It is generally acknowledged that once the nosology of psychiatric disorders finds a basis in neurobiology, the field will be more successful at specifying, finding treatments, and predicting outcomes for psychiatric conditions.
The need for biology-based parsing in psychosis
Leaders in psychiatry have called for biologic characteristics to be the basis for designating psychiatric diagnostic groups. Most recent efforts have focused on genetics for biological classification, despite the weak genetic effects for the psychoses [1]. Geneticists themselves have concluded that their results “strongly suggest that our diagnostic categories do not define pathophysiological entities” [2]. Therefore, seeking clues to biology, we have turned to a comprehensive characterization of quantitative behavioral, cognitive, electro-physiologic, and imaging traits (each with some but not complete biological understanding) in a cross-diagnostic psychosis population.
B-SNIP experience and replication
The Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP) began by seeking quantitative markers for DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition)-defined psychoses in 933 probands, and 1059 relatives with 459 healthy controls (HCs) [3]. Finding no markers strong enough for diagnostic distinctions [4], we proceeded to use computational approaches to develop biologically based clusters of psychosis probands from these deep phenotyping data. Using principal component analysis and k-means clustering, three distinct groups emerged, named Biotype-1, -2, and -3 (B-1; B-2; B-3). The groups show distinct biological characteristics: B-1 has low electroencephalography (EEG) power, profound cognitive dysfunction, gray matter reductions throughout neocortex, and reduced abstract thinking. B-2 has high EEG power, moderate cognitive dysfunction, a reduction in fronto-temporal gray matter volume, and increased thought disorder. B-3 is mostly normal on these features, with EEG power and cognition similar to the HCs and a distribution of gray matter reduction limited to limbic cortex, albeit with mild psychosis [5]. Four years later in an entirely new, well-phenotyped sample with 919 probands, remarkably high Biotype replication was found with the original sample with intraclass correlation coefficients of 0.85–0.90 [6].
Phenomenological and biological groups are orthogonal
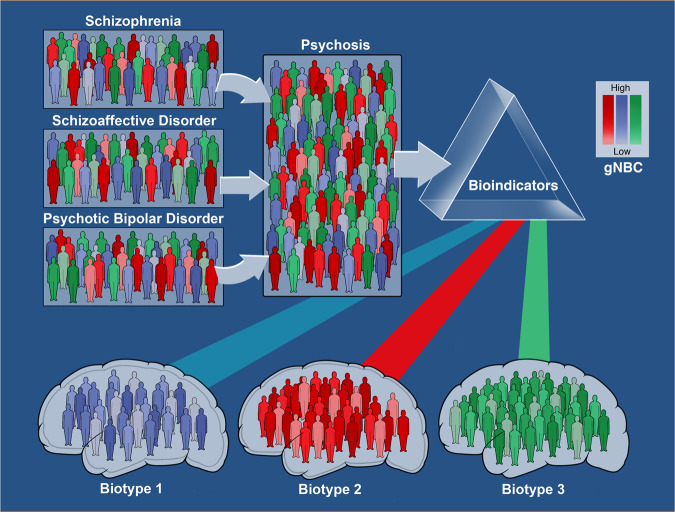
Each psychosis Biotype includes individuals with representative DSM-5 psychosis diagnoses, with biomarker characteristics falling orthogonal to conventional diagnoses, with each of the Biotypes containing all conventional diagnoses. Biological variability is still not low within Biotypes and we predict more subgroups within each Biotype. We describe, as well, a common complex cognition measure that includes cognition determinants, cognition measures, and biological correlates of cognition, potentially creating distinctive sub-classes within Biotypes (Fig. 1).
Fig. 1.
Psychosis is a defining characteristic of schizophrenia, schizoaffective disorder, and psychotic bipolar disorder, all mechanistically complex syndromes. These clinical syndromes were not distinguishable by the B-SNIP biomarker panel, with >70 individual biomarkers. Psychosis subjects, therefore, were combined into a single group, independent of conventional diagnosis. Then, biomarker variables were used to define subgroups with shared neurobiological variance. Two biomarker dimensions, “cognitive control” and “sensorimotor reactivity,” provided a means for creating biomarker-defined subgroups (called psychosis Biotypes). Biotype-1, -2, and -3 had distinctive neurobiological characteristics, including on variables not used in their definition (external validators). Within each Biotype, the level of cognition (see color intensity) can vary from low to high. Neural characteristics as they segregate in these groups could be the basis for distinct molecular and therapeutic targets
It takes a biomarker battery for comprehensive coverage
To develop these large cross-cutting psychosis Biotypes took a biomarker battery, not just single biomarkers, a battery broad enough to capture neural complexity in the psychosis phenotype. Many different disorders likely reside within each Biotype, suggesting that these three organizing Biotypes are analogous to finding the overarching categories of Dropsey, with “cardiac,” “pulmonary,” and “renal” determinants.
Moving forward with biologically based hypotheses
The usefulness of these Biotypes for clinical care and disease understanding needs to be demonstrated, to establish that biomarker clusters predict treatments, correlate with disease, or define a genetic fingerprint. Moreover, we predict that these Biotypes will guide our search for pathophysiology, even though biomarker biology is not completely worked out. We see all of these applications as the goal of this work and anticipate breakthroughs based on biomarker-guided studies.
Funding and disclosure
B-SNIP collaborators, in addition to the authors, include Drs. Elliot Gershon (Chicago), Matcheri S. Keshavan (Boston), Godfrey D. Pearlson (Hartford), and John A. Sweeney (Cincinnati). All PIs do equal work. The research reported here was funded by NIMH: MH077851, MH077862, MH078113, MH077945, and MH077852.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ripke S, Neale BM, Corvin A. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan PF, Agrawal A, Bulik CM, et al. Psychiatric genomics: an update and an agenda. Am J Psychiatry. 2018;175:15–27. doi: 10.1176/appi.ajp.2017.17030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1263–74. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- 4.Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–84. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1285–96. doi: 10.1176/appi.ajp.2013.13010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clementz BA, Parker D, Ivleva EC, McDowell, J, Hudgens-Hane M, Thomas A, et al. Replication and extension of B-SNIP Biotypes— evolution of personalized medicine in psychiatry. 2019. In preparation.