Abstract
Purpose
Among younger men, lower body mass is associated with fewer urinary symptoms, including incontinence and nocturia. However, lower body mass may have different implications for older men due to age-associated muscle atrophy and decreased strength.
Materials and Methods
We conducted a prospective analysis of community-dwelling men, aged 70 to 79, in the multicenter Health, Aging, and Body Composition Study who underwent measurement of body mass (physical examination), composition (dual x-ray absorptiometry), and strength (grip and lower leg dynamometry). Associations with prevalent incontinence and nocturia (from structured questionnaires), as well as concurrent changes in urinary symptoms over 3 years was evaluated using multivariate logistic regression.
Results
Of the 1,298 men analyzed, 22% reported incontinence and 52% reported nocturia at baseline. Higher body mass index and fat mass, as well as lower appendicular lean mass, grip and quadriceps strength (corrected for body mass index), were associated with increased prevalence of incontinence (P<0.05 for all). Higher body mass index and fat mass were also associated with increased nocturia prevalence (P<0.05 for all). Concurrent ≥5% decrease in body or fat mass was not associated with lower odds of new or worsening incontinence or nocturia, whereas ≥5% decrease in maximum grip strength was associated with higher odds of new or worsening incontinence.
Conclusions
Older men with higher body mass index and fat mass are more likely to report prevalent incontinence and nocturia. Whereas, late-life decreases in strength, but not increases in body or fat mass, were associated with concurrent increase in urinary incontinence.
Keywords: Lower Urinary Tract Symptoms, Body Mass Index, Body Composition, Muscle Strength (1–5 MESH)
INTRODUCTION
Lower urinary tract symptoms affect over 45% of men over age 65 and can have a major impact on functioning and health-related quality of life.1,2 Higher BMI is associated with increased risk of both male UI3 and nocturia4, but prior studies included few men over age 70, in whom changes in body mass are more complicated5. Understanding the relationship between body mass, composition, strength, and urinary symptoms in older men could provide a pathway to improved prevention and treatment for this high-risk population.
Prior studies have reported higher rates of resolution of UI among overweight men with diabetes enrolled in weight loss interventions6 and among men with severe obesity undergoing bariatric surgery7. However, these studies were conducted in a younger population and benefits for UI were not necessarily accompanied by benefits for other urinary symptoms such as nocturia. Among older men, weight loss can accelerate the replacement of lean mass with fat over time8, which may have different implications for risk of urinary symptoms.
To address this gap, we evaluated associations of body mass, composition, and strength with both prevalent and worsening UI and nocturia among older men in a large, prospective cohort study. We hypothesized that higher BMI, waist circumference, and fat mass, as well as lower ALM and strength, would be associated with an increased prevalence of UI and nocturia. We further hypothesized that concurrent increases in body and fat mass and decreases in strength would be associated with new or worsening UI and nocturia. Our goal was to provide new insights to guide future clinical recommendations regarding weight loss or physical conditioning as potential strategies for improving urinary function among older men.
MATERIALS AND METHODS
Participants
The Health, Aging, and Body Composition Study was a prospective, multicenter study of community-dwelling, black and white men and women, aged 70 to 79 years. Participants without initial difficulty with activities of daily living or mobility were recruited from a random sample of Medicare beneficiaries in 1997, with enhanced community recruitment of black individuals.9
Of the 1,488 men originally enrolled, this analysis includes 1,298 men (87%) who provided complete data on body mass, composition, strength, and urinary symptoms at baseline, and had no history of Parkinson’s disease, stroke, or genitourinary cancer. Longitudinal data were available for 1,005 men (77% of analytic sample); 99 men died, 51 were lost to follow-up for other reasons, and 143 were missing repeated exposure or outcome measurements. This study was approved by the Institutional Review Boards of participating institutions, and all participants provided signed informed consent.
Anthropomorphic Measurements
At baseline and after 3 years, weight was measured with balance beams or digital scales and height was measured with stadiometers9 for calculation of BMI in kg/m2. Waist circumference was measured in cm using a tape measure at the level of largest circumference.
Body Composition Assessment
ALM was measured with whole-body dual-energy X-ray absorptiometry (DEXA; QDR4500A, software version 8.21, Hologic, Waltham, MA) by calculating the sum of lean mass in the arms and legs, excluding fat and bone tissue. ALM was reported in kg as well as corrected for BMI (ALM/BMI) in accordance with the Foundations of the National Institutes of Health guidelines.10 Total fat mass was measured in kg using DEXA and was also expressed as a percentage of total body mass.
