Short abstract
Metastasis is the most challenging issue for gastric cancer, and identification of the molecular mechanism and suitable targets for treatment is the major purpose of recent research. In this study, we found the long non-coding RNA ANRIL was critical for the progression of gastric cancer. Knockdown of ANRIL (also known as CDKN2B-AS) with shRNA increased apoptosis, inhibited tumor growth, and suppressed migration of cancer cells. TET2 (Tet Methylcytosine Dioxygenase 2), a methylcytosine dioxygenase suppressed ANRIL function and prevented cancer progression. Patients with higher TET2 expression survived better, while with higher ANRIL survived worse. Furthermore, expressions of TET2 and ANRIL were negatively correlated in the patient samples. The mechanistic study suggested that ANRIL promoted tumor progression mainly by enhancing NF-kB signaling.
Impact statement
Gastric cancer is one of the leading causes of cancer-related death. The lack of curative therapeutic options ascribes to the complex genetic background and heterogeneity of gastric cancer. Understanding the molecular details of the disease and identifying the therapeutic targets would offer additional treatment options. Long non-coding RNA ANRIL was involved in the progression of many cancers, including gastric cancer, but the mechanism was unknown. The current study indicated that ANRIL supported tumor cell survival by inhibiting apoptosis and promoted metastasis by enhancing NF-kB signaling. NF-kB signaling was critical in tumor progression, and this study proved another long non-coding RNA that could regulate NF-kB signaling. ANRIL would be a potential biomarker and therapeutic target for gastric cancer prognosis and treatment.
Keywords: Gastric cancer, ANRIL, TET2, NF-kB signaling
Introduction
Gastric cancer is a type of common cancer, and surgery is the primary option for curing the disease at early stages. Patients often have a poor quality of life with a shortage of effective therapy when at later stages. Metastasis and resistance of chemotherapy are the major challenging issues in the treatment of gastric cancer,1 and the knowledge of the properties and molecular details of gastric cancer was still limited. Identification of the mechanisms of cancer progression and novel targets is urgently required.
Recent researches indicated that the long non-coding RNA (lncRNA) was involved in cancer progress.2 The lncRNA ANRIL is 3.8 kb transcription3 and has properties related to cancer progression.4 ANRIL is implicated in many cancers including lung cancer,5 liver cancer,6 breast cancer,7 esophageal squamous cell carcinoma,8 colon cancer,9 and gastric cancer.10 NF-kB signaling is well known for regulating immune response to infection, and incorrect regulation was reported widely linked to cancer progression, especially for metastasis.11 Our previous study indicated low expression of TET2 promoted gastric cancer progress,12 here we further proved that TET2 inhibited ANRIL to decrease cancer cell survival and suppress metastasis, and ANRIL increases metastasis by enhancing NF-kB signaling.
Method
Patients
There are 192 gastric cancer patients with full case history involved in this study. The patients were enrolled in the Beijing Friendship Hospital from January 2009 to December 2016. All patients and donors signed the informed consent forms. The whole research was approved by the Clinical Research Ethics Committee of Capital Medical University.
Apoptosis assay
For apoptosis, cells were collected and stained with Annexin V-FITC, according to the protocol of the Apoptosis Detection Kit (Abcam).
Quantitative PCR
The RNA was extracted with TRIZOL (Invitrogen) according to the manufacturer’s protocol. The cDNAs were synthesized with One-Step RT-PCR Kit (TAKARA). The quantitative real-time PCR was conducted with the SYBR Green master mix (TAKARA) according to the standard protocol.
Western blotting
Briefly, total protein was prepared with RIPA buffer containing protease inhibitor mixture. Equal amounts of protein in SDS-PAGE loading buffer were loaded into each well, and after electrophoresis, transfer the protein from gel to PVDF membrane. The membrane was incubated with primary antibodies overnight at 4 degrees celsius after blocking and HRP-conjugated secondary antibodies for 1 h at room temperature. Anti-p65 (Abcam) and anti-beta-actin (Abcam) were used in the experiment.
