Abstract
Background:
Macrophages have been known to have diverse roles either after tissue damage or during the wound healing process; however, their roles in flap wound healing are poorly understood. In this study, we aimed to evaluate how macrophages contribute to the flap wound regeneration.
Methods:
A murine model of a pedicled flap was generated, and the time-course of the wound healing process was determined. Especially, the interface between the flap and the residual tissue was histopathologically evaluated. Using clodronate liposome, a macrophage-depleting agent, the functional role of macrophages in flap wound healing was investigated. Coculture of human keratinocyte cell line HaCaT and monocytic cell line THP-1 was performed to unveil relationship between the two cell types.
Results:
Macrophage depletion significantly impaired flap wound healing process showing increased necrotic area after clodronate liposome administration. Interestingly, microscopic evaluation revealed that epithelial remodeling between the flap tissue and residual normal tissue did not occurred under the lack of macrophage infiltration. Coculture and scratch wound healing assays indicated that macrophages significantly affected the migration of keratinocytes.
Conclusion:
Macrophages play a critical role in the flap wound regeneration. Especially, epithelial remodeling at the flap margin is dependent on proper macrophage infiltration. These results implicate to support the cellular mechanisms of impaired flap wound healing.
Electronic supplementary material
The online version of this article (10.1007/s13770-019-00214-x) contains supplementary material, which is available to authorized users.
Keywords: Reconstruction, Flap, Wound healing, Macrophage, Inflammatory cell
Introduction
Flap surgery, either a microvascular free-tissue transfer or a pedicled flap, is pivotal for reconstruction of considerable tissue defects following oncologic ablative surgery or trauma [1]. With advances in the microvascular technique, reconstructive surgery using tissue flaps are increasingly being performed for various kinds of tissue defects [2]. However, it has been reported that even experienced surgeons can encounter 0.6–6.6% of flap wound complications, which often cause devastating clinical sequelae [3, 4]. Patients with comorbidities including diabetes mellitus often show an increased rate of flap wound complications [5]. Moreover, prior radiation or chemoradiation therapy have been known to induce a less favorable tissue environment leading to a higher risk of complications [6, 7]. Efforts are required to understand the detailed mechanisms of flap wound regeneration and to decrease flap wound complications.
There is unique tissue microenvironment during the process of wound healing following flap reconstruction which consists of the vascularized flap tissue and the residual normal tissue. In general, it has been widely accepted that the wound healing process has three overlapping but distinct phases including inflammation, proliferation, and remodeling [8, 9]. The inflammatory phase, characterized by abundant neutrophil infiltration and platelet activation, starts immediately after tissue damage, and lasts for about 48 h. The second phase of wound healing occurs 2–10 days after injury and is characterized by cellular proliferation and migration of different cell types [8]. This process includes epithelial cell migration over the injured area, angiogenesis, macrophages infiltration, and lastly fibroblast proliferation. The third phase of wound healing is characterized by disappearance of endothelial cells, macrophages, and fibroblast leading to a chronic remodeling of collagen and other extracellular matrix proteins. Interactions of different cell types and secretory molecules play a critical role in all stages of wound healing.
It is well known that inflammatory cells play an important role in the wound healing process [10]. Once tissue damage occurs, a robust inflammatory response is induced and modulates tissue regeneration [10]. Among the inflammatory cell populations, tissue-resident as well as newly recruited macrophages have been shown to exhibit critical regulatory activity at all stages of tissue repair and regeneration, serving as an important therapeutic target [11, 12]. Their roles are not only scavenging and phagocytizing cellular debris and invading organisms but also generating chemokines, matrix metalloproteinases (MMPs), and cytokines during tissue repair [11]. However, it has not yet been evaluated how macrophages contribute to wound healing following flap surgery.
In this study, we developed a preclinical murine model of the pedicled flap reproducing the clinical flap reconstruction. Through a functional study using pharmacologic depletion of macrophages as well as histophathologic evaluation of the flap wound margin, we found a unique phenotypic changes (impaired epithelial remodeling) of the flap wound after macrophage depletion, indicating a critical role of the proper macrophage infiltration during flap wound regeneration process.
