Abstract
The military lifestyle often includes continuous operations whether in training or deployed environments. These stressful environments present unique challenges for service members attempting to achieve consolidated, restorative sleep. The significant mental and physical derangements caused by degraded metabolic, cardiovascular, skeletomuscular, and cognitive health often result from insufficient sleep and/or circadian misalignment. Insufficient sleep and resulting fatigue compromises personal safety, mission success, and even national security. In the long-term, chronic insufficient sleep and circadian rhythm disorders have been associated with other sleep disorders (e.g., insomnia, obstructive sleep apnea, and parasomnias). Other physiologic and psychologic diagnoses such as post-traumatic stress disorder, cardiovascular disease, and dementia have also been associated with chronic, insufficient sleep. Increased co-morbidity and mortality are compounded by traumatic brain injury resulting from blunt trauma, blast exposure, and highly physically demanding tasks under load. We present the current state of science in human and animal models specific to service members during- and post-military career. We focus on mission requirements of night shift work, sustained operations, and rapid re-entrainment to time zones. We then propose targeted pharmacological and non-pharmacological countermeasures to optimize performance that are mission- and symptom-specific. We recognize a critical gap in research involving service members, but provide tailored interventions for military health care providers based on the large body of research in health care and public service workers.
Subject terms: Post-traumatic stress disorder, Sleep, Behavioural genetics, Translational research
Introduction
Sleep must be properly calibrated and carefully considered during nearly every aspect of one’s military career. Service members are notorious for sleeping at any given opportunity, and often fall asleep quickly in non-traditional, noisy environments. Achieving adequate amounts of restorative sleep is an ongoing problem that is critical for reasons of personal safety, unit performance, and even a matter of national security. Although there are educational resources in place for optimizing sleep in military personnel (e.g., Army Office of the Surgeon General’s Performance Triad (P3) initiative), poor sleep hygiene in the long-term can increase sleep problems co-morbid with physiological and psychological issues that include: cardiovascular disease [1], substance abuse [2], post-traumatic stress disorder (PTSD), and mood disorders [3–5].
This review is written at a time when US military leaders are giving increased attention to the critical importance of sleep for next-day performance and long-term physical and mental health across a military career. For instance, the US Army’s P3 highlights that adequate sleep, physical activity and nutrition are essential for increasing and sustaining mental and physical performance. The US Army Field Manual (FM) 6–22.5 further recognizes the importance of sleep and provides guidance on how to implement preventative measures for decreasing sleep disturbances, as well as providing military leaders direction on how to cope and counterbalance periods of insufficient sleep/disrupted sleep due to operational contingencies through evidence-based pharmacological and non-pharmacological treatment strategies.
Here, we cover results from both clinical and preclinical studies, highlighting military relevant literature where possible. A majority of military-focused studies on the impact of sleep on next-day operating performance are limited to self-report and/or only capture impact pre- and post- deployment or at a single time point. Fortunately, there are numerous human studies of cognitive, endocrine, and molecular function during reverse sleep cycles (i.e., night shift work), 24 h work schedules, and rotating (rapidly shifting) work schedules in healthcare (e.g. nurses) and public service (e.g., firefighters) workers with translational value to military personnel. Military-focused studies on sleep disorders are comprehensive, and capture problems across a single deployment (pre- and post), re-deployment, and biological sex. Areas where preclinical animal research can be improved or tailored for military relevance are also emphasized. Lastly, we examine new tools developed by defense laboratories that provide timely performance predictions (2B-Alert), coupled with potential countermeasures and techniques, to combat fatigue during sleep loss in the operating environment.
Overview of sleep
Sleep wake states can be classified as waking, rapid eye movement (REM) and non-rapid eye movement (NREM) sleep based on characteristics of electroencephalography (EEG) and electromyography (EMG) signals. Waking is defined by high-frequency, lower amplitude EEG signals and a robust EMG signal, owing to active postural and kinetic muscle activity. In contrast, NREM sleep EEG exhibits lower frequency, higher amplitude signals following more synchronized neural firing patterns and a reduced amplitude EMG. REM sleep EEG reverts back to high-frequency, lower amplitude signals reminiscent of waking, yet the EMG signals are devoid of activity, referred to as atonia, which is used to differentiate REM from waking in polysomnographic recordings [6].
Sleep and waking are both active processes in the brain, yet different mechanisms drive each state. Cognitive arousal is largely mediated by excitatory brainstem structures and neurochemicals such as the peduncolopontine nucleus (acetylcholine), locus coeruleus (norepinephrine) and raphe (serotonin), as well as the midbrain tuberomamillary nucleus (histamine) that activate thalamic and cortical structures. In contrast, inhibitory cells in the hypothalamus (ventrolateral preoptic area) send γ-aminobutyric acid (GABA) and galanin projections to inhibit these structures, amongst others, to initiate sleep. Recent evidence from another midbrain inhibitory structure, the rostromedial tegmental nucleus [7], suggests a distributed ventral inhibitory system may exist that promotes sleep (Jhou and Good et al., unpublished data) [8]. One explanation of this reciprocal relationship is referred to the “flip-flop circuit” theory proposed by Saper et al. to finitely explain hypothalamic regulation of NREM sleep and NREM transitions to REM and waking states [9, 10].
During development, sleep/wake patterns change such that infants require approximately twice the amount of sleep time as mature adults, but do so in a fragmented pattern throughout the day and night [11–13]. As development progresses, the number of sleep bouts gradually consolidate to the adult cycle consisting of a single ~8 h nightly sleep episode during which the individual transitions through NREM and REM stages. In adolescents and early adulthood there is an increase in sleep pressure that can escalate sleep need and duration to ~9 h per night [14, 15], and shift bedtimes and awakenings to later hours. This time period coincides with the age group who typically enlists in the United States (US) military post-high school (17–20 years of age) [16]. At this ontogenetic time, sleep/wake cycles are still transitioning to adult patterns [11]. This runs counter to the sleep/wake schedules of military training to which these adolescents enter, where <6 h of sleep per night and rise times of 04:30 are commonplace [17–20]. Unfortunately, insufficient sleep <6 h per night becomes chronic and normative during early training and is pervasive even at elite military academies [17, 19]. To test the impact of sleep schedules on health and performance, Miller et al. shifted the sleep schedules of US Army trainees during Basic Combat Training to better align with the natural sleep drive and habits of adolescents. Results showed reductions in mood disturbances, improvements in marksmanship and less fatigue, as well as overall improvements in scores of sleep quality [18], highlighting the significant impact of natural sleep drive on human performance. It’s hypothesized that these early adolescent improvements in sleep hygiene during basic training, a time when most trainees sleep patterns are still developing, could carry forward to increased resiliency against future circadian/sleep disruptions or mental illness. Longitudinal studies suggest that sleep disturbances in young adults serve as an early risk factor for developing major depression [21–23], and this risk could persevere for many years [24]. Additionally, pre-deployment insomnia is a significant contributor to post-deployment PTSD and suicidal ideation [25–27]. A recent model of insomnia in US military veterans identifies early sleep problems as an initial, precipitating stressor that may reduce resilience to subsequent stressors, including persistent sleep problems [28]. This feed-forward pattern could impair an individual’s adaptive capacity to cope with additional or larger stressful events, predisposing them to depression, anxiety or PTSD. Whereas it remains unknown if mitigating sleep disturbances early during basic training could afford protection against future mental illness in military populations, effort should be directed to determining if early intervention to minimize sleep debt could reduce subsequent negative outcomes in this susceptible population. Although, given the military culture where sleeping less is common throughout ones career, and is largely viewed as a sign of mental and physical toughness, it is unclear if early improvements in sleep hygiene could overcome many subsequent years of disordered sleep.
Nightly sleep duration for US service members is truncated, as compared to civilian counterparts. The National Sleep Foundation, American Academy of Sleep Medicine, and Sleep Research Society all recommend a minimum of 7 h of sleep per night for adults 18 years of age or older [15, 29]. Indeed, a large national US epidemiology study found that 63% of Americans slept 7–8 h per night, whereas only 28% slept 6 or fewer hours per night, consistent with short sleep duration (SSD) [30]. This contrasts with two US military studies which found 72 and 69% of service members were classified as SSD with less than 6 h of sleep per night, and only 27 and 30% obtained the recommended 7–8 h of sleep, respectively [3, 31]. Redeployed US Army Soldiers with prior combat exposures were most likely to have SSD, whereas being wounded or injured during combat was a strong predictor of sleeping less than 5 h per night post-injury [31]. Operationally, SSD truncates opportunities to maximize the recuperative value of sleep required of highly mentally and physically demanding tasks inherent of military operations. SSD also degrades next-day performance as found in a sleep duration study of artillery marksmanship [32, 33], compromising personal safety as well as resources.
Consequences of military work schedules
The biological impact of rotating work schedules has been extensively studied, yet examples focused on military personnel and operations are sparse despite operational work cycles of 12 h and 24 h being common-place. This is due in part to the non-controlled dynamic settings in which data would have to be collected, where the mission comes first and duties cannot be routinely interrupted for data collection efforts. Even prospective studies evaluating the effects of shift schedules are challenging in the military population due to the variable and temporary nature of most work schedules and environments, and are more likely to yield less conclusive results than civilian studies where work schedules tend to be more consistent. Further, the confounding and variable factors that must be statistically accounted for across subjects would be immense given the situation and task specific nature of service member’s duties. Fortunately, there are numerous human studies of cognitive, endocrine, and molecular function during reverse sleep cycles (i.e., night shift work), 24 h work schedules, and rotating (rapidly shifting) work schedules in healthcare (e.g. nurses) and public service (e.g., firefighters) workers with translational value to military personnel.
One military population that is more conducive to short-term studies of shift work is the Navy, where Sailors are deployed on ships for days to weeks at a time and tend to have more defined watch duties while at sea. Researchers located at the Naval Postgraduate School have published a number of studies that leveraged this population, with particular emphasis on deriving watch schedules that better align with circadian rhythms [34] and offer more time for dedicated sleep [35] than the common rotating 5 h on/10 h off watch schedule [20]. These studies consistently suggest that Sailors prefer the 3 h on/ 9 h off watch schedule, with less reported daytime sleepiness and improved mood and reaction times with fewer errors. Although this military population is more amenable to studying the effects of shift schedules, care still must be taken when generalizing sleep and physiology results across ship departments [36, 37]. For instance, salivary cortisol levels can vary across individuals due to increased stress in noisy departments (e.g., engine rooms and gun turrets), whereas light intensity can shift melatonin levels depending on duty station (e.g., interior control room versus outdoor ship bridge) [37]. In another military population, surveyed US Army Aviation personnel working a reverse sleep cycle reported that they did not achieve adequate daytime sleep when working this schedule [38], which can lead to pilot errors [39].
Night shift work (reverse sleep cycles)
In general, night shift work of any kind and duration increases morbidity and mortality, negatively impacting both physiological and psychological health. Regarding physiological health, night shift work in female nurses leads to unhealthy lifestyles that excludes exercise [40] and increases overall caloric intake and craving for high-fat, high-sugar, and protein-deficient foods [41] by means of altering gut-derived release of hormones regulating hunger and satiety (ghrelin) [42]. Interestingly, positive stress such as exercise has even been shown to exacerbate clinically significant endocrine disruption of ghrelin, leptin, insulin, and triglyceride levels [43] induced by night shift work [44, 45], indicating that the timing of exercise must also be properly calibrated in night shift workers. These results have direct corollaries with military populations, leading to reduced ability to control one’s weight despite semi-annual evaluations in order to maintain current military occupation and to determine odds of deployability (AR 600-9). In the long-term, a higher body mass index (BMI) “set point” in service members manifest from shift work may lead to gastrointestinal tract issues and type 2 diabetes as found in civilian counterparts [40, 46, 47]. Night shift work can also reduce skeletomuscular (isometric) strength by 20% in civilian populations [48], can contribute to increases in musculoskeletal symptoms in US Navy crewmembers [49], and lead to cardiovascular stress through an increase of the blood-borne marker, cyclooxygenase-2 (COX-2) [50]. As a longitudinal consequence of night shift work (20,142 shift workers studied across 22 years), new cases of hypertension increased by >10% [51]. This trifecta of hypertension, reduced isomeric strength, and higher BMI increase the risk for cardiovascular disease. Finally, night shift work in police officers – a civilian occupation with many overlapping responsibilities as military personnel – have heightened blood-borne markers of inflammation (increased white blood cell counts, lymphocytes, and monocytes) [52], potentially leading to new cancer cases specific to biological sex (breast [female] and prostate [male]). In the short-term for military personnel, this trifecta could increase the risk of musculoskeletal injuries [49] and/or myocardial infarction while engaging in physically demanding tasks specific to military occupation. Post-military service, this trifecta could contribute to the increased risk for heart disease in veterans [53]; a finding that could be compounded by the lack of mandatory early morning exercise sessions and field training that are no longer part of a service members routine, as well as injuries or neurological disorders incurred during service, amongst other factors.
Regarding psychological health and performance, basic and advanced cognitive processes such as reaction time to respond to visual cues and the ability to quickly and correctly perform mental calculations are impaired in nighttime healthcare workers compared to daytime counterparts [54, 55]. Similar to healthcare workers, military occupations have unique skill sets requiring service members to accurately and rapidly attend to, process and integrate new information specific to one’s occupational duties as part of a military mission. Cognitive shortfalls in the ability to quickly learn, remember, and execute a specific task can lead to mission failure and can compromise individual and unit safety as well as national security. Further, night shift work leads to long-term elevations in psychological stress and “burnout” that can be detrimental to sustained performance [56]. The extent of reduced attention and vigilance during night shift work can be predicted by, and is directly related to, the extent of misalignment between working schedules and two distinct physiological processes: (a) daytime peaks in core body temperature (when alertness is higher); and (b) nighttime peaks in dim-light melatonin onset (DLMO; when alertness is lower) [57].
