Short abstract
Hypoxic–ischemic brain damage (HIBD) is one of the leading causes of brain injury in infant with high risk of mortality and disability; therefore, it is important to explore more feasible and effective treatment strategies. Here, we assessed the neuroprotective effects of different hydrogen inhalation times for the treatment of HIBD. We induced hypoxia–ischemia in Sprague–Dawley rats (postnatal day 7, both sexes), followed by treatment with hydrogen inhalation for 30, 60, or 90 min. Morphological brain injury was assessed by Nissl and TUNEL staining. Acute inflammation was evaluated by examining the expression of interleukin-1β (IL-1β) and NF-κB p65, as well as Iba-1 immunofluorescence in the brain. Neural apoptosis was evaluated by examining the expression of P-JNK and p53 as well as NeuN immunofluorescence. Neurobehavioral function of rats was evaluated by Morris water maze test at 36 days after surgery. The results showed that hypoxia–ischemia injury induced the inflammatory response of microglia; however, these changes were inhibited by hydrogen inhalation. The inhibitory effects became more apparent as the treatment duration increased (P < 0.05). Furthermore, hypoxia–ischemia induced neuronal damage and increased the expression of the apoptotic factors, P-JNK, and p53, which were attenuated by hydrogen inhalation (P < 0.05). Hypoxia–ischemia caused long-term spatial memory deficits during brain maturation, which were ameliorated by hydrogen inhalation (P < 0.01). In conclusion, hypoxia–ischemia induced severe long-term damage to the brain, which could be alleviated by hydrogen inhalation in a time-dependent manner.
Impact statement
Oxidative stress is known to be involved in the main pathological progression of neonatal hypoxic–ischemic brain damage (HIBD). Hydrogen (H2) is an antioxidant that can be used to treat HIBD; however, the mechanism by which hydrogen may be used as a promising treatment for neonates with HIBD is not very clear. This study demonstrated that inhaled H2 is neuroprotective against HIBD in SpragueDawley rats by inhibiting the brain’s inflammatory response and neuronal apoptosis or damage and protecting against spatial memory decline. Further, this study showed that inhaled H2 has potential as a therapeutic approach for HIBD. This is relevant to clinical treatment protocols when hypoxia–ischemia is suspected in neonates.
Keywords: Cerebral ischemia–hypoxia, hydrogen, spatial memory, inflammation, apoptosis
Introduction
Hypoxic–ischemic brain damage (HIBD) refers to brain damage caused by asphyxia due to hypoxia and ischemia during the perinatal period, which is proved to be the main cause of mortality and disability in neonates.1,2 The global incidence of HIBD among live births is approximately 1–8‰, reaching up to 26‰ in developing countries and up to 60% among newborns with very low birth weight. The mortality rate for children with HIBD is approximately 20–25% in the neonatal period, and approximately 25% of surviving children experience permanent neurological deficits, such as mental retardation, developmental retardation, motor or cognitive impairment, cerebral palsy, and epilepsy.2,3 Mild hypothermia therapy can alleviate the brain damage caused by hypoxia–ischemia (HI); however, some neonatal patients exhibit persistent neurological defects even after treatment.4–6 Therefore, it is essential to develop more feasible and effective therapeutic strategies for reducing brain damage caused by HI in neonates.
Hydrogen (H2) is a colorless, odorless, non-toxic antioxidant that is protective against diseases mediated by oxidative stress, including ischemia–reperfusion injury in the adult rat brain, heart, liver, kidney, small intestine, spinal cord, and other organs.7–12 In 2008, Kajiyama et al.13 investigated H2 in clinical trials, observing that drinking H2-rich water could effectively improve the symptoms in patients with diabetes. At present, there are three primary forms of H2 administration: H2 inhalation, injection of H2-rich saline, and oral ingestion of H2-saturated water.14,15 One study reported that oral H2-rich water may result in harmful alterations to some biochemical indices.16 Oral ingestion or injection of H2-rich water or saline, respectively, can increase the allostatic load. In addition, it is difficult to control the concentration of H2 using these methods. Moreover, the curative effect associated with these treatments are limited by short exposure times and other factors, and the complex protocols required are not suitable for newborns.17,18 In contrast, H2 inhalation treatment is safe for the human body at concentrations < 4%, is not associated with a risk of explosion when exposed to air, and the concentration is easy to control.19,20 H2 has a low molecular weight and strong permeability, and it easily penetrates the biofilm and diffuses into the cytoplasm, mitochondria, and nucleus.12 Thus, researchers have focused more heavily on this method of administration.
Cai et al.21 demonstrated that inhalation of H2 has a neuroprotective effect in HIBD rats; however, Matchett et al.22 reported that H2 inhalation does not protect against HIBD and aggravates brain damage in rats. To identify a safe and effective method, we examined the effect of 3% H2 inhalation for different durations in HIBD rats. Furthermore, the potential mechanisms underlying the neuroprotective effect of H2 inhalation will be assessed as well.
Materials and methods
Animals
Seven-day-old female and male Sprague–Dawley (SD) rats (body weight range from 9 to13 g) were purchased from Slac Corporation (Changsha Slac Animal Corporation, Hunan, China). The rats’ pups were housed in cages with their mothers. All experiments were approved by the Center for Medical Ethics of Central South University and were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (eight edition).
Hypoxia–ischemia model in neonatal rats
We induced HIBD in rats using the Rice–Vannucci model.23 The pups were anesthetized with isoflurane, and the left common carotid artery (CCA) was carefully exposed. Afterwards, the CCA was double-ligated using a 6–0 surgical silk tie, which was cut between sutures. The skin incision was then sutured, and pups were permitted to recover for 2 h with their dam in their home cages. Following this, rats were placed in a glass anoxic chamber (constant temperature of 37°C; 8% O2+ 92% N2) for 100 min to induce brain injury.
H2 inhalation and experimental design
H2 was generated via water electrolysis in a hydrogen gas supply device (MiZ Co. Ltd, Kanagawa, Japan). Following HI injury, the rat pups were treated with mixed gas (3% hydrogen and 97% air) in a device. The device was 30 × 13 × 12.5 cm in size, white, and transparent, allowing us to observe the activity of the rats. A total of 140 rats were divided randomly into seven groups. The four experimental groups were classified according to the length of H2 inhalation. The rats divided in the first three groups listed below were interfered with H2 inhalation immediately after HI, while the rats in the last group were started at 2 h after HI: (1) 30-min (n = 22; female: 12, male: 10); (2) 60 min (n = 22; female: 11, male: 11); (3) 90 min (n = 22; female: 10, male: 12); (4) 90 min (start at 2 h after HI, n = 8; female: 4, male: 4). The three control groups were classified as follows: (1) naive (no processing) (n = 22; female: 10, male: 12); (2) sham-surgery (left common carotid artery was separated without ligation then sutured, and no hypoxia) (n = 22; female: 13, male: 9); and (3) HIBD (carotid ligation and hypoxia) (n = 22; female: 11, male: 11). Rats were interfered with H2 inhalation twice daily then euthanized at 24 h or 72 h after surgery for 2,3,5- triphenyltetrazolium chloride (TTC) staining or immunoblot and histological staining, respectively. Twelve rats per group were used for pathology and molecular biology experiments, and ten rats per group were used to evaluate neurobehavioral function.
