Abstract
Pain can be both a cause and a consequence of sleep deficiency. This bidirectional relationship between sleep and pain has important implications for clinical management of patients, but also for chronic pain prevention and public health more broadly. The review that follows will provide an overview of the neurobiological evidence of mechanisms thought to be involved in the modulation of pain by sleep deficiency, including the opioid, monoaminergic, orexinergic, immune, melatonin, and endocannabinoid systems; the hypothalamus-pituitary-adrenal axis; and adenosine and nitric oxide signaling. In addition, it will provide a broad overview of pharmacological and non-pharmacological approaches for the management of chronic pain comorbid with sleep disturbances and for the management of postoperative pain, as well as discuss the effects of sleep-disturbing medications on pain amplification.
Subject terms: Neurophysiology, Diseases
Introduction
Chronic pain is highly comorbid with sleep that is deficient in duration or quality, such as is seen in sleep disorders. Moreover, the pain-sleep relationship is a bidirectional one: pain can disrupt sleep, and short or disturbed sleep in turn lowers pain thresholds and increases spontaneous pain.
Epidemiological studies have shown that poor sleep quality and insufficient sleep duration are risk factors for the development of chronic pain [1]. Furthermore, there is strong evidence that having short or disturbed sleep can cause hyperalgesia (i.e., an increased sensitivity to painful stimulation) and the development or exacerbation of spontaneous pain symptoms (e.g., muscle pain, headache) [2]. This association has been demonstrated in a number of experimental models of sleep loss, where sleep is restricted or disrupted over one or more days, and effects are assessed using subjective pain reports and/or quantitative sensory testing. This bidirectional relationship between sleep deficiency and pain serves to perpetuate and amplify sleep deficiency and pain via a vicious cycle in chronic pain populations; for example, a bad night’s sleep enhances pain, which in turn disturbs sleep, and the cycle then perpetuates and amplifies over time.
In spite of this well-established bidirectional relationship between deficient sleep and pain, there is very little scientific knowledge about the basic neurochemical mechanisms underlying this reciprocal relationship. Such understanding is sorely needed to spur the development of new drugs and perhaps behavioral interventions that could help to manage or alleviate pain, potentially through targeting shared pathways modulating both sleep and pain.
Potential mechanisms underlying the relationship between sleep deficiency and chronic pain
Our current understanding of the neurobiological mechanisms of pain [3] implicate involvement of neuronal, as well as non-neuronal, components of the opioid, monoaminergic, orexinergic, immune, melatonin, and endocannabinoid systems; the hypothalamus-pituitary-adrenal axis; and adenosine and nitric oxide signaling, among others. These systems that have been hypothesized to mediate the effects of deficient sleep on pain are reviewed below.
Opioid system
Opioids exert their pharmacological actions through three opioid receptors (mu, delta and kappa), which can be activated by endogenous opioid peptides, including enkephalins, dynorphins, and endorphins. Both opioid receptors and associated peptides are broadly expressed throughout the peripheral and central nervous system. Opioid receptors can also be activated by exogenous opioids, such as morphine. In addition to the opioid system’s central role in pain modulation, it is also involved in the regulation of multiple other systems, including the stress response, immune function, and emotional modulation (for reviews, see [4, 5]).
With respect to opioid-regulated nociception and analgesia, experimental studies in healthy adults using PET imaging have shown that endogenous opioid peptides are released in various brain areas in response to experimentally-induced sustained pain, and that the degree of mu-opioid receptor activation is associated with reduced pain intensity and unpleasantness ratings [6]. In addition, PET imaging studies demonstrate that patients with chronic pain have decreased mu-opioid receptor transmission in response to painful challenges compared to a control condition [7]. It has been hypothesized that decreased central nervous system (CNS) opioid receptor availability leads to the reduced ability to inhibit pain, thereby facilitating chronic pain conditions [8].
A potential role of the opioid system in sleep deprivation induced pain hypersensitivity was first hypothesized in the late 1970s [9]. More recently, the analgesic effects of the mu-opioid agonist morphine and enkephalinase inhibition have been shown to decrease in rodent models of REM sleep deprivation [10, 11]. While the role of the opioid system in sleep-wake regulation and in mediating the hyperalgesic effects of deficient sleep has not been directly tested in humans, some studies have investigated the effects of sleep deprivation or disruption on the descending pain inhibition system. This system is in part mediated by the endogenous opioid and monoaminergic systems, and it has been shown that acute experimental sleep disruption in healthy individuals impairs the endogenous pain inhibition system [12]. Diminished pain inhibition capacity has also been observed in patients with insomnia disorder (i.e., a disorder of difficulty falling asleep or maintaining sleep that is associated with impairment in daytime functioning [13]). Together, these findings suggest that inadequate sleep deteriorates functioning of the opioid antinociceptive system.
Monoaminergic system
The monoaminergic (serotonin, norepinephrine, dopamine) and opioid systems are closely related and can interact to modulate neurobiological functions such as nociception. The monoamine serotonin is widely distributed in the periphery and the central nervous system. Along with noradrenergic neurons, the serotonergic system appears to be necessary for mu-opioid analgesic function involved in endogenous pain inhibition [14]. The involvement of serotonin receptors in the modulation of pain is further suggested by the effectiveness of serotonin reuptake inhibitors in the management of various clinical pain conditions, such as fibromyalgia [8]. While serotonin can exert analgesic as well as proalgesic effects in the CNS, it is well established for its proalgesic effects in the periphery and is an active ingredient of the ‘inflammatory soup’ (i.e., a combination of proinflammatory mediators that stimulates and sensitizes nociceptors [15]). Dual actions of serotonin with proalgesic effects in the periphery and pro- and/or analgesic effects in the CNS are likely related to the type of receptor(s) activated by serotonin, emphasizing the complex role of the serotonergic system in pain modulation [16].
The serotonergic system is also important for the control of sleep-wake behavior. In the 1970s, it was thought that serotonin initiated and maintained deep non-REM sleep; however, recent studies have shown that serotonin predominantly functions to promote wakefulness and to inhibit REM sleep [17]. The inhibition of serotonin through systemic administration of serotonin 2A/2C antagonists (e.g., ritanserin) increases deep non-REM sleep in laboratory animals, healthy sleepers, as well as in patients with insomnia [17]. Sleep deficiency has also been associated with changes in the serotonin system. In animals, acute sleep deprivation increased extracellular serotonin metabolites in the basal forebrain [18], while more chronic forms of sleep restriction reduce the sensitivity of the serotonin 1A receptor (reviewed in [19]). In humans, acute sleep deprivation has been reported to increase serotonin plasma metabolites, which have been suggested to mediate the antidepressive effect of acute sleep deprivation [20].
Given the involvement of the serotonergic system in both pain and sleep-wake control, disturbances of this system may mediate the hyperalgesic effects of deficient sleep; however, this hypothesis warrants further investigation.
Norepinephrine (also called noradrenaline) functions as a neurotransmitter in the brain, where it is synthesized by neurons located in the locus coeruleus (LC) and a few other nuclei with projections to many other brain areas. In the periphery, norepinephrine is produced and released from the adrenal glands into the bloodstream as a hormone, and also functions as a neurotransmitter in the sympathetic nervous system. Norepinephrine activates adrenergic receptors and, depending on the receptor subtype (alpha- or beta-adrenergic), causes an increase in vigilance, readiness for action, vasoconstriction, increase in heart rate, among many other effects. Thus, norepinephrine plays a key role in promoting wakefulness and arousal (reviewed in [21]).
With respect to the sleep-wake cycle, noradrenergic neurons in the LC are active during wake, less active during non-REM sleep, and almost silent during REM sleep of rats [22]. In humans, norepinephrine levels in the blood circulation decline with sleep onset and are lower during sleep compared to wakefulness (reviewed in [19]. Experimental induction of sleep deprivation increased the levels of norepinephrine transporter mRNA in the LC of rodents [23] and of norepinephrine in the blood circulation in rodents and humans [24, 25]. Increased activity of the noradrenergic system in the LC is incompatible with sleep and may contribute to insomnia and conditions characterized by elevated arousal, such as stress-related disorders (reviewed in [21]).
With respect to nociception, preclinical data suggest that enhancement of norepinephrine transmission has an analgesic effect in murine models of persistent or neuropathic pain, with a stronger analgesic effect produced when both noradrenergic and serotonergic transmissions are enhanced. In accordance, medications that inhibit the reuptake of both norepinephrine and serotonin (e.g., the serotonin-norepinephrine reuptake inhibitors [SRNI] duloxetine) are efficacious agents in the treatment of various chronic pain conditions, including fibromyalgia and chronic headaches (reviewed in [26, 27]).
The involvement of the noradrenergic system in mediating the pain-promoting effects of sleep deficiency has not been directly addressed. However, it has been demonstrated that experimentally-induced neuropathic pain in rodents increase the activity of noradrenergic neurons in the LC, which may underlie the sleep disturbances caused by neuropathic pain [28]. However, based on our current knowledge, it is unlikely that this system contributes to the pain-promoting effect of sleep disturbances, given that deficient sleep appears to increase activity in this system, which would suggest an analgesic effect.
Several findings implicate that dopamine signaling may play a mechanistic role in linking sleep deficiency and pain [29]. Dopamine is a wake-promoting mediator, and pharmacologically induced increases in dopamine tone (e.g., through administration of amphetamines or modafinil) have potent wake-promoting effects (reviewed in [30]).