Strength Assessment
Maximum grip strength was measured in kg using the average of 2 trials with hand-held dynamometers (JAMAR Technologies, Inc., Hatfield, PA) in the stronger hand.11 Concentric knee extensor torque (quadriceps strength), was measured on the right leg at 60 degrees per second (Kin-Con Isokinetic Dynameter, Chattanooga, TN); the left leg was used if limited by pain or history of joint replacement. Both grip and quadriceps strength were reported as both uncorrected and corrected for BMI, consistent with prior research.10
Urinary Symptom Assessment
UI and nocturia were assessed at baseline and after 3 years using interviewer-administered questionnaires from prior large observational studies12 and the exact questions are included in the Appendix. For baseline analyses, the primary outcomes were 1) prevalence of at least monthly UI of any type, compared to none or less than monthly UI, and 2) prevalence of nocturia, defined as ≥2 voids per night, compared to <2 voids per night. For longitudinal outcomes, new or worsening UI was defined as at least monthly UI among men who reported none or less than monthly UI at baseline, or any increase in frequency of UI among men with at least monthly UI at baseline; new or worsening nocturia was defined as ≥2 voids per night among men who reported <2 voids per night at baseline, or any increase in frequency among men who initially reported ≥2 voids per night at baseline.
Statistical Analysis
We separately examined the association of each body mass, composition, and strength measure, per 1 standard deviation increment, with: 1) prevalent UI, and 2) prevalent nocturia, using multivariate logistic regression. Exposures of interest included BMI (kg/m2), waist circumference (cm), ALM (kg) with and without correction for BMI, total fat mass (kg), percent fat mass, maximum grip strength (kg) with and without correction for BMI, and quadriceps strength (Nm/kg) with and without correction for BMI.
To assess longitudinal associations with new or worsening UI and nocturia, we modeled concurrent changes in body mass, composition, and strength measures as categorical variables using thresholds of percent change based on prior body composition literature.13 We created 3 categories of concurrent change in each exposure: ≥5% decrease, <5% increase or decrease, and ≥5% increase after 3 years of follow-up compared to baseline. Waist circumference was only measured at baseline and therefore was not evaluated in longitudinal analyses. The final adjusted models included age, race, diabetes mellitus, and self-reported general health status. We also assessed but did not include the following variables in final multivariate models because they were not significantly associated with our primary outcomes in bivariate models (P>0.10): education, smoking, alcohol intake, physical activity, and history of coronary artery disease, myocardial infarction, congestive heart failure, and depression.
We conducted sensitivity analyses examining relationships with urgency UI, mixed UI, at least weekly UI, and severe nocturia (≥4 voids/night); stress UI was too rare to evaluate separately in our study population. We also evaluated associations with BMI as a categorical variable to consider non-linear associations and gait speed as an exploratory exposure that is influenced by body mass, composition, and strength. For longitudinal analyses, we conducted sensitivity analyses with a lower threshold of 2% change in each exposure. We also examined whether race modified associations by including a cross-product term in our multivariate models. P<0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
General characteristics of the 1,298 men in the analytic sample are reported in Table 1. At baseline, 20% of men reported UI at least monthly and 11% reported UI at least weekly. Urgency (63%) and mixed UI (30%) were the most common subtypes, whereas stress UI was rare (7%). Almost half of men reported prevalent nocturia (48%), and fewer men reported severe nocturia (11%). Prevalence of UI and nocturia did not vary by race (Supplemental Table 1).
Table 1.
General Characteristics of 1,298 Men at Baseline.
