Abstract
Background:
Congenital defects of the urinary bladder (micro- or contracted bladder, bladder exstrophy) remain a challenging problem for pediatric surgeons. Even when conservative treatment options are fully exhausted, irreversible renal dysfunction can be observed in a large number of cases that can even lead to chronic renal failure and the need for kidney transplantation. To protect kidney function bladder augmentation using intestinal tissue is commonly applied as the standard treatment method. However due to the unphysiological nature of intestinal tissue a number of problems and complications such as urinary tract infections or bladder stone formation limit the clinical success of this approach. Moreover a number of substitutes for the implementation of a bladder augmentation have been tested without success to date. Here we used an experimental model to test wether the biocompatible collagen mesh Lyoplant may be a suitable candidate for bladder augmentation.
Methods:
We implanted a biocompatible collagen mesh (Lyoplant®) in a bladder defect rat model for bladder augmentation (Lyoplant®-group: n = 12; sham group n = 4). After 6 weeks the abdomen was reopened and the initial implant as well as the bladder were resected for histological and immunohistochemical examination.
Results:
All but one rat exhibited physiological growth and behaviour after the operation without differences between the Lyoplant®-group (n = 12) and the sham group (n = 3). One rat from the sham group had to be excluded because of a suture leakage. No wound healing complications, wound infections and no herniation were observed. After 5 weeks the implants showed an adequate incorporation in all cases. This was confirmed by immunohistological analyses where a significant cell infiltration and neovascularization was observed.
Conclusion:
In summary, Lyoplant® appears to be a promising tool in experimental bladder augmentation/regeneration in rats.
Keywords: Biocompatible collagen mesh, Bladder regeneration/augmentation, Rat model
Introduction
Patients affected by the “bladder-exstrophy-epispadias” complex suffer from severe uro-rectal malformations. Due to an abdominal wall defect, the patients are born with a bladder that is exposed to the external environment [1–3]. The therapy of congenital urogenital malformations such as bladder exstrophy is variable and includes e.g. a reconstruction of the bladder wall or establishing a continent urinary diversion [1, 2, 4, 5]. Both methods result in a number of complications. Patients treated with a bladder wall reconstruction often remain incontinent or they suffer from hypercontinence [2, 5].
A continent urinary diversion can be carried out in different ways. By using an ileocecal pouch continence can be achieved but clinical success is dependent on high patient compliance, a big surgical effort and a high rate of re-surgery is common. Alternatively, the method of using a sigma rectum pouch has the advantage that the patients do not have to catheterise themselves because the urine is emitted with the stool. However, sequelae, such as the damage of the upper urinary tract, appear commonly and are described to develop in 90% of cases in children. In addition, infections, hyperchloraemic acidosis and a higher risk to develop colon cancer may be consequences of this procedure [4–6].
Clearly, the complications and the rate of continence between the different treatment options for bladder exstrophy have to be weighed up in order to achieve an optimal quality of life outcome for the patients. Thanks to the recent advances in tissue engineering some alternative materials for the augmentation of bladders have been tested, but unfortunately no clear alternatives have been developed that provide the basis for reproducible treatment [7]. A number of studies using synthetic materials such as polypropylene and xenogenous materials or such as small intestinal submucosa (SIS) or Pelvicol® have been performed. It was demonstrated that a number of synthetic and xenogenous materials are not suitable for urinary bladder augmentation. Synthetic materials were observed to trigger strong inflammation response followed by progressive massive fibrosis around the implant [8–10]. The implantation of xenogenous materials resulted in acute inflammation as well as microcalcification and abscess formation [11, 12].
In view of all these problems we hypothesized that the biocompatible collagen mesh Lyoplant® (B. Braun, Germany) which has previously been used in neurosurgery for reconstructing dura defects could be an alternative approach to perform urinary bladder augmentation [13]. Studies using Lyoplant® for the therapy of congenital abdominal wall defects in animal models reveal positive results [8–10]. Therefore we used an experimental model in rats to test this hypothesis in order to provide a basis for a novel applicable clinical approach.
Materials and methods
A bladder augmentation model was created using 16 Wistar WU rats (Harlan-Winkelmann, Borchen, Germany). The rats weighed between 75 and 100 g. All animals were housed in a well ventilated, climate-controlled environment, fed laboratory chow and given free access to water. The study was performed according to a protocol approved by the local committee for animal use and care (Regierung von Unterfranken: 55.2-2531.01-01/12).
For bladder augmentation a collagen mesh (Lyoplant®, B.Braun, Melsungen, Germany) was used. Lyoplant® is made of bovine pericardium and it is free of non-collagen material [13]. During manufacturing it is ensured, that a transfer of viruses or priones is impossible [10].