In mice, administration of modafinil normalized sleep deprivation induced increases in pain sensitivity, while administration of the anti-inflammatory drug ibuprofen and the opiate morphine did not [31]. A recent study extended these findings by showing that the hyperalgesic effect of acute REM sleep deprivation in rats relates to decreased activity at dopamine D2 receptors in the nucleus accumbens, which is involved in both pain and sleep-wake cycle control [32]. Experimental administration of a D2 agonist is shown to block the hyperalgesic effect of sleep deprivation, indicating that sleep deprivation increases pain by decreasing dopaminergic activity. Clinically, such findings suggest that in patients with chronic pain comorbid with sleep disturbances, the responsiveness to analgesic drugs may be enhanced by co-administration of dopaminergic drugs [32]. Given the wake-promoting properties of dopamine, proper timing of drug administration may play a crucial role in preventing amplification of sleep disturbances by dopamine.
Adenosine signaling
Adenosine is a neuromodulator that is involved in the regulation of a wide array of diverse functions, including energy metabolism, inflammatory and immune responses, sleep-wake regulation, and nociception. It physiological effects are mediated by action on four distinct adenosine receptors that are widely expressed in cells and tissues throughout the body (reviewed in [33]). The adenosine A2A receptor, for example, is located on both neurons and glia cells in the CNS and on immune cells in the periphery [34]. Adenosine has sleep-regulatory and sleep-promoting properties, and extracellular adenosine levels in the basal forebrain and cortex have been shown to increase in proportion to wake time (reviewed in [30, 35]). Additionally, adenosine levels in the basal forebrain have been shown to increase in response to sleep fragmentation in rats, demonstrating a potential mediating effect of adenosine in the context of sleep disturbances [36].
With respect to the role of adenosine in nociception, the effects of adenosine receptor agonists and antagonists have been examined in multiple inflammatory and neuropathic pain models. Proalgesic and analgesic effects of the adenosine A2A receptor have been reported, such that systemic administration of a selective A2A receptor antagonist has been shown to produce an analgesic profile in several preclinical pain models, but also to block the analgesic effect of opioids (reviewed in [37].
To our knowledge, three animal studies have addressed the mechanistic role of adenosine in linking sleep deficiency with increased pain sensitivity. In mice, administration of caffeine, a nonselective adenosine receptor antagonist widely used as a waking stimulant, reversed pain hypersensitivity caused by sleep deprivation [31]. Caffeine also prevented the postsurgical pain hypersensitivity in rats caused by sleep deprivation prior to surgery [38]. In the same study, microinjection of a adenosine A2A receptor antagonist into the median preoptic nucleus, an area involved in sleep regulation, blocked postsurgical pain hypersensitivity caused by pre-surgical sleep deprivation, and prevented pain hypersensitivity caused by sleep deprivation alone [38]. In accordance, the hyperalgesic effect of acute REM sleep deprivation in rats correlated with increased activity at adenosine A2A receptor in the nucleus accumbens, and administration of an A2A receptor antagonist prevented the hyperalgesic effect of sleep deprivation [32]. These findings suggest that one pathway through which sleep deficiency enhances pain is through increased adenosinergic activity.
Nitric oxide signaling
Nitric oxide (NO), formed by the oxidation of nitrogen, is a potent vasodilator produced by cells in the central and peripheral nervous system that are involved in regulating immune, cardiovascular and nervous system functions. In the immune system, NO is produced by phagocytic white blood cells such as macrophages, and is involved in the killing of engulfed host-invading bacteria. In the cardiovascular system, NO is induced by a number of triggers and is synthesized in vascular endothelium by the enzyme endothelial nitric oxide synthase (eNOS) and produces smooth muscle relaxation, vasodilation, resulting in slowing of heart rate and lowering of blood pressure.
With respect to nociception, a study using a mouse genetic knockout of nitric oxide synthase (NOS), found that neuronal NOS (nNOS), particularly in the dorsal root ganglion (DRG), is very important for mechanical hypersensitivity after nerve injury. These investigators found further, that in these knockout animals systemic or spinal administration of pharmacological nNOS inhibitors attenuated the hypersensitivity. Interestingly, the nerve injury led to upregulated nNOS protein expression in the DRG but not the spinal cord, suggesting the DRG is a key site for the development of hypersensitivity [39]. The treatment of patients with complex chronic neuropathic pain with DRG stimulation is an area where clinical interventions are currently under development (reviewed in [40]).
Studies have shown that NO is another important player in the homeostatic regulation of sleep and wakefulness; in murine models, markers of NOs level in the basal forebrain were found to double during sleep deprivation [41, 42]. It was further discovered that NO production occurs upstream of adenosine increases [42]. These investigators also found that inducible NOS (iNOS) produced in wake-active neurons in the basal forebrain is positively correlated with sleep pressure [43], and that basal forebrain NO increases during sleep deprivation begin before frontal cortex increases in iNOS and NO, and are followed by increases in adenosine [44]. These results demonstrate that changes in the NO system appear to occur in advance of changes in the adenosine system and together regulate sleep pressure.
Damasceno and colleagues [45] investigated REM sleep deprivation to modulate nociception in the rat and found that NOS was increased in the periaqueductal gray matter area (PAG) in the brainstem as a result of sleep deprivation. Other studies have shown spinal level modulation of pain due to REM sleep deprivation in rats [46–48]. In addition, in a rat model of chronic pain, Tomim and colleagues [49] found that descending pain inhibitory and pain facilitatory activity in the brainstem PAG was intensified by REM sleep deprivation.
While we are aware of no studies that have investigated the role of sleep deprivation or disruption on the development of spontaneous pain or pain hypersensitivity in the genetic NOS knockout mouse model, the existing literature suggests that NO increases in the basal forebrain and frontal cortex during sleep deprivation and that it is also an important mediator of pain in the PAG. Studies of NOS knockout mice in conjunction with pharmacological testing may be helpful in furthering our site-specific understanding of how sleep disruption impacts the development of chronic pain.
Orexinergic system
Discovered in the late 1990, the orexinergic system consists of two neuropeptides (orexins A and B—also known as hypocretins 1 and 2) and the orexin-1 and -2 receptors. Orexin-producing neurons are located in the lateral hypothalamic area and project to multiple brain regions, many of which are involved in the regulation of sleep and wakefulness [50], as well as to the spinal cord [51]. Indicative of orexin’s role in sleep-wake regulation are findings of orexin deficiency associated with the sleep disorder narcolepsy in animals and humans (reviewed in [52]). Orexins are also involved in a broad range of other physiological and behavioral functions, including pain control. In the following, associations of the orexinergeric system with sleep-wake states and with pain control will be outlined.
Across the sleep-wake cycle, orexin neurons are active during wake and almost silent during sleep. This finding has been demonstrated in both murine and canine animal models [53]. Photo- and chemical stimulation of orexin neurons have been linked to wakefulness and inhibition of orexin neurons have been shown to promote sleep in animal models, supporting the hypothesis that the activity of orexin neurons is related to the behavioral states of sleep and wake [54, 55].
Experimental REM sleep deprivation in animals increases orexin A in discrete brain regions [56]. In humans, cerebrospinal fluid (CSF) orexin concentration increased after several nights of experimental sleep restriction [57], further supporting its involvement in maintaining wakefulness. Conversely, sleep fragmentation in atherosclerosis-prone mice has been recently shown to decrease expression of orexin in the hypothalamus, with a corresponding decrease of orexin A levels in plasma and bone marrow [58]. These findings suggest that sleep deficiency affects the orexinergic system, and that short and disturbed sleep may potentially have differential effects on this system. Clinically, orexin receptors are a target in the pharmacological treatment of narcolepsy and insomnia disorder. The dual orexin receptor antagonists suvorexant, for example, is currently approved for the treatment of insomnia disorder in the US and Japan [59].
Orexins neurons innervate brain regions that are also involved in nociception, such as the periaqueductal grey [60]. Further, orexin A immunoreactive fibers have been found in layers of the spinal dorsal horn, making it likely that the orexinergic system is also involved in pain transmission and modulation. Indeed, several studies have shown that intrathecal injection of orexin A produces anti-hyperalgesic and anti-allodynic effects in neuropathic, postsurgery, and inflammatory pain models in animals (reviewed in [61]. Furthermore, it has been demonstrated that the analgesic effect can be reversed by administration of a selective orexin-1 receptor antagonist, but not, for example, by administration of the opioid antagonist naloxone [62–64]. These findings indicate that the analgesic effect is mediated by activation of the orexin-1 receptor. Destruction of orexin neurons in narcolepsy patients has also been shown to be associated with a pro-inflammatory immune signature, including increased production of pro-inflammatory cytokines by T cells [65]. In light of the proalgesic effects of inflammatory molecules, the orexin-immune connection may underlie the link between sleep deficiency and pain. However, orexin neurons in the perifornical area (PeF) do not receive direct nociceptive input from the lamina I nociceptive second order neurons in the spinal cord [66], and a recent study by Asano and colleagues [67], suggested that the nociceptive information from the spinal cord is transmitted to the PeF in hypothalamus via the glutamatergic lateral parabrachial (LPB) neurons. A subpopulation of the LPB neurons that express the calcitonin gene related peptide (CGRP) encodes and processes many noxious and aversive stimuli like cutaneous pain, aversive food [68], and hypercapnia [69]. These neurons are then involved in sensory processing of these signals by its projections to the forebrain areas [68, 69]. This newly discovered circuitry to PeF via LPB and the role of the descending pain pathways in modulating pain and arousal warrants further investigations.