| Demographic and Health-related Characteristics | |
| Age, years, mean ± SD | 73.7 ± 3 |
| Race, n (%) | |
| White | 830 (64) |
| Black | 468 (36) |
| Moderate-Vigorous Physical Activity, METs/week, mean ± SD | 6.0 ± 17 |
| Alcohol Intake ≥8 drinks/week, n (%) | 70 (5) |
| Self-Reported Fair-Poor Health, n (%) | 214 (17) |
| Current Smoking, n (%) | 139 (11) |
| Comorbidities, n (%) | |
| Diabetes Mellitus | 217 (17) |
| Myocardial Infarction | 196 (15) |
| Congestive Heart Failure | 45 (4) |
| Depression | 92 (7) |
| Body Mass, Composition, and Strength Measures (mean ± SD) | |
| Body Mass Index (BMI), kg/m2, mean ± SD and n(%) | 26.9 ± 4 |
| <18.5 | 8 (1) |
| 18.5 to <25 | 406 (31) |
| 25 to <30 | 622 (48) |
| ≥35 | 262 (20) |
| Waist Circumference, cm | 100.6 ± 13 |
| Appendicular Lean Mass, kg | 25.3 ± 4 |
| Appendicular Lean Mass/BMI | 1.0 ± 0.1 |
| Total Fat Mass, kg | 24.0 ± 7 |
| Total Fat Mass, % | 29.1 ± 5 |
| Grip Strength, kg | 40.9 ± 9 |
| Maximum Grip Strength/BMI | 1.5 ± 0.4 |
| Quadriceps Strength, Nm/kg | 131.6 ± 34 |
| Quadriceps Strength/BMI | 4.9 ± 1.3 |
| Baseline Prevalence of Urinary Symptoms | |
| Urinary Incontinence, n (%) | |
| None or less than once per month | 1040 (80) |
| At least monthly | 115 (9) |
| At least weekly | 103 (8) |
| Daily | 30 (3) |
| Subtype of Urinary Incontinence, n (%) | |
| Urgency | 163 (63) |
| Stress | 17 (7) |
| Mixed | 78 (30) |
| Nocturnal Voids, n (%) | |
| 0 | 154 (12) |
| 1 | 516 (40) |
| 2 | 339 (26) |
| 3 | 138 (11) |
| 4 | 58 (4) |
| ≥5 | 86 (7) |
| Missing | 7 (<1) |
Abbreviations: SD Standard Deviation; n Sample Size; BMI Body Mass Index; kg Kilogram; cm Centimeter; Nm Newton Meter.
Associations with UI Prevalence
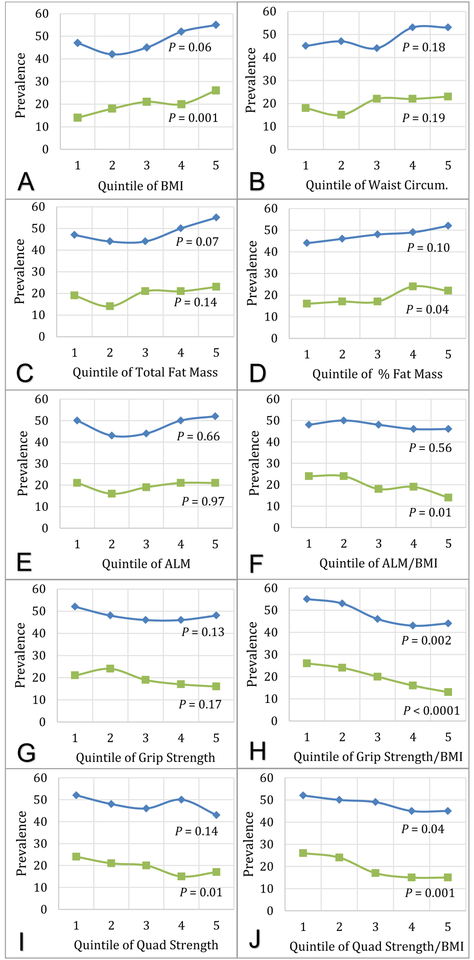
Figure 1 displays the unadjusted prevalence of UI within quintiles of body mass, composition, and strength. Higher BMI and total fat mass were associated with higher odds of prevalent UI, as was lower ALM/BMI (Table 2). When UI subtypes were examined separately, significant associations were observed for urgency UI, but not mixed UI. Higher percent fat mass and waist circumference were borderline associated with prevalent UI at least monthly, but were more strongly associated with increased odds of prevalent UI at least weekly in sensitivity analyses (Supplemental Table 2).
Figure 1.
Baseline Prevalence of Urinary Incontinence and Nocturia among 1,298 men, by quintile of A) Body Mass Index (BMI), B) Waist Circumference, C) Appendicular Lean Mass (ALM), D) ALM/BMI, E) Total Fat Mass, F) % Fat Mass, G) Grip Strength, H) Grip Strength/BMI, I) Quadriceps Strength, and J) Quadriceps Strength/BMI. Blue lines with diamond markers represent Nocturia prevalence. Green lines with square markers indicate Urinary Incontinence prevalence. P value calculated using Mantel-Haenszel Chi-Square test.
Table 2.
Cross-sectional Associations of Body Mass, Composition, and Strength Measures With Urinary Incontinence among 1,298 Men, by Subtype.
