Abstract
Background:
Tendon and ligament injuries accounted for 30% of all musculoskeletal consultations with 4 million new incidences worldwide each year and thus imposed a significant burden to the society and the economy. Damaged tendon and ligament can severely affect the normal body movement and might lead to many complications if not treated promptly and adequately. Current conventional treatment through surgical repair and tissue graft are ineffective with a high rate of recurrence.
Methods:
In this review, we first discussed the anatomy, physiology and pathophysiology of tendon and ligament injuries and its current treatment. Secondly, we explored the current role of tendon and ligament tissue engineering, describing its recent advances. After that, we also described stem cell and cell secreted product approaches in tendon and ligament injuries. Lastly, we examined the role of the bioreactor and mechanical loading in in vitro maturation of engineered tendon and ligament.
Results:
Tissue engineering offers various alternative ways of treatment from biological tissue constructs to stem cell therapy and cell secreted products. Bioreactor with mechanical stimulation is instrumental in preparing mature engineered tendon and ligament substitutes in vitro.
Conclusions:
Tissue engineering showed great promise in replacing the damaged tendon and ligament. However, more study is needed to develop ideal engineered tendon and ligament.
Keywords: Tendon, Ligament, Tissue engineering, Stem cell, Bioreactor, Exosomes
Introduction
In the United States, more than 300,000 patients underwent surgery to repair their injured tendon and ligament annually [1]. Usually, the torn tendon and ligament will be ligated and the affected joint will be immobilized for several weeks until it is healed. For some cases, the torn tissues will be replaced either with a tendon from another area of the body (an autograft), from another person (an allograft), from different species (a xenograft) or a synthetic graft [2]. The major disadvantage of autograft is donor site morbidity. The allograft and xenograft are limited by the tissue availability as well as potential risk of immune rejection and pathogen transmission [3]. The earlier generation of synthetic grafts suffer from the shortcomings of early rupture, loss of mechanical strength over time, insufficient tissue ingrowth and deposition of graft debris [4]. Although the new generation showed better performance, however, these use of these synthetic grafts remain controversial as variable results reported in different clinical reports [5]. The re-rupture rate after surgical repair can be high for certain tendon and ligament. Randelli et al. [6] found that for the 41 out of 56 studies that reported complications related to arthroscopic rotator cuff repair, the re-rupture rate ranges from 11.4 to 94%. The re-rupture rate after anterior crucial ligament (ACL) reconstruction is 6.9% [7]. For Achilles tendon, the re-rupture rate after surgical repair has been reported to be between 1.7 and 5.6% [8]. For other commonly injured tendon and ligament, the re-rupture rate of distal biceps tendon and lateral ankle sprain (anterior talofibular, calcaneofibular, and posterior talofibular ligaments) is 1.6% and 18.1% respectively [9–11]. Due to these issues, there is a growing interest in the past decade to prepare tissue-engineered tendon/ligament to replace the autograft, allograft, xenograft and synthetic graft. The concept of tissue engineering is to produce a tissue substitute with improved safety and effectiveness in tissue repair/regeneration in a biologic environment [12]. One of the approaches that are being used is the fabrication of engineered scaffolds which provide a physical environment that modulates the repair and regeneration of injured tissue [13].
The extracellular matrix (ECM) is the non-cellular component composed of a mixture of proteins and polysaccharides, mainly collagen, elastin, hyaluronic acid, proteoglycans, and glycosaminoglycans that present within all tissues and organs. They provide not only essential physical scaffolding for the cellular constituents but also initiates important biochemical and biomechanical cues that are required for tissue morphogenesis, differentiation and homeostasis [14]. Yang et al. [15] have reported that tendon-derived ECM enhances the tenogenic differentiation of mesenchymal stem cells (MSCs) induced with transforming growth factor-β3 (TGF-β3). Thus, for tendon and ligament tissue engineering, the preparation of an ideal scaffold that could function as ECM analogue is essential to create a microenvironment that mimics the native tendon and ligament biological constituents and can trigger specific cellular responses in order to expedite the tissue regeneration.
Stem cell is commonly incorporated into the engineered scaffolds to enhance the tissue repair and regeneration capability. For tendon and ligament tissue engineering, tenocytes, ligamentocytes and MSCs are more often used as they are the native cells of these tissues. The usage of MSCs in tendon and ligament tissue engineering is very popular as these cells are easy to harvest from various tissue sources (such as umbilical cord, bone marrow and adipose tissues), possesses anti-inflammatory property that reduce tissue inflammation, secrete a myriad of trophic factors that promote tissue regeneration and can differentiated into tenocytes and ligamentocytes to re-cellularize the regenerating tissue [16–18]. The cell secreted products such as exosomes and secretomes as well as platelet-rich plasma (PRP) also can be incorporated into the engineered tissue to enhance the therapeutic effect [19–21].
In this review, we will extensively discuss the current progress in tissue-engineered tendon and ligament using the scaffolds, stem cells and cell secretory products for tendon and ligament repair. Furthermore, we will discuss the use of bioreactor and mechanical loading for in vitro engineered tendon and ligament maturation. Finally, an overview on future direction in tendon and ligament tissue engineering is proposed.
Tendon/ligament structure and function
Tendon is a flexible but inelastic cord attaching a muscle to bone. Its primary function is to transmit forces generated from muscle to the rigid bone that will produce joint movement [22]. Tendons are stronger than muscles and can resist against tensile and compressive forces. The Achilles tendon, which is the strongest and largest tendon in the body, can sustain loads up to 17 times of the body weight [23]. As muscles are closely related to the joint movement, each muscle usually consists of two ends of the tendon, proximal and distal. Proximal insertion of the tendon to muscle is the myotendinous junction while the distal insertion to the bone is the osteotendinous junction [24].
Ligament is a connective tissue that connects bone to bone for joint stability and support. It consists of defined bands of collagen fibers that anchored to the bone at joint at either end. They passively stabilize joint and guide it through the joint’s range of motion when load is applied [25]. Tendon and ligament are both similar in structure and ECM content and slightly differ in term of resident cells. Microscopically, ligament is characterized by multiple layers of connective tissues that contain the ECM and resident cells in hierarchical architecture (Fig. 1).
Fig. 1.
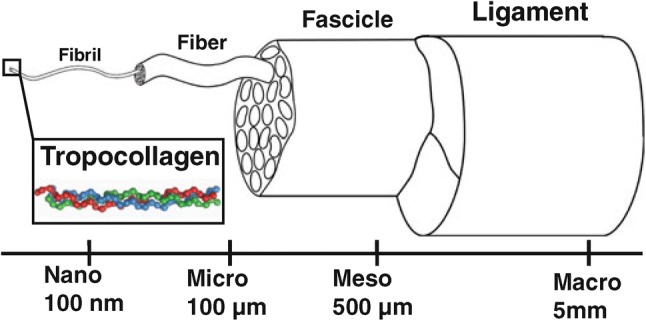
Ligament structure. (Reproduced from Ref. [190] with permission from Springer Nature)
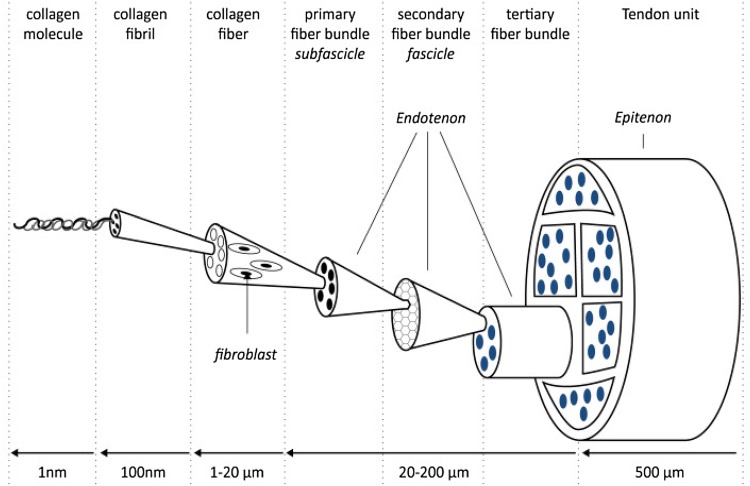
Paratenon is considered as the outermost layer of the tendon that is made up of type I and III collagen elastic fibrils that cause free movement of the tendon against surrounding tissue [26]. Underneath it lays the second layer, epitenon, which consist of a dense fibrillary network of collagen that housed multiple bundles of compact collagen fibrils embedded with the primary resident cells, tenoblasts and tenocytes and together they form primary fiber bundle (subfascicles). Collections of these subfascicles will be ensheathed by connective tissue layer called endotenon and they make the secondary fiber bundle. These secondary bundles then were grouped and formed tertiary fiber bundles and these will make up the solid tendon structure (Fig. 2) [27].
Fig. 2.
Tendon structure. (Reproduced from Ref. [191])
Tendon and ligament have excellent resistance to mechanical loads attributed to its special collagen fibers arrangement. Collagen fibers are arranged longitudinally, transversely and horizontally in a manner that provide good buffer capacity and rotational forces during movement. Ultrastructurally, collagen fibers constitute from networks of collagen fibrils, the basic unit of tendon and ligament [27]. Figure 3 showing the histology of tendon and ligament. Collagen represents 65–80% of the dry mass of the tendon while other fibers such as elastin accounted for only 1–2% [22, 28]. Under the light microscope, in resting phase, these fibrils showed a wavy pattern but disappeared when stretch forces are applied and resumed to its original configuration when the force is removed [27]. When the stretch forces and elongation exceeded the fibers’ limitation, the wavy configuration is not retained and subsequent deformation will compromise the tissue function [29].
Fig. 3.
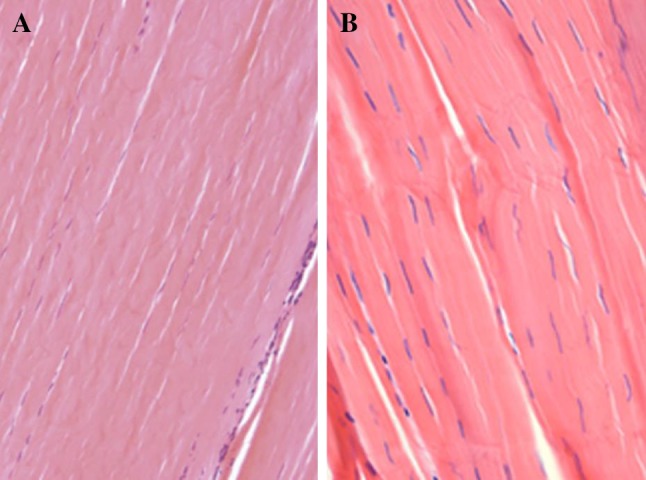
Histology of tendon and ligament. A, B The collagen fibers of tendon and ligament are highly aligned. (Reproduced from Ref. [192] with permission from Springer Nature and Ref. [193])
The tendon and ligament ECM are composed of internal networks of collagen fibers, elastin and ground substances that make up the most of the proteoglycan-water matrix although the percentage of the composition slightly differed among another (Table 1) [27]. Ligament has a lower percentage of collagen but higher with elastin, proteoglycans and water [30]. The ground substances that surround the collagen are made up of proteoglycans, glycosaminoglycans, glycoproteins which have good water binding capacity [22]. This shared property is crucial as it will improve the elasticity against compressive forces and maintain collagen homeostasis and structural stability.
Table 1.
Differences between tendon and ligament structure [30]
| Content/feature | Ligament | Tendon |
|---|---|---|
| Resident cell | Ligamentocyte | Tenoblast and tenocyte |
| Ground substance | 20–30% | Lower |
| Collagen | 70–80% | Slightly higher |
| Collagen type I | 90% | 95–99% |
| Collagen type III | 10% | 1–5% |
| Elastin | Up to 2 × of collagen | Scarce |
| Water | 60–80% | 60–80% |
| Organization | More random | Organized |
| Orientation | Weaving pattern | Long axis orientation |
Cellular components inside the tendon and ligament play an important role in maintaining the homeostasis of the microenvironment and also involved in pathological injury and healing process after an insult or rupture. In tendon, the resident cells, which consist of tenoblasts and tenocytes accounted for 90–95% of the cellular components, the rest are chondrocytes, synovial cells and vascular cells [27]. In ligament, the resident cells are made up of ligamentocytes that represent only a small percentage of the total ligament volume [25]. Tenoblast, a round shaped cell, is the primary cellular constituent in tendon during the young age. As the tendon aged, the tenoblast matured to the elongated tenocyte [27]. Both the tendon and ligament fibroblasts, i.e. tenocytes and ligamentocyte, involved in ECM synthesis and has low metabolic rate with efficient anaerobic glycolysis [31]. Given to these characteristics, they are able to withstand loads and maintain tension for a long period without the risk of ischemia.
Tendon/ligament biological and mechanical properties
Tendon tissues are well known for their flexibility, optimum elasticity and excellent mechanical strength. Collagen fibers influenced the mechanical behavior of the tendon. The characteristics of tendon collagen fibers are listed in Table 2. The number and type of bond formed either inter or intra-molecular among collagen fibers correlated with their configuration, strength, and resistance [32]. Although it possesses high performance in term of tensile strength and elasticity, tendon is also subjected to deformation resulted from the extreme strain. Excessive stretch caused sustained elongation of the collagen fibrils which also translated to molecular elongation. At a certain point, the gap between the molecules also increased as the strain increased, causing breakdown of connected molecules leading to the destruction of functional collagen [33].
Table 2.
Characteristic of collagen fibrils in tendon [27]
| Parameter | Collagen fibrils |
|---|---|
| Diameter | 20–150 nm |
| Alignment | Unilateral |
The tensile strength of tendon/ligament is very much dependent on the collagen fiber crosslinking [34]. Achilles tendon, which is the most commonly damaged tendon in sports injuries, has an ultimate tensile strength of approximately 1200 N [35]. As the largest tendon in the body, Achilles tendon can bear load up to 3500 N [36]. Due to acute acceleration and deceleration in sports such as football, basketball, tennis and soccer, Achilles tendon is prone to be injured and ruptured as a result of rapid changes of tension loading [37].
Interestingly, studies in animal have correlated exercise training with tendon efficiency. Better tensile strength with improved elastic stiffness, tendon weight and tendon cross-sectional area were observed in trained animals [38]. These are partly due to increased collagen and ECM synthesis by tenocytes [39]. Due to the effects of aging, collagen synthesis, content and cross-linking were reduced and tendon stiffness increased. These changes in tendon and sarcopenia are the main reasons for the reduction in muscle strength and power upon ageing [40]. In addition, reduction in level of physical activity and hormonal changes in the older adults also lead to deterioration of muscle strength and power.
