Abstract
Vigilant attention is a major component of a wide range of cognitive performance tasks. Vigilant attention is impaired by sleep deprivation and restored after rest breaks and (more enduringly) after sleep. The temporal dynamics of vigilant attention deficits across hours and days are driven by physiologic, sleep regulatory processes—a sleep homeostatic process and a circadian process. There is also evidence of a slower, allostatic process, which modulates the sleep homeostatic setpoint across days and weeks and is responsible for cumulative deficits in vigilant attention across consecutive days of sleep restriction. There are large inter-individual differences in vulnerability to sleep loss, and these inter-individual differences constitute a pronounced human phenotype. However, this phenotype is multi-dimensional; vulnerability in terms of vigilant attention impairment can be dissociated from vulnerability in terms of other cognitive processes such as attentional control. The vigilance decrement, or time-on-task effect—a decline in performance across the duration of a vigilant attention task—is characterized by progressively increasing response variability, which is exacerbated by sleep loss. This variability, while crucial to understanding the impact of sleep deprivation on performance in safety-critical tasks, is not well explained by top-down regulatory mechanisms, such as the homeostatic and circadian processes. A bottom-up, neuronal pathway-dependent mechanism involving use-dependent, local sleep may be the main driver of response variability. This bottom-up mechanism may also explain the dissociation between cognitive processes with regard to trait vulnerability to sleep loss.
Subject terms: Circadian rhythms and sleep, Cognitive neuroscience
Introduction
The study of vigilant attention in the context of sleep deprivation has yielded far-reaching insights into the effects of sleep deprivation on cognition and the brain. The primary focus of this review is to provide an overview of inter-related findings on sleep deprivation and vigilant attention, and review how these findings have shaped our understanding of the neurocognitive effects of sleep deprivation and, by extension, sleep.
Vigilant attention, also called sustained attention, refers to the ability to maintain stable, focused attention across a time interval [1]. Vigilant attention is typically measured with (computerized) performance tasks requiring responses to target signals. In the psychology literature, deficits in vigilant attention have been studied in the context of Signal Detection Theory [2], which provides a “discriminability” measure of an individual’s performance on a stimulus detection task while accounting for the individual’s willingness to respond. The concept of discriminability is directly related to the concept of signal-to-noise ratio [3], which may be seen as a measure of the fidelity of information processing in the brain [4]. Deficits in vigilant attention are also commonly described in terms of the time-on-task effect, or vigilance decrement, which is a decline in timely or correct responses across the duration of a vigilant attention performance task [5].
A variety of task paradigms have been used to measure vigilant attention, including simple reaction time tasks, go/no-go tasks, and two-alternative forced choice tasks, all of which typically require subjects to make speeded, accurate responses to visual or auditory stimuli. Historically, performance tasks employed to study vigilant attention required responding to relatively infrequently appearing target signals or events (e.g., occurring less than once a minute) across extended periods of time (an hour or more) [6]. High workload—operationalized as a relatively high rate of target signals—can increase the vigilance decrement, so that sensitivity to deficits in vigilant attention can be retained in shorter tasks by increasing the stimulus density (although the vigilance decrement may be reduced again if stimuli are presented as often as every few seconds) [7]. As such, shorter tasks (less than an hour in duration) are currently favored in studies of vigilant attention.
Vigilance deficits have previously been posited to be the result of neural habituation to repeated stimulation [8]. However, studies based on event-related potentials have cast doubt on this idea [9]. It has also been proposed that the vigilance decrement arises from under-stimulation or boredom due to the monotonous nature of stereotypical vigilance tasks [10, 11]. At the level of brain functioning, though, it is not evident why under-stimulation would lead to a vigilance decrement. Even more puzzling, from this perspective, is the pivotal observation that sleep deprivation causes significant impairment in vigilant attention [12–15] and acceleration of the vigilant decrement [16–19]. As we shall see, this phenomenon suggests an alternative explanation for the vigilance decrement (and an alternative view of what constitutes “boredom” at the level of brain function).
Central to several of the findings on sleep deprivation and vigilant attention is the psychomotor vigilance test (PVT) [20]. The PVT is a 10-min, one-choice reaction time task that requires responding—as quickly as possible—to a stimulus appearing at random, 2–10 s inter-trial intervals. Performance on the task can be quantified by means of the number of lapses of attention (traditionally defined as the number of response times >500 ms) [21] and a variety of other outcome measures [22] including the time-on-task effect (vigilance decrement) [15, 23]. The high stimulus load and the varying inter-trial intervals require a level of vigilant attention that appears to be near-optimally sensitive to impairment due to sleep deprivation. The task has been characterized in depth [24]. It has no learning curve [25], and baseline aptitude for the task varies little among individuals [21]. As such, it is well suited for use in studies pursuing frequent, repeated measurements to probe the temporal dynamics of vigilant attention.
Temporal dynamics
The timing and duration of periods of sleep and wakefulness are governed by two key physiological processes [26]. A homeostatic process—for which the underlying neurobiology is yet to be elucidated—serves to balance time spent awake with time spent asleep by building up a pressure for sleep across time spent awake and dissipating that pressure across time spent asleep. Simultaneously, a circadian process—originating in the biological clock in the suprachiasmatic nuclei (SCN) of the hypothalamus—produces a physiological drive for wakefulness during the day and sleep at night (in humans and other diurnal species) by generating a pressure for wakefulness during the afternoon and evening and withdrawing that pressure during the night and early morning.
The difference between the pressure for sleep from the homeostatic process and the pressure for wakefulness from the circadian process is a primary determinant of the level of sleepiness experienced while awake [27]. Although this was first shown on the basis of self-reported fatigue [28], the interplay between the homeostatic and circadian processes also drives the temporal dynamics of vigilant attention, as can be readily observed in performance on the PVT across a period of total sleep deprivation [15]. As shown in Fig. 1, vigilant attention deficits increase across time awake due to the build-up of homeostatic pressure for sleep, with strong modulation over time of day due to the waxing and waning of circadian pressure for wakefulness [29]. As a result of these effects acting in tandem, performance impairment during a period of acute total sleep deprivation is greatest during the early to late morning hours following a night awake, whereas performance is partially restored during the subsequent afternoon in spite of continuing sleep deprivation. While these temporal dynamics can be understood principally as an additive interaction between the homeostatic and circadian processes, there is evidence that the interaction is actually synergistic in nature, with the influence of the circadian process on vigilant attention increasing when homeostatic pressure is high [30, 31].
Fig. 1.
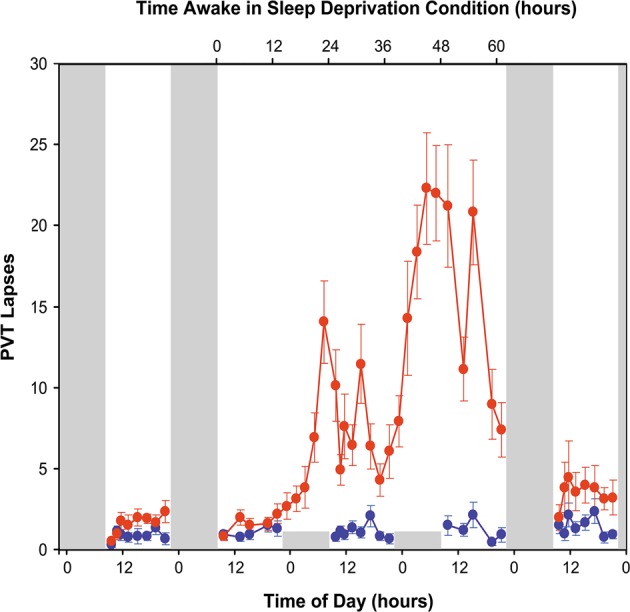
Effects of acute total sleep deprivation and recovery sleep on vigilant attention. Twenty-six healthy young adults were randomized to a total sleep deprivation condition (n = 13) or a control condition (n = 13) in a laboratory study. Subjects in the sleep deprivation condition had two baseline days with 10-h sleep opportunities, a 62-h period of total sleep deprivation, and two recovery days with 10-h sleep opportunities. Subjects in the control condition had 10-h sleep opportunities every night. A 10-min PVT was administered repeatedly during scheduled waking periods to measure vigilant attention. Data show the mean (± standard error) number of lapses (defined as response times greater than 500 ms) on the PVT. Performance was stable across days in the control group (blue line). In contrast, during the 62-h sleep deprivation period, subjects had significantly impaired performance, with deficits increasing across days of total sleep deprivation—modulated by circadian rhythmicity, such that the number of lapses was highest during the morning hours; and performance was quickly recuperated following recovery sleep (red line). Tall gray bars represent sleep opportunities (22:00–08:00) in both conditions; short gray bars represent sleep opportunities in the control condition only. Figure adapted from Whitney et al. [122] with permission
In comparison with studies of acute total sleep deprivation, studies of sustained sleep restriction (i.e., daily sleep curtailment) have revealed that this two-process understanding of the temporal dynamics of vigilant attention is incomplete. That is, based on the effects of the homeostatic and circadian processes alone (or various other theories of sleep regulation no longer widely considered), it would be predicted that people could adapt to sustained sleep restriction rapidly, reaching a steady state of only mild impairment within a few days and returning to baseline after only one or two nights of extended recovery sleep [32]. Whereas that is indeed what is observed for self-reported sleepiness, data from other performance tasks including the PVT tell a different story [33–36].