Materials and methods
Design of murine model of pedicled flap
Animal care and procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and all experiments were approved by the Committee for Ethics in Animal Experiments of the Ajou University School of Medicine. Six-week-old male C57BL/6N mice (~ 20 g) were obtained from Orient Bio (Seoul, Korea) and maintained in the pathogen-free animal laboratory for at least 1 week.
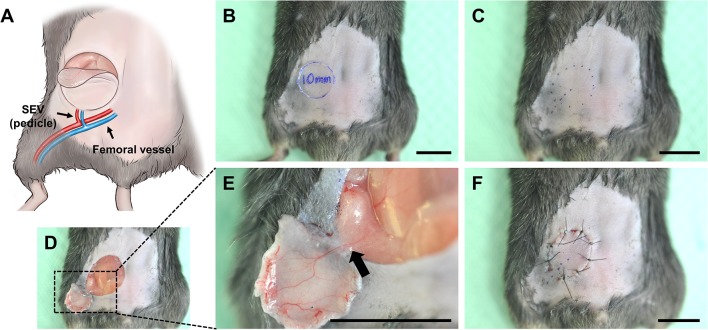
For surgical procedures, anesthesia was administered with intramuscular injections of Zoletile 50 (11.45 mg/kg; Vibac, Carros, France) and Rumpun (0.58 mg/kg; Bayer, Seoul, Korea). For newly developed murine pedicled flap model, following surgical procedures were performed (Fig. 1). The hair over the ventral surface of the abdominal wall was removed with depilatory cream. After hair removal, the depilatory cream was cleaned off with warm saline. Using a surgical skin marker, a 1-cm sized superficial inferior epigastric vessel-based flap was designed on the basis of anatomical landmarks, including the vertical midline, xyphoid process, and pubic symphysis (Fig. 1B, C). The skin was incised and dissected with micro scissors until the panniculus carnosus layer was reached. The flap was raised from cranial to caudal and from medial to lateral, carefully exposing the flap’s pedicle. The flap was elevated, as shown in Fig. 1D, E; the right superficial inferior epigastric artery (SIEA) and superficial inferior epigastric vein (SIEV) were preserved as the pedicle. Each flap was returned to the original harvest area and sutured using 6-0 non-absorbable nylon sutures (Fig. 1F) (Ethicon Inc., Somerville, NJ, USA). The flaps were photographed on postoperative days 3, 7, and 10, after which the mice were sacrificed and the flaps, harvested.
Fig. 1.
A Scheme depicting a mouse model of a superficial epigastric vessel (SEV)-based skin flap. B–F Gross images showing consecutive surgical procedures for a mouse model of the pedicled flap. The arrow indicates the vascular pedicle. Scale bars 1 mm
Macrophage depletion protocol
Clodronate liposome and control liposome were purchased from FormuMax Scientific (Sunnyvale, CA, USA). Our in vivo macrophage depletion protocol was followed as previously described [13–15]. In detail, 200 µL of clodronate liposome was intraperitoneally injected at 1 day before flap surgery and on every other day before analyses. Control mice were injected with 200 µL of control liposome using the same schedule. The depletion of infiltrated macrophages at the flap wound was evaluated by immunofluorescence imaging, as described below.
Histopathologic evaluation
On the indicated days after flap surgery, the mice were anesthetized as previously described. Flap tissue and the surrounding normal tissue were meticulously harvested and fixed in 1–4% paraformaldehyde. The fixed tissues were embedded with a tissue freezing medium (Leica, Wetzlar, Germany) or paraffin and sectioned. The paraffin-sectioned tissues were stained according to the standard hematoxylin and eosin (H&E) staining protocol. Images of the H&E-stained sections were captured on a microscope equipped with the CCD camera (Carl Zeiss, Oberkochen, Germany). Degree of acute/chronic inflammatory cell infiltrates, vessel infiltrates, fibrosis was evaluated and rated as minimal, mild, moderate, and severe or marked by a pathologist (J.R., Table 1).
Table 1.