The mechanical properties of different types of human tendon vary accordingly [41]. Each tendon has its own mechanical properties such as tendon stress, strain and Young’s modulus (elasticity). The range of tendon and ligament tensile strength properties is represented in Table 3. Tendon mechanical properties could be further demonstrated through the stress–strain curve. The stress–strain curve consists of three distinct regions. The first region is called the “toe” region that corresponds with straightening of crimped collagen fiber bundles that disappear under tension and reappear after the stress is released. The second region is the linear region, whereby the slope is constant and referred to as Young’s modulus or stiffness. At this point, the collagen bundles are stretched and oriented themselves in the direction of tensile mechanical load. The third region is the yield and failure region in which the bundles are stretched beyond its physiological limit and loss of intramolecular cross-links between fibers occurred (Fig. 4) [42].
Table 3.
Mechanical properties of native tendon and ligament [170]
| Mechanical property | Native tendon and ligament |
|---|---|
| Ultimate tensile strength | 5–100 MPa |
| Strain at failure | 10–15% |
| Young’s modulus | 20–1200 MPa |
MPa megapascal (1 MPa = 1 Newton (N)/mm2)
Fig. 4.
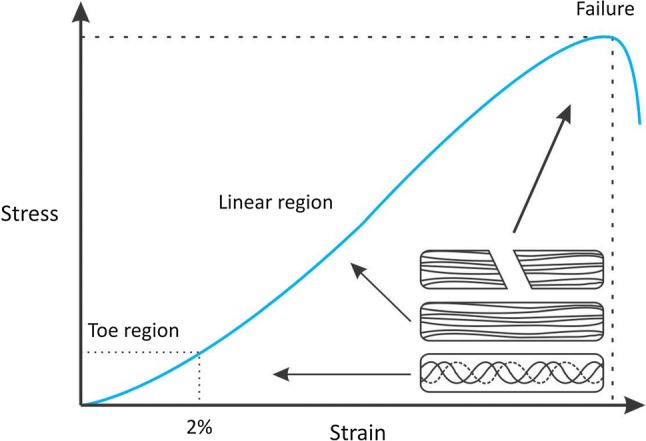
Stress–strain curve. (Reproduced from Ref. [30])
Tendon/ligament injury
A recent report on Global Burden of Disease 2016 has stated the prevalence and incidence of musculoskeletal disorders accounted around 1.27 billion and 652 million respectively [43]. The impact of the musculoskeletal disorder on the global economy was 213 billion US dollars in 2011 [44]. In the United States, 50% of the musculoskeletal disorders are related to tendon and ligament injuries that caused huge burden towards the economy and society [45].
Tendon injuries could be classified into tendinopathy and tendon rupture with the latter raised a concern due to treatment challenges faced by the orthopedic surgeons. Tendon injuries could be caused by hyperpronation, excessive loading, and microtrauma [46, 47]. Achilles tendon is the most commonly ruptured or injured tendon [48].
Ligament injuries usually involve partial or complete disruption of ligaments especially in major joints such as the knee joints. According to the National Collegiate Athletic Association Injury Surveillance System, anterior cruciate ligament (ACL) injury is most prominent especially in men’s football and women’s gymnastics [49]. According to Robi et al., ligament injuries are classified into three grades; grade I instated mild sprain, grade II with moderate sprain or partial tear and grade III is complete ligament tear. Avulsion of the ligament from its bony insertion is also considered as part of ligament injuries. Clinically, patients experienced from mild to severe pain with some cases affected the joint stability [30].
Pathophysiologically, damages towards tendon were responded with inflammation of sheath followed with degeneration of body of tendon. Several theories have surfaced to discuss the possible mechanism on what influence and factors that caused tendon to degenerate. Robi and colleagues in their review have elaborated on four theories on the mechanism of tendon degeneration; (1) mechanical (2) vascular, (3) neural, and (4) alternative theory. The mechanical theory discussed on the impact of overload to the tendon that triggers the pathologic process while vascular theory blamed the hypovascularity of tendon that contributed towards poor healing response. The neural theory stated that substance P released by neutrally mediated mast cell degranulation together with inflammatory cascades localized around the vessels of tendon could implicate the condition. Lastly, the alternative theory denoted that localized hyperthermia induced by exercise could jeopardize tenocytes’ survival [30].
Throughout the pathologic process, tenocytes responded with ECM production and degeneration. Histologically, collagen degeneration and disordered healing with the absence of inflammatory cells were seen [29]. The degeneration would later lead to reduced tensile strength and predisposed to tendon rupture [42]. Such process could also be observed in ligament injury.
Tendon/ligament repair and healing
Following injury, a cascade of healing process was initiated in an attempt to reduce damage and repair the tissue breakdown through the formation of scar tissue. The processes were stratified into 4 phases; inflammatory phase, proliferative phase, remodeling phase and modelling phase [31, 42].
Inflammatory phase
After introduction of external injury, a constellation of chemotactic factors was released to initiate inflammation. Red blood cells, neutrophils were among the first to be recruited which later were followed by monocytes and macrophages. At this phase, the cytokines and growth factors by these cells will initiate angiogenesis, stimulate tenocyte proliferation, synthesis of collagen type III and more recruitment of inflammatory cells.
Proliferative phase
During the proliferative phase, fibroblasts recruitment and proliferation were seen. The fibroblasts were responsible for the synthesis of collagen, proteoglycan, and other components of ECM. At the end of the phase, the wound would have a scar-like appearance with extensive blood network is apparent.
Remodelling phase
A few days after proliferative phase, remodeling phase followed with further increased in synthesis of type III collagen, high water content and glycosaminoglycans that last for several weeks.
Modelling phase
6 weeks following injury, the modelling phase constituted, where healing tissue undergone resized and reshaped. The phase is categorized into two distinct stages, i.e. consolidation and maturation. Consolidation, which occurred between 6 and 10 weeks of injury, portrayed increased production of type I collagen. Tenocyte metabolism increased and the collagen and tenocytes were aligned in the direction of stress. Maturation stage commenced after 10 weeks, whereby fibrous tissue was gradually transformed into scar tissue over the course of 1 year. At this stage, tenocytes metabolism was decreased and tendon vascularity was reduced.
Despite serious attempt of repair and healing of injured tendon/ligament, the newly formed healed tendon/ligament would never match the intact pre-injured tendon/ligament in term of biochemical and mechanical properties. In comparison, the rupture force for healed tendon tissue was only 56.7% of normal tendon [50]. In addition, healed tendon also has poorer strength (approximately 80%), stiffness (approximately 80%), stress (approximately 40%) and Young’s modulus (approximately 40%) compared to the normal tendon [51].
Current treatments and limitations in tendon/ligament injuries and repairs
Depending on the severity of tendon injuries, currently the treatments are limited to either conservative management or surgical intervention.
Conservative management
Mild or moderate tendon and ligament injuries that do not pose clinical difficulties are generally managed conservatively. For example, partially ruptured ulnar collateral ligament of the thumb is commonly treated conservatively and the surgical management is only recommended for complete rupture [52]. Generally, the goal of the treatment is to control the pain and to reduce the inflammation and swelling of the injured tissue. Among approaches that are well recognized include rest, usage of non-steroidal anti-inflammatory drugs, cryotherapy, injection therapy with corticosteroids, laser therapy, continuous passive motion and restrictive bracing [53–55]. Treatment with non-steroidal anti-inflammatory drugs and corticosteroids provides pain relieve but does not help in tendon/ligament healing [56–58]. Conservative approaches frequently have high failure rates and limited success when treating severe tendon and ligament injuries such as those with complete rupture. A systematic review performed by Monk et al. [59] showed that 50% of the patients with ACL injury received conservative treatment opted for surgical intervention within 5 years due to poor healing. Nonetheless, conservative management also gives very good results for certain tendon and ligament injuries, such as the anterior talofibular ligament, calcaneofibular ligament, posterior talofibular ligament and medial collateral ligament in the ankle. Surgical management of these ligaments are not recommended as it does not provide any benefits in term of recovery time and complication rate compared to conservative management [60]. Similarly, for the peroneal tendon injuries, surgical treatment will only be considered when the conservative management failed [61].
Surgical management
Most of the severe cases of tendon/ligament injuries with complete rupture or multiple torn tendon/ligament would require surgical interventions. In addition, surgery is also performed when the conservative treatment failed. Surgical management is also recommended for patients (e.g. athletes and patients with impaired activity of daily living) that wish to minimize the loss or regain the function and strength [62]. The aim is to reduce the symptoms, stabilize and improve articular function. Surgical interventions that are usually applied include re-suturing ruptured tendon, removal of the damaged tissue and replacement of the tissue with grafts [55]. Clinicians will select the suitable intervention according to the clinical factors, including age, mode of injury, size of defect and tendon location. Tissue grafting will be performed to fill large defect and to treat chronic tendon injury as well as when suturing technique failed [63].
Tissue grafting
Tissue grafting in tendon repair could be classified into three types; autograft, allograft, and xenograft. Limitations of each types of graft are listed in Table 4.
Table 4.
Limitations of autograft, allograft and xenograft
| Tissue graft | Limitations |
|---|---|
| Autograft | Complications at the donor site |
| Tissue that can be harvested is limited | |
| Poor tissue integration/non-anatomic placement | |
| Graft impingement or tension | |
| Allograft/xenograft | Immunosuppression to avoid tissue rejection |
| Poor tissue integration/non-anatomic placement | |
| Risk of disease transmission | |
| Graft impingement or tension | |
| Zoonotic transmission (xenograft) |
Autograft is a method of harvesting another part of tissue in the body as the replacement for the injured one. For example, reconstruction of ACL required hamstring and patellar tendon harvesting. The reconstruction would allow almost 50% of the ligament function from the pre-injured state. However, despite the success rate, the donor site morbidity and functional disability could not be disregarded since long term outcome that include pain, instability, mechanical incompetence will be suffered by the patients [64].
Allograft and xenograft for reconstruction also provides an alternative to autograft replacement and thus prevents donor site morbidity. Allograft could be classified into artificial or biological-derived. Ligament Advanced Reinforcement System (LARS ligament) and Kennedy ligament augmentation device (Kennedy LAD) are examples of artificial allograft commonly used in ACL tissue reconstruction with improved knee stability and full weight bearing documented [65]. LARS ligament are made from polyethylene terephthalate (PET) fibers that are separated into 2 compartments; an intraosseous segment with longitudinal fibers bound together by a transverse knitted structure and an intra-articular segment with parallel longitudinal fibers twisted at 90° which support growth of surrounding tissue [66]. Kennedy LAD is a cylindrical prosthesis made of braided polypropylene [67]. Biological-derived allograft such as GraftJacket™ (human derived) and xenograft such as Restore™ (porcine derived) were also used [68]. While artificial allografts are limited by its mechanical failure and mismatch with native tissue, biological allografts and xenografts increase the risk of immune-rejection and zoonotic transmission. Table 5 demonstrates the mechanical strength of commercial graft and native tendon and ligament.
Table 5.
Mechanical properties of commercial graft and native tendon and ligament
| Type of graft/tissue | Name | Origin/material | Mechanical strength (N) | References |
|---|---|---|---|---|
| Biological graft | GraftJacket™ | Human dermal matrix | 157–229 | [171] |
| Restore™ | Porcine small intestine | 38 | [171] | |
| TissueMend® | mucosa | 70–76 | [171] | |
| CuffPatch™ | Bovine dermal matrix | 32 | [171] | |
| Permacol™ | Porcine small intestine mucosa | 128 | [171] | |
| Porcine dermal matrix | ||||
| Synthetic graft | LARS ligament | Polyethylene terephthalate | 998 ± 148 | [172] |
| Leeds-Keio ligament | Polyethylene terephthalate | 780 ± 200 | [173] | |
| Native tendon and ligament | Rotator cuff | – | 1978 ± 301 | [174] |
| ACL | – | 1246 ± 243 | [175] | |
| Patellar | – | 3855 ± 550 | [175] | |
| Achilles | – | 5098 ± 1199 | [176] |
Autograft, allograft, and xenograft all imposed several limitations on achieving the best functional outcomes in comparison with the original tissue. Tissue grafting has also increased the risk of failure and recurrence, the formation of scar tissue, the risk of nerve damage, infection, and also mechanical and tissue mismatch [53–55]. Patient with biological allograft or xenograft would require life-long immune-therapy in order to prevent graft rejection while possible pathogen transmission should be taken into precaution.
Limitations involving tissue grafting have led researchers to find new revolutionary techniques that could serve a functional tissue replacement while waiting for in-body tissue regeneration process.
Tendon/ligament tissue engineering
In 1997, the world was introduced with the human ear on the back of a mouse created by Charles Vacanti laboratory in the University of Massachusetts Medical Center. The idea of creating a viable tissue externally has stemmed a new era in biomedical science with the revolutionary field called tissue engineering. The idea of tissue engineering was first introduced in 1987 and was defined as the application of the principles and methods of engineering and life sciences toward the fundamental understanding of structure–function relationships in normal and pathologic mammalian tissue and the development of biological substitutes to restore, maintain, or improve function [69].
Tissue engineering is a multidisciplinary approach with the aim to induce repair and replacement or regeneration of tissue. Tissue engineering involves the use a combination of cells, scaffolds and biologically active molecules to produce a functional tissue [70]. Cells serve as the building blocks of tissue which made the organ. Scaffold provides mechanical stability and 3-D template for growth of regenerative tissue and biologically active molecules such as growth factors drive the process of cell differentiation and maturation. Together, they made a newly formed viable tissue outside the human body to be applied in tissue replacement techniques.
To develop a viable tissue for tissue replacement, an engineered substitute should have similar and comparable characteristic towards native tissue. Stem from the principles of tissue engineering, newly engineered tendon/ligament tissues should have an environment that mimics the native tendon/ligament tissues in term of cells population, ECM components, and mechanical properties.
Collagen and elastin are the major components of the ECM of tendon and ligament tissue and the usage of biomaterials that have comparable properties towards both collagen and elastin are logically sensible [22]. The construct of the native ECM is relatable to the building of the scaffold in tissue engineering. Thus, to develop tissue engineered tendon/ligament, the tissue should also provide scaffold structures that are suitable for tenocyte or ligamentocyte attachment, differentiation and growth.