Collectively, these findings suggest that the orexinergic system is involved in pain transmission and modulation. Accordingly, orexin peptides and their receptors offer opportunities for developing analgesic drugs [70]. In light of the involvement of the orexinergic system in both pain and sleep-wake control, the orexinergic system may mediate the hyperalgesic effects of sleep deficiency, a dual effect yet to be investigated.
Hypothalamus-pituitary-adrenal axis
The Hypothalamus-pituitary-adrenal (HPA) axis mediates the response to both physical and psychological stress. The release of corticotropin-releasing hormone (CRH) from the hypothalamus stimulates the secretion of adrenocorticotropin hormone (ACTH) from the pituitary, which then stimulates the secretion of glucocorticoids from the adrenal cortex (cortisol in humans and corticosterone in rats). The HPA axis is tightly inter-related with the immune system such that pro-inflammatory cytokines activate the HPA axis resulting in increased production of cortisol [71]. Cortisol, in turn, inhibits pro-inflammatory cytokine production, such as interleukin (IL)-1 and IL-6, which are known to sensitize nociceptors in the periphery or pain transmission neurons in the CNS (reviewed in [72]). Thus, cortisol and synthetic glucocorticoids (e.g., prednisolone and dexamethasone) are likely to modulate the nociceptive system indirectly through changes in the secretion of proinflammatory and proalgesic cytokines and prostaglandins (for details on cytokines and prostaglandins, see section g. Immune System).
In patients with chronic pain conditions, such as rheumatoid arthritis, fibromyalgia, headaches, or low-back pain, a dysfunctional HPA axis has been reported, including HPA hypo-reactivity and basal hypocortisolism, as well as HPA hyper-reactivity and basal hypercortisolism (reviewed in [73]). Such dysfunctions disrupt the balance between the HPA and immune systems and result in weakened immunoregulation and a state of low-grade inflammation in the body. Further, most studies in human have shown that sleep deficiency causes small elevations of basal cortisol levels (reviewed in [74]) and a greater surge of cortisol response to stress [75]. For example, an experimental study design consisting of restricting sleep on weeknights and catching up on sleep over the weekend over a three-week period progressively increased morning cortisol levels in healthy participants [76]. In these healthy participants, the monocytes were much more sensitive to the counter-inflammatory cortisol signal, but this did not inhibit them from producing less of the pro-inflammatory IL-6 signal, suggesting a marked disruption in the interplay between the HPA and the immune system caused by recurrent episodes of sleep restriction and recovery [76].
Among individuals suffering from insomnia symptoms, mild increases in basal cortisol levels (reviewed in [74]) and a hyper-reactivity of the HPA axis to stressors [77] have been reported. Of interest, such hyper-reactivity has been found to mediate the relationship between deficient sleep and higher pain sensitivity [78]. This suggests that dysregulations of HPA axis responses may potentially serve as a marker for chronic pain risk associated with chronic deficient sleep.
Immune system
The immune system’s response to infection and tissue injury is inflammation and includes the production of inflammatory mediators such as prostaglandins and cytokines. Small elevations of these inflammatory mediators (identified as low-grade inflammation) can also be found in the absence of the classical inducers of a typical inflammatory response (i.e., infection and tissue injury), and are likely due to cellular stresses and malfunction (reviewed in [79]). An upregulation of inflammatory mediators can be observed in various types of pain conditions, and in response to short or disturbed sleep, as discussed below.
Prostaglandins
Prostaglandins (PGs) are classic inflammatory markers that mediate some of the cardinal symptoms of inflammation, such as fever and pain. Their involvement in the production of such symptoms is demonstrated by the therapeutic effects of nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or acetylsalicylic acid (aspirin), which primarily prevent the synthesis of PGs through inhibition of cyclooxygenase (COX-1 and/or COX-2 enzymes [80]). With respect to the effects of PGs on sleep, inhibition of PG production by COX-2 inhibitors reduced spontaneous and cytokine-induced increases in non-REM sleep in animals [81]. In humans, inhibition of PG production through acute administration of aspirin at the recommended daily dose range has been shown to disrupt sleep (i.e., decreased sleep efficiency, increased number of awakenings) and decrease slow-wave sleep (SWS) [82, 83], supporting a role of PGs in sleep modulation. The effects of chronic administration of NSAIDs on sleep are unknown Recently, the PG system has been shown to not only promote inflammation, but also to play a role in the resolution of inflammation [84]. Thus, under certain circumstances, inhibiting this system by NSAIDs may contribute to ongoing inflammation and potentially disturb sleep. Given that a large proportion of the population uses NSAIDs on a regular basis [85], future research should address their long-term effects on sleep.
Regarding the effects of sleep deficiency on prostaglandin production, experimental sleep deprivation leads to increased levels of various PGs in the CSF of animals [86] and to increased urinary PGE2 metabolite levels in humans [87]. The latter increase was associated with greater pain reporting, suggesting that PGE2 mediates the hyperalgesic effects of sleep deficiency.
Cytokines
In addition to PGs, cytokines (e.g., IL-1, IL-6, tumor necrosis factor [TNF] alpha), have also been identified as potent pronociceptive factors capable of sensitizing peripheral sensory and central nociceptive neurons, thereby promoting hyperalgesia [72]. IL-6, for example, is a small protein that is produced mainly by circulating monocytes and macrophages but also by CNS immune cells, such as glial cells. Elevations in IL-6 in the periphery and spinal cord have been shown in various animal pain models [88]. Administration of IL-6 can lead to hyperalgesia, and IL-6 blockers (e.g., IL-6 receptor antibody tocilizumab) can reduce pain hypersensitivity [88], supporting an important role of IL-6 in the induction or amplification of pain. With respect to sleep, IL-6 and other inflammatory cytokines increase in response to short or disturbed sleep, and such cytokine elevations indicate a state of low-grade inflammation in the body [79]. Ongoing or chronic low-grade inflammation is thought to increase risk of a number of chronic health diseases, including cardiovascular, metabolic, neurodegenerative, and chronic pain conditions [79]. In the human experimental setting, elevations in blood IL-6 levels correlate with increased pain reporting after prolonged sleep restriction [89]. Thus, low-grade inflammation may constitute a mechanism that links short or disturbed sleep to chronic pain; however, causality is yet to be demonstrated.
Pineal melatonin system
The synthesis of melatonin, the main hormone secreted by the pineal gland, is stimulated by darkness and suppressed by light. In humans, peak melatonin levels occur during the night. The threshold to suppress melatonin has been estimated to be as low as 30 lux [90], which is well below normal fluorescent light of about 300–400 lux. Decreased suppression at night has been observed in shift workers exposed to artificial lighting at night, but also in individuals using light-emitting electronic devices, such as smartphones or tablets, before bedtime [91].
Melatonin has many actions and properties, including anti-inflammatory, analgesic, and sleep-promoting effects (reviewed in [92]). For example, melatonin can downregulate inflammatory mediators including prostaglandins and cytokines (reviewed in [93]), both markers known for their pain-sensitizing actions. Mechanisms of melatonin’s analgesic properties are not entirely clear but appear to involve endorphins, GABA receptor, opioid receptors, and the nitric oxide-arginine pathway [94]. Potentiating the melatonin signal with exogenous melatonin administration can have a beneficial effect on sleep in certain sleep disorders, including insomnia disorder (i.e., a disorder of difficulty falling asleep or maintaining sleep that is associated with impairment in daytime functioning) and circadian rhythm sleep disorder of the delayed sleep phase type (i.e., a disorder characterized by delayed habitual bedtime and delayed rising time that is in conflict with work or lifestyle requirements (reviewed in [95]. In these patients, melatonin improves sleep by reducing sleep onset latency or by regulating sleep-wake times (reviewed in [95].
In patients suffering from chronic pain conditions, such as fibromyalgia and irritable bowel syndrome, exogenous administration of melatonin has been shown to reduce subjective pain [96, 97] and improve endogenous pain inhibition in fibromyalgia [98]. In animals, the administration of melatonin attenuated the development of neuropathic pain following nerve injury [99], suggesting that the melatonin system presents another potential mechanism through which deficient sleep facilitates pain.
Endocannabinoid system
The endocannabinoid system is a phylogenetically ancient system that appears to date back to the unicellular common ancestor of animals and plants. Endocannabinoids are lipid mediators that bind to cannabinoid receptors expressed in the central and peripheral nervous system. Besides endogenous cannabinoids (e.g., anandamide), exogenous cannabinoids target these receptors, including constituents of the cannabis plant (i.e., the psychoactive delta(9)-tetrahydrocannbinol [THC] and the nonpsychoactive cannabidiol [CBD]). The endocannabinoid system is involved in the regulation of a wide range of biological functions, including the modulation of pain and sleep, as reviewed below. Recently, cannabinoids have been increasingly used in the treatment of chronic pain. The cannabinoid system is known to play a modulating role in analgesia and sleep. A recent systematic review including over 6000 chronic pain patients showed that the average number of patients who reported a reduction in pain of at least 30% was greater with cannabinoids than with placebo [100].
In preclinical studies using diverse inflammatory and neuropathic pain models, cannabinoids have been found to exert antinociceptive effects [101]. The endocannabinoid system has been shown to attenuate the inflammatory response in inflammatory pain models, suggesting that inflammation constitutes a pathway by which endocannabinoids reduce the experience of pain (reviewed in [101]. Cannabinoids appear to also have an opioid-sparing action. For example, cannabinoid receptor agonists reduced the opioid dose needed to produce anti-nociception in pre-clinical inflammatory pain models (reviewed in [102]). This suggests an interplay between endogenous opioid and cannabinoid systems. However, the opioid-sparing effect of cannabinoids is less clear from the findings of large controlled clinical trials (reviewed in [102]). Whether the promotion of the endocannabinoid system is an effective therapeutic strategy to reduce opioid use is still an open question.