Transwell migration
The upper chamber was seeded with 5 × 104 gastric cancer cells in RPMI1640 without FBS, and the low chamber contained RPMI1640 with 20% FBS. The cells on the membranes after migration were counted under the microscope after HE staining 24 h later. All of the experiments were performed in triplicate.
Xenograft tumor mouse model
Gastric cancer cells, AGS, were cultured for three days in vitro and harvested for subcutaneous transplantation on the flank of nude mice. From six days after transplantation, the size of tumors was measured for every three days. Pictures of the tumors were taken at the end of the experiment.
In vivo metastasis mouse model
Female C57 BL/6J and NOD-SCID IL2Rγ-null (NSG) mice (aged 6–8 weeks) were purchased from Charles River Laboratories in China (Beijing). Metastatic assays were performed as reported.13 NSG mice were transplanted with luciferase-labeled SGC-7901-Luc shRNA-transduced cells (1 × 107 cells in 200 µL PBS) via their tail injection. The metastasis was visualized six weeks after transplantation. Animal experiments were approved by Capital Medical University Animal Experiments and Experimental Animals Management Committee.
Statistics
The differences between two groups were compared with Student’s t-test. The overall survival of the patients with different TET2 or lncRNA-ANRIL expression was analyzed with the log-rank test. The relationship between lncRNA-ANRIL and TET2, p21, cIAP1, uPA, or MMP2 expression in patient samples was estimated with linear regression analysis. The results were presented as mean ± SEM of no less than three independent experiments.
Results
TET2 promotes apoptosis and ANRIL suppresses apoptosis in gastric cancer cell
The methylcytosine dioxygenase TET2 and lncRNA ANRIL were knocked down with specific shRNA in gastric cancer cells, and there were more cells in apoptosis with ANRIL knockdown and fewer cells in apoptosis with TET2 knockdown (Figure 1). However, in the absence of ANRIL, knockdown of TET2 cannot result in a decrease of apoptosis (Figure 1). These results suggested that TET2 decreased gastric cancer cell survival and ANRIL enhanced cell survival, and the function of TET2 depended on ANRIL, implying that ANRIL was a downstream target of TET2.
Figure 1.
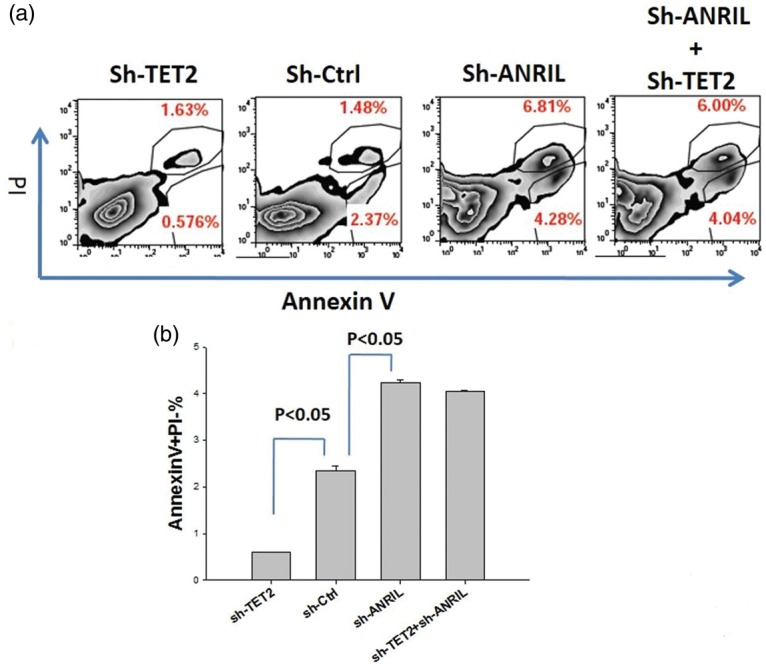
Knockdown of TET2 inhibits apoptosis, and knockdown ANRIL increases apoptosis in gastric cancer cell line MG803. (a) Representative image and (b) the summary of the results (each group includes three experiments). (A color version of this figure is available in the online journal.)