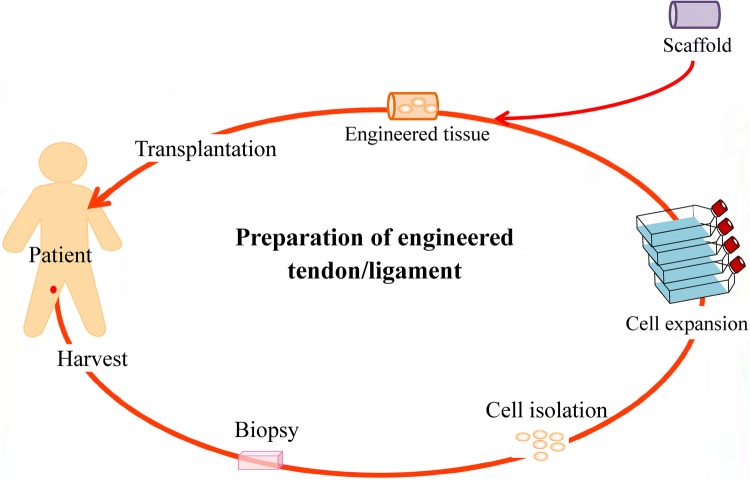
The goal of any tissue-engineered substitute is to provide temporary functional tissue to give time for own body’s natural regeneration to take place. For tissue engineered tendon/ligament, the scaffold would have comparable mechanical strength and also similar cell population of tendon/ligament. Figure 5 showing the process of preparing engineered tendon/ligament.
Fig. 5.
Preparation of engineered tendon/ligament
Current tissue engineering substitute of tendon and ligament
High failure and recurrence rates have implicated the usage of autograft, allograft and xenograft in tissue replacement. Tissue-engineered substitute has served a promising future for the next generation of tissue replacement that could overcome such limitations. For decades, several attempts have been made in a quest to find the best engineered viable tissue for tendon and ligament. Challenges faced by the bioengineers are to find the best suitable biomaterials and combination recipes that could mimic the anisotropic structure of tendon and ligament tissue and promote cell adhesion and differentiation of tenocyte or ligamentocyte, while possessing the right mechanical properties.
Carefully selecting the best suitable biomaterial and advanced scaffold-producing technique are needed to ensure that the tissue-engineered substitute has the best characteristic and function for a particular tissue. Choosing the suitable biomaterials for a scaffold is challenging as the ideal scaffold need to have several features that could translate it into functional tissue. To mediate the cellular attachment, the biomaterials used to construct the scaffold should have important characteristics. This includes hydrophilicity, wettability, biocompatibility, biodegradability, less cytotoxicity and good mechanical strength. Apart from important properties of biomaterials that mediate the cellular attachment, the structural framework and mechanical properties of the scaffold also should be considered. Among parameters that should be measured for a scaffold include porosity, pore size, degradation rates, mechanical strength and elasticity [71, 72].
Biocompatibility of the scaffold is important to support the appropriate cellular activity. Scaffold need to have high porosity and contain interconnected pores with suitable pore size in order to provide an ideal physical environment for the cell to grow and proliferate, to distribute uniformly and to support neovascularization. The biostability of the scaffold depends on factors such as mechanical strength, elasticity and biodegradation. Matching the mechanical properties of a scaffold to that of the native ECM is important to ensure the tissue growth is not limited by mechanical failure of the scaffold. Controlling the scaffold biodegradation and new ECM formation is very important in order to prevent loss of scaffold mechanical strength and architecture. An ultimate and ideal scaffold should serve as a template and provide biochemical cues for cellular attachment, proliferation, migration and differentiation just like the native ECM [71, 72].
Many biomaterials including collagen, silk, alginate, chitosan, polycaprolactone, polyglycolic acid and polylactic acid have been fabricated into a scaffold for tendon/ligament tissue engineering using techniques such as electrospinning, electrochemical alignment, knitting and freeze-drying. The biggest issue with current tissue engineered tendon/ligament is it showed promising short-term results but failed over time. Santos et al. 2017 in their review have discussed several examples of scaffold biomaterials that have been studied for tendon and ligament, and their advantages (Table 6) [73]. Table 7 showed some of the current developed tendon/ligament substitutes with their mechanical properties.
Table 6.
Examples of scaffold biomaterials in tendon/ligament tissue engineering
| Source | Biomaterials | Advantages | Reference |
|---|---|---|---|
| Natural | Collagen | Slow degradation rate | [177] |
| Main component of ECM | |||
| Reasonable mechanical strength | |||
| Silk | Good mechanical strength | [178] | |
| Slow degradation rate | |||
| Alginate | Biocompatible and hydrophilic properties helped to sustain and control delivery of biological factors | [179] | |
| Synthetic | PCL | Easier to process | [77] |
| PLGA | Mass production with low cost | [180] | |
| PLLA | High mechanical strength | [181] | |
| Synthetic/natural | PCL/CHT | Combination of the best properties of both natural and synthetic polymers | [182] |
| PLCL/Collagen | [102] | ||
| PCL/CHT/HA | [183] | ||
| PLLA/CHT-collagen/alginate | [184] |
PCL polycaprolactone, PLGA poly (lactic-co-glycolic acid), CHT chitosan, PLCL poly (l-lactide-co-ε-caprolactone), PLLA poly-l-lactic acid, HA hydroxyapatite
Table 7.
Recently developed tissue engineered tendon/ligament substitutes and their mechanical properties
| Composite | Ultimate tensile strength (MPa) | Strain at failure (%) | Young’s modulus (MPa) | References |
|---|---|---|---|---|
| PLCL/collagen | 6a | 150a | 4.5a | [102] |
| PLA-braided | 77 ± 0.7 | 4 ± 0.5 | 1370 ± 87 | [185] |
| PLA-poloxamer | 24 ± 3 | 78 ± 23 | 346 ± 109 | [186] |
| PLA-poloxamine | 29 ± 1 | 20 ± 2 | 440 ± 99 | [186] |
| PCL/gelatin | 1.41 ± 0.15 | – | 2.51 ± 1.33 | [187] |
| 1.45 ± 0.19 | ||||
| PLA/collagen | 0.075 ± 0.009 | 348 ± 43 | 0.084 ± 0.003 | [188] |
| PLLA/CHT-collagen hydrogel | 7.89 ± 1.5 | – | – | [184] |
| PCL–HA/CHT | 250.1 | – | 215.5 | [183] |
| PU/collagen/silk | 13.5 | 55 | 21.7 | [189] |
PLA polylactic acid, PCL polycaprolactone, PLCL poly(l-lactide-co-ɛ-caprolactone), CHT chitosan, HA hydroxyapatite, PLLA poly-l-lactic acid, PU polyurethane
aRelatively from graph
While the idea of replacing ruptured tendon/ligament with a tissue-engineered substitute is the most feasible and logical currently, however to date, there is no tissue-engineered substitute that has been successfully implanted in humans. Nonetheless, several studies have shown the promising effect of these substitutes in animals. Most of the in vivo studies have focused on the reconstruction of the ACL as the primary target for tissue-engineered replacement. ACL plays an important role in knee stability and is a common sport-related injury.
Fan and colleagues have tested a composite substitute consists of MSCs and silk scaffold for ACL regeneration in rabbits. In their studies, MSCs were cultured in vitro with the silk scaffold and high cell proliferation and increased amount of collagen were seen. The substitute was implanted subsequently in rabbits and it was shown that the MSCs exhibited fibroblast morphology with components of ECM prominently produced after 24 weeks. The tissue-engineered substitute also performed well in term of mechanical strength and direct ligament-bone insertion was reconstructed successfully [74]. The same group repeated the study in a pig model and similar results were obtained after 24 weeks [75]. Importantly, the mechanical strength of the regenerated ligament was maintained after 24 weeks of implantation despite significant degradation of the implanted scaffold.
Another study by Petrigliano and colleagues has demonstrated the use of polycaprolactone (PCL) as a biomaterial for ligament reconstruction in rodents. PCL was electrospun to produce highly aligned nanofibers. In vivo gradual collagen depositions were seen with some infiltration of collagen-producing fibroblast and inflammatory cells. However, the strength of the implanted PCL scaffold was insufficient compared to the native ligament and less biocompatibility were observed through modest inflammatory cell infiltrations [76].
Later, Leong and team investigated the PCL scaffold incorporated with basic fibroblast growth factor (bFGF) and human foreskin fibroblast (HFF). The scaffold has resulted in successful bony integration with gradual increased in strength although still has not achieved up to the standard of the native ACL. However, they speculated that HFFs had an unfavorable effect on aligned collagen production and mechanical properties and bFGF could contribute in term of mechanical properties [77].
A recent study by Lee et al. showed that decellularized porcine tibialis tendons re-cellularized with human bone marrow-derived MSCs (BM-MSCs) subjected to mechanical loading similar to the ACL in the knee joint implanted to the pig restored 80% of the ACL mechanical strength. These results indicated the importance of bioreactor and mechanical stresses in vitro to stimulate engineered tendon/ligament tissue maturation and subsequently enhance the tendon/ligament regeneration in vivo [78].
Achilles tendon at the heel is another popular target of in tendon/ligament tissue engineering. Deng et al. found that poly(glycolic acid)/poly(lactic acid) (PGA/PLA) scaffold implanted for 45 weeks unable to improve the mechanical strength of rabbit Achilles tendon. However, the same scaffold seeded with adipose stem cells gradually forms neo-tendon and improves the mechanical strength of tendon [79]. In a separate study, Chen et al. tested a chitosan-based scaffold with a asymmetric structure on Achilles tendon defect in rats. They found that the scaffold promotes ECM production, tenogenic differentiation and tissue maturation. Furthermore, they also found that incorporation of rat tendon stem/progenitor cells to the scaffold enhance the tissue regeneration in vivo [80]. Results from Deng et al. and Chen et al. clearly showed that stem cell is a very important component in engineered tendon/ligament to ensure the success of the graft upon transplantation.
Human amniotic membrane as a scaffold for tendon/ligament tissue engineering
Recent progress in tissue engineering has explored the potential of human amniotic membrane (AM) application in its regenerative capability. For the past years, it was discovered that AM could be used in tissue engineering to construct a scaffold. AM is the innermost layer of the placenta located next to foetus consisting of a thick basement membrane and an avascular stromal matrix [81]. AM is a redundant tissue that can be collected from millions of birth every year. Thus, there is not ethical concerns with the collected and use of AM. AM have been shown to contain growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-α (TGF-α), TGF-β1, basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), placental growth factor (PLGF) and granulocyte-colony stimulating factor (G-CSF) [82]. AM possesses biological properties that have great significance in tissue engineering including anti-microbial, anti-inflammatory, anti-scarring, anti-fibrosis in addition to satisfactory mechanical property and low immunogenicity [83].
Decellularization technique is an important step for building the AM scaffold as this technique will reduce immunoreactivity from the host and create space for recellularization with target cells. E.g. epithelial cells are removed from the AM and recellularized with tenocytes to prepare a tissue-engineered tendon. When AM is decellularized, it is left with the matrix that rich in ECM proteins such as collagen from various type including type I and type IV, fibronectin, laminin, and ground substances such as proteoglycans, that provide important cues for cellular proliferation, growth, signaling and communication [84]. Decellularization AM has been reported to promote cell adhesion, proliferation, differentiation and stratification as well as more uniform cell growth compared to intact AM [85]. Apart from bearing unique biological and mechanical qualities which render it desirable as scaffolding materials, AM also provide most important factors that promote wound healing such as epidermal growth factor and keratocyte growth factor [86, 87]. Basically, a decellularized AM is a scaffold with a template of the ECM. Together with natural ECM properties and growth factors, AM could play an important role in the microenvironment of the tissue scaffold.
Currently, AM, whether with intact cells or without is widely used in ophthalmology field in reconstructing ocular surface from burn injuries or epithelial defects [81]. AM also has been demonstrated to be able to serve as a nerve conduit in peripheral nerve regeneration and as a carrier matrix for cartilage regeneration [88, 89].
Viscoelasticity property of AM can be attributed to its ECM protein content. The amount of collagen and elastin in AM determine its mechanical properties in term of strength and elasticity [90]. A tissue scaffold when first transplanted to a damaged site must bear loads close to the physiological levels. Together, with a combination of other biomaterials fabricated on to the AM, they would provide a better structure to withstand the tension and loads to the joint.
To date, limited literatures have reported the use of AM in tendon/ligament tissue engineering. Previous literatures have shown that amniotic membrane wrapped around the partially lacerated tendon/ligament reduces inflammation, increases collagen fibers alignment and improves the mechanical strength of tendon [91, 92]. Studies also found that AM can prevent the formation of peritendinous adhesion which is a major contributor to poor tendon surgery outcome [93, 94]. However, a clinically study terminated after 5 patients due to unsatisfactory results hinted that AM might not be able to improve the outcome of tendon repair [95].
Seo and colleagues in 2016 have demonstrated the application of silk scaffold, composite silk (CS) scaffold consists of silk, collagen-hyaluronan and chondroitin-6-sulfate, and CS wrapped with AM (CS-AM) scaffold in Achilles tendon regeneration. The silk scaffold was prepared from silk suture using a weaving machine and sericin was removed from the silk scaffold using a detergent. CS scaffold was prepared by soaking the silk scaffold in collagen-hyaluronic acid-chondroitin-6-sulfate solution and air-dried. The scaffold was implanted into Achilles tendon injury in rabbit. Under histological examination, collagen fibrils were found to be more organized, and collagen bundles were denser, parallel, and linear in CS-AM compared to silk and CS group alone. In addition, neoangiogenesis were noted through CD34 staining in CS-AM group with increased fibroblast-like cell migration [96]. Moreover, CS-AM group was better in term of mechanical strength. Results from this study suggested that AM can be used with extra scaffolding materials to maximize its therapeutic potential in augmenting the healing of tendon/ligament injury. Reinforcement of CS with AM enhanced the performance of the scaffold probably due to the improved mechanical strength and biological property as AM was known to be rich in growth factors.
A recent case study reported the used of dehydrated AM as a patch with hamstring autograft in a patient with primary ACL tear through arthroscopic-assisted ACL reconstruction. Early MRI scan postoperative 3 months indicated early vascularization and maturation of repaired tendon at 6 months. Patient also noted to gain a normal range of motion and gait faster than usual [97].
In a randomized controlled trial, micronize (powdered) AM was tested for its efficacy in patients with plantar fasciitis. According to the guideline, no single treatment is guaranteed to reduce the pain. 45 patients were randomized and blinded to receive either 0.5 or 1.25 cc micronize AM or 1.25 cc saline (control). The outcome measures used were The American Orthopedic Foot and Ankle Society (AOFAS) Hindfoot Scale and The Wong–Baker FACES Pain Rating Scale to assess the pain and Quality Metric’s SF-36v2 Standard Health Survey to assess functional health and well-being from the patient’s point of view during the study period. Results showed that single injection of micronize AM is sufficient to alleviate pain and improved functionality within 1 week. Both groups of patients receiving micronize AM improved the AOFAS Hindfoot scale and the effect seen may be due to the anti-inflammatory effect of AM and also growth factors that stimulate epithelial cell migration and proliferation thus together promote tendon healing [98].