Several important observations have stood out. First, there is a steady build-up of vigilant attention deficits (but not self-reported sleepiness) across consecutive days of sleep restriction [35]. Second, this build-up is sleep dose-dependent, such that the balance between wakefulness and prior sleep determines the build-up rate [37, 38] (see Fig. 2). Third, the deficits are expressed primarily in the morning hours, while performance in the afternoon and evening is comparatively little affected (like what is seen during total sleep deprivation, cf. Fig. 1) [39] (see Fig. 3). Fourth, recuperation across consecutive days of recovery sleep following a period of sustained sleep restriction can be slow [34] (see Fig. 4)—compared to recuperation from much greater vigilant attention deficits due to acute total sleep deprivation (cf. Fig. 1). Fifth, the build-up rate of impairment across consecutive days of sleep restriction [40], or across a period of total sleep deprivation [41, 42], and the recuperation rate across subsequent days of recovery sleep [40, 42] depends on prior sleep/wake history (see Fig. 4).
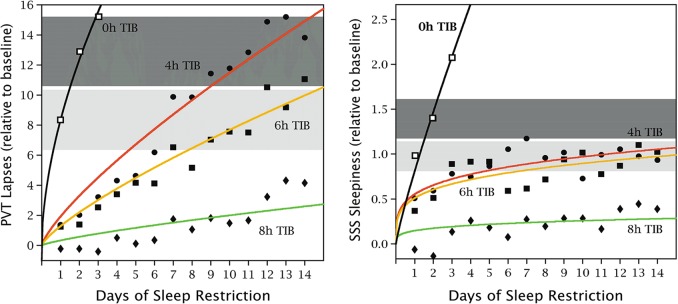
Fig. 2.
Vigilant attention and self-reported sleepiness under conditions of sustained sleep restriction. Forty-eight healthy young adults were assigned to 3 days of acute total sleep deprivation (0 h time in bed [TIB]; n = 13; black) or 14 days of sustained sleep restriction with randomization to 4 h TIB per day (n = 13; red), 6 h TIB per day (n = 13; yellow), or 8 h TIB per day (n = 9; green), in the laboratory. The 10-min PVT and the Stanford Sleepiness Scale (SSS) [123] were administered repeatedly during scheduled waking periods to measure vigilant attention and subjective sleepiness, respectively. Data show the daily means (±standard error) for the number of lapses (defined as response times >500 ms) on the PVT and the self-reported sleepiness score on the SSS, relative to baseline. The horizontal gray bands represent the mean (±standard error) in the total sleep deprivation condition following 1 night and 2 nights with 0 h TIB. In the 8-h TIB condition, lapses of attention were relatively rare and subjective sleepiness was stable and low across the study duration. In the 6 and 4-h TIB conditions, there was a steady build-up of vigilant attention deficits on the PVT across the 14 days of sleep restriction, in a sleep dose–response manner—such that impairment in the 4-h TIB condition reached levels equivalent to 2–3 days of acute total sleep deprivation. However, there was no steady build-up of self-reported sleepiness on the SSS, and no systematic dose–response effect—such that subjective sleepiness in the 4 and 6 h TIB conditions stabilized at a much lower level than seen in the total sleep deprivation condition. Figure adapted from Van Dongen et al. [35] with permission
Fig. 3.
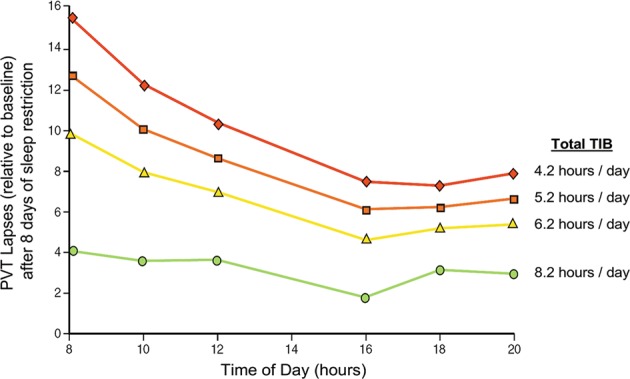
Vigilant attention deficits after sustained sleep restriction as a function of sleep dose and time of day. In a laboratory study, 90 healthy adults were randomized to one of 18 sustained nocturnal sleep restriction conditions with or without daytime naps of various durations, with the total sleep opportunity ranging from 4.2 to 8.2 h per day. A 10-min PVT was administered repeatedly during scheduled waking periods to measure vigilant attention. Data show the estimated means for the number of PVT lapses (defined as response times >500 ms) after 8 days of sleep restriction, relative to baseline, for daily total time in bed (TIB) of 4.2 h (red), 5.2 h (orange), 6.2 h (yellow), and 8.2 h (green). The data reveal that the dose–response effect of sleep restriction is most pronounced in the morning hours, while the circadian drive for wakefulness provides a degree of protected against vigilant attention deficits in the afternoon (the “wake maintenance zone” [124]). Figure adapted from Mollicone et al. [39] with permission
Fig. 4.
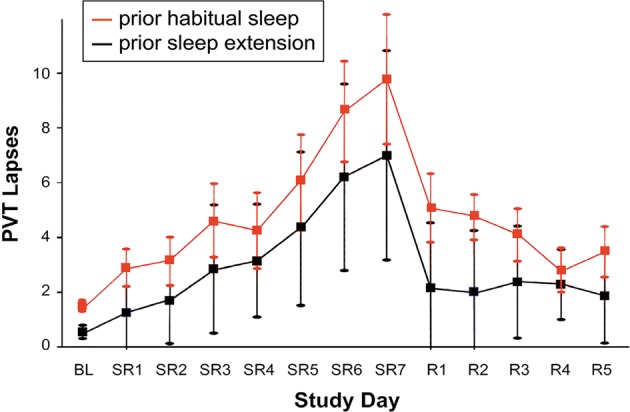
Effects of sustained sleep restriction and prior wake extension on vigilant attention. In a laboratory study, 24 healthy young adults were assigned to 7 days of sustained sleep restriction to 3 h time in bed (TIB) per day (SR1–7), followed by 5 days of recovery sleep at 8 h TIB per day (R1–5). In the days prior to the laboratory study, they were randomized to a week of sleep extension to 10 h TIB per day (n = 12; black) or keeping their habitual sleep schedule (n = 12; red). A 5-min PVT was administered repeatedly during scheduled waking periods in the laboratory to measure vigilant attention. Data show the daily means (±standard error) for the number of PVT lapses (defined as response times >500 ms). In the prior habitual sleep condition, there was a steady build-up of vigilant attention deficits across days of sleep restriction, and a gradual recuperation across recovery days. In the prior sleep extension condition, however, the build-up of deficits across days of sleep restriction was attenuated, and recuperation across recovery days was accelerated. These results show long-term effects of sleep restriction and extension indicative of an allostatic process modulating the setpoint of the sleep homeostatic process. Figure adapted from Rupp et al. [40] with permission
These observations point to the involvement of another key physiological process regulating vigilant attention, for which the temporal dynamics play out more slowly than the homeostatic and circadian processes (i.e., over days and weeks instead of hours and days). Mathematical modeling [37] has indicated that this third process can be seen as an allostatic process [43], which serves to preserve homeostatic and circadian regulation in the face of chronic sleep insufficiency. Another, equivalent way to understand the third process is that it shifts the setpoint of the homeostatic process around which the interplay of the pressures for sleep and wakefulness takes place. Thus, sustained sleep restriction gradually shifts the homeostatic setpoint, while consecutive days of extended recovery sleep gradually shift it back. Interestingly, this means that people’s sleep/wake history may enduringly influence their baseline state and, as such, their vulnerability to vigilant attention deficits due to sleep loss [40, 42, 44].