Infarct volume
TTC (Sigma, St. Louis, MO, USA) staining was used to evaluate cerebral infarcted area in rats. At 24 h post-surgery, rats were sacrificed via decapitation, and brains were sliced into five sequential coronal sections (thickness, 2 mm), followed by immersed in the 1% TTC solution for 30 min at 37°C and turned every 5 min. The sections were sequentially photographed (Sony, Tokyo, Japan) and the cerebral infarction/non-infarction volume was calculated by Image J software. The calculation of the percentage of infarct volume was the same as Cai et al. described.21 The percentage of infarct volume (%) = the infarct volume/the total volume of the sections.
Nissl staining
Rats’ pups were deeply anaesthetized and the brains were obtained following perfusion and fixed. Then, the fixed brain tissue was paraffin embedded, followed by serial sectioning into 5-μm slices. After deparaffinization, the slices were immersed in 2% Nissl working solution for 5 min. Afterwards, the slices were rinsed in differentiation solution until the Nissl body was clarified (Wellbio, Beijing, China). The slices were photographed under a microscope (magnification 200×; Nikon, Tokyo, Japan).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
TUNEL staining assay kit (Roche, Basel, Switzerland) was applied to detect apoptosis. Briefly, the slices were incubated with TUNEL reaction solution for 1 h. Afterwards, washed the slices with PBS and counterstained the nucleus with DAPI for 10 min. The sections were visualized under a fluorescence microscope (Nikon) with 400× magnification. Four slide fields within the left frontal and parietal cortex and hippocampal CA1 were randomly examined and the results are expressed as the percentage of TUNEL-positive cells in selected field (TUNEL-positive cells/total number of nuclei).
Immunofluorescence
After deparaffinization, sections were under antigen retrieval process. Followed by blocking with 5% BSA for 1 h, the slices were immersed overnight at 4°C in primary antibodies. The antibodies examined in the experiment are listed below: IL-1β (1:100; Proteintech, Rosemont, Maryland, USA), NF-κB p65 (1:100; Abcam, Cambridge, CA, USA), P-JNK (1:100; Abcam), p53 (1:200; Proteintech), Iba-1 (1:500; Abcam) and NeuN (1:500; Millipore, Billerica, MA, USA). Next day, the sections were rinsed with PBST three times, and visualized with Alexa Fluor-labeled secondary antibodies (Alexa Fluor 594, 1:800; Thermo Scientific, Rockford, IL, USA; Alexa Fluor 488, 1:800; Thermo Scientific, Rockford, IL, USA) for 2 h. DAPI were used for counterstaining the nucleus. The micro- and macro-images from the frontal and parietal cortex region or hippocampal CA1 were acquired under a microscope (magnification 400×; Nikon), and merged using Image J.
Western blot analysis
The tissue proteins of the frontal and parietal cortex region were extracted at 72 h after surgery and quantified with a BCA kit (CWBIO, Beijing, China). Protein samples were separated by using SDS-PAGE gels under different concentrations range from 8% to 12%, and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA). After blocking with 5% skimmed milk in PBS for 2 h, membranes were incubated overnight at 4°C with the following primary antibodies: IL-1β (1:500, Bioss, Beijing, China), NF-κB p65 (1:500, Abcam, Cambridge, CA, USA), P-JNK (1:500, Abcam), p53 (1:1000, Proteintech, Rosemont, Maryland, USA), β-actin (1:1000, Proteintech), and GAPDH (1:1000, Proteintech). The next day, membranes were washed with PBST. Afterwards, membranes were immersed in the secondary antibody (IRDye 800CW, 1:10,000). Immunoblotting bands were automatically photographed by the Odyssey-CLX infrared imaging system (Li-COR®, USA). The relative intensities of the bands were analyzed using Image J.
Morris water maze
The MWM test was performed during adolescence (postnatal days 43–47) to assess neurobehavioral function of rats mainly related to the abilities including the spatial memory and learning.24 The experiment was carried out in a cylindrical pool (Panlab, Spain), and water temperature of 22 ± 1°C. The pool included four equal quadrants: southeast, southwest, northeast, and northwest. A platform was hidden in the middle of the northeast quadrant. Visual reference objects were located around the pool for spatial localization, such as fixed experimental items, lights, and cards of different shapes, which were unchanged throughout the experiment.
The experiment included two parts: (1) Examination of spatial learning ability (location–navigation test). All rats underwent four trials per day, lasting four days. In a trial, animals were required to find the hidden platform in less than 1 min. An experimenter recorded the time to reach the platform, which was defined as the “escape latency.” If the rats could not reach the hidden platform in less than 1 min, the experimenter guided them to the hidden platform, and recorded the escape latency for 1 min. Each rat was required to stay on the hidden platform for 20 s and allowed a 20 min intertrial interval. The escape latency was automatically tracked and analyzed using tracking software (SMART 3.0 video-tracking system, Panlab, Spain). The average escape latency recorded for each of the four trials was taken as the learning achievement of the rats on the same day, and the trial was repeated for four days. (2) Examination of spatial memory (space exploration test). In probe trials, the platform was taken away, and each rat was placed in the same selected quadrant (opposite quadrant to the original hidden platform). The tracking software recorded the time (residence time) to stay in the probe quadrant and swimming path over 1 min. The residence time was used as a measure of spatial memory.
Statistical analysis
The quantified data were shown as means ± SD. One-way analyses of variance (ANOVA) followed by Tukey’s post hoc tests were used for multiple comparisons of different groups. The univariate repeated measures ANOVA was used to analyze the MWM results for independent group comparisons. Statistical analysis was performed using SPSS 22.0 Software and the bar graphs were drawn by GraphPad Prism 6 software. Differences with P < 0.05 were defined as statistically significant. In the Nissl staining experiment, TTC staining, and immunofluorescence tests, no significantly differences were obtained between the naive group and the sham-surgery group. So, the results of the experiment in the naive group are not shown in the picture.