Electrospinning to produce nanofibrous scaffold for tendon/ligament tissue engineering
The electrospinning technique has been widely studied for the manufacturing of biomimetic nanoscale fibrous structure for tissue engineering applications. Through electrospinning technique, finer fibers ranging from 15 nm to 10 μm or greater could be generated. The technique generally involved electrostatic force that eventually pressured fine fiber formation, collected as a non-woven mesh with high surface area/unit mass and high volume of interconnected porosity [99].
Briefly, in electrospinning technique, the desired solution of biomaterial in a syringe is subjected to a strong electric field through the syringe needle and ultra-fine fiber ranging from few micrometers to tens of nanometers in diameter is formed via stretching by the electric force when the polymer solution ejected from the nozzle travel across the distance to deposit on the collector. The fiber is collected in a rotating collector to produce highly aligned nanofibers. Theoretically, the applied electrical potential between the polymer source and collector would create an accumulation of charges at the surface of an emerging polymeric droplet at the end of the syringe needle. The cohesive force of the solution would be overcome by the force of the electric field and an electrically charged jet of polymer-containing solution erupts. As the jet moves toward the collector plate, it is elongated by electrostatic interactions between charges on nearby segments of the same jet (Fig. 6) [71, 99].
Fig. 6.
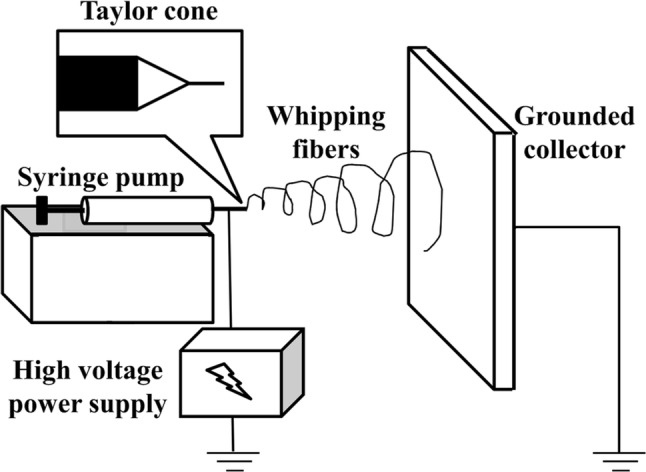
Schematic diagram of electrospinning equipment. (Reproduced from Ref. [71] with permission from Springer Nature)
Characteristics of electrospun fiber are affected by the parameters such as viscosity of the polymer solution, distance between the needle tip and the collector, the voltage applied, the flow rate, the spinning temperature, the rotation speed of collector, the spinning duration, the environment temperature and humidity [71, 100]. Viscosity of the polymer solution is the most important parameter in controlling the fiber diameter whereby increased viscosity leads to the formation of fibers with larger diameter. Distance between the needle and rotating collector could affect the electrospun fiber diameter as well. In theory, collecting distance is inversely proportional to fiber diameter due to the fact that more time is needed for the jet to stretch in the electric field before it is collected and the polymer solution has more time to evaporate. However, some studies claimed that increasing the distance would increase the fiber diameter. Some also reported that changing the distance would not affect the fiber diameter. Applied voltage also plays an important role in controlling the fiber diameter. By increasing the voltage, the electrical field strength and electrostatic stretching force become larger, thus decreasing the fiber diameter. However, some also believed that the mass flow rate from the needle tip to a collector would be increased when a higher voltage is applied thus creating thicker fiber diameter. In term of flow rate, increasing the volume per hour would eject the solution at larger amount in a given time that could result in thicker diameter. Achieving finest fiber diameter is crucial as it will also produce a higher surface-to-volume ratio of the scaffold that could promote and increase cell proliferation and adhesion [71, 100, 101]. An example of electrospun fibers is shown in Fig. 7.
Fig. 7.
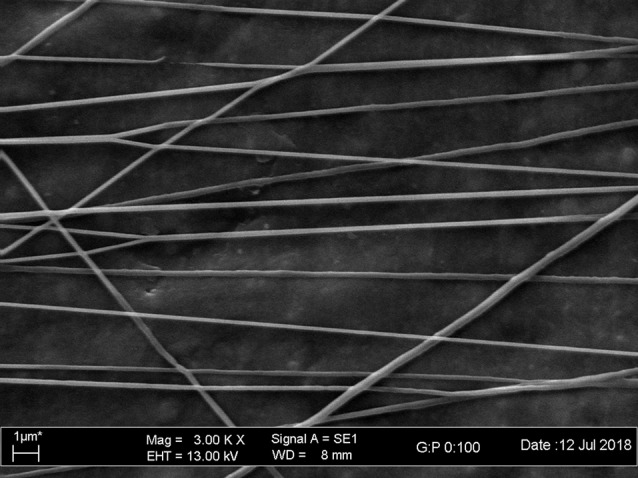
Electrospun polycaprolactone nanofibers
Several studies have successfully fabricated align nanofibers using electrospinning method. Xu et al. fabricated poly(L-lactide-co-e-caprolactone)/collagen nanoyarn network that significantly enhanced tenocyte proliferation and expression of tendon related genes [102]. At the same year, Barber et al. used the braided technique to form scaffold from electrospun poly-(l-lactic acid). Surprisingly, the scaffold could mimic the mechanical behavior of native tendon/ligament during loading [103]. Orr et al. [104] later developed multilayered electrospun polycaprolactone scaffold that was found to increase gene expression of tenogenic differentiation markers and collagen alignment. More recently, Maghdouri-White et al. [105] fabricated electrospun silk/collagen scaffold that could support adipose stem cell attachment and proliferation while maintaining its favorable biocompatibility and biodegradability properties to support tendon/ligament regeneration. Sensini et al. [106] prepared electrospun poly-(l-lactic acid)/collagen scaffold with mechanical properties comparable to native tendon and found that this scaffold promotes adhesion and proliferation of human tenocytes.
Stem cells for tendon/ligament tissue engineering
Cell is one of the components of tissue engineering triad. Both differentiated cells and undifferentiated stem cells can be used in tissue engineering. Most of the studies used stem cells that can self-renew and differentiate into multiple cell types, while showing low immunogenicity upon transplantation. Multiple studies have involved the usage of different types of stem cells including embryonic, fetal, and adult stem cells from various sources in tendon/ligament repair with promising results. Most of the studies used a single type of cells. However, co-culture might be the way forward as it has been found to increase the tenogenic markers, hence the formation of tendon-like tissue [107].
A few types of cells have showed great potential in tendon and ligament tissue engineering, i.e. induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), MSCs, tenocytes and ligamentocytes. Normally, there is no ethical issue with the use of autologous cells as the cells were harvested from the patient and reintroduced to the same patient after cell expansion in vitro. The use of allogeneic iPSCs, MSCs, tenocytes and ligamentocytes also has minimal ethical issues as the cells were normally collected from cadaver or donor with informed consent. The use of ESCs is controversial and argument for the use of these cells has been going on for a long time. This is because the collection of ESCs involved the destruction of embryo that can develop into a human. Regardless of the types of cells and applications (research and clinical), compliance with the regulation set by the regulatory authority is very important to avoid any ethical issues that may arise.
Pluripotent stem cells
Pluripotent stem cells, comprise of embryonic stem cells (ESCs) and induced-pluripotent stem cells (iPSCs) are both stem cells that have the unlimited proliferative capability and ability to differentiate into any cell type. Stepwise differentiation of ESCs towards MSCs has regenerated tendon tissue with better structural and mechanical properties than control in patellar tendon repair in rat [108]. Self-regeneration of tendon tissue was also activated from the paracrine secretion of differentiation factors and fetal tendon-specific matrix [68]. Xu et al. [109] experimented the iPSC-derived neural crest stem cells on rat patellar tendon window defect and it was found to enhance tendon healing. As ESCs and iPSCs are pluripotent and have great proliferative capability, there is an increased risk of teratoma formation post-administration. In addition, derivation of ESCs may raise ethical consideration as the cells are isolated from the embryo.
Multipotent stem cells
MSCs are multipotent stem cells with better self-renewal and multilineage differentiation ability compared to terminally differentiated cells such as tenocytes and ligamentocytes. MSCs can be derived from the bone marrow, umbilical cord, adipose tissue, amniotic tissue and even tendon tissue itself [110–113]. Compared to ESCs, MSCs do not increase the risk of developing teratoma and is easier to obtain without ethical concern. However, MSCs have to be used with cautious as the cells have been reported to influence tumor development and cancer drug resistance. Manipulation of the culture environment during in vitro cell expansion is also very important in ensuring the safety of MSC therapy as factors like medium supplement and oxygen partial pressure can modulate the cell differentiation potential. This is very important to avoid the formation of unwanted tissue at the implantation site, e.g. bone tissue in tendon and ligament.
Many studies have reported the potential of MSCs from bone marrow, umbilical cord, adipose tissue, synovial tissue in tendon/ligament healing especially in the small animal models [114–118]. MSC therapeutic area of the tendon has been focusing on specific tendon site including rotator cuff tendon and superficial digital flexor tendon (SDFT). Rotator cuff is a group of four muscles that come together as a tendon that surround the shoulder joint. Intervention through direct injection of umbilical cord-derived MSCs (UC-MSCs) has been shown to promote healing of rotator cuff tear in rabbit [114, 118]. Furthermore, a polyglycolic acid scaffold seeded with BM-MSCs were able to regenerate infraspinatus tendon-bone insertion as well as increase type I collagen content and mechanical strength of the regenerated tendon [115]. On the other hand, a single administration of adipose tissue-derived MSCs (AT-MSCs) also positively affects the collagen crosslinking and remodeling of scar tissue in SDFT lesion [116]. However, another study has shown that implanted synovial MSCs in tendon graft only affect early remodeling (first 1–2 weeks) as no histological changes were observed after 4 weeks [117]. Improved neovascularization during tendon healing could also be observed when horses were injected with AT-MSCs for SDFT repair [119]. Doppler ultrasonography recorded more intense signal after 2 weeks treatment with AT-MSCs and more vascularity seen on the tissue 22 weeks post-injection.
Even though the studies on MSCs in tendon/ligament regeneration are mostly done in animals, the potential therapeutic effect of MSC therapy on human also have been reported in several studies. Lee et al. [120] performed injection of AT-MSCs into the hypoechoic common extensor tendon lesion in lateral epicondylosis of 12 patients and found that the tendon defects were significantly reduced and elbow pain improved. On the other hand, Hernigou and colleagues recruited 90 patients with symptomatic rupture of the rotator cuff and divided them into the treatment group that received BM-MSCs as an adjunct following surgical repair and control group received the surgical repair only [121]. Faster healing rate was observed in the patients received MSCs by 6 months. Furthermore, those in the treatment group also showed improved tendon integrity in the long run. These findings indicated that MSC transplantation could be an effective adjunct therapy for surgical repair in tendon/ligament injuries.
The efficacy of MSCs in term of survival and ability to attach to the lesion site also has been demonstrated. MSCs can survive 6 weeks after the transplantation and increased the expression of type I collagen [122]. In addition, MSCs were found to remain at the tendon up to 9 weeks after the injection [123]. However, the administered MSCs cannot migrate towards another lesion site on a contralateral limb as the labelled MSCs were not detected in the untreated tendon [124]. This might be due to insufficient chemotaxis signal produced at the control lesion site to direct the migration of MSCs.
Several key factors have been identified to direct the tenogenic differentiation of MSCs upon implanted. Among others, early growth response 1 (EGR1) transcription factor has been shown to be involved in tendon differentiation [125]. Tendon-derived MSCs (T-MSCs) overexpressing EGR1 have been recently discovered to have higher tenogenic differentiation and better than T-MSCs in promoting the repair of rotator cuff injury [126]. The study further elaborated that EGR1 induced expression of tendon-related genes, including scleraxis (SCX), tenomodulin (TNMD), tenascin-C (TNC) and type I collagen. The author also found that EGR1 induced the tenogenic differentiation via the bone morphogenic protein 12 (BMP12) pathway. BMP12 has been reported to augment the gene expression of tenogenic markers in BM-MSCs and transplantation of these cells improved the formation of tendon-like tissue in tendon defect in rats [127]. More recently, Mohawk (Mkx) activation was discovered to lead tendon development during embryogenesis and may play a role in tenogenic differentiation of MSCs [128]. Furthermore, BM-MSCs overexpressed SCX are better in improving the mechanical properties of rotator cuff injury [129]. Taken together, augmentation of EGR1, BMP12, Mkx and SCX expression in MSCs prior administration for tendon repair can promote tenogenic differentiation, thus improving collagen and matrix synthesis.
Unipotent tenocytes and ligamentocytes
Previously, we have discussed that tenocytes and ligamentocytes are the major cell types in tendon and ligament, respectively, and also the one that drives tissue repair through ECM synthesis and growth factor production. Considering these facts, it might be feasible to use these cells to augment tendon/ligament regeneration. However, these highly differentiated cells have limited ability to proliferate and differentiate, thus limiting its use in tendon/ligament repair. Furthermore, autologous transplantation of these cells would also introduce donor site morbidity during harvest and the donor site intrinsic ability to heal upon biopsy is relatively poor.
Long-term efficacy of autologous tenocytes in human tendon repair have been reported by Wang et al. in their first paper published in the year 2013 and the follow-up paper published 2 years later [130, 131]. They treated 17 patients with chronic resistant lateral epicondylitis that was not improved after received the nonsurgical treatment with autologous tenocytes. They found that at 12 months follow-up, the patients received the autologous tenocyte injection shown significant improvement in visual analogue scale (VAS) score, Quick Disabilities of the Arm, Shoulder and Hand (QuickDASH) score, grip strength and magnetic resonance imaging (MRI) score. Encouragingly, these improvements sustained up to 5 years. Similar findings have been reported in small animal studies whereby autologous tenocyte therapy improved histological and biomechanical properties as well as the type I collagen synthesis and locomotion abilities without inciting adverse immune reactions [132–134]. Furthermore, implantation of collagen scaffold seeded with tenocytes also resulted in better rotator cuff tendon healing and remodeling [135].