Vigilant attention is affected by a spectrum of other, predominantly transient, factors, including ambient temperature, light exposure, physical activity and posture, hunger, and environmental noise and other distractions [29]. The influences of these and other factors are integrated with homeostatic, circadian, and allostatic processes; recent evidence suggests that this may occur through orexinergic/hypocretinergic neurons in the lateral hypothalamus [45]. Vigilant attention is also significantly affected by sleep inertia, a brief period of disorientation and cognitive impairment immediately after awakening from (deep, non-REM) sleep [46]. The temporal dynamics and underlying mechanisms of these factors are generally poorly understood, and they are outside the scope of this paper. They have considerable practical relevance, though, especially in work settings where managing vigilant attention is critically important for productivity and safety.
Inter-individual differences
There is wide variability among people in how much vigilant attention performance impairment they exhibit while deprived of sleep [15, 47–50]. The same inter-individual differences in vigilant attention performance impairment are seen under conditions of total sleep deprivation and sustained sleep restriction [51], and they are quite substantial (see Fig. 5). Importantly, these inter-individual differences in vulnerability to sleep loss are highly replicable and stable within individuals [41, 51], indicating that they are a trait, or phenotype. Furthermore, a study in twins demonstrated that vulnerability to sleep loss is heritable [52].
Fig. 5.
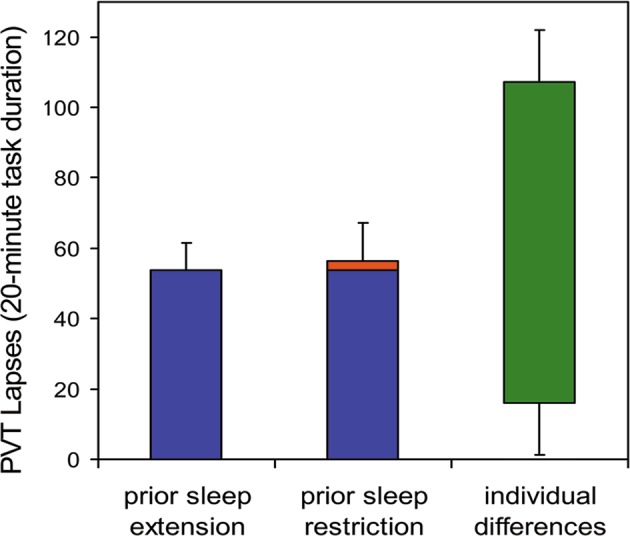
Inter-individual differences in vigilant attention deficits during periods of total sleep deprivation following two different conditions for prior sleep/wake history. Twenty-one healthy young adults were each exposed to 36 h of acute total sleep deprivation in the laboratory on three separate occasions. In the week prior to each laboratory sleep deprivation session, subjects were randomized to a week of sleep restriction to 6 h time in bed (TIB) per day (one session) or sleep extension to 12 h TIB per day (two sessions). A 20-min PVT was administered repeatedly during scheduled waking periods in the laboratory to measure vigilant attention. Data show mean (±standard error) lapses of attention (response times ≥500 ms), averaged across the final 24 h of each 36-h sleep deprivation period. While the effect of prior sleep restriction was evident (red), and consistent with the idea of a shifting homeostatic setpoint due to prior sleep/wake history, the effect was small compared to idiosyncratic, trait inter-individual differences in vulnerability to sleep deprivation (green; 95% range). Trait inter-individual differences in vulnerability to vigilant impairment due to sleep deprivation dominated the data set, explaining 67.5% of the variance [41]
Extensive attempts have been made to find predictors of phenotypic vulnerability to sleep loss. Baseline levels of vigilant attention performance are to some (limited) extent predictive of performance impairment during total sleep deprivation [53]; as are several aspects of brain functioning as characterized through functional neuroimaging [54], which continue to be the subject of investigation [55].
Much attention has been paid to genetic predictors of phenotypic vulnerability to sleep loss, of which several have been discovered—predominantly genetic variants of genes associated with adenosinergic mechanisms that may underlie the homeostatic process [56, 57] and genetic variants of clock genes involved in circadian rhythmicity [58, 59]. Complex biobehavioral traits tend to involve multitudes of genes, generally making it difficult to explain even a small percentage of the observed phenotypic variance [60]. Nonetheless, vigilant attention impairment due to sleep loss is a strongly expressed phenotype [61] (see Fig. 5), and at least one gene has been identified that predicts a relatively substantial portion of inter-individual variability in psychomotor vigilance impairment due to sleep loss. This concerns a single nucleotide polymorphism of the TNFα gene, which was found to explain 6.4% of the variance in PVT performance during total sleep deprivation [62]. Moreover, two genetic variants of the dopaminergic system—a variable number tandem repeat polymorphism of the DAT1 gene and a single nucleotide polymorphism of the DRD2 gene— together explained 15% of the variance in PVT performance during total sleep deprivation [63].
However, the phenotype of cognitive vulnerability due to sleep deprivation is not a unitary phenomenon. A surprise finding in the study that first established inter-individual differences in vulnerability to sleep loss as a phenotype [41] was the task-dependence of the trait. That is, trait inter-individual differences in vigilant attention deficits as measured on the PVT were not congruent with inter-individual differences in performance deficits on a number of other cognitive tasks, and also not with inter-individual differences in self-reported sleepiness. This paradoxical result exposed the issue that overall performance impairment on a cognitive task may be caused by deficits in any of the underlying cognitive processes—a conundrum known as the “task impurity problem” [64]. This highlighted the importance of decomposing task performance into the constituent cognitive processes and examining the effects of sleep deprivation on these processes separately [65, 66].
Efforts to explore this issue further revealed that various cognitive processes, such as working memory scanning, resisting proactive interference, semantic encoding, and motor action planning, may be resilient to degradation caused by sleep deprivation [67–70]—suggesting that performance impairment from sleep loss on a range of cognitive tasks may be driven primarily by underlying deficits in vigilant attention [71]. Interestingly, a recent study of human gene expression during total sleep deprivation suggested a distinction between a large number of genes for which the expression profiles were non-specifically influenced by time awake, and a smaller group of genes for which the expression profiles appeared to be more closely related to the level of performance impairment on the PVT in particular [72]. It is currently unknown whether similar gene expression patterns would emerge with respect to other performance tasks for which performance impairment reflects deficits in vigilant attention.
These intriguing results aside, at least one other cognitive process is also greatly affected by sleep deprivation, independently from vigilant attention: top-down attentional control [73]. Sleep deprivation-induced impairments in this process are believed to underlie deficits in cognitive flexibility, which are not linked to deficits in vigilant attention and not predicted by the same genes [74, 75]. More research is underway to better understand what is unique about attentional control and the impact of sleep deprivation thereon [76]. At the same time, though, the question may be asked: what is unique about vigilant attention?
Top-down and bottom-up regulation
Crucial for a deeper understanding of the effects of sleep deprivation on vigilant attention, and on the brain mechanisms that subserve vigilant attention performance, is the observation that sleep loss-induced deficits in performance on vigilant attention tasks, such as the PVT, are characterized by increased moment-to-moment variability. This was first recognized as an increase in exceptionally slow responses (of, say, more than twice the mean response time) interspersed among otherwise normal, fast responses on reaction time tasks performed during sleep deprivation. This observation led to the “lapse hypothesis,” which posited that performance during sleep deprivation is disrupted by brief moments of reduced arousal that prevent timely responding to the task [77]. The lapse hypothesis provides a simple heuristic for how sleep deprivation leads to increased errors and accidents in real-world settings [78]. This useful feature notwithstanding, careful inspection of the distribution of response times on the PVT (and other vigilant attention tasks) shows a more nuanced picture. That is, during sleep deprivation the whole response time distribution skews to the right (see Fig. 6). As such, all response times are affected by sleep deprivation, with the right tail of the distribution showing the greatest change [14].
Fig. 6.