Surgical procedure
Lyoplant® group (n = 12): After inducing anasthesia using isoflurane (1-chloro-2,2,2-trifluoroethyl difluoromethyl ether, Abbot GmbH, Wiesbaden, Germany), the abdominal skin was shaved, desinfected and covered with a sterile compress. After a median laparotomy and mobilising the intestine, the urinary bladder was presented. A 0.8 × 0.8 cm sized stripe of the bladder was resected and replaced with a 0.8 × 0.8 cm sized collagen-mesh (Lyoplant®, B. Braun, Melsungen, Germany) which was stitched off using six non-absorbable prolene simple interrupted sutures (Prolene® 8/0, Ethicon, Norderstedt, Germany) and covered by a fibrinogen patch (TachoSil®, Nycomed, Linz, Austria). The fascia was closed using non-absorbable polypropylene sutures (4/0) and the skin closure was performed using continuous polypropylene suturing (7/0) in a continuous technique.
Postoperative analgesia using Tramal® (tramadol, Grünenthal, Aachen, Germany) was administered and the animals were kept under observation under a heat lamp for a period of 2 h.
Sham group (n = 4)
In order to exclude influences of the anaesthesia and suture material, a sham-operation with identical conditions was performed in a control group (n = 4). The defect of the bladder was closed primarily with direct sutures (Prolene® 8/0, Ethicon, Norderstedt, Germany) and covered by a fibrinogen patch (TachoSil®, Nycomed, Linz, Austria) as well.
All animals underwent postoperative inspections daily for 6 weeks where bodyweight, impairment of wound healing and postoperative complications such as incisional hernia, ileus or any infections were monitored. At the end of the trial the laboratory animals wereen anaesthetised for a final laparotomy. The adhesions to the intestines were rated as shown in Table 1.
Table 1.
Division of different degrees of adhesion
| Adhesion degree | Description of adhesion |
|---|---|
| Adhesion degree 0 | No adhesion |
| Adhesion degree 1 | Adhesion is detachable by finger |
| Adhesion degree 2 | Adhesion is very difficult detachable by finger |
| Adhesion degree 3 | Adhesion is only detachable by scalpel |
Histological and immunohistochemical assessment
Histological examination of the whole bladder and its surrounding tissue was performed after fixation in 10% formalin and embedded in paraffin as described previously [8, 9]. Paraffin-embedded tissue blocks were sectioned into 10-µm slices and stained with haematoxylin/eosin (H&E). Slides were coded and microscopically analyzed by two different investigators using a BX-50 light microscope (Olympus, Düsseldorf, Germany).
For immunohistochemical staining the slides were hydrated using a standard protocol, washed three times with PBS and fixed for 30 min at 37 °C in acetone. The slides were then washed three times with TRIS-Buffer and incubated for 12 h at 4 °C with various non-labeled monoclonal mouse anti-rat primary CD-antibodies (CD4, CD8, CD68, Collagen I, Collagen III and Collagen IV; see Table 2). The secondary antibody, a prediluted biotinylated rabbit anti-mouse IgG antibody (P0161; DAKO, Hamburg, Germany) was incubated for 1 h at room temperature. The second antibody was washed off the slides, 3 × with PBS. Then, peroxidase-conjugated streptavidin (DAKO, Hamburg, Germany) was added. The immune reaction was visualized using 3,3′-diaminobenzidine (DAB, 40 mg/200 ml), dissolved in 0.05 M Tris/HCl buffer (pH 7.6) with 0.006% (v/v) H2O2. Finally the slides were washed, stained with haematoxylin, washed again and dehydrated using a standard protocol in a graded series of ethanol and xylol. The coverslips were mounted with Pertex (Histolab Products, Gotheburg, Sweden). Slides were coded and microscopically analyzed by two different investigators as well.
Table 2.