ANRIL: CDKN2B-AS; PI, Propidium Iodide; TET2: Tet Methylcytosine Dioxygenase 2.
TET2 suppressed metastasis and ANRIL enhances metastasis
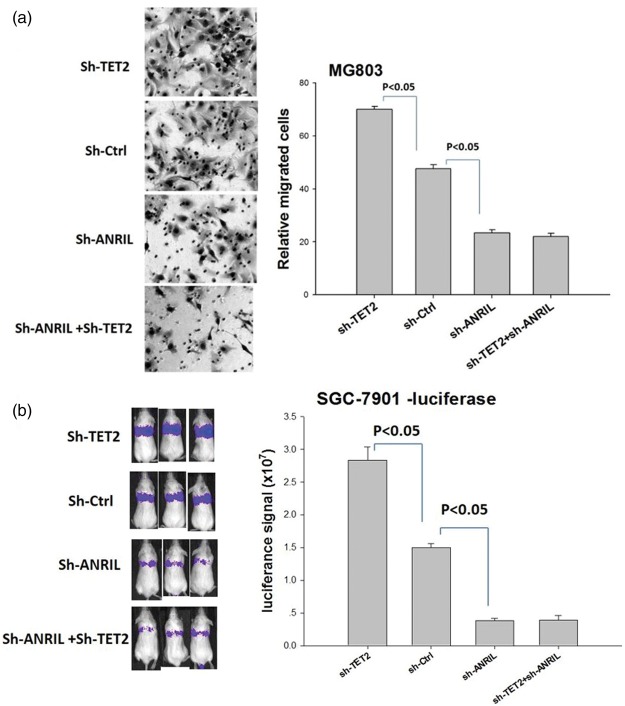
Metastasis is critical in gastric cancer progression. For testing the functions of TET2 and ANRIL in gastric cancer metastasis, specific shRNAs were introduced into gastric cancer cell MG803. Knockdown of TET2 significantly enhanced migration of MG803 cells in transwell migration assay, while knockdown of ANRIL decreased the migration (Figure 2). However, in the absence of ANRIL, knockdown of TET2 did not affect the migration of MG803 cells. To confirm the functions of TET2 and ANRIL in vivo, gastric cancer cell SGC-7901 stably expressing luciferase was knocked down of TET2 or ANRIL with a specific shRNA. The SGC-7901-luciferase cells were tail vein injected into NSG mice. Three weeks later, SGC-7901-luciferase cells were detected in vivo by injected luciferin. Knockdown of TET2 significantly increased lung metastasis and knockdown of ANRIL decreased lung metastasis (Figure 2). The above results indicate that TET2 suppresses metastasis and ANRIL promotes metastasis, and the suppression of TET2 depends on the presence of ANRIL, suggesting that ANRIL is a downstream target of TET2.
Figure 2.
Knockdown of TET2 increases metastasis and knockdown of ANRIL decreases metastasis of gastric cancer cell line. (a) Left panel is a representative result of transwell migration assay of MG803 cell line, and right panel is the summary of the results (each group includes three experiments) and (b) in vivo metastasis experiment with SGC-7901 cells stably expressing luciferase, the left panel is the image of NSG mice bearing gastric cancer cell line and the right panel is the summary of the results. (A color version of this figure is available in the online journal.)
ANRIL: CDKN2B-AS; TET2: Tet Methylcytosine Dioxygenase 2.
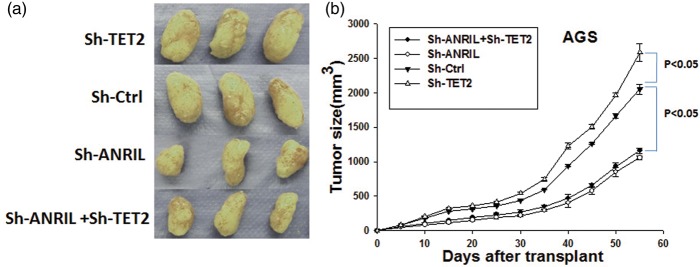
Figure 3.