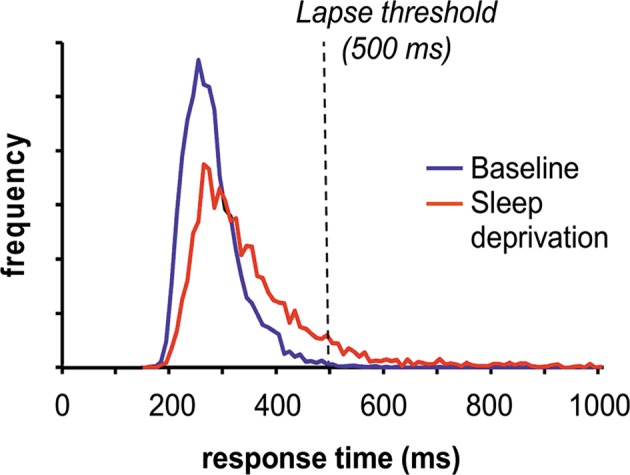
Effect of sleep deprivation on the PVT response time distribution. Sixteen healthy young adults were each exposed to 38 h of acute total sleep deprivation in the laboratory. A 10-min PVT was administered repeatedly during the sleep deprivation period to measure vigilant attention. A histogram of the response times, in bins of 10 ms each, is shown for daytime performance test times at baseline (blue) and for the same daytime performance test times 24 h later during sleep deprivation (red). The data show that the main effect of sleep deprivation is a skewing of the response time distribution to the right, such that many more response times end up in the right tail of the distribution. The dashed line denotes the commonly used threshold defining lapses of attention (i.e., response times >500 ms). The graph illustrates that the skewing of the distribution due to sleep deprivation lengthens the slowest response times and increases the number of lapses considerably. In contrast, the effects of sleep deprivation on the peak of the distribution and the fastest response times is much more modest, and the majority of responses remains in the baseline range (~200–300 ms for well-rested, healthy young adults). Figure adapted from Grant et al. [90] with permission
This finding gave rise to the “state instability hypothesis,” which purported that sleep deprivation makes cognitive performance progressively more variable due to the interaction of escalating homeostatic pressure for sleep with the waxing and waning of circadian pressure for wakefulness and a person’s compensatory effort to continue to perform [15]. This hypothesis is consistent with the documented neurobiology of global, top-down sleep/wake regulation. Notable components of this neurobiology include the ascending arousal system, which mediates the brain’s arousal and, as such, reflects the homeostatic sleep drive and the prevailing amount of compensatory effort; the SCN, which orchestrates circadian rhythmicity and the associated wake drive; and the ventrolateral preoptic (VLPO) nucleus or “sleep switch,” which blocks arousal from the ascending arousal system to initiate sleep [79]. When homeostatic sleep drive is high due to sleep deprivation, the interaction of these components may result in rapid fluctuations between wake and sleep states (i.e., state instability) [80], which could explain the observed skewing of response time distributions.
Results of neuroimaging studies of the effects of sleep deprivation on PVT performance support a global (albeit regionally distributed), top-down perspective on state instability and the skewing of response time distributions in vigilant attention performance [54, 81–86]. The restorative effects of wake-promoting drugs (stimulants) that target neurotransmitter systems in the ascending arousal system, such as modafinil and amphetamine [87], are also consistent with this view. However, the well-known restorative effect of caffeine [88], which at typical doses (in the 100–200 mg range) acts predominantly as an adenosine antagonist, is not readily explained in terms of global, top-down sleep/wake regulation.
Moreover, if the effects of sleep deprivation are solely globally mediated, the impact of sleep deprivation would not be expected to differ fundamentally between cognitive processes, nor should there be any profound task-dependence of inter-individual differences in vulnerability to sleep loss. A global, top-down perspective on vigilant attention also does not provide a parsimonious explanation for one of the most prominent features of impaired vigilant attention, i.e., the vigilance decrement or time-on-task effect. The key to understanding these issues may lie in a more detailed investigation of the time-on-task effect during sleep deprivation and adapting a bottom-up perspective on vigilant attention.
Vigilance decrement and local, use-dependent sleep
The vigilance decrement involves a gradual degradation of performance across the duration of a vigilant attention performance task [5]. The homeostatic and circadian processes that drive the temporal dynamics of vigilant attention interact with the time-on-task effect, amplifying the time-on-task effect when the homeostatic drive for sleep is high and the circadian drive for wakefulness is low [15, 16, 18, 89, 90] and as a function of consecutive days of sleep restriction [19] (see Fig. 7). Rest breaks provide recuperation from the time-on-task effect [91, 92], as do brief periods of engagement in a different task [92, 93]. The temporal dynamics of such breaks have not been well characterized—e.g., it is unclear how much time is needed to reset the time-on-task effect—but even relatively short breaks can be quite effective. The underlying mechanisms are a topic of debate [94, 95].
Fig. 7.
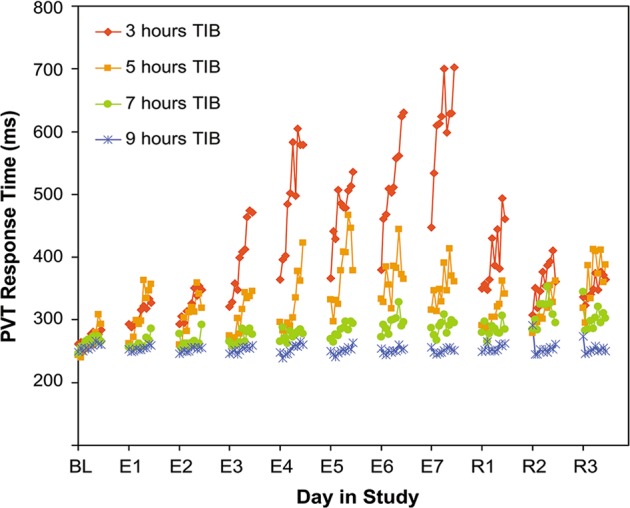
Effect of sustained sleep restriction on the time-on-task effect. Following a baseline period, 66 healthy young adults were assigned to 7 days of sustained sleep restriction or extension, with daily time in bed (TIB) randomized to 3 h (n = 18; red), 5 h (n = 16; orange), 7 h (n = 16; green), or 9 h (n = 16; blue), which was followed by 3 days of recovery sleep at 8 h TIB daily. A 10-min PVT was administered repeatedly during scheduled waking periods to measure vigilant attention. Data show average response times per 1-min bin (not drawn to scale on the time axis) during a baseline day (BL), during the 7 days of experimental sleep restriction or extension (E1–7), and during the 3 recovery days (R1–3). In each of the conditions, there was a general increase of the 1-min average response time across the 10-min task duration, which was reset by the rest breaks between the test bouts. This time-on-task effect was exacerbated as a function of consecutive days of sleep restriction, with shorter sleep durations corresponding to greater time-on-task effects in a dose–response manner. The time-on-task effect was diminished across consecutive recovery days. Figure adapted from Van Dongen et al. [19] with permission
Although often described as a gradual decline in the mean level of performance across the duration of a vigilant attention task, the vigilance decrement actually entails an increase in response variability as a function of time-on-task [96]. Under conditions of sleep deprivation, the increase in response variability over time-on-task is accelerated [15]. The effects of sleep deprivation and time-on-task on response variability are thus similar and interacting—and also involve overlapping brain areas as seen in functional neuroimaging experiments [97]—which has been interpreted as evidence that these effects may essentially be the same [19]. However, whereas the time-on-task effect can be overcome by a mere rest break, the effect of sleep deprivation can only be undone by a period of sleep.
This paradox may be resolved by considering “local sleep theory,” which posits that sleep may be expressed locally in the brain—at the level of neuronal/glial assemblies, such as cortical columns—in a bottom-up, use-dependent manner [98]. In the local sleep state, neuronal/glial assemblies show synchronized firing patterns typical of the sleeping brain, with short bursts of high activity followed by brief periods of inactivity that are characteristic of slow wave sleep [99–101]. Furthermore, they show altered neuronal input–output relationships resulting in high-amplitude evoked responses typical of the sleeping brain [102], which are reversible and show homeostatic and use-dependent properties that are also typical of the sleeping brain [102–104]. However, they can do so independent of the states of neighboring neuronal/glial assemblies and independent of the global brain state [102]. For a review of local sleep theory, see ref. [98].
Local sleep theory’s relevance for vigilant attention has been illustrated in a whisker-twitching experiment in rats, in which specific whisker barrels (i.e., cortical columns) exhibited evoked responses characteristic of sleep, while other whisker barrels simultaneously exhibited evoked responses characteristic of wakefulness and while the whole organism was functionally awake. The probability of a whisker barrel entering the local sleep state increased with time spent in the wake state and the intensity of stimulation of the associated whisker, indicating a homeostatic, use-dependent process [102]. Notably, rats trained to monitor and respond to stimulation of a specific whisker showed greater performance impairment (more failures to respond) when the corresponding whisker barrel had been driven into the local sleep state [103]. Similarly, in humans implanted with intracranial electrodes to record single neurons, local changes in neuronal activity were observed immediately prior to lapses of attention on the PVT [105]. Collectively, these results suggest that repeated stimulation of the same neuronal circuitry produces local sleep in that circuitry, which results in degraded information processing and increased variability in task performance [19].