Overview of the primary antibodies used in this trial
| Serial number | Company | Dilution | |
|---|---|---|---|
| Mouse-anti-Rat | |||
| CD4 | GTX76103-250 | Genetex | 1:100 |
| CD8a | EXB-11-678-C100 | Exbio | 1:200 |
| CD68 | Genetex | Genetex | 1:300 |
| Rabbit-anti-Rat | |||
| Collagen I | CED-CL50141AP-1 | Cedarlane | 1:500 |
| Collagen III | CED-CL50341AP-1 | Cedarlane | 1:500 |
| Collagen IV | CED-CL50441AP-1 | Cedarlane | 1:500 |
Results
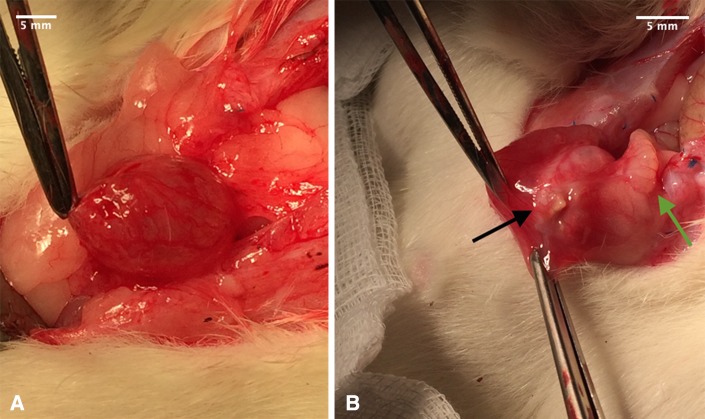
One of the 16 Wistar WU rats from the sham group was excluded from the trial on its second day postoperative because of suture leakage followed by a 4-quadrant peritonitis. The other 15 animals (Lyoplant®-group: n = 12; sham-group: n = 3) showed a physiological growth curve in their bodyweight protocol (data not shown). Throughout the trial no signs of infections and no incisional hernia or wound infection, neither clinically/macroscopically nor postoperative macroscopically, were observed. The operation scar did not show any signs of irritation. One third of the rats of the total population showed no macroscopic adhesions at all, 60% showed 1st degree adhesions and one rat (corresponding to 6.7%) exhibited 2nd degree adhesions. During explantation of the bladder from the latter rat with 2nd degree adhesion the intestine and the bladder’s wall were found to have fusioned tightly (Fig. 1).
Fig. 1.
Macroscopic evaluation of adhesions in labaratory rats with A adhesion degree 0 and B adhesion degree 2 (black arrow points at macroscopic visible collagen-mesh, green arrow points at intestine and bladder wall grown together)
Lyoplant®-group
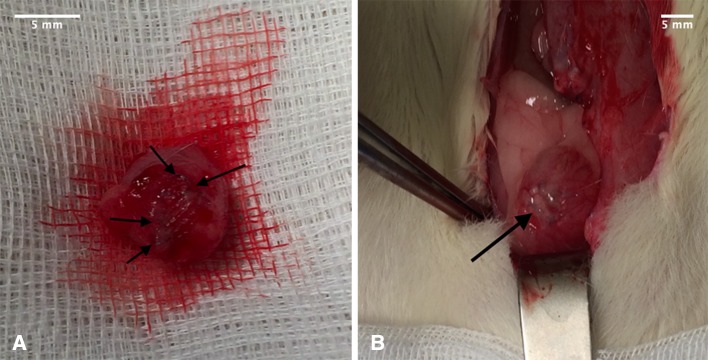
In 45% of Wistar WU rats the implants appeared completely incorporated macroscopically but the sutures remained visible. In 20% of the animals, the implants were only partially visible and in 33% of rats, the implants remained clearly visible. The urinary bladder wall of the three animals with only partially visible implants left, showed higher signs of vascularisation around the implant, which was interpreted as an indication of neovascularisation (see Figs. 2, 3). None of the cases developed an incisional hernia. Histologically, the bladder wall remained trilaminar, consisting of epithelium, detrusor urinae muscle and the serosa. In the area of Lyoplant®, instead of the muscle layer, connective tissue was found. Only small parts of the implant were left, because Lyoplant® had been transformed into connective tissue, built by fibroblasts (see Fig. 4). A lot of small blood vessels appeared to be in the layer of connective tissue, representing the neovascularisation. Some inflammatory cells, like macrophages and neutrophil leucocytes, had invaded. The sutures that had been used for implantation were non-absorbable and stayed visible in histological sections. At the sites of the sutures an accumulation of macrophages was observed.
Fig. 2.
A macroscopically completely incorporated collagen-mesh and B macoscopically visible collagen-mesh (Lyoplant®; black arrow)
Fig. 3.
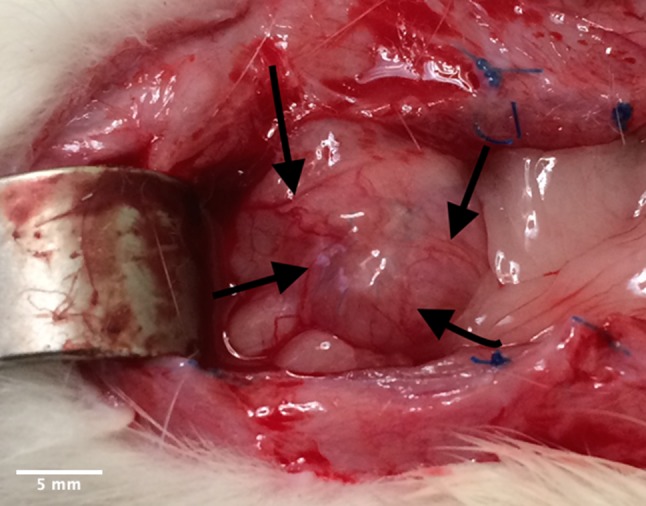
Vascularisation in urinary bladder (black arrows) with incompletely incorporated collagen mesh (Lyoplant®)
Fig. 4.