| Any Urinary Incontinence | Urgency Urinary Incontinence | Mixed Urinary Incontinence | ||||
|---|---|---|---|---|---|---|
| No. of Cases/Total | OR (95% CI)* | No. of Cases/Total | OR (95% CI)* | No. of Cases/Total | OR (95% CI)* | |
| Body Mass Index, kg/m2 | 258/1298 | 163/1203 | 78/1118 | |||
| Age/Race-Adjusted Model | 1.36 (1.16, 1.63)‡ | 1.49 (1.21, 1.83)‡ | 1.23 (0.93, 1.64) | |||
| Fully Adjusted Model | 1.37 (1.15, 1.63)‡ | 1.49 (1.21, 1.83)‡ | 1.28 (0.96, 1.69) | |||
| Waist Circumference, cm | 257/1293 | 163/1199 | 77/1113 | |||
| Age/Race-Adjusted Model | 1.11 (0.97, 1.27) | 1.17 (1.00, 1.38)† | 1.16 (0.94, 1.43) | |||
| Fully Adjusted Model | 1.10 (0.96, 1.26) | 1.15 (0.98, 1.35) | 1.18 (0.95, 1.46) | |||
| Appendicular Lean Mass, kg | 237/1208 | 149/1120 | 74/1045 | |||
| Age/Race-Adjusted Model | 1.10 (0.95, 1.28) | 1.18 (0.99, 1.42) | 1.00 (0.78, 1.29) | |||
| Fully Adjusted Model | 1.11 (0.96, 1.29) | 1.19 (0.99, 1.42) | 1.04 (0.81, 1.33) | |||
| Appendicular Lean Mass/BMI | 237/1208 | 149/1120 | 74/1045 | |||
| Age/Race-Adjusted Model | 0.80 (0.69, 0.94)‡ | 0.75 (0.63, 0.91)‡ | 0.88 (0.69, 1.12) | |||
| Fully Adjusted Model | 0.81 (0.69, 0.95)† | 0.78 (0.64, 0.95)† | 0.87 (0.68, 1.13) | |||
| Total Fat Mass, kg | 253/1289 | 161/1197 | 76/1112 | |||
| Age/Race-Adjusted Model | 1.16 (1.01, 1.33)† | 1.23 (1.05, 1.45)† | 1.10 (0.87, 1.38) | |||
| Fully Adjusted Model | 1.15 (1.00, 1.32)† | 1.21 (1.03, 1.43)† | 1.10 (0.87, 1.38) | |||
| Total Fat Mass, % | 244/1256 | 153/1165 | 76/1088 | |||
| Age/Race-Adjusted Model | 1.14 (0.99, 1.32) | 1.15 (1.00, 1.32)† | 1.10 (0.87, 1.39) | |||
| Fully Adjusted Model | 1.13 (0.98, 1.30) | 1.16 (0.98, 1.38) | 1.09 (0.86, 1.38) | |||
| Maximum Grip Strength, kg | 252/1281 | 158/1187 | 77/1106 | |||
| Age/Race-Adjusted Model | 0.93 (0.81, 1.08) | 0.90 (0.75, 1.07) | 1.01 (0.79, 1.28) | |||
| Fully Adjusted Model | 0.95 (0.82, 1.10) | 0.91 (0.76, 1.10) | 1.04 (0.82, 1.33) | |||
| Maximum Grip Strength/BMI | 252/1281 | 158/1187 | 77/1106 | |||
| Age/Race-Adjusted Model | 0.81 (0.70, 0.94)‡ | 0.74 (0.61, 0.88)‡ | 0.91 (0.72, 1.16) | |||
| Fully Adjusted Model | 0.82 (0.70, 0.95)† | 0.75 (0.62, 0.91)‡ | 0.91 (0.71, 1.16) | |||
| Quadriceps Strength, Nm/kg | 224/1152 | 141/1069 | 68/996 | |||
| Age/Race-Adjusted Model | 0.88 (0.76, 1.02) | 0.86 (0.72, 1.04) | 0.92 (0.71, 1.18) | |||
| Fully Adjusted Model | 0.89 (0.76, 1.03) | 0.87 (0.72, 1.05) | 0.93 (0.72, 1.21) | |||
| Quadriceps Strength/BMI | 224/1152 | 141/1069 | 68/996 | |||
| Age/Race-Adjusted Model | 0.77 (0.66, 0.90)‡ | 0.70 (0.58, 0.85)‡ | 0.88 (0.68, 1.13) | |||
| Fully Adjusted Model | 0.77 (0.66, 0.90)‡ | 0.71 (0.58, 0.86)‡ | 0.87 (0.67, 1.14) | |||
Abbreviations: BMI Body Mass Index; OR Odds Ratio; CI Confidence Interval; kg Kilogram; cm Centimeter; Nm Newton Meter.
Odds ratios were calculated per 1 standard deviation increment, except BMI which was per 5 kg/m2. Fully adjusted logistic models includes age, race, diabetes mellitus, and self-reported general health status.
P < 0.05
P < 0.005
Greater grip strength/BMI and quadriceps strength/BMI were both associated with lower odds of prevalent UI (Table 2); when separated by UI subtype, associations were limited to urgency UI. However, grip and quadriceps strength uncorrected for BMI were not associated with prevalent UI.