Two separate population-based cohorts derived from the Swedish Twin Registry (largest twin registry in the world) demonstrated a clinically significant, dose-response relationship between duration of shift work and night shift work (in years) and incident risk for neurocognitive disorder (as determined from International Classification of Disease [ICD] and Anatomical Therapeutic Chemical Classification System [ATC] codes from patient registers) [58]. This risk was amplified in carriers of the APOE4 mutation [58], common in individuals of European descent [59], who worked a shift or night schedule for more than 20 years. Results from this study suggest that shift work of any duration could increase the risk for neurocognitive disorder many years later, although this needs to be confirmed in other populations. Similar studies should be explored in military veterans, where medical histories, occupation, and work histories, and deployments are documented. The interplay between shift work, night shift work, and traumatic brain injury is another avenue that could yield insight into increased risk for subsequent neurocognitive disorders.
Rapid time-zone, light/dark shifts
In general, light is the most potent environmental stimulus capable of entraining, but also phase-shifting, the mammalian sleep/wake cycle. Depending on when white- or blue-enriched light (but not red-enriched light) of any duration is presented at night, light will phase-delay or advance the sleep/wake cycle, as characterized by the photic phase-response curve [60, 61]. This physiological response to timed light exposure can help ameliorate the negative consequences of night shift work as first shown >20 years ago [62]. Light timing and quality does matter as evidenced by the implementation of light “recipes” [63–65] and the efficacy of light-block wearables for optimizing immediate physiological alertness under various night shift schedules [66]. For military personnel, timed light “recipes” can be a solution for phase-shifting sleep/wake cycles for the purpose of “owning the night,” and executing a successful night mission that maximizes performance and reduces risk for injury.
Poor quality and limited artificial incandescent light is common of military installations with 24 h operation centers, necessitating an understanding of the short-term and long-term impact of these conditions on physiological and psychological health in military populations. Incandescent lighting compared to natural sunlight (>10,000 lux) was shown to reduce sleep quality through a dampening in the amplitude of nighttime melatonin release [67], and natural light compared to artificial light results in better consolidation of a sleep/wake cycle through more robust release of brain-derived melatonin [67]. Rapid changes in sleep/wake patterns can shift melatonin release out-of-phase with military duty schedules and result in increased sleepiness during work hours when vigilance needs are high [37]. Whereas a constant dim light routine typically yields benign impact on physiological and psychological health, chronic bright light was shown to cause sleep/wake arrhythmia and sleep/wake rhythm splitting [68], dampen rhythmic gene expression [69], and functionally lead to reduced insulin sensitivity and immune deficiency in rodent models [70, 71]. Nighttime light pollution is also a significant problem in operating environments. Nearly all service members who have to relieve bodily functions in the middle of the night have to get dressed and walk more than 250 m outside in order to use the latrines [39], which can be located inside a facility that is lit 24 h using incandescent lighting. Stadium-type lighting is also used to illuminate these trekked paths on operating bases, further compounding light pollution in already poor living quarters for restorative sleep. In both instances, the bright light can serve as a zeitgeber that reduces an individual’s ability to fall back asleep upon returning to their bunk, further dysregulating their sleep patterns. With better integration of more natural lighting on military installations, service members could maximize their opportunities for achieving restorative sleep on non-training days and, most importantly, to maximize this opportunity for “sleep banking” leading up to sleep loss during multi-day training exercises or operations [72].
Sub-acute physical and psychological stress can act as non-photic zeitgebers in rodent models by means of altering circadian-controlled rhythms of corticosterone [73, 74]. Physiological stress reactivity in humans is circadian-controlled [75], suggesting that stressors may potentially alter circadian rhythms at specific times of the day. It is well known that service members are exposed to more acute physiological and psychological stress in operating environments compared to the general population. Therefore, amplitudinal and phase-shifts in cortisol rhythms induced by acute stressors experienced by service members may also serve as a zeitgeber [37], similar to bright light exposure, but certainly warrants further investigation. Nonphotic stimuli such as exercise [76–79] and social interaction [80] are also capable of entraining and phase-shifting the mammalian sleep/wake cycle, [78, 79, 81, 82], yet nonphotic cues are highly complex and can cancel out the phase-shifting actions of light in animal models [81]. In humans, exercise can phase-shift circadian rhythms; an effect that is additive to bright light exposure [83]. Time of day also plays a role, with recent evidence suggesting multiple time windows to include exercise to adjust circadian rhythms [84]. These exciting results suggest the timing of novel non-photic stimuli (e.g., military warrior tasks and battle drills) must be carefully controlled when seeking to purposefully and effectively phase-shift a sleep/wake cycle with enriched light for greater performance operability at night. Although, this level of circadian coordination is not currently adopted by military units.
To add to the problem, around-the-clock military operations often result in routine back-to-back phase-advances and subsequent phase-delays of traveling from “safety bases” >2 time zones away from the front line of troops. Recent research in an animal model demonstrated that phase-advances and subsequent phase-delays (or vice versa) can be a “zero sum game” by means of creating circadian decoupling and dampening rhythmic gene expression [85] and functionally impairing memory encoding and recall [86, 87]. Therefore, there is a critical need to better understand these biological underpinnings of human performance in response to novel photic/non-photic stimuli specifically for military personnel in order to better operate at night, while also ensuring that the physiological and psychological health of military personnel are being optimized in the short- (combat) and long-term (career and retirement).
24 h operational tempos
In contrast to the majority of civilian occupations, US military operations are around-the-clock. Unpredictable and ever changing duty schedules and >24 h operations are not only common during deployment to a combat zone (i.e., operating environment), but are also seen stateside (i.e., in garrison) in order to create a continuous information flow to and from the operating environment. Service members must often engage in mid-night teleconferences with a unit operating >8 time zones away in the Middle East or Pacific Islands (anti-phasic coordination), and then still perform their daily duties the next day under shortened and fragmented sleep. The reverse is also common; higher military headquarters communicating during daylight hours in Washington, DC with units engaging in nighttime operations in the Middle East often means that service members executing nighttime operations are awake >12 h preparing for the mission even prior to the overnight mission itself. This scenario is exemplified by the US military’s pride in “owning the night,” meaning that high-risk operations against enemy forces are prioritized at night instead of during the day (e.g., mid-night capture of Osama bin Laden in Abbottabad, Pakistan in 2011). Although we, as a nation, will also often remember and glorify these highly publicized overnight missions, many smaller scale and lesser publicized nighttime missions have significantly compromised the health and safety of US service members and have resulted in mission failure as a consequence of long-standing nighttime shift work, coupled with chronic insufficient sleep, that affords many opportunities for performance decrements and errors following this operational schedule. Outside of combat operations, it is also very common for service members to go on multi-day training exercises and sleep <5 h per night (Skeiky et al., unpublished data). Thus, around-the-clock military operations continue to place added risk on life-and-death decisions made by sleep-deprived service members working in highly stressful environments across many geographical regions and battlefield domains (i.e., land, sea, and air) with increasingly advanced weapon and data systems requiring a high degree of cognitive throughput. Given the requirement to complete physically and mentally demanding tasks on a consistent basis during mission execution, the inability to perform and recover due to the inability to achieve restorative sleep, is a setup for mission failure.
Studies examining the consequences of a single night of >24 h sleep deprivation and chronic sleep restriction (<7 days) have yielded immediate, short-term compromises in physiological and brain health at levels of neural connectivity, endocrine and molecular signaling cascades, and psychological/behavioral outcomes. For instance, a study of real-time brain function via positron-emission tomography (PET) found that a single night of >24 h sleep deprivation rapidly increased tau- and amyloid plaque accumulation – two gold-standard signatures of brain inflammation and risk for Alzheimer’s disease – that did not fully return to baseline levels after recovery sleep [88]. Immediate, clinically significant declines in anabolic processes such as decreases in blood-borne testosterone and growth hormone release [89, 90], concurrent with increases in catabolic processes such as blood-borne cortisol release [89–93] and oxidative stress [94] have been reported following acute sleep deprivation. Extended sleep deprivation also phase-shifts and dampens the amplitude of biochemical substrates of glucose, fatty acid, and amino acid metabolism: including cholesterol [95], acylcarnitines [96], oxalic acid [97, 98], and diacylglycerol [97, 98]. At the most microscopic, cellular levels of study, sleep deprivation alters the human DNA transcriptome [99] and ATP synthesis [94]. These human studies show the broad neural, endocrine, tissue, and cellular impacts of a single night of >24 h sleep deprivation and chronic sleep restriction (<7 days) on both physiological and psychological health that are highly relevant to the duties of service members.
Sleep and psychiatric disorders
It is estimated that over 60% of service members who never, previously, or were currently deployed sleep less than 6 h per night, with those previously or currently deployed more likely to report sleeping <5 h per night [100]. There are several factors contributing to the lack of sufficient sleep in the military, including combat operations, shift work, and the comorbidity of psychiatric disorders in service members. Although real-time physiological and psychological health data in active duty personnel during deployed military operations is sparse, sleep disturbances, namely insomnia and obstructive sleep apnea (OSA), have been broadly studied pre- and post-deployment and across sexes in medical treatment facilities [3–5, 101, 102]. Insomnia is often a principal symptom in many psychiatric disorders, most notably in PTSD, depression, and mild traumatic brain injury (mTBI). In a study of service members with combat-related head injuries, 55.2% experienced insomnia symptoms, and 90.5% had at least one comorbid psychiatric condition [103]. Whereas sleep disturbances may result from psychiatric injury, pre-morbid disordered sleep may also predispose a service member to developing PTSD and other psychiatric conditions [104].
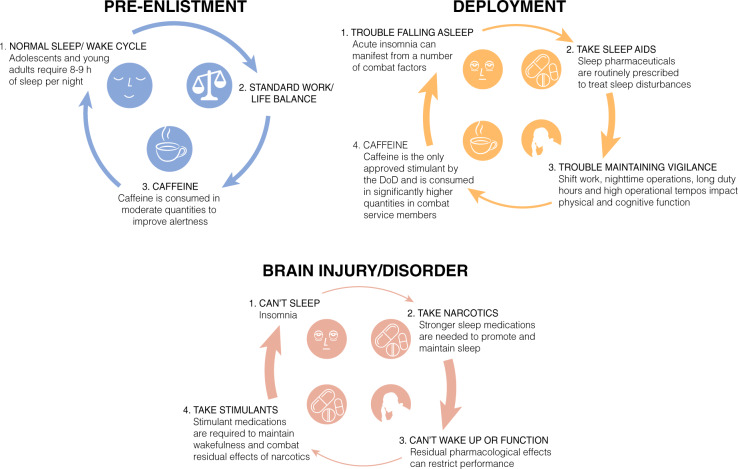
Comprehensive reviews have been written on the integral correlations between sleep and traumatic brain injury (TBI) [105, 106] and PTSD [3, 100, 107, 108], including a review by Neylan et al. appearing in this volume. Although discussing the surrounding literature is outside the scope of the current article, it should be noted that the majority of service members that endorse PTSD will also endorse significant insomnia or other sleep disturbances, (i.e. nightmares), posing a unique challenge to treating this patient population (Fig. 1).
Fig. 1.
Three sleep/wake scenarios are represented based on discussion throughout the manuscript. 1. As illustrated in blue, enlistees generally join the military with normal sleep/wake habits that must rapidly transition to an environment where restricted and fragmented sleep is the norm. 2. Upon deployment, as shown in orange, a number of precipitating factors can cause acute insomnia that require prescription sleep aids to help service members fall asleep. Residual pharmaceutical effects, combined with long duty hours and irregular sleep schedules, result in statistically higher amounts of caffeine intake to maintain alertness and performance. If taken at the wrong time, the awake-promoting effects of caffeine can cause further sleep disruptions and prevent restorative sleep during an already limited sleep window. This feed-forward cycle can necessitate service members being prescribed an increasingly potent sleep aid to overcome the stimulant effects, further necessitating stimulants to transition to wakefulness and maintain vigilance throughout subsequent duty hours, as illustrated by the increasing thickness of the arrows in the cycle. 3. Following injury or diagnosis of neurological disorder, as shown in red, persistent insomnia occurs in a large number of service members. Stronger narcotic sleep aids are routinely prescribed to overcome insomnia, creating a stronger dependence on pharmacology to drive sleep/wake states, as depicted by the even thicker red arrows within the cycle
Insomnia
Insomnia is the inability to fall asleep or stay asleep resulting in impaired daytime function and subjective patient distress. Generally, insomnia is characterized as either transient (lasting less than a month) or chronic (>1 month). The first step in treating insomnia is identification of the underlying cause or causes contributing to the patient’s complaint, as often a symptom of an underlying psychiatric condition: PTSD, depression, generalized anxiety disorder, panic disorder, bipolar disorder, and substance dependence. In fact, several studies of active duty military personnel (ADMP) referred to a sleep clinic had insomnia co-morbid with chronic pain, PTSD, anxiety and/or depression [3–5].
Military providers screen for mood, anxiety, and substance use disorders prior to referral to a sleep specialist [109]. The causes for patients presenting with transient or short-term insomnia are more easily identified than patients with chronic insomnia. A service member may experience insomnia at the beginning of a deployment because of changes in their sleeping environment, excessive noise, jet lag, shift work, the stress of being separated from their families, unpleasant room temperature, or anxiety about death or injury during deployment [110]. For instance, a cross-sectional study of deployed US Air Force Airmen found 40% of respondents had a sleep efficiency of <85% or extended sleep-onset latency >30 minutes, whereas 75% reported diminished sleep quality as compared to at home [39]. Further, night shift workers were statistically more likely to have lower sleep efficiency, greater sleep latencies, and report disturbed sleep during daylight hours due to loud noises in their surroundings. Overuse of psychostimulants such as caffeine or modafinil prescribed for shift work can further disrupt sleep patterns, and can create a feed-forward pattern where more caffeine or prescription medication is required to maintain vigilance during the day, despite the negative consequences it has on falling asleep that night (Fig. 1). This cycle is confounded by the additional stressors one endures during deployment, as discussed above. Unfortunately, insomnia often does not resolve when the service member leaves a combat zone. Difficulties initiating and maintaining sleep often persist for months after a service member returns home. Other causes of insomnia for the service member include:
Sleep state misperception
Inadequate sleep hygiene
Altitude Insomnia
General medical disorders
Neurologic disorders
Neurologic conditions such as strokes, TBI, headache syndromes (i.e., migraine and cluster headache), trigeminal neuralgia, and neurodegenerative disorders. (i.e., Alzheimer’s disease and Parkinson’s disease) all can contribute to insomnia and change sleep architecture [27, 129, 130].