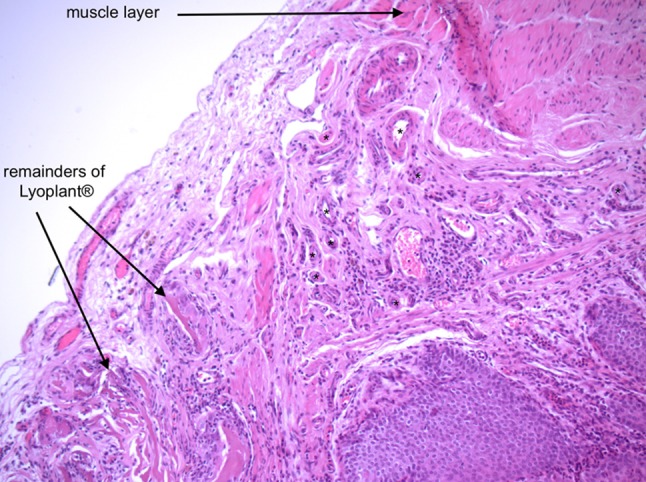
Zone of transformation of untreated bladder wall with muscle layer to the area of implanted Lyoplant®. Stars (*) mark small blood vessels in between the two layers, representing neovascularization (magnification: ×10)
Immunohistological staining showed a strong infiltration of CD-4 positive cells around the resorbable Lyoplant® material (see Fig. 5) compared to a moderate infiltration of CD8-positive cells, as well as the presence of connective tissue as revealed by collagen III- and collagen IV-positive staining patterns, especially near the blood vessels. CD68-positive cells were only found around suture materials (Prolene®; data not shown). The stronger immunohistological reactions of cells against collagen I were found to be distributed all over the sections (data not shown).
Fig. 5.
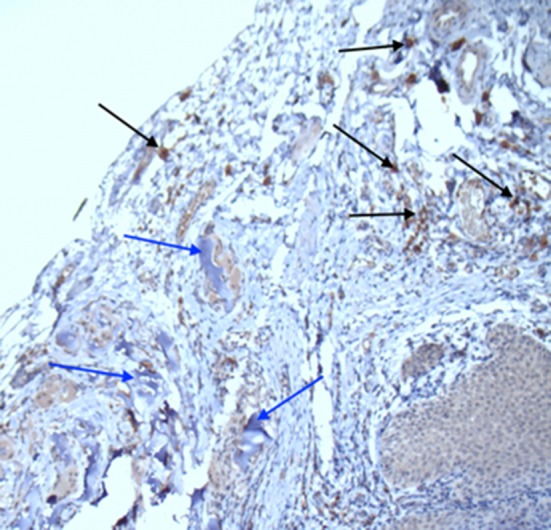
Immunohistochemical staining: CD4 positive cells (black arrows) around the remodulate Lyoplant® (blue arrows; magnification: ×10)
Sham group
In the primary closed (= sham) group scar tissue had resolved and architecture of the tissue was normal, including smooth muscle formation (data not shown). Only some infiltrations of CD4 and CD8-positive cells were detected in the bladder wall. CD68 positive cells were only traceable in areas next to the suture material (see Fig. 6).
Fig. 6.
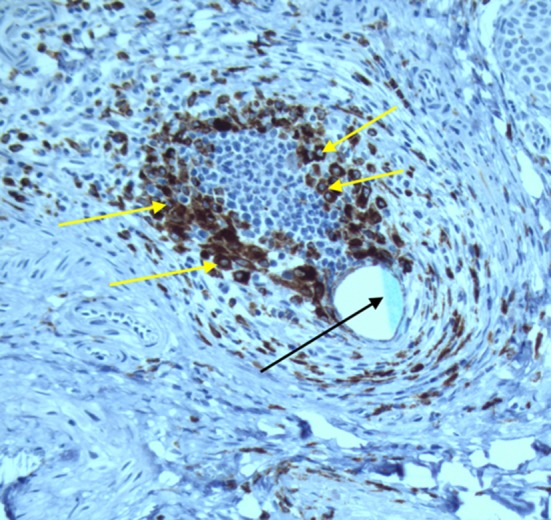
Immunohistochemical staining: CD68 positive cells (yellow arrows) around the suture material (Prolene®, black arrow; magnification ×20)
Discussion
The therapy of bladder exstrophy is a tremendous challenge for the surgeons in charge [14, 15]. Many different treatment options were tried, such as bladder closure with reconstructing the vesical cervix, bladder augmentation and continent urinary diversion [1, 2, 16, 17]. Even though those methods can achieve urinary continence, they were accompanied by a lot of complications. There are risks for developing anastomosis stenoses between the urinary and intestinal tract, as well as metabolic acidosis due to physiological resorption in the intestinal segment. Urinary tract infection including an ascending infection leading to a renal dysfunction may occur. Chologenic diarrhoea and formation of urolithiasis, stoma stenoses and bladder stones were also described.