Results
H2 inhalation improves neuronal injury and cerebral infarction volume
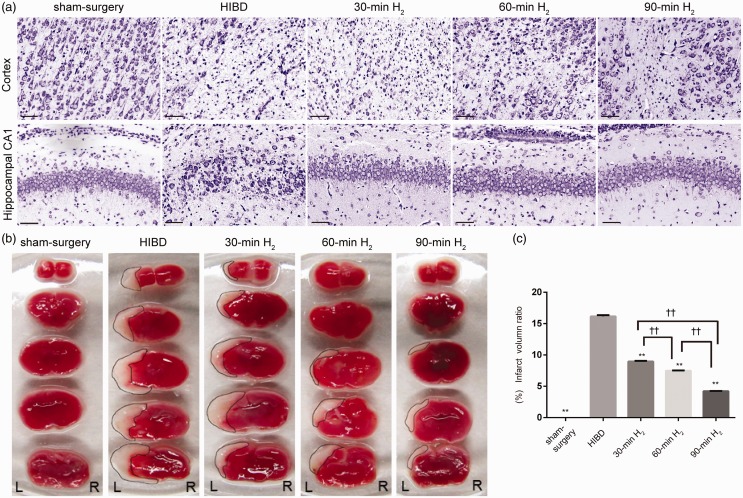
In the present study, Nissl staining was used to observe neuronal damage in the left frontal and parietal cortex and hippocampal CA1 of rats. In the sham-surgery group, neurons had a complete morphological structure, large cell bodies, and abundant cytoplasm and Nissl bodies; however, the HIBD group exhibited extensive neuronal damage, such as karyopyknosis, dark-blue staining of nuclei, neuronal structure and Nissl’s body disappeared. Interestingly, this neuronal damage was obviously alleviated after H2 inhalation, especially in the 90-min H2 group (Figure 1(a)).
Figure 1.
Nissl and TTC staining following H2 inhalation in rats with HIBD. (a) Representative images of Nissl staining in the left frontal and parietal cortex and hippocampal CA1 region of the sham-surgery, HIBD, 30-min H2, 60-min H2, and 90-min H2 groups at 72 h after surgery. Scale bar = 100 μm. n = 4 per group. (b) Representative images of TTC staining on the first day after surgery from coronal brain sections of the sham-surgery, HIBD, 30-min H2, 60-min H2 and 90-min H2 groups at 24 h after surgery. (c) The percentage of the cerebral infarct volume in the ipsilateral hemisphere of different groups. n = 4 per group. **P < 0.001 vs. HIBD group, ††P < 0.001.
In addition, TTC staining was used to analyze cerebral infarction volume. There was a dramatically higher cerebral infarction rate in HIBD rats than those in sham-surgery rats (Figure 1(a) and (b)). Infarction rates (8.96 ± 0.08%, 7.50 ± 0.02%, and 4.22 ± 0.04%, respectively) were significantly lower in the 30-min H2, 60-min H2, and 90-min H2 groups than in the HIBD group (16.16 ± 0.16%, P < 0.001; Figure 1(c)). These results indicate that H2 inhalation ameliorated neuronal injury and reduced cerebral infarction volume, which was most evident after 90-min of H2 inhalation (30-min H2 vs. 60-min H2, P < 0.001; 60-min H2 vs. 90-min H2, P < 0.001). And we found that the effects of inhaling H2 immediately after HI were better than that inhalation of H2 at 2 h after HI (P < 0.05, Supplementary Figure 1).
H2 inhalation inhibits microglial activation and inflammation
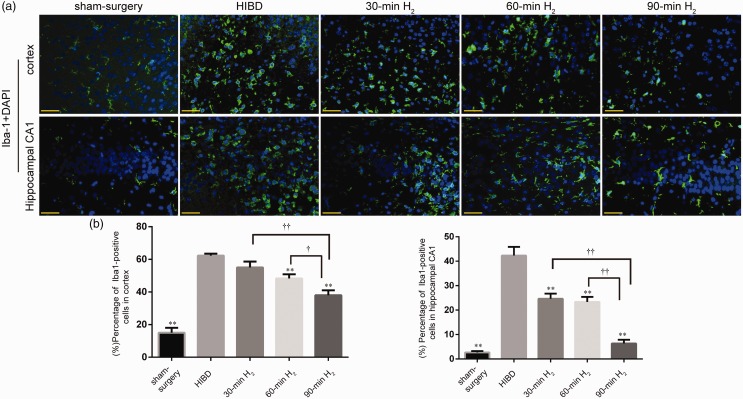
Microglia are immune cells. Iba-1 is a marker of resting and activated microglia. At 72 h after surgery, we examined Iba-1 fluorescence in the left frontal and parietal cortex and left hippocampal CA1 regions to investigate microglial activation. In the sham-surgery group, microglia were dendritic, and few positive cells were observed, indicative of resting microglia. However, in the HIBD group, microglia were oval or amoeboid in shape, and the number of positive cells had increased significantly, indicative of activated microglia (Figure 2(a) and (b)). Immunofluorescence staining showed that H2 inhalation remarkably reduced the activation of microglia after HI (Cortex, 30-min H2 vs. HIBD, P = 0.055; 60-min H2 vs. HIBD, P < 0.001; 90-min H2 vs. HIBD, P < 0.001. Hippocampal CA1, 30-min H2, 60-min H2 or 90-min H2 vs. HIBD, P < 0.001), and decreases in Iba-1 expression became more obvious as the duration of H2 inhalation increased (Cortex, P < 0.01; Hippocampal CA1, P < 0.001) (Figure 2(a) and (b)). These findings indicate that H2 inhalation significantly inhibited the activation and proliferation of microglia induced by hypoxia–ischemia, and this inhibition was most pronounced in the 90-min H2 group.
Figure 2.
Microglial activation changes following H2 inhalation in rats with HIBD. (a) Representative immunofluorescence staining Iba-1 (green, activated microglia) and DAPI (blue, all nuclei) in the left frontal and parietal cortex and hippocampal CA1 region of rats in sham-surgery, HIBD, 30-min H2, 60-min H2, and 90-min H2 groups at 72 h after surgery. Scale bar = 50μm. (b) Differences in the percentage of Iba-1-positive cells in the cortex and hippocampal CA1 between groups. n = 4 per group. **P < 0.01 vs. HIBD group, ††P < 0.01, †P < 0.05.