Knockdown of TET2 increases tumor growth and knockdown of ANRIL decreases tumor growth in vivo. (a) Image of tumors at the endpoint in the xenograft model of AGS gastric cancer cells with nude mice and (b) the summary of the tumor growth curve. (A color version of this figure is available in the online journal.)
ANRIL: CDKN2B-AS; TET2: Tet Methylcytosine Dioxygenase 2.
TET2 suppresses tumor growth and ANRIL promotes tumor growth
The above results indicated that TET2 increased apoptosis of gastric cancer cells and ANRIL inhibited apoptosis. To confirm their functions for cell survival in vivo, AGS cells stably expressing specific shRNA for TET2 or ANRIL were subcutaneously injected into nude mice, and the tumor size was monitored every three days (Figure 3). The tumor expressing shRNA targeting TET2 grew faster than the control cells, and the tumor expressing shRNA targeting ANRIL grew slower than the control. In addition, tumors knocked down with ANRIL and TET2 had the same growth rate with the cells knocked down with ANRIL alone, suggesting suppression of tumor growth by TET2 depended on ANRIL.
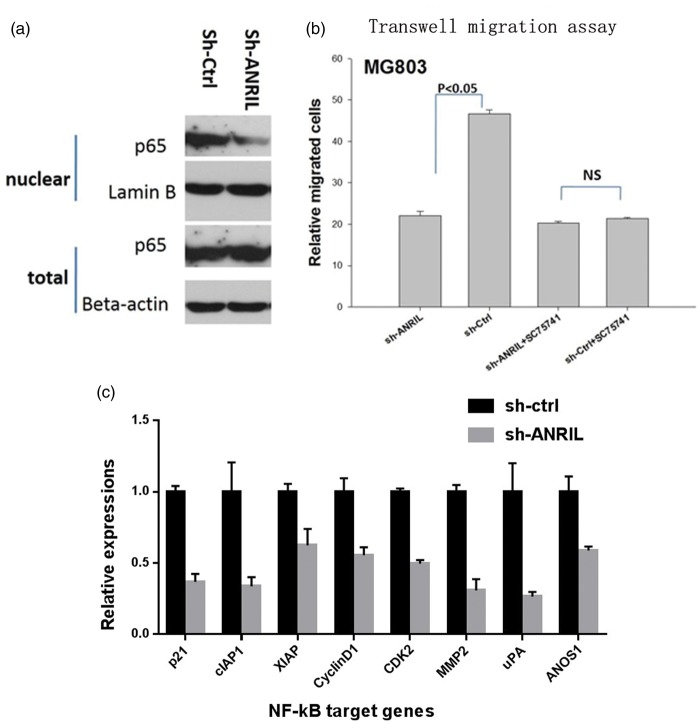
ANRIL promotes metastasis by activating NF-kB signaling
TET2 suppressed tumor growth by regulating ANRIL, and ANRIL supported tumor progress as indicated above, but its mechanism was unclear. NF-kB signaling was critical in cancer progression. To identify the relationship between NF-kB and ANRIL, we evaluated p65 protein levels in the nucleus of MG803 cells. Unlike the total protein level in the whole cell, knockdown of ANRIL resulted in a decrease of p65 protein in the nucleus (Figure 4(a)), suggesting that ANRIL could promote p65 to enter nucleus from the cytoplasm. Furthermore, NF-kB inhibitor sc75741 could inhibit migration of gastric cancer cells, and ANRIL could not further suppress migration in the presence of sc75741 (Figure 4(b)). Real-time qPCR indicated knockdown of ANRIL resulted in a decrease in the mRNA levels of NF-kB target genes (Figure 4(c)). All the above results suggested that the function of ANRIL depended on NF-kB signaling.
Figure 4.