Gearhart et al. [18] claim that due to a primary failure of the treatment, the chances of urinary continence are lowered to 60%, and in cases of a secondary failure, the chances of urinary continence are lowered to 40%. Additionally, the period of hospitalisation is prolonged, because of a higher number of operations and corrective operations leading to higher costs.
The failure of primary operations of patients with bladder exstrophy leads to increased costs of 9000$ for the clinical treatment and additional 11,000$ for surgery [19].
Based on recent studies, dealing with tissue engineering in pediatric urology [7, 20] and also on studies that claim Lyoplant® to be a suitable material in the therapy of congenital abdominal wall defects [8–10], it was evaluated if Lyoplant® is also suitable for bladder augmentation. Important criteria for being a suitable material are: (1) biocompatibility (in order not to generate a foreign tissue reaction of the body), (2) resorbability (as the body should be exposed to external material for a preferably short period of time), (3) mechanical stability in order to guarantee the consistency even under increased bladder pressure, (4) support of regenerative processes and cell differentiation and (5) anticarcinogenic and antiallergic properties [7, 9, 21].
During the 6 weeks trial, the animals showed a physiological curve of growing and no complications, like infections, hernia formation and adhesion. Thus, the stability of Lyoplant® seemed to be adequate enough to resist the high pressure in the lower abdomen. The mechanical stability of the implant has also been tested in studies, comparing the mechanical stability of Lyoplant® with other materials like polypropylene (PPP) and polytetrafluorethylene (PTFE), using a tensiometer as an objective method [8–10, 22]. Both macroscopically and histologically, only remnants of the implant were visible Lyoplant® is a biocompatible material, that has been converted into epithelial and connective tissue, as defined as a remodelling process. This process starts with neovascularisation. Histologically, a lot of small blood vessels were found in the area of transformation. The used fibrin patch (TachoSil®, Nycomed, Austria) in both groups did not seem to have an influence on this remodelling process.
Those blood vessels enable cells, like fibroblasts, to infiltrate the manipulated area. They produce collagenous fibres and develop to fibrocytes which form a stable collagenous reticulum [23]. Therefore, cell differentiation is supported by the implanted material. The remodelling process is supported by a microporous structure and a loose structure of collagenous fibres of Lyoplant®, making it easier for surrounding cells to infiltrate the implant [13]. As a result, the conditions are set for a good adaption in the bladder wall. The duration of incorporating foreign material is short within the body. This reduces the risk of transplant rejection.
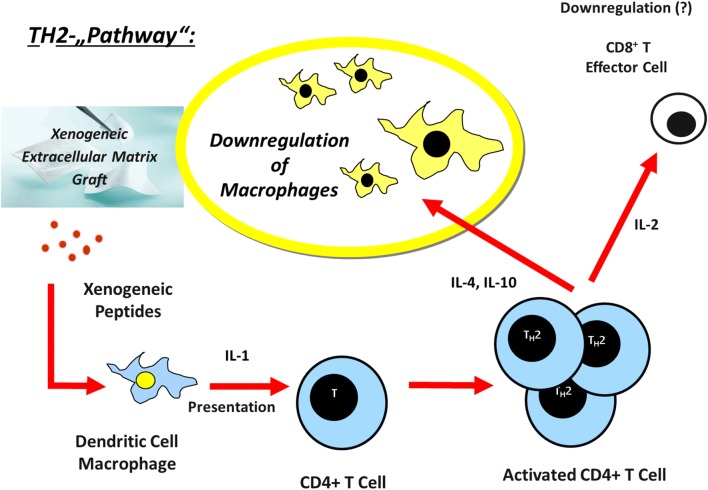
Furthermore, Lyoplant® is edited to an avascular and acellular material, preserving the immunocompetence and supporting the transplant acceptance [8, 13, 24, 25]. Immunhistologically no strong signs of inflammation were observed. CD68-positive cells (CD 68 is a surface marker for macrophages) accumulated around the suture materials. Thus, Prolene®-sutures seem to cause a strong rejection reaction of the body, but it is in no relation to the used collagen-mesh. As a result, Lyoplant® fulfills several criteria for being a suitable material (see above). The reason for the absence of rejection is not sufficiently clarified. It is presumed, that biocompatible materials like Lyoplant®, small intestinal submucous membrane (SIS) or porcine dermal collagen mesh (Pelvicol®) support the transplant acceptance by activating the TH2-pathway [8, 9, 22, 26].