This bottom-up perspective on performance impairment in vigilant attention tasks may explain a number of otherwise unexplained phenomena at the intersection of human vigilant attention and sleep deprivation [106]. Although task performance in humans is unlikely to rely critically on information processing by only a single cortical column, there are components of cognitive pathways with relatively sparse circuitry—such as visuospatial mental operations in the precuneus and posterior cingulate cortex [107]—which represent a potential bottleneck for cognitive processing. Extended and/or intensive use of such circuitry through sleep deprivation and/or task performance may result in expression of local sleep and consequent degradation of information processing, leading to a steady increase in performance instability [19]. This would explain the pervasive link between the vigilance decrement and monotony (i.e., persistent use of the same brain circuitry) [108, 109], and suggests that what is commonly experienced as “boredom” during monotonous tasks may actually be an epiphenomenon of the occurrence of local sleep.
During a rest break (or after switching to another task not critically affected by the same bottleneck for cognitive processing), the specific circuitry involved in the prior task can recover through local sleep (without any overt impact, since that circuitry is no longer being relied upon). Thus, a rest break may be conceptualized as an opportunity for local sleep to overcome the time-on-task effect, functionally equivalent to how global sleep overcomes the overall effect of sleep deprivation on cognitive performance.
People may vary in the degree of sparsity of circuits that are potential bottlenecks for cognitive processing. That is, they may differ from each other in how much or how little redundancy there is in the capacity to process information—and this level of redundancy (or “cognitive capacity”) may be dissimilar across distinct circuits in a given individual’s brain. This would provide a plausible explanation for why different cognitive processes are found to be differentially affected by sleep deprivation [66] and why there is considerable task-dependence in the inter-individual differences in vulnerability to sleep deprivation [55]. It has also been suggested as an explanation for developmental changes in vulnerability to vigilant attention performance impairment in adolescents [110].
Furthermore, local sleep theory predicts that the effect of sleep deprivation on cognitive performance is modulated by information processing load (or “task load”). For example, it has been shown that performance deficits on a visual short-term memory task occur especially in individuals who fail to maintain baseline levels of brain activation in the precuneus and posterior cingulate cortex (as observed through functional neuroimaging) while sleep deprived and under high processing load [111]—presumably because these individuals have less redundancy in that task-critical neuronal circuitry and thus suffer the consequences of local sleep induced by sleep deprivation and information processing load more strongly [55].
This same line of reasoning may provide an explanation for the counterintuitive observation that performance on the relatively brief (i.e., 10-min) PVT is more sensitive to sleep deprivation than performance on classical vigilant attention performance tasks with infrequent critical signals and much longer task duration. Specifically, the PVT has a much higher stimulus density and thus presents a greater information processing load, persistently engaging the same neuronal circuitry [19]. Intriguingly, it would follow that the PVT has been such a useful tool for research on sleep deprivation and vigilant attention [24] because it probes the susceptibility of a key attentional network in the brain to local sleep by very effectively inducing local sleep in that network, with definite, readily interpretable consequences for the response time distribution.
This theoretical account of sleep deprivation and vigilant attention has been supported by research based on cognitive modeling [112, 113]. Additionally, a (simplified) model for the neurobiological underpinnings, which combines bottom-up and top-down views on vigilant attention, has been proposed (see Fig. 8).
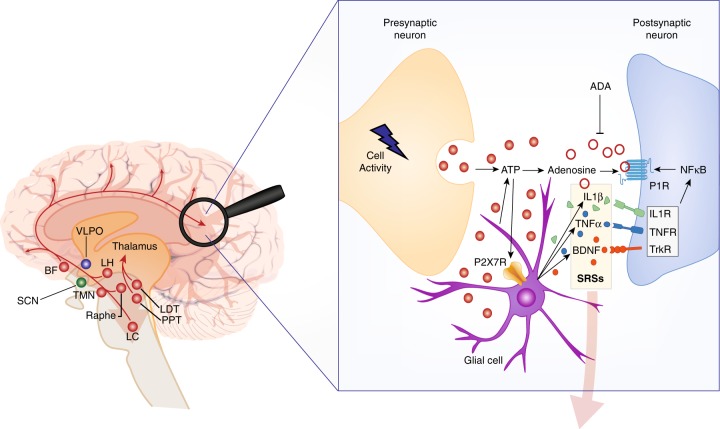
Fig. 8.
Conceptual model pertaining to the effects of sleep deprivation on vigilant attention. Left: The ascending arousal system (AAS) promotes global arousal throughout the cortex by means of wide-ranging projections (red pathways). These projections originate from cholinergic structures in the brainstem (PPT, pedunculopontine tegmental nucleus; LDT, laterodorsal tegmental nucleus) and basal forebrain (BF), monoaminergic structures in the BF and hypothalamus (e.g., LC locus coeruleus; TMN tuberomammillary nucleus; raphe nuclei), and orexinergic/hypocretinergic neurons in the lateral hypothalamus (LH), modulated by circadian rhythmicity generated in the suprachiasmatic nucleus (SCN). The strength of arousal from these projections, through interaction between cortical glutamatergic excitatory neurons, and GABAergic inhibitory neurons in the cortex (not shown), instantiates the homeostatic and circadian processes reflecting prior wakefulness and time of day and mediates the propensity for local sleep at the level of neuronal/glial assemblies. This propensity is manifested as a consequence of intense neuronal use in support of information processing during vigilant attention task performance, in task-activated cortical areas such as the precuneus (magnifying glass). Right: Information processing in a neuronal/glial assembly triggers a series of biochemical processes that induce the local sleep state. Synaptic transmission is associated with increased local metabolic activity and energy transfer and release of adenosine triphosphate (ATP) from presynaptic neurons and glial cells into the extracellular space. Breakdown of extracellular ATP to recover the energy captured in the phosphoryl groups results in use-dependent accumulation of adenosine. Binding of adenosine at postsynaptic adenosine receptors (P1R purine type 1 receptors) promotes local sleep, thereby fundamentally altering the neuronal assembly’s synaptic transmission. As a consequence, the fidelity of task-relevant information processing is degraded in a use-dependent manner, modulated by the strength of subcortical arousal from the AAS. This gives rise to the time-on-task effect (vigilance decrement) in interaction with the homeostatic and circadian processes. A rest break (or switching to a task that does not intensively use the same neuronal/glial assemblies) allows adenosine levels to decay (e.g., through the enzymatic action of ADA, adenosine deaminase), thereby resetting the time-on-task effect. Binding of ATP, prior to breakdown, to purine type 2X7 receptors (P2X7R) leads to release of a cascade of sleep regulatory substances (SRSs), such as interleukin-1β (IL-1β), tumor necrosis factor α (TNFα), and brain-derived neurotrophic factor (BDNF). Sustained wakefulness allows SRSs to accumulate and, via their receptors and nuclear factor κB (NF-κB), causes the density of postsynaptic adenosine receptors to increase. This leads to a build-up of the propensity for use-dependent local sleep across consecutive days of wake extension. Across days of recovery sleep, adenosine receptors downregulate and the baseline propensity for local sleep is gradually restored. Left and right: Through mechanisms yet to be elucidated, accumulation of SRSs across the cortex reflecting the collective states of neuronal/glial assemblies is signaled to subcortical circuits (transparent downward arrows), influencing in particular the ventrolateral preoptic nucleus (VLPO). In response, the VLPO blocks the AAS and induces local sleep across the whole cortex (i.e., global sleep), which enables restoration of baseline SRS concentrations and allows recuperation from prior information processing deficits across neuronal/glial assemblies. Figure adapted from a schematic in Van Dongen et al. [19], with visual elements derived from Saper et al. [79] and Davis et al. [125]
Future research directions
The conceptual model shown in Fig. 8 of the neurobiological underpinnings of vigilant attention deficits and the impact of sleep deprivation, time-on-task, rest breaks, and recovery sleep is, of course, an oversimplification of the mechanisms underlying sleep regulation and cognition. Modern experimental techniques such as optogenetics and chemogenetics are sketching an increasingly complex picture of the regulatory mechanisms involved [45, 114]. Nonetheless, evidence has been accumulating for many of the individual components of the model in Fig. 8, such as the top-down regulation of neuronal activity by the homeostatic and circadian processes [115], the bottom-up, use-dependent drive for local sleep in neuronal/glial assemblies [102], the variability in cognitive processing due to local sleep [103], the binding of extracellular ATP to purine type 2 (X7) receptors [116], key aspects of the signaling mechanisms involving sleep regulatory substances [117, 118], and the upregulation of purine type 1 (A1) receptors in response to prior sleep loss [119].
