Several lines of scientific data suggest a correlation between neuroinflammation and CNS disorders such as multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, epilepsy, chronic pain and neuropsychiatric disorders (depression, bipolar disorder and schizophrenia). Many of these CNS diseases are comorbid with classical peripheral inflammatory disorders such as rheumatoid arthritis, inflammatory bowel disease, psoriasis, to name a few. The scientific underpinning of this comorbidity in patients is perhaps driven by a compromised immune surveillance system wherein the immune cells and cytokines/chemokines in the periphery cross-talk to the CNS either directly (BBB integrity compromise) or indirectly via modulating meningeal immunity. In addition, there is an inherent ‘central’ component of neuroinflammation that is driven by microglial cells within the CNS. As such, neuroinflammatory drug targets on microglia cells within the CNS have been an emerging area of research within the neuroscience community, both in academia, industry and in public-private consortia.
P2X7 is an ATP activated ion channel, that is expressed abundantly on microglia (and peripheral immune cells) and is a critical mediator of neuroinflammation via release of NLRP3 dependent IL-1β and IL-18 [1]. In several preclinical models of stress in rodents, independent research groups have demonstrated an activated NLRP3-IL1β pathway; as such intervention of this pro-inflammatory pathway by targeting the P2X7 ion channel with CNS penetrant antagonists have demonstrated efficacy in models of psychiatric diseases [2] and provides optimism for novel therapeutic options for patients with mood disorders. When ATP is present in high concentrations in extracellular space as a danger signal, the P2X7-NLRP3- IL1β signaling is activated initiating the microglial neuroinflammatory cascade. The functional expression of this pathway may or may not be associated with enhanced expression of P2X7 at the protein level and an absence of P2X7 upregulation should not be taken as proof of absence of P2X7 in the disease process. Patients with inflammatory burden and activated immune cells with enhanced IL-1β and IL-18 signaling, ought to be tested in controlled clinical studies with doses of P2X7 antagonists that satisfy target engagement of brain P2X7 channels in humans.
We have recently disclosed two brain penetrant P2X7 antagonists in JNJ-54175446 [3] and JNJ-55308942 [4]. In a phase I single ascending dose study in human subjects, JNJ-54175446 demonstrated dose dependent increases in plasma exposure, CSF exposure and ex-vivo inhibition of IL-1β from human blood (Fig. 1) [5]; more importantly, the P2X7 antagonist demonstrated robust occupancy at the human brain P2X7 as was assessed by a PET study (Fig. 1) [6]. Preliminary data from two Phase 1 human experimental medicine studies indicates a central effect of JNJ-54175446 in sleep deprived and amphetamine challenged human subjects (ACNP 2018) setting the stage for proof of concept testing in treatment resistant depression (http://www.isrctn.com/ISRCTN44411633). We remain optimistic based on the preclinical body of evidence that P2X7 antagonism will bring in novel therapeutic options for patients and their physicians, either as mono/adjunctive therapies to address the unmet need in neuropsychiatry.
Fig. 1.
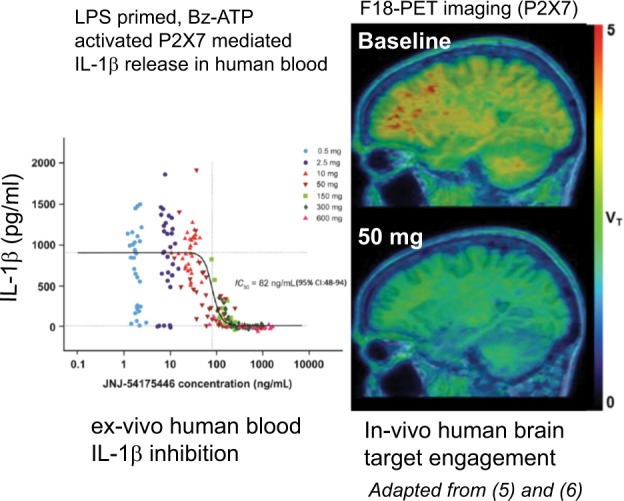
Incremental human plasma exposure of the P2X7 antagonist JNJ-54175446 caused a dose dependent attenuation of P2X7 dependent ex-vivo IL-1β release (ex-vivo LPS primed, ex-vivo Bz-ATP as 2nd stimulus) from human subjects (left). The dose of 50 mg JNJ-54175446 produced central target engagement as assessed by displacing the P2X7 PET ligand, [18F]JNJ-64413739 (right)
Funding and Disclosure
The research and development was funded by Janssen; both authors are full time employees of Janssen R&D.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia. 2016;64:1772–87. doi: 10.1002/glia.23001. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya A, Lord B, Grigoleit JS, He Y, Fraser I, Campbell SN, et al. Neuropsychopharmacology of JNJ-55308942: evaluation of a clinical candidate targeting P2X7 ion channels in animal models of neuroinflammation and anhedonia. Neuropsychopharmacology. 2018;43:2586–96. doi: 10.1038/s41386-018-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letavic MA, Savall BM, Allison BD, Aluisio L, Andres JI, De Angelis M, et al. 4-Methyl-6,7-dihydro-4H-triazolo[4,5-c]pyridine-Based P2X7 Receptor Antagonists: Optimization of Pharmacokinetic Properties Leading to the Identification of a Clinical Candidate. J Med Chem. 2017;60:4559–72. doi: 10.1021/acs.jmedchem.7b00408. [DOI] [PubMed] [Google Scholar]
- 4.Chrovian CC, Soyode-Johnson A, Peterson AA, Gelin CF, Deng X, Dvorak CA, et al. A dipolar cycloaddition reaction to access 6-Methyl-4,5,6,7-tetrahydro-1H-[1,2,3]triazolo[4,5-c]pyridines enables the discovery synthesis and preclinical profiling of a P2X7 antagonist clinical candidate. J Med Chem. 2018;61:207–23. doi: 10.1021/acs.jmedchem.7b01279. [DOI] [PubMed] [Google Scholar]
- 5.Timmers M, Ravenstijn P, Xi L, Triana-Baltzer G, Furey M, Van Hemelryck S, Biewenga J, et al. Clinical pharmacokinetics, pharmacodynamics, safety, and tolerability of JNJ-54175446, a brain permeable P2X7 antagonist, in a randomised single-ascending dose study in healthy participants. J Psychopharmacol. 2018;32:1341–50. doi: 10.1177/0269881118800067. [DOI] [PubMed] [Google Scholar]
- 6.Koole M, Schmidt ME, Hijzen A, Ravenstijn P, Vandermeulen C, Van Weehaeghe D, et al. (18)F-JNJ-64413739, a novel PET ligand for the P2X7 ion channel: radiation dosimetry, kinetic modeling, test-retest variability, and occupancy of the P2X7 antagonist JNJ-54175446. J Nucl Med. 2019;60:683–90. doi: 10.2967/jnumed.118.216747. [DOI] [PubMed] [Google Scholar]