Time-course analyses of inflammatory cell infiltrates, vessel infiltrates, degree of fibrosis after flap surgery
| No. | Post-operative days | Acute inflammatory cell infiltrates | Chronic inflammatory cell infiltrates | Vessel | Fibrosis |
|---|---|---|---|---|---|
| 01 | 3 | Severe | Minimal | Marked | No |
| 02 | 3 | Severe | Mild | Moderate | Mild |
| 03 | 3 | Severe | Mild | Marked | Minimal |
| 04 | 7 | Mild | Moderate | Moderate | Moderate |
| 05 | 7 | Minimal | Moderate | Moderate | Mild |
| 06 | 10 | Minimal | Moderate | Mild | Moderate |
| 07 | 10 | No | Mild | Minimal | Moderate |
| 08 | 10 | Minimal | Mild | Mild | Mild |
For immunofluorescence imaging of F4/80-positive macrophages, the frozen samples were blocked with 5% gout serum in PBST (0.1% Triton X-100 in PBS) and incubated for 3 h at room temperature with the primary antibodies, anti-F4/80 (rat monoclonal, eBioscience, San Diego, CA, USA). After several washes with PBS, the sections were incubated for 2 h at room temperature with the secondary antibodies: FITC-conjugated anti-rat IgG (Jackson ImmunoResearch, Baltimore, PA, USA). A Zeiss LSM 710 confocal microscope (Carl Zeiss, Oberkochen, Germany) was used to visualize the fluorescent signals and obtain digital images. Morphometric analysis was performed using ImageJ software (http://rsb.info.nih.gov/ij). The discolored area of the flap was measured and divided by the total area of the flap. The percentage of F4/80-positive area was measured and quantified.
Cell culture
The immortalized human keratinocyte cell line HaCaT was maintained in Dulbecco’s modified Eagle’s medium (DMEM, Welgene, Daegu, Korea), supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and 1% antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin). THP-1 cells, the human monocytic cell line, was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI-1640 (Welgene, Gyeongsan-si, Republic of Korea) supplemented with 10% FBS and 1% antibiotics. THP-1 cells were induced to differentiate into macrophages using phorbol 12-myristate 13-acetate (PMA, Sigma-aldrich, St. Louis, MO, USA) 250 nM in basal medium for 24 h. Then, THP-1 macrophages were stimulated with lipopolysaccharide (LPS, Sigma-aldrich, St. Louis, MO, USA) 1 μg/mL in basal medium for 24 h.
Scratch wound healing assay
For evaluating cell migration, scratch wound healing assay was performed. In detail, HaCaT cells (1 × 105 cells/well) cultured in the lower compartment in 24 transwell plates. About 24 h after seeding to permit cell adhesion and the formation of a confluent monolayer, a 700 μm wide wound was created by scratching the cell monolayer with a sterilized 1000 μL pipette tip. After washing three times with PBS, the THP-1 cell insert was then placed on top of the HaCaT cells to avoid physical contact and incubation continued for 24 h. Cells were fixed with 10% neutral buffered formaldehyde (NBF), incubated with 1% crystal violet for 60 min at room temperature. Digital images of the scratched cell cultures were photographed (EVOS™ FL Color Imaging System, Thermo Fisher Scientific, Waltham, MA, USA), and the width of the wound was measured using ImageJ software. The results were expressed as percentage of the control wound area.
Statistical analysis
Values are presented as mean ± standard deviation. Significant differences between the groups were determined by the two-sided Mann–Whitney U test. Statistical analyses were performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA). A p value less than 0.05 was considered statistically significant.
Results
Time-course of the flap wound healing process in an experimental murine flap model
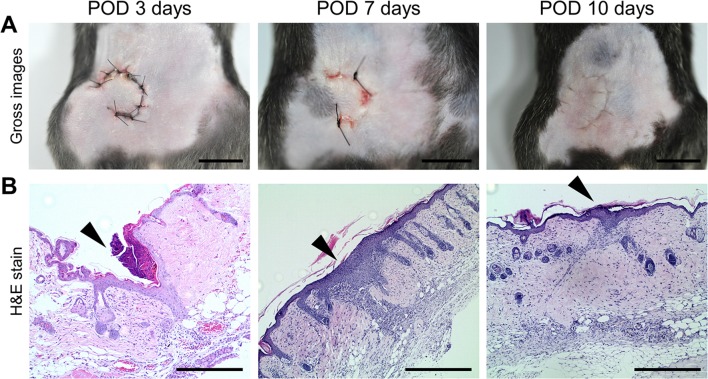
First, the time course of wound healing process were evaluated in our newly developed mouse model of pedicled flap. The flap margins were grossly and histopathologically evaluated on the post-operative 3, 7, and 10 days (Fig. 2). Flap margins were almost completely healed by 10 days after flap surgery and all experimental mice survived without any complications (Fig. 2A). On the 3rd postoperative day, acute inflammatory cell infiltrates such as leukocytes were severe and marked vessel infiltrates were observed while epithelial cells did not cover the defect area (Fig. 2B, Table 1). On the 7th postoperative day, robust chronic inflammatory cell infiltrates (mostly monocytes/macrophages) were observed especially at the skin flap margin. The epithelial layer was thickened and covered the flap margin area. On the 10th postoperative day, inflammatory cell infiltrates were decreased and fibrosis were increased at the wound margin.