Even so, the proposed neurobiological integration of the bottom-up and top-down aspects depicted in Fig. 8 awaits experimental confirmation. This facet of the model could be investigated, for example, by measuring performance on a sequence of alternating cognitive tasks, one being a vigilant attention task, such as the PVT, and another being a task associated with intense use of a different neuronal pathway, during sleep deprivation. By comparing the result with sleep-deprived performance on just the PVT, extended to match for total duration of performance testing, the interaction between bottom-up, use-dependent regulation of the time-on-task effect and top-down, homeostatic regulation of the sleep deprivation effect would be exposed.
The model of Fig. 8 would predict that continued testing on the extended PVT results in progressively worsening performance (per the time-on-task effect), as has been found [120]. However, even if the other task exhibits a time-on-task effect also, the model would not predict that performance would continue to worsen across the sequence of alternating tasks. Rather, it would predict that temporarily engaging in the other task allows the neurons specifically used intensively in the PVT to recover so as to reset the time-on-task effect. Thus, the other task would effectively serve as a “rest break” for the PVT. If this prediction does not hold true, then the model needs to be revised.
Several other important implications to the specifics of the interplay between local and global processes remain to be investigated. For example, if performance of a vigilant attention task, such as the PVT, induces the local sleep state, does that then also influence the global homeostatic process, such that repeated performance of the PVT across a period of sleep deprivation would accelerate the build-up of sleep pressure (as compared to not performing the task during sleep deprivation)? And if so, what is the signaling mechanism for this local-to-global interaction? Also, would it work the same way for performance tasks that do not require much vigilant attention, but rather rely critically on, say, attentional control or emotional control? Might this explain why a day full of social interaction can make one feel particularly sleepy? And could addressing these issues shed any light on why sustained sleep restriction leads to cumulative deficits in cognitive performance, but not in homeostatic sleep markers (e.g., delta power in the non-REM EEG or theta power in the waking EEG [32, 35])—suggesting a fundamental distinction between the regulation of sleep and wakefulness versus the regulation of waking alertness and performance?
Sleep deprivation represents a powerful, reversible intervention that allows for the probing of vigilant attention and other aspects of cognition, as well as the underlying mechanisms [61, 73]. Ultimately, research on sleep deprivation, vigilant attention, and brain function may help to determine fixed and/or malleable connections between specific neuronal pathways with specific cognitive processes, which may yield new insights with respect to the elusive mind-body problem [121].
Funding and disclosure
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Medical Research Program under award no. W81XWH-16-1-0319 and through the FY17, Broad Agency Announcement for Extramural Medical Research under award no. W81XWH-18-1-0100. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. This work was also supported by Defense University Research Instrumentation Program grant N00014-17-1-2990 and in part by NIH grant R01HL105768 (Fig. 1) and NASA grant NAG9-1161 (Fig. 5). The authors declare that they have no conflict of interest.
Acknowledgements
We thank James Krueger, Radhika Basheer, and Christopher Davis for their helpful suggestions with regard to local sleep theory and Fig. 8.
Footnotes
Shared senior authorship: Kimberly A. Honn, Hans P. A. Van Dongen.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Warm J, Parasuraman R, Matthews G. Vigilance requires hard mental work and is stressful. Hum Factors. 2008;50:433–41. doi: 10.1518/001872008X312152. [DOI] [PubMed] [Google Scholar]
- 2.Green DM, Swets JA. Signal detection theory and psychophysics. New York City, NY: Wiley; 1966. [Google Scholar]
- 3.Rieke F, Warland D, de Ruyter van Steveninck RR, Bialek W. Spikes: exploring the neural code. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 4.Chavali VP, Riedy SM, Van Dongen HPA. Signal-to-noise ratio in PVT performance as a cognitive measure of the effect of sleep deprivation on the fidelity of information processing. Sleep. 2017;40:zsx016. doi: 10.1093/sleep/zsx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackworth JF. Performance decrement in vigilance, threshold, and high-speed perceptual motor tasks. Can J Psychol. 1964;18:209–23. doi: 10.1037/h0083302. [DOI] [PubMed] [Google Scholar]
- 6.Broadbent DE. Perception and communication. London: Pergamon; 1958. [Google Scholar]
- 7.Parasuraman R, Davies DR. A taxonomic analysis of vigilance. In: Mackie RR, editor. Vigilance: theory, operational performance and physiological correlates. New York, NY: Plenum; 1977. pp. 559–74. [Google Scholar]
- 8.Mackworth JF. Vigilance and habituation. Baltimore: Penguin; 1969. [Google Scholar]
- 9.Parasuraman R. Sustained attention: a multifactorial approach. In: Posner M, Marin O, editors. Attention and performance XI. Hillsdale, NJ: Lawrence Erlbaum; 1985. pp. 493–511. [Google Scholar]
- 10.Manly T, Robertson IH, Galloway M, Hawkins K. The absent mind: further investigations of sustained attention to response. Neuropsychologia. 1999;37:661–70. doi: 10.1016/s0028-3932(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 11.Pattyn N, Neyt X, Henderickx D, Soetens E. Psychophysiological investigation of vigilance decrement: boredom or cognitive fatigue? Physiol Behav. 2008;93:369–78. doi: 10.1016/j.physbeh.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Kjellberg A. Sleep deprivation and some aspects of performance II. Lapses and other attentional effects. Waking Sleep. 1977;1:145–8. [Google Scholar]
- 13.Horne JA, Anderson NR, Wilkinson RT. Effects of sleep deprivation on signal detection measures of vigilance: implications for sleep function. Sleep. 1983;6:347–58. doi: 10.1093/sleep/6.4.347. [DOI] [PubMed] [Google Scholar]
- 14.Dinges DF, Kribbs NB. Performing while sleepy: Effects of experimentally-induced sleepiness. In: Monk TF, editor. Sleep, sleepiness, and performance. New York, NY: Wiley; 1991. pp. 97–128. [Google Scholar]
- 15.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: Evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 16.Wilkinson RT. Effects of up to 60 hours’ sleep deprivation on different types of work. Ergonomics. 1964;7:175–86. [Google Scholar]
- 17.Kribbs NB, Dinges DF. Vigilance decrement and sleepiness. In: Harsh JR, Ogilvie RD, editors. Sleep onset mechanisms. Washington, DC: American Psychological Association; 1994. pp. 113–25. [Google Scholar]
- 18.Wesensten NJ, Belenky G, Thorne DR, Kautz MA, Balkin TJ. Modafinil vs. caffeine: effects on fatigue during sleep deprivation. Aviat Space Environ Med. 2004;75:520–5. [PubMed] [Google Scholar]
- 19.Van Dongen HPA, Belenky G, Krueger JM. Investigating the temporal dynamics and underlying mechanisms of cognitive fatigue. In: Ackerman PL, editor. Cognitive fatigue: multidisciplinary perspective on current research and future applications. Washington, DC: American Psychological Association; 2011. pp. 127–47. [Google Scholar]
- 20.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Ins C. 1985;17:652–5. [Google Scholar]
- 21.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: neurocognitive assay sensitive sleep loss. In: Kushida CA, editor. Sleep deprivation: clinical issues, pharmacology, and sleep loss effects. New York, NY: Marcel Dekker; 2005. pp. 39–70. [Google Scholar]
- 22.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep Loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim J, Wu WC, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49:3426–35. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 25.Basner M, Hermosillo E, Nasrini J, McGuire S, Saxena S, Moore TM, et al. Repeated administration effects on psychomotor vigilance test performance. Sleep. 2018;41:zsx187. doi: 10.1093/sleep/zsx187. [DOI] [PubMed] [Google Scholar]
- 26.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 27.Achermann P, Borbély AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biol Cybern. 1994;71:115–21. doi: 10.1007/BF00197314. [DOI] [PubMed] [Google Scholar]
- 28.Daan S, Beersma DG, Borbély AA. Timing of human sleep: recovery process gated by circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 29.Gabehart RJ, Van Dongen HPA. Circadian rhythms in sleepiness, alertness, and performance. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6th ed. Philadelphia: Elsevier; 2016. pp. 388–95. [Google Scholar]
- 30.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Dongen HPA, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res. 2003;12:181–7. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Dongen HPA, Rogers NL, Dinges DF. Sleep debt: theoretical and empirical issues. Sleep Biol Rhythms. 2003;1:5–13. [Google Scholar]