ANRIL enhances migration of gastric cancer cells by increasing NF-kB signaling. (a) Knockdown of ANRIL decreases NF-kB signaling; (b) knockdown of ANRIL inhibits migration of MG803 cells in transwell assay, and NF-kB signaling inhibitor sc75741 abolishes the effect; and (c) knockdown of ANRIL inhibits expression of NF-kB target genes. ANRIL: CDKN2B-AS.
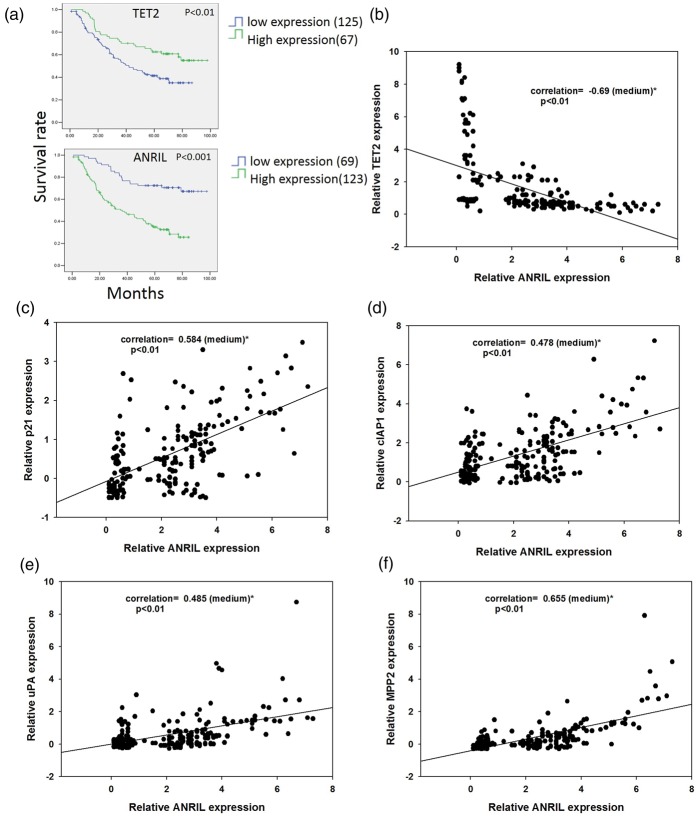
TET2 improves gastric cancer patient survival and ANRIL decreases patient survival
To confirm the functions of TET2 and ANRIL in human patients, 192 gastric cancer patients were followed up, and the mRNA levels of TET2, ANRIL, and NF-kB target genes in the tumor samples were evaluated with real-time qPCR. There were 125 patients grouped to low TET2 expression and 67 patients arranged to high TET2 expression, and patients with high TET2 expression had significantly longer survival time. For analysis of the relationship between ANRIL and patient survival (Figure 5(a)), 123 patients were grouped to low ANRIL expression, and 69 patients were high ANRIL expression. On the contrary, patients with low ANRIL had a longer survival time (Figure 5(a)). In the tumor samples, expression of TET2 and ANRIL was negatively correlated (Figure 5(b)), and ANRIL was expressed positively related to NF-kB target genes p21, cIAP1, uPA, and MMP2 (Figure 5(c) to (f)). These results further indicated that ANRIL enhanced NF-kB signaling.
Figure 5.
The relationship of TET2, ANRIL, and patient survival time in gastric cancer. (a) Patients have longer survival time with high TET2 expression or low ANRIL expression (there are 192 patients analyzed, and the patient numbers classified with low or high expression are indicated), (b) the mRNA expression levels of TET2 and ANRIL are negatively correlated, (c) the mRNA expression levels of p21 and ANRIL are positively correlated, (d) the mRNA expression levels of cIAP1 and ANRIL are positively correlated, (e) the mRNA expression levels of uPA and ANRIL are positively correlated, and (f) the mRNA expression levels of MMP2 and ANRIL are positively correlated. (A color version of this figure is available in the online journal.)
ANRIL: CDKN2B-AS; TET2: Tet Methylcytosine Dioxygenase 2.