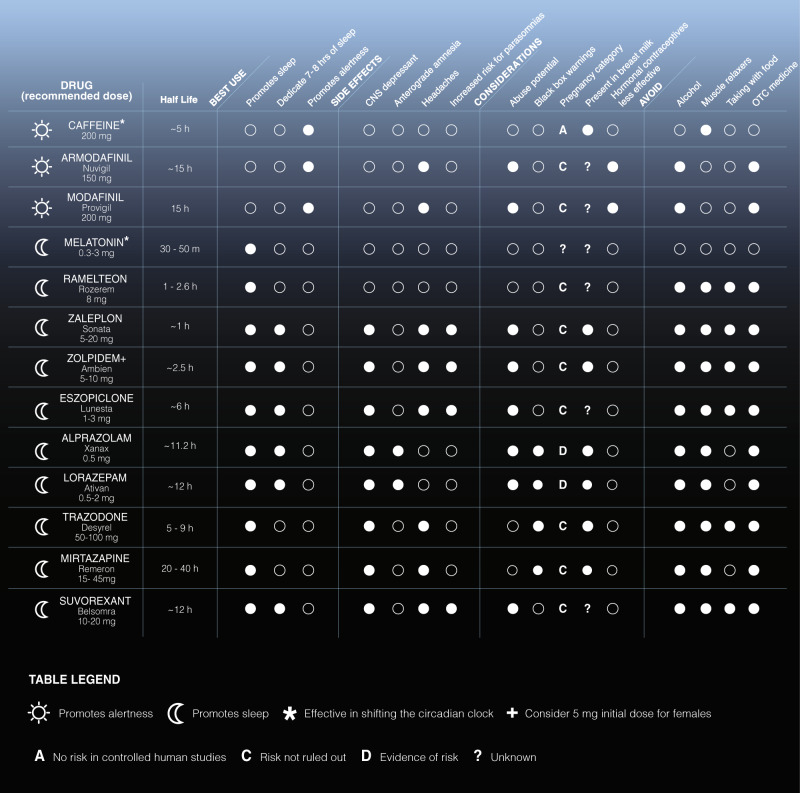
The most effective treatments for chronic insomnia are non-pharmacological, such as cognitive-behavioral therapy (individual and group) [110, 131–133], as well as motivational interviewing and mobile health delivery of sleep recommendations [134]. Service members are also asked to complete a sleep diary during treatment for insomnia and complete education about sleep hygiene and stimulus control measures. Despite this, use of sleep pharmaceuticals by military personnel is very high. In 2018, we found that nearly half of active duty Army personnel (>100,000 service members) were prescribed zolpidem (trade name: Ambien) or eszopiclone (trade name: Lunesta) as part of a deployment or routine visit to a military treatment facility for sleep complaints (Devine et al., unpublished data). Sleep pharmaceuticals largely target inhibitory pathways through GABA signaling, resulting in reduced short-term and long-term neurobehavioral performance in attention and reaction time that can manifest in a dose-dependent manner [135, 136]. A large list of sleep/wake medications approved for use by military personnel are found in Fig. 2 (half-lives and side effects derived from a Department of Defense formulary search tool). In lieu of residual, inhibitory side effects and increased sleep inertia common of sleep pharmaceuticals, the trade-off is that military personnel must have a high level of personal accountability for use. Missions require service members to be “on call” for 24 h, especially in combat zones. A service member who used a sleep medication the night prior to a planned mission in the early morning or an unplanned mission after mid-night awakening would likely have reduced combat effectiveness and compromised safety. As such, US Army guidelines (ATP 6–22.5) dictate that a Soldier have at least 8 h of non-work time after taking a prescription sleep aid, although it is unknown how well this is followed.
Fig. 2.
List of common wake (sun) and sleep (moon) medications prescribed to military personnel; all Food and Drug Administration (FDA) approved medications are available for prescription at military treatment facilities. In general, over-the-counter drugs (OTC; e.g. melatonin) are preferred first, followed by non-benzodiazepines. Benzodiazepines are least preferred given their pronounced side effects and deleterious impact on function and performance. Use of trademarked names does not imply endorsement by the U.S. Government and is intended only to assist in identification of specific medications
To date, one of the most comprehensive studies of sleep amounts, disrupted sleep, and co-morbidity with physiological and psychological health states and persistent sleep disturbances in military personnel comes from the Millennium Cohort Study (MCS) [137, 138]. MCS was a 7-year study of self-reported physiological and psychological health pre- and post-deployment and across multiple deployments in >55,000 service members from all US military service branches who were active duty, Reserve, or National Guard. 22% of this study population were deployed in support of Iraq and Afghanistan. MCS found co-morbidity of insomnia symptoms with lower self-rated health, more lost work days, lower odds of deployment, higher odds of early discharge from military service, and more health care utilization [138]. Co-morbidity was highest for “short sleepers” (<6 h per night) and “long sleepers” (>8 h per night), showing intra-individual variability in a service member’s sleep “homeostat” (set point). Although, the co-morbidity with long sleepers could be explained by an underlying mood disorder (e.g. depression) that was not fully captured by the questionnaires [138]. Further, the MCS determined that sleep duration and insomnia symptoms pre-deployment were risk factors for new-onset mental health disorders following military deployment [25]. Finally, a separate study determined that the prevalence (and impact) of short sleep duration persisted during re-deployment to Iraq and Afghanistan [31].
Obstructive sleep apnea
OSA is pervasive in ADMP [5, 101, 102], similar to studies of OSA prevalence and severity with respect to biological sex in civilian populations [139–141]. However, salient differences between the patient populations of civilian studies [139–142], as compared to ADMP studies [5, 102], exist. Namely, ADMP diagnosed with OSA are younger (<35 compared to >40 years of age), have lower BMI (<28 compared to >30), and are more physically active than civilian counterparts; age, BMI, and physical activity are three key predictors of risk for OSA, in general. Greater than 30% of ADMP who present with a sleep disturbance have insomnia co-morbid with OSA [5, 101, 102]. Of greatest concern in the OSA studies in ADMP is the high degree of co-morbidity of insomnia and OSA with other physiological (chronic pain) and psychological (anxiety and depression) health states, specifically for women [5, 102].
Although there are far fewer female service members compared to males, recent US government regulations now allow females to serve as combat arms officers and in forward operating units. Whereas studies of insomnia co-morbid with OSA in ADMP do not show any sex differences in self-reported sleepiness and insomnia [5, 102], polysomnography revealed a two-fold longer sleep-onset latency for women with an insomnia-OSA diagnosis and differential time spent in light NREM and REM sleep compared to males [5, 102]. In addition, female service members with a sleep disturbance had greater co-morbidity of an insomnia-OSA diagnosis with anxiety/depression not seen in male counterparts [5, 102]. Of the 100 ADMP females from Capener et al. who had a sleep evaluation, 35% had a diagnosis of insomnia-OSA (compared to 37% with insomnia and 15% with OSA, only) that was additionally co-morbid with pain at a rate of 59%, 49% for anxiety, 47% for depression, and 22% for PTSD. Thus, sex differences in the co-morbidity of insomnia-OSA with physiological and psychological health will continue to be pervasive and a critical decision point for the military as the rate of females serving in forward operating units continues to rise.
Military relevant animal models
Recapitulating the military sleep environment, particularly a combat scenario, in the laboratory using rodents is a challenging endeavor. Imagine a scenario where a service member has been deployed to a combat zone where they’ve been consistently operating on little sleep, varied operational schedules, and are under heavy stress daily. Five months after initial deployment and while on dismounted patrol, a large improvised explosive device (IED) detonates nearby, resulting in TBI to the service member. To understand the human impact of restricted sleep on TBI symptoms or severity, long-term prospective studies would be required to routinely monitor a large number of troops throughout deployment for clinical sleep disorders and exposure to other risk factors, followed by a diagnosis of TBI or PTSD, for example. Rodent studies offer an option to accelerate these studies and control test variables and treatment options. Yet, how can we accurately model this combat scenario, or any number like it, in the laboratory? On one hand, we have to develop and validate paradigms of longer-term and complete sleep cycle disruption that better model operating environments. We then must merge these paradigms with relevant models of blast, impact or stress to induce TBI or PTSD-like symptoms in these animals, acknowledging that each aspect of these models in of themselves are likely to result in physiological and psychological changes to the animals that must be understood and placed in the broader context of human sleep conditions. How the two manipulations interact is unknown, but should be emphasized in future studies.
Lower mammalian species permit invasive studies on the neural underpinning of sleep and sleep disruption that are not possible using human subjects. Rodents have inherently different sleep/wake patterns than humans (fragmented versus consolidated sleep/wake patterns, respectively), yet certain features of the sleep/wake system have been conserved across species that permit results to be translated. At present, the laboratory mouse (Mus musculus) has become the most prevalent rodent model for studying sleep/wake neurocircuitry due in part to conserved genetic regulation of sleep/wake states and sleep/circadian rhythm disorders that are similar to humans, including:
baseline (normal) sleep amounts and homeostatic responsiveness to sleep loss (Bmal1) [143] [mouse]; [144][mouse]; [145] [human]);
sex-linked regulation of sleep/wake processes (Ube3a: [149] [mouse]; [150] [human]; Sry: (Brager et al., unpublished data), [mouse]; and
broad quantitative trait loci studies in mice [151] and equivalent genome wide studies in humans [152, 153].
Further, widespread integration of genetic tools and techniques (e.g. optogenetics) now allow cell type and pathway specific studies into the brain regions that control sleep/wake states [154–157]. This is a critical advance, as early research into the neuroanatomical basis of these states relied on gross surgical techniques to sever pathways, as well as electrolytic or chemolytic ablation of a brain area (rodent), or as a consequence of stroke or injury (humans) to isolate and assess the influence of distinct brain regions.
Sleep as a metric in military relevant animal models
Environmental circadian disruption (ECD) in animal models, achieved through routine phase-shifts in the light-dark cycle, is of great relevance to better understand the consequences of military operations on physiological and psychological health. In rodents, regimens of ECD have residual impact on total sleep time and amplify misalignment of activity rhythms with sleep rhythms [158]. The consequence of this misalignment is a heightened pro-inflammatory response exclusively due to the phase-shift in the light-dark cycle, rather than concurrent sleep loss [158]. Regimens of ECD also impact metabolic processes on whole-body, macroscopic [159] and microscopic scales (heart [160]; skeletal muscle [161]; liver [162]) similar to what has been reported in human models of sleep loss compounded by shift work [54, 163, 164]. Animal models of constant light and constant darkness can desynchronize the suprachiasmatic nucleus (SCN) and alter gene activity [68, 69], and can further our understanding of the consequences of extended shift work and night operations in military personnel.
Whereas TBI research utilizes a variety of tools and techniques to simulate an injurious event to the brain [165], compressed air driven shock (blast) tubes are routinely used to simulate a concussive event like the one described above [166]. In a generic shock tube experiment, animals are removed from their home cages, anesthetized, and placed in a restraint device at the end of the tube. Following a shockwave event of set magnitude, the animals are removed from the tube and generally treated in some manner in an effort to mitigate the effects of shock loading. Behavioral assays are run days to weeks later to evaluate treatment efficacy, followed by neuropathology assays of neural tissue to quantify cell damage [167]. A key missing variable from this study design is the effect of sleep deprivation prior to the blast event, or how the simulated blast event disrupts sleep patterns post-blast. This is despite sleep disorders occurring in a high percentage of deployed military personnel [3], as well as clinical sleep disorders being a common symptom in TBI [105, 168–170].
Relatively few animal studies have investigated the effect of TBI on post-injury sleep, and to our knowledge none have used the military relevant shock tube to simulate blast TBI [171]. Similarly, no known published studies have chronically disrupted sleep prior to causing TBI. However, using the fluid percussion injury model to induce mild TBI in mice, Lim et al. demonstrated an inability for injured animals to maintain consistent waking during the dark (active) phase, a chronic fragmented sleep pattern with an increase in the number of wake to sleep transitions, and a decrease in sleep bout duration [172]. A similar study of moderate TBI in rats also reported a significant increase in wake to NREM transitions and a corresponding decrease in sleep/wake bout lengths that persisted chronically out to 29 days post-injury [173]. Both studies implicated deficits in the orexin system, which is consistent with the destabilization of waking during the active period [174]. These animal studies, amongst others, illustrate the similarities in excessive daytime sleepiness found between this model and civilian [175, 176] and military [177] cases of TBI. Integrating sleep measures into blast tube experiments, as well as other animal models of TBI, is critical to uncovering the link between neural injury and the sleep/wake system, as well as designing new treatment options for service members.