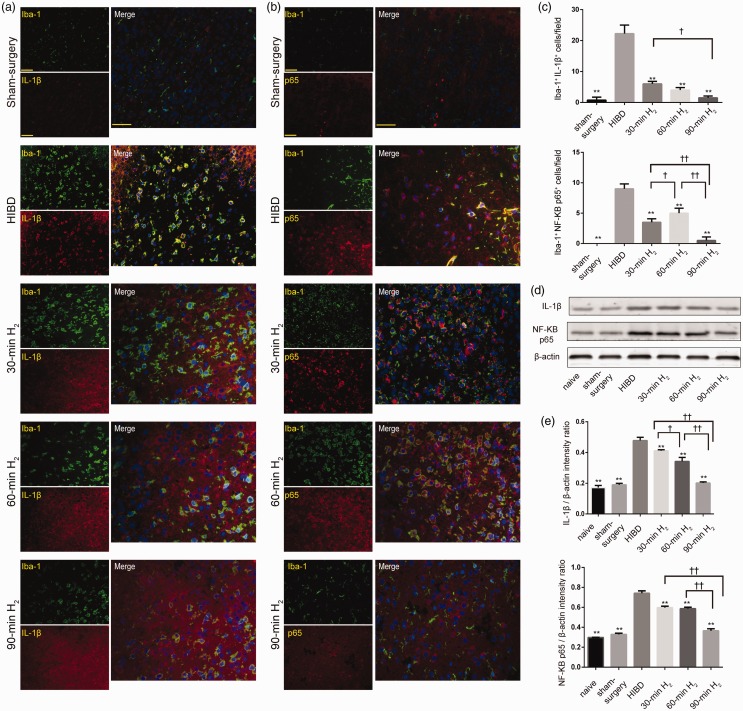
Furthermore, to verify whether H2 can inhibit the inflammatory response of microglia, we examined the expression of Iba-1, IL-1β, and NF-кB p65 in the left frontal and parietal cortex. There were dramatically increased Iba-1+/IL-1β+ and Iba-1+/NF-кB p65+ cells at 72 h after surgery, which suggested that HI induced microglial inflammatory responses. However, immediate inhalation of H2 after HI injury notably reversed these changes, and the effects were most pronounced in the 90-min H2 group (P < 0.05, Figure 3(a) to (c)). The patterns of IL-1β and NF-кB p65 expression observed via Western blot analysis were consistent with those detected via fluorescence (Figure 3(d) and (e)). The expression of IL-1β and NF-кB p65 was dramatically increased after HI injury (P < 0.001), and significantly decreased by H2 inhalation when compared with HIBD rats at 72 h post-surgery (IL-1β, 30-min H2 vs. HIBD, P = 0.0026; 60-min H2, 90-min H2 vs. HIBD, P < 0.0001. NF-кB p65, P < 0.0001). Decreases became more obvious as the duration of H2 inhalation increased (IL-1β, 30-min H2 vs. 60-min H2, P = 0.0019; 60-min H2 vs. 90-min H2, P < 0.001. NF-кB p65, 30-min H2 vs. 60-min H2, P = 0.9710; 60-min H2 vs. 90-min H2, P < 0.001).
Figure 3.
Inflammatory changes following H2 inhalation in rats with HIBD. (a, b) Representative images of IL-1β (red) or NF-κB p65 (red) and Iba-1 (green) immunofluorescence staining, and merged immunofluorescence in the left frontal and parietal cortex of rats from sham-surgery, HIBD, 30-min H2, 60-min H2, and 90-min H2 groups. Scale bar = 50 μm. (c) Quantitative analyses of Iba-1+/IL-1β+ and Iba-1+/NF-κB p65+ cells. n = 4 per group. (d) Representative western blot bands of IL-1β and NF-κB p65 in the cortex of rats from the naive, sham-surgery, HIBD, 30-min H2, 60-min H2, 90-min H2 groups. β-actin protein levels were used as internal loading controls. (e) Data represent the mean ± SD of the normalized densitometry values of IL-1β and NF-κB p65. Quantitative protein levels were normalized with β‐actin. n = 4 per group. **P < 0.01 vs. HIBD group, ††P < 0.01, †P < 0.05.
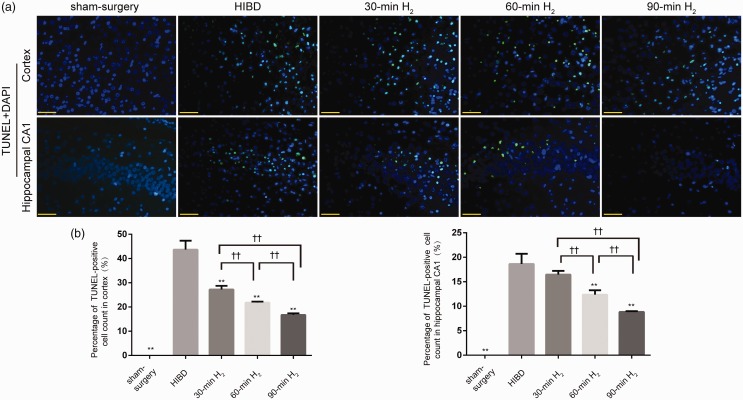
H2 inhalation inhibits neuronal apoptosis in HIBD rats
TUNEL staining assay was applied to examine the inhibition effect of apoptosis by H2 inhalation. The results illustrated that numbers of TUNEL-positive cells were obviously raised after HI, while they were reversed by H2 inhalation (P < 0.001, Figure 4(a) and (b)), especially in the 90-min H2 group (Cortex, 30-min H2 vs. 60-min H2, P = 0.0258, 60-min H2 vs. 90-min H2, P = 0.0381. Hippocampal CA1, 30-min H2 vs. 60-min H2, P = 0.0058, 60-min H2 vs. 90-min H2, P = 0.0148). However, 30 min of H2 inhalation did not reduce the number of TUNEL-positive cells in HI condition in the hippocampal CA1 region (30-min H2 vs. HIBD, P = 0.1617), indicating that 30 min of H2 inhalation had no obvious effect on neuronal apoptosis induced by HI injury. Nonetheless, the inhibitory effects of H2 inhalation on apoptosis were obvious in the 90-min H2 group. And we also found that the effects of inhaling H2 immediately after HI injury were better than that inhalation of H2 started at 2 h after HI (P < 0.05, Supplementary Figure 2(a) and (b)).