The endocannabinoid system has been shown to exhibit a diurnal rhythm across the normal sleep-wake cycle, with higher levels during wakefulness (reviewed in [103]. Furthermore, sleep restriction increased circulating levels of endocannabinoids in healthy humans [104, 105]. This increase may explain greater appetite induced by deficient sleep. However, it may not explain greater pain reporting and pain sensitivity commonly observed in response to deficient sleep [2], suggesting that mediators other than cannabinoids contribute to pain amplification following sleep deficiency.
Research on exogenous cannabinoids on sleep is still in early stages and the results to date have been inconsistent. Preliminary findings suggest that the cannabinoid cannabidiol (CBD, the nonpsychoactive constituent of cannabis), does not affect sleep parameters in healthy sleepers [106]; however, it may have a beneficial effect in the treatment of insomnia disorder. For example, high-dose CBD (unlike low-dose CBD) has been shown to increase total sleep time and reduce arousal frequency in individuals suffering from insomnia disorder (reviewed in [107].
Some studies have addressed the potential role of cannabinoids in patients with chronic pain conditions comorbid with sleep disturbances. Based on a recent systematic review, 22 out of 29 randomized clinical trials showed that cannabinoid use is associated with a modest analgesic effect in the management of chronic non-cancer pain, with some trials reporting a concomitant improvement in subjective sleep [108]. For example, in patients with diabetic peripheral neuropathic pain, the synthetic cannabinoid nabilone was associated with improvements in pain relief and subjective sleep when compared to placebo [109]. Similarly, in various neuropathic pain conditions, a THC and CBD oromucosal spray treatment improved pain and sleep quality when compared to placebo treatment [110]. Administration of THC also appears to have a beneficial effect on muscle stiffness, pain and sleep in multiple sclerosis patients [111]. Further, Sativex, a 1:1 TCH/CBD compound, has demonstrated significant beneficial effects on both pain and sleep quality among a small sample of patients with rheumatoid arthritis [112]. In summary, the research of cannabinoids effect on sleep and pain is relatively new and controlled longitudinal studies are needed to better understand both the underlying mechanisms and their clinical implications.
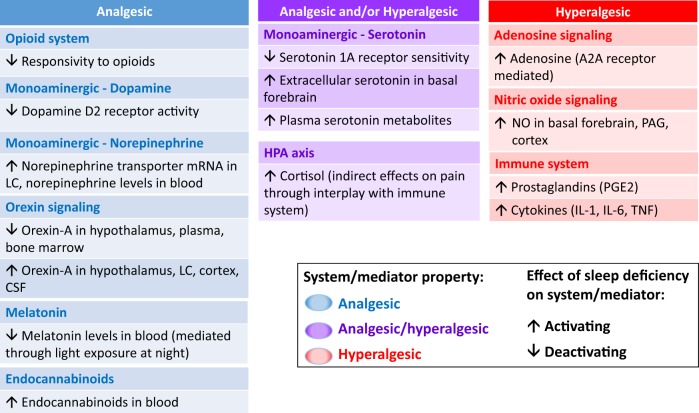
Figure 1 summarizes findings on the effects of sleep deficiency on neurobiological systems and mediators with predominantly analgesic, hyperalgesic, or dual analgesic and hyperalgesic properties. Collectively, sleep deficiency appears to have a de-activating effect on several systems/mediators with predominantly analgesic properties, including the opioid system, the orexinergic system, the melatonin system, and dopamine signaling, while activating systems/mediators with predominantly hyperalgesic properties, including NO and adenosine signaling, and inflammatory mediators of the immune system.
Fig. 1.
Effects of sleep deficiency on neurobiological systems/mediators with predominantly analgesic or hyperalgesic properties. Systems/mediators with predominantly analgesic properties are presented in blue, those with analgesic and/or hyperalgesic properties (depending on site of action and receptors involved) are presented in purple, and those with predominantly hyperalgesic properties are presented in red. The effect of sleep deficiency on systems/mediators is indicated by up- and down- arrows, with up-arrows reflecting an activating influence and down-arrows reflecting a deactivating influence on systems/mediators. For example, activation of the opioid system has analgesic effects (blue), and sleep deficiency leads to reduced responsivity to exogenous opioids (down-arrow), suggesting that the pain-promoting effect of sleep deficiency is mediated by the opioid system. Not depicted are the many interactions between these systems and mediators, further emphasizing the complexity of the neurobiological mechanisms involved in the pain-promoting effects of sleep deficiency. LC: Locus coeruleus; CSF: cerebrospinal fluid; HPA: hypothalamus-pituitary adrenal; NO: nitric oxide, NOS: NO synthase; PAG: periaqueductal gray; IL: interleukin; TNF: tumor necrosis factor
Clinical implications
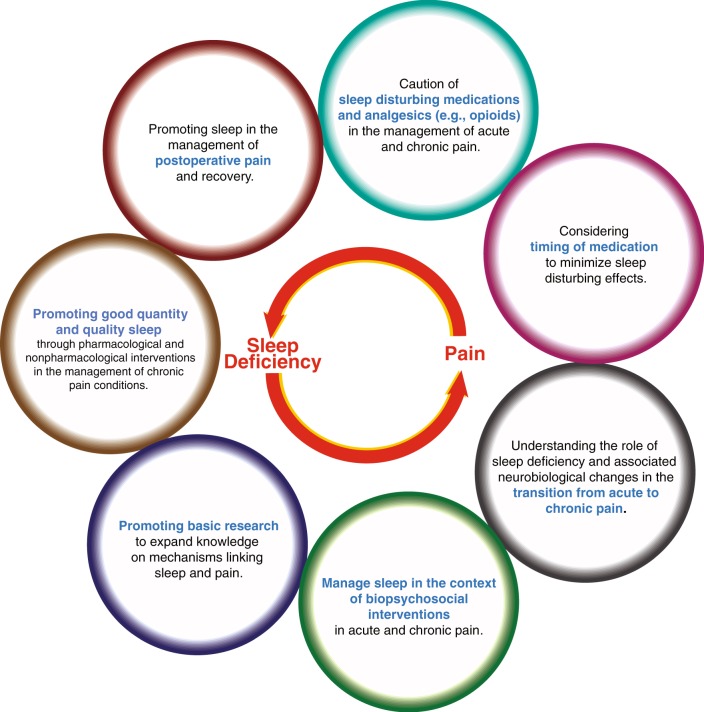
In the following section, clinical implications of the bidirectional relationship between sleep and pain and its underlying mechanisms will be discussed, including behavioral and pharmacological approaches to manage chronic pain comorbid with sleep disturbances, interventional approaches to reduce postoperative pain, as well as sleep-disturbing and sleep promoting medications to be considered in pain management (Fig. 2). This domain is of great clinical interest, as demonstrated by a large scale study demonstrating that short term improvements in insomnia symptoms predict long-term improvement in both sleep and pain, regardless of the treatment modality pharmacological or non-pharmacological [113]. This finding emphasizes the hypothesis that improving sleep might yield a better pain relief, particularly as sleep appears to be a more reliable predictor of pain than pain is that of sleep [114].
Fig. 2.
Clinical implications and research opportunities based on current knowledge of the sleep-pain relationship
Interventions to manage chronic pain comorbid with sleep deficiency
Non-pharmacological approaches
Non-pharmacological sleep interventions, such as sleep hygiene (i.e., good sleep habits), mindfulness, and relaxation training, are effective strategies to improve sleep quality in populations reporting poor sleep health (reviewed in [115]). In clinical populations meeting diagnostic criteria for insomnia disorder, cognitive behavioral therapy for insomnia (CBT-I), is considered the first-line treatment [116]. CBT-I is a multi-component intervention that includes sleep hygiene education, time in bed restriction, stimulus control, targeting of negative cognitions about sleep, and relaxation tools; and has been shown to be an effective intervention in populations with both chronic pain and insomnia. For example, in patients with comorbid insomnia and osteoarthritis, CBT-I led to objective reductions in the time spent awake during the night, which in turn predicted reductions in clinical pain [117]. In patients with comorbid insomnia and fibromyalgia, combined CBT-I and CBT-P (P stands for pain) was found to improve both subjective sleep measures and pain severity [118].
In recent years, studies on CBT-I have begun to incorporate physiological assessments, which allows for a better understanding of biological changes associated with CBT-I. In adults suffering from insomnia disorder, CBT-I has been found to result in lower levels of C-reactive protein (CRP), an acute phase protein regulated by IL-6, and this decrease was associated with remission of insomnia [119]. Additionally, TNF and IL-6 expression by monocytes were lower post-CBT-I treatment, and gene transcripts involved in inflammation were downregulated [120]. These immune effects suggest that CBT-I in adults suffering from insomnia disorder reduces both insomnia symptoms and inflammation. Recently, the impact of CBT-I on immune markers has also been investigated in populations with insomnia comorbid with chronic pain. In patients with osteoarthritis knee pain, improvement of insomnia was paralleled by improved physical functioning, a decline in knee pain, and a reduced IL-6 response to an experimental pain challenge [121]. These findings suggest that improving sleep has a beneficial effect on various inflammatory mediators, which may underlie the observed improvement of chronic pain following CBT-I. However, further research is needed to substantiate the mechanistic role of these and other mediators in the association between sleep deficiency and pain.