Similar to the TBI field, rodent models of stress and PTSD largely ignore the effects of pre-existing sleep dysregulation, or sleep alterations in response to stress exposure, on disorder severity or treatment outcome. A recent review authored by researchers at the Walter Reed Army Institute of Research (WRAIR) highlights the myriad methods used to model PTSD, as well as their respective strengths and weaknesses (Lowery-Gionta et al., unpublished data). Missing from these models are the integral ties to sleep and the role of pre-existing sleep disorders. Another timely review on the status of animal models of PTSD concluded that sleep alterations, amongst other metrics, should be included to increase translation of the results to human populations [178]. In fact, few studies have evaluated the impact on sleep architecture using stress or PTSD models, relative to the larger volume of literature. Initial publications using single bouts of social defeat stress in rats reported significant increases in acute NREM following conflict, regardless of winner or loser of the bout [179–182]. Sleep fragmentation was also increased 4 days after conflict, as compared to baseline and one day post-conflict, suggesting a time-dependent dysregulation of sleep architecture [182]. Limited to no changes in REM were found in these studies. A mouse study using a ten day chronic social defeat stress (CSDS) model found statistical increases in total REM time and number of bouts, as well as increases in NREM, during the ten days of CSDS, with only the increase in REM bouts remaining elevated following the protocol during the recovery phase, indicative of sleep fragmentation [183]. Pretreatment with a kappa opioid receptor antagonist prevented the increase in REM sleep bouts (reduced sleep fragmentation), alterations in circadian body temperature changes, as well as blocked alterations of the Clock gene mPer2 in mesolimbic structures, illustrating the benefit of integrating sleep markers into behavioral and treatment study designs.
Using the single-prolonged stress (SPS) model in rats, acute increases in total REM time and number of transitions to REM were reported in the active dark phase (lights off) on the day of SPS exposure, which then returned to baseline levels out to day 9 post-exposure [184]. The number of REM sleep bouts trended downward from days 5 to 9 during the sleep phase, whereas REM bout duration statistically increased, suggesting a time-evolving consolidation of REM sleep following exposure [184]. In another study using SPS in rats, an acute increase in waking was seen during the lights on sleep phase that coincided with statistically significant decreases in NREM and REM sleep [185]. Immediately following during the dark phase, both NREM and REM rebounded above baseline levels; REM sleep exhibited the largest increase, similar to findings from the Vanderheyden (2015) report. During the light phase, acute increases in body temperature were also reported during all phases of the sleep/wake cycle. Collectively, these studies highlight the ability of continuous EEG monitoring in rodent models of stress and PTSD to detect both immediate and delayed changes in sleep architecture that may offer clues to PTSD symptom development, and offer new translatable metrics to assess treatment efficacy.
Wireless telemetry
Sleep disruption paradigms (e.g. ECD) must be regularly employed prior to experimental manipulations to better simulate the military environment, whereas new wireless technology and software solutions are required to lower the entry burden into this field and promote more widespread adoption. Doing so will promote more ethologically relevant study designs with greater military relevance. These data will further our understanding of the role of sleep and sleep disruption in neural injury and recovery and guide development of more effective treatment regimens for service members.
Most sleep studies still rely on “tethered” EEG data acquisition systems that require animals to be removed from their homecages at set experimental intervals, placed in a recording chamber and connected to an amplifier via surgically implanted headmounts that allow access to brain and muscle activity. The majority of these tethered studies only take snapshots of an animals sleep/wake state during discrete time windows without accounting for changes that could be occurring during other periods of the day or night, missing large amounts of data in the process. Relying on specialized recording chambers limit study environments, particularly military relevant models and when using interactive studies such as the CSDS model of stress and depression. To circumvent these issues, fully implantable EEG telemetry systems are gaining traction across the research community (reviewed by Lundt et al. [186]). In-house military laboratories were an early adopter of the technology, and telemetry systems are routinely used to monitor recurring nerve-agent induced seizure activity [187–189], amongst other studies throughout the US Army Medical Command. These implants rely on small integrated batteries to wirelessly stream EEG, EMG and body temperature data to a nearby acquisition system, negating the need to physically connect the animals during each recording session. Importantly, continuous physiological sleep monitoring can be performed in the animals’ homecage, as well as in social environments [183, 190], without worrying about entangled wires. Unfortunately, their long-term use is constrained by battery life (~1.5 months, mouse; ~3 months, rat) and their physical size may also result in nuanced changes to animal behavior that can impact results in unanticipated ways [183]. That said, body temperature measurements streamed from these devices proved to be significantly altered in two of the previously discussed sleep studies [183, 185]; these findings would not be possible using traditional tethered EEG approaches and highlight the need for advanced technology to further understand sleeps’ role in brain disorders.
Dramatic reductions in miniaturized electronic platforms and advances in radio-frequency technology have the potential to revolutionize this field [191–194], as was demonstrated by wireless operation of implantable µLED’s for optogenetic studies [195]. Adapting this technology to include advanced electronics capable of wirelessly transmitting EEG, EMG, and body temperature would allow long-term continuous monitoring of sleep/wake states from baseline through recovery (Good and Lu et al., unpublished data). Advanced software capable of semi-automating the data processing and analysis pipeline will allow additional exploration of changes in EEG from these comprehensive datasets. Work is underway to integrate advanced EEG and sleep metrics into the predator exposure model of PTSD to understand the psychological ramifications of threat-to-life on sleep architecture [196]. Moving forward, our goal is to further integrate novel wireless technology into military relevant sleep disruption study designs for enhanced translation of results to service members.
Sleep and performance
At present, there is a paradox in military regulations for optimal and minimal sleep amounts. The Leader’s Guide to Soldier Health and Fitness developed by the Office of the Army Surgeon General (ATP 6–22.5) recommends a Soldier sleep >7 h per night whenever possible. This regulation also recommends >9 h per night in preparation for episodes of inadequate sleep. In contrast, the minimum amount of sleep a US Army Soldier is required to achieve during field training exercises is >4 h per night per Army regulation TR 350–6, for example. A recent analysis of sleep amounts using wrist-worn actigraphy in armored (Army) battalions revealed the commonality of <5 h of sleep per night during training (at the National Training Center near Death Valley, CA) and deployment (Kuwait; Skeiky et al., unpublished data). In most cases, these Soldiers did not have consolidated sleep of <5 h per night but rather multiple bouts of <2 h. Twenty years before this study, the same group of researchers demonstrated a strong exponential relationship between nighttime sleep amounts and next-day performance on an artillery exercise at the same training location (National Training Center). For every hour of sleep lost, combat effectiveness degraded by 15–25% with Soldiers being at 15% total effectiveness with 4 hours per night (the current minimum per Army regulation TR 350-6) [33]. Reductions in combat effectiveness, as evidenced by cognitive performance [20] and marksmanship [197–199] decrements, as well as musculoskeletal symptoms [20], have been found following reduced or deprived sleep in recruits and trained warfighters. Whereas individual differences in how sleep loss impacts alertness and performance have been reported [200–202], only recently has the military begun exploring means to leverage these findings.
Sleep inertia is the transition period between sleeping and waking in which individuals display decrements in reaction performance and alertness, and can be seen as a reorganization of neural activity and connectivity patterns upon awakening from sleep states [203]. Rapid transitions from states of sleep to wakefulness across the nighttime and in the morning after a full night of sleep can degrade vigilance/alertness, as demonstrated by Balkin et al. in a military science laboratory [204], and are important aspects of human physiology to consider for military planning purposes. In fact, performance decrements resulting from sleep inertia experienced in the middle of a nighttime sleep episode after a few hours of sleep is similar to performance decrements under total sleep deprivation (>24 h extended wakefulness) [204]. Sleep inertia is maximal in the middle of the biological night when the circadian signal to sleep is highest, even if sleep requirements via the homeostatic system are met [205]. Sleep inertia is thought to be an operationally significant, yet overlooked problem. In deployed situations, service members are often rapidly awoken from sleep and must immediately attend to mission requirements that involve high levels of vigilance and life-or-death decision making (e.g. defensively returning fire following a base attack). To mitigate the effects of sleep inertia, military researchers at WRAIR demonstrated that performance decrements can be minimized through immediate caffeine intake upon waking [206]. It has also been found that sustained low-level caffeine consumption, combined with short naps, may be enough to stabilize performance and minimize sleep inertia under conditions of chronic sleep loss similar to that experienced in operating environments [207].
The ability to “bank sleep” to optimize performance under partial/total sleep deprivation, as well as enhance performance under normal conditions, is a burgeoning area of research that originated out of a military science laboratory (WRAIR) [72]. In Rupp et al., the authors showed that 10 h of time in bed (sleep extension) improved alertness and performance during subsequent sleep restriction (3 h per night) and recovery, as compared to individuals who slept their normal duration prior to sleep restriction. Mah et al. determined that when high-level collegiate basketball players extended their sleep duration by ~2 h (similar to [72], the players’ sprint times and shooting accuracies statistically improved, and their reaction times decreased, as measured using the psychomotor vigilance test (PVT) [208]. During partial sleep deprivation, napping has also been shown to reduce heart rate and boost short-term memory in athletes [209], as well as improve endurance in runners [210]. In an observational study of habitual sleep amounts in Reserve Officers’ Training Corps (ROTC) tactical athletes, measured through wrist-worn actigraphy, Ritland et al. determined that longer nighttime sleep durations correlated with increased motivation levels and better cognitive processing performance compared to military cadets with shorter sleep durations [211]; although other performance improvements were not found [211]. In another study, the same group experimentally extended sleep duration in ROTC tactical athletes by ~1.5 h and demonstrated statistical improvements in reaction time, athletic performance and motivation [212]. Importantly, some of these improvements persisted for up to four days following the end of sleep extension [212]. Collectively, these studies suggest that extending or “banking” sleep prior to operating missions in which sleep will be restricted could confer critical improvements in reaction time and performance for service members. Given the encouraging results from these studies, additional intervention-based studies of sleep extension are warranted, as the current state of the science regarding sleep duration and long-term health [213–215] and performance [216, 217] is heavily driven by large-scale observational/epidemiological studies of the general population.
Psychomotor vigilance test
Despite what occurs in field training environments, sleep research in military laboratories have shown that <5 h sleep per night is not sufficient to sustain performance [72, 218]. These same laboratories have also shown that countermeasures used to stabilize performance across sleep loss are ineffective after 3 days of <6 h sleep per night [218]. Broadly, the temporal relationship between sleep loss and performance decrements has been operationalized in laboratory studies using the PVT. The PVT is a computerized test of reaction time with a means to track inter-individual variability in neurobehavioral performance in real-time [219]. The test has high ecological reliability and validity [220] and translates very well to current military demands in numerous operating scenarios, including watch duty aboard US Navy ships [221]; it can be completed to monitor real-time vigilance in any environment, harsh or benign, with the availability of a smartphone-based version funded by the Army [222]. Current warfare, termed the multi-domain battle space, is one of cognition not attrition. Service members must endure long periods of sustained attention/vigilance during low-level, monotonous activities (e.g., driving in a convoy long distances from one operating base to another), knowing that the current weapon system of choice of our nation’s enemies are IED’s placed along roadsides or rocket-propelled grenades that can injure and kill numerous individuals at a moment’s notice. In this scenario, the PVT is ideal for monitoring ones reaction time and could serve as an early predictor of fatigue or loss of attention that could be detrimental to the entire unit.
With the PVT, researchers have been able to show temporal changes in performance across total sleep deprivation (forced wakefulness) and recovery sleep in order to determine “tipping points” in neurobehavioral decline [223]. In general, performance is stabilized across 24 h forced wakefulness [223] followed by a precipitous decline with >24 h of forced wakefulness [223, 224]. This performance decline can be protected, in part, by sleep extension prior to sleep loss [72], or having a PVT session coincide with a circadian-driven peak in alertness during increasing homeostatic pressure to sleep [224]. Finally, PVT performance is also predictive of the extent of recovery from total sleep deprivation [223].
The PVT has also been widely used to determine inter-individual variability in resiliency (or sensitivity) to sleep loss under both conditions of total sleep deprivation [223–225] and chronic sleep restriction [218, 226]. Whereas sleep extension prior to sleep loss helps to protect against performance decline, in general [72], inter-individual variability in resiliency to sleep loss is largely biologically regulated. Genetic association studies in humans revealed that select genotypes of an adenosine receptor (A2A) mediate the homeostatic drive to sleep [227], whereas select genotypes of dopamine metabolism (COMT) [228], and select genotypes of a clock gene (PER) known to mediate circadian timekeeping [229], can confer enhanced resiliency or sensitivity to varied conditions of sleep loss (total sleep deprivation, chronic sleep restriction; [230–235].
Resiliency to sleep loss can also be pharmacologically regulated. Caffeine’s half-life ranges from 3 to 7 h in adults [236], and can protect against performance decline during sleep loss in a dose- [237] and time-dependent manner [238]. Studies demonstrated that the ability of caffeine to sustain vigilance/alertness is genetically dependent. Habitual caffeine intake has been associated with reduced (self-rated) sleep quality in caffeine-sensitive individuals, but not in caffeine-insensitive individuals [239], regulated in part by single-nucleotide polymorphisms of the adenosine A2A receptor gene (ADORA2A; [239]. Further, ADORA2A polymorphisms not only mediate the extent of habitual caffeine use [240], but also mediate the extent of insomnia-like changes in the EEG induced by caffeine intake [231, 239]. Select genotypes of the adenosine receptor that additionally intersect with genotypes conferring sleep resiliency also confer enhanced (or reduced) sensitivity to the ability of caffeine to stabilize performance across sleep loss [231, 232]. However, it has been shown that the performance-enhancing effects of caffeine are ineffective for stabilizing performance under chronic sleep restriction [218] (<5 h sleep per night) which is the most common type of sleep loss that service members are exposed to (Choynowski et al., unpublished data) [241].
With this information, we have developed a working model to compartmentalize individual variability in sensitivity to sleep loss to determine “fitness for military duty” (Fig. 3). In our model, A1 individuals (blue box) would be most suited to perform in military operations. These individuals would have a genetic predisposition that allows their performance to be stabilized with sleep loss, but also sensitive to the performance-enhancing actions of caffeine. B2 individuals (red box) would be least suited to perform military operations. These individuals would have a genetic predisposition that degrades performance across sleep loss, but also linked to high caffeine tolerance. A2 and B1 individuals (gray boxes) would be moderately suited for military operations. There individuals would have stabilized performance with sleep loss (B1) but with a genetic trade-off of high caffeine tolerance (A2). Since individual variability in performance with sleep loss is high, this lends for the ability of military commanders to capitalize on genetic heterogeneity for the benefit of combat assignment and effectiveness. In fact, the Defense Advanced Research Projects Agency is currently focused on identifying novel genetic targets in order to eliminate subject bias for the candidate selection process, as well as recognizing persons who otherwise would not have been identified using high-throughput gene sequencing techniques. Through partnerships with academia, industry, and government, the intent is to use these rapid and non-invasive high-throughput sequencing techniques to determine fitness for duty and select mission assignment in these populations through a better understanding of genes regulating sleep and circadian synchrony.