Several studies have analyzed the role of TH1 and TH2 lymphocytes in cell-mediated immune responses to xenografts. TH1 lymphocytes produce interleukin- (IL)2, interferon (IFN), and tumor necrosis factor (TNF) leading to macrophage activation, stimulation of complement fixing antibody isotypes (IgG2a and IgG2b in mice) and differentiation of CD81 cells to a cytotoxic phenotype [8]. Activation of this pathway is associated with both allogeneic and xenogeneic transplant rejection [8]. TH2 lymphocytes produce IL-4, IL-5, IL-6, and IL-10, cytokines that do not activate macrophages and lead to production of noncomplement-fixing antibody isotypes (IgG1 in mice). Activation of the TH2 pathway is associated with transplant acceptance. Extracelluar matrix (ECM) grafts serve as bioscaffolds for appropriate host tissue growth and remodeling and are resorbed during thisprocess. In addition, extracellular matrix materials are acellular and avascular which makes immune-mediated destruction of tissue blood supply unlikely. The cells that populate the ECM graft are host-derived and thus nonimmunogenic. Nevertheless, it has been unclear why such xenogeneic material does not appear to induce an inflammatory response that leads to rejection [26]. Lyoplant® (B/Braun Aesculap, Germany) is a bovine-derived, acellular and avascular material. Our previous findings and the literature data suggest that the biocompatibility of the xenogeneic matrix (Lyoplant®) is different from the TH1-pathway [8, 26].
Our study shows, that there is a vigorous host response to the implanted ECM-xenografts. Allmann et al. [26] had demonstrated an early, acute inflammatory response at the graft site, which consists mostly of polymorphonuclear leukocytes (PMNs). Cytokine and antibody isotype analysis demonstrated the presence of a TH2 response but the absence of a TH1 response. These results differ from typical cytokine and antibody responses to xenografts, which induce a TH1 cytokine response. Clearly, xenogeneic ECM, such as Lyoplant®, SIS® or Pelvicol®, is not an inert material, but is associated with the production of antiinflammatory cytokines and noncomplement-fixing antibodies, which is compatible with graft acceptance (see Fig. 7).
Fig. 7.
Hypothetic TH2-Immunresponse to xenogeneic extracellular matrix grafts, such as Lyoplant®
The TH2 dominant immune response to ECM may play a pivotal role in the favourable clinical response to xenogeneic biomaterials. Our group found in further studies that a TH2 response was associated with acceptance of xenogeneic transplants and a TH1-mediated response was associated with rejection. A TH2 dominant response may prevent cell-mediated inflammation of the extracellular matrix graft through the suppressive action of IL-10 on TH1 cells. The secretion of pro-inflammatory cytokines by macrophages and eosinophils is also inhibited by IL-10 [8]. Thus, TH2-mediated antiinflammatory activity may be an important factor in promoting extracellular matrix remodeling by modulating postsurgical inflammation [8].
In this way, interleukin (IL)-4, IL-5, IL-6 and IL-10 are distributed and inhibit the TH1-pathway which uses IL-2, interferon (IFN) and tumor necrosis factor (TNF) in order to activate macrophages, causing a cytotoxic immune response. This would lead to an inflammatory response against the implanted material [8–10, 27]. By producing IL-10 and activating the TH2-pathway, the TH1-pathway triggered inflammatory reaction of the macrophages is suppressed [27]. Collagen type I is the most common type of collagen. It is present in dermis, tendons and ligaments [23]. As collagen type I is very common in different tissues, the strong immunological reaction of the antibodies against collagen type I is probably caused by higher physiological amounts of collagen I in the bladder wall. Collagen III is found in reticular fibres, which are components of basal laminae. Collagen IV is provided in basal laminae, too [23].