Cell-secreted products for tendon/ligament tissue engineering
Aforementioned discussion has explored the potential of stem cells in tendon/ligament repair. However, a recent paradigm shift has arisen, implying that their beneficial effects may not be restricted to cellular regeneration alone, but also through their transient paracrine activity. Stem cells can secrete growth factors, cytokines and extracellular vesicles that can modulate the molecular composition of the environment to evoke responses from resident cells. In tissue engineering applications, secretory products of cells such as secretome and growth factors have been tested for tendon/ligament repair.
Exosomes
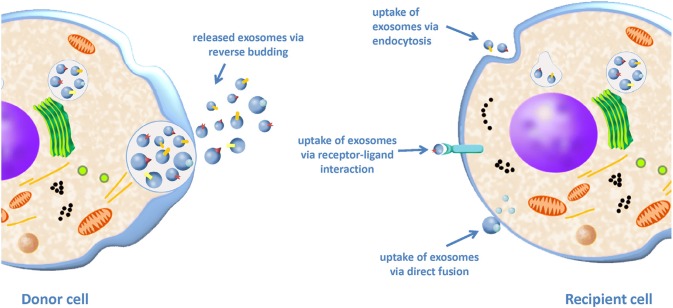
Exosome is a type of extracellular microvesicles with diameter ranging around 40-100 nm [136]. Exosome contains protein, lipid, growth factors, and genetic materials such as RNA and microRNA [137]. The lipid bilayer membrane of exosome contains certain protein markers that are specific to certain types of cells and also cholesterol and sphingolipid that are important in cell communication [138]. Exosomes can be up-taken by neighboring cells through surface receptor-mediated interaction, endocytosis or by a process of membrane fusion (Fig. 8) [139]. Due to the risk of capillary blockade after stem cell infusion, many have believed that stem cell-derived exosomes that are smaller in size could be beneficial in tissue repair and may replace stem cells as cell-free therapy. However, just like the stem cells, exosomes prepared from the cells varies from batch-to-batch and this situation as worrisome as it will greatly affect the biological function of the exosomes.
Fig. 8.
Communication between the donor cells and recipient cells via exosomes. Exosomes can be up-taken by the neighbouring cells via endocytosis, receptor-ligand interaction and membrane fusion. (Reproduced from Ref. [194] with permission from Elsevier)
In recent years, stem cell-derived exosomes have demonstrated its potential to treat several diseases, including cardiovascular ischemia, liver fibrotic disease, kidney injury, and cutaneous wounds healing and could have the potential for tendon/ligament repair and regeneration. Currently, the effect of exosomes per se on tendon/ligament regeneration is still scarce. In a horse study, exosomes secreted by horse amniotic-derived MSCs regulated the pro-inflammatory cytokines expression by down-regulating matrix metallopeptidase (MMP) 1, MMP 9, MMP 13 and tumor necrosis factor-α (TNFα) expression suggesting that exosomes might be beneficial in tendon healing [140].
Secretomes/growth factors
It is well known that proteins secreted by cells play a key role in the modulation of many biological functions such as cell signaling, differentiation and growth. Secretome, which is rich in a complex set of molecules secreted by living cells, could have potential in tendon/ligament healing. Recent advances in the field of proteomics have characterized hundreds of growth factors and cytokines secreted especially from stem cells to elucidate its regenerative capability. A well-known growth factor, platelet-derived growth factor (PDGF) has been extensively studied for its role in tendon/ligament repair.
Uggen et al. first investigated the role of PDGF as a coating substance for FiberWire sutures for rotator cuff repairs in sheep. Coated PDGF suture enhanced histologic scores of the repaired tendon. However, no significant differences were reported in term of its mechanical strength particularly load to failure [141]. Later, Hee and colleagues reported that PDGF/collagen scaffold treatment significantly increase the load to failure of rotator cuff repair in sheep compared to the suture only control. The PDGF treated group also had improved anatomical appearance but no differences were seen in term of inflammation or cellularity. Interestingly, increasing the PDGF concentration from 75 µg and 150 µg to 500 µg resulted in poorer outcomes [142]. This indicated that setting an ideal therapeutic dose is very important for optimum tendon recovery. In 2014, Kovacevic et al. incorporates PDGF into a collagen scaffold and found that cellularity and vascularity increase in the rotator cuff repair in rats, but only at the early phase of healing. No differences were found in collagen fiber organization and mechanical strength at the later stage of healing [143]. Tokunaga et al. studied the PDGF loaded gelatin hydrogel sheet and found that it was able to promote better collagen fiber orientation and ultimate failure load, but not the cellularity and vascularity [144]. The discrepancy seen in these studies were partly attributed to the differences in the animal model used, mechanism of delivery and preparation of PDGF itself.
To current knowledge, three studies have reported the use of secretome or conditioned medium for tendon/ligament healing. Basically, cells were cultured in a medium for a specified time and the medium was then collected and filtered. The filtered medium would be either undergone proteomic analysis or be incorporated into treatments to test its efficacy. MSC condition medium (MSC-CM) has effectively improved the healing process of massive rotator cuff tear by increasing fibrocartilage formation, organization and cellular density. In addition, the regenerated tendon with MSC-CM has higher elasticity, allowing better elongation when subjected to higher force of disruption. Moreover, in vitro study also showed that MSC-CM increase tendon cell viability [20]. Myoblast conditioned medium (Myo-CM) was also reported to upregulate tendon/ligament genes, TNMD, tenascin C and COMP, and enhanced collagen production in vitro. When tested on rats for ACL reconstruction, Myo-CM accelerated femoral tunnel closure but not tibial tunnel closure [145]. Physiologically, tenocytes synthesize the ECM including collagen and other proteins for tendon formation [146]. As expected, tenocyte conditioned medium (T-CM) induces proliferation and stimulates tenogenesis as well as reduces secretion of inflammatory proteins.
Platelet-rich plasma for tendon/ligament tissue engineering
PRP is a blood plasma with high concentration of platelet obtained by removing the red blood cells through centrifugation of whole blood (Fig. 9) [147, 148]. These platelets can release various growth factors including PDGF, insulin-like growth factor, fibroblast growth factor and vascular endothelial growth factor [149]. Extensive preclinical studies have reported the beneficial effects of PRP in tendon/ligament healing. Even though some athletes have been reported to gain beneficial effects from this therapy, there hasn’t been an established guideline for the use of PRP for tendon/ligament healing.
Fig. 9.
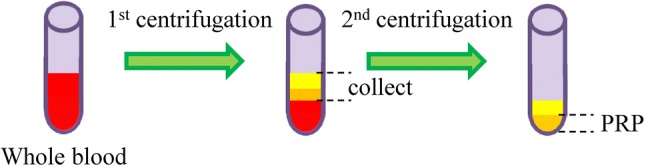
Preparation of platelet-rich plasma
In a rat model, administration of PRP has been shown to improve biomechanical properties of the repaired tear, increase angiogenesis and produce better orientation of collagen fibers. Unfortunately, the load to failure was not increased and PRP also increased fibroblastic response [150]. However, Dolkart et al. reported contradictory results whereby PRP treatment improves the load to failure. They showed that single PRP administration as an adjuvant to surgical repair produced higher maximal load and stiffness and hence suggested that PRP improved tendon-bone healing [151]. Improved tendon continuity and thickness as well as lower vascularity and inflammation were reported in a study by Hapa and colleagues. In term of mechanical strength, treatment of PRP only give positive impact at the earlier phase at week 2 [149]. This phenomenon was partly due to transient effect of platelet in tendon healing and the robust healing of tendon in rats that made the differences undetectable at 4 weeks after injury.
Administration of PRP also enhances tendon repair in large animals. The repaired tendon in PRP group has been reported to has higher cellularity, collagen and glycosaminoglycan content, along with higher strength at failure and elastic modulus compared to the control group in a placebo-controlled study in horses [152]. The subsequent study reported that PRP also significantly increased angiogenesis as proven through Doppler ultrasonography [153]. Taken together, a single administration of PRP is sufficient to produce significant positive effect in small and large animals.
The positive effects seen in small and large animals have yet to be fully translatable to human. Although some may recommend the administration of PRP in patients who cannot tolerate corticosteroid as both treatments are comparable in term of effectiveness [154], others may disagree as a randomized-controlled trial on the usage of PRP in tendon/ligament healing failed to demonstrate improvement in tendon healing, including tissue vascularity, muscle strength and clinical rating scales [155]. Furthermore, a systematic review on the usage of PRP in musculoskeletal soft tissue injury published in 2014 also concluded that there were insufficient evidences to support the use of PRP for treating musculoskeletal soft tissue injuries and stressed that there should be a proper standardization of PRP preparation methods [156]. The differences in the PRP prepared from different clinics resulted in the variation in the reported results.
Bioreactor and mechanical loading to enhance tendon/ligament substitute maturation in vitro
The structure of tendon and ligament is complex, consisting of millions of dense collagen fibrils in a highly organized manner. This complex structure is designed so that it can resist tension and compressive force. During the development, tenocytes and ligamentocytes synthesize ECM and collagen that made the tissue, and factors such as mechanical force could influence their behaviors. This was demonstrated by several studies identifying the effects of mechanical stimulation on tenocyte or ligamentocyte behavior.
Collagen secretion of ligamentocytes and MSCs increased when then cells were subjected to mechanical stretch. Nonetheless, the optimal amplitude and frequency of the stretching to stimulate collagen production and cell proliferation differed between the 2 types of cells [157]. These suggested that cells dynamically adjusted its behavior according to specific parameter of the mechanical stretch. Increased collagen production in response to mechanical stimulation also has been reported in other studies [158–160]. To relate to clinical scenario, early passive motion of the joint after treatment could help to promote remodeling of the repaired tendon/ligament tissue.
It is challenging for the researcher to study the behavior of tenocytes and ligamentocytes in a 2D model culture due to insufficient influence, especially mechanical cues. With the purpose of simulating the environment of the native tissue itself, a tightly controlled local environment with the ability to support and propagate cells while providing biological and mechanical signals in aseptic condition is needed. These functions could be provided by modern bioreactor that can be tailored to support a specific tissue such as tendon/ligament [161]. Tissue-engineered tendon/ligament can be cultured in 3D inside a bioreactor with controlled environment while also receiving mechanical stimulus and chemical signal to guide tissue maturation. Carefully selecting the biomaterials as the scaffolding agent is important, as some biomaterial may not be able to withstand the mechanical loading due to poor mechanical properties. From the literature search, we have found several reports that studied the modern bioreactor for tendon/ligament engineering.
M. Hohlrieder and colleagues have constructed bioreactor for the physical stimulation of ACL grafts. The bioreactor was capable to cyclically stretch and rotate the scaffold, with controlled perfusion flow and environmental and culture parameters (temperature, pH, oxygen, humidity). The mechanical loading to the scaffold was claimed to closely resemble the ACL mechanical conditions as the mechanical stimulation can be accurately and precisely controlled [162]. Thus, the well-controlled mechanical, biological and fluidic control system of the bioreactor can assure an optimal environment for the engineered tissue.
Laurent et al. developed a multi-chamber tension–torsion bioreactor to accommodate the physiological simulation in ligament tissue engineering. BM-MSCs were seeded in the scaffold and cultured dynamically inside the bioreactor. The BM-MSCs were able to adhere to the scaffold even after application of cyclic loading (2.5% strain and 10° rotation at 1 Hz) [163]. Youngstrom et al. studied the effect of cyclic loading (0, 3 and 5% strain at 0.33 Hz) on graft maturation and cellular phenotype. Decellularized equine tendon was clamped in the bioreactor vessel while BM-MSC suspension was deposited and subjected to cyclical mechanical stimulation. Cyclic strain promotes tenocyte specific gene, SCX at 0% and 3%, COL-I and COL-III at 3%. In term of mechanical properties, 3% strain constructs have better load to failure and elastic moduli compared to 0% and 5% and almost similar to the native tissue. The concentration of glycosaminoglycan in the constructs exceeded the one in native tissue suggesting that BM-MSCs were able to colonize and function properly inside the scaffold. In addition, histological analysis proved that BM-MSCs integrated into the scaffold at high density and adopt tenocyte morphology [164]. Similarly, Bourdón-Santoyo et al. found that bioreactor providing mechanical stimulation helps in the maturation of engineered ligament tissue [165].
Other studies also confirmed the increased expression of SCX, TNMD and tenascin C (tenogenic differentiation) after stimulation with cyclic stretching [107, 166, 167]. Furthermore, cyclic strain also promotes activation of ECM related genes, COL-1, COL-III, DCN, and COMP. COL-III is a crucial element for tendon healing whereby it catalyzes the formation of rapid crosslinks to stabilize the repair site while DCN is a regulator of collagen fibrils assembly during tendon development [107, 167–169].
Future perspectives
Introduction of new technology and discovery of new knowledge have enabled researchers to develop engineered tissue that is more closely resemble the native tissue. Recently introduced bioreactor with mechanical stimulation has enabled the physiological regulation of engineered tendon and ligament, with the aim of stimulating their maturation in vitro. Furthermore, the bioreactor also provides a platform to study the cellular behavior of transplanted engineered tendon and ligament. With these understandings, a more precise engineered tissue could be developed. Perhaps in the future, cells could be cultured inside bioreactor with complex stimuli to form tendon and ligament organoids. Furthermore, multiple types of cells can be co-culture on the scaffold to achieve functional tissue formation in vitro. Thus far, we are depending on the scaffold to support the cells and to form the engineered tissue. However, will we really need that in the future? If we have a complete understanding of various biological and mechanical factors that influence the cells to produce tendon and ligament organoids, it will solve some of the limitations that we are facing in tendon and ligament tissue engineering.
Conclusion
High failure and recurrence rates of the current tendon and ligament grafts have prompted researchers to explore new potential in tendon and ligament repair via tissue engineering. Although this field has provided limitless possibilities, many are not able to be translated into clinical applications. A better understanding of how native tissue developed and functioned could enlighten us on which are the best mixtures of the tissue engineering triad (cell, scaffold, biological factor) to prepare the engineered tendon and ligament that resemble the native tissue. Electrospinning method has demonstrated its ability to generate highly aligned nanofibers that is similar to collagen fibrils arrangement in native tendon and ligament. Stem cells and cell secreted products are promising, but its application in clinical practice is still vague. Proper standardization of clinical trials, preparation of stem cell source and secretory products are needed to prove its efficacy. Bioreactor, extended with mechanical loading features could dynamically affect the behavior of cells within scaffold and could mimic the physiological situation of tendon and ligament. This technology brings the potential for more precise understanding of tissue formation and maturation in vitro and the ability for mass production. Overall, more research is needed to satisfy our thirst in developing an ideal engineered tissue. The future is about to become interesting as the development of new technology is rapid and robust.