Associations with Nocturia Prevalence
Unadjusted nocturia prevalence varied within categories of grip strength/BMI and quadriceps strength/BMI (P < 0.05 for both; Figure 1). In fully adjusted models, men with higher BMI and fat mass (both total and percent) were more likely to report prevalent nocturia (Table 3). No significant associations were observed between ALM, ALM/BMI, or waist circumference and prevalent nocturia. Sensitivity analyses examining associations with severe nocturia (≥4 voids/night) demonstrated similar effect estimates but confidence intervals were wider (Supplemental Table 2).
Table 3.
Cross-sectional Associations of Body Mass, Composition, and Strength Measures With Nocturia among 1,298 Men.
| Nocturia | ||
|---|---|---|
| No. of Cases/Total | OR (95% CI)* | |
| Body Mass Index, kg/m2 | 630/1307 | |
| Age/Race-Adjusted Model | 1.28 (1.11, 1.47)‡ | |
| Fully Adjusted Model | 1.26 (1.09, 1.46)‡ | |
| Waist Circumference, cm | 629/1302 | |
| Age/Race-Adjusted Model | 1.12 (1.00, 1.26)† | |
| Fully Adjusted Model | 1.10 (0.98, 1.23) | |
| Appendicular Lean Mass, kg | 580/1217 | |
| Age/Race-Adjusted Model | 1.11 (0.99, 1.25) | |
| Fully Adjusted Model | 1.12 (0.99, 1.26) | |
| Appendicular Lean Mass/BMI | 580/1217 | |
| Age/Race-Adjusted Model | 0.90 (0.79, 1.01) | |
| Fully Adjusted Model | 0.92 (0.81, 1.04) | |
| Total Fat Mass, kg | 624/1298 | |
| Age/Race-Adjusted Model | 1.20 (1.08, 1.35)‡ | |
| Fully Adjusted Model | 1.19 (1.06, 1.33)‡ | |
| Total Fat Mass, % | 605/1265 | |
| Age/Race-Adjusted Model | 1.16 (1.04, 1.30)† | |
| Fully Adjusted Model | 1.15 (1.02, 1.29)† | |
| Maximum Grip Strength, kg | 620/1289 | |
| Age/Race-Adjusted Model | 0.94 (0.84, 1.06) | |
| Fully Adjusted Model | 0.98 (0.88, 1.10) | |
| Maximum Grip Strength/BMI | 620/1289 | |
| Age/Race-Adjusted Model | 0.86 (0.77, 0.96)† | |
| Fully Adjusted Model | 0.89 (0.79, 1.00) | |
| Quadriceps Strength, Nm/kg | 552/1159 | |
| Age/Race-Adjusted Model | 0.97 (0.86, 1.09) | |
| Fully Adjusted Model | 1.01 (0.89, 1.14) | |
| Quadriceps Strength/BMI | 552/1159 | |
| Age/Race-Adjusted Model | 0.88 (0.78, 1.00)† | |
| Fully Adjusted Model | 0.92 (0.81, 1.05) | |
Abbreviations: BMI Body Mass Index; OR Odds Ratio; CI Confidence Interval; kg Kilogram; cm Centimeter; Nm Newton Meter.
Odds ratios were calculated per 1 standard deviation increment, except BMI which was per 5 kg/m2. Fully adjusted logistic models includes age, race, diabetes mellitus, and self-reported general health status.
P < 0.05
P < 0.005
Maximum grip strength and quadriceps strength were not associated with odds of prevalent nocturia (Table 3). In sensitivity analyses, higher maximum grip strength with and without correction for BMI was modestly associated with lower odds of severe nocturia (Supplemental Table 2).
Prospective Associations with UI and Nocturia over 3 years
We observed a different pattern of associations between changes in body mass, composition, or strength measures and concurrent changes in UI or nocturia over 3 years (Table 4). Older men with 5% or greater decrease in maximum grip strength had greater odds of concurrent new or worsening UI. Conversely, men with 5% or greater increase in BMI were less likely to report new or worsening nocturia. These associations were in the same direction, but attenuated and with larger confidence intervals, in sensitivity analyses using a smaller threshold of 2% change in BMI (Supplemental Table 3).
Table 4.