Fig. 3.
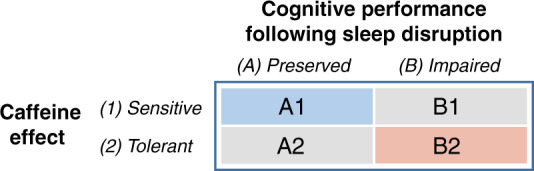
A working model of individual variation in the ability to perform in an operating environment under limited sleep conditions. The first principle is cognitive resilience to sleep disruption. Some individuals, due to biological modifiers, are able to preserve their level of vigilance and cognitive performance while sleep-deprived. The second principle is the degree of individual sensitivity to a caffeine countermeasure. This working model is thought to create a framework for group (unit-level) performance that can be optimized by stabilizing performance at the individual level and assigning duties accordingly
Caffeine and sleep medications for fatigue management
Because the military has thrived on 24 h shift work operations, it has a rich history of stimulant and depressant use to maintain alertness or consolidate sleep, respectively. For instance, “Go” medications such as dextroamphetamine (Dexedrine) were once used to counter fatigue, but are no longer authorized (AFI 11–202, v3; AFSOCSUP 28 JULY 2017). However, it is widely known that military personnel over-consume caffeine (usually through energy drinks) in order to counteract the negative consequences of shift work, which in turn, amplifies acute stress responses [242]. Caffeine abuse and overuse during training and combat is a chief reason why caffeine-dosing strategies were recently developed by Army research laboratories [238, 243, 244] and implemented into regulations (ATP 6–22.5). Caffeine, taken at the right time and in correct dosage, has the ability to improve performance on certain tasks. For instance, 200 and 300 mg of caffeine improved marksmanship accuracy and sighting time, as compared to placebo and 100 mg, in sleep-deprived Navy SEAL trainees during Hell Week. The 200 mg dose group showed rapid improvements at 1 h, whereas the 300 mg dose suggested improvements in accuracy could last past 8 h based on salivary caffeine levels at that time point [198]. Another reason why caffeine is often abused and overused by military personnel is that only select units, such as aviation [245], are permitted access to pharmaceutical-grade wake-promoting agents (modafinil [100–200 mg; Trade name: Provigil] and armodafinil [150 mg; Trade name: Nuvigil]), despite modafinil stabilizing neurobehavioral performance during simulated shift work in a military science laboratory [246]. Whereas in the general population modafinil and armodafinil are prescribed to treat daytime sleepiness co-morbid with narcolepsy [247] and shift work disorder [248], a narcolepsy diagnosis requires medical discharge from the military.
Stimulants and depressants also have the ability to shift sleep/wake rhythms. Independent of genetic regulation of caffeine use and psychoactive effects, one study discovered that caffeine intake (200 mg; equivalent to a 16 ounce energy drink) 3 h before bedtime phase-delayed human endocrine (melatonin) rhythms by 40 min [249] with similar extrapolated findings in human cell culture (osteosarcoma U20S; [249]) and a mouse model [250]. However, beyond elevating daytime alertness, caffeine appears unable to entrain endocrine rhythms, as demonstrated in blind individuals [251]. In humans, caffeine has been shown to alter clock-driven melatonin release [249], but more research is warranted in this area in order develop better wake- and sleep-pharmacologic dosing strategies sensitive to individual and occupational demands.
The SCN is the central circadian pacemaker and has projections onto the mesolimbic system [252], and dopamine signaling in the ventral tegmental area is clock-driven [253, 254]. In animal models, GABA-derived depressants (alcohol) and monoamine-derived stimulants (cocaine) can block the ability of the circadian system to phase-shift to photic and non-photic stimuli [158, 255, 256]. The psychoactive effects of commonly prescribed sleep aids on human circadian rhythms are mild. Trazadone, a tricyclic antidepressant, and zolpidem, a non-benzodiazepine, have both been shown to marginally advance human circadian rhythms of core body temperature [257] and improve re-entrainment to shifted light-dark cycles [258] without affecting the circadian-controlled rhythm of REM sleep propensity [257].
Although research on the ability of vigilance-promoting nootropics and hypnotics to shorten/lengthen, phase-shift, and re-entrain human circadian-controlled rhythms is limited, it warrants further investigation within the context of military operations. Circadian desynchrony has recently been recognized as an operational threat in official military reports regarding future warfare, particularly when taking longer trans-meridian travel in the current geopolitical space into consideration, and more work is needed on means to counter this desynchronization.
Conclusions
There are several factors contributing to the lack of sufficient, restorative sleep in the military, including combat operations, shift work, and comorbidities of psychiatric disorders and TBI in service members. Although real-time physiological and psychological health data in active duty personnel during deployed military operations is sparse, sleep disturbances, namely insomnia and OSA, have been broadly studied pre- and post-deployment and across biological sex in medical treatment facilities [5, 101, 102]. It is critical to understand these substrates and mechanisms - both physiological and psychological in nature -underlying comorbidity of sleep and psychiatric disorders as a path forward for sleep in the military. Understanding inter-individual variability in resiliency to sleep loss and stress can determine “fitness for military duty,” and can assist in placing a service member in an optimized military occupational specialty (MOS) and schedule that minimizes operational and personal risk and maximizes group performance and safety. The military is in the best position possible for changing cultural attitudes about sleep compared to civilian organizations. There are published guidelines all military leaders must recognize (The Leader’s Guide to Soldier Health and Fitness developed by the Office of the Army Surgeon General (ATP 6–22.5)) that provide strategies for achieving >7 h of sleep and maintaining vigilance during combat for the >400,000 ADMP. The military also possess machine learning-based tools to predict and counteract fatigue (through caffeine supplementation) in real-time (2B-Alert) [244]. The intent of these initiatives is to protect against immediate physiological and psychological decline during combat exposure in the short-term and optimize physiological and psychological health across one’s military career and into retirement.
Future research directions
Long-term and comprehensive animal and human studies that include continuous sleep disruption designs are required to fully understand the physiological changes in neural circuitry following repeated circadian disruptions, and how these changes are intertwined with TBI and PTSD symptoms, amongst other neurological disorders. Studies of this nature must include numerous control groups that track the entire time-course of the experimental designs (and stages of the military life cycle), including matched healthy controls, circadian disrupted-only subjects and trauma exposed-only subjects; in order to properly dissect the role of pre-existing sleep disorders in disease severity and treatment outcome, as well as the restorative and protective effects of sleep following neurological insult and traumatic stress exposure. Using advanced technology capable of tracking physiology and behavior and integrating real-time countermeasures for many months in military personnel (e.g., Army’s 2B-alert) is critical to achieving these goals. Future research efforts will continue to focus on inter-individual variability, rather than group (unit-level) performance, in order to clearly identify individuals who are most sensitive and resilient to environmental stress and then use appropriately timed countermeasures in order to stabilize performance. Success of these comprehensive studies will additionally require consistent commitment from funding agencies, military organization leaders, academia and industry in order to realize the long-term benefits to help service members optimize their physiological and psychological health in the short-term (combat) and long-term (military career and retirement) from this approach. Lastly, it is our hope and anticipation that future iterations of animal models such as the CSDS [259], single prolonged stress, and blast TBI protocols, amongst other models of neurological disorders [178], will integrate sleep more readily into study designs.
Funding and disclosure
CHG is supported by a Laboratory University Collaboration Initiative (LUCI) Fellowship sponsored by the Office of the Under Secretary of Defense for Research & Engineering. AJB and VFC are supported by the Military Operational Medicine Research Program. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The authors declare no competing interests.
Acknowledgements
We thank Courtney Campbell and Folarin Adewale for their technical assistance in constructing this manuscript, as well as Summer Abdoh and Amy Wegener for illustrative support.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Allison J. Brager, Vincent F. Capaldi, Vincent Mysliwiec
References
- 1.Ulmer CS, et al. Associations between sleep difficulties and risk factors for cardiovascular disease in veterans and active duty military personnel of the iraq and afghanistan conflicts. J Behav Med. 2015;38:544–55. doi: 10.1007/s10865-015-9627-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson IG, et al. Alcohol use and alcohol-related problems before and after military combat deployment. JAMA. 2008;300:663–75. doi: 10.1001/jama.300.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mysliwiec V, et al. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36:167–74. doi: 10.5665/sleep.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mysliwiec V, et al. Trauma associated sleep disorder: A proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and rem without atonia in trauma survivors. J Clin Sleep Med. 2014;10:1143–8. doi: 10.5664/jcsm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capener DC, Brock MS, Hansen SL, Matsangas P, Mysliwiec V. An initial report of sleep disorders in women in the U.S. Military. Mil Med. 2018;183:e266–e271. doi: 10.1093/milmed/usx116. [DOI] [PubMed] [Google Scholar]
- 6.Iber C & Iber C. The aasm manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Vol. 1 IL: American Academy of Sleep Medicine Westchester; 2007.
- 7.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (rmtg), a gabaergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SR, et al. The rostromedial tegmental nucleus is essential for non-rapid eye movement sleep. PLoS Biol. 2018;16:e2002909. doi: 10.1371/journal.pbio.2002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 10.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–19. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 12.Miller K, Danner F, Staten R. Relationship of work hours with selected health behaviors and academic progress among a college student cohort. J Am Coll Health. 2008;56:675–9. doi: 10.3200/JACH.56.6.675-679. [DOI] [PubMed] [Google Scholar]
- 13.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 14.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- 15.Watson NF, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the american academy of sleep medicine and sleep research society. Sleep. 2015;38:843–4. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jan I. Maby POK, William W, Teresa D. Pearce, David N. Cowan, Amanda L. Kelley, Camille B. Wada, et al. Accession medical standards analysis and research activity (amsara) 2017 annual report. Silver Spring, MD: Walter Reed Army Institute of Research; 2018.
- 17.Miller NL, Shattuck LG. Sleep patterns of young men and women enrolled at the united states military academy: results from year 1 of a 4-year longitudinal study. Sleep. 2005;28:837–41. doi: 10.1093/sleep/28.7.837. [DOI] [PubMed] [Google Scholar]
- 18.Miller NL, Tvaryanas AP, Shattuck LG. Accommodating adolescent sleep-wake patterns: the effects of shifting the timing of sleep on training effectiveness. Sleep. 2012;35:1123–36. doi: 10.5665/sleep.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller NL, Shattuck LG, Matsangas P. Longitudinal study of sleep patterns of united states military academy cadets. Sleep. 2010;33:1623–31. doi: 10.1093/sleep/33.12.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shattuck NL, Matsangas P. Operational assessment of the 5-h on/10-h off watchstanding schedule on a us navy ship: sleep patterns, mood and psychomotor vigilance performance of crewmembers in the nuclear reactor department. Ergonomics. 2016;59:657–64. doi: 10.1080/00140139.2015.1073794. [DOI] [PubMed] [Google Scholar]
- 21.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 22.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. an opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. BMC Psychiatry. 2016;16:375. doi: 10.1186/s12888-016-1075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146:105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 25.Gehrman P, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36:1009–18. doi: 10.5665/sleep.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HE, et al. Pre-deployment insomnia is associated with post-deployment post-traumatic stress disorder and suicidal ideation in us army soldiers. Sleep. 2019; 42, zsy229, 10.1093/sleep/zsy229. [DOI] [PMC free article] [PubMed]
- 27.Taylor DJ, et al. Prevalence, correlates, and predictors of insomnia in the us army prior to deployment. Sleep. 2016;39:1795–806. doi: 10.5665/sleep.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes JM, Ulmer CS, Gierisch JM, Nicole Hastings S, Howard MO. Insomnia in united states military veterans: an integrated theoretical model. Clin Psychol Rev. 2018;59:118–25. doi: 10.1016/j.cpr.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirshkowitz M, et al. National sleep foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Krueger PM, Friedman EM. Sleep duration in the united states: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luxton DD, et al. Prevalence and impact of short sleep duration in redeployed oif soldiers. Sleep. 2011;34:1189–95. doi: 10.5665/SLEEP.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorne D, Genser S, Sing H, & Hegge F. Plumbing human performance limits during 72 h of high task load. In: Proceedings of the twenty-fourth nato defense research group seminar on the human as limiting element in military systems. Brussels: NATO. Vol. I. 1983. p. 17–40.
- 33.Belenky G et al. The effects of sleep deprivation on performance during continuous combat operations. In: Food components to enhance performance: an evaluation of potential performance-enhancing food componenets for operational rations. Washington, DC: National Academies Press (US); 1994. p. 127–35. [PubMed]
- 34.Brown S, Matsangas P & Shattuck NL. Improved sleep hygiene and psychomotor vigilance performance following crew shift to a circadian-based watch schedule. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. p. 1167–71. Los Angeles, CA: SAGE Publications Sage CA.
- 35.Shattuck NL & Matsangas P. Comparison of the 3/9 and 6/6 watchstanding schedules for crewmembers of a US Navy destroyer. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. p. 881–5. Los Angeles, CA: SAGE Publications Sage CA.
- 36.Matsangas P & Shattuck NL. The effect of ship department on crew sleep patterns and psychomotor vigilance performance. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. p. 1182–6. Los Angeles, CA: SAGE Publications Sage CA.