Acknowledgements
This work was supported by research grants from Universiti Kebangsaan Malaysia Medical Centre (FF-2017-368) and Universiti Kebangsaan Malaysia (GGPM-2017-050).
Authors’ contributions
All the authors participate in drafting the article and revising it critically for important intellectual content. All the authors give final approval of the version to be published.
Compliance with ethical standards
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99:203–222. doi: 10.1002/bdrc.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chainani A, Hippensteel KJ, Kishan A, Garrigues NW, Ruch DS, Guilak F, et al. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng Part A. 2013;19:2594–2604. doi: 10.1089/ten.tea.2013.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docheva D, Müller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev. 2015;84:222–239. doi: 10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhammi IK, Rehan-Ul-Haq, Kumar S. Graft choices for anterior cruciate ligament reconstruction. Indian J Orthop. 2015;49:127–128. doi: 10.4103/0019-5413.152393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Jiang J, Chen S. Status and headway of the clinical application of artificial ligaments. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2015;2:15–26. doi: 10.1016/j.asmart.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randelli P, Spennacchio P, Ragone V, Arrigoni P, Casella A, Cabitza P. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9–16. doi: 10.1007/s12306-011-0175-y. [DOI] [PubMed] [Google Scholar]
- 7.Schilaty ND, Nagelli C, Bates NA, Sanders TL, Krych AJ, Stuart MJ, et al. Incidence of second anterior cruciate ligament tears and identification of associated risk factors from 2001 to 2010 using a geographic database. Orthop J Sports Med. 2017;5:2325967117724196. doi: 10.1177/2325967117724196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanada M, Takahashi M, Matsuyama Y. Open re-rupture of the Achilles tendon after surgical treatment. Clin Pract. 2011;1:e134. doi: 10.4081/cp.2011.e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin NH, Volpi A, Lynch TS, Patel RM, Cerynik DL, Schickendantz MS, et al. Complications of distal biceps tendon repair: a meta-analysis of single-incision versus double-incision surgical technique. Orthop J Sports Med. 2016;4:2325967116668137. doi: 10.1177/2325967116668137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinchey JW, Aronowitz JG, Sanchez-Sotelo J, Morrey BF. Re-rupture rate of primarily repaired distal biceps tendon injuries. J Shoulder Elbow Surg. 2014;23:850–854. doi: 10.1016/j.jse.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Kemler E, Thijs KM, Badenbroek I, van de Port IG, Hoes AW, Backx FJ. Long-term prognosis of acute lateral ankle ligamentous sprains: high incidence of recurrences and residual symptoms. Fam Pract. 2016;33:596–600. doi: 10.1093/fampra/cmw076. [DOI] [PubMed] [Google Scholar]
- 12.Oryan A, Moshiri A, Meimandi-Parizi A. Graft selection in ACL reconstructive surgery: past, present, and future. Curr Orthop Pract. 2013;24:321–333. [Google Scholar]
- 13.Aldana AA, Abraham GA. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int J Pharm. 2017;523:441–453. doi: 10.1016/j.ijpharm.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 14.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang G, Rothrauff BB, Lin H, Yu S, Tuan RS. Tendon-derived extracellular matrix enhances transforming growth factor-β3-induced tenogenic differentiation of human adipose-derived stem cells. Tissue Eng Part A. 2017;23:166–176. doi: 10.1089/ten.tea.2015.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J, Razi ZRM, Law JX, Nawi AM, Idrus RBH, Chin TG, et al. Mesenchymal stromal cells from the maternal segment of human umbilical cord is ideal for bone regeneration in allogenic setting. Tissue Eng Regen Med. 2018;15:75–87. doi: 10.1007/s13770-017-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai L, Hu X, Zhang X, Zhu J, Zhang J, Fu X, et al. Different tenogenic differentiation capacities of different mesenchymal stem cells in the presence of BMP-12. J Transl Med. 2015;13:200. doi: 10.1186/s12967-015-0560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatia R, Hare JM. Mesenchymal stem cells: future source for reparative medicine. Congest Hear Fail. 2005;11:87–91. doi: 10.1111/j.1527-5299.2005.03618.x. [DOI] [PubMed] [Google Scholar]
- 19.Yuan T, Zhang CQ, Wang JH. Augmenting tendon and ligament repair with platelet-rich plasma (PRP) Muscles Ligaments Tendons J. 2013;3:139–149. [PMC free article] [PubMed] [Google Scholar]
- 20.Sevivas N, Teixeira FG, Portugal R, Direito-Santos B, Espregueira-Mendes J, Oliveira FJ, et al. Mesenchymal stem cell secretome improves tendon cell viability in vitro and tendon-bone healing in vivo when a tissue engineering strategy is used in a rat model of chronic massive rotator cuff tear. Am J Sports Med. 2018;46:449–459. doi: 10.1177/0363546517735850. [DOI] [PubMed] [Google Scholar]
- 21.Tetta C, Consiglio AL, Bruno S, Tetta E, Gatti E, Dobreva M, et al. The role of microvesicles derived from mesenchymal stem cells in tissue regeneration; a dream for tendon repair? Muscles Ligaments Tendons J. 2012;2:212–221. [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkendall DT, Garrett WE. Function and biomechanics of tendons. Scand J Med Sci Sports. 1997;7:62–66. doi: 10.1111/j.1600-0838.1997.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 23.Norris CM. Sports and soft tissue injuries: A guide for students and therapists. 5. London: Taylor & Francis; 2018. [Google Scholar]
- 24.O’Brien M. Anatomy of tendons. In: Maffulli N, Renstrom P, Leadbetter WB, editors. Tendon injuries. London: Springer; 2005. pp. 3–13. [Google Scholar]
- 25.Frank CB. Ligament structure, physiology and function. J Musculoskelet Neuronal Interact. 2004;4:199–201. [PubMed] [Google Scholar]
- 26.Franchi M, Trirè A, Quaranta M, Orsini E, Ottani V. Collagen structure of tendon relates to function. ScientificWorldJournal. 2007;7:404–420. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 28.Józsa L, Lehto M, Kvist M, Bálint JB, Reffy A. Alterations in dry mass content of collagen fibers in degenerative tendinopathy and tendon-rupture. Matrix. 1989;9:140–146. doi: 10.1016/s0934-8832(89)80032-0. [DOI] [PubMed] [Google Scholar]
- 29.Józsa L, Kannus P. Human tendons: anatomy, physiology and pathology. 1. Champaign: Human Kinetics Publisher; 1997. [Google Scholar]
- 30.Robi K, Jakob N, Matevz K, Matjaz V. The physiology of sports injuries and repair processes. In: Hamlin M, Draper N, Kathiravel Y, editors. Current issues in sports and exercise medicine. London: InTech; 2013. [Google Scholar]
- 31.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- 32.Depalle B, Qin Z, Shefelbine SJ, Buehler MJ. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater. 2015;52:1–13. doi: 10.1016/j.jmbbm.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki N, Shukunami N, Matsushima N, Izumi Y. Time-resolved X-ray diffraction from tendon collagen during creep using synchrotron radiation. J Biomech. 1999;32:285–292. doi: 10.1016/s0021-9290(98)00174-2. [DOI] [PubMed] [Google Scholar]
- 34.Xu B, Chow MJ, Zhang Y. Experimental and modeling study of collagen scaffolds with the effects of crosslinking and fiber alignment. Int J Biomater. 2011;2011:172389. doi: 10.1155/2011/172389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis-Ugbo J, Leeson B, Hutton WC. Tensile properties of fresh human calcaneal (Achilles) tendons. Clin Anat. 2004;17:30–35. doi: 10.1002/ca.10126. [DOI] [PubMed] [Google Scholar]
- 36.Freedman BR, Gordon JA, Soslowsky LJ. The Achilles tendon: fundamental properties and mechanisms governing healing. Muscles Ligaments Tendons J. 2014;4:245–255. [PMC free article] [PubMed] [Google Scholar]
- 37.Kannus P, Natri A. Etiology and pathophysiology of tendon ruptures in sports. Scand J Med Sci Sports. 1997;7:107–112. doi: 10.1111/j.1600-0838.1997.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 38.Vilarta R, Vidal Bde C. Anisotropic and biomechanical properties of tendons modified by exercise and denervation: aggregation and macromolecular order in collagen bundles. Matrix. 1989;9:55–61. doi: 10.1016/s0934-8832(89)80019-8. [DOI] [PubMed] [Google Scholar]
- 39.Kannus P, Józsa L, Natri A, Järvinen M. Effects of training, immobilization and remobilization on tendons. Scand J Med Sci Sports. 1997;7:67–71. doi: 10.1111/j.1600-0838.1997.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 40.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9:3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521 Pt 1:307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briggs AM, Woolf AD, Dreinhöfer K, Homb N, Hoy DG, Kopansky-Giles D, et al. Reducing the global burden of musculoskeletal conditions. Bull World Health Organ. 2018;96:366–368. doi: 10.2471/BLT.17.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu F, Nerlich M, Docheva D. Tendon injuries: basic science and new repair proposals. EFORT Open Rev. 2017;2:332–342. doi: 10.1302/2058-5241.2.160075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nigg BM. The role of impact forces and foot pronation: a new paradigm. Clin J Sport Med. 2001;11:2–9. doi: 10.1097/00042752-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Selvanetti A, Cipolla M, Puddu G. Overuse tendon injuries: basic science and classification. Oper Tech Sports Med. 1997;5:110–117. [Google Scholar]
- 48.Pedowitz D, Kirwan G. Achilles tendon ruptures. Curr Rev Musculoskelet Med. 2013;6:285–293. doi: 10.1007/s12178-013-9185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42:311–319. [PMC free article] [PubMed] [Google Scholar]
- 50.Bruns J, Kampen J, Kahrs J, Plitz W. Achilles tendon rupture: experimental results on spontaneous repair in a sheep-model. Knee Surg Sports Traumatol Arthrosc. 2000;8:364–369. doi: 10.1007/s001670000149. [DOI] [PubMed] [Google Scholar]
- 51.Geremia JM, Bobbert MF, Casa Nova M, Ott RD, Lemos Fde A, Lupion Rde O, et al. The structural and mechanical properties of the Achilles tendon 2 years after surgical repair. Clin Biomech (Bristol, Avon) 2015;30:485–492. doi: 10.1016/j.clinbiomech.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Sen S, Badge R, Murali R. Ligament injuries of the hand. Orthop Trauma. 2019;33:38–44. [Google Scholar]
- 53.Ackermann PW. Tendinopathy I: understanding epidemiology, pathology, healing, and treatment. In: Gomes ME, Reis RL, Rodrigues MT, editors. Tendon regeneration. London: Elsevier; 2015. pp. 113–147. [Google Scholar]
- 54.Rodrigues MT, Reis RL, Gomes ME. Engineering tendon and ligament tissues: present developments towards successful clinical products. J Tissue Eng Regen Med. 2013;7:673–686. doi: 10.1002/term.1459. [DOI] [PubMed] [Google Scholar]
- 55.Siegel L, Vandenakker-Albanese C, Siegel D. Anterior cruciate ligament injuries: anatomy, physiology, biomechanics, and management. Clin J Sport Med. 2012;22:349–355. doi: 10.1097/JSM.0b013e3182580cd0. [DOI] [PubMed] [Google Scholar]
- 56.Chan KM, Fu SC. Anti-inflammatory management for tendon injuries—friends or foes? Sports Med Arthrosc Rehabil Ther Technol. 2009;1:23. doi: 10.1186/1758-2555-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ziltener JL, Leal S, Fournier PE. Non-steroidal anti-inflammatory drugs for athletes: an update. Ann Phys Rehabil Med. 2010;53:278–288. doi: 10.1016/j.rehab.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Connizzo BK, Yannascoli SM, Tucker JJ, Caro AC, Riggin CN, Mauck RL, et al. The detrimental effects of systemic Ibuprofen delivery on tendon healing are time-dependent. Clin Orthop Relat Res. 2014;472:2433–2439. doi: 10.1007/s11999-013-3258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monk AP, Davies LJ, Hopewell S, Harris K, Beard DJ, Price AJ. Surgical versus conservative interventions for treating anterior cruciate ligament injuries. Cochrane Database Syst Rev. 2016;4:CD011166. doi: 10.1002/14651858.CD011166.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Mohrej OA, Al-Kenani NS. Acute ankle sprain: conservative or surgical approach? EFORT Open Rev. 2016;1:34–44. doi: 10.1302/2058-5241.1.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Dijk PAD, Kerkhoffs GMMJ, van Dijk CN. Peroneal tendon injuries. In: Canata GL, D’Hooghe P, Hunt KJ, Kerkhoffs GMMJ, Longo UG, editors. Sports injuries of the foot and ankle: a focus on advanced surgical techniques. Berlin: Springer; 2019. pp. 317–326. [Google Scholar]
- 62.LaBella CR, Hennrikus W, Hewett TE, Brenner JS, Brookes MA, Demorest RA, et al. Anterior cruciate ligament injuries: diagnosis, treatment, and prevention. Pediatrics. 2014;133:e1437–e1450. doi: 10.1542/peds.2014-0623. [DOI] [PubMed] [Google Scholar]
- 63.Sakabe T, Sakai T. Musculoskeletal diseases—tendon. Br Med Bull. 2011;99:211–225. doi: 10.1093/bmb/ldr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mascarenhas R, Tranovich MJ, Kropf EJ, Fu FH, Harner CD. Bone-patellar tendon-bone autograft versus hamstring autograft anterior cruciate ligament reconstruction in the young athlete: a retrospective matched analysis with 2–10 year follow-up. Knee Surgery Sport Traumatol Arthrosc. 2012;20:1520–1527. doi: 10.1007/s00167-011-1735-2. [DOI] [PubMed] [Google Scholar]
- 65.Batty LM, Norsworthy CJ, Lash NJ, Wasiak J, Richmond AK, Feller JA. Synthetic devices for reconstructive surgery of the cruciate ligaments: a systematic review. Arthroscopy. 2015;31:957–968. doi: 10.1016/j.arthro.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 66.Parchi PD, Ciapini G, Paglialunga C, Giuntoli M, Picece C, Chiellini F, et al. Anterior cruciate ligament reconstruction with LARS artificial ligament-clinical results after a long-term follow-up. Joints. 2018;6:75–79. doi: 10.1055/s-0038-1653950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mascarenhas R, MacDonald PB. Anterior cruciate ligament reconstruction: a look at prosthetics–past, present and possible future. Mcgill J Med. 2008;11:29–37. [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J, Xu J, Wang A, Zheng M. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert Rev Med Devices. 2009;6:61–73. doi: 10.1586/17434440.6.1.61. [DOI] [PubMed] [Google Scholar]