- 33.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 34.Belenky G, Wesensten N, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose–response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 35.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose–response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 36.Banks S, Van Dongen HPA, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose–response effects of one night for recovery. Sleep. 2010;33:1013–26. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HPA. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256:227–39. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McHill AW, Hull JT, Wang W, Czeisler CA, Klerman EB. Chronic sleep curtailment, even without extended (>16-h) wakefulness, degrades human vigilance performance. Proc Natl Acad Sci USA. 2017;115:6070–5. doi: 10.1073/pnas.1706694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mollicone DJ, Van Dongen HPA, Rogers NL, Banks S, Dinges DF. Time of day modulates the effects of chronic sleep restriction on neurobehavioral performance. Aviat Space Environ Med. 2010;81:735–44. doi: 10.3357/asem.2756.2010. [DOI] [PubMed] [Google Scholar]
- 40.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–21. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 42.Arnal PJ, Sauvet F, Leger D, van Beers P, Bayon V, Bougard C, et al. Benefits of sleep extension on sustained attention and sleep pressure before and during total sleep deprivation and recovery. Sleep. 2015;38:1935–43. doi: 10.5665/sleep.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15:576–84. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Grant DA, Van Dongen HPA. Individual differences in sleep duration and responses to sleep loss. In: Shaw P, Tafti M, Thorpy MJ, editors. The genetic basis of sleep and sleep disorders. Cambridge, UK: Cambridge University Press; 2013. pp. 189–96. [Google Scholar]
- 45.Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43:937–52. doi: 10.1038/npp.2017.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinges DF. Are you awake? Cognitive performance and reverie during the hypnopompic state. In: Bootzin RR, Kihlstrom JF, Schacter DL, editors. Sleep and cognition. Washington, DC.: American Psychological Association; 1990. pp. 159–75. [Google Scholar]
- 47.Wilkinson RT. Interaction of lack of sleep with knowledge of results, repeated testing, and individual differences. J Exp Psychol. 1961;62:263–71. doi: 10.1037/h0048787. [DOI] [PubMed] [Google Scholar]
- 48.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–90. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 49.Frey DJ, Badia P, Wright KP. Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13:305–15. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 50.Van Dongen HPA, Caldwell JA, Jr., Caldwell JL. Investigating systematic individual differences in sleep-deprived performance on a high-fidelity flight simulator. Beh Res Methods. 2006;38:333–43. doi: 10.3758/bf03192785. [DOI] [PubMed] [Google Scholar]
- 51.Rupp TL, Wesensten NJ, Balkin TJ. Trait-like vulnerability to total and partial sleep loss. Sleep. 2012;35:1163–72. doi: 10.5665/sleep.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuna ST, Maislin G, Pack FM, Staley B, Hachadoorian R, Coccaro EF, et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012;35:1223–33. doi: 10.5665/sleep.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patanaik A, Chee KK, Chua ECP, Gooley JJ, Chee MWL. Classifying vulnerability to sleep deprivation using baseline measures of psychomotor vigilance. Sleep. 2015;38:723–34. doi: 10.5665/sleep.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chee MWL, Tan JC. Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. Neuroimage. 2010;51:835–43. doi: 10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 55.Chee MWL, Van Dongen HPA. Functional imaging of inter-individual differences in response to sleep deprivation. In: Nofzinger E, Maquet P, Thorpy MJ, editors. Neuroimaging of sleep and sleep disorders. Cambridge, UK: Cambridge University Press; 2013. pp. 154–62. [Google Scholar]
- 56.Rétey JV, Martin A, Gottselig JM, Khatami R, Dürr R, Achermann P, et al. Adenosinergic mechanisms contribute to individual differences in sleep deprivation-induced changes in neurobehavioral function and brain rhythmic activity. J Neurosci. 2006;26:10472–79. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bachmann V, Klaus F, Bodenmann S, Schäfer N, Brugger P, Huber S, et al. Functional ADA polymorphism increases sleep depth and reduces vigilant attention in humans. Cereb Cortex. 2012;22:962–70. doi: 10.1093/cercor/bhr173. [DOI] [PubMed] [Google Scholar]
- 58.Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 59.Pellegrino R, Halil Kavakli O, Goel N, Cardinale CJ, Dinges DF, Kuna ST, et al. A novel BHLHE41 variant is associated with short sleep and resistance to sleep deprivation in humans. Sleep. 2014;37:1327–36. doi: 10.5665/sleep.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibson G. Rare and common variants: twenty arguments. Nat Rev Genet. 2012;13:135–45. doi: 10.1038/nrg3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satterfield BC, Stucky B, Landolt HP, Van Dongen HPA. Unraveling the genetic underpinnings of sleep deprivation-induced impairments in human cognition. Prog Brain Res. 2019;246:127–58. doi: 10.1016/bs.pbr.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 62.Satterfield BC, Wisor JP, Field SA, Schmidt MA, Van Dongen HPA. TNFα G308A polymorphism is associated with resilience to sleep deprivation-induced psychomotor vigilance performance impairment in healthy young adults. Brain Beh Immun. 2015;47:66–74. doi: 10.1016/j.bbi.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holst SC, Müller T, Valomon A, Seebauer B, Berger W, Landolt HP. Functional polymorphisms in dopaminergic genes modulate neurobehavioral and neurophysiological consequences of sleep deprivation. Sci Rep. 2017;7:45982. doi: 10.1038/srep45982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitney P, Hinson JM. Measurement of cognition in studies of sleep deprivation. Prog Brain Res. 2010;185:37–48. doi: 10.1016/B978-0-444-53702-7.00003-8. [DOI] [PubMed] [Google Scholar]
- 65.Ratcliff R, Van Dongen HPA. Sleep deprivation affects multiple distinct cognitive processes. Psychon Bull Rev. 2009;16:742–51. doi: 10.3758/PBR.16.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson ML, Gunzelmann G, Whitney P, Hinson JM, Belenky G, Rabat A, et al. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev. 2013;17:215–25. doi: 10.1016/j.smrv.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Cogn Brain Res. 2004;18:306–21. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 68.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HPA. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Honn KA, Grant DA, Hinson JM, Whitney P, Van Dongen HPA. Total sleep deprivation does not significantly degrade semantic encoding. Chronobiol Int. 2018;35:746–9. doi: 10.1080/07420528.2017.1411361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fournier LR, Hansen DA, Stubblefield AM, Van Dongen HPA. Action plan interrupted: resolution of proactive interference while coordinating execution of multiple action plans during sleep deprivation. Psychol Res. 2018; epub ahead of print, 10.1007/s00426-018-1054-z. [DOI] [PMC free article] [PubMed]
- 71.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–89. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uyhelji HA, Kupfer DM, White VL, Jackson ML, Van Dongen HPA. Exploring gene expression biomarker candidates for neurobehavioral impairment from total sleep deprivation. BMC Genom. 2018;19:341. doi: 10.1186/s12864-018-4664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitney P, Hinson JM, Satterfield BC, Grant DA, Honn KA, Van Dongen HPA. Sleep deprivation diminishes attentional control effectiveness and impairs flexible adaptation to changing conditions. Sci Rep. 2017;7:16020. doi: 10.1038/s41598-017-16165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satterfield BC, Hinson JM, Whitney P, Schmidt MA, Wisor JP, Van Dongen HPA. Catechol-O-methyltransferase (COMT) genotype affects cognitive control during total sleep deprivation. Cortex. 2018;99:179–86. doi: 10.1016/j.cortex.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Honn KA, Hinson JM, Whitney P, Van Dongen HPA. Cognitive flexibility: a distinct element of performance impairment due to sleep deprivation. Accid Anal Prev. 2019;126:191–7. doi: 10.1016/j.aap.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 76.Whitney P, Hinson JM, Nusbaum AT. A dynamic attentional control framework for understanding sleep deprivation effects on cognition. Prog Brain Res. 2019;246:111–26. doi: 10.1016/bs.pbr.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 77.Williams HL, Lubin A, Goodnow JJ. Impaired performance with acute sleep loss. Psychol Monogr. 1959;73:1–26. [Google Scholar]