- 37.Goh VH, Tong TY, Lim CL, Low EC, Lee LK. Circadian disturbances after night-shift work onboard a naval ship. Mil Med. 2000;165:101–5. [PubMed] [Google Scholar]
- 38.Caldwell JL, Gilreath SR. Work and sleep hours of u.S. Army aviation personnel working reverse cycle. Mil Med. 2001;166:159–66. [PubMed] [Google Scholar]
- 39.Peterson AL, Goodie JL, Satterfield WA, Brim WL. Sleep disturbance during military deployment. Mil Med. 2008;173:230–5. doi: 10.7205/milmed.173.3.230. [DOI] [PubMed] [Google Scholar]
- 40.Shan Z, et al. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large us cohorts of female nurses. BMJ. 2018;363:k4641. doi: 10.1136/bmj.k4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Lauren S, Chang BP, Shechter A. Objective food intake in night and day shift workers: a laboratory study. Clocks Sleep. 2018;1:42–49. doi: 10.3390/clockssleep1010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian J, Morris CJ, Caputo R, Garaulet M & Scheer F. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int J Obes. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 43.Morris CJ, et al. Paradoxical post-exercise responses of acylated ghrelin and leptin during a simulated night shift. Chronobiol Int. 2010;27:590–605. doi: 10.3109/07420521003663819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bescos R, et al. Four days of simulated shift work reduces insulin sensitivity in humans. Acta Physiol (Oxf) 2018;223:e13039. doi: 10.1111/apha.13039. [DOI] [PubMed] [Google Scholar]
- 45.Wefers J, et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci USA. 2018;115:7789–94. doi: 10.1073/pnas.1722295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vetter C, et al. Night shift work, genetic risk, and type 2 diabetes in the uk biobank. Diabetes Care. 2018;41:762–9. doi: 10.2337/dc17-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson BJ, Stock MS, Banuelas VK. Effects of accumulating work shifts on performance-based fatigue using multiple strength measurements in day and night shift nurses and aides. Hum Factors. 2017;59:346–56. doi: 10.1177/0018720816677814. [DOI] [PubMed] [Google Scholar]
- 49.Lewis Shattuck N, Matsangas P, Moore J, Wegemann L. Prevalence of musculoskeletal symptoms, excessive daytime sleepiness, and fatigue in the crewmembers of a u.S. Navy ship. Mil Med. 2016;181:655–62. doi: 10.7205/MILMED-D-15-00279. [DOI] [PubMed] [Google Scholar]
- 50.Nakao T, et al. The impact of night-shift work on platelet function in healthy medical staff. J Occup Health. 2018;60:324–32. doi: 10.1539/joh.2018-0027-FS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hublin C, et al. Shift-work and cardiovascular disease: A population-based 22-year follow-up study. Eur J Epidemiol. 2010;25:315–23. doi: 10.1007/s10654-010-9439-3. [DOI] [PubMed] [Google Scholar]
- 52.Wirth MD, et al. Association of shiftwork and immune cells among police officers from the buffalo cardio-metabolic occupational police stress study. Chronobiol Int. 2017;34:721–31. doi: 10.1080/07420528.2017.1316732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assari S. Veterans and risk of heart disease in the united states: A cohort with 20 years of follow up. Int J Prev Med. 2014;5:703–9. [PMC free article] [PubMed] [Google Scholar]
- 54.Molzof Hylton E., Prapanjaroensin Aoyjai, Patel Vivek H., Mokashi Mugdha V., Gamble Karen L., Patrician Patricia A. Misaligned core body temperature rhythms impact cognitive performance of hospital shift work nurses. Neurobiology of Learning and Memory. 2019;160:151–159. doi: 10.1016/j.nlm.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Muzio M, et al. Not only a problem of fatigue and sleepiness: changes in psychomotor performance in italian nurses across 8-h rapidly rotating shifts. J Clin Med. 2019;8:e47. [DOI] [PMC free article] [PubMed]
- 56.Mollart L, Skinner VM, Newing C, Foureur M. Factors that may influence midwives work-related stress and burnout. Women Birth. 2013;26:26–32. doi: 10.1016/j.wombi.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 57.McCauley P, et al. Dynamic circadian modulation in a biomathematical model for the effects of sleep and sleep loss on waking neurobehavioral performance. Sleep. 2013;36:1987–97. doi: 10.5665/sleep.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bokenberger K, et al. Shift work and risk of incident dementia: A study of two population-based cohorts. Eur J Epidemiol. 2018;33:977–87. doi: 10.1007/s10654-018-0430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cedernaes J, et al. Candidate mechanisms underlying the association between sleep-wake disruptions and alzheimer’s disease. Sleep Med Rev. 2017;31:102–11. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimomura K, Menaker M. Light-induced phase shifts in tau mutant hamsters. J Biol Rhythms. 1994;9:97–110. doi: 10.1177/074873049400900201. [DOI] [PubMed] [Google Scholar]
- 61.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa G, Ghirlanda G, Minors DS, Waterhouse JM. Effect of bright light on tolerance to night work. Scand J Work Environ Health. 1993;19:414–20. doi: 10.5271/sjweh.1453. [DOI] [PubMed] [Google Scholar]
- 63.Stone JE, et al. Temporal dynamics of circadian phase shifting response to consecutive night shifts in healthcare workers: Role of light-dark exposure. J Physiol. 2018;596:2381–95. doi: 10.1113/JP275589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sletten TL, et al. Randomised controlled trial of the efficacy of a blue-enriched light intervention to improve alertness and performance in night shift workers. Occup Environ Med. 2017;74:792–801. doi: 10.1136/oemed-2016-103818. [DOI] [PubMed] [Google Scholar]
- 65.Bajaj A, Rosner B, Lockley SW, Schernhammer ES. Validation of a light questionnaire with real-life photopic illuminance measurements: the harvard light exposure assessment questionnaire. Cancer Epidemiol Biomark Prev. 2011;20:1341–9. doi: 10.1158/1055-9965.EPI-11-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lockley SW. Safety considerations for the use of blue-light blocking glasses in shift-workers. J Pineal Res. 2007;42:210–1. doi: 10.1111/j.1600-079X.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 67.Stothard ER, et al. Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr Biol. 2017;27:508–13. doi: 10.1016/j.cub.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butler MP, Rainbow MN, Rodriguez E, Lyon SM, Silver R. Twelve-hour days in the brain and behavior of split hamsters. Eur J Neurosci. 2012;36:2556–66. doi: 10.1111/j.1460-9568.2012.08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caldelas I, Poirel VJ, Sicard B, Pevet P, Challet E. Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal arvicanthis ansorgei. Neuroscience. 2003;116:583–91. doi: 10.1016/s0306-4522(02)00654-1. [DOI] [PubMed] [Google Scholar]
- 70.Coomans CP, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27:1721–32. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 71.Mizutani H, Tamagawa-Mineoka R, Minami Y, Yagita K, Katoh N. Constant light exposure impairs immune tolerance development in mice. J Dermatol Sci. 2017;86:63–70. doi: 10.1016/j.jdermsci.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 72.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–21. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tahara Y, et al. Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Sci Rep. 2015;5:11417. doi: 10.1038/srep11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weibel L, Maccari S, Van Reeth O. Circadian clock functioning is linked to acute stress reactivity in rats. J Biol Rhythms. 2002;17:438–46. doi: 10.1177/074873002237138. [DOI] [PubMed] [Google Scholar]
- 75.Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute hpa axis responses, heart rate, and mood changes to psychosocial stress (tsst) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–92. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Reebs SG, Mrosovsky N. Large phase-shifts of circadian rhythms caused by induced running in a re-entrainment paradigm: The role of pulse duration and light. J Comp Physiol A. 1989;165:819–25. doi: 10.1007/BF00610880. [DOI] [PubMed] [Google Scholar]
- 77.Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of syrian hamsters: entrainment and phase response curve. J Biol Rhythms. 1989;4:39–48. doi: 10.1177/074873048900400103. [DOI] [PubMed] [Google Scholar]
- 78.Mrosovsky N, Ralph MR. Phase response curve to anisomycin in tau mutant hamsters. Experientia. 1992;48:875–7. doi: 10.1007/BF02118423. [DOI] [PubMed] [Google Scholar]
- 79.Mrosovsky N, Salmon PA, Menaker M, Ralph MR. Nonphotic phase shifting in hamster clock mutants. J Biol Rhythms. 1992;7:41–49. doi: 10.1177/074873049200700104. [DOI] [PubMed] [Google Scholar]
- 80.Scheibler E, Wollnik F. Interspecific contact affects phase response and activity in desert hamsters. Physiol Behav. 2009;98:288–95. doi: 10.1016/j.physbeh.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 81.Ralph MR, Mrosovsky N. Behavioral inhibition of circadian responses to light. J Biol Rhythms. 1992;7:353–9. doi: 10.1177/074873049200700408. [DOI] [PubMed] [Google Scholar]
- 82.Klerman EB, et al. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;274:R991–996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- 83.Youngstedt SD, et al. Circadian phase-shifting effects of bright light, exercise, and bright light + exercise. J Circadian Rhythms. 2016;14:2. doi: 10.5334/jcr.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Youngstedt SD, Elliott JA & Kripke DF. Human circadian phase-response curves for exercise. J. Physiol. 2019;597:2253–68. [DOI] [PMC free article] [PubMed]
- 85.Grone BP, et al. Acute light exposure suppresses circadian rhythms in clock gene expression. J Biol Rhythms. 2011;26:78–81. doi: 10.1177/0748730410388404. [DOI] [PubMed] [Google Scholar]
- 86.Hermans EJ, et al. How the amygdala affects emotional memory by altering brain network properties. Neurobiol Learn Mem. 2014;112:2–16. doi: 10.1016/j.nlm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 87.Ruby NF, et al. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci USA. 2008;105:15593–8. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shokri-Kojori E, et al. Beta-amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci USA. 2018;115:4483–8. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leproult R, Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305:2173–4. doi: 10.1001/jama.2011.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spiegel K, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol. 2000;279:R874–883. doi: 10.1152/ajpregu.2000.279.3.R874. [DOI] [PubMed] [Google Scholar]
- 91.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–70. [PubMed] [Google Scholar]
- 92.Leproult R, Van Reeth O, Byrne MM, Sturis J, Van Cauter E. Sleepiness, performance, and neuroendocrine function during sleep deprivation: Effects of exposure to bright light or exercise. J Biol Rhythms. 1997;12:245–58. doi: 10.1177/074873049701200306. [DOI] [PubMed] [Google Scholar]
- 93.Van Cauter E, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young men. J Clin Invest. 1997;100:745–53. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trivedi MS, Holger D, Bui AT, Craddock TJA, Tartar JL. Short-term sleep deprivation leads to decreased systemic redox metabolites and altered epigenetic status. PLoS One. 2017;12:e0181978. doi: 10.1371/journal.pone.0181978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aho V, et al. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci Rep. 2016;6:24828. doi: 10.1038/srep24828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van den Berg R, et al. A single night of sleep curtailment increases plasma acylcarnitines: novel insights in the relationship between sleep and insulin resistance. Arch Biochem Biophys. 2016;589:145–51. doi: 10.1016/j.abb.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 97.Weljie AM, et al. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc Natl Acad Sci USA. 2015;112:2569–74. doi: 10.1073/pnas.1417432112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davies SK, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci USA. 2014;111:10761–6. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilms B, et al. Sleep loss disrupts morning-to-evening differences in human white adipose tissue transcriptome. J Clin Endocrinol Metab. 2019;104:1687–96. doi: 10.1210/jc.2018-01663. [DOI] [PubMed] [Google Scholar]
- 100.Troxel WM, et al. Sleep in the military: promoting healthy sleep among u.S. Servicemembers. Rand Health Q. 2015;5:19. [PMC free article] [PubMed] [Google Scholar]
- 101.Mysliwiec V, et al. Sleep disorders in us military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144:549–57. doi: 10.1378/chest.13-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mysliwiec V, et al. Comorbid insomnia and obstructive sleep apnea in military personnel: correlation with polysomnographic variables. Mil Med. 2014;179:294–300. doi: 10.7205/MILMED-D-13-00396. [DOI] [PubMed] [Google Scholar]
- 103.Collen J, Orr N, Lettieri CJ, Carter K, Holley AB. Sleep disturbances among soldiers with combat-related traumatic brain injury. Chest. 2012;142:622–30. doi: 10.1378/chest.11-1603. [DOI] [PubMed] [Google Scholar]
- 104.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12:185–95. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wickwire EM, et al. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a national working group. Neurotherapeutics. 2016;13:403–17. doi: 10.1007/s13311-016-0429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoge CW, et al. Mild traumatic brain injury in u.S. Soldiers returning from iraq. N Engl J Med. 2008;358:453–63. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 107.Germain A, McKeon AB, Campbell RL. Sleep in ptsd: conceptual model and novel directions in brain-based research and interventions. Curr Opin Psychol. 2017;14:84–89. doi: 10.1016/j.copsyc.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 108.Pace-Schott EF, Germain A, Milad MR. Sleep and rem sleep disturbance in the pathophysiology of ptsd: the role of extinction memory. Biol Mood Anxiety Disord. 2015;5:3. doi: 10.1186/s13587-015-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Capaldi II VF & Capaldi M. Military psychologists’ desk reference. New York: Oxford University Press; 2013. p. 246–50.