Pharmacological approaches
Pharmacological interventions targeting sleep
Pharmacological interventions in the treatment of insomnia include GABAA receptor agonists non-benzodiazepine hypnotics (e.g., zolpidem, zopiclone), melatonin receptor agonists (e.g., ramelteon), antidepressants (e.g., trazodone, doxepin), orexin receptor antagonists (e.g., suvorexant), GABAA receptors agonists benzodiazepines (e.g., lorazepam), and gabapentin-receptor α2δ subunit antagonist gabapentinoids, such as gabapentin and pregabalin [59]. Several studies have investigated the effects of pharmacotherapy on both sleep and pain in patients with sleep disturbances comorbid with chronic pain, and addressed the question whether successfully managing sleep disturbances can improve pain symptoms (reviewed in [122]). For example, in patients with rheumatoid arthritis, the non-benzodiazepine zopiclone improved sleep, but this effect was not paralleled by an improvement in pain, while the benzodiazepine triazolam improved both sleep and pain parameters (reviewed in [123]. These discrepant results could be related to the more targeted impact of non-benzodiazepine selective agonists at GABAA receptors in sleep-promoting neural domains compared to the broader impact of benzodiazepines in the brain. In patients with temporomandibular joint dysfunction, melatonin has been shown to improve sleep quality, decrease pain scores, and reduce analgesic consumption [124]. To date, sleep promoting pharmacotherapy studies have not shown consistent improvement in comorbid chronic pain, suggesting a potentially complex reciprocal sleep-pain relationship across chronic pain disorders.
Recently, cannabinoids have been increasingly used in the treatment of chronic pain. The cannabinoid system has been proposed to play a favorable modulating role on analgesia and sleep. A recent systematic review including over 6000 chronic pain patients showed that the average number of patients who reported a reduction in pain of at least 30% was greater with cannabinoids than with placebo [100]. A review on the effects of cannabinoids on objective sleep measures showed inconsistent findings in the general population, though in clinical populations, findings suggest that cannabinoids may improve sleep via reducing pain [125]. The research of cannabinoid effects on sleep in pain conditions is relatively new and controlled and longitudinal studies are needed to advance our understanding and clinical implications.
Pharmacological interventions targeting inflammation
Few studies have targeted inflammatory pathways in the management of chronic pain, with some also assessing the effects on sleep. Immune dysregulation plays a pathophysiological role in various chronic pain conditions and can be the cause or the consequence of deficient sleep (reviewed in [79]). Consequently, immunotherapy may not only be effective to improve disease activity, but also to improve sleep. In patients with inflammatory bowel diseases (i.e., Crohn’s disease and ulcerative colitis), administration of the anti-inflammatory agents anti-integrin (vedolizumab) or anti-TNF (infliximab or adalimumab) resulted in improved sleep quality within six weeks of therapy initiation, although associations with pain intensity were not reported [126]. Similarly, in patients with ankylosing spondylitis, a chronic inflammatory disease affecting the axial skeleton, thoracic cage, and muscle-joint pain, anti-TNF therapies improved subjective sleep quality, and this improvement was associated with a reduction in disease activity and pain [127]. In patients with rheumatoid arthritis, a chronic progressive autoimmune disease involving excessive production of various cytokines, in particular TNF, several studies have reported subjective and objective (polysomnography) sleep improvements in response to anti-TNF therapy. For example, anti-TNF infusion treatment with infliximab in patients with active disease decreased time to fall asleep and increased sleep efficiency, but these sleep improvements did not relate to joint pain amelioration. Thus, the effect on sleep may independently resulted from the inhibition of TNF actions in the CNS [128]. Treatment with an IL-6 receptor inhibitor (tocilizumab) in rheumatoid arthritis patients with active disease improved self-reported sleep quality and daytime sleepiness. The observed sleep improvement could not be explained by a reduction in disease activity, further suggesting a direct effect of cytokines on sleep regulation that is independent of disease activity [129].
The above findings suggest that cytokine therapy has a beneficial effect on sleep in chronic inflammatory diseases, which may be independent from its effect on improvement in disease activity in certain conditions and populations.
Interventions to reduce postoperative pain
Postoperative pain is a major health care challenge that remains undermanaged (reviewed in [130]). Sleep patterns in the postoperative period can be severely disrupted and shortened with a suppression of both slow-wave and rapid-eye-movement (REM) sleep [131]. The quantity and quality of sleep after surgery are influenced by a multitude of factors, including hospital-related environmental factors (e.g., noise, light), interruptions in sleep due to nurse checks or other medical interventions, the extent of tissue injury, the effectiveness of the analgesics, and the activation of the surgical stress response, as well as pain [132].
Patients experiencing postsurgical pain are often provided with analgesic medication. Opioids, while a potentially effective form of short term pain management, have significant risks for both dependence and mortality [133]. Opioids also have a broad physiological action, including both significant sedative effects [134] and sleep-disruptive effects (reviewed in [135, 136]. This suggests opposing actions of opioids alleviating pain while also disrupting sleep. Opioids also potentially aggravate pain by inducing pain hypersensitivity, or hyperalgesia, via administration of select short-acting opioids or intermediate-acting opioids when administered in high doses or for extended time [137]. It has also been observed that opioids may specifically exaggerate postoperative sleep disturbances [131], thereby potentially contributing to greater postoperative pain observed following opioid treatment [138]. A mild sleep-disrupting effect has been reported for acute treatment with NSAIDs (e.g., aspirin, ibuprofen). Given their beneficial effect in acute pain management [138], the potentially mildly sleep disturbing effect of NSAIDs is outweighed by the effect of reducing the interference of pain in sleep processes.
Gabapentinoids (gabapentin, pregabalin) have been reported to improve postoperative pain management as demonstrated by reduced opioid consumption and pain scores [138], and have also been shown to improve insomnia symptoms in patients with fibromyalgia and anxiety disorders [59]. In a clinical study that investigated the effects of perioperative use of pregabalin on both sleep and pain in patients after intracranial surgery, preoperative sleep quality improved and postoperative pain scores and analgesic usage were reduced compared to placebo [139]. However, the relationship between changes in sleep quality and pain was not examined (e.g., whether improvements in sleep preceded reduced analgesic use or vice versa). Of note, both gabapentin and pregabalin are associated with significant side effects, including sedation and dizziness [140], which need to be considered in balancing the clinical benefits and risks in the management of acute postoperative pain.
Several studies have investigated the effect of melatonin on sleep in the postoperative period, and most of those studies reported a beneficial effect on sleep [141]. In breast cancer patients, for example, melatonin administered pre- and post-operatively increased sleep efficiency as objectively measured by actigraphy; however, subjectively assessed postoperative pain did not differ between melatonin and placebo administration [142]. Given melatonin’s favorable side effect profile and its sleep promoting and analgesic properties reported in many studies, rigorous and methodologically well-designed clinical investigations are needed to better understand the role of melatonin on the relationship between sleep and postoperative pain.
Preoperative sleep disturbances the night before surgery have also been shown to increase postoperative pain. In breast cancer patients, lower sleep efficiency the night prior to surgery was associated with higher self-reported pain after surgery, and this association was independent of factors such as use of perioperative analgesics, psychosocial distress, or depression [143]. In animals, sleep loss the night prior to surgery caused a marked increase in mechanical hypersensitivity after surgery and prolonged postoperative recovery time [38]. Thus, obtaining good quantity and quality sleep the night prior to surgery may serve as an interventional target in the management of surgical pain.
In summary, pre- and postoperative sleep and pain management with pharmacological and non-pharmacological approaches are likely to improve sleep, reduce postoperative pain, and accelerate recovery processes with variable influences on the relationship between pain and sleep. However, there are risks of some medications (e.g., opioids) for both increasing daytime sedation as well as disrupting nighttime sleep. In view of the complex influence of the extent of tissue injury and the perioperative psychosocial factors on both pain and sleep, further investigations are needed to better understand the complex biological nature of a combination of nociceptive, neuropathic and inflammatory processes and psychosocial factors on postoperative pain and its relationship to sleep disruption.
Sleep-disturbing medications and pain management
A number of medications for the treatment of various diseases have sleep disturbing effects, thereby potentially augmenting pain in patients with acute and chronic pain. Sleep disturbing or altering effects have not only been shown for some analgesics (in particular opioids; see above), but also other classes of drugs, including psychotropic medications (e.g., antidepressants), cardiovascular drugs (e.g., beta-blocking agents), and corticosteroids (reviewed in [144]). As mentioned earlier, despite transient benefits for pain management, chronic and acute opioid use generally disrupt sleep, as indicated by reduced slow wave sleep, REM sleep suppression, and increased awakenings and arousal during sleep [144]. In addition to the known risks for dependence and abuse, chronic opioid use increases both daytime sedation and the prevalence of breathing-related disorders, particularly central sleep apnea.
Antidepressants used in the treatment of chronic pain conditions can have variable effects on sleep, depending on the drug class and dose. For example, low-dose tricyclic antidepressants (e.g., amitriptyline, doxepin) have sleep-promoting effects, including increased slow wave sleep and sleep continuity, while activating tricyclic antidepressants (e.g., imipramine) have sleep-disrupting properties (reviewed in [145]. Mirtazapine, a medication with antihistamine, α2-blocker and antiserotonergic activity is effective for both treatment of depression and insomnia, albeit in different dose ranges [146], has been demonstrated to be effective on both sleep and pain in patients with fibromyalgia [147]. Reported side effects of beta-adrenergic blocking agents (e.g., propranolol, atenolol) include insomnia. These agents also inhibit melatonin production, thereby interfering with melatonin’s sleep-promoting and circadian phase regulatory properties, which may underlie the sleep-disturbing effect of beta-blocker [148].