The immunological reaction against areas with collagen III and IV was very low and localised in walls of blood vessels, which are highly represented in the area of neovascularisation. This leads to the assumption that only the endothelium of blood vessels contain both types of collagen. Therefore, the areas with a lot of collagen-IV are especially located around blood vessels. CD4 and CD8a are specific for T-lymphocytes [27, 28]. As the reaction against CD4 and CD8a positive cells has been very little, this result is interpreted as a sign of accepting the transplant. This remodeling process is also supported by the work of Roelofs et al [29]. They showed in a lamb animal model, that bladder tissue engineering with a highly porous collagen scaffold was possible in a disease model. Regeneration of the bladder was also comparable to regeneration in healthy bladder, when using an ovine model for bladder exstrophy, and results in tissue of good quality [29]. Leonhäuser et al. showed also good properties of collagen scaffolds in Göttinger minipigs. In this in vivo model the collagen scaffold had good ingrowth capacity into the bladder wall including a quick lining with urothelial cells. The ingrowth of detrusor muscle tissue, along with the degradation of the scaffolds, could also be observed throughout the study period [30].
In conclusion, the biocompatible collagen-mesh (Lyoplant®) convinces macroscopically, microscopically and immunhistologically in our trial. The material seems to be immunocompetent. The transplant acceptance is based on the remodelling process, initiated by neovascularisation. Lyoplant® stimulated the formation of epithelial and connective tissue for 6 weeks observation. To what extent Lyoplant® can be used treating congenital urogenital malformations in clinical practice, has to be tested and confirmed in further animal and human studies.
Acknowledgement
The author’s thanks Mrs. Chodnesvska for the technical support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study was performed according to a protocol approved by the local committee for animal use and care (Regierung von Unterfranken: 55.2-2531.01-01/12).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Langer S, Radtke C, Györi E, Springer A, Metzelder ML. Bladder augmentation in children: current problems and experimental strategies for reconstruction. Wien Med Wochenschr. 2019;169:61–70. doi: 10.1007/s10354-018-0645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrer F, Gearhart JP. Bladder exstrophy: considerations and management of the newborn patient. In: Puri P, editor. Newborn Surgery. Oxford: Oxford University Press; 2003. pp. 619–627. [Google Scholar]
- 3.Pokrywczynska M, Adamowicz J, Sharma AK, Drewa T. Human urinary bladder regeneration through tissue engineering: an analysis of 131 clinical cases. Exp Biol Med (Maywood) 2014;239:264–271. doi: 10.1177/1535370213517615. [DOI] [PubMed] [Google Scholar]
- 4.Diamond DA, Chan IHY, Holland AJA, Kurtz MP, Nelson C, Estrada CR, Jr, et al. Advances in paediatric urology. Lancet. 2017;390:1061–1071. doi: 10.1016/S0140-6736(17)32282-1. [DOI] [PubMed] [Google Scholar]
- 5.Hoen L’, Ecclestone H, Blok BFM, Karsenty G, Phé V, Bossier R, et al. Long-term effectiveness and complication rates of bladder augmentation in patients with neurogenic bladder dysfunction: a systematic review. Neurourol Urodyn. 2017;36:1685–1702. doi: 10.1002/nau.23205. [DOI] [PubMed] [Google Scholar]
- 6.Smeulders N, Woodhouse CR. Neoplasia in adult exstrophy patients. BJU Int. 2001;87:623–628. doi: 10.1046/j.1464-410x.2001.02136.x. [DOI] [PubMed] [Google Scholar]
- 7.Kollhoff DM, Cheng EY, Sharma AK. Urologic applications of engineered tissue. Regen Med. 2011;6:757–765. doi: 10.2217/rme.11.91. [DOI] [PubMed] [Google Scholar]
- 8.Meyer T, Meyer B, Schwarz K, Höcht B. Immune response to xenogeneic matrix grafts used in pediatric surgery. Eur J Pediatr Surg. 2007;17:420–425. doi: 10.1055/s-2007-989306. [DOI] [PubMed] [Google Scholar]
- 9.Meyer T, Schwarz K, Ulrichs K, Höcht B. A new biocompatible material (Lyoplant) for the therapy of congenital abdominal wall defects: first experimental results in rats. Pediatr Surg Int. 2006;22:369–374. doi: 10.1007/s00383-006-1658-z. [DOI] [PubMed] [Google Scholar]
- 10.Meyer T, Seifert A, Meyer B, Ulrichs K, Germer CT. PAUL procedure. A new biocompatible concept for the therapy of congenital abdominal wall defects. Chirurg. 2010;81:236–242. doi: 10.1007/s00104-009-1791-z. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer M, Kaiser A, Stehr M, Beyer HJ. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol. 2013;9:878–883. doi: 10.1016/j.jpurol.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Ayyildiz A, Nuhoglu B, Huri E, Ozer E, Gurdal M, Germiyanoglu C. Using porcine acellular collagen matrix (Pelvicol) in bladder augmentation: experimental study. Int Braz J Urol. 2006;32:88–92. doi: 10.1590/S1677-55382006000100015. [DOI] [PubMed] [Google Scholar]
- 13.AG, B.B.M. Lyoplant. 2015; Available from: https://www.bbraun.de/de/products/b0/lyoplant-onlay.html.