Multivariate Associations of 3-Year Changes in Body Mass, Composition, and Strength Measures With Concurrent Changes in Urinary Incontinence and Nocturia.
| New or Worsening UI† | New or Worsening Nocturia‡ | |||
|---|---|---|---|---|
| No. of Events/Total | AOR (95% CI)* | No. of Events/Total | AOR (95% CI)* | |
| Body Mass Index, kg/m2 | ||||
| ≥5% decrease | 118/737 | 0.72 (0.43, 1.21) | 170/744 | 0.97 (0.65, 1.47) |
| <5% decrease or increase | 20/160 | Reference | 37/162 | Reference |
| ≥5% increase | 17/108 | 0.98 (0.56, 1.72) | 15/111 | 0.51 (0.30, 0.91)§ |
| Appendicular Lean Mass, kg | ||||
| ≥5% decrease | 91/564 | 0.89 (0.60, 1.33) | 121/570 | 1.12 (0.80, 1.57) |
| <5% decrease or increase | 43/300 | Reference | 72/304 | Reference |
| ≥5% increase | 5/25 | 1.27 (0.46, 3.5) | 3/26 | 0.45 (0.13, 1.54) |
| Appendicular Lean Mass/BMI | ||||
| ≥5% decrease | 91/580 | 0.98 (0.66, 1.45) | 127/583 | 0.98 (0.70, 1.38) |
| <5% decrease or increase | 45/290 | Reference | 65/297 | Reference |
| ≥5% increase | 3/19 | 0.94 (0.26, 3.5) | 4/20 | 0.83 (0.27, 2.6) |
| Total Fat, kg | ||||
| ≥5% decrease | 66/400 | 0.89 (0.57, 1.38) | 87/403 | 1.11 (0.76, 1.63) |
| <5% decrease or increase | 37/239 | Reference | 58/245 | Reference |
| ≥5% increase | 49/347 | 0.82 (0.55, 1.23) | 68/350 | 0.87 (0.61, 1.25) |
| Total Fat, % | ||||
| ≥5% decrease | 76/505 | 0.79 (0.45, 1.38) | 107/510 | 1.17 (0.75, 1.83) |
| <5% decrease or increase | 18/139 | Reference | 34/142 | Reference |
| ≥5% increase | 50/294 | 1.14 (0.77, 1.69) | 66/298 | 1.08 (0.76, 1.53) |
| Maximum Grip Strength, kg | ||||
| ≥5% decrease | 29/261 | 1.64 (1.04, 2.60)§ | 58/266 | 1.08 (0.76, 1.55) |
| <5% decrease or increase | 88/512 | Reference | 122/517 | Reference |
| ≥5% increase | 35/218 | 1.46 (0.85, 2.50) | 37/219 | 0.70 (0.44, 1.11) |
| Maximum Grip Strength/BMI | ||||
| ≥5% decrease | 33/253 | 1.36 (0.88, 2.10) | 60/260 | 0.92 (0.64, 1.32) |
| <5% decrease or increase | 83/500 | Reference | 112/504 | Reference |
| ≥5% increase | 35/236 | 1.16 (0.69, 1.94) | 44/236 | 0.74 (0.48, 1.15) |
| Quadriceps Strength, Nm/kg | ||||
| ≥5% decrease | 19/136 | 1.02 (0.60, 1.76) | 32/135 | 0.93 (0.60, 1.46) |
| <5% decrease or increase | 85/575 | Reference | 131/582 | Reference |
| ≥5% increase | 23/125 | 1.35 (0.69, 2.60) | 19/127 | 0.57 (0.30, 1.08) |
| Quadriceps Strength/BMI | ||||
| ≥5% decrease | 18/143 | 1.17 (0.67, 2.00) | 28/144 | 1.24 (0.78, 1.96) |
| <5% decrease or increase | 83/569 | Reference | 133/575 | Reference |
| ≥5% increase | 26/124 | 1.84 (0.95, 3.60) | 21/125 | 0.84 (0.45, 1.57) |
Abbreviations: UI Urinary Incontinence; BMI Body Mass Index; AOR Adjusted Odds Ratio; CI Confidence Interval; kg Kilogram; cm Centimeter; Nm Newton Meter.
Adjusted odds ratios were calculated using multivariate logistic regression models age, race, diabetes mellitus, and self-reported general health status.
New or worsening urinary incontinence was defined as at least monthly urinary incontinence among men who reported none at baseline or an increase in frequency among men with any incontinence at baseline.
New or worsening nocturia was defined as two or more voids per night among men who reported less than two voids per night or an increase in frequency to four or more voids per night among men with nocturia at baseline.
P < 0.05.
There was no evidence for effect modification of associations reported above by race (P>0.05 for all except for quadriceps strength and UI which was P = 0.03). In exploratory analyses, gait speed was not associated with prevalence of or concurrent change in UI or nocturia (Supplemental Table 4).