Discussion
Gastric cancer is one of the leading causes of cancer-related death.14 This is mainly because of limited treatment options for the later emergence of symptoms in the development of the disease. The lack of curative therapeutic options was ascribed to the complex genetic background and heterogeneity of gastric cancer.15 Understanding of the pathogenesis of the disease and identifying potential therapeutic targets would offer additional treatment options. Previously, we have shown TET2 inhibits gastric cancer progression by inhibiting cell proliferation and promoting apoptosis.12 Here, besides to promote apoptosis, in vitro and in vivo experiments indicated that TET2 also suppressed metastasis. Mechanistically, TET2 suppressed ANRIL and ANRIL inhibited apoptosis and increased metastasis by enhancing NF-kB signaling. Furthermore, analysis of gastric cancer patient samples indicated that expression of TET2 and ANRIL was negatively correlated, and ANRIL was positively associated with NF-kB target genes.
LncRNAs are involved in epigenetic modifications, mRNA processing and translation, and protein activities.16 These non-coding RNAs previously thought non-functional were recently reported to be involved in multiple disease and cancers.16 Recent report targeting MALAT1 lncRNA with antisense oligonucleotides in breast cancer provided lncRNA a possible therapeutic target in cancer treatment.17,18 LncRNA-ANRIL is identified initially as a genetic locus associated with intracranial aneurysm, type 2 diabetes, and coronary disease.4 Recent studies indicated that ANRIL was involved in multiple cancers,4–10 including gastric cancer.10 In the current research, the ANRIL expression was identified significantly negatively correlated with the survival of gastric cancer patients (Figure 5(a)), suggesting that ANRIL promoted gastric cancer progress and it might be a potential target for cancer treatment.
NF-kB signaling is critical in immune response and is widely proved to be important in cancer progression, especially for tumor cell proliferation, survival, and metastasis.11 Multiple reports show that lncRNA regulated NF-kB signaling.19–22 LncRNA NKILA formed a complex with NF-kB/IkB to suppress NF-kB signaling in breast cancer.20 LncRNA MIR31HG could bind to IkBa directly and activated NF-kB signaling.21 In ovarian cancer, NF-kB up-regulated lncRNA HOTAIR, and HOTAIR down-regulated Ik-Ba expression and activated NF-kB signaling.22 LncRNA BANCR increased gastric cancer cell proliferation by up-regulating NF-kB1.19 ANRIL is regulated by NF-kB signaling and involved in the regulation of NF-κB pathway in coronary artery disease.23 Our results indicated that ANRIL could also enhance NF-kB signaling by helping p65 enter the nucleus in gastric cancer cells (Figure 4(a)), suggesting that positive feedback might exist in gastric cancer, namely NF-kB activates ANRIL expression and ANRIL enhances NF-kB signaling.
In conclusion, this study explores the function of ANRIL in gastric cancer, identifying TET2 as an upstream factor for suppressing its expression and NF-kB signaling as the downstream target for enhancing gastric cancer progress. ANRIL would be a potential biomarker and therapeutic target for gastric cancer prognosis and treatment.