Corticosteroids (e.g., cortisone, prednisone) are used as an immunosuppressant drug in a wide array of medical conditions, including certain inflammatory and autoimmune diseases and some types of cancer. Greater endogenous cortisol secretion and/or dysregulation of the diurnal cortisol rhythm have been frequently reported in insomnia and may be responsible for insomnia symptoms [149]. Based on cross-sectional studies, about 50% of patients exposed to systemic corticosteroids report sleep disturbances [150, 151]. Currently, there are no studies on objectively measured sleep changes in response to corticosteroid therapy in patients, or how these changes may depend on dose and duration of use.
To summarize, adjustments of dosage and timing of pharmacological treatments for both pain and sleep should be considered in chronic pain patients with multiple comorbidities in order to keep associated sleep-disturbing effects at a minimum, thereby preventing or reducing the pain-augmenting effect of sleep disturbances.
Conclusions and future research directions
Sleep deficiency affects various systems known to influence nociceptive processing, including the opioid, monoaminergic, orexinergic, immune, melatonin, and endocannabinoid systems; the hypothalamus-pituitary-adrenal axis; and adenosine and nitric oxide signaling, among others. Based on our current knowledge, sleep deficiency appears to have a de-activating effect on several systems/mediators with predominantly analgesic properties, including the opioid system, the orexinergic system, the melatonin system, and dopamine signaling, while activating systems/mediators with predominantly hyperalgesic properties, including NO and adenosine signaling, and inflammatory mediators of the immune system.
Within the highly complex and reciprocal interactions among these organized neurobiological systems, potential modulating pathways may exist by which short and disturbed sleep promote pain amplification. The current knowledge on the interplay between sleep and pain apparently plays a role in enhancing pain in various clinical acute and chronic conditions. As described, clinical implications are that both behavioral and pharmacological approaches are necessary for the optimal management of the coexistence of chronic pain with insomnia. Interventional approaches in the management of acute postoperative pain is complicated by the release of proinflammatory mediators, nociception, psychosocial and environmental stressors, as well as confounded by the administration of sleep disturbing analgesics, therefore requiring multimodal management. In chronic pain disorders, CBT-I is most effective in addressing sleep disturbance. The incorporation of physiological measures (e.g., immune mediators) in interventional studies will further our understanding of mechanisms through which interventions influence sleep and pain. Clarification of these mechanisms is crucial for the development of more refined therapeutic strategies to improve both sleep and pain control in future research.
Funding and disclosure
This work was supported by grant NIH/NINDS NS112175, NIH/NHLBI HL125379, NIH/NHLBI HL105544, NIH/NINDS NS091177 and NIH/NHLBI HL136310. The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Afolalu EF, Ramlee F, Tang NKY. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. 2018;39:82–97. doi: 10.1016/j.smrv.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–52. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolf CJ. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441–51. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 4.Ferdousi M, Finn DP. Stress-induced modulation of pain: role of the endogenous opioid system. Opioid Syst Interface Brain’S Cogn Motiv Syst. 2018;239:121–77. doi: 10.1016/bs.pbr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zubieta JK, Smith YR, Bueller JA, Xu YJ, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 7.Martikainen IK, Pecina M, Love TM, Nuechterlein EB, Cummiford CM, Green CR, et al. Alterations in endogenous opioid functional measures in chronic back pain. J Neurosci. 2013;33:14729–37. doi: 10.1523/JNEUROSCI.1400-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Yvonne C, Nassikas Nicholas J, Clauw Daniel J. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Research & Therapy. 2011;13(2):211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hicks RA, Moore JD, Findley P, Hirshfield C, Humphrey V. REM-sleep deprivation and pain thresholds in rats. Percept Mot Skills. 1978;47:848–50. doi: 10.2466/pms.1978.47.3.848. [DOI] [PubMed] [Google Scholar]
- 10.Ukpanmwan OE, Rupreht J, Dzoljic MR. REM-sleep deprivation decreases the antinociceptive property of enkephalinase-inhibition, morphine and cold-water-swim. Gen Pharm. 1984;15:255–8. doi: 10.1016/0306-3623(84)90170-8. [DOI] [PubMed] [Google Scholar]
- 11.Nascimento DC, Andersen ML, Hipolide DC, Nobrega JN, Tufik S. Pain hypersensitivity induced by paradoxical sleep deprivation is not due to altered binding to brain mu-opioid receptors. Behav Brain Res. 2007;178:216–20. doi: 10.1016/j.bbr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 13.Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16:522–33. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 15.Steen KH, Steen AE, Reeh PW. A dominant role of acid ph in inflammatory excitation and sensitization of nociceptors in rat skin, in-vitro. J Neurosci. 1995;15:3982–9. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viguier F, Michot B, Hamon M, Bourgoin S. Multiple roles of serotonin in pain control mechanisms -Implications of 5-HT7 and other 5-HT receptor types. Eur J Pharm. 2013;716:8–16. doi: 10.1016/j.ejphar.2013.01.074. [DOI] [PubMed] [Google Scholar]
- 17.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011;15:269–81. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Zant JC, Leenaars CHC, Kostin A, van Someren EJW, Porkka-Heiskanen T. Increases in extracellular serotonin and dopamine metabolite levels in the basal forebrain during sleep deprivation. Brain Res. 2011;1399:40–8. doi: 10.1016/j.brainres.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci USA. 2014;111:10761–6. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16:187–97. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basheer R, Magner M, Mccarley RW, Shiromani PJ. REM sleep deprivation increases the levels of tyrosine hydroxylase and norepinephrine transporter mRNA in the locus coeruleus. Mol Brain Res. 1998;57:235–40. doi: 10.1016/s0169-328x(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 24.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 25.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin- 2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 26.Clauw DJ. Fibromyalgia a clinical review. Jama-J Am Med Assoc. 2014;311:1547–55. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 27.Marks DM, Shah MJ, Patkar AA, Masand PS, Park GY, Pae CU. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol. 2009;7:331–6. doi: 10.2174/157015909790031201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh K, Hamada A, Hamada Y, Yanase M, Sakaki M, Someya K, et al. Possible involvement of activated locus coeruleus-noradrenergic neurons in pain-related sleep disorders. Neurosci Lett. 2015;589:200–6. doi: 10.1016/j.neulet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17:173–83. doi: 10.1016/j.smrv.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown RE, Basheer R, McKenna JT, Strecker RE, Mccarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexandre C, Latremoliere A, Ferreira A, Miracca G, Yamamoto M, Scammell TE, et al. Decreased alertness due to sleep loss increases pain sensitivity in mice. Nat Med. 2017;23:768–74. doi: 10.1038/nm.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sardi NF, Tobaldini G, Morais RN, Fischer L. Nucleus accumbens mediates the pronociceptive effect of sleep deprivation: the role of adenosine A(2A) and dopamine D-2 receptors. Pain. 2018;159:75–84. doi: 10.1097/j.pain.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 33.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98:1591–625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- 34.Antonioli L, Csoka B, Fornai M, Colucci R, Kokai E, Blandizzi C, et al. Adenosine and inflammation: what’s new on the horizon? Drug Discov Today. 2014;19:1051–68. doi: 10.1016/j.drudis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15:123–35. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 36.McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, Mccarley RW, et al. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–73. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2016;338:1–18. doi: 10.1016/j.neuroscience.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Hambrecht-Wiedbusch VS, Gabel M, Liu LJ, Imperial JP, Colmenero AV, Vanini G. Preemptive caffeine administration blocks the increase in postoperative pain caused by previous sleep loss in the rat: a potential role for preoptic adenosine A(2A) receptors in sleep-pain interactions. Sleep. 2017;40:zsx116. doi: 10.1093/sleep/zsx116. [DOI] [PubMed] [Google Scholar]
- 39.Guan Y, Yaster M, Raja SN, Tao YX. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain. 2007;3:29. doi: 10.1186/1744-8069-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison C, Epton S, Bojanic S, Green AL, FitzGerald JJ. The efficacy and safety of dorsal root ganglion stimulation as a treatment for neuropathic. Pain: A Lit Rev Neuromodulation. 2018;21:225–33. doi: 10.1111/ner.12685. [DOI] [PubMed] [Google Scholar]
- 41.Kalinchuk AV, Lu Y, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem. 2006;99:483–98. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- 42.Kalinchuk AV, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur J Neurosci. 2006;24:1443–56. doi: 10.1111/j.1460-9568.2006.05019.x. [DOI] [PubMed] [Google Scholar]