H2 inhalation inhibits upregulation of P-JNK/p53 signaling in HIBD rats
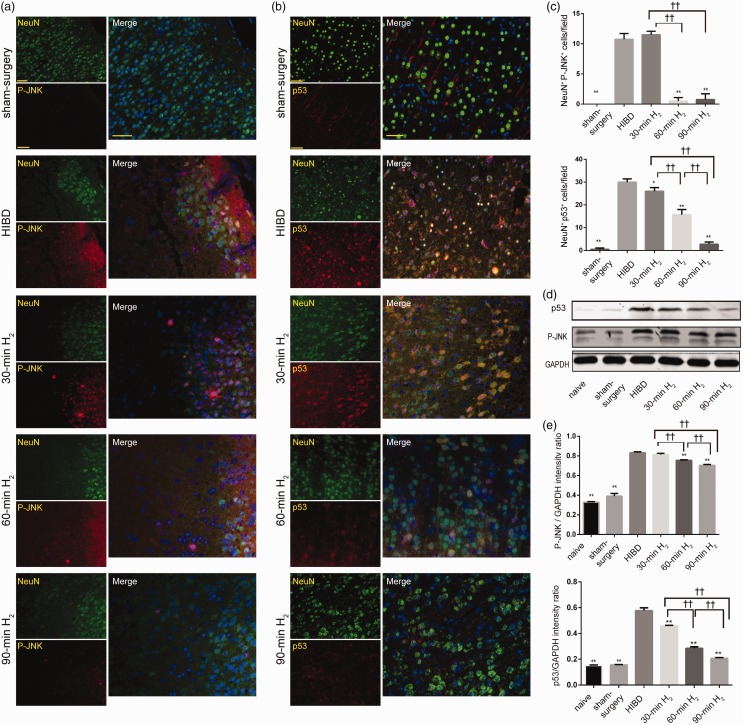
C-Jun N-terminal kinase (JNK) activates transcription factor p53 and induces apoptosis of the mitochondrial pathway. NeuN is neuronal-specific. In this study, we examined the double immunofluorescence of NeuN and P-JNK or p53 in the left frontal and parietal cortex. Double-immunofluorescence staining revealed that NeuN+/P-JNK+ and NeuN+/p53+ cells were notably raised in the HIBD rats, indicating that P-JNK and p53 expression in neurons increased after HI injury, thereby inducing apoptosis. However, hydrogen inhalation reversed this increase, and these effects were most pronounced in the 90-min H2 group (P < 0.05, Figure 5(a) to (c)). Similar results were observed via immunoblot. The expression of P-JNK and p53 protein was remarkably increased by HI injury when compared with naive and sham-surgery rats at 72 h after surgery (P < 0.001; Figure 5(d) and (e)). However, H2 inhalation attenuated these increases (P-JNK, 30-min H2 vs. HIBD, P = 0. 6215, 60-min H2 vs. HIBD, P = 0. 002, 90-min H2 vs. HIBD, P < 0.001. p53, 30-min H2, 60-min H2 or 90-min H2 vs. HIBD, P < 0.001), and such attenuation became more obvious as the duration of H2 inhalation increased (P < 0.05). The result of this section illustrated that H2 inhalation inhibited the P-JNK/p53 signaling in a time-dependent manner, thus, it inhibited neuronal apoptosis.
Figure 5.
H2 inhalation decreases the protein expression of P-JNK and p53 in the cortex of rats with HIBD. (a, b) Representative images of P-JNK (red) or p53 (red) and NeuN (green) staining, and merged immunofluorescence in the left frontal and parietal cortex of rats from the sham-surgery, HIBD, 30-min H2, 60-min H2, and 90-min H2 groups at 72 h after surgery. Scale bar = 50μm. (c) Quantitative analyses of NeuN+/P-JNK+ and NeuN+/p53+ cells. n = 4 per group. (d) Representative Western blot bands of P-JNK and p53 in the cortex of rats from the naive, sham-surgery, HIBD, 30-min H2, 60-min H2, and 90-min H2 groups. GAPDH protein levels were used as internal loading controls. (e) Data represent the mean ± SD of the normalized densitometry values of P-JNK and p53. Quantitative protein levels were normalized with GAPDH. n = 4 per group. **P < 0.01 vs. HIBD group, ††P < 0.01.
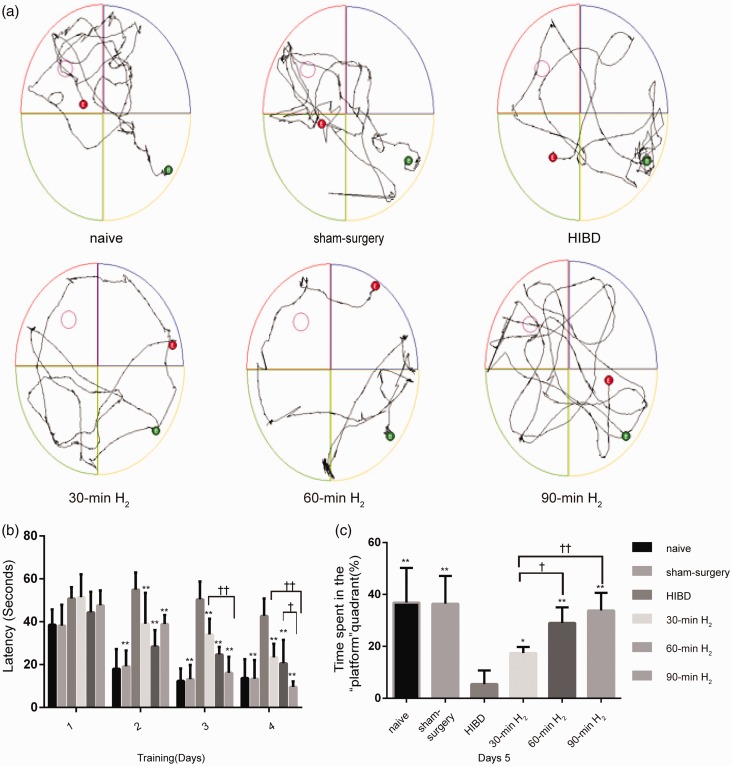
H2 inhalation improves long-term neurobehavioral deficits in HIBD rats
To further assess the neuroprotective effects of H2 inhalation, the long-term neurobehavioral function was examined by MWM experiment in HI rats during adolescence (P43–47). From the first to fourth day, the mean escape latency gradually decreased in all groups. On the first day, H2 inhalation could not shorten the escape latency after HI (P > 0.05). However, from days 2 to 4, H2 inhalation significantly shortened the mean escape latency (main effects of group, P < 0.001; main effects of time, P < 0.001; the interaction between group and time, P < 0.001; days 2–4, P < 0. 001) (Figure 6(b)), compared with the HIBD group. Furthermore, the mean escape latency was shortest in the 90-min H2 group. These results indicated that all rats showed the abilities of learning and remembering the location of the platform; however, this learning ability was impaired because of hypoxia and ischemia. Furthermore, the findings suggest that H2 inhalation rescued spatial learning in HIBD rats. On the fifth day, the swimming path was located mainly around the initial quadrant (Figure 6(a)) in the HIBD group, which also exhibited the shortest time in the target quadrant (Figure 6(c)). These findings suggested that HI damage during the neonatal period resulted in memory impairment in adolescent rats. However, H2 inhalation obviously improved the duration spent in the platform quadrant (30-min H2 vs. HIBD, P = 0.0231, 60-min H2 or 90-min H2 vs. HIBD, P < 0.0001), which was longest in the 90-min H2 group (Figure 6(c)). These results suggested that H2 inhalation effectively improved memory impairments induced by HI.
