar]
- 129.Vlassenko AG, Gordon BA, Goyal MS, Su Y, Blazey TM, Durbin TJ, et al. Aerobic glycolysis and tau deposition in preclinical Alzheimer’s disease. Neurobiol Aging. 2018;67:95–98. doi: 10.1016/j.neurobiolaging.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Montal V, Vilaplana E, Alcolea D, Pegueroles J, Pasternak O, Gonzalez-Ortiz S, et al. Cortical microstructural changes along the Alzheimer’s disease continuum. Alzheimers Dement. 2018;14(3):340–351. doi: 10.1016/j.jalz.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 131.Femminella GD, Dani M, Wood M, Fan Z, Calsolaro V, Atkinson R, et al. Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology. 2019;92(12):e1331–e1e43. doi: 10.1212/WNL.0000000000007133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Q, Wang Y, Liu J, Sutphen CL, Cruchaga C, Blazey T, et al. Quantification of white matter cellularity and damage in preclinical and early symptomatic Alzheimer’s disease. Neuroimage Clin. 2019;22:101767. doi: 10.1016/j.nicl.2019.101767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park J, Wetzel I, Marriott I, Dreau D, D’Avanzo C, Kim DY, et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci. 2018;21(7):941–951. doi: 10.1038/s41593-018-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sosna J, Philipp S, Albay R, 3rd, Reyes-Ruiz JM, Baglietto-Vargas D, LaFerla FM, et al. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener. 2018;13(1):11. doi: 10.1186/s13024-018-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Terada Tatsuhiro, Yokokura Masamichi, Obi Tomokazu, Bunai Tomoyasu, Yoshikawa Etsuji, Ando Ichiro, Shimada Hitoshi, Suhara Tetsuya, Higuchi Makoto, Ouchi Yasuomi. In vivo direct relation of tau pathology with neuroinflammation in early Alzheimer’s disease. Journal of Neurology. 2019;266(9):2186–2196. doi: 10.1007/s00415-019-09400-2. [DOI] [PubMed] [Google Scholar]
- 136.Parbo P, Ismail R, Sommerauer M, Stokholm MG, Hansen AK, Hansen KV, et al. Does inflammation precede tau aggregation in early Alzheimer’s disease? A PET study Neurobiol Dis. 2018;117:211–216. doi: 10.1016/j.nbd.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 137.Dani M, Wood M, Mizoguchi R, Fan Z, Walker Z, Morgan R, et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain. 2018;141(9):2740–2754. doi: 10.1093/brain/awy188. [DOI] [PubMed] [Google Scholar]
- 138.Querol-Vilaseca M, Colom-Cadena M, Pegueroles J, San Martin-Paniello C, Clarimon J, Belbin O, et al. YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer’s disease and other tauopathies. J Neuroinflammation. 2017;14(1):118. doi: 10.1186/s12974-017-0893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Falcon C, Monte-Rubio GC, Grau-Rivera O, Suarez-Calvet M, Sanchez-Valle R, Rami L, et al. CSF glial biomarkers YKL40 and sTREM2 are associated with longitudinal volume and diffusivity changes in cognitively unimpaired individuals. Neuroimage Clin. 2019;23:101801. doi: 10.1016/j.nicl.2019.101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Janelidze S, Mattsson N, Stomrud E, Lindberg O, Palmqvist S, Zetterberg H, et al. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology. 2018;91(9):e867–ee77. doi: 10.1212/WNL.0000000000006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mattsson N, Carrillo MC, Dean RA, Devous MD, Sr, Nikolcheva T, Pesini P, et al. Revolutionizing Alzheimer’s disease and clinical trials through biomarkers. Alzheimers Dement (Amst) 2015;1(4):412–419. doi: 10.1016/j.dadm.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boche D., Nicoll J. A. R. Neuroinflammation in ageing and in neurodegenerative disease. Neuropathology and Applied Neurobiology. 2013;39(1):1–2. doi: 10.1111/nan.12009. [DOI] [PubMed] [Google Scholar]
- 143.Satoh Jun-ichi, Kino Yoshihiro, Asahina Naohiro, Takitani Mika, Miyoshi Junko, Ishida Tsuyoshi, Saito Yuko. TMEM119 marks a subset of microglia in the human brain. Neuropathology. 2015;36(1):39–49. doi: 10.1111/neup.12235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 124 kb)