- 78.Van Dongen HPA, Balkin TJ, Hursh SR. Performance deficits during sleep loss and their operational consequences. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6th ed. Philadelphia: Elsevier; 2016. pp. 682–8. [Google Scholar]
- 79.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 80.Phillips AJK, Robinson PA. A quantitative model of sleep-wake dynamics based on the physiology of the brainstem ascending arousal system. J Biol Rhythms. 2007;22:167–79. doi: 10.1177/0748730406297512. [DOI] [PubMed] [Google Scholar]
- 81.Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18:8979–89. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drummond SPA, Bischoff-Grethe A, Dinges DF, Ayalon I, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 83.Chee MWL, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorondnov V, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomasi D, Wang RL, Telang F, Boronikolas V, Jayne MC, Wang GJ, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2009;19:233–40. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tucker AM, Rakitin BC, Basner RC, Gazes Y, Steffener J, Stern Y. fMRI activation during failures to respond key to understanding performance changes with sleep deprivation. Behav Brain Res. 2011;218:73–9. doi: 10.1016/j.bbr.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 87.Killgore WD, Rupp TL, Grugle NL, Reichardt RM, Lipizzi EL, Balkin TJ. Effects of dextroamphetamine, caffeine and modafinil on psychomotor vigilance test performance after 44 h of continuous wakefulness. J Sleep Res. 2008;17:309–21. doi: 10.1111/j.1365-2869.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 88.Paech M, Banks S, Pajcin M, Grant C, Johnson K, Kamimori GH, et al. Caffeine administration at night during extended wakefulness effectively mitigates performance impairment but not subjective assessments of fatigue and sleepiness. Pharm Biochem Behav. 2016;145:27–32. doi: 10.1016/j.pbb.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Angus RG, Heslegrave RJ. Effects of sleep loss on sustained cognitive performance during a command and control simulation. Behav Res Meth Ins C. 1985;17:55–67. [Google Scholar]
- 90.Grant DA, Honn KA, Layton ME, Riedy SM, Van Dongen HPA. 3-minute smartphone-based and tablet based psychomotor vigilance tests for the assessment of reduced alertness due to sleep deprivation. Behav Res Methods. 2017;49:1020–9. doi: 10.3758/s13428-016-0763-8. [DOI] [PubMed] [Google Scholar]
- 91.Bergum BO, Lehr DJ. Vigilance performance as a function of interpolated rest. J Appl Psychol. 1962;46:425–7. [Google Scholar]
- 92.Ralph BC, Onderwater K, Thomson DR, Smilek D. Disrupting monotony while increasing demand: benefits of rest and intervening tasks on vigilance. Psychol Res. 2017;81:432–44. doi: 10.1007/s00426-016-0752-7. [DOI] [PubMed] [Google Scholar]
- 93.Ariga A, Lleras A. Brief and rare mental “breaks” keep you focused: deactivation and reactivation of task goals preempt vigilance decrements. Cognition. 2011;118:439–43. doi: 10.1016/j.cognition.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Helton WS, Russell PN. Rest is best: the role of rest and task interruptions on vigilance. Cognition. 2015;134:165–173. doi: 10.1016/j.cognition.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 95.Haubert A, Walsh M, Boyd R, Morris M, Wiedbusch M, Krusmark M, et al. Relationship of event-related potentials to the vigilance decrement. Front Psychol. 2018;9:237. doi: 10.3389/fpsyg.2018.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bills AG. Blocking: A new principle in mental fatigue. Am J Psychol. 1931;43:230–45. [Google Scholar]
- 97.Asplund CL, Chee MW. Time-on-task and sleep deprivation effects are evidenced in overlapping brain areas. Neuroimage. 2013;82:326–35. doi: 10.1016/j.neuroimage.2013.05.119. [DOI] [PubMed] [Google Scholar]
- 98.Krueger JM, Rector DM, Roy S, Van Dongen HPA, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hinard V, Mikhail C, Pradervand S, Curie T, Houtkooper RH, Auwerx J, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci. 2012;32:12506–17. doi: 10.1523/JNEUROSCI.2306-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jewett KA, Taishi P, Sengupta P, Roy S, Davis CJ. Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia. Eur J Neurosci. 2015;42:2078–90. doi: 10.1111/ejn.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortial columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Krueger JM, Huang YH, Rector DM, Buysse DJ. Sleep: a synchrony of cell activity-driven small network states. Eur J Neurosci. 2013;38:2199–209. doi: 10.1111/ejn.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murphy M, Huber R, Esser S, Riedner BA, Massimini M, Ferrarelli F, et al. The cortical topography of local sleep. Curr Top Med Chem. 2011;11:2438–46. doi: 10.2174/156802611797470303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nir Y, Andrillon T, Marmelshtein A, Suthana N, Cirelli C, Tononi G, et al. Selective neuronal lapses precede human cognitive lapses following sleep deprivation. Nat Med. 2017;23:1474–80. doi: 10.1038/nm.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Dongen HPA, Belenky G, Krueger JM. A local, bottom-up perspective on sleep deprivation and neurobehavioral performance. Curr Top Med Chem. 2011;11:2414–22. doi: 10.2174/156802611797470286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cavanna A, Trimble M. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 108.Robertson IH, Garavan H. Vigilant attention. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge, MA: MIT Press; 2004. pp. 631–40. [Google Scholar]
- 109.Hancock PA. In search of vigilance: the problem of iatrogenically created psychological phenomena. Am Psychol. 2013;68:97–109. doi: 10.1037/a0030214. [DOI] [PubMed] [Google Scholar]
- 110.Campbell IG, Van Dongen HPA, Gainer M, Karmouta E, Feinberg I. Differential and interacting effects of age and sleep restriction on daytime sleepiness and vigilance in adolescence: a longitudinal study. Sleep. 2018;41:zsy177. doi: 10.1093/sleep/zsy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chee MW, Chuah YM. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc Natl Acad Sci USA. 2007;104:9487–92. doi: 10.1073/pnas.0610712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ratcliff R, Van Dongen HPA. Diffusion model for one-choice reaction-time tasks and the cognitive effects of sleep deprivation. Proc Natl Acad Sci USA. 2011;108:11285–90. doi: 10.1073/pnas.1100483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walsh MM, Gunzelmann G, Van Dongen HPA. Computational cognitive modeling of the temporal dynamics of fatigue from sleep loss. Psychon Bull Rev. 2017;24:1785–807. doi: 10.3758/s13423-017-1243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varin Christophe, Bonnavion Patricia. Sleep-Wake Neurobiology and Pharmacology. Cham: Springer International Publishing; 2018. Pharmacosynthetic Deconstruction of Sleep-Wake Circuits in the Brain; pp. 153–206. [DOI] [PubMed] [Google Scholar]
- 115.Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21:482–93. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- 116.Krueger JM, Taishi P, De A, Davis CJ, Winters BD, Clinton J, et al. ATP and the purine type 2 X7 receptor affect sleep. J Appl Physiol. 2010;109:1318–27. doi: 10.1152/japplphysiol.00586.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yasuda K, Churchill L, Yasuda T, Blindheim K, Falter M, Krueger JM. Unilateral cortical application of interleukin-1β (IL1β) induces asymmetry in Fos- and IL1β-immunoreactivity: implication for sleep regulation. Brain Res. 2007;1131:44–59. doi: 10.1016/j.brainres.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 118.Vanderheyden WM, Goodman AG, Taylor RH, Frank MG, Van Dongen HPA, Gerstner JR. Astrocyte expression of the Drosophila TNF-alpha homologue, Eiger, regulates sleep in flies. PLoS Genet. 2018;14:e1007724. doi: 10.1371/journal.pgen.1007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–5. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Veksler BZ, Gunzelmann G. Functional equivalence of sleep loss and time on task effects in sustained attention. Cogn Sci. 2018;42:600–32. doi: 10.1111/cogs.12489. [DOI] [PubMed] [Google Scholar]
- 121.Young RM. The mind–body problem. In: Olby RC, Cantor GN, Christie JR, Hodges MJS, editors. Companion to the history of modern science. London: Taylor and Francis; 1996. pp. 702–11. [Google Scholar]
- 122.Whitney P, Hinson JM, Jackson ML, Van Dongen HPA. Feedback blunting: total sleep deprivation impairs decision making that requires updating based on feedback. Sleep. 2015;38:745–54. doi: 10.5665/sleep.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 124.Strogatz SH, Kronauer RE, Czeisler CA. Circadian pacemaker interferes with sleep onset at specific times each day: role in insomnia. Am J Physiol. 1987;253:R172–8. doi: 10.1152/ajpregu.1987.253.1.R172. [DOI] [PubMed] [Google Scholar]
- 125.Davis CJ, Taishi P, Honn KA, Koberstein JN, Krueger JM. P2X7 receptors in body temperature, locomotor activity, and brain mRNA and IncRNA responses to sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2016;311:R1004–12. doi: 10.1152/ajpregu.00167.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]