- 14.Kouame BD, Kouame GS, Sounkere M, Koffi M, Yaokreh JB, Odehouri-Koudou T, et al. Aesthetic, urological, orthopaedic and functional outcomes in complex bladder exstrophy-epispadias’s management. Afr J Paediatr Surg. 2015;12:56–60. doi: 10.4103/0189-6725.150985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertin KD, Serge KY, Moufidath S, Maxime K, Hervé OK, Baptiste YJ, et al. Complex bladder-exstrophy-epispadias management: causes of failure of initial bladder closure. Afr J Paediatr Surg. 2014;11:334–340. doi: 10.4103/0189-6725.143149. [DOI] [PubMed] [Google Scholar]
- 16.Inouye BM, Tourchi A, Di Carlo HN, Young EE, Gearhart JP. Modern management of the exstrophy-epispadias complex. Surg Res Pract. 2014;2014:587064. doi: 10.1155/2014/587064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodhouse CR, North AC, Gearhart JP. Standing the test of time: long-term outcome of reconstruction of the exstrophy bladder. World J Urol. 2006;24:244–249. doi: 10.1007/s00345-006-0053-7. [DOI] [PubMed] [Google Scholar]
- 18.Gearhart JP, Ben-Chaim J, Sciortino C, Sponseller PD, Jeffs RD. The multiple reoperative bladder exstrophy closure: what affects the potential of the bladder? Urology. 1996;47:240–243. doi: 10.1016/S0090-4295(99)80424-5. [DOI] [PubMed] [Google Scholar]
- 19.Hesh CA, Young E, Intihar P, Gearhart JP. The cost of failure: the economic impact of failed primary closure in classic bladder exstrophy. J Pediatr Surg. 2016;51:1312–1316. doi: 10.1016/j.jpedsurg.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Stein R, Hohenfellner M, Pahernik S, Roth S, Thüroff JW, Rübben H. Übersichtsarbeit-Therapiekonzepte und Konsequenzen der Harnableitung. Dtsch Arztebl Ausg A. 2012;109:617–622. doi: 10.3238/arztebl.2012.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Taji OM, Khattak AQ, Hussain SA. Bladder reconstruction: the past, present and future. Oncol Lett. 2015;10:3–10. doi: 10.3892/ol.2015.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wünsch L, Ehlers EM, Russlies M. Matrix testing for urothelial tissue engineering. Eur J Pediatr Surg. 2005;15:164–169. doi: 10.1055/s-2004-830356. [DOI] [PubMed] [Google Scholar]
- 23.Cranidis A, Nestoridis G, Delakas D, Lumbakis P, Kanavaros P. Bladder autoaugmentation in the rabbit using de-epithelialized segments of small intestine, stomach and lyophilized human dura mater. Br J Urol. 1998;81:62–67. doi: 10.1046/j.1464-410x.1998.00475.x. [DOI] [PubMed] [Google Scholar]
- 24.Bolland F, Korossis S, Wilshaw SP, Ingham E, Fisher J, Kearney JN, et al. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials. 2007;28:1061–1070. doi: 10.1016/j.biomaterials.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Yang B, Zhang Y, Zhou L, Sun Z, Zheng J, Chen Y, et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods. 2010;16:1201–1211. doi: 10.1089/ten.tec.2009.0311. [DOI] [PubMed] [Google Scholar]
- 26.Allman A, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann SHE. T-Zellen. In: Kaufmann SHE, editor. Basiswissen immunologie. Berlin: Springer; 2014. pp. 63–83. [Google Scholar]
- 28.Gulbins E, Lang KS. Immunsystem. In: Schmidt RF, Lang F, Heckmann M, editors. Physiologie des menschen. Berlin: Springer; 2007. pp. 550–562. [Google Scholar]
- 29.Roelofs LA, Kortmann BB, Oosterwijk E, Eggink AJ, Tiemessen DM, Crevels AJ, et al. Tissue engineering of diseased bladder using a collagen scaffold in a bladder exstrophy model. BJU Int. 2014;114:447–457. [PubMed] [Google Scholar]
- 30.Leonhäuser D, Stollenwerk K, Seifarth V, Zraik IM, Vogt M, Srinivasan PK, et al. Two differentially structured collagen scaffolds for potential urinary bladder augmentation: proof of concept study in a Göttingen minipig model. J Transl Med. 2017;15:3. doi: 10.1186/s12967-016-1112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]