DISCUSSION
In this multicenter, prospective cohort study, we found that older men with lower BMI and fat mass were less likely to report prevalent UI and nocturia. Appendicular lean mass and strength measures were also associated with UI, but only after correction for BMI. We did not detect a consistent pattern of associations between concurrent changes in body mass, composition, or strength with new or worsening UI or nocturia, although we did observe an increased risk of new or worsening UI among men with concurrent decreases in grip strength. This study provides additional evidence that higher body mass and fat are associated with symptomatic urinary tract dysfunction among older men, similar to younger men, and that older men with declining strength, rather than increasing body or fat mass, may have higher risk of UI.
Few prior studies have evaluated the association between BMI and UI prevalence among men over age 70.14–17 Evidence from randomized controlled trials suggests that younger overweight and obese men who lose weight via lifestyle or surgical interventions experience reduced UI.6,7 In our study, older men with higher BMI and fat mass were more likely to report prevalent UI, but we did not observe similar improvements in UI with concurrent decreases in BMI or fat mass. ALM/BMI was inversely associated with prevalent UI, but neither ALM without BMI correction nor waist circumference were independently associated with prevalent UI. Our findings therefore suggest that the association between BMI and UI in older men might be primarily driven by higher fat mass, rather than lower ALM or isolated central adiposity.
To our knowledge, this is the first examination of associations between strength and urinary symptoms in older men. We observed inverse associations between both grip and quadriceps strength measures and prevalent UI, although these associated were not observed without BMI correction. In longitudinal models, men with 5% or greater decrease in maximum grip strength corrected for BMI were more likely to report concurrent new or worsening UI after 3 years of follow-up. Given the inconsistent associations with and without BMI correction, and since both grip and quadriceps strength are strongly correlated with BMI18, it remains unknown if these relationships are predominantly driven by BMI. Strength measures were not associated with nocturia. Although confirmation is needed, these findings provide preliminary evidence that community-dwelling older men who maintain higher grip strength may be at lower risk of new or worsening UI.
Our results are consistent with prior cross-sectional studies demonstrating a lower prevalence of nocturia among younger men with lower BMI19–22, as well as a small number of studies conducted among older men4. However, our study is also consistent with prior research suggesting that weight loss is not consistently associated with improvements in nocturia.6 In fact, we observed that older men with 5% or greater increase in BMI were less likely to report new or worsening nocturia, although this isolated finding needs to be confirmed. We also report a novel association between higher fat mass and prevalent nocturia. Since higher weight is associated with increased fat mass as well as lean body mass in older men8, this finding provides preliminary evidence that the association between BMI and nocturia may be driven by higher fat mass rather than BMI alone but that older men with late-life increases in BMI are not more likely to experience new or worsening nocturia.
Several possible mechanisms may explain these associations of body mass, composition, and strength with urinary symptoms among older men. Obesity and increased adiposity may represent a non-specific inflammatory state, in which oxidative stress, bladder ischemia and fibrosis, and pro-inflammatory cytokines lead to detrusor muscle instability and urgency symptoms.23 Prior studies have also suggested that obesity, particularly central adiposity as measured by waist circumference, leads to urinary symptoms via increased abdominal pressure transmitted to the bladder12, however our findings do not support this hypothesis. Older men who maintain greater body mass, muscle mass, and strength may have stronger pelvic floor muscles and a functional urethral sphincter, both of which can deteriorate with age and lead to UI.24 However, changes in body mass or composition may have limited effects on urinary symptoms once men reach older age, since changes to the bladder or prostate may become permanent rather than reversible with weight loss.25
We recognize several limitations to our study. This is an observational study with potential for unmeasured confounding, such as unintentional weight loss due to underlying malignancy or chronic disease progression, although we did adjust for confounding from known comorbidities. This study also had limited power to evaluated small effect sizes for longitudinal associations because a minority of men experienced significant change in body composition or strength measures during the 3 years of follow-up. It is possible that longer duration of follow-up is needed to evaluate these longitudinal relationships. Lastly, we were unable to validate our findings using patient-reported outcomes with other objective measures such as voiding diaries or pad tests.
CONCLUSIONS
Our study suggests that older men with lower body mass and fat mass are less likely to report prevalent UI and nocturia. Although concurrent decreases in body mass and fat mass were not associated with meaningful decreases in UI or nocturia, older men who avoided declines in grip strength were less likely to develop new or worsening nocturia. These findings suggest that variations in fat mass, and perhaps strength, may be as important as BMI in determining susceptibility to male lower urinary tract symptoms.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Bauer was supported by grant 1K12DK111028 from the National Institute of Diabetes, Digestive, and Kidney Disorders. This research was supported by NIA Contracts N01-AG-6–2101; N01-AG-6–2103; N01-AG-6–2106; NIA Grant R01-AG028050; and National Institute of Nursing Research Grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. Dr. Huang has had research funding from Pfizer, Inc. and Astellas Pharma through grants awarded to the University of California to conduct research unrelated to this report.