- 43.Kalinchuk AV, Mccarley RW, Porkka-Heiskanen T, Basheer R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J Neurosci. 2010;30:13254–64. doi: 10.1523/JNEUROSCI.0014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalinchuk AV, Mccarley RW, Porkka-Heiskanen T, Basheer R. The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J Neurochem. 2011;116:260–72. doi: 10.1111/j.1471-4159.2010.07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damasceno F, Skinner GO, Araujo PC, Ferraz MMD, Tenorio F, de Almeida OMMS. Nitric oxide modulates the hyperalgesic response to mechanical noxious stimuli in sleep-deprived rats. Bmc Neurosci. 2013;14:92. doi: 10.1186/1471-2202-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei H, Hao B, Huang JL, Ma AN, Li XY, Wang YX, et al. Intrathecal administration of a gap junction decoupler, an inhibitor of Na+−K+−2C1(−) cotransporter 1, or a GABA(A) receptor agonist attenuates mechanical pain hypersensitivity induced by REM sleep deprivation in the rat. Pharmacol Biochem Behav. 2010;97:377–83. doi: 10.1016/j.pbb.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Wei H, Ma A, Wang YX, Pertovaara A. Role of spinal 5-HT receptors in cutaneous hypersensitivity induced by REM sleep deprivation. Pharm Res. 2008;57:469–75. doi: 10.1016/j.phrs.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Wei H, Zhao WJ, Wang YX, Pertovaara A. Pain-related behavior following REM sleep deprivation in the rat: Influence of peripheral nerve injury, spinal glutamatergic receptors and nitric oxide. Brain Res. 2007;1148:105–12. doi: 10.1016/j.brainres.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 49.Tomim DH, Pontarolla FM, Bertolini JF, Arase M, Tobaldini G, Lima MMS, et al. The pronociceptive effect of paradoxical sleep deprivation in rats: evidence for a role of descending pain modulation mechanisms. Mol Neurobiol. 2016;53:1706–17. doi: 10.1007/s12035-014-9059-0. [DOI] [PubMed] [Google Scholar]
- 50.Peyron Christelle, Tighe Devin K., van den Pol Anthony N., de Lecea Luis, Heller H. Craig, Sutcliffe J. Gregor, Kilduff Thomas S. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. The Journal of Neuroscience. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Date Y, Mondal MS, Matsukura S, Nakazato M. Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neurosci Lett. 2000;288:87–90. doi: 10.1016/s0304-3940(00)01195-2. [DOI] [PubMed] [Google Scholar]
- 52.Mahoney CE, Cogswell A, Koralnik IJ, Scammell TE. The neurobiological basis of narcolepsy. Nat Rev Neurosci. 2019;20:83–93. doi: 10.1038/s41583-018-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Front Pharmacol. 2014;5:16. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4U9. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. Plos One. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta R, Khanday MA, Mallick BN. REM sleep loss associated changes in orexin-A levels in discrete brain areas in rats. Neurosci Lett. 2015;590:62–67. doi: 10.1016/j.neulet.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 57.Olsson M, Arlig J, Hedner J, Blennow K, Zetterberg H. Sleep deprivation and cerebrospinal fluid biomarkers for Alzheimer’s disease. Sleep. 2018;41:zsy025. doi: 10.1093/sleep/zsy025. [DOI] [PubMed] [Google Scholar]
- 58.McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566:383–7. doi: 10.1038/s41586-019-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197–245. doi: 10.1124/pr.117.014381. [DOI] [PubMed] [Google Scholar]
- 60.Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci. 2011;31:14600–10. doi: 10.1523/JNEUROSCI.2671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Razavi BM, Hosseinzadeh H. A review of the role of orexin system in pain modulation. Biomed Pharm. 2017;90:187–93. doi: 10.1016/j.biopha.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 62.Toyama S, Shimoyama N, Shimoyama M. The analgesic effect of orexin-A in a murine model of chemotherapy-induced neuropathic pain. Neuropeptides. 2017;61:95–100. doi: 10.1016/j.npep.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T, Nozaki-Taguchi N, Chiba T. Analgesic effect of intrathecally administered orexin-A in the rat formalin test in the rat hot plate test. Br J Pharm. 2002;137:170–6. doi: 10.1038/sj.bjp.0704851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng JK, Chou RCC, Hwang LL, Chiou LC. Antiallodynic effects of intrathecal orexins in a rat model of postoperative pain. J Pharmacol Exp Ther. 2003;307:1065–71. doi: 10.1124/jpet.103.056663. [DOI] [PubMed] [Google Scholar]
- 65.Hartmann FJ, Bernard-Valnet R, Queriault C, Mrdjen D, Weber LM, Galli E, et al. High-dimensional single-cell analysis reveals the immune signature of narcolepsy. J Exp Med. 2016;213:2621–33. doi: 10.1084/jem.20160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468:24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- 67.Asano H, Arima Y, Yokota S, Fujitani M. New nociceptive circuits to the hypothalamic perifornical area from the spinal cord and spinal trigeminal nucleus via the parabrachial nucleus. Biochem Biophys Res Commun. 2019;512:705–11. doi: 10.1016/j.bbrc.2019.02.153. [DOI] [PubMed] [Google Scholar]
- 68.Campos CA, Bowen AJ, Roman CW, Palmiter RD. Encoding of danger by parabrachial CGRP neurons. Nature. 2018;555:617–+. doi: 10.1038/nature25511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, et al. A genetically defined circuit for arousal from sleep during hypercapnia. Neuron. 2017;96:1153–67. doi: 10.1016/j.neuron.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilron I, Dickenson AH. Emerging drugs for neuropathic pain. Expert Opin Emerg Drugs. 2014;19:329–41. doi: 10.1517/14728214.2014.915025. [DOI] [PubMed] [Google Scholar]
- 71.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinology. 2009;5:374–81. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 72.Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39:240–55. doi: 10.1016/j.it.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 73.Woda A, Picard P, Dutheil F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology. 2016;71:127–35. doi: 10.1016/j.psyneuen.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 74.Balbo M, Leproult R.Van Cauter E, Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010:759234. [DOI] [PMC free article] [PubMed]
- 75.Minkel J, Moreta M, Muto J, Htaik O, Jones C, Basner M, et al. Sleep deprivation potentiates hpa axis stress reactivity in healthy adults. Health Psychol. 2014;33:1430–4. doi: 10.1037/a0034219. [DOI] [PubMed] [Google Scholar]
- 76.Simpson NS, Diolombi M, Scott-Sutherland J, Yang H, Bhatt V, Gautam S, et al. Repeating patterns of sleep restriction and recovery: do we get used to it? Brain Behav Immun. 2016;58:142–51. doi: 10.1016/j.bbi.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Devine J.K., Bertisch S.M., Yang H., Scott-Sutherland J., Wilkins A., Molina V., Henrikson K., Haack M. Glucocorticoid and inflammatory reactivity to a repeated physiological stressor in insomnia disorder. Neurobiology of Sleep and Circadian Rhythms. 2019;6:77–84. doi: 10.1016/j.nbscr.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. 2012;91:36–41. doi: 10.1016/j.biopsycho.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99:1325–80. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat-New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida H, Kubota T, Krueger JM. A cyclooxygenase-2 inhibitor attenuates spontaneous and TNFa-induced non-rapid eye movement sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 2003;285:R99–109. doi: 10.1152/ajpregu.00609.2002. [DOI] [PubMed] [Google Scholar]
- 82.Horne JA, Percival JE, Traynor JR. Aspirin and human sleep. Electro Clin Neurophysiol. 1980;49:409–13. doi: 10.1016/0013-4694(80)90238-2. [DOI] [PubMed] [Google Scholar]
- 83.Murphy PJ, Badia P, Myers BL, Boecker MR, Wright KP., Jr Nonsteroidal anti-inflammatory drugs affect normal sleep patterns in humans. Physiol Behav. 1994;55:1063–6. doi: 10.1016/0031-9384(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 84.Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31:1273–88. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis CJ, Lee HY, Kim J, Avani SM, Peng HL, Banfield E, et al. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4:e000550. doi: 10.1136/openhrt-2016-000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ram A, Pandey HP, Matsumura H, Kasahara-Orita K, Nakajima T, Takahata R, et al. CSF levels of prostaglandins, especially the level of prostaglandin D2 are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751:81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- 87.Haack M, Lee E, Cohen D, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145:136–41. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflamm. 2016;13:141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rea MS, Figueiru MG. A working threshold for acute nocturnal melatonin suppression from “white” light sources used in architectural applications. J Carcinog Mutagen. 2013;4:1000150. [Google Scholar]
- 91.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015;112:1232–7. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin-A pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–84. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 93.Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez-Gallego J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54:1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 94.Chen WW, Zhang X, Huang WJ. Pain control by melatonin: physiological and pharmacological effects. Exp Ther Med. 2016;12:1963–8. doi: 10.3892/etm.2016.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. 2017;34:10–22. doi: 10.1016/j.smrv.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Citera G, Arias MA, Maldonado-Cocco JA, Lazaro MA, Rosemffet MG, Brusco LI, et al. The effect of melatonin in patients with fibromyalgia: a pilot study. Clin Rheumatol. 2000;19:9–13. doi: 10.1007/s100670050003. [DOI] [PubMed] [Google Scholar]
- 97.Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome: a systematic review. Curr Pharm Des. 2010;16:3646–55. doi: 10.2174/138161210794079254. [DOI] [PubMed] [Google Scholar]