Fig. 2.
A Gross images showing the pedicled flap wound on the postoperative days 3, 7, and 10. Scale bars, 1 mm. B Images of the H&E-stained sections showing flap margin healing. Arrowheads indicate the area of flap wound margin. Scale bars 400 µm
Macrophage depletion impaired flap wound healing
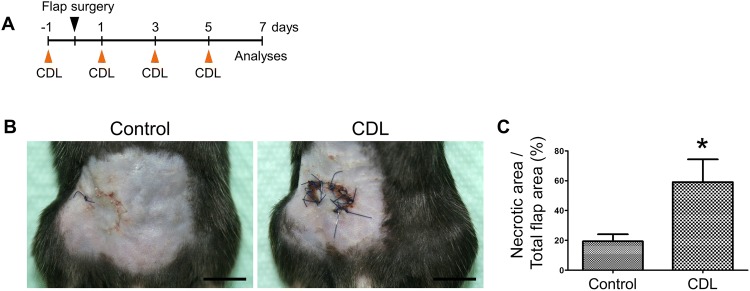
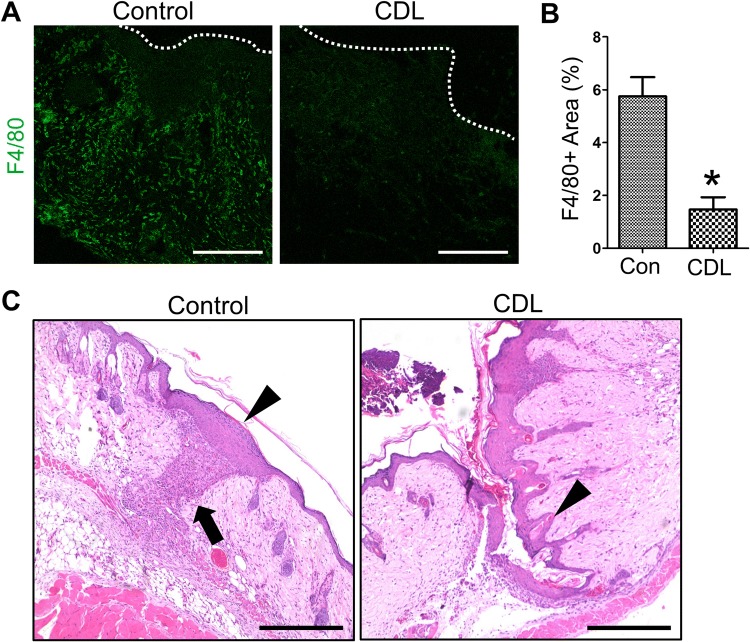
On the basis of the observation of robust inflammatory cell infiltrates at the flap margin on the postoperative day 7, we aimed to evaluate the role of macrophages in the flap wound healing process by macrophage depletion experiments. Clodronate liposome (CDL) was intraperitoneally administered on the day before flap surgery (Fig. 3A). After the flap surgery, CDL was administered every other day, followed by evaluation of the flap wound on the postoperative day 7. Interestingly, compared with that in control mice, CDL-injected mice showed significantly delayed wound healing, especially at the flap margin (Fig. 3B, C). Flap margins of CDL-injected mice were necrotic compared to those of control mice. Immunofluorescence imaging showed significantly decreased F4/80+ macrophage infiltration in CDL-injected mice compared with controls, indicating the proper macrophage depletion following CDL injection (Fig. 4).