- 110.Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401. doi: 10.1007/s11920-013-0401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hermans LWA, et al. Sleep EEG characteristics associated with sleep onset misperception. Sleep Med. 2019;57:70–79. doi: 10.1016/j.sleep.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 112.Maes J, et al. Sleep misperception, eeg characteristics and autonomic nervous system activity in primary insomnia: a retrospective study on polysomnographic data. Int J Psychophysiol. 2014;91:163–71. doi: 10.1016/j.ijpsycho.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 113.Centers for Disease Control and Prevention (CDC). Energy drink consumption and its association with sleep problems among u.S. Service members on a combat deployment - Afghanistan, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:895–8. [PubMed] [Google Scholar]
- 114.Lincoln ML, Moore RS, Ames GM. Sleep disturbances after deployment: National guard soldiers’ experiences and strategies. Sleep Health. 2018;4:377–83. doi: 10.1016/j.sleh.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller MB, DiBello AM, Carey KB, Pedersen ER. Insomnia moderates the association between alcohol use and consequences among young adult veterans. Addict Behav. 2017;75:59–63. doi: 10.1016/j.addbeh.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McLellan TM, Riviere LA, Williams KW, McGurk D & Lieberman HR. Caffeine and energy drink use by combat arms soldiers in afghanistan as a countermeasure for sleep loss and high operational demands. Nutr Neurosci. 2018: 1–10. [Epub ahead of print]. [DOI] [PubMed]
- 117.LaJambe CM, Kamimori GH, Belenky G, Balkin TJ. Caffeine effects on recovery sleep following 27 h total sleep deprivation. Aviat Space Environ Med. 2005;76:108–13. [PubMed] [Google Scholar]
- 118.Stephens MB, Attipoe S, Jones D, Ledford CJ, Deuster PA. Energy drink and energy shot use in the military. Nutr Rev. 2014;72(Suppl 1):72–77. doi: 10.1111/nure.12139. [DOI] [PubMed] [Google Scholar]
- 119.Killgore WD, et al. Effects of dextroamphetamine, caffeine and modafinil on psychomotor vigilance test performance after 44 h of continuous wakefulness. J Sleep Res. 2008;17:309–21. doi: 10.1111/j.1365-2869.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 120.Nicholson AN, Smith PA, Stone BM, Bradwell AR, Coote JH. Altitude insomnia: studies during an expedition to the himalayas. Sleep. 1988;11:354–61. doi: 10.1093/sleep/11.4.354. [DOI] [PubMed] [Google Scholar]
- 121.Heinzer R, et al. Comparison of sleep disorders between real and simulated 3,450-m altitude. Sleep. 2016;39:1517–23. doi: 10.5665/sleep.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beaumont M, et al. Effect of zolpidem on sleep and ventilatory patterns at simulated altitude of 4,000 meters. Am J Respir Crit Care Med. 1996;153:1864–9. doi: 10.1164/ajrccm.153.6.8665047. [DOI] [PubMed] [Google Scholar]
- 123.Szymczak RK, et al. Subjective sleep quality alterations at high altitude. Wilderness Environ Med. 2009;20:305–10. doi: 10.1580/1080-6032-020.004.0305. [DOI] [PubMed] [Google Scholar]
- 124.Taylor DJ, et al. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 125.Wei Y, Blanken TF, Van Someren EJW. Insomnia really hurts: Effect of a bad night’s sleep on pain increases with insomnia severity. Front Psychiatry. 2018;9:377. doi: 10.3389/fpsyt.2018.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taylor SS, et al. Prevalence of and characteristics associated with insomnia and obstructive sleep apnea among veterans with knee and hip osteoarthritis. BMC Musculoskelet Disord. 2018;19:79. doi: 10.1186/s12891-018-1993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ulmer CS, et al. Associations between sleep difficulties and risk factors for cardiovascular disease in veterans and active duty military personnel of the iraq and afghanistan conflicts. J Behav Med. 2015;38:544–55. doi: 10.1007/s10865-015-9627-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34:859–67. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ulmer CS, et al. A comparison of sleep difficulties among iraq/afghanistan theater veterans with and without mental health diagnoses. J Clin Sleep Med. 2015;11:995–1005. doi: 10.5664/jcsm.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barone P, et al. The priamo study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in parkinson’s disease. Mov Disord. 2009;24:1641–9. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 131.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164:1888–96. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 132.Morin, C. M. Treatment manuals for practitioners. Insomnia: Psychological assessment and management. New York, NY, US: Guilford Press. (1993).
- 133.Taylor DJ, et al. Impact of cognitive behavioral therapy for insomnia disorder on sleep and comorbid symptoms in military personnel: A randomized clinical trial. Sleep. 2018; 41, zsy069, 10.1093/sleep/zsy069. [DOI] [PubMed]
- 134.Pulantara IW, Parmanto B, Germain A. Clinical feasibility of a just-in-time adaptive intervention app (irest) as a behavioral sleep treatment in a military population: feasibility comparative effectiveness study. J Med Internet Res. 2018;20:e10124. doi: 10.2196/10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dinges DF, Basner M, Ecker AJ, Baskin P & Johnston S. Effects of zolpidem and zaleplon on cognitive performance after emergent tmax and morning awakenings: a randomized placebo-controlled trial. Sleep. 2018, zsy258, 10.1093/sleep/zsy258. [DOI] [PubMed]
- 136.Matsunaga Y, et al. Effects of zolpidem/triazolam on cognitive performance 12 h after acute administration. Sleep Med. 2018;52:213–8. doi: 10.1016/j.sleep.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 137.Seelig AD, et al. Sleep patterns before, during, and after deployment to iraq and afghanistan. Sleep. 2010;33:1615–22. doi: 10.1093/sleep/33.12.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Seelig AD, et al. Sleep and health resilience metrics in a large military cohort. Sleep. 2016;39:1111–20. doi: 10.5665/sleep.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quintana-Gallego E, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98:984–9. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 140.Pracharktam N, Hans MG, Strohl KP, Redline S. Upright and supine cephalometric evaluation of obstructive sleep apnea syndrome and snoring subjects. Angle Orthod. 1994;64:63–73. doi: 10.1043/0003-3219(1994)064<0063:UASCEO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 141.O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–72. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 142.Kump K, et al. Assessment of the validity and utility of a sleep-symptom questionnaire. Am J Respir Crit Care Med. 1994;150:735–41. doi: 10.1164/ajrccm.150.3.8087345. [DOI] [PubMed] [Google Scholar]
- 143.Ehlen JC, et al. Bmal1 function in skeletal muscle regulates sleep. Elife. 2017;6:e26557. [DOI] [PMC free article] [PubMed]
- 144.Toyota H, et al. Behavioral characterization of mice lacking histamine h(3) receptors. Mol Pharm. 2002;62:389–97. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- 145.Pellegrino R, et al. A novel bhlhe41 variant is associated with short sleep and resistance to sleep deprivation in humans. Sleep. 2014;37:1327–36. doi: 10.5665/sleep.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kopp C, Albrecht U, Zheng B, Tobler I. Homeostatic sleep regulation is preserved in mper1 and mper2 mutant mice. Eur J Neurosci. 2002;16:1099–106. doi: 10.1046/j.1460-9568.2002.02156.x. [DOI] [PubMed] [Google Scholar]
- 147.Carpen JD, Archer SN, Skene DJ, Smits M, von Schantz M. A single-nucleotide polymorphism in the 5’-untranslated region of the hper2 gene is associated with diurnal preference. J Sleep Res. 2005;14:293–7. doi: 10.1111/j.1365-2869.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 148.He Y, et al. The transcriptional repressor dec2 regulates sleep length in mammals. Science. 2009;325:866–70. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ehlen JC, et al. Maternal ube3a loss disrupts sleep homeostasis but leaves circadian rhythmicity largely intact. J Neurosci. 2015;35:13587–98. doi: 10.1523/JNEUROSCI.2194-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.den Bakker H, et al. Abnormal coherence and sleep composition in children with angelman syndrome: a retrospective eeg study. Mol Autism. 2018;9:32. doi: 10.1186/s13229-018-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Winrow CJ, et al. Uncovering the genetic landscape for multiple sleep-wake traits. PLoS One. 2009;4:e5161. doi: 10.1371/journal.pone.0005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lane JM, et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat Genet. 2017;49:274–81. doi: 10.1038/ng.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dashti HS, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10:1100. doi: 10.1038/s41467-019-08917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carter ME, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jego S, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–43. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weber F, et al. Regulation of rem and non-rem sleep by periaqueductal gabaergic neurons. Nat Commun. 2018;9:354. doi: 10.1038/s41467-017-02765-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsunematsu T, et al. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 2011;31:10529–39. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brager AJ, Stowie AC, Prosser RA, Glass JD. The mper2 clock gene modulates cocaine actions in the mouse circadian system. Behav Brain Res. 2013;243:255–60. doi: 10.1016/j.bbr.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wolff G, Duncan MJ, Esser KA. Chronic phase advance alters circadian physiological rhythms and peripheral molecular clocks. J Appl Physiol. 2013;115:373–82. doi: 10.1152/japplphysiol.01139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McGinnis GR, et al. Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the heart. J Mol Cell Cardiol. 2017;110:80–95. doi: 10.1016/j.yjmcc.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc. 2012;44:1663–70. doi: 10.1249/MSS.0b013e318255cf4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Christie S, et al. A rotating light cycle promotes weight gain and hepatic lipid storage in mice. Am J Physiol Gastrointest Liver Physiol. 2018;315:G932–G942. doi: 10.1152/ajpgi.00020.2018. [DOI] [PubMed] [Google Scholar]
- 163.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–9. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reinhardt É, PACM Fernandes, Markus RP, Fischer FM. Night work effects on salivary cytokines tnf, il-1β and il-6. Chronobiol Int. 2019;36:11–26. doi: 10.1080/07420528.2018.1515771. [DOI] [PubMed] [Google Scholar]
- 165.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–42. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hue CD, et al. Blood-brain barrier dysfunction after primary blast injury in vitro. J Neurotrauma. 2013;30:1652–63. doi: 10.1089/neu.2012.2773. [DOI] [PubMed] [Google Scholar]
- 167.Wang Y, et al. Tightly coupled repetitive blast-induced traumatic brain injury: development and characterization in mice. J Neurotrauma. 2011;28:2171–83. doi: 10.1089/neu.2011.1990. [DOI] [PubMed] [Google Scholar]
- 168.Mathias JL, Alvaro PK. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med. 2012;13:898–905. doi: 10.1016/j.sleep.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 169.Shekleton JA, et al. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 2010;74:1732–8. doi: 10.1212/WNL.0b013e3181e0438b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Grima N, Ponsford J, Rajaratnam SM, Mansfield D, Pase MP. Sleep disturbances in traumatic brain injury: a meta-analysis. J Clin Sleep Med. 2016;12:419–28. doi: 10.5664/jcsm.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sandsmark DK, Elliott JE & Lim MM. Sleep-wake disturbances after traumatic brain injury: synthesis of human and animal studies. Sleep. 2017;40, zsx044, 10.1093/sleep/zsx044. [DOI] [PMC free article] [PubMed]
- 172.Lim MM, et al. Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Sci Transl Med. 2013;5:215ra173. doi: 10.1126/scitranslmed.3007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Skopin MD, Kabadi SV, Viechweg SS, Mong JA, Faden AI. Chronic decrease in wakefulness and disruption of sleep-wake behavior after experimental traumatic brain injury. J Neurotrauma. 2015;32:289–96. doi: 10.1089/neu.2014.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baumann CR, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–9. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Watson NF, Dikmen S, Machamer J, Doherty M, Temkin N. Hypersomnia following traumatic brain injury. J Clin Sleep Med. 2007;3:363–8. [PMC free article] [PubMed] [Google Scholar]
- 176.Masel BE, Scheibel RS, Kimbark T, Kuna ST. Excessive daytime sleepiness in adults with brain injuries. Arch Phys Med Rehabil. 2001;82:1526–32. doi: 10.1053/apmr.2001.26093. [DOI] [PubMed] [Google Scholar]
- 177.Capaldi VF, 2nd, Guerrero ML, Killgore WD. Sleep disruptions among returning combat veterans from iraq and afghanistan. Mil Med. 2011;176:879–88. doi: 10.7205/milmed-d-10-00440. [DOI] [PubMed] [Google Scholar]
- 178.Deslauriers J, Toth M, Der-Avakian A, Risbrough VB. Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol Psychiatry. 2018;83:895–907. doi: 10.1016/j.biopsych.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meerlo P, Pragt BJ, Daan S. Social stress induces high intensity sleep in rats. Neurosci Lett. 1997;225:41–44. doi: 10.1016/s0304-3940(97)00180-8. [DOI] [PubMed] [Google Scholar]
- 180.Meerlo P, de Bruin EA, Strijkstra AM, Daan S. A social conflict increases eeg slow-wave activity during subsequent sleep. Physiol Behav. 2001;73:331–5. doi: 10.1016/s0031-9384(01)00451-6. [DOI] [PubMed] [Google Scholar]
- 181.Kamphuis J, Lancel M, Koolhaas JM, Meerlo P. Deep sleep after social stress: NREM sleep slow-wave activity is enhanced in both winners and losers of a conflict. Brain Behav Immun. 2015;47:149–54. doi: 10.1016/j.bbi.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 182.Kinn AM, et al. A double exposure to social defeat induces sub-chronic effects on sleep and open field behaviour in rats. Physiol Behav. 2008;95:553–61. doi: 10.1016/j.physbeh.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 183.Wells AM, et al. Effects of chronic social defeat stress on sleep and circadian rhythms are mitigated by kappa-opioid receptor antagonism. J Neurosci. 2017;37:7656–68. doi: 10.1523/JNEUROSCI.0885-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vanderheyden WM, et al. Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of ptsd. Exp Brain Res. 2015;233:2335–46. doi: 10.1007/s00221-015-4302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nedelcovych MT, et al. A rodent model of traumatic stress induces lasting sleep and quantitative electroencephalographic disturbances. ACS Chem Neurosci. 2015;6:485–93. doi: 10.1021/cn500342u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lundt A, et al. EEG radiotelemetry in small laboratory rodents: a powerful state-of-the art approach in neuropsychiatric, neurodegenerative, and epilepsy research. Neural Plast. 2016;2016:8213878. doi: 10.1155/2016/8213878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Marrero-Rosado Brenda, de Araujo Furtado Marcio, Schultz Caroline R., Stone Michael, Kundrick Erica, Walker Katie, O’Brien Sean, Du Fu, Lumley Lucille A. Soman-induced status epilepticus, epileptogenesis, and neuropathology in carboxylesterase knockout mice treated with midazolam. Epilepsia. 2018;59(12):2206–2218. doi: 10.1111/epi.14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.de Araujo Furtado M, et al. Analyzing large data sets acquired through telemetry from rats exposed to organophosphorous compounds: An eeg study. J Neurosci Methods. 2009;184:176–83. doi: 10.1016/j.jneumeth.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 189.de Araujo Furtado M, et al. Spontaneous recurrent seizures after status epilepticus induced by soman in sprague-dawley rats. Epilepsia. 2010;51:1503–10. doi: 10.1111/j.1528-1167.2009.02478.x. [DOI] [PubMed] [Google Scholar]