- 98.de Zanette SA, Vercelino R, Laste G, Rozisky JR, Schwertner A, Machado CB, et al. Melatonin analgesia is associated with improvement of the descending endogenous pain-modulating system in fibromyalgia: a phase II, randomized, double-dummy, controlled trial. Bmc Pharmacol Toxicol. 2014;15:Article Number 40. doi: 10.1186/2050-6511-15-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang CT, Chiang RPY, Chen CL, Tsai YJ. Sleep deprivation aggravates median nerve injury-induced neuropathic pain and enhances microglial activation by suppressing melatonin secretion. Sleep. 2014;37:1513–23. doi: 10.5665/sleep.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use a systematic review and meta-analysis. Jama-J Am Med Assoc. 2015;313:2456–73. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 101.Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, et al. The Endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43:52–79. doi: 10.1038/npp.2017.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, et al. Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology. 2017;42:1752–65. doi: 10.1038/npp.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43:155–72. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cedernaes J, Fanelli F, Fazzini A, Pagotto U, Broman JE, Vogel H, et al. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology. 2016;74:258–68. doi: 10.1016/j.psyneuen.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 105.Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, et al. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep. 2016;39:653–64. doi: 10.5665/sleep.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Linares IMP, Guimaraes FS, Eckeli A, Crippa ACS, Zuardi AW, Souza JDS, et al. No acute effects of cannabidiol on the sleep-wake cycle of healthy subjects: a randomized, double-blind, placebo-controlled, crossover study. Front Pharmacol. 2018;9:Article No. 315. doi: 10.3389/fphar.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19:23. doi: 10.1007/s11920-017-0775-9. [DOI] [PubMed] [Google Scholar]
- 108.Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol. 2015;10:293–301. doi: 10.1007/s11481-015-9600-6. [DOI] [PubMed] [Google Scholar]
- 109.Toth C, Mawani S, Brady S, Chan C, Liu CX, Mehina E, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain. 2012;153:2073–82. doi: 10.1016/j.pain.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 110.Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18:999–1012. doi: 10.1002/j.1532-2149.2013.00445.x. [DOI] [PubMed] [Google Scholar]
- 111.Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–32. doi: 10.1136/jnnp-2012-302468. [DOI] [PubMed] [Google Scholar]
- 112.Blake DR, Robson P, Ho M, Jubb RW, Mccabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology. 2006;45:50–52. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- 113.Vitiello MV, McCurry SM, Shortreed SM, Baker LD, Rybarczyk BD, Keefe FJ, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. 2014;155:1547–54. doi: 10.1016/j.pain.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang NKY, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35:675–87. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murawski B, Wade L, Plotnikoff RC, Lubans DR, Duncan MJ. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med Rev. 2018;40:160–9. doi: 10.1016/j.smrv.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 116.van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3–16. doi: 10.1016/j.smrv.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A, et al. Cognitive-behavior therapy for insomnia in knee osteoarthritis: a double-blind, randomized, active placebo controlled clinical trial. Arthritis Rheuma. 2015;67:1221–33. doi: 10.1002/art.39048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lami MJ, Martinez MP, Miro E, Sanchez AI, Prados G, Caliz R, et al. Efficacy of combined cognitive-behavioral therapy for insomnia and pain in patients with fibromyalgia: a randomized controlled trial. Cogn Ther Res. 2018;42:63–79. [Google Scholar]
- 119.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, et al. Cognitive behavioral therapy vs. tai chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 2014;37:1543–U361. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: a randomized controlled trial. Biol Psychiatry. 2015;78:721–9. doi: 10.1016/j.biopsych.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Heffner KL, France CR, Ashrafioun L, Quinones M, Walsh P, Maloney MD, et al. Clinical pain-related outcomes and inflammatory cytokine response to pain following insomnia improvement in adults with knee osteoarthritis. Clin J Pain. 2018;34:1133–40. doi: 10.1097/AJP.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Almoznino G, Haviv Y, Sharav Y, Benoliel R. An update of management of insomnia in patients with chronic orofacial pain. Oral Dis. 2017;23:1043–51. doi: 10.1111/odi.12637. [DOI] [PubMed] [Google Scholar]
- 123.Roehrs TA. Does effective management of sleep disorders improve pain symptoms? Drugs. 2009;69:5–11. doi: 10.2165/11531260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 124.Vidor LP, Torres ILS, de Souza ICC, Fregni F, Caumo W. Analgesic and sedative effects of melatonin in temporomandibular disorders: A Double-Blind, Randomized, Parallel-Group, Placebo-Controlled Study. J Pain Symptom Manag. 2013;46:422–32. doi: 10.1016/j.jpainsymman.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 125.Gates PJ, Albertella L, Copeland J. The effects of cannabinoid administration on sleep: a systematic review of human studies. Sleep Med Rev. 2014;18:477–87. doi: 10.1016/j.smrv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 126.Stevens BW, Borren NZ, Velonias G, Conway G, Cleland T, Andrews E, et al. Vedolizumab therapy is associated with an improvement in sleep quality and mood in inflammatory bowel diseases. Dig Dis Sci. 2017;62:197–206. doi: 10.1007/s10620-016-4356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Karatas G, Bal A, Yuceege M, Firat H, Gurcay E, Ardic S, et al. Evaluation of sleep quality in patients with ankylosing spondylitis and efficacy of anti-TNF- therapy on sleep problems: A polisomnographic study. Int J Rheum Dis. 2018;21:1263–9. doi: 10.1111/1756-185X.13102. [DOI] [PubMed] [Google Scholar]
- 128.Zamarron C, Maceiras F, Mera A, Gomez-Reino JJ. Effect of the first infliximab infusion on sleep and alertness in patients with active rheumatoid arthritis. Ann Rheum Dis. 2004;63:88–90. doi: 10.1136/ard.2003.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fragiadaki K, Tektonidou MG, Konsta M, Chrousos GP, Sfikakis PP. Sleep disturbances and interleukin 6 receptor inhibition in rheumatoid arthritis. J Rheuma. 2012;39:60–62. doi: 10.3899/jrheum.110617. [DOI] [PubMed] [Google Scholar]
- 130.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–25. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- 131.Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. 2014;18:273–82. doi: 10.1016/j.smrv.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Wesselius HM, van den Ende ES, Alsma J, ter Maaten JC, Schuit SCE, Stassen PM, et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. Jama Intern Med. 2018;178:1201–8. doi: 10.1001/jamainternmed.2018.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. Mmwr-Morb Mortal Wkly Rep. 2016;64:1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 134.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–20. [PubMed] [Google Scholar]
- 135.Shaw IR, Lavigne G, Mayer P, Choiniere M. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary study. Sleep. 2005;28:677–82. doi: 10.1093/sleep/28.6.677. [DOI] [PubMed] [Google Scholar]
- 136.Cao M, Javaheri S. Effects of chronic opioid use on sleep and wake. Sleep Med Clin. 2018;13:271–81. doi: 10.1016/j.jsmc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112:991–1004. doi: 10.1093/bja/aeu137. [DOI] [PubMed] [Google Scholar]
- 138.Richebe P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. 2018;129:590–607. doi: 10.1097/ALN.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 139.Shimony N, Amit U, Minz B, Grossman R, Dany MA, Gonen L, et al. Perioperative pregabalin for reducing pain, analgesic consumption, and anxiety and enhancing sleep quality in elective neurosurgical patients: a prospective, randomized, double-blind, and controlled clinical study. J Neursurg. 2016;125:1513–22. doi: 10.3171/2015.10.JNS151516. [DOI] [PubMed] [Google Scholar]
- 140.Fabritius ML, Strom C, Koyuncu S, Jaeger P, Petersen PL, Geisler A, et al. Benefit and harm of pregabalin in acute pain treatment: a systematic review with meta-analyses and trial sequential analyses. Br J Anaesth. 2017;119:775–91. doi: 10.1093/bja/aex227. [DOI] [PubMed] [Google Scholar]
- 141.Andersen LPH, Werner MU, Rosenberg J, Gogenur I. A systematic review of peri-operative melatonin. Anaesthesia. 2014;69:1163–71. doi: 10.1111/anae.12717. [DOI] [PubMed] [Google Scholar]
- 142.Madsen MT, Hansen MV, Andersen LT, Hageman I, Rasmussen LS, Bokmand S, et al. Effect of melatonin on sleep in the perioperative period after breast cancer surgery: a randomized, double-blind, placebo-controlled trial. J Clin Sleep Med. 2016;12:225–33. doi: 10.5664/jcsm.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wright CE, Bovbjerg DH, Montgomery GH, Weltz C, Goldfarb A, Pace B, et al. Disrupted sleep the night before breast surgery is associated with increased postoperative pain. J Pain Symptom Manag. 2009;37:352–62. doi: 10.1016/j.jpainsymman.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Van Gastel A. Drug-induced insomnia and excessive sleepiness. Sleep Med Clin. 2018;13:147–59. doi: 10.1016/j.jsmc.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 145.Wichniak A, Wierzbicka A, Walecka M, Jernajczyk W. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19:63. [DOI] [PMC free article] [PubMed]
- 146.Alam A, Voronovich Z, Carley JA. A review of therapeutic uses of mirtazapine in psychiatric and medical conditions. Prim Care Companion CNS Disord. 2013;15:PCC.13r01525. doi: 10.4088/PCC.13r01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ottman AA, Warner CB, Brown JN. The role of mirtazapine in patients with fibromyalgia: a systematic review. Rheumatol Int. 2018;38:2217–24. doi: 10.1007/s00296-018-4068-3. [DOI] [PubMed] [Google Scholar]
- 148.Schweitzer Paula K., Randazzo Angela C. Principles and Practice of Sleep Medicine. 2017. Drugs that Disturb Sleep and Wakefulness; p. 480-498.e8. [Google Scholar]
- 149.Vargas I, Vgontzas AN, Abelson JL, Faghih RT, Morales KH, Perlis ML. Altered ultradian cortisol rhythmicity as a potential neurobiologic substrate for chronic insomnia. Sleep Med Rev. 2018;41:234–43. doi: 10.1016/j.smrv.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum-Arthritis Care Res. 2006;55:420–6. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]
- 151.Morin C, Fardet L. Systemic glucocorticoid therapy: risk factors for reported adverse events and beliefs about the drug. A cross-sectional online survey of 820 patients. Clin Rheumatol. 2015;34:2119–26. doi: 10.1007/s10067-015-2953-7. [DOI] [PubMed] [Google Scholar]