- 69.Meyer U. The history of tissue engineering and regenerative medicine in perspective. In: Meyer U, Handschel J, Wiesmann HP, Meyer T, editors. Fundamentals of tissue engineering and regenerative medicine. Berlin: Springer; 2009. pp. 5–12. [Google Scholar]
- 70.Law JX, Liau LL, Aminuddin BS, Ruszymah BH. Tissue-engineered trachea: A review. Int J Pediatr Otorhinolaryngol. 2016;91:55–63. doi: 10.1016/j.ijporl.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 71.Law JX, Liau LL, Saim A, Yang Y, Idrus R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng Regen Med. 2017;14:699–718. doi: 10.1007/s13770-017-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229–258. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santos ML, Rodrigues MT, Domingues RMA, Reis RL, Gomes ME. Biomaterials as tendon and ligament substitutes: current developments. In: Oliveira JM, Reis RL, editors. Regenerative strategies for the treatment of knee joint disabilities. Cham: Springer; 2016. pp. 349–371. [Google Scholar]
- 74.Fan H, Liu H, Wong EJ, Toh SL, Goh JC. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials. 2008;29:3324–3337. doi: 10.1016/j.biomaterials.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 75.Fan H, Liu H, Toh SL, Goh JC. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30:4967–4977. doi: 10.1016/j.biomaterials.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 76.Petrigliano FA, Arom GA, Nazemi AN, Yeranosian MG, Wu BM, McAllister DR. In vivo evaluation of electrospun polycaprolactone graft for anterior cruciate ligament engineering. Tissue Eng Part A. 2015;21:1228–1236. doi: 10.1089/ten.tea.2013.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leong NL, Kabir N, Arshi A, Nazemi A, Wu B, Petrigliano FA, et al. Evaluation of polycaprolactone scaffold with basic fibroblast growth factor and fibroblasts in an athymic rat model for anterior cruciate ligament reconstruction. Tissue Eng Part A. 2015;21:1859–1868. doi: 10.1089/ten.tea.2014.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee KI, Lee JS, Kang KT, Shim YB, Kim YS, Jang JW, et al. In vitro and in vivo performance of tissue-engineered tendons for anterior cruciate ligament reconstruction. Am J Sports Med. 2018;46:1641–1649. doi: 10.1177/0363546518759729. [DOI] [PubMed] [Google Scholar]
- 79.Deng D, Wang W, Wang B, Zhang P, Zhou G, Zhang WJ, et al. Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials. 2014;35:8801–8809. doi: 10.1016/j.biomaterials.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 80.Chen E, Yang L, Ye C, Zhang W, Ran J, Xue D, et al. An asymmetric chitosan scaffold for tendon tissue engineering: in vitro and in vivo evaluation with rat tendon stem/progenitor cells. Acta Biomater. 2018;73:377–387. doi: 10.1016/j.actbio.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 81.Meller D, Pauklin M, Thomasen H, Westekemper H, Steuhl KP. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011;108:243–248. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10:493–500. doi: 10.1111/iwj.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 84.Francisco JC, Cunha RC, Simeoni RB, Guarita-Souza LC, Ferreira RJ, Irioda AC, et al. Amniotic membrane as a potent source of stem cells and a matrix for engineering heart tissue. J Biomed Sci Eng. 2013;6:1178–1185. [Google Scholar]
- 85.Gobinathan S, Zainol SS, Azizi SF, Iman NM, Muniandy R, Hasmad HN, et al. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J Biomater Sci Polym Ed. 2018;29:2051–2067. doi: 10.1080/09205063.2018.1485814. [DOI] [PubMed] [Google Scholar]
- 86.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- 87.Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, et al. How does amniotic membrane work? Ocul Surf. 2004;2:177–187. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 88.Mligiliche N, Endo K, Okamoto K, Fujimoto E, Ide C. Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J Biomed Mater Res. 2002;63:591–600. doi: 10.1002/jbm.10349. [DOI] [PubMed] [Google Scholar]
- 89.Boo L, Sofiah S, Selvaratnam L, Tai CC, Pingguan-Murphy B, Kamarul T. A preliminary study of human amniotic membrane as a potential chondrocyte carrier. Malays Orthop J. 2009;3:16–23. [Google Scholar]
- 90.Benson-Martin J, Zammaretti P, Bilic G, Schweizer T, Portmann-Lanz B, Burkhardt T, et al. The Young’s modulus of fetal preterm and term amniotic membranes. Eur J Obstet Gynecol Reprod Biol. 2006;128:103–107. doi: 10.1016/j.ejogrb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Yang JJ, Jang EC, Song KS, Lee JS, Kim MK, Chang SH. The effect of amniotic membrane transplantation on tendon-healing in a rabbit achilles tendon model. Tissue Eng Regen Med. 2010;7:323–329. [Google Scholar]
- 92.Nicodemo MC, Neves LR, Aguiar JC, Brito FS, Ferreira I, Sant’Anna LB, et al. Amniotic membrane as an option for treatment of acute Achilles tendon injury in rats. Acta Cir Bras. 2017;32:125–139. doi: 10.1590/s0102-865020170205. [DOI] [PubMed] [Google Scholar]
- 93.Ozgenel GY. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86:301–307. doi: 10.1302/0301-620x.86b2.14435. [DOI] [PubMed] [Google Scholar]
- 94.Dogramaci Y, Duman IG. Reinforcement of the flexor tendon repair using human amniotic membrane: a biomechanical evaluation using the modified kessler method of tendon repair. J Am Podiatr Med Assoc. 2016;106:319–322. doi: 10.7547/15-036. [DOI] [PubMed] [Google Scholar]
- 95.Leppänen OV, Karjalainen T, Göransson H, Hakamäki A, Havulinna J, Parkkinen J, et al. Outcomes after flexor tendon repair combined with the application of human amniotic membrane allograft. J Hand Surg Am. 2017;42:474.e1–474.e8. doi: 10.1016/j.jhsa.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Seo YK, Kim JH, Eo SR. Co-effect of silk and amniotic membrane for tendon repair. J Biomater Sci Polym Ed. 2016;27:1232–1247. doi: 10.1080/09205063.2016.1188349. [DOI] [PubMed] [Google Scholar]
- 97.Levengood GA. Arthroscopic-assisted anterior cruciate ligament reconstruction using hamstring autograft augmented with a dehydrated human amnion/chorion membrane allograft: a retrospective case report. Orthop Muscular Syst. 2016;5:213. [Google Scholar]
- 98.Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis—a feasibility study. Foot Ankle Int. 2013;34:1332–1339. doi: 10.1177/1071100713502179. [DOI] [PubMed] [Google Scholar]
- 99.Lannutti J, Reneker D, Ma T, Tomasko D, Farson D. Electrospinning for tissue engineering scaffolds. Mater Sci Eng C Mater Biol Appl. 2007;27:504–509. [Google Scholar]
- 100.Ngadiman NHA, Noordin MY, Idris A, Kurniawan D. A review of evolution of electrospun tissue engineering scaffold: From two dimensions to three dimensions. Proc Inst Mech Eng H. 2017;231:597–616. doi: 10.1177/0954411917699021. [DOI] [PubMed] [Google Scholar]
- 101.Pillay V, Dott C, Choonara YE, Tyagi C, Tomar L, Kumar P, et al. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J Nanomater. 2013;2013:789289. [Google Scholar]
- 102.Xu Y, Wu J, Wang H, Li H, Di N, Song L, et al. Fabrication of electrospun poly(L-lactide-co-ɛ-caprolactone)/collagen nanoyarn network as a novel, three-dimensional, macroporous, aligned scaffold for tendon tissue engineering. Tissue Eng Part C Methods. 2013;19:925–936. doi: 10.1089/ten.tec.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barber JG, Handorf AM, Allee TJ, Li WJ. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng Part A. 2013;19:1265–1274. doi: 10.1089/ten.tea.2010.0538. [DOI] [PubMed] [Google Scholar]
- 104.Orr SB, Chainani A, Hippensteel KJ, Kishan A, Gilchrist C, Garrigues NW, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117–126. doi: 10.1016/j.actbio.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maghdouri-White Y, Petrova S, Sori N, Polk S, Wriggers H, Ogle R, et al. Electrospun silk–collagen scaffolds and BMP-13 for ligament and tendon repair and regeneration. Biomed Phys Eng Express. 2018;4:025013. [Google Scholar]
- 106.Sensini A, Gualandi C, Cristofolini L, Tozzi G, Dicarlo M, Teti G, et al. Biofabrication of bundles of poly (lactic acid)-collagen blends mimicking the fascicles of the human Achille tendon. Biofabrication. 2017;9:015025. doi: 10.1088/1758-5090/aa6204. [DOI] [PubMed] [Google Scholar]
- 107.Wu S, Wang Y, Streubel PN, Duan B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater. 2017;62:102–115. doi: 10.1016/j.actbio.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen X, Song XH, Yin Z, Zou XH, Wang LL, Hu H, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27:1276–1287. doi: 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 109.Xu W, Wang Y, Liu E, Sun Y, Luo Z, Xu Z, et al. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng Part A. 2013;19:2439–2451. doi: 10.1089/ten.tea.2012.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liau LL, Makpol S, Azurah AGN, Chua KH. Human adipose-derived mesenchymal stem cells promote recovery of injured HepG2 cell line and show sign of early hepatogenic differentiation. Cytotechnology. 2018;70:1221–1233. doi: 10.1007/s10616-018-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tan Q, Lui PP, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18:840–851. doi: 10.1089/ten.tea.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lim J, Razi ZR, Law J, Nawi AM, Idrus RB, Ng MH. MSCs can be differentially isolated from maternal, middle and fetal segments of the human umbilical cord. Cytotherapy. 2016;18:1493–1502. doi: 10.1016/j.jcyt.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 113.Hafez P, Chowdhury SR, Jose S, Law JX, Ruszymah BHI, Mohd Ramzisham AR, et al. Development of an in vitro cardiac ischemic model using primary human cardiomyocytes. Cardiovasc Eng Technol. 2018;9:529–538. doi: 10.1007/s13239-018-0368-8. [DOI] [PubMed] [Google Scholar]
- 114.Kwon DR, Park GY, Lee SC. Treatment of full-thickness rotator cuff tendon tear using umbilical cord blood-derived mesenchymal stem cells and polydeoxyribonucleotides in a rabbit model. Stem Cells Int. 2018;2018:7146384. doi: 10.1155/2018/7146384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow–derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40:1259–1268. doi: 10.1177/0363546512442343. [DOI] [PubMed] [Google Scholar]
- 116.Geburek F, Roggel F, van Schie HTM, Beineke A, Estrada R, Weber K, et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial. Stem Cell Res Ther. 2017;8:129. doi: 10.1186/s13287-017-0564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ju YJ, Muneta T, Yoshimura H, Koga H, Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332:469–478. doi: 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 118.Park GY, Kwon DR, Lee SC. Regeneration of full-thickness rotator cuff tendon tear after ultrasound-guided injection with umbilical cord blood-derived mesenchymal stem cells in a rabbit model. Stem Cells Transl Med. 2015;4:1344–1351. doi: 10.5966/sctm.2015-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conze P, van Schie HT, van Weeren R, Staszyk C, Conrad S, Skutella T, et al. Effect of autologous adipose tissue-derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen Med. 2014;9:743–757. doi: 10.2217/rme.14.55. [DOI] [PubMed] [Google Scholar]
- 120.Lee SY, Kim W, Lim C, Chung SG. Treatment of lateral epicondylosis by using allogeneic adipose-derived mesenchymal stem cells: a pilot study. Stem Cells. 2015;33:2995–3005. doi: 10.1002/stem.2110. [DOI] [PubMed] [Google Scholar]
- 121.Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 122.Kim YS, Lee HJ, Ok JH, Park JS, Kim DW. Survivorship of implanted bone marrow-derived mesenchymal stem cells in acute rotator cuff tear. J Shoulder Elbow Surg. 2013;22:1037–1045. doi: 10.1016/j.jse.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 123.Geburek F, Mundle K, Conrad S, Hellige M, Walliser U, van Schie HT, et al. Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions-a pilot study. Stem Cell Res Ther. 2016;7:21. doi: 10.1186/s13287-016-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carvalho AM, Yamada AL, Golim MA, Álvarez LE, Hussni CA, Alves AL. Evaluation of mesenchymal stem cell migration after equine tendonitis therapy. Equine Vet J. 2014;46:635–638. doi: 10.1111/evj.12173. [DOI] [PubMed] [Google Scholar]
- 125.Lejard V, Blais F, Guerquin MJ, Bonnet A, Bonnin MA, Havis E, et al. EGR1 and EGR2 involvement in vertebrate tendon differentiation. J Biol Chem. 2011;286:5855–5867. doi: 10.1074/jbc.M110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tao X, Liu J, Chen L, Zhou Y, Tang K. EGR1 induces tenogenic differentiation of tendon stem cells and promotes rabbit rotator cuff repair. Cell Physiol Biochem. 2015;35:699–709. doi: 10.1159/000369730. [DOI] [PubMed] [Google Scholar]
- 127.Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li Y, Akinbiyi T, et al. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One. 2011;6:e17531. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu H, Zhang C, Zhu S, Lu P, Zhu T, Gong X, et al. Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells. 2015;33:443–455. doi: 10.1002/stem.1866. [DOI] [PubMed] [Google Scholar]
- 129.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow—derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39:1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 130.Wang A, Breidahl W, Mackie KE, Lin Z, Qin A, Chen J, et al. Autologous tenocyte injection for the treatment of severe, chronic resistant lateral epicondylitis: a pilot study. Am J Sports Med. 2013;41:2925–2932. doi: 10.1177/0363546513504285. [DOI] [PubMed] [Google Scholar]
- 131.Wang A, Mackie K, Breidahl W, Wang T, Zheng MH. Evidence for the durability of autologous tenocyte injection for treatment of chronic resistant lateral epicondylitis: mean 4.5-year clinical follow-up. Am J Sports Med. 2015;43:1775–1783. doi: 10.1177/0363546515579185. [DOI] [PubMed] [Google Scholar]
- 132.Güngörmüş C, Kolankaya D, Aydin E. Histopathological and biomechanical evaluation of tenocyte seeded allografts on rat Achilles tendon regeneration. Biomaterials. 2015;51:108–118. doi: 10.1016/j.biomaterials.2015.01.077. [DOI] [PubMed] [Google Scholar]