Fig. 3.
Effects of macrophage depletion on flap wound healing. A Experimental protocol. B Gross images showing the flap wound healing status in control and clodronate liposome (CDL)-injected mice. Scale bars 1 mm. C Comparisons of the relative percentage of necrotic area in flap wounds between control- and CDL-injected mice (below). Each group, n = 4–5. *p < 0.05
Fig. 4.
A, B Comparisons of F4/80+ macrophage distribution in flap wound between control and clodronate liposome (CDL)-injected mice. Dotted lines indicate the wound margin. Scale bars 100 µm. *p < 0.05. C Representative images showing impaired epithelial remodeling of flap margin in CDL-injected mice. The arrow indicates infiltrated macrophages at the flap margin. The arrowheads indicates remodeled epithelium at the flap margin. Scale bars 400 µm
Macrophage depletion impaired epithelial remodeling during flap margin healing
Importantly, images of the H&E-stained sections revealed that the epithelial cells between flap tissue and residual normal tissue were not attached to each other in CDL-injected mice, whereas active epithelial remodeling (thickened epithelium, coverage of flap margin defect) was observed in control mice (Fig. 4B, Supplementary Fig. 1). To evaluate direct effects of macrophage on epithelial cells, coculture experiments were performed using immortalized human keratinocyte cell line HaCaT and human monocytic cell line THP-1. Scratch wound healing assay was performed to evaluate cell migration ability. As a result, coculture with stimulated THP-1 cells significantly increased the migration of HaCaT cells (Fig. 5), indicating that the secretory factors form macrophages affects keratinocyte migration.
Fig. 5.
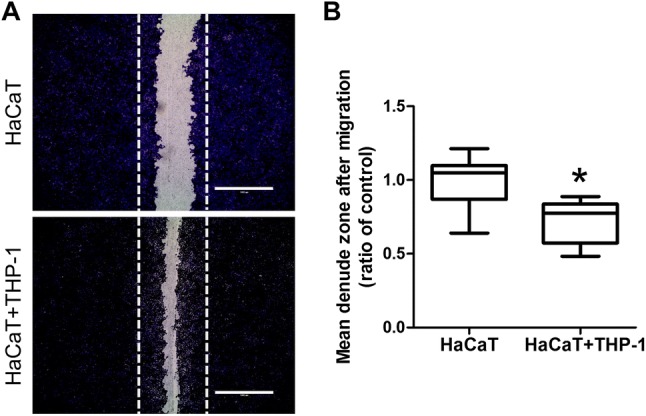
A Coculture with THP-1 cells increases cell migration of HaCaT cells. HaCaT cells were plated in 24 transwell plate and a scratch wound healing assay was performed under condition of HaCaT cell alone and coculture of HaCaT cells with THP-1 cells (HaCaT + THP-1). B Mean denuded zone was obtained by calculating the ratio of average area of denuded zone to the area in the control. Scale bars 1000 µm. Each group, n = 10. *p < 0.05
Discussion
Pedicled or free tissue transfer is frequently used in the reconstruction of considerable tissue defects, with over 95% of success rates [1, 2]. In the immediate postoperative period following flap reconstruction, survival of transferred tissue mainly depends on the patency of the vascular pedicle. Thereafter, neovascularization and tissue integration between the transferred flap and the surrounding recipient wound bed may support flap viability. The time and molecular process for successful flap healing has not yet been fully clarified. Clinically, diabetes mellitus, previous chemoradiation, or systemic vasculitis are reported to affect these processes without the support of exact molecular mechanisms [5, 6, 16]. Damage to the microenvironment and the microvasculature and consequent inflammatory response leading to tissue remodeling might play a pivotal role in flap wound healing [10]. In this investigation, we intended to focus on the inflammatory responses seen in flap wound healing, especially on the role of macrophages.