Figure 6.
H2 inhalation improves long-term spatial learning and memory in rats with HIBD. Rats were trained from postnatal days 43–47. Each rat underwent four trials per day for four consecutive days and a probe trial on the fifth day. (a) Representative swimming paths of rats on day 5 of testing in the naive, sham-surgery, HIBD, 30-min H2, 60-min H2, and 90-min H2 groups of rats. (b) Latencies to reach the hidden platform for each group of rats. (c) The time spent (%) in the target quadrant on day 5 of testing for each group of rats. Data represent mean ± SD from independent experiments. n = 10 per group. ** P < 0.01 vs. HIBD group, ††P < 0.01, †P < 0.05.
Discussion
In summary, our present findings confirmed the neuroprotective effect of H2 inhalation in HIBD rats. H2 inhaling reduced the activation of microglia in HIBD rats, reduced the accumulation of IL-1β and NF-KB p65, inhibited the microglial inflammatory response, reduced the cerebral infarction rate, inhibited neuronal apoptosis by downregulating the expression of P-JNK and p53, reduced neuronal damage, and improved long-term neurobehavioral deficits induced by HI in a time-dependent manner.
The common clinical treatment methods for neonatal HIBD include mild hypothermia, hyperbaric oxygen, and stem cell transplantation26,26; however, this effect is not consistent.4,27,28 Previous researches have indicated that oxidative stress, apoptosis, and inflammation are associated with neonatal HIBD.12,25 Oxidative stress is involved in the main pathological progression of HIBD. Hypoxia–ischemia injury induces the activation of microglia to produce oxygen free radicals, which then promote the secretion of inflammatory mediators.29 Both H2 and hydrogen sulfide (H2S) have shown neuroprotective effect by anti-oxidative stress and selectively scavenge oxygen free radicals.12,30 However, the discrepancy of H2S after brain injury needs to be pointed. For example, Li et al.31 reported that 0.1 mmol/L protected against ischemic brain damage in rats; however, the absolutely opposite effect was obtained by Qu et al.32 when the concentration of H2S reached 0.18 mmol/L in the same model. These results indicated that the effective window of H2S is extremely small. Considering that H2S is a poisonous gas having a pungent odor, it is not suitable for newborns. Moreover, neither is easy to control the therapeutic concentration, nor can instrument accurately measure the concentration of H2S in cells, it is difficult to track and analyze the effects of H2S.33 Compared to H2S, H2 is a safe and stable molecular free radicals’ scavenger odor, which can be measured easily and accurately. Therefore, H2 was chosen for treatment in the present study.
We performed three different durations of H2 inhalation, i.e. 30 min, 60 min, and 90 min. We compared the effects of three different H2 inhalation durations on HIBD and found that inhaling H2 for 90 min was the most effective. The therapeutic effect of inhaling H2 continuously several times was more effective than single short-term inhalation of H2.22 Simultaneously, we performed H2 inhalation at two different time intervals after HI injury, i.e. immediately, and 2 h after HI injury. We found that the effects of inhaling H2 immediately after HI were better than that inhalation of H2 start at 2 h after HI. It seems to imply that the earlier H2 intervention is performed, the better neuroprotective effect will be obtained.
Our MWM test results indicated that neonatal rats exposed to HI exhibit poor neurobehavioral outcomes during adolescence, suggesting that hippocampal and cortex injury during the stage of development impairs spatial memory and learning abilities. However, inhalation of H2 immediately after HI injury significantly improved neurobehavioral function (Figure 6). The neuroprotective effect of H2 on HIBD appears to be associated with the anti-inflammatory and anti-apoptotic effects after scavenging oxygen-free radicals, as H2 treatment significantly diminished the activation of microglia, in addition to IL-1β and NF-кB p65 protein expression. In addition, we observed that H2 inhalation significantly increased neuronal survival, decreased the infarct ratio and level of apoptosis, and reduced P-JNK and p53 activity.
Microglia are derived from the hematopoietic system and constitute immune cells in the central nervous system, serving immune and self-defense functions.34,35 Microglia are first activated after cerebral ischemia and produce proinflammatory cytokines, which participate in the inflammatory response after ischemia injury.36 Therefore, we investigated inflammatory responses based on microglial activation.
IL-1β interacts with activated microglia, producing persistent inflammatory products that enhance inflammation and cause neuronal damage.37,38 NF-кB upregulates the expression of inflammatory cytokines after activation under various extracellular stresses, thereby initiating the inflammatory response and apoptosis, which includes p65 and p50 subunits.39–41 The secretion of the proinflammatory cytokine IL-1β activates NF-кB, which in turn upregulates the expression of IL-1β, thus enhancing the neuroinflammatory response. In the present study, we observed microglial activation, and a corresponding increase in IL-1β and NF-кB p65 expression, following HI injury, which were significantly attenuated following H2 treatment in HIBD rats (Figures 2 and 3). Moreover, these inhibitory effects became more apparent as the treatment duration increased. Furthermore, the therapeutic effects of H2 were possibly mediated by the inhibition of IL-1β and NF-кB p65 activities.
Activated microglia produce proinflammatory cytokines that have been proven to be critical mediators of neurodevelopment.42 However, over-reactive microglia and inflammatory mediators may cause neuronal damage.38 In the present study, the loss of Nissl bodies following HI injury was associated with vacuole formation, and there was a remarkable rise in infarction rates and apoptosis (Figures 1 and 4). These findings indicate that the increased inflammatory response results in neuronal damage; however, inhalation of H2 after HI injury obviously attenuated neuronal damage. Taken together, these data further indicate that inhalation of H2 attenuates neuronal damage induced by HI.
Figure 4.
H2 inhalation reduces neuronal apoptosis in the cortex and hippocampal CA1 of rats with HIBD. (a) Representative TUNEL staining in the left frontal and parietal cortex and hippocampal CA1 region of rats from the sham-surgery, HIBD, 30-min H2, 60-min H2, and 90-min H2 groups at 72 h after surgery (green, TUNEL-positive cells; blue, cell nuclei). Scale bar = 50 μm. (b) The percentage of TUNEL-positive staining in each group. n = 4 per group. **P < 0.01 vs. HIBD group, ††P < 0.01.