Footnotes
Conflict of Interest:
Dr. Huang has had research funding from Pfizer, Inc. and Astellas Pharma through grants awarded to the University of California to conduct research unrelated to this report.
REFERENCES
- 1.Taylor BC, Wilt TJ, Fink HA, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006;68(4):804–809. [DOI] [PubMed] [Google Scholar]
- 2.Holroyd-Leduc JM, Mehta KM, Covinsky KE. Urinary incontinence and its association with death, nursing home admission, and functional decline. Journal of the American Geriatrics Society. 2004;52(5):712–718. [DOI] [PubMed] [Google Scholar]
- 3.Tsui A, Kuh D, Cardozo L, Davis D. Vascular risk factors for male and female urgency urinary incontinence at age 68 years from a British birth cohort study. BJU international. 2018;122(1):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirayama A, Torimoto K, Mastusita C, et al. Evaluation of factors influencing the natural history of nocturia in elderly subjects: results of the Fujiwara-kyo Study. The Journal of urology. 2013;189(3):980–986. [DOI] [PubMed] [Google Scholar]
- 5.Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Archives of internal medicine. 2005;165(9):1035–1040. [DOI] [PubMed] [Google Scholar]
- 6.Breyer BN, Phelan S, Hogan PE, et al. Intensive lifestyle intervention reduces urinary incontinence in overweight/obese men with type 2 diabetes: results from the Look AHEAD trial. The Journal of urology. 2014;192(1):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subak LL, King WC, Belle SH, et al. Urinary Incontinence Before and After Bariatric Surgery. JAMA internal medicine. 2015;175(8):1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. The American journal of clinical nutrition. 2005;82(4):872–878; quiz 915–876. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2003;51(3):323–330. [DOI] [PubMed] [Google Scholar]
- 10.McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(5):576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. Journal of the American Geriatrics Society. 2010;58(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suskind AM, Cawthon PM, Nakagawa S, et al. Urinary Incontinence in Older Women: The Role of Body Composition and Muscle Strength: From the Health, Aging, and Body Composition Study. Journal of the American Geriatrics Society. 2017;65(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes care. 2011;34(7):1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goode PS, Burgio KL, Redden DT, et al. Population based study of incidence and predictors of urinary incontinence in black and white older adults. The Journal of urology. 2008;179(4):1449–1453; discussion 1453–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bortolotti A, Bernardini B, Colli E, et al. Prevalence and risk factors for urinary incontinence in Italy. European urology. 2000;37(1):30–35. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly DW, Donnelly C, Kearney T, et al. Urinary, bowel and sexual health in older men from Northern Ireland. BJU international. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim IH, Chun H, Kwon JW. Gender differences in the effect of obesity on chronic diseases among the elderly Koreans. Journal of Korean medical science. 2011;26(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomlinson DJ, Erskine RM, Morse CI, Winwood K, Onambele-Pearson G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. 2016;17(3):467–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgio KL, Johnson TM, 2nd, Goode PS, et al. Prevalence and correlates of nocturia in community-dwelling older adults. Journal of the American Geriatrics Society. 2010;58(5):861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madhu C, Coyne K, Hashim H, Chapple C, Milsom I, Kopp Z. Nocturia: risk factors and associated comorbidities; findings from the EpiLUTS study. International journal of clinical practice. 2015;69(12):1508–1516. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa S, Sakai T, Niiya T, et al. Obesity and the prevalence of nocturia in Japanese elderly patients with type 2 diabetes mellitus: The Dogo study. Geriatrics & gerontology international. 2017;17(12):2460–2465. [DOI] [PubMed] [Google Scholar]
- 22.Shiri R, Hakama M, Hakkinen J, et al. The effects of lifestyle factors on the incidence of nocturia. The Journal of urology. 2008;180(5):2059–2062. [DOI] [PubMed] [Google Scholar]
- 23.Nomiya M, Sagawa K, Yazaki J, et al. Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis-induced chronic bladder ischemia. Neurourology and urodynamics. 2012;31(1):185–189. [DOI] [PubMed] [Google Scholar]
- 24.Chung E, Katz DJ, Love C. Adult male stress and urge urinary incontinence - A review of pathophysiology and treatment strategies for voiding dysfunction in men. Australian family physician. 2017;46(9):661–666. [PubMed] [Google Scholar]
- 25.Suskind AM. Frailty and Lower Urinary Tract Symptoms. Current urology reports. 2017;18(9):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.