Importantly, our study revealed that macrophages critically affect epithelial remodeling at the flap margin. In the CDL-injected mice, the epithelium of the pedicled flap and the recipient tissue had not migrated and remodeled, resulting in failure of re-epithelialization at the flap margin (Fig. 4). The epithelium is a highly selective barrier present in the skin or on mucosal surfaces, and epithelial resurfacing is a pivotal step for re-establishing homeostasis following injury [17]. Various factors including growth factor-receptor interactions and immune cell infiltration may affect keratinocyte migration and proliferation [18]. The epithelial–macrophage interactions have been known to be closely associated with the wound healing process [17, 18]. Our data show unique phenotypic changes during the flap healing process, indicating the indispensable role of macrophages in epithelial remodeling. Clinically, while pedicle patency is important for the early survival of the transferred flap, impaired flap margin healing is also frequently associated with increased complications such as pharyngeal fistula after head and neck reconstruction. Our results may help clarify the cellular mechanisms in impaired flap margin healing.
It has been reported that clodronate liposome was reliably used for macrophage-depletion in various organs or tissues to explore the functional role of macrophages [13, 14]. Clodronate liposome consists of phospholipid bilayers and clodronate. When clodronate liposome is absorbed into the macrophage, the lysosomal phospholipases disintegrate the liposome and release clodronate into the cell. Increased clodronate concentration in cells induces apoptosis.
During wound repair, macrophages are an important component of the innate immune response that acts as a source of chemokines, matrix metalloproteinases (MMPs), and other inflammatory mediators that drive the initial cellular response [11]. Diverse phenotypes of macrophages have been known to promote extracellular-matrix (ECM) synthesis, angiogenesis, epithelial cell migration, and proliferation [11]. In general, macrophages have been classified into two subtypes, which include inflammatory monocytes that rapidly differentiate into classically activated macrophages (M1 macrophages) and tissue-resident macrophages (M2 macrophages) [17, 19]. In our coculture experiments (Fig. 5), THP-1 monocytes differentiated into macrophages using PMA. Once differentiated (M0 macrophages), they were incubated with lipopolysaccharide for classical macrophage activation (M1 macrophages) that had been shown to promote epithelial cell migration [20]. Proinflammatory cytokines including tumor necrosis factor, IL-1, IL-6, IL-12, and reactive oxygen species (ROS), the secretory factors from M1 macrophages, could be responsible for the flap wound healing [17]. The underlying molecular mechanisms describing the unique role of macrophages in flap-epithelial remodeling needs to be investigated more deeply in future studies.
Most experimental research on flap survival or wound healing has been carried out in rats or larger animal models of random-pattern skin flaps [21–23]. In this study, we used an axial-pattern murine flap based on superficial inferior epigastric vessels. Compared with the random-pattern flap, this flap showed more consistent, definable, and reproducible flap survival and healing even in the distal margins and represented the clinical conditions of flap reconstruction more closely. Therefore, we could precisely compare the wound healing status of CDL-injected or not-injected mice in this investigation. Furthermore, a murine model has the advantages of low cost, ease of handling and housing, availability of transgenic mice, and availability of murine-specific immunologic reagents. Growing knowledge of mouse genetics might enable us to understand basic mechanisms regarding flap wound healing in future investigations using transgenic mice models. However, in this study, the time-course of wound healing process was determined using only H&E stained images (Fig. 2B) that limits the investigations of detailed cellular information despite of an evaluation of the pathologist (J.R.). Analyses with detailed immunohistochemical staining might be helpful for future validation of this murine model.
In conclusion, we generated a reliable mouse model of a pedicled flap for evaluation of the wound margin healing process. We report the occurrence of unique phenotypic changes during the flap healing process, indicating the indispensable role of macrophages in epithelial remodeling at the flap margin. As far as we know, this is the first attempt to investigate contributions of the immune microenvironment in the flap wound healing process. This platform can be expected to be utilized in various preclinical disease models, such as diabetes mellitus.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 10542 kb)
Acknowledgements
This study was supported by research Grants from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2018R1D1A1A02043691, NRF-2016R1D1A1B03932867).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Ajou University (IACUC approval No. 2017-0025).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jeon Yeob Jang and Yoo Seob Shin have contributed equally to this work.
Contributor Information
Jeon Yeob Jang, Phone: +82-31-219-5138, Email: manup1377@gmail.com.
Yoo Seob Shin, Phone: +82-31-219-5369, Email: ysshinmd@ajou.ac.kr.