- 133.Liang JI, Lin PC, Chen MY, Hsieh TH, Chen JJ, Yeh ML. The effect of tenocyte/hyaluronic acid therapy on the early recovery of healing Achilles tendon in rats. J Mater Sci Mater Med. 2014;25:217–227. doi: 10.1007/s10856-013-5036-9. [DOI] [PubMed] [Google Scholar]
- 134.Chen J, Yu Q, Wu B, Lin Z, Pavlos NJ, Xu J, et al. Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue Eng Part A. 2011;17:2037–2048. doi: 10.1089/ten.TEA.2010.0492. [DOI] [PubMed] [Google Scholar]
- 135.Chen JM, Willers C, Xu J, Wang A, Zheng MH. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007;13:1479–1491. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 136.Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khalyfa A, Gozal D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J Transl Med. 2014;12:162. doi: 10.1186/1479-5876-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 139.McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomark. 2015;4:7. doi: 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lange-Consiglio A, Perrini C, Tasquier R, Deregibus MC, Camussi G, Pascucci L, et al. Equine amniotic microvesicles and their anti-inflammatory potential in a tenocyte model in vitro. Stem Cells Dev. 2016;25:610–621. doi: 10.1089/scd.2015.0348. [DOI] [PubMed] [Google Scholar]
- 141.Uggen C, Dines J, McGarry M, Grande D, Lee T, Limpisvasti O. The effect of recombinant human platelet-derived growth factor BB–coated sutures on rotator cuff healing in a sheep model. Arthroscopy. 2010;26:1456–1462. doi: 10.1016/j.arthro.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 142.Hee CK, Dines JS, Dines DM, Roden CM, Wisner-Lynch LA, Turner AS, et al. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am J Sports Med. 2011;39:1630–1639. doi: 10.1177/0363546511404942. [DOI] [PubMed] [Google Scholar]
- 143.Kovacevic D, Gulotta LV, Ying L, Ehteshami JR, Deng XH, Rodeo SA. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin Orthop Relat Res. 2015;473:1644–1654. doi: 10.1007/s11999-014-4020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tokunaga T, Ide J, Arimura H, Nakamura T, Uehara Y, Sakamoto H, et al. Local application of gelatin hydrogel sheets impregnated with platelet-derived growth factor BB promotes tendon-to-bone healing after rotator cuff repair in rats. Arthroscopy. 2015;31:1482–1491. doi: 10.1016/j.arthro.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 145.Ghebes CA, Groen N, Cheuk YC, Fu SC, Fernandes HM, Saris DBF. Muscle-secreted factors improve anterior cruciate ligament graft healing: an in vitro and in vivo analysis. Tissue Eng Part A. 2018;24:322–334. doi: 10.1089/ten.TEA.2016.0546. [DOI] [PubMed] [Google Scholar]
- 146.Rubio-Azpeitia E, Sánchez P, Delgado D, Andia I. Adult cells combined with platelet-rich plasma for tendon healing: cell source options. Orthop J Sports Med. 2017;5:2325967117690846. doi: 10.1177/2325967117690846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Law JX, Chowdhury SR, Saim AB, Idrus RBH. Platelet-rich plasma with keratinocytes and fibroblasts enhance healing of full-thickness wounds. J Tissue Viability. 2017;26:208–215. doi: 10.1016/j.jtv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 148.Xian LJ, Chowdhury SR, Bin Saim A, Idrus RB. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy. 2015;17:293–300. doi: 10.1016/j.jcyt.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 149.Hapa O, Cakıcı H, Kükner A, Aygün H, Sarkalan N, Baysal G. Effect of platelet-rich plasma on tendon-to-bone healing after rotator cuff repair in rats: an in vivo experimental study. Acta Orthop Traumatol Turc. 2012;46:301–307. doi: 10.3944/aott.2012.2664. [DOI] [PubMed] [Google Scholar]
- 150.Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40:2037–2044. doi: 10.1177/0363546512453300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134:1271–1277. doi: 10.1007/s00402-014-2026-4. [DOI] [PubMed] [Google Scholar]
- 152.Bosch G, van Schie HT, de Groot MW, Cadby JA, van de Lest CH, Barneveld A, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: a placebo-controlled experimental study. J Orthop Res. 2010;28:211–217. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- 153.Bosch G, Moleman M, Barneveld A, van Weeren PR, van Schie HT. The effect of platelet-rich plasma on the neovascularization of surgically created equine superficial digital flexor tendon lesions. Scand J Med Sci Sports. 2011;21:554–561. doi: 10.1111/j.1600-0838.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 154.Shams A, El-Sayed M, Gamal O, Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837–842. doi: 10.1007/s00590-016-1826-3. [DOI] [PubMed] [Google Scholar]
- 155.Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40:1234–1241. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 156.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4:CD010071. doi: 10.1002/14651858.CD010071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun L, Qu L, Zhu R, Li H, Xue Y, Liu X, et al. Effects of mechanical stretch on cell proliferation and matrix formation of mesenchymal stem cell and anterior cruciate ligament fibroblast. Stem Cells Int. 2016;2016:9842075. doi: 10.1155/2016/9842075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lohberger B, Kaltenegger H, Stuendl N, Rinner B, Leithner A, Sadoghi P. Impact of cyclic mechanical stimulation on the expression of extracellular matrix proteins in human primary rotator cuff fibroblasts. Knee Surg Sports Traumatol Arthrosc. 2016;24:3884–3891. doi: 10.1007/s00167-015-3790-6. [DOI] [PubMed] [Google Scholar]
- 159.Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell–collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13:1219–1226. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 160.Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell–collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12:2291–2300. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 161.Mace J, Wheelton A, Khan WS, Anand S. The role of bioreactors in ligament and tendon tissue engineering. Curr Stem Cell Res Ther. 2016;11:35–40. doi: 10.2174/1574888x10666150904113827. [DOI] [PubMed] [Google Scholar]
- 162.Hohlrieder M, Teuschl AH, Cicha K, van Griensven M, Redl H, Stampfl J. Bioreactor and scaffold design for the mechanical stimulation of anterior cruciate ligament grafts. Biomed Mater Eng. 2013;23:225–237. doi: 10.3233/BME-130746. [DOI] [PubMed] [Google Scholar]
- 163.Laurent CP, Vaquette C, Martin C, Guedon E, Wu X, Delconte A, et al. Towards a tissue-engineered ligament: design and preliminary evaluation of a dedicated multi-chamber tension-torsion bioreactor. Processes (Basel) 2014;2:167–179. [Google Scholar]
- 164.Youngstrom DW, Rajpar I, Kaplan DL, Barrett JG. A bioreactor system for in vitro tendon differentiation and tendon tissue engineering. J Orthop Res. 2015;33:911–918. doi: 10.1002/jor.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bourdón-Santoyo M, Quiñones-Uriostegui I, Martínez-López V, Sánchez-Arévalo F, Alessi-Montero A, Velasquillo C, et al. Preliminary study of an in vitro development of new tissue applying mechanical stimulation with a bioreactor as an alternative for ligament reconstruction. Rev Invest Clin. 2014;66:S100–S110. [PubMed] [Google Scholar]
- 166.Burk J, Plenge A, Brehm W, Heller S, Pfeiffer B, Kasper C. Induction of tenogenic differentiation mediated by extracellular tendon matrix and short-term cyclic stretching. Stem Cells Int. 2016;2016:7342379. doi: 10.1155/2016/7342379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grier WK, Moy AS, Harley BA. Cyclic tensile strain enhances human mesenchymal stem cell Smad 2/3 activation and tenogenic differentiation in anisotropic collagen-glycosaminoglycan scaffolds. Eur Cell Mater. 2017;33:227–239. doi: 10.22203/eCM.v033a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu SH, Yang RS, al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res. 1995;318:265–278. [PubMed] [Google Scholar]
- 169.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 170.LaCroix AS, Duenwald-Kuehl SE, Lakes RS, Vanderby R., Jr Relationship between tendon stiffness and failure: a metaanalysis. J Appl Physiol (1985) 2013;115:43–51. doi: 10.1152/japplphysiol.01449.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22:534–538. doi: 10.1016/j.arthro.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 172.Leduc S, Yahia L, Boudreault F, Fernandes JC, Duval N. Mechanical evaluation of a ligament fixation system for ACL reconstruction at the tibia in a canine cadaver model. Ann Chir. 1999;53:735–741. [PubMed] [Google Scholar]
- 173.Schindhelm K, Rogers GJ, Milthorpe BK, Hall PJ, Howlett CR, Sekel R, et al. Autograft and Leeds-Keio reconstructions of the ovine anterior cruciate ligament. Clin Orthop Relat Res. 1991;267:278–293. [PubMed] [Google Scholar]
- 174.Nightingale EJ, Allen CP, Sonnabend DH, Goldberg J, Walsh WR. Mechanical properties of the rotator cuff: response to cyclic loading at varying abduction angles. Knee Surg Sports Traumatol Arthrosc. 2003;11:389–392. doi: 10.1007/s00167-003-0404-5. [DOI] [PubMed] [Google Scholar]
- 175.Handl M, Drzík M, Cerulli G, Povýsil C, Chlpík J, Varga F, et al. Reconstruction of the anterior cruciate ligament: dynamic strain evaluation of the graft. Knee Surg Sports Traumatol Arthrosc. 2007;15:233–241. doi: 10.1007/s00167-006-0175-x. [DOI] [PubMed] [Google Scholar]
- 176.Wren TA, Yerby SA, Beaupré GS, Carter DR. Mechanical properties of the human achilles tendon. Clin Biomech (Bristol, Avon) 2001;16:245–251. doi: 10.1016/s0268-0033(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 177.Gurkan UA, Cheng X, Kishore V, Uquillas JA, Akkus O. Comparison of morphology, orientation, and migration of tendon derived fibroblasts and bone marrow stromal cells on electrochemically aligned collagen constructs. J Biomed Mater Res A. 2010;94:1070–1079. doi: 10.1002/jbm.a.32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Font Tellado S, Bonani W, Balmayor ER, Foehr P, Motta A, Migliaresi C, et al. Fabrication and characterization of biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Tissue Eng Part A. 2017;23:859–872. doi: 10.1089/ten.TEA.2016.0460. [DOI] [PubMed] [Google Scholar]
- 179.Yoon JP, Lee CH, Jung JW, Lee HJ, Lee YS, Kim JY, et al. Sustained delivery of transforming growth factor β1 by use of absorbable alginate scaffold enhances rotator cuff healing in a rabbit model. Am J Sports Med. 2018;46:1441–1450. doi: 10.1177/0363546518757759. [DOI] [PubMed] [Google Scholar]
- 180.Sahoo S, Toh SL, Goh JC. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31:2990–2998. doi: 10.1016/j.biomaterials.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 181.Rothrauff BB, Lauro BB, Yang G, Debski RE, Musahl V, Tuan RS. Braided and stacked electrospun nanofibrous scaffolds for tendon and ligament tissue engineering. Tissue Eng Part A. 2017;23:378–389. doi: 10.1089/ten.tea.2016.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Leung M, Jana S, Tsao CT, Zhang M. Tenogenic differentiation of human bone marrow stem cells via a combinatory effect of aligned chitosan–poly-caprolactone nanofibers and TGF-β3. J Mater Chem B. 2013;1:6516–6524. doi: 10.1039/c3tb20825g. [DOI] [PubMed] [Google Scholar]
- 183.Wu G, Deng X, Song J, Chen F. Enhanced biological properties of biomimetic apatite fabricated polycaprolactone/chitosan nanofibrous bio-composite for tendon and ligament regeneration. J Photochem Photobiol B. 2018;178:27–32. doi: 10.1016/j.jphotobiol.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 184.Deepthi S, Nivedhitha Sundaram M, Deepti Kadavan J, Jayakumar R. Layered chitosan-collagen hydrogel/aligned PLLA nanofiber construct for flexor tendon regeneration. Carbohydr Polym. 2016;153:492–500. doi: 10.1016/j.carbpol.2016.07.124. [DOI] [PubMed] [Google Scholar]
- 185.Araque-Monrós MC, Gamboa-Martínez TC, Santos LG, Bernabé SG, Pradas MM, Estellés JM. New concept for a regenerative and resorbable prosthesis for tendon and ligament: physicochemical and biological characterization of PLA-braided biomaterial. J Biomed Mater Res A. 2013;101:3228–3237. doi: 10.1002/jbm.a.34633. [DOI] [PubMed] [Google Scholar]
- 186.Leroy A, Nottelet B, Bony C, Pinese C, Charlot B, Garric X, et al. PLA-poloxamer/poloxamine copolymers for ligament tissue engineering: sound macromolecular design for degradable scaffolds and MSC differentiation. Biomater Sci. 2015;3:617–626. doi: 10.1039/c4bm00433g. [DOI] [PubMed] [Google Scholar]
- 187.Yang G, Lin H, Rothrauff BB, Yu S, Tuan RS. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 2016;35:68–76. doi: 10.1016/j.actbio.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bellini D, Cencetti C, Sacchetta AC, Battista AM, Martinelli A, Mazzucco L, et al. PLA-grafting of collagen chains leading to a biomaterial with mechanical performances useful in tendon regeneration. J Mech Behav Biomed Mater. 2016;64:151–160. doi: 10.1016/j.jmbbm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 189.Sharifi-Aghdam M, Faridi-Majidi R, Derakhshan MA, Chegeni A, Azami M. Preparation of collagen/polyurethane/knitted silk as a composite scaffold for tendon tissue engineering. Proc Inst Mech Eng H. 2017;231:652–662. doi: 10.1177/0954411917697751. [DOI] [PubMed] [Google Scholar]
- 190.Reese SP, Ellis BJ, Weiss JA. Multiscale modeling of ligaments and tendons. In: Gefen A, editor. Multiscale computer modeling in biomechanics and biomedical engineering. Berlin: Springer; 2013. pp. 103–147. [Google Scholar]
- 191.Barfod KW. Acute Achilles tendon rupture: assessment of non-operative treatment. Dan Med J. 2014;61:B4837. [PubMed] [Google Scholar]
- 192.Richardson LE, Dudhia J, Clegg PD, Smith R. Stem cells in veterinary medicine—attempts at regenerating equine tendon after injury. Trends Biotechnol. 2007;25:409–416. doi: 10.1016/j.tibtech.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 193.Nguyen DT, Dellbrügge S, Tak PP, Woo SL, Blankevoort L, van Dijk NC. Histological characteristics of ligament healing after bio-enhanced repair of the transected goat ACL. J Exp Orthop. 2015;2:4. doi: 10.1186/s40634-015-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Steinbichler TB, Dudás J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–181. doi: 10.1016/j.semcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]