The JNK signaling pathway can be activated by proinflammatory cytokines, and associated with oxidative stress, inflammation, and apoptosis.43,44 The JNK pathway mediates apoptosis via the death receptor, mitochondrial apoptosis, and endoplasmic reticulum stress apoptotic pathways. At the early stage of apoptosis, calcium ions in the cytoplasm continuously increase the activation of JNK [activated JNK (P-JNK)] in the nucleus, further activating transcription factors, such as c-Jun or p53, and regulating the activity of the Bcl-2 family of proteins in the cytoplasm.45 This results in an increase in mitochondrial membrane permeability, which activates the mitochondrial apoptosis pathway. P53, a tumor suppressor protein, acts as a transcription factor to induce cell cycle arrest, DNA damage and repair, and cell senescence.46 Therefore, we chose JNK as the major indicator, which was validated by the JNK/p53 pathway in our experiments. Our results indicate that HI injury activated the JNK signaling pathway and increased P-JNK expression, promoting the expression of the downstream pro-apoptotic protein p53, which induced apoptosis (Figure 5).
Our findings demonstrated that inhalation of 3% H2 could reduce the activation of microglia. Furthermore, inhibition of the JNK/p53 pathway significantly improved learning and memory functions in HIBD rats. However, there are some limitations to our study that must be acknowledged. For example, we focused on microglia in this study. Astrocytes, other immune cells, and other brain regions should be assessed in future studies to further our understanding of the protective mechanism of H2. We can further study the therapeutic effect of hydrogen on different sexes of rats.
Taken together, our study demonstrated that cerebral HI activates microglia, promotes the secretion of IL-1β and NF-кB p65, induces neuronal damage, upregulates P-JNK/p53 signal transduction, and induces long-term spatial memory defects. Inhalation of 3% H2 immediately after HI injury reduced inflammation and inhibited cell apoptosis. In summary, H2 may play a neuroprotective role and improve long-term spatial memory in HIBD rats by reducing inflammation and inhibiting apoptosis. Therefore, H2 may be an effective method for the treatment of HIBD.
Supplemental Material
Supplemental material, Supplemental Material1 for Hydrogen inhalation protects hypoxic–ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats by Guojiao Wu, Zhiheng Chen, Peipei Wang, Mingyi Zhao, Masayuki Fujino, Chen Zhang, Wenjuan Zhou, Shin-Ichi Hirano, Xiao-Kang Li and Lingling Zhao: on behalf of the Heinz Nixdorf Recall Study Investigative Group in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplemental Material2 for Hydrogen inhalation protects hypoxic–ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats by Guojiao Wu, Zhiheng Chen, Peipei Wang, Mingyi Zhao, Masayuki Fujino, Chen Zhang, Wenjuan Zhou, Shin-Ichi Hirano, Xiao-Kang Li and Lingling Zhao: on behalf of the Heinz Nixdorf Recall Study Investigative Group in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplemental Material3 for Hydrogen inhalation protects hypoxic–ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats by Guojiao Wu, Zhiheng Chen, Peipei Wang, Mingyi Zhao, Masayuki Fujino, Chen Zhang, Wenjuan Zhou, Shin-Ichi Hirano, Xiao-Kang Li and Lingling Zhao: on behalf of the Heinz Nixdorf Recall Study Investigative Group in Experimental Biology and Medicine
ACKNOWLEDGMENTS
We are grateful to the staff from the Medical Center Laboratory of the Third Xiangya Hospital for their excellent technical assistance.
Authors’ contributions
The designation of the experiments as well as the interpretation and analysis of the data was contributed by all authors. The manuscript was drafted by Wu and reviewed and approved by all authors; GW, ZC, PW, MZ, and WZ conducted the experiments; MF and SH supplied critical reagents.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Project for Xiangya famous doctor in Central South University (2014[68]), New xiangya talent of the third Xiangya hospital (2014[97]), and the Natural Science Foundation of Hunan Province of China (2018 JJ 2620).
References
- 1.Lawn J, Blencowe H, Oza S, You D, Lee A, Waiswa P, Lalli M, Bhutta Z, Barros A, Christian P, Mathers C, Cousens S. Every newborn: progress, priorities, and potential beyond survival. Lancet 2014; 384:189–205 [DOI] [PubMed] [Google Scholar]
- 2.Kurinczuk J, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010; 86:329–38 [DOI] [PubMed] [Google Scholar]
- 3.Douglas-Escobar M, Weiss M. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr 2015; 169:397–403 [DOI] [PubMed] [Google Scholar]
- 4.Edwards A, Brocklehurst P, Gunn A, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010; 340:c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzopardi D, Strohm B, Edwards A, Halliday H. Moderate Hypothermia to Treat Perinatal Asphyxial Encephalopathy. N Engl J Med 2009; 361:1349–58 [DOI] [PubMed] [Google Scholar]
- 6.Thoresen M. Who should we cool after perinatal asphyxia? Semin Fetal Neonatal Med 2015; 20:66–71 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X. Anti-inflammatory effect of hydrogen- rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol 2011; 148:91–5 [DOI] [PubMed] [Google Scholar]
- 8.Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun 2007; 361:670–4 [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Yu G, Liu S, Li J. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res 2011; 167:339–44 [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Sun Y, Hu P, Liu W, Xiang H, Li Y, Yan R, Su N, Ruan C, Sun X, Wang Q. The effects of hydrogen-rich saline on the contractile and structural changes of intestine induced by ischemia-reperfusion in rats. J Surg Res 2011; 167:316–22 [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Xie K, Li J, Xu N, Gong G, Wang G, Yu Y, Dong H, Xiong L. Beneficial effects of hydrogen gas against spinal cord ischemia-reperfusion injury in rabbits. Brain Res 2011; 1378:125–36 [DOI] [PubMed] [Google Scholar]
- 12.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007; 13:688–94 [DOI] [PubMed] [Google Scholar]
- 13.Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, Nakamura N, Kitawaki J, Imai S, Nakano K, Ohta M, Adachi T, Obayashi H, Yoshikawa T. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res 2008; 28:137–43 [DOI] [PubMed] [Google Scholar]
- 14.Kurokawa R, Seo T, Sato B, Hirano S, Sato F. Convenient methods for ingestion of molecular hydrogen: drinking, injection, and inhalation. Med Gas Res 2015; 5:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terawaki H, Zhu W, Matsuyama Y, Terada T, Takahashi Y, Sakurai K, Kabayama S, Miyazaki M, Itami N, Nakazawa R, Ito S, Era S, Nakayama M. Effect of a hydrogen (H2)-enriched solution on the albumin redox of hemodialysis patients. Hemodial Int 2014; 18:459–66 [DOI] [PubMed] [Google Scholar]
- 16.Saitoh Y, Harata Y, Mizuhashi F, Nakajima M, Miwa N. Biological safety of neutral-pH hydrogen-enriched electrolyzed water upon mutagenicity, genotoxicity and subchronic oral toxicity. Toxicol Ind Health 2010; 26:203–16 [DOI] [PubMed] [Google Scholar]