References
- 1.Hanasono MM, Matros E, Disa JJ. Important aspects of head and neck reconstruction. Plast Reconstr Surg. 2014;134:968e–980e. doi: 10.1097/PRS.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 2.Cannady SB, Rosenthal EL, Knott PD, Fritz M, Wax MK. Free tissue transfer for head and neck reconstruction: a contemporary review. JAMA Facial Plast Surg. 2014;16:367–373. doi: 10.1001/jamafacial.2014.323. [DOI] [PubMed] [Google Scholar]
- 3.Chang EI, Zhang H, Liu J, Yu P, Skoracki RJ, Hanasono MM. Analysis of risk factors for flap loss and salvage in free flap head and neck reconstruction. Head Neck. 2016;38:E771–E775. doi: 10.1002/hed.24097. [DOI] [PubMed] [Google Scholar]
- 4.Pattani KM, Byrne P, Boahene K, Richmon J. What makes a good flap go bad? A critical analysis of the literature of intraoperative factors related to free flap failure. Laryngoscope. 2010;120:717–723. doi: 10.1002/lary.20825. [DOI] [PubMed] [Google Scholar]
- 5.Rosado P, Cheng HT, Wu CM, Wei FC. Influence of diabetes mellitus on postoperative complications and failure in head and neck free flap reconstruction: a systematic review and meta-analysis. Head Neck. 2015;37:615–618. doi: 10.1002/hed.23624. [DOI] [PubMed] [Google Scholar]
- 6.Herle P, Shukla L, Morrison WA, Shayan R. Preoperative radiation and free flap outcomes for head and neck reconstruction: a systematic review and meta-analysis. ANZ J Surg. 2015;85:121–127. doi: 10.1111/ans.12888. [DOI] [PubMed] [Google Scholar]
- 7.Paderno A, Piazza C, Bresciani L, Vella R, Nicolai P. Microvascular head and neck reconstruction after (chemo)radiation: facts and prejudices. Curr Opin Otolaryngol Head Neck Surg. 2016;24:83–90. doi: 10.1097/MOO.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 8.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 9.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 10.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11. doi: 10.1016/j.semcdb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Kim KE, Koh YJ, Jeon BH, Jang C, Han J, Kataru RP, et al. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol. 2009;175:1733–1745. doi: 10.2353/ajpath.2009.090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Li B, Jiang H, Wang Y, Qu M, Duan H, et al. Macrophage depletion impairs corneal wound healing after autologous transplantation in mice. PLoS One. 2013;8:e61799. doi: 10.1371/journal.pone.0061799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawanishi N, Mizokami T, Niihara H, Yada K, Suzuki K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem Biophys Rep. 2016;5:146–151. doi: 10.1016/j.bbrep.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TY, Serletti JM, Kolasinski S, Low DW, Kovach SJ, Wu LC. A review of 32 free flaps in patients with collagen vascular disorders. Plast Reconstr Surg. 2012;129:421e–427e. doi: 10.1097/PRS.0b013e3182412a0b. [DOI] [PubMed] [Google Scholar]
- 17.Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune–epithelial interactions. Mucosal Immunol. 2015;8:959–968. doi: 10.1038/mi.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2018 doi: 10.1016/j.addr.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15:577. doi: 10.1186/s12885-015-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto NM, Aoki M, Nakao J, Peng WX, Takami Y, Umezawa H, et al. Experimental rat skin flap model that distinguishes between venous congestion and arterial ischemia: the reverse u-shaped bipedicled superficial inferior epigastric artery and venous system flap. Plast Reconstr Surg. 2017;139:79e–84e. doi: 10.1097/PRS.0000000000002900. [DOI] [PubMed] [Google Scholar]
- 22.Casal D, Pais D, Iria I, Mota-Silva E, Almeida MA, Alves S, et al. A model of free tissue transfer: the rat epigastric free flap. J Vis Exp. 2017 doi: 10.3791/55281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MS, Ahmad T, Lee J, Awada HK, Wang Y, Kim K, et al. Dual delivery of growth factors with coacervate-coated poly(lactic-co-glycolic acid) nanofiber improves neovascularization in a mouse skin flap model. Biomaterials. 2017;124:65–77. doi: 10.1016/j.biomaterials.2017.01.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 10542 kb)