- 190.Makinodan M, et al. Effects of the mode of re-socialization after juvenile social isolation on medial prefrontal cortex myelination and function. Sci Rep. 2017;7:5481. doi: 10.1038/s41598-017-05632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim NY, Dhakal R, Adhikari KK, Kim ES, Wang C. A reusable robust radio frequency biosensor using microwave resonator by integrated passive device technology for quantitative detection of glucose level. Biosens Bioelectron. 2015;67:687–93. doi: 10.1016/j.bios.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 192.Kim J, Basham E, Pedrotti KD. Geometry-based optimization of radio-frequency coils for powering neuroprosthetic implants. Med Biol Eng Comput. 2013;51:123–34. doi: 10.1007/s11517-012-0975-8. [DOI] [PubMed] [Google Scholar]
- 193.Gutruf P, Rogers JA. Implantable, wireless device platforms for neuroscience research. Curr Opin Neurobiol. 2018;50:42–49. doi: 10.1016/j.conb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 194.Gutruf P, Good CH, Rogers JA. Perspective: implantable optical systems for neuroscience research in behaving animal models—current approaches and future directions. J APL Photonics. 2018;3:120901. [Google Scholar]
- 195.Shin G, et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron. 2017;93:509–21 e503. doi: 10.1016/j.neuron.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Moore NL, Altman DE, Gauchan S, Genovese RF. Adulthood stress responses in rats are variably altered as a factor of adolescent stress exposure. Stress. 2016;19:295–302. doi: 10.1080/10253890.2016.1191465. [DOI] [PubMed] [Google Scholar]
- 197.Haslam DR. Sleep loss, recovery sleep, and military performance. Ergonomics. 1982;25:163–78. doi: 10.1080/00140138208924935. [DOI] [PubMed] [Google Scholar]
- 198.Tharion WJ, Shukitt-Hale B, Lieberman HR. Caffeine effects on marksmanship during high-stress military training with 72 h sleep deprivation. Aviat Space Environ Med. 2003;74:309–14. [PubMed] [Google Scholar]
- 199.McLellan TM, et al. Caffeine maintains vigilance and marksmanship in simulated urban operations with sleep deprivation. Aviat Space Environ Med. 2005;76:39–45. [PubMed] [Google Scholar]
- 200.Leproult R, et al. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–290. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 201.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 202.Rupp TL, Wesensten NJ, Balkin TJ. Trait-like vulnerability to total and partial sleep loss. Sleep. 2012;35:1163–72. doi: 10.5665/sleep.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Braun AR, et al. The process of awakening: a pet study of regional brain activity patterns mediating the re‐establishment of alertness and consciousness. Brain. 2002;125:2308–19. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- 204.Balkin TJ, Badia P. Relationship between sleep inertia and sleepiness: cumulative effects of four nights of sleep disruption/restriction on performance following abrupt nocturnal awakenings. Biol Psychol. 1988;27:245–58. doi: 10.1016/0301-0511(88)90034-8. [DOI] [PubMed] [Google Scholar]
- 205.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23:353–61. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Newman RA, Kamimori GH, Wesensten NJ, Picchioni D, Balkin TJ. Caffeine gum minimizes sleep inertia. Percept Mot Skills. 2013;116:280–93. doi: 10.2466/29.22.25.PMS.116.1.280-293. [DOI] [PubMed] [Google Scholar]
- 207.Van Dongen HP, et al. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep. 2001;24:813–9. doi: 10.1093/sleep/24.7.813. [DOI] [PubMed] [Google Scholar]
- 208.Mah CD, Mah KE, Kezirian EJ, Dement WC. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep. 2011;34:943–50. doi: 10.5665/SLEEP.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Waterhouse J, Atkinson G, Edwards B, Reilly T. The role of a short post-lunch nap in improving cognitive, motor, and sprint performance in participants with partial sleep deprivation. J Sports Sci. 2007;25:1557–66. doi: 10.1080/02640410701244983. [DOI] [PubMed] [Google Scholar]
- 210.Blanchfield AW, Lewis-Jones TM, Wignall JR, Roberts JB, Oliver SJ. The influence of an afternoon nap on the endurance performance of trained runners. Eur J Sport Sci. 2018;18:1177–84. doi: 10.1080/17461391.2018.1477180. [DOI] [PubMed] [Google Scholar]
- 211.Ritland BM, et al. Sleep health and its association with performance and motivation in tactical athletes enrolled in the reserve officers’ training corps. J Sleep Health. 2019. [Epub ahead of print]. [DOI] [PubMed]
- 212.Ritland Bradley M., Simonelli Guido, Gentili Rodolphe J., Smith J. Carson, He Xin, Mantua Janna, Balkin Thomas J., Hatfield Bradley D. Effects of sleep extension on cognitive/motor performance and motivation in military tactical athletes. Sleep Medicine. 2019;58:48–55. doi: 10.1016/j.sleep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 213.Grandner M, et al. Sleep duration and hypertension: analysis of > 700,000 adults by age and sex. J Clin Sleep Med. 2018;14:1031–9. doi: 10.5664/jcsm.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Grandner MA, Schopfer EA, Sands-Lincoln M, Jackson N, Malhotra A. Relationship between sleep duration and body mass index depends on age. Obesity. 2015;23:2491–8. doi: 10.1002/oby.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Petrov ME, et al. Sleep duration and risk of incident stroke by age, sex, and race: the regards study. Neurology. 2018;91:e1702–e1709. doi: 10.1212/WNL.0000000000006424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kirschen GW, Jones JJ & Hale L. The impact of sleep duration on performance among competitive athletes: a systematic literature review. Clin J Sport Med. 2018. [Epub ahead of print]. [DOI] [PubMed]
- 217.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 218.Doty TJ, et al. Limited efficacy of caffeine and recovery costs during and following 5 days of chronic sleep restriction. Sleep. 2017;40:39–45. doi: 10.1093/sleep/zsx171. [DOI] [PubMed] [Google Scholar]
- 219.Khitrov MY, et al. Pc-pvt: a platform for psychomotor vigilance task testing, analysis, and prediction. Behav Res Methods. 2014;46:140–7. doi: 10.3758/s13428-013-0339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (pvt) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shattuck NL, Matsangas P. Psychomotor vigilance performance predicted by epworth sleepiness scale scores in an operational setting with the united states navy. J Sleep Res. 2015;24:174–80. doi: 10.1111/jsr.12243. [DOI] [PubMed] [Google Scholar]
- 222.Reifman J, Kumar K, Khitrov MY, Liu J, Ramakrishnan S. Pc-pvt 2.0: an updated platform for psychomotor vigilance task testing, analysis, prediction, and visualization. J Neurosci Methods. 2018;304:39–45. doi: 10.1016/j.jneumeth.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 223.Belenky G, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 224.McMahon WR, et al. The wake maintenance zone shows task dependent changes in cognitive function following one night without sleep. Sleep. 2018;41, zsy148, 10.1093/sleep/zsy148. [DOI] [PubMed]
- 225.Lamp A, Chen JMC, McCullough D, Belenky G. Equal to or better than: the application of statistical non-inferiority to fatigue risk management. Accid Anal Prev. 2019;126:184–90. doi: 10.1016/j.aap.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 226.St Hilaire MA, et al. Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 2017; 40, zsw009, 10.1093/sleep/zsw009. [DOI] [PMC free article] [PubMed]
- 227.Porkka-Heiskanen T, et al. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bodenmann S, et al. Pharmacogenetics of modafinil after sleep loss: Catechol-o-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharm Ther. 2009;85:296–304. doi: 10.1038/clpt.2008.222. [DOI] [PubMed] [Google Scholar]
- 229.Jones CR, et al. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–5. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 230.Viola AU, et al. Per3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 231.Bodenmann S, et al. Polymorphisms of adora2a modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep eeg after sleep deprivation. Br J Pharmacol. 2012;165:1904–13. doi: 10.1111/j.1476-5381.2011.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Landolt HP. “No thanks, coffee keeps me awake”: individual caffeine sensitivity depends on adora2a genotype. Sleep. 2012;35:899–900. doi: 10.5665/sleep.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Satterfield BC, Wisor JP, Schmidt MA & Van Dongen. HPA Time-on-task effect during sleep deprivation in healthy young adults is modulated by dopamine transporter genotype. Sleep. 2017;40, zsx167, 10.1093/sleep/zsx167. [DOI] [PMC free article] [PubMed]
- 234.Goel N, Banks S, Lin L, Mignot E, Dinges DF. Catechol-o-methyltransferase val158met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS One. 2011;6:e29283. doi: 10.1371/journal.pone.0029283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rupp TL, Wesensten NJ, Newman R, Balkin TJ. Per3 and adora2a polymorphisms impact neurobehavioral performance during sleep restriction. J Sleep Res. 2013;22:160–5. doi: 10.1111/j.1365-2869.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 236.Wishart DS, et al. Drugbank 5.0: a major update to the drugbank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ramakrishnan S, et al. Dose-dependent model of caffeine effects on human vigilance during total sleep deprivation. J Theor Biol. 2014;358:11–24. doi: 10.1016/j.jtbi.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 238.Vital-Lopez FG, Ramakrishnan S, Doty TJ, Balkin TJ, Reifman J. Caffeine dosing strategies to optimize alertness during sleep loss. J Sleep Res. 2018;27:e12711. doi: 10.1111/jsr.12711. [DOI] [PubMed] [Google Scholar]
- 239.Rétey JV, et al. A genetic variation in the adenosine a2a receptor gene (adora2a) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharm Ther. 2007;81:692–8. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 240.Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine a2a receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86:240–4. doi: 10.1093/ajcn/86.1.240. [DOI] [PubMed] [Google Scholar]
- 241.Choynowski J, et al. 0204 advantage of single-effort ultra-marathons to examine homeostatic loads and circadian properties of human endurance under total sleep deprivation. Sleep. 2018;41:A79–A80. [Google Scholar]
- 242.Toblin RL, Adrian AL, Hoge CW, Adler AB. Energy drink use in u.S. Service members after deployment: associations with mental health problems, aggression, and fatigue. Mil Med. 2018;183:e364–e370. doi: 10.1093/milmed/usy205. [DOI] [PubMed] [Google Scholar]
- 243.Hansen, D.A., Ramakrishnan, S., Satterfield, B.C. et al. Randomized, double-blind, placebo-controlled, crossover study of the effects of repeated-dose caffeine on neurobehavioral performance during 48 h of total sleep deprivation. Psychopharmacology. 2018: 1–10. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 244.Reifman J, et al. 2b-alert web: an open-access tool for predicting the effects of sleep/wake schedules and caffeine consumption on neurobehavioral performance. Sleep. 2016;39:2157–9. doi: 10.5665/sleep.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Air Force Instruction 11-202, Volume 3; General Flight Rules; Supplement 28 July 2017.
- 246.Wesensten NJ, Reichardt RM, Balkin TJ. Ampakine (cx717) effects on performance and alertness during simulated night shift work. Aviat Space Environ Med. 2007;78:937–43. doi: 10.3357/asem.2055.2007. [DOI] [PubMed] [Google Scholar]
- 247.Dauvilliers Y, et al. Effect of sodium oxybate, modafinil, and their combination on disrupted nighttime sleep in narcolepsy. Sleep Med. 2017;40:53–57. doi: 10.1016/j.sleep.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 248.Erman MK, Rosenberg R, for the U.S. Modafinil Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with chronic shift work sleep disorder: Effects on patient functioning and health-related quality of life. Prim Care Companion J Clin Psychiatry. 2007;9:188–94. doi: 10.4088/pcc.v09n0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Burke TM, et al. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med. 2015;7:305ra146. doi: 10.1126/scitranslmed.aac5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Oike H, Kobori M, Suzuki T, Ishida N. Caffeine lengthens circadian rhythms in mice. Biochem Biophys Res Commun. 2011;410:654–8. doi: 10.1016/j.bbrc.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 251.Hilaire MAS, Lockley SW. Caffeine does not entrain the circadian clock but improves daytime alertness in blind patients with non-24-hour rhythms. Sleep Med. 2015;16:800–4. doi: 10.1016/j.sleep.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. Eur J Neurosci. 2009;29:748–60. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci USA. 2009;106:16493–8. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.McClung CA, et al. Regulation of dopaminergic transmission and cocaine reward by the clock gene. Proc Natl Acad Sci USA. 2005;102:9377–81. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Glass JD, Brager AJ, Stowie AC, Prosser RA. Cocaine modulates pathways for photic and nonphotic entrainment of the mammalian scn circadian clock. Am J Physiol Regul Integr Comp Physiol. 2012;302:R740–750. doi: 10.1152/ajpregu.00602.2011. [DOI] [PubMed] [Google Scholar]
- 256.Brager AJ, Prosser RA, Glass JD. Circadian and acamprosate modulation of elevated ethanol drinking in mper2 clock gene mutant mice. Chronobiol Int. 2011;28:664–72. doi: 10.3109/07420528.2011.601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Suzuki H, Yamadera H, Nakamura S, Endo S. Effects of trazodone and imipramine on the biological rhythm: an analysis of sleep eeg and body core temperature. J Nippon Med Sch. 2002;69:333–41. doi: 10.1272/jnms.69.333. [DOI] [PubMed] [Google Scholar]
- 258.Hirschfeld U, et al. Progressive elevation of plasma thyrotropin during adaptation to simulated jet lag: effects of treatment with bright light or zolpidem. J Clin Endocrinol Metab. 1996;81:3270–7. doi: 10.1210/jcem.81.9.8784082. [DOI] [PubMed] [Google Scholar]
- 259.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]