- 17.Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome – an open label pilot study. J Clin Biochem Nutr 2010; 46:140–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohno K, Ito M, Ichihara M, Ito M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid Med Cell Longev 2012; 2012:31–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, Makino S, Ohta S, Ogawa S, Fukuda K. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun 2008; 373:30–5 [DOI] [PubMed] [Google Scholar]
- 20.Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Kaneko K, Nakao A, Imaoka T. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patientsfor safety check with physiological parameters and measurement of blood H2 level. Med Gas Res 2012; 2:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai J, Kang Z, Liu W, Luo X, Qiang S, Zhang J, Ohta S, Sun X, Xu W, Tao H, Li R. Hydrogen therapy reduces apoptosis in neonatal hypoxia–ischemia rat model. Neurosci Lett 2008; 441:167–72 [DOI] [PubMed] [Google Scholar]
- 22.Matchett G, Fathali N, Hasegawa Y, Jadhav V, Ostrowski R, Martin R, Dorotta I, Sun X, Zhang J. Hydrogen gas is ineffective in moderate and severe neonatal hypoxia–ischemia rat models. Brain Res 2009; 1259:90–7 [DOI] [PubMed] [Google Scholar]
- 23.Rice J, Vannucci R, Brierley J. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981; 9:131–41 [DOI] [PubMed] [Google Scholar]
- 24.Vorhees C, Williams M. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1:848–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao M, Zhu P, Fujino M, Zhuang J, Guo H, IdrisAhmed S, Zhao L, Li X-K. Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies. Int J Mol Sci 2016; 17:E2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu M, Lu M, Li Q, Zhang Z, Wu Z, Li J, Qian L, Xu Y, Wang Z. Hyperbaric oxygen suppresses hypoxic-ischemic brain damage in newborn rats. J Child Neurol 2015; 30:75–82 [DOI] [PubMed] [Google Scholar]
- 27.Gould L, Leong M, Mushkudiani I. Hyperbaric oxygen delays healing in an ischemic wound model. J Surg Res 2003; 114:262 [Google Scholar]
- 28.McMonnies C. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin Exp Optom 2015; 98:122–5 [DOI] [PubMed] [Google Scholar]
- 29.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87:779–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y, Xiao X. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti- inflammatory and anti-apoptotic effects in rats. Brain Res 2013; 1491:188–96 [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Wang Y, Xie Y, Yang Z, Zhang T. Protective effects of exogenous hydrogen sulfide on neurons of hippocampus in a rat model of brain ischemia. Neurochem Res 2011; 36:1840–9 [DOI] [PubMed] [Google Scholar]
- 32.Qu K, Chen C, Halliwell B, Moore P, Wong P. Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke 2006; 37:889–93 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Li Y, Song J, Pang H. Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int 2014; 64:37–47 [DOI] [PubMed] [Google Scholar]
- 34.Lassmann H, Schmied M, Vass K, Hickey W. Bone marrow derived elements and resident microglia in brain inflammation. Glia 1993; 7:19–24 [DOI] [PubMed] [Google Scholar]
- 35.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler M, Conway S, Ng L, Stanley E, Samokhvalov I, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330:841–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17:796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen T, Kim Y, Kim T, Le O, Kim J, Kang H, Hasegawa H, Kanaho Y, Jou I, Lee S. Phosphatidylinositol 4-phosphate 5-kinase alpha facilitates Toll-like receptor 4-mediated microglial inflammation through regulation of the Toll/interleukin-1 receptor domain-containing adaptor protein (TIRAP) location. J Biol Chem 2013; 288:5645–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giles J, Greenhalgh A, Davies C, Denes A, Shaw T, Coutts G, Rothwell N, McColl B, Allan S. Requirement for interleukin-1 to drive brain inflammation reveals tissue-specific mechanisms of innate immunity. Eur J Immunol 2015; 45:525–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crews F, Qin L, Sheedy D, Vetreno R, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry 2013; 73:602–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung H, Joo J, Kim D, In J, Roh M, Jeong J, Noh S, Choi J. Effect of milrinone on the inflammatory response and NF-kB activation in renal ischemia-reperfusion injury in mice. Korean J Anesthesiol 2014; 66:136–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian J, Dai H, Deng Y, Zhang J, Li Y, Zhou J, Zhao M, Zhao M, Zhang C, Zhang Y, Wang P, Bing G, Zhao L. The effect of HMGB1 on sub-toxic chlorpyrifos exposure-induced neuroinflammation in amygdala of neonatal rats. Toxicology 2015; 338:95–103 [DOI] [PubMed] [Google Scholar]
- 42.Kimoto H, Eto R, Abe M, Kato H, Araki T. Alterations of glial cells in the mouse hippocampus during postnatal development. Cell Mol Neurobiol 2009; 29:1181–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim E, Choi E. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010; 1802:396–405 [DOI] [PubMed] [Google Scholar]
- 44.Iwayama H, Sakamoto T, Nawa A, Ueda N. Crosstalk between Smad and mitogen-activated protein kinases for the regulation of apoptosis in cyclosporine A-induced renal tubular injury. Nephron Extra 2011; 1:178–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Zhang H, Zhao B, Fei H. IL-1beta caused pancreatic beta-cells apoptosis is mediated in part by endoplasmic reticulum stress via the induction of endoplasmic reticulum Ca2+ release through the c-Jun N-terminal kinase pathway. Mol Cell Biochem 2009; 324:183–90 [DOI] [PubMed] [Google Scholar]
- 46.Vousden K, Prives C. Blinded by the light: the growing complexity of p53. Cell 2009; 137:413–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Hydrogen inhalation protects hypoxic–ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats by Guojiao Wu, Zhiheng Chen, Peipei Wang, Mingyi Zhao, Masayuki Fujino, Chen Zhang, Wenjuan Zhou, Shin-Ichi Hirano, Xiao-Kang Li and Lingling Zhao: on behalf of the Heinz Nixdorf Recall Study Investigative Group in Experimental Biology and Medicine
Supplemental material, Supplemental Material2 for Hydrogen inhalation protects hypoxic–ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats by Guojiao Wu, Zhiheng Chen, Peipei Wang, Mingyi Zhao, Masayuki Fujino, Chen Zhang, Wenjuan Zhou, Shin-Ichi Hirano, Xiao-Kang Li and Lingling Zhao: on behalf of the Heinz Nixdorf Recall Study Investigative Group in Experimental Biology and Medicine
Supplemental material, Supplemental Material3 for Hydrogen inhalation protects hypoxic–ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats by Guojiao Wu, Zhiheng Chen, Peipei Wang, Mingyi Zhao, Masayuki Fujino, Chen Zhang, Wenjuan Zhou, Shin-Ichi Hirano, Xiao-Kang Li and Lingling Zhao: on behalf of the Heinz Nixdorf Recall Study Investigative Group in Experimental Biology and Medicine