Authors’ contributions
WD conducted the experiments, collected the data, and wrote the manuscript; ZZ participated in the study design and reviewed the manuscript; YZ, JC, JZ, XL, JY, ZB, and HY participated in the study.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Beijing Natural Science Foundation (Grant No. 7174293), and Natural Science Foundation of China (Grant No. 81702345).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Shi X, Sun M, Wu Y, Yao Y, Liu H, Wu G, Yuan D, Song Y. Post-transcriptional regulation of long noncoding RNAs in cancer. Tumor Biol 2015; 36:503–13 [DOI] [PubMed] [Google Scholar]
- 3.Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou M-M. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell 2010; 38:662–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasmant E, Sabbagh A, Vidaud M, Bièche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J 2011; 25:444–8 [DOI] [PubMed] [Google Scholar]
- 5.Nie F-Q, Sun M, Yang J-S, Xie M, Xu T-P, Xia R, Liu Y-W, Liu X-H, Zhang E-B, Lu K-H. Long noncoding RNA ANRIL promotes non-small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther 2015; 14:268–77 [DOI] [PubMed] [Google Scholar]
- 6.Chen W-M Qi F-Z Xia R Sun M Xu T-P Yin L Zhang E-B De W,Shu Y-Q.. Long non-coding RNA ANRIL is upregulated in hepatocellular carcinoma and regulates cell proliferation by epigenetic silencing of KLF2. J Hematol Oncol 2015; 8:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meseure D, Vacher S, Alsibai KD, Nicolas A, Chemlali W, Caly M, Lidereau R, Pasmant E, Callens C, Bieche I. Expression of ANRIL–polycomb complexes–CDKN2A/B/ARF genes in breast tumors: identification of a two-gene (EZH2/CBX7) signature with independent prognostic value. Mol Cancer Res 2016; 14:623–33 [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Zhang Z, Mao C, Zhou Y, Yu L, Yin Y, Wu S, Mou X, Zhu Y. ANRIL inhibits p15INK4b through the TGFβ1 signaling pathway in human esophageal squamous cell carcinoma. Cell Immunol 2014; 289:91–6 [DOI] [PubMed] [Google Scholar]
- 9.Sun Z, Ou C, Ren W, Xie X, Li X, Li G. Downregulation of long non-coding RNA ANRIL suppresses lymphangiogenesis and lymphatic metastasis in colorectal cancer. Oncotarget 2016; 7:47536–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang E-B, Kong R, Yin D-D, You L-H, Sun M, Han L, Xu T-P, Xia R, Yang J-S, De W. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget 2014; 5:2276–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinkenbaugh A, Baldwin A. The NF-κB pathway and cancer stem cells. Cells 2016; 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng W, Wang J, Zhang J, Cai J, Bai Z, Zhang Z. TET2 regulates LncRNA‐ANRIL expression and inhibits the growth of human gastric cancer cells. IUBMB Life 2016; 68:355–64 [DOI] [PubMed] [Google Scholar]
- 13.Huang JZ, Chen M, Zeng M, Xu SH, Zou FY, Chen D, Yan GR. Down‐regulation of TRPS1 stimulates epithelial–mesenchymal transition and metastasis through repression of FOXA1. J Pathol 2016; 239:186–96 [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. Cancer incidence and mortality worldwide: IARC CancerBase. International Agency for Research on Cancer, Lyon, France. GLOBOCAN 2012; 11 [Google Scholar]
- 15.McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol 2014; 11:664–74 [DOI] [PubMed] [Google Scholar]
- 16.Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol 2018; 19:143–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arun G, Diermeier S, Akerman M, Chang K-C, Wilkinson JE, Hearn S, Kim Y, MacLeod AR, Krainer AR, Norton L. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev 2016; 30:34–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendell JT. Targeting a long noncoding RNA in breast cancer. N Engl J Med 2016; 374:2287–9 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z-X, Liu Z-Q, Jiang B, Lu X-Y, Ning X-F, Yuan C-T, Wang A-L. BRAF activated non-coding RNA (BANCR) promoting gastric cancer cells proliferation via regulation of NF-κB1. Biochem Biophys Res Commun 2015; 465:225–31 [DOI] [PubMed] [Google Scholar]
- 20.Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, Lin L, Yao H, Su F, Li D. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 2015; 27:370–81 [DOI] [PubMed] [Google Scholar]
- 21.Jin C, Jia L, Huang Y, Zheng Y, Du N, Liu Y, Zhou Y. Inhibition of lncRNA MIR31HG promotes osteogenic differentiation of human adipose‐derived stem cells. Stem Cells 2016; 34:2707–20 [DOI] [PubMed] [Google Scholar]
- 22.Özeş AR, Miller DF, Özeş ON, Fang F, Liu Y, Matei D, Huang T, Nephew KP. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene 2016; 35:5350–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, Han X, Wittfeldt A, Sun J, Liu C, Wang X, Gan L-M, Cao H, Liang Z. Long non-coding RNA ANRIL regulates inflammatory responses as a novel component of NF-κB pathway. RNA Biol 2016; 13:98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]