Short abstract
Acquired tamoxifen-resistance is an important cause of death in patients with hormone-dependent breast tumors. Therefore, understanding the molecular mechanisms underlying the development of tamoxifen-resistance is critical for successful endocrine therapy. This study aimed to define the role of WW domain-containing oxidoreductase (WWOX) in acquired tamoxifen-resistance. Our results show that low WWOX expression was significantly related to tamoxifen-resistance. Moreover, WWOX-knockdown increased resistance to tamoxifen, while WWOX overexpression decreased the resistance. Furthermore, WWOX silencing decreased Yes-associated protein (YAP) phosphorylation and increased YAP nuclear translocation. Finally, YAP silencing decreased tamoxifen-resistance in WWOX-knockdown cells. Our findings demonstrate that WWOX downregulation can lead to the development of tamoxifen-resistance by inactivating Hippo signaling. Thus, WWOX might be a valuable target and prognostic marker for tamoxifen-resistance.
Impact statement
Understanding the molecular pathways leading to the development of tamoxifen-resistance is an important research focus as acquired tamoxifen-resistance is the main cause of death in patients with benign primary prognosis. Although WW domain-containing oxidoreductase (WWOX) has been related to breast tumorigenesis, its role in acquired tamoxifen-resistance has not yet been demonstrated. Our findings show that WWOX might be a valuable therapeutic target and prognostic marker for tamoxifen-resistance.
Keywords: Breast cancer, Hippo, tamoxifen, WW domain-containing oxidoreductase, cell, resistance
Introduction
Endocrine therapy has been around for more than a century. It suppresses accelerated cell proliferation by attenuating estrogen receptor (ER) activity and inhibiting estrogen synthesis.1,2 Tamoxifen, as a selective ER modulator blocks the binding of estrogen with its receptor.3,4 However, five years is the standard duration for endocrine therapy based on tamoxifen treatment. Due to the development of acquired resistance, prolonged treatment cannot improve therapeutic efficacy. Therefore, it is necessary to understand the molecular mechanisms underlying the tamoxifen-resistance of breast cancer.
Recently, abnormal changes in Hippo signaling have been confirmed to be responsible for the development of drug resistance. The Hippo signaling pathway regulates cell stemness, apoptosis, and proliferation under the action of extensive intracellular and extracellular signaling.5,6 However, the molecular mechanism underlying the development of Hippo-regulated tamoxifen-resistance is largely unknown. Understanding the detailed mechanism of Hippo pathway-promoted development of tamoxifen-resistance would help in the development of strategies to prevent the development of tamoxifen-resistance and in the adoption of appropriate treatment methods to improve prognosis.
WWOX is tumor suppressor, and its dysregulation or loss leads to the development of various cancers.7–11 WWOX knockout mice show abnormal mammary gland branching.12 The reduction or loss of WWOX lead to breast cancer progression and poor prognosis. In addition, some evidence suggests that high WWOX expression is correlated with successful tamoxifen therapy. In patients with breast cancer, high expression of WWOX might predict beneficial effects of adjuvant tamoxifen.13 However, thus far, there is no clear evidence for the role of WWOX in tamoxifen-resistance. This study aimed to reveal the role of WWOX in acquired resistance to endocrine therapy.
Materials and methods
Immunohistochemistry and scoring
Tumor specimens were provided by the First Affiliated Hospital of Jiaotong University. Breast cancer relapsed after tamoxifen 20 mg/d conventional hormone therapy. Immunohistochemistry (IHC) was performed on nine pairs of paraffin sections of metastatic tamoxifen-resistant tissues and matched primary tumor tissues obtained from the same patients (Table 1) and 10 pairs of tamoxifen-resistant breast cancer tissues and their matching primary tumors obtained from different patients (Table 2). Tumor specimens were embedded in paraffin wax. Five-micrometer thick sections were mounted on glass slides and deparaffinized in xylene. Heat-induced epitope retrieval was performed with 0.01mmol/L sodium citrate for 10 min in a microwave oven (100w, 6min), followed by 20 min of cool down.The slides were incubated in blocking solution (10% goat serum) for 15 min. Then the slides were incubated with the primary WWOX antibody (1:200, SC-20528, Santa Cruz) at 4°C overnight. After washing for three times, the slides were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 30 min and then stained with 3,3ʹ-diaminidin. The sections were observed under a Leica microscope (SCN400; Mannheim, Germany) and images were obtained.
Table 1.
Clinicopathologic characteristics of nine pairs of tamoxifen-resistant breast cancer tissues and their matching primary tumors from the same patients.
| Sample ID | Type of breast cancer tissues | Site of breast cancer tissues | ER | PR | TAM therapy received |
|---|---|---|---|---|---|
| TamM-1 | Primary tumors | RT-left breast | − | + | Sep 2009 |
| TamR-1 | Metastatic breast cancer | Recurrence to left chest wall and diffuse lung metastasis | − | − | Mar 2011 |
| TamM-2 | Primary tumors | RT-left breast | + | + | May 2008 |
| TamR-2 | Metastatic breast cancer | Recurrence to left chest wall and right breast | − | − | Mar 2011 |
| TamM-3 | Primary tumors | RT-right breast | + | − | Feb 2009 |
| TamR-3 | Metastatic breast cancer | Recurrence to right chest wall | − | − | Apr 2011 |
| TamM-4 | Primary tumors | RT-left breast | + | + | Jan 2007 |
| TamR-4 | Metastatic breast cancer | Recurrence to left chest wall | − | − | Jan 2011 |
| TamM-5 | Primary tumors | RT-left breast | + | + | Jul 2007 |
| TamR-5 | Metastatic breast cancer | Recurrence to left clavicular lymph node | − | − | Jan 2010 |
| TamM-6 | Primary tumors | RT-left breast | + | + | Apr 2007 |
| TamR-6 | Metastatic breast cancer | Recurrence to left chest wall | + | + | Feb 2011 |
| TamM-7 | Primary tumors | RT-right breast | + | + | Oct 2013 |
| TamR-7 | Metastatic breast cancer | Recurrence to bone | − | − | Aug 2015 |
| TamM-8 | Primary tumors | RT-right breast | + | + | Feb 2014 |
| TamR-8 | Metastatic breast cancer | Recurrence to right chest wall | − | − | Sep 2015 |
| TamM-9 | Primary tumors | RT-right breast | + | + | Jan 2009 |
| TamR-9 | Metastatic breast cancer | Recurrence to bone | − | − | Mar 2011 |
Table 2.
Clinicopathologic characteristics of tamoxifen-resistant breast cancer tissues and primary tumors from the different patients.
| Sample ID | Type of breast cancer tissues | Site of breast cancer tissues | ER | PR |
|---|---|---|---|---|
| TamM-10 | primary tumors | RT-Left Breast | + | + |
| TamM-11 | primary tumors | RT-Left Breast | + | - |
| TamM-12 | primary tumors | RT-Right Breast | + | + |
| TamM-13 | primary tumors | RT-Left Breast | + | + |
| TamM-14 | primary tumors | RT-Right Breast | + | + |
| TamM-15 | primary tumors | RT-Left Breast | + | + |
| TamM-16 | primary tumors | RT-Left Breast | + | - |
| TamM-17 | primary tumors | RT-Left Breast | + | + |
| TamM-18 | primary tumors | RT-Right Breast | + | + |
| TamM-19 | primary tumors | RT-Right Breast | + | - |
| TamR-10 | metastatic breast cancer | Recurrence to Right Breast | + | - |
| TamR-11 | metastatic breast cancer | Recurrence to Right Breast | - | - |
| TamR-12 | metastatic breast cancer | Recurrence to Left Breast | - | - |
| TamR-13 | metastatic breast cancer | Recurrence to liver | + | + |
| TamR-14 | metastatic breast cancer | Recurrence to liver | + | - |
| TamR-15 | metastatic breast cancer | Recurrence to liver | + | - |
| TamR-16 | metastatic breast cancer | Recurrence to right chest wall | - | - |
| TamR-17 | metastatic breast cancer | Recurrence to right chest wall | - | - |
| TamR-18 | metastatic breast cancer | Recurrence to right chest wall | - | - |
| TamR-19 | metastatic breast cancer | Recurrence to bone | - | + |
Single-blind image analysis was performed. Three different microscopic fields on each image were scored twice by a pathologist who was unaware of the grouping. WWOX expression was measured by the positively stained tumor cells. IHC staining scores were assigned as 3, 2, 1, 0 and respectively correspond strongly positive, moderately positive, weakly positive, and negative. Staining range was defined as positively staining cells and scored as 3 (>70%), 2 (40–70%), 1 (10–40%), or 0 (<10%).
Cell culture
MCF7 and T47D were provided by Jianmin Zhang (Roswell Park Cancer Institute, Buffalo, NY, USA). The tamoxifen-resistant (TamR) cell lines (MCF7-TR-1 and MCF7-TR-2) were generated from MCF7 by continuous exposure to tamoxifen as previously described.14 MCF7-TR-1/2 cells were exposed to a final concentration of 5 μM 4-hydroxytamoxifen (4-OHT) as previously reported.15,16
The WWOX overexpression vector was purchased from GeneChem (Shanghai, China). Knockdown WWOX siRNA was purchased from GenePharma (Shanghai, China) and sequences (5ʹ–3ʹ) were as follows: WWOX-1 sense, GGA GAA GAC UCA GUG GGA ATT, WWOX-2 sense, GCA CCA CUG CCA UGG AAA UTT.
Real-time PCR
The Script RT reagent Kit was used to synthesize the cDNA. The primers were designed by TaKaRa. SYBR Premix TapTM was purchased from TaKaRa. The primers used are listed below.
WWOX-F, 5ʹ-GAACGCAGTGCATCCTGGAA-3ʹ
WWOX-R: 5ʹ-AGCGGCAGCAGTTGTTGAAGTA-3ʹ
CTGF-F, 5ʹ-AGGTGTGGCTTTAGGAGCAG-3ʹ
CTGF-R, 5ʹ-TCTTGATGGCTGGAGAATGC-3ʹ
CYR61-F, 5ʹ-TGGAACTGGTATCTCCACACG-3ʹ
CYR61-R, 5ʹ-TACACTGGCTGTCCACAAGG-3ʹ
GAPDH-F, 5ʹ-CTCCTCCACCTTTGACGCTG-3ʹ
GAPDH-R, 5ʹ-TCCTCTTGTGCTCTTGCTGG-3ʹ
Western blotting
Western blotting was carried out as described previously.17 Proteins were separated by SDS/PAGE. The polyvinylidene difluoride (PVDF) membranes were incubated with primary antibodies at 4°C overnight. After washing three times with Tris-buffered saline with Tween-20, the PVDF membranes were incubated with HRP-conjugated secondary antibodies (Santa Cruz, CA, USA) for 2 h at room temperature. Chemiluminescent signals were detected using ECL Plus (Millipore, Temecula, CA, USA). Anti-WWOX antibody (1:200, #sc-20528) was obtained from Santa Cruz (Dallas, TX, USA). Anti-Mst2 (#3952), anti-Mst1 (#3682), antiMob1 (#3863), anti-Lats1 (#3477), anti-p-Lats1 (Thr1079, #8654), anti-p-Mst1/2 (#3681), anti-Sav1 (#13301), anti-p-Mob1 (Thr35, #8699), anti-p-YAP (Ser127, #13008), and anti-YAP (#4912) antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA) and the anti-GAPDH antibody was from Proteintech (Wuhan, China).
Immunofluorescence
The cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 for 10 min. The cells were incubated with the primary antibodies anti-WWOX (1:50, #sc-20528) and anti-YAP (1:200, #4912) overnight at 4°C. The cells were incubated with the secondary antibodies (donkey anti-goat 488 (1:200, #A-11055) and goat anti-rabbit 546 (1:200, #A-11010) secondary antibodies B (Thermo, San Jose, CA, USA), and then cells were observed under a Leica TCS SP5 II Microscope (Leica, Wetzlar, Germany) and the images were captured.
Statistical analysis
SPSS software (version 22) was used for statistical analysis. The statistical differences between the two groups were compared by double-tailed Student’s t test or Wilcoxon signed-rank test; one-way ANOVA and Sidak multiple comparison tests were used for comparison of three groups. The results are represented as mean ± SEM from at least three independent experiments (***P < 0.001, **P < 0.01, *P < 0.05).
Results
Low expression of WWOX was closely related to tamoxifen-resistance
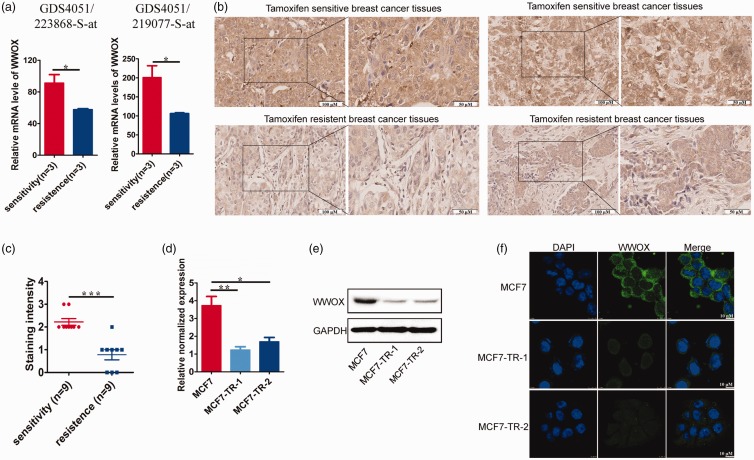
To study the relationship between WWOX gene expression and tamoxifen-resistance, we used the GEO profile database of the National Center for Biotechnology Information. This database provides the mRNA expression profile of WWOX in tamoxifen-sensitive MCF7 and tamoxifen-resistant subclone of MCF7. The expression profile of WWOX was compared in the gene expression profiles of GDS4051 223868_S_at and 219077_S_at provided in the database of the mRNA expression of WWOX in tamoxifen-sensitive and tamoxifen-resistant MCF7 lines. The sample numbers of tamoxifen-sensitive lines were GSM649484/485/486, and the sample numbers of tamoxifen-resistant lines were GSM649496/497/498 in the gene expression profiles GDS4051 223868_S_at and GDS4051 219077_S_at. The expression of WWOX gene in the tamoxifen-resistant MCF7 subclone was significantly lower than the MCF7 subclone sensitive to tamoxifen (P < 0.05; Figure 1(a)).
Figure 1.
Tamoxifen-resistance is associated with loss of WWOX protein. (a) Tamoxifen-sensitive and tamoxifen-resistant MCF7 cells were cultured in estrogen-depleted medium with or without 4-OHT for 4 h. We compared the WWOX expression profile in the gene expression profiles of GDS4051 223868_S_at and 219077_S_at in the mRNA expression database of WWOX in tamoxifen-sensitive and tamoxifen-resistant MCF7 lines. The sample numbers of tamoxifen-sensitive lines are GSM649484/485/486, and the sample numbers of tamoxifen-resistant lines are GSM649496/497/498 in the gene expression profiles GDS4051 223868_S_at and GDS4051 219077_S_at. (b) Micrographs showing WWOX staining in tamoxifen-resistant tissues and primary tumor counterparts. (c) WWOX total intensity (scored as 0: absent, 1: weak, 2: medium, and 3: strong expression) and WWOX protein expression in tamoxifen-resistant tissues and primary tumor counterparts were analyzed. (d) Analysis of WWOX protein expression in ER-positive and ER-negative breast tissue. (e) WWOX protein expression was analyzed in PR positive breast tissues and PR negative breast tissues. (f) RT-PCR analyses of WWOX mRNA expression in MCF7 and corresponding tamoxifen-resistant cells (MCF7-TR-1 and MCF7-TR-2). (g) Immunoblot analyses of WWOX protein expression in MCF7 and MCF7-TR-1/MCF7-TR-2 cells. (h) Immunofluorescence analyses of WWOX protein expression in MCF7 and MCF7-TR-1/MCF7-TR-2 cells. (A color version of this figure is available in the online journal.)
To further examine this phenomenon, we assessed the expression of WWOX protein in nine pairs of tamoxifen-resistant breast cancer tissues and matching primary tumors from the same patients (Table 1) and 10 pairs of tamoxifen-resistant breast cancer tissues and matching primary tumors from different patients (Table 2).
IHC showed that the expression of WWOX in tamoxifen-resistant tissues was lower than that in the primary tumor tissues (P < 0.05; Figure 1(b) and (c)).
Moreover, we statistically analyzed the relationship between WWOX expression level and the ER/PR status of the primary and relapsed lesion. The expression levels of WWOX and ER/PR status showed a positive correlation in tamoxifen-resistant and primary tumors (Figure 1(d) and (e)). These results suggest that WWOX downregulation is significantly related to tamoxifen-resistance.
To examine whether the observed expression pattern of WWOX could be reproduced in vitro, we determined mRNA and protein expression of the WWOX gene in MCF7 and MCF7-TR-1 and MCF7-TR-2 (corresponding tamoxifen-resistant cells). We had demonstrated the tamoxifen-resistance of MCF7-TR-1 and MCF7-TR-2 in an earlier study.15,16 Results from the current study showed that MCF7-TR-1 and MCF7-TR-2 cells had lower levels of WWOX mRNA and protein expression (Figure 1(f)–(h)). This expression pattern significantly suggests that WWOX may be associated with resistance to tamoxifen.
Suppression of WWOX expression conferred tamoxifen-resistance to breast cancer cells
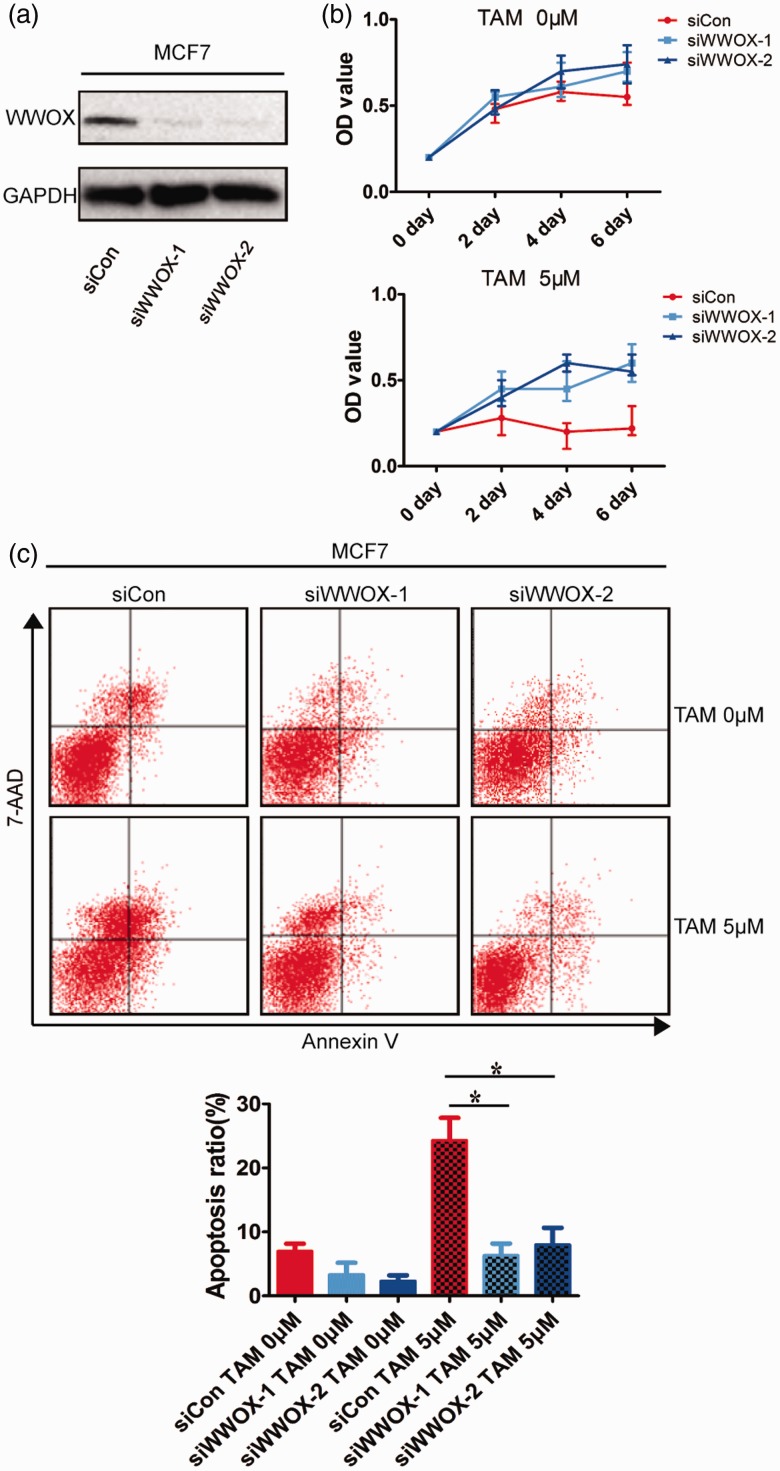
To further investigate the effect of WWOX on tamoxifen-resistance, siRNA was used to knock out the expression of WWOX in MCF7 cells (Figure 2(a)). The sensitivity of the WWOX knockout MCF7 cells to tamoxifen was evaluated using MTT assay and apoptosis. MCF7 cells were transfected with siCon/siWWOX-1/siWWOX-2 and treated with 5 μM 4-OHT for two, four, and six days. The results showed that depletion of WWOX increased the viability of MCF7 cells during tamoxifen treatment (Figure 2(b)). In addition, WWOX-knockdown reduced 4-OHT treatment-induced apoptosis (Figure 2(c)). These results indicate that the downregulation of WWOX induced the development of tamoxifen-resistance in the same ER-positive cells.
Figure 2.
WWOX downregulation induces tamoxifen-resistance in tamoxifen-sensitive cells. (a) Control of WWOX protein levels in non-targeted siRNA (siCon) and siWWOX-1/siWWOX-2 transfected MCF7 cells were detected by immunoblot. (b) The survival rates of siCon and siWWOX-1/siWWOX-2 cells were assayed after treatment with 4-OHT for 2, 4, and 6 days. (c) siCon and siWWOX-1/siWWOX-2 were treated with 4-OHT (5 μΜ) for 2 days and examined by FACS. (A color version of this figure is available in the online journal.)
Overexpression of WWOX in tamoxifen-resistant cells enhanced tamoxifen sensitivity
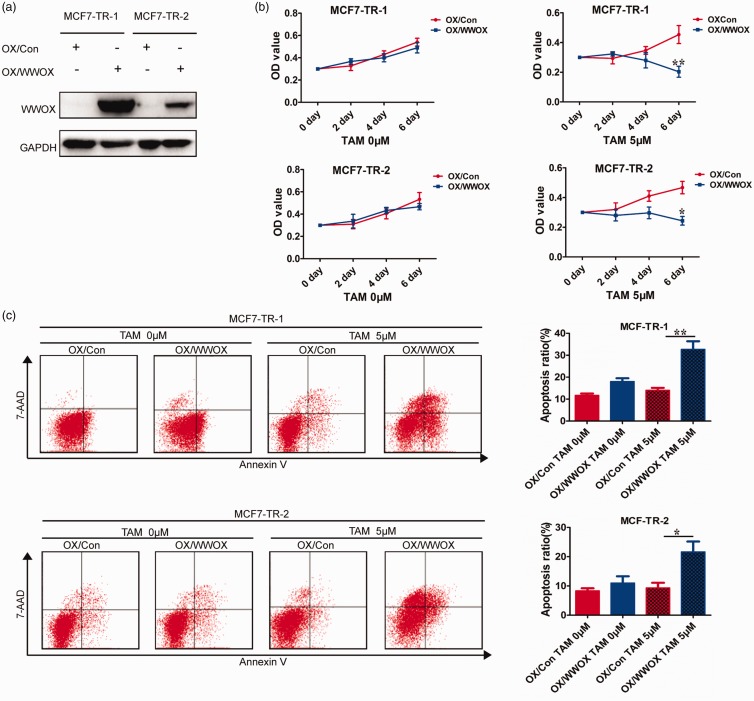
We next examined whether the altered expression of WWOX in tamoxifen-resistant cells was correlated with changes in tamoxifen-resistance. We overexpressed WWOX in tamoxifen-resistant cells (MCF7-TR-1 and MCF7-TR-2) by transfecting the pCDNA3.1 vector (Figure 3(a)) and then measured the cellular response to 4-OHT. WWOX overexpression led to decreased viability of MCF7-TR-1/MCF7-TR-2 cells (Figure 3(b)) and increased apoptosis under 4-OHT treatment (Figure 3(c)). These results indicate that WWOX overexpression in tamoxifen-resistant cells re-sensitizes to tamoxifen and could provide a novel therapeutic strategy against the development of tamoxifen-resistance.
Figure 3.
Overexpression of WWOX in tamoxifen-resistant cells re-sensitizes them to tamoxifen. (a) Tamoxifen-resistant cells (MCF7-TR-1 and MCF7-TR-2) were transfected with pCDNA3.1 vector (OX/Con) and pCDNA3.1-WWOX (OX/WWOX). (b) The cell survival rates of OX/Con and OX/WWOX cells after treatment with 4-OHT for 2, 4, or 6 days. (c) OX/Con and OX/WWOX cells were treated with 4-OHT (5 μΜ) for 2 days and then examined by FACS. (A color version of this figure is available in the online journal.)
WWOX downregulation conferred tamoxifen-resistance through Hippo signaling
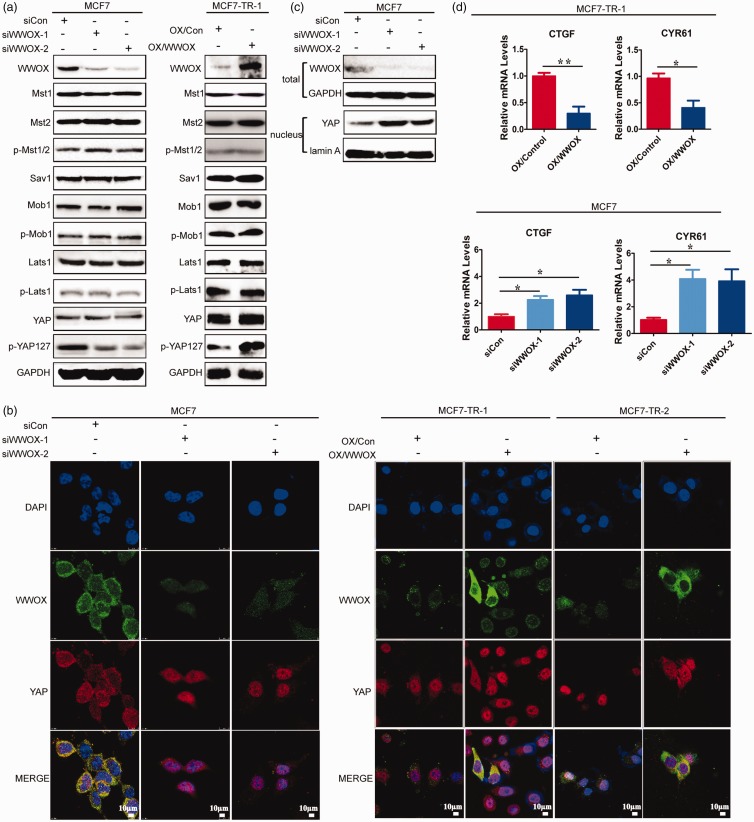
The Hippo signaling has been shown to be associated with drug resistance, such as resistance to 5-fluorouracil and doxorubicin.18,19 In addition, the Hippo pathway is rich in components in WW domains and their cognate proline-interacting motifs.20–22 The role of WWOX is closely related toWWdomains. WWOX has been demonstrated to interact with proteins containing the PPxY motif involved in cell cycle and apoptosis, such as p73, AP-2γ, ErbB4, and C-Jun.23–26 More importantly, WWOX has been shown to compete with Yes-associated protein (YAP; downstream transcription co-activator of Hippo signaling) to interact with p73, Ap-2g, and ErbB-4 to inhibit their transcription factor activity.27 Therefore, we evaluated whether WWOX could regulate tamoxifen-resistance via the Hippo pathway. First, we examined the expression of Hippo pathway components in siWWOX cells and OX/WWOX cells. WWOX-knockdown reduced YAP phosphorylation in tamoxifen-sensitive cells. However, the overexpression of WWOX increased YAP phosphorylation in tamoxifen-resistant cells. The expression of Mst1/2, Sav1, Mob1, and Lats1 and the phosphorylation of Mst1/2, Mob1, and Lats1 were not significantly altered in siWWOX and OX/WWOX cells (Figure 4(a)). In addition, WWOX-knockdown increased the relative level of nuclear YAP (Figure 4(b)). YAP was localized in the cytoplasm of siCon cells and in the nucleus of siWWOX cells (Figure 4(c)). Furthermore, WWOX-knockdown significantly increased the mRNA levels of YAP-targeted Cyr61 and CTGF in MCF7 cells, whereas enhanced WWOX expression decreased the mRNA levels of Cyr61 and CTGF in MCF7-TR-1 cells (Figure 4(d)). Thus, WWOX was confirmed to be an upstream regulator of the Hippo pathway and regulate YAP localization.
Figure 4.
WWOX regulates Hippo signaling pathways. (a) The expression of Hippo pathway components in MCF7-siCon, MCF7-siWWOX-1, MCF7-siWWOX-2, MCF7-TR-1-OX/Con, and MCF7-TR-1-OX/WWOX cells were analyzed by immunoblot. (b) Immunofluorescence analysis of YAP location in MCF7-siCon, MCF7-siWWOX-1, MCF7-siWWOX-2, MCF7-TR-1-OX/Con, MCF7-TR-1-OX/WWOX, MCF7-TR-2-OX/Con, and MCF7-TR-2-OX/WWOX cells. (c) Immunoblot analysis of the distribution of YAP in MCF7-siCon, MCF7-siWWOX-1, and MCF7-siWWOX-2 cells. (d) RT-PCR analysis of CTGF and CYR61 mRNA levels in MCF7-siCon, MCF7-siWWOX-1, MCF7-siWWOX-2, MCF7-TR-1-OX/Con, and MCF7-TR-1-OX/WWOX cells. (A color version of this figure is available in the online journal.)
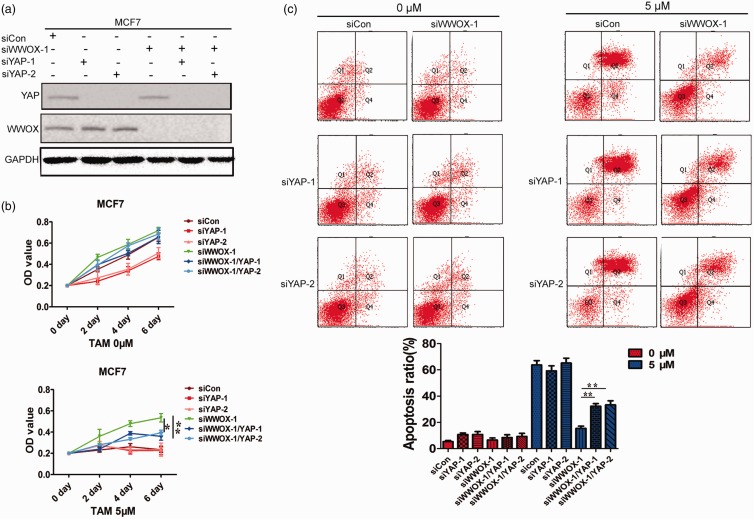
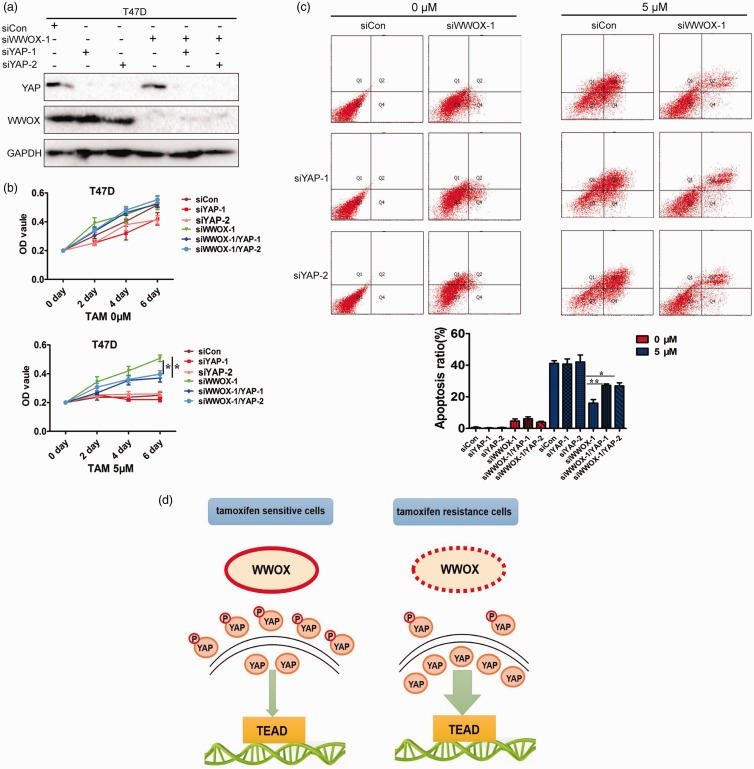
We next examined whether the altered expression of YAP in siWWOX cells was correlated with the changes in tamoxifen-resistance. Endogenous YAP was knocked down in MCF7-siCon and MCF7-siWWOX-1 cells using siRNA (Figure 5(a)). We examined the viability of MCF7-siCon, MCF7-siYAP-1, MCF7-siYAP-2, MCF7-siWWOX-1, MCF7-siWWOX-1/YAP-1, and MCF7-siWWOX-1/YAP-2 cells in the presence of 4-OHT. The knockdown of YAP in MCF7-siWWOX-1 (MCF7-siWWOX-1/YAP-1 and MCF7-siWWOX-1/YAP-2) cells significantly reversed the tamoxifen-induced increase in cell viability and enhanced apoptosis under 4-OHT treatment compared to that in siRNA control cells (MCF7-siWWOX-1; Figure 5(b) and (c)). To further verify the results, we used another tamoxifen-sensitive breast cancer line, T47D, to examine whether the downregulation of YAP decreased tamoxifen-resistance of siWWOX cells. The results showed that the knockdown of WWOX induced tamoxifen-resistance in T47D breast cancer lines, and YAP downregulation in T47D-siWWOX-1 reversed the tamoxifen sensitivity (Figure 6(a)–(c)). This evidence supports the notion that the downregulation of WWOX confers tamoxifen-resistance by the inactivation of Hippo signaling (Figure 6(d)).
Figure 5.
Downregulation of WWOX confers tamoxifen-resistance by inactivating Hippo signaling pathway. (a) Immunoblot analyses of WWOX and YAP expression following siRNA-mediated WWOX and YAP knockdown in MCF7 cells. (b) The survival rates of siCon, siYAP-1, siYAP-2, siWWOX, siWWOX/YAP-1, and siWWOX/YAP-2 cells after treatment with 4-OHT for 2, 4, or 6 days. (c) siCon, siYAP-1, siYAP-2, siWWOX-1, siWWOX-1/YAP-1, and siWWOX-1/YAP-2 cells were treated with 4-OHT (5 μΜ) for 4 days and examined by FACS. (A color version of this figure is available in the online journal.)
Figure 6.
(a) Immunoblot analysis of WWOX and YAP expression following siRNA-mediated WWOX and YAP knockdown in T47D cells. (b) The survival rates of siCon, siYAP-1, siYAP-2, siWWOX, siWWOX/YAP-1, and siWWOX/YAP-2 cells after treatment with 4-OHT for 2, 4, or 6 days. (c) siCon, siYAP-1, siYAP-2, siWWOX-1, siWWOX-1/YAP-1, and siWWOX-1/YAP-2 cells were treated with 4-OHT (5 μΜ) for 4 days and examined by FACS. (d) Schematic diagram of the role of WWOX in tamoxifen-resistance. (A color version of this figure is available in the online journal.)
Discussion
WWOX is a tumor suppressor associated with the development of endocrine carcinomas. When a single dose of the enhanced mutagen ethylideneuron was used to treat WWOX+/− mice, 80% of them developed mammary, lymphoblastic or lung tumors.11,12,28 On the other hand, it has also been reported that WWOX expression was absent in 29% of breast cancer tissues and decreased in 55–63.2% of breast cancer tissues.29–31 In addition, WWOX expression has been found to be negatively related to clinical stages of breast cancer and positively correlated with hormone receptor status.32,33 Subgroup analysis of WWOX showed that its expression was significantly reduced in invasive breast cancers and triple-negative breast cancer compared to that in normal subgroups.33–35 WWOX expression is highly correlated with ER/PR status, suggesting that it may affect the tamoxifen treatment in breast cancer. These conclusions specifically led us to hypothesize that WWOX could be of value in endocrine treatment.
Tamoxifen is the most prolific drug used for endocrine therapy at present. However, tamoxifen-resistance has become a hindrance in the success of this treatment. Thus, identifying the molecular mechanism that induces tumor cells to resist endocrine therapy is urgently warranted. In this report, we identified significant downregulation of WWOX in tamoxifen-treated breast cancer tissues, which corresponds with the results of Guler et al.36 Consistent with a causal role of WWOX in tamoxifen-resistance, WWOX-knockdown conferred tamoxifen-resistance to breast cancer cells, whereas tamoxifen-resistant cells overexpressing WWOX were more responsive to tamoxifen treatment. However, the basis of the relationship between tamoxifen-resistance and WWOX remains unclear. Nevertheless, a novel finding of this study was that the downregulation of WWOX conferred tamoxifen-resistance through the inactivation of Hippo signaling.
The Hippo signaling plays a key role in regulating cell stemness, apoptosis, and proliferation under the action of extensive intracellular and extracellular signaling.19,20 This pathway has been shown to be associated with drug resistance, such as resistance to 5-fluorouracil and doxorubicin.37,38 LATS1/2 regulates YAP by regulating protein stability and localization. Phosphorylated YAP is bound to 14-3-3 and located in the cytoplasm.38–40 Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) is key downstream transcription coactivators of Hippo signaling pathway. When the Hippo pathway is inactivated, the dephosphorylated YAP/TAZ complex is transferred to the nucleus and binds to transcription factors to promote cell proliferation and survival.41,42 However, the role of YAP in breast cancer remains controversial. In 2014, Göran Landberg and co-workers published a report showing that the decreased expression of YAP1 was an independent prognostic factor of luminal A breast cancer and that YAP1 downregulation possibly conferred decreased tamoxifen sensitivity.43 The present study showed that WWOX-knockdown reduced YAP phosphorylation, increased the levels of nuclear YAP, and increased the expression of the YAP-downstream molecules CCCTC-binding factor and Cyr61 in tamoxifen-sensitive cells. However, enhanced WWOX expression decreased nuclear YAP, Cyr61, and CTGF expression but increased YAP phosphorylation in tamoxifen-resistant cells. These studies indicate that WWOX can increase YAP phosphorylation to suppress YAP activity. Furthermore, the downregulation of YAP resulted in partial recovery of tamoxifen sensitivity in siWWOX tamoxifen-resistant cells, confirming an oncogenic role of YAP in tamoxifen-resistant cells. Collectively, these findings indicate that the downregulation of WWOX confers tamoxifen-resistance by inactivating Hippo signaling. However, the regulatory mechanism of WWOX-mediated phosphorylation of YAP in breast cancer remains to be clarified. Therefore, we analyzed the regulatory network of WWOX using the STRING database (https://string-db.org/cgi/). The database predicted the top 10 functional partners of WWOX. Mitogen-activated protein kinase 8 (MAPK8) a serine/threonine-protein kinase was one of the predicted proteins.44–46 Muranen and Laura identified that p38 MAPK kinase cascade regulates YAP activity and Hippo target expression by modulating F-actin and Jub (Ajuba LIM protein) and is a new upstream branch of the Hippo pathway.47 Meanwhile, the inhibition of p38 upregulated YAP through the stabilization of cAMP response element-binding protein.48 The relationship between MAPK8 and WWOX leads us to speculate that MAPK8 is a link between WWOX and the Hippo pathway and that WWOX-knockdown would increaseMAPK kinase activity and regulate YAP phosphorylation by promoting the phosphorylation of Ajuba family proteins or the F-actin. In addition to MAPK8, the network analysis provided many other functional partners of WWOX, such as SMAD4 and SRC, which are important components of TGFβ and epidermal growth factor receptor signaling. The regulation of YAP activity by the above signaling pathways has been reported previously.49–52 Thus, the regulation of WWOX through YAP occurs via multiple channels and pathways.
In summary, our data suggested that WWOX downregulation is significantly associated with tamoxifen-resistance. WWOX-knockdown in tamoxifen-sensitive cells led to tamoxifen-resistance, and WWOX overexpression in tamoxifen-resistant cells re-sensitized them to tamoxifen. The downregulation of WWOX conferred tamoxifen-resistance by inactivating Hippo signaling. Our findings may provide new insights into the molecular mechanisms of tamoxifen-resistance in breast cancer.
Authors’ contributions
JL and XF conducted experiments, analyzed data including statistical analysis, and revised the manuscript. CL, RW, and HC provided technical support. JL and PL revised the manuscript. PL was involved in the design of the study.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The project was funded by the National Natural Science Foundation of China (Grant Numbers: 81502620, 81703002, 81702631).
References
- 1.Lumachi F, Luisetto G, Basso SM, Basso U, Brunello A, Camozzi V. Endocrine therapy of breast cancer. Curr Med Chem 2011; 18:513–22 [DOI] [PubMed] [Google Scholar]
- 2.Orlando L, Schiavonea P, Fedelea P, Calvania N, Naccia A, Rizzoa P, Marinoa A, D’Amicoa M, Sponzielloa F, Mazzoni E, Cinefraa M, Faziob N, Maielloc E, Silvestrisd N, Coluccid G, Cinieria S. Molecularly targeted endocrine therapies for breast cancer. Cancer Treat Rev 2010; 36:S67–71 [DOI] [PubMed] [Google Scholar]
- 3.Group* EBCTC. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998; 351:1451–67 [PubMed] [Google Scholar]
- 4.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998; 95:927–37 [DOI] [PubMed] [Google Scholar]
- 5.Zhao B, Ye X, Yu J, Li L, Li W, Li S. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008; 22:1962–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene 2012; 31:5117–22 [DOI] [PubMed] [Google Scholar]
- 7.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13:12–22 [DOI] [PubMed] [Google Scholar]
- 8.Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci U S A 2007; 104:3949–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JT, Tzai TS, Liao CY, Wang JS, Wu TT, Wang HY. WWOX protein expression varies among RCC histotypes and downregulation of WWOX protein correlates with less-favorable prognosis in clear RCC. Ann Surg Oncol 2013; 20:193–9 [DOI] [PubMed] [Google Scholar]
- 10.Nunez MI, Rosen DG, Ludes-Meyers JH, Abba MC, Kil H, Page R. WWOX protein expression varies among ovarian carcinoma histotypes and correlates with less favorable outcome. BMC Cancer 2005; 5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paige AJW, Zucknick M, Janczar S, Paul J, Mein CA, Taylor K, Stewant M, Gourley C, Richardson S, Perren T, Ganeasan TS, Smyth JF, Brown R, Gabra H. WWOX tumour suppressor gene polymorphisms and ovarian cancer pathology and prognosis. Eur J Cancer 2010; 46:818–25 [DOI] [PubMed] [Google Scholar]
- 12.Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PLoS One 2009; 4:e7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gothlin Eremo A, Wegman P, Wegman P, Stal O, Nordenskjold B, Fornander T, Wingren S. Wwox expression may predict benefit from adjuvant tamoxifen in randomized breast cancer patients. Oncol Rep 2013; 29:1467–74 [DOI] [PubMed] [Google Scholar]
- 14.Coser KR, Wittner BS. Antiestrogen-resistant subclones of MCF-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor. Proc Natl Acad Sci U S A 2009; 106:14536–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Z, Li W, Liu P. Simvastatin suppresses DNA replication licensing factor MCM7 and inhibits growth of tamoxifen resistant breast cancer cells. Sci Rep 2017; 7:41776–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma G, Pan Y, Zhou C, Liu P, Ren Y, He J. Mitogen-activated protein kinase phosphatase 1 is involved in tamoxifen resistance in MCF7 cells. Oncol Rep 2015; 34:2423–30 [DOI] [PubMed] [Google Scholar]
- 17.Li J, Liu J. Loss of LKB1 disrupts breast epithelial cell polarity and promotes breast cancer metastasis and invasion. J Exp Clin Cancer Res 2014; 33:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Touil Y, Igoudjil W, Corvaisier M, Dessein AF, Vandomme J, Monte D, Stechly L, Skrypek N, Langlois C, Grard G. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res 2014; 20:837–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salah Z, Aqeilan RI. WW domain interactions regulate the Hippo tumor suppressor pathway. Cell Death Dis 2011; 2:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang DY, Wu YN, Huang JQ, Wang W, Xu M, Jia JP, Han G, Mao BB, Bi WZ. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin J Cancer 2016; 35:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudol M, Harvey KF. Modularity in the Hippo signaling pathway. Trends Biochem Sci 2010; 35:627–33 [DOI] [PubMed] [Google Scholar]
- 22.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 2011; 13:877–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aqeilan RI, Donati V, Gaudio E, Nicoloso MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H. Association of Wwox with ErbB4 in breast cancer. Cancer Res 2007; 67:9330–6 [DOI] [PubMed] [Google Scholar]
- 24.Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res 2004; 64:8256–61 [DOI] [PubMed] [Google Scholar]
- 25.Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K, Croce CM. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci U S A 2004; 101:4401–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res 2006; 66:11585–9 [DOI] [PubMed] [Google Scholar]
- 27.Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, Sudol M, Croce CM. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res 2005; 65:6764–72 [DOI] [PubMed] [Google Scholar]
- 28.Nunez MI, Ludes-Meyers J, Aladz CM. WWOX protein expression in normal human tissues. J Mol Histol 2006; 37:115–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guler G, Uner A, Guler N, Han SY, Lliopoulos D, McCue P, Huebner K. Concordant loss of fragile gene expression early in breast cancer development. Pathol Int 2005; 55:471–8 [DOI] [PubMed] [Google Scholar]
- 30.Ekizoglu S, Muslumanoglu M, Dalay N, Buyru N. Genetic alterations of the WWOX gene in breast cancer. Med Oncol 2012; 29:1529–35 [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Chao L, Ma G, Chen L, Zang Y, Sun J. The prognostic significance of WWOX expression in patients with breast cancer and its association with the basal-like phenotype. J Cancer Res Clin Oncol 2011; 137:271–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pluciennik E, Kusinska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX – the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol 2006; 32:153–7 [DOI] [PubMed] [Google Scholar]
- 33.Ferguson BW, Gao X, Kil H, Lee J, Benavides F, Abba MC, Aldaz CM. Conditional Wwox deletion in mouse mammary gland by means of two CRE recombinase approaches. PLoS One 2012; 7:e36618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salah Z, Aqeilan R, Huebner K. WWOX gene and gene product: tumor suppression through specific protein interactions. Future Oncol 2010; 6:249–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guler G, Uner A, Guler N, Han SY, Lliopoulos D, Hauck WW, McCue P, Huebner K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer 2004; 100:1605–14 [DOI] [PubMed] [Google Scholar]
- 36.Guler G, Iliopoulos D, Guler N, Himmetoglu C, Hayran M, Huebner K. Wwox and Ap2gamma expression levels predict tamoxifen response. Clin Cancer Res 2007; 13:6115–21 [DOI] [PubMed] [Google Scholar]
- 37.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res 2011; 71:2728–38 [DOI] [PubMed] [Google Scholar]
- 38.Chang JY, He RY, Lin HP, Hsu LJ, Lai FJ, Hong Q, Chen SJ, Chang NS. Signaling from membrane receptors to tumor suppressor WW domain-containing oxidoreductase. Exp Biol Med 2010; 235:796–804 [DOI] [PubMed] [Google Scholar]
- 39.Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med 2015; 21:212–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 2010; 24:72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem 2010; 285:37159–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 2008; 14:388–98 [DOI] [PubMed] [Google Scholar]
- 43.Lehn S, Tobin NP, Sims AH, Stål O, Jirström K, Axelson H, Landberg G. Decreased expression of Yes-associated protein is associated with outcome in the luminal A breast cancer subgroup and with an impaired tamoxifen response. BMC Cancer 2014; 14:119–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci 2006; 31:268–75 [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Li N. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem Sci 2014; 39:268–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang D, Li X. Regulation of Hippo signalling by p38 signalling. J Mol Cell Biol 2016; 8:328–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muranen T, Laura M. ERK and p38 MAPK activities determine sensitivity to PI3K/mTOR inhibition via regulation of MYC and YAP. Cancer Res 2016; 76:7168–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun G, Irvine KD. Ajuba family proteins link JNK to Hippo signaling. Sci Signal 2014; 6:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito A, Nagase T. Hippo and TGF-β interplay in the lung field. Am J Physiol Lung Cell Mol Physiol 2015; 309:756–67 [DOI] [PubMed] [Google Scholar]
- 50.Grannas K, Arngården L. Crosstalk between Hippo and TGFβ: subcellular localization of YAP/TAZ/Smad complexes. J Mol Biol 2015; 427:3407–15 [DOI] [PubMed] [Google Scholar]
- 51.Elshaer N, Piulachs MD. Crosstalk of EGFR signalling with Notch and Hippo pathways to regulate cell specification, migration and proliferation in cockroach panoistic ovaries. Biol Cell 2015; 107:273–85 [DOI] [PubMed] [Google Scholar]
- 52.Zhao K, Wang Q. EGFR/c-myc axis regulates TGFβ/Hippo/Notch pathway via epigenetic silencing miR-524 in gliomas. Cancer Lett 2017; 406:12–21 [DOI] [PubMed